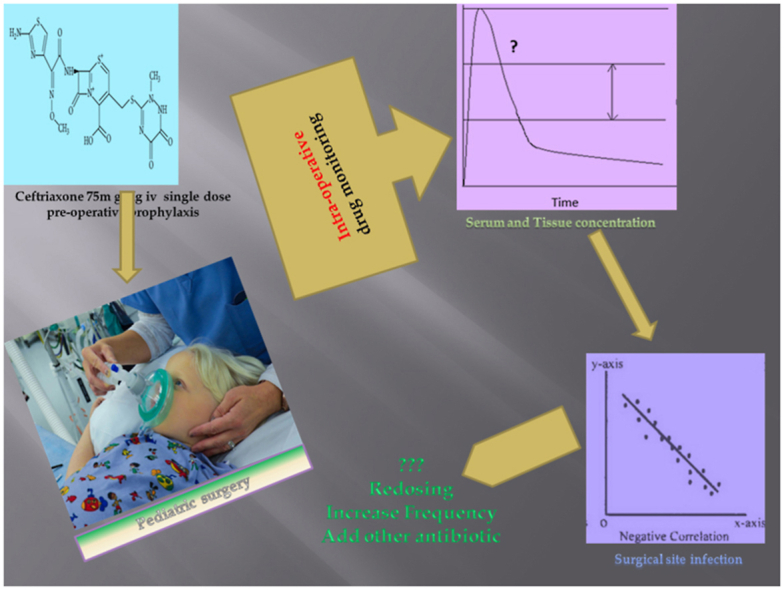
Abstract
Studies have determined the serum concentration of ceftriaxone in the adult population, but there are only a few studies that measured the tissue concentrations. However, no studies have concurrently evaluated the serum and tissue concentrations of ceftrixaone in elective pediatric surgery patients. Therefore, our study was planned to evaluate the serum and tissue concentrations of single dose intravenous prophylactic ceftriaxone intra-operatively during an ongoing pediatric surgery and the outcome of surgical-site infections (SSIs). We did a correlation analysis to determine the relationship of various concentrations and surgery related risk factors with the outcome of SSIs. It was an open label prospective study in 50 patients who underwent elective pediatric surgery under prophylactic cover of ceftriaxone. Serum and tissue concentration were estimated by High Pressure Liquid Chromatography (HPLC). Subjects were observed for post operative complications including SSI. Serum and tissue concentrations of ceftriaxone were significant at test value of 4 mg/L. Tissue concentrations of ceftriaxone at incision (p = 0.02) and closure (p = 0.04) were significantly correlated with SSI but there was no significant association. The measured serum ceftriaxone concentrations were more than 20 times the susceptible minimum inhibitory concentration (MIC) at any given point of the surgery. On the other hand, this target level was achieved at the tissue levels in the majority of the patient. The factors associated with SSI were duration of surgery, wound category of contaminated clean type, the use of urinary catheter and implants in the surgery. An intra-operative re-dose, extension of dose or addition of another antibiotic may be considered for such patients.
Keywords: Antibiotic prophylaxis, Ceftriaxone, Pediatric surgery, Surgical site infection
Graphical abstract
Highlights
-
•
Currently, no standard guidelines are available for the pre-operative prophylactic antibiotic use in pediatric surgery.
-
•
This study addresses the issue about antibiotic dose and frequency in context of elective pediatric surgery patients.
-
•
The tissue concentration of ceftriaxone at incision and at closure of surgery are important predictors for SSI.
-
•
For optimizing the antibiotic use and re-dosing in SSI prophylaxis, tissue concentration of the antibiotic should be preferred over the plasma concentrations.
1. Introduction
SSIs are the most frequently associated nosocomial infections in the surgical patients, ranging from 2.5 to 20% of the surgical pediatric patients (Uludag et al., 2000; Davenport and Doig, 1993; Bhattacharyya and Kosloske, 1990; Sharma and Sharma, 1986; Davis et al., 1984; Doig and Wilkinson, 1976; Bhattacharyya et al., 1993). The risk factors for SSIs are generally derived from adult patients (Haley et al., 1985; Gaynes et al., 2001). There are limited studies identifying the association of risk factors in the pediatric population (Bucher et al., 2011; Casanova et al., 2006a). Surgery-related events or procedures are more closely related with development of SSIs than the physiological state of the child per se (Horwitz et al., 1998). In spite of contradictory views, one of the widely accepted intervention for preventing SSIs is the use of perioperative antibiotic prophylaxis (Bratzler and Hunt, 2006; Bratzler and Houck, 2005; Stulberg et al., 2010).
No specific guidelines are available for prophylactic antibiotic use in pediatric surgeries, due to which the choice of anti-microbial, dose, timing and frequency are based mainly on the guidelines for adult patients and the surgeon's experience (Berríos-Torres et al., 2017; Allegranzi et al., 2016; Scottish Intercollegiate, 2008; Ban et al., 2017; Anderson et al., 2014; Bratzler et al., 2013; Hawn et al., 2013; Steinberg et al., 2009; van Kasteren et al., 2007; Classen et al., 1992). Third generation cephalosporin, ceftriaxone is used preferentially because of its easy availability, intravenous route, relatively good safety profile, broad-spectrum and long elimination half-life with a time-dependent effect as per the concentration in the blood (Salkind and Rao, 2011; Brunton et al., 2018; Salim et al., 2021).
The objective for administration of prophylactic antibiotic is to achieve an optimal concentration of the drug throughout the surgery in the plasma and at the surgical site as well (Tarchini et al., 2017). Tissue concentrations are more direct measure of the pharmacodynamic effect of the drug than the plasma concentrations (Liu and Derendorf, 2003). Several studies have determined the serum concentration of ceftriaxone in adult population, but there are only few studies that measured the tissue concentrations (Kundra et al., 2018; Leone et al., 2003; Martin et al., 1992; Shinagawa, 1989). Simultaneous determination of the serum and the tissue concentration of ceftriaxone had not been done in elective pediatric surgery patients. Therefore, our study was planned to evaluate the serum and tissue concentrations of single dose intravenous prophylactic ceftriaxone intra-operatively during an ongoing pediatric surgery and the outcome of SSIs.
2. Material and methods
2.1. Ethical considerations
The study was conducted after obtaining ethical clearance from the Institutional Ethics Committee of the Safdarjung Hospital, New Delhi. Our study conformed to the Helsinki Declaration of 1975, revised in 2000. The ethical code followed was the Indian Council of Medical Research (ICMR) National Ethical Guidelines for Biomedical Research on human participants 2006, updated in 2017. A written informed consent was obtained from the legally acceptable representative of the study participants.
2.2. Study settings
50 consecutive subjects were recruited from the pediatric ward of a tertiary care teaching hospital, New Delhi. It was an open label prospective study in consecutive patients of pediatric surgery under the prophylactic cover of ceftriaxone.
2.3. Drug
All participants received single dose of 75 mg per kilogram body weight upto a maximum dose of 1000 mg of ceftriaxone (Monocef, Aristo Pharmaceuticals, India) in 10 ml saline intravenously over 5 min, after induction.
2.4. Inclusion criteria
Patients of either sex of less than 12 years of age, planned for surgery and whose legally acceptable representative gave written informed consent.
2.5. Exclusion criteria
Those with any clinically significant renal, hepatic, or cardiac disease, any clinical or laboratory sign of infection, history of allergy to beta lactams/cephalosporins and having received any antibiotic in the previous 1 week were excluded from the study.
2.6. Blood and tissue sampling
3 venous blood samples of 1 ml each per patient were collected for drug concentration estimation from the anti-cubital vein. First blood sample was taken after the start of injection ceftriaxone (t = 0) at the time of taking incision (8.8 ± 2.1 min). Second sample was taken at the mid way of surgery as per experience of the operating surgeon (50.6 ± 13.7 min). Third sample was taken at the time of closure of the surgical wound (104.6 ± 25.94 min). Additionally, 3 more tissue (fat) samples from the incision site, the surgical site (during the surgery) and the suturing site (at the time of closure of the wound) were collected. Tissue samples were taken simultaneously along with the blood samples.
2.7. Drug estimation
Serum concentration of ceftriaxone was measured via an isocratic high performance liquid chromatography (Waters Corporation, Milford, Massachusetts) assay, as described earlier (Martin et al., 1992). The separation was done on a spherisorb C18, 5 μm, 250 mm analytical column (Waters Corporation, Milford, Massachusetts). The mobile phase comprised of a mixture of acetonitrile (Fisher Scientific, Loughborough, England), hexadecyl-trimethylammonium bromide (0.4 g) (Fluka Laboratories), buffer (pH 7.0) (titrisol) (Merck, Darmstadt, Germany) and HPLC-grade water (50:5:45 ml). Probenecid (Merck, Darmstadt, Germany) served as the internal standard. Ceftriaxone concentration in the tissue sample was done after adequate pretreatment of the sample. Sample was crushed and then homogenized in the magnetic sonificator for 5 min. The 100 mg sample and 1 ml of normal saline was centrifuged for 10 min, supernatant was used for ceftriaxone assay. The mixture of the sample (0.1 ml), water (0.3 ml) and internal standard (2 μl, 200 μg/ml) in methanol was vortexed vigorously for 5 min and then centrifuged. HPLC column was injected with 25 μl of the supernatant. The UV spectrophotometer detector was set at wavelength 254 nm. The flow rate was 1 ml per minute and the retention time was 10 min. Calibration curves were prepared with standard solutions of ceftriaxone (Sigma-Aldrich, St. Louis, Missouri). The assay response was linear between 1 and 200 mg/L with ‘r2’ value more than 0.98. The accuracy of quality control samples varied from 94% to 104% and the intra-day and inter-day coefficients of variation varied from 1% to 6%. Samples were analyzed on the same day of collection.
2.8. Data collection
Various concentrations of ceftriaxone were evaluated with the outcome of SSIs and procedure related factors. The factors evaluated were age less than 5 years, American Society of Anaesthesiologists (ASA) class 1 and 2, wounds classification (clean or clean, contaminated), use of other medical/surgical material (urinary catheter, intravenous cannula, implants or drains) and pre hospital stay.
2.9. Statistical analysis
Data was analyzed using SPSS version 26 (IBM Corporation, New York, USA). Continuous data was presented as the mean with standard deviation, unless specified. One sample Student's t-test was performed on various serum and tissue concentrations of ceftriaxone at the test value of 4 mg/L. We conducted correlation between the serum and tissue concentrations and the patient characteristics. Categorical and continuous variables in the outcome (SSI or No SSI) groups were compared by the Fisher's exact test and the Mann-Whitney test, respectively. Correlation tests were conducted with serum and tissue concentrations of ceftriaxone and patient characteristics as independent variables, and SSI as the dependent variable. We also analyzed the association of the SSI with each individual predictable variable and various concentrations of the drug by binary logistic regression. P < 0.05 was considered to be statistically significant. The figures were created using R platform (R Core Team, 2021).
3. Results
The mean age of the study group was 6.1 years, the youngest being 2 years and the oldest 12 years. Twenty-nine patients were male, and rests twenty one were female. The average weight of the patients was 24.4 kgs with the range between 9 kgs and 39 kgs. The mean for the height of the patients were 1.01 m ranging from 0.60 m to 1.38 m. Patients were categorized and grouped as per high risk and surgical events (Table 1).
Table 1.
Patients characteristics and surgical procedure related factors. Yrs-years, Kg-kilograms, min-minutes & ASA- American Society of Anaesthesiology.
| Mean ± SD | ||
|---|---|---|
| Age (yrs) | 6.1 ± 3.06 | |
| Weight (kg) | 24.4 ± 8.18 | |
| Height (meters) | 1.01 ± 0.17 | |
| Duration of Surgery (min) |
104.6 ± 25.94 |
|
| Number |
||
| Sex | Female | 21 |
| Male | 29 | |
| Age | <5 years | 18 |
| >5 years | 32 | |
| ASA Classification | Class 1 | 28 |
| Class 2 | 22 | |
| Wound Category | Clean | 33 |
| Clean contaminated | 17 | |
| Use of medical equipment | Urinary Catheter | 16 |
| IV Cannula | 14 | |
| Drain | 11 | |
| Implants | 7 | |
| Pre-surgery hospital stay | 37 | |
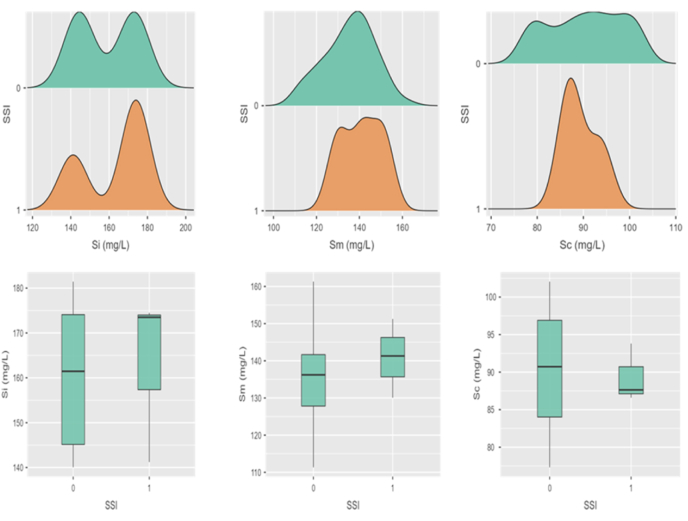
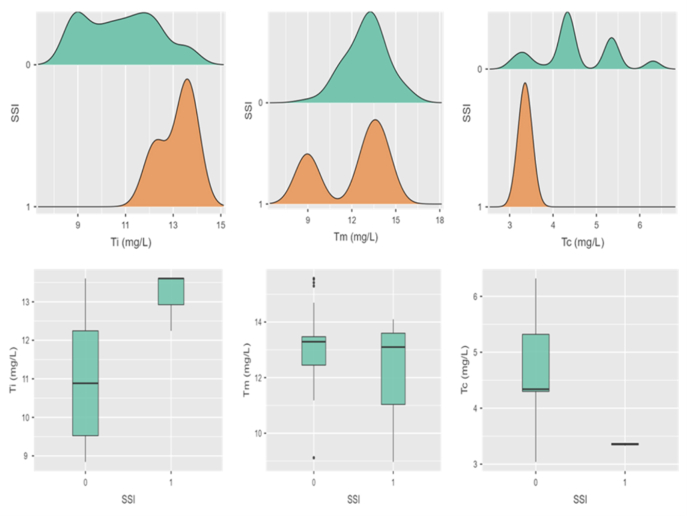
The mean serum concentration of ceftriaxone at incision (Si), midway (Sm) and closure (Sc) was 159.1 ± 14.8, 135.7 ± 11.5 and 90.3 ± 7.7 mg/L respectively; whereas corresponding mean tissue concentration (Ti, Tm & Tc) was 10.9 ± 1.5, 12.9 ± 1.4 and 4.4 ± 0.8 mg/L (Fig. 1, Fig. 2)
Fig. 1.
The density distribution and box plots of serum concentrations in outcome groups (0 = absence & 1 = presence of SSI). Si, Sm & Sc are concentrations of ceftriaxone at incision, midway and at closure respectively. mg/L- Milligram per litre.
Fig. 2.
The density distribution and box plots of tissue concentrations in outcome groups (0 = absence & 1 = presence of SSI). Ti, Tm & Tc are concentrations of ceftriaxone at incision, midway and at closure respectively. mg/L- Milligram per litre.
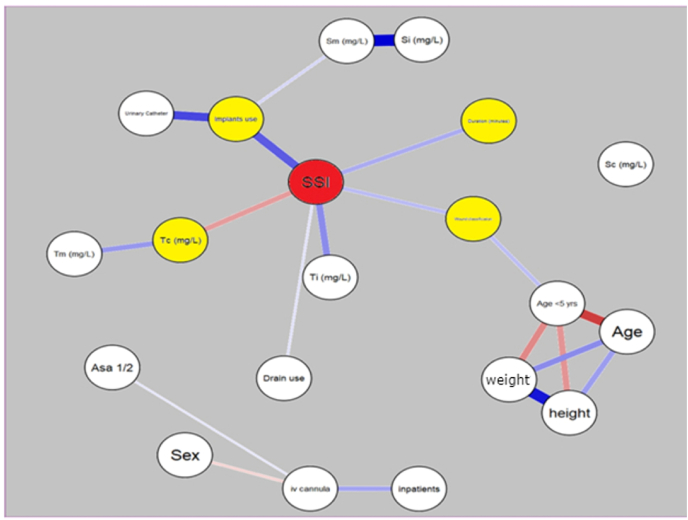
Serum and tissue concentrations of ceftriaxone were significant at test value of 4 mg/L (p < 0.001). Significant correlation of the serum concentrations at midway was found with the use of implants (r = 0.288, p = 0.043), whereas the serum concentrations of ceftriaxone at closure of surgery were significantly correlated negatively with the sex (r = −0.283, p = 0.047) and duration of the surgery (r = −0.295, p = 0.038). Tissue concentrations of ceftriaxone at incision and closure were significantly correlated with the outcome of SSI but there was no significant association (Table 2). No significant correlations were found between the other patient's characteristics or risk factor with the concentrations of ceftriaxone at serum and tissue level. Network plotting of inter-variables, dependent and independent both is depicted in Fig. 3.
Table 2.
Analysis of serum and tissue concentrations of ceftriaxone and SSI. . (Si, Sm & Sc are concentrations of ceftriaxone at incision, midway and at closure respectively. Ti, Tm & Tc are concentrations of ceftriaxone at incision, midway and at closure respectively.) r-correlation coefficient, n-number & CI-confidence interval. ∗P < 0.05
| Correlation Test |
Mann-Whitney test |
Binary logistic regression |
|||
|---|---|---|---|---|---|
| r(P) | No SSI (n = 47) | SSI (n = 3) | P | Odds Ratio (CI) | |
| Si | 0.06(0.67) | 158.87 ± 14.73 | 163.08 ± 18.93 | 0.68 | 1.02 (0.93–1.11) |
| Sm | 0.09(0.52) | 135.41 ± 11.59 | 140.87 ± 10.61 | 0.52 | 1.05 (0.94–1.17)) |
| Sc | −0.05(0.75) | 90.41 ± 7.97 | 89.35 ± 3.90 | 0.75 | 0.98 (0.84–1.14) |
| Ti | 0.34(0.02)∗ | 10.81 ± 1.53 | 13.15 ± 0.79 | 0.02 | 4.75 (0.97–23.2) |
| Tm | −0.07(0.64) | 12.98 ± 1.33 | 12.06 ± 2.72 | 0.65 | 0.66 (0.31–1.40) |
| Tc | −0.29(0.04)∗ | 4.51 ± 0.85 | 3.36 ± 0.03 | 0.04 | 0.04 (0.001–1.43) |
Fig. 3.
Network plot depicting correlations between individual variables and with outcome of SSI. Yellow colored nodes are significantly correlated with SSI. The thickness of edges relates to the strength of correlation, pink color depicts a negative relationship whereas blue depicts a positive relationship. (Si, Sm & Sc are concentrations of ceftriaxone at incision, midway and at closure respectively. Ti, Tm & Tc are concentrations of ceftriaxone at incision, midway and at closure respectively.) mg/L- Milligram per litre. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Evaluation of independent risk factors revealed a significant positive correlation and significant association between SSI with duration of surgery, clean contaminated wound, use of urinary catheters and use of implants in surgery. Other factors did not show any significant correlation with SSI (Table 3).
Table 3.
Parameters evaluated for association with SSI. ∗P < 0.05 aHaldane Ascombe correction applied. r-correlation coefficient, n-number & CI-confidence interval.
| Parameter | Correlation Test |
Fisher's exact or Mann-Whitney Test |
Binary logistic regression |
|||
|---|---|---|---|---|---|---|
| r(P) | No SSI (n = 47) | SSI (n = 3) | P | Odds Ratio(CI) | ||
| Age (year) | −0.04(0.81) | 6.09 ± 2.97 | 6.0 ± 5.2 | 0.82 | 0.99 (0.67–1.46) | |
| Sex | Males | −0.13(0.38) | 28 | 1 | 0.57 | 0.34 (0.03–4.01) |
| Females | 19 | 2 | ||||
| Weight (kg) | −0.07(0.61) | 24.6 ± 8.03 | 22.0 ± 12.1 | 0.62 | 0.96 (0.83–1.11) | |
| Height (m) | −0.07(0.64) | 1.01 ± 0.17 | 0.95 ± 0.27 | 0.67 | 0.15 (0.0–115) | |
| Duration (min)∗ | 0.34(0.017) | 102 ± 25 | 140 ± 9.29 | 0.02 | 1.08 (0.99–1.18) | |
| Age <5year | 0.16(0.26) | 16 | 2 | 0.29 | 3.9 (0.33–46.01) | |
| ASA | Class 1 | 0.12(0.43) | 27 | 1 | 0.58 | 2.7 (0.23–31.9) |
| Class 2 | 20 | 2 | ||||
| Wound Category∗ | Clean | 0.35(0.01) | 33 | 0 | 0.035 | 16.2a (0.07–334) |
| Clean Contaminated | 14 | 3 | ||||
| Use of medical equipment | Urinary Catheter∗ | 0.37(0.008) | 13 | 3 | 0.029 | 17.9a (0.87–370) |
| IV Cannula | 0.22(0.13) | 12 | 2 | 0.19 | 5.8a (0.48–70.2) | |
| Drain | 0.27(0.056) | 9 | 2 | 0.12 | 8.4a (0.68–104) | |
| Implants∗ | 0.63(0.001) | 4 | 3 | 0.002 | 67.7a (2.9–1529) | |
| Pre-surgery hospital stay | 0.15(0.30) | 34 | 3 | 0.56 | 2.74a (0.13–56) | |
4. Discussion
Ceftriaxone is an antibiotic with a time dependent efficacy (Brunton et al., 2018). Achieving and maintaining optimal concentrations in the plasma and at the tissue level during an ongoing surgery is an essential pre-requisite to avoid the risk of postoperative infection including SSI (Casanova et al., 2006b). Tissue concentrations are more direct measure of the pharmacodynamic effect of the drug than the plasma concentrations (Liu and Derendorf, 2003). The peculiarity of our study is the concomitant quantitative exploration of both the serum and the tissue concentrations of single dose intravenous ceftriaxone with specifically focusing its use as a pre-incisional prophylactic antibiotic within the scope of pediatric surgery.
Due to lack of specific guidelines for anti-microbial prophylaxis for surgery in pediatric population, the choice of anti-microbial, timing, dose and frequency are based mainly on the guidelines for adult patients and the surgeon's experience (Berríos-Torres et al., 2017; Allegranzi et al., 2016; Scottish Intercollegiate, 2008; Ban et al., 2017; Anderson et al., 2014; Bratzler et al., 2013). We favored ceftriaxone because of its easy availability, intravenous route, relatively good safety profile, broad spectrum and long elimination half life with a time dependent effect as per the concentration in the blood (Salkind and Rao, 2011; Brunton et al., 2018; Salim et al., 2021). It was administered as a single injection prior to skin incision within time frame of 30 min (Hawn et al., 2013; Steinberg et al., 2009; van Kasteren et al., 2007; Classen et al., 1992). The recommended dose of ceftriaxone for children is 50–75 mg per kg of body weight. Based on the advisory of the hospital committee for control of hospital infections, data about culture and sensitivity reports and breakpoint Minimum Inhibitory Concentration (MIC) of 4 mg/L for common susceptible bacterias (including methicillin resistant Staphylococcus and Gram negative bacterias), the dose of 75 mg per kg of body weight with maximum dose of 1000 mg was administered (Nordbring, 1978; The European Committee on, 2019). The measured serum ceftriaxone concentrations were more than 20 times the susceptible MIC at any given point of the surgery. On the other hand, this target level was achieved at the tissue levels of majority of the patients. Interestingly, the outcome of SSI was present only in those patients in whom the tissue levels were less than 4 mg/L at the time of closure.
In our study, the rate of SSI was 6%, which was on the lower side of the range found in other studies (Uludag et al., 2000; Davenport and Doig, 1993; Bhattacharyya and Kosloske, 1990; Sharma and Sharma, 1986; Davis et al., 1984; Doig and Wilkinson, 1976; Bhattacharyya et al., 1993). In concordance with earlier studies (Porras-Hernández et al., 2003), the factors associated with SSIs in our study were duration of surgery, wound category of contaminated clean type, the use of urinary catheter and use of implants in the surgery. Increased duration of the surgery is a significant risk factor for SSIs as it is a surrogate co-variate for increased bacterial exposure, tissue damage, skill of the surgeon, complexity of surgery and blood loss (Altemeier et al., 1968; Garibaldi et al., 1991). Use of implants or instrumentation during surgery will require more time resulting in increased duration of the surgery. In addition, it will be a foreign material in the body of the recipients, increasing the risk of bacterial infections. Similarly, the use of urinary catheter during hospital stay is an independent risk factor for nosocomial infections including SSI (Guggenbichler et al., 2011). The risk for SSI increases with the category of the wound (Cheng et al., 2015). In our study, we included patients of elective surgery only. SSI developed only in the clean, contaminated type of wounds. Evaluation of other factors did not find any significant relationship with the outcome of SSI.
The key finding of our study is that the tissue to concentrations of ceftriaxone at incision and at closure of the elective pediatric surgery were significantly correlated with SSIs and are an important predictor for SSI. We observed a negative correlation with tissue concentration at closure and the development of SSI. No such association was found with the serum concentrations. It implies that for evaluating the pharmacodynamic effect of an antibiotic for surgical prophylaxis in the paediatric population, the tissue concentrations of the antibiotic should be preferred over the plasma concentrations.
Our study had a few limitations, which need to be addressed. We did not measure the free plama ceftriaxone. It is a highly protein bound drug (>98%) and the tissue ceftriaxone is in equilibrium with the free plasma level (Liu and Derendorf, 2003). The choice and dose of ceftriaxone selection was on the basis of observations of the local hospital data as there are no global guidelines for anti-microbial prophylaxis use in pediatric surgeries. There was no control group to compare the intervention with other doses of same drug or another antibiotic. Sample size was not determined by formal method which limited the study from conductance of multi-variate analysis to identify all the predictors associated with the outcome of SSI (Peduzzi et al., 1996). There is chance of residual confounding as several factors like use of drains, anti-septic dressing, use of other antibiotics or any other concomitant drugs were not considered in the study. The total number of cases of SSI was only 3 in the study, which may limit the valid interperation of the analysis. Multicollinearity between individual independent variable was on the higher side of acceptable range of VIF (upto 5), which may be a cause of concern in inadequately sampled study (O'brien, 2007). More case controlled, randomized studies incorporating more factors are warranted to develop an optimum guideline for the use of antibiotic of surgical prophylaxis.
5. Conclusion
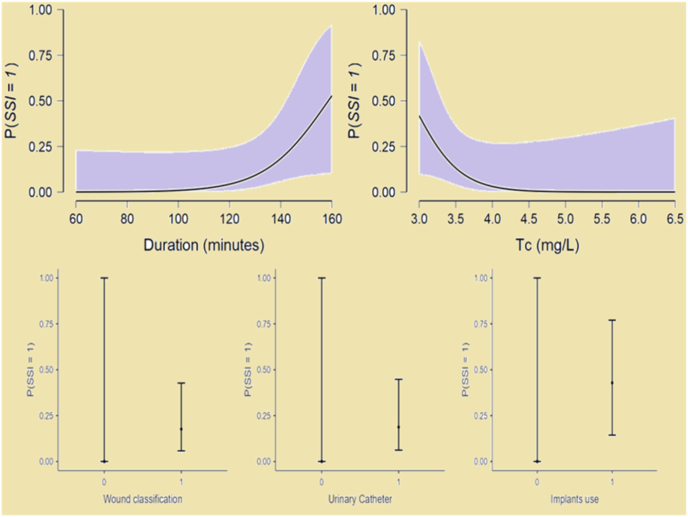
The tissue concentration of ceftriaxone at the closure of pediatric surgery is a better marker for monitoring of prophylactic antibiotic and development of SSI. Duration of surgery, wound category of contaminated clean type, the use of urinary catheter and implants in the pediatric surgery are predictors of SSI (Fig. 4). Therefore, an intra-operative re-dose, extension of dose or addition of another antibiotic may can be considered for such patients.
Fig. 4.
Estimated marginal mean plots of significant predictors of SSI. P(SSI = 1) indicates the probability of Surgical-site Infections. mg/L- Milligram per litre.
CRediT authorship contribution statement
Salim Sheikh: Visualization, Data curation, Investigation, Methodology, Software, Writing – original draft. Ravinder Majoka: Data curation, Investigation. Chakra Dhar Tripathi: Conceptualization, Supervision, Validation, Resources. Veena Verma: Supervision, Validation, Project administration. Deepak Bagga: Supervision, Validation. Bushra Ahmed Karim: Formal analysis, Software, Writing – review & editing. Girish Gulab Meshram: Writing – review & editing.
Declaration of competing interest
None Declared.
Acknowledgments
None.
References
- Allegranzi B., Zayed B., Bischoff P., Kubilay N.Z., de Jonge S., de Vries F., et al. WHO Guidelines Development Group. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect. Dis. 2016;16:e288–e303. doi: 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- Altemeier W.A., Culbertson W.R., Hummel R.P. Surgical considerations of endogenous infections- sources, types, and methods of control. Surg. Clin. 1968;48:227–240. doi: 10.1016/s0039-6109(16)38448-1. [DOI] [PubMed] [Google Scholar]
- Anderson D.J., Podgorny K., Berríos-Torres S.I., Bratzler D.W., Dellinger E.P., Greene L., et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014;35:605–627. doi: 10.1086/676022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban K.A., Minei J.P., Laronga C., Harbrecht B.G., Jensen E.H., Fry D.E., et al. American College of Surgeons and Surgical Infection Society: surgical site infection guidelines, 2016 update. J. Am. Coll. Surg. 2017;224:59–74. doi: 10.1016/j.jamcollsurg.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Berríos-Torres S.I., Umscheid C.A., Bratzler D.W., Leas B., Stone E.C., Kelz R.R., et al. Healthcare Infection Control Practices Advisory Committee Centers for Disease Control and Prevention guideline for the prevention of surgical site infection. JAMA Surg. 2017;152:784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya N., Kosloske A.M. Postoperative wound infection in pediatric surgical patients: a study of 676 infants and children. J. Pediatr. Surg. 1990;25:125–129. doi: 10.1016/s0022-3468(05)80177-0. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya N., Kosloske A.M., Macarthur C. Nosocomial infection in pediatric surgical patients: a study of 608 infants and children. J. Pediatr. Surg. 1993;28:338–344. doi: 10.1016/0022-3468(93)90228-d. [DOI] [PubMed] [Google Scholar]
- Bratzler D.W., Houck P.M. Antimicrobial prophylaxis for surgery: an advisory statement from the national surgical infection prevention project. Am. J. Surg. 2005;189:395–404. doi: 10.1016/j.amjsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Bratzler D.W., Hunt D.R. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin. Infect. Dis. 2006;43:322–330. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- Bratzler D.W., Dellinger E.P., Olsen K.M., Perl T.M., Auwaerter P.G., Bolon M.K., et al. American Society of Health-System pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013;70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- Brunton L.L., Hilal-Dandan R., Knollmann B.C. thirteenth ed. McGraw Hill Education; New York: 2018. The Pharmacological Basis of Therapeutics; pp. 1023–1037. (Chapter 57), Penicillins, Cephalosporins, and Other B-Lactam Antibiotics. [Google Scholar]
- Bucher B.T., Guth R.M., Elward A.M., Hamilton N.A., Dillon P.A., Warner B.W., Keller M.S. Risk factors and outcomes of surgical site infection in children. J. Am. Coll. Surg. 2011;212:1033–1038. doi: 10.1016/j.jamcollsurg.2011.01.065. [DOI] [PubMed] [Google Scholar]
- Casanova J.F., Herruzo R., Diez J. Risk factors for surgical site infection in children. Infect. Control Hosp. Epidemiol. 2006;27:709–715. doi: 10.1086/504938. [DOI] [PubMed] [Google Scholar]
- Casanova J.F., Herruzo R., Diez J. Risk factors for surgical site infection in children. Infect. Control Hosp. Epidemiol. 2006;27:709–715. doi: 10.1086/504938. [DOI] [PubMed] [Google Scholar]
- Cheng K., Li J., Kong Q., Wang C., Ye N., Xia G. Risk factors for surgical site infection in a teaching hospital: a prospective study of 1,138 patients. Patient Prefer. Adherence. 2015;14:1171–1177. doi: 10.2147/PPA.S86153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen D.C., Evans R.S., Pestotnik S.L., Horn S.D., Menlove R.L., Burke J.P. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N. Engl. J. Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- Davenport M., Doig C.M. Wound infection in pediatric surgery: a study of 1,094 neonates. J. Pediatr. Surg. 1993;28:26–30. doi: 10.1016/s0022-3468(05)80348-3. [DOI] [PubMed] [Google Scholar]
- Davis D.S., Sobocinski K., Hoffman R.G., Mohr B., Nelson D.B. Postoperative wound infections in a children's hospital. Pediatr. Infect. Dis. 1984;3:114–116. doi: 10.1097/00006454-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Doig C.M., Wilkinson A.W. Wound infection in a children's hospital. Br. J. Surg. 1976;63:647–650. doi: 10.1002/bjs.1800630822. [DOI] [PubMed] [Google Scholar]
- Garibaldi R.A., Cushing D., Lerer T. Risk factors for postoperative infection. Am. J. Med. 1991;91:158S–163S. doi: 10.1016/0002-9343(91)90362-2. [DOI] [PubMed] [Google Scholar]
- Gaynes R.P., Culver D.H., Horan T.C., et al. Surgical site infection (SSI) rates in the United States, 1992–1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin. Infect. Dis. 2001;33:S69–S77. doi: 10.1086/321860. [DOI] [PubMed] [Google Scholar]
- Guggenbichler J.P., Assadian O., Boeswald M., Kramer A. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials - catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip. 2011;6:Doc18. doi: 10.3205/dgkh000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley R.W., Culver D.H., Morgan W.M., et al. Identifying patients at high risk of surgical wound infection. A simple multivariate index of patient susceptibility and wound contamination. Am. J. Epidemiol. 1985;121:206–215. doi: 10.1093/oxfordjournals.aje.a113991. [DOI] [PubMed] [Google Scholar]
- Hawn M.T., Richman J.S., Vick C.C., Deierhoi R.J., Graham L.A., Henderson W.G., et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013;148:649–657. doi: 10.1001/jamasurg.2013.134. [DOI] [PubMed] [Google Scholar]
- Horwitz J.R., Chwals W.J., Doski J.J., Suescun E.A., Cheu H.W., Lally K.P. Pediatric wound infections. A prospective multicenter study. Ann. Surg. 1998;227:553–558. doi: 10.1097/00000658-199804000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra P., Vaithilingam B., Vinayagam S., Adithan C., Nema S. Ceftriaxone concentration at the surgical site following systemic and isolated upper limb injection. J. Anaesthesiol. Clin. Pharmacol. 2018;34:314–317. doi: 10.4103/joacp.JOACP_28_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone M., Albanèse J., Tod M., Savelli V., Ragni E., Rossi D., Martin C. Ceftriaxone (1 g intravenously) penetration into abdominal tissues when administered as antibiotic prophylaxis during nephrectomy. J. Chemother. 2003;15:139–142. doi: 10.1179/joc.2003.15.2.139. [DOI] [PubMed] [Google Scholar]
- Liu P., Derendorf H. Antimicrobial tissue concentrations. Infect. Dis. Clin. 2003;17:599–613. doi: 10.1016/s0891-5520(03)00060-6. [DOI] [PubMed] [Google Scholar]
- Martin C., Ragni J., Lokiec F., Guillen J.C., Auge A., Pecking M., et al. Pharmacokinetics and tissue penetration of a single dose of ceftriaxone (1,000 milligrams intravenously) for antibiotic prophylaxis in thoracic surgery. Antimicrob. Agents Chemother. 1992;36:2804–2847. doi: 10.1128/aac.36.12.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordbring F. Tissue penetration of antibiotics. Introduction. Focus on some problems involved in the treatment of infectious diseases. Scand. J. Infect. Dis. 1978;S14:21–22. [PubMed] [Google Scholar]
- O’brien R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quantity. 2007;41:673–690. [Google Scholar]
- Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Porras-Hernández J.D., Vilar-Compte D., Cashat-Cruz M., Ordorica-Flores R.M., Bracho-Blanchet E., Avila-Figueroa C. A prospective study of surgical site infections in a pediatric hospital in Mexico City. Am. J. Infect. Control. 2003;31:302–308. doi: 10.1067/mic.2003.85. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A Language and environment for statistical computing version 4.0. 2021. https://cran.r-project.org 19 March 2021. [Google Scholar]
- Salim S., Kumar M.N., Tripathi C.D., Arya S.V., Verma V., Ahmed K.B., Meshram G.G. Pharmacological evaluation of prophylactic anti-microbial use in laparoscopic cholecystectomy; an open labelled study evaluating the concentrations of single dose intravenous ceftriaxone at serum and tissue level. Eur. J. Clin. Pharmacol. 2021;77:1011–1016. doi: 10.1007/s00228-021-03093-1. [DOI] [PubMed] [Google Scholar]
- Salkind A.R., Rao K.C. Antiobiotic prophylaxis to prevent surgical site infections. Am. Fam. Physician. 2011;83:585–590. [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (SIGN) Antibiotic prophylaxis in surgery. Edinburgh: SIGN. 2008. http://www.sign.ac.uk SIGN publication no.104. July 2008.
- Sharma L.K., Sharma P.K. Postoperative wound infection in a pediatric surgical service. J. Pediatr. Surg. 1986;21:889–891. doi: 10.1016/s0022-3468(86)80016-1. [DOI] [PubMed] [Google Scholar]
- Shinagawa N. Comparative pharmacokinetics of ceftriaxone, cefmetazole and moxalactam during abdominal surgery. J. Chemother. 1989;1:524–525. [PubMed] [Google Scholar]
- Steinberg J.P., Braun B.I., Hellinger W.C., Kusek L., Bozikis M.R., Bush A.J., et al. Trial to reduce antimicrobial prophylaxis Errors (TRAPE) study group timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the trial to reduce antimicrobial prophylaxis Errors. Ann. Surg. 2009;250:10–16. doi: 10.1097/SLA.0b013e3181ad5fca. [DOI] [PubMed] [Google Scholar]
- Stulberg J.J., Delaney C.P., Neuhauser D.V., Aron D.C., Fu P., Koroukian S.M. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- Tarchini G., Liau K.H., Solomkin J.S. Antimicrobial stewardship in surgery: challenges and opportunities. Clin. Infect. Dis. 2017;64:S112–S114. doi: 10.1093/cid/cix087. [DOI] [PubMed] [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters version 9.0. 2019. http://www.eucast.org
- Uludag O., Rieu P., Niessen M., Voss A. Incidence of surgical site infections in pediatric patients: a 3-month prospective study in an academic pediatric surgical unit. Pediatr. Surg. Int. 2000;16:417–420. doi: 10.1007/s003830000389. [DOI] [PubMed] [Google Scholar]
- van Kasteren M.E., Manniën J., Ott A., et al. Antibiotic prophylaxis and the risk of surgical site infections following total hip arthroplasty: timely administration is the most important factor. Clin. Infect. Dis. 2007;44:921–927. doi: 10.1086/512192. [DOI] [PubMed] [Google Scholar]