Abstract
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus, Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2), led to the ongoing global public health crisis. Existing clinical data suggest that COVID-19 patients with acute respiratory distress syndrome (ARDS) have worse outcomes and increased risk of intensive care unit (ICU) admission. The rapid increase in the numbers of patients requiring ICU care may imply a sudden and major challenge for affected health care systems. In this narrative review, we aim to summarize current knowledge of pathophysiology, clinical and morphological characteristics of COVID-19-associated ARDS and ARDS caused by other factors (classical ARDS) as defined by Berlin criteria, and therefore to elucidate the differences, which can affect clinical management of COVID-19-associated ARDS. Fully understanding the characteristics of COVID-19-associated ARDS will help identify its early progression and tailor the treatment, leading to improved prognosis in severe cases and reduced mortality. The notable mechanisms of COVID-19-associated ARDS include severe pulmonary infiltration/edema and inflammation, leading to impaired alveolar homeostasis, alteration of pulmonary physiology resulting in pulmonary fibrosis, endothelial inflammation and vascular thrombosis. Despite some distinct differences between COVID-19-associated ARDS and classical ARDS as defined by Berlin criteria, general treatment principles, such as lung-protective ventilation and rehabilitation concepts should be applied whenever possible. At the same time, ventilatory settings for COVID-19-associated ARDS require to be adapted in individual cases, depending on respiratory mechanics, recruitability and presentation timing.
Keywords: SARS-CoV-2, COVID-19 pandemic, Respiratory distress syndrome, Respiratory mechanics
INTRODUCTION
The epidemic of coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) has rapidly progressed into a pandemic (1–3). While the first case of COVID-19 was recorded in the Hubei province of China on December 8, 2019 (4), 22 months later, by November 11, 2021, WHO reported 252,328,767 cases of COVID-19, resulting in 5,092,154 deaths.
In Ukraine, the first case of COVID-19 was reported in Chernivtsi on February 29, 2020 (confirmed on March 3, 2020) in a man who has traveled from Italy. A month later, by March 25, 2020, the number of confirmed cases has reached 113, including four deaths, with the majority of infections resulting from contacts abroad, and the early COVID-19 progression in the number of cases similar to that of other European countries such as Sweden and Poland (5). By November 11, 2021, the Ukrainian Public Health Center reported that the number of confirmed COVID-19 cases in the country had reached 3,155,519, and 74,857 of them were lethal.
Drawing parallels with other respiratory viral infections, scientists predict that up to 80% of the human population may become infected by SARS-CoV-2 (6). The highest risk of death from this disease affects elderly and those with pre-existing comorbidities. This susceptibility of the elderly is an especially acute issue in Ukraine, since it has the sixth largest elderly population among Eastern European countries. Almost all of those aged over 60 in Ukraine have at least one chronic disease, making them extremely vulnerable to COVID-19 and high risk for development of acute respiratory failure and death appeared to be related to acute respiratory distress syndrome (ARDS), which is the most common cause of admission to the intensive care unit (ICU) (7). The rapid increase in the numbers of patients requiring ICU care may imply a sudden and major challenge for affected health care systems (8). Early diagnosis, application of effective therapies and adequate strategies of clinical stratification are needed for better outcomes in COVID-19 patients (9).
At the same time descriptions of the pathophysiological characteristics of COVID-19 respiratory failure are limited. That is why today is highly important to identify key questions that could be addressed in clarification of notable COVID-19-associated ARDS mechanisms and clinical presentation, the implementation of which could lead to optimising ventilatory management and reducing the mortality and disability rates in COVID-19 patients.
In this narrative review, we aim to summarize current knowledge of pathophysiology, clinical and morphological characteristics of COVID-19-associated ARDS and ARDS caused by other factors (classical ARDS) as defined by Berlin criteria, and therefore to elucidate the differences, which can affect clinical management of COVID-19-associated ARDS.
MATERIALS AND METHODS
We provide a summary of the published literature based on a Google and PubMed search using the terms “SARS-CoV-2”, “COVID-19”, “respiratory failure”, “respiratory mechanics”, and “acute respiratory distress syndrome”. The studies used were those published from 1 January 2020 to 1 March 2021.
Risk factors associated with the development of ARDS in patients with COVID-19.
COVID-19 has a broad spectrum of clinical presentations. The majority of patients with SARS-CoV-2 infection are either asymptomatic or present with mild upper respiratory symptoms such as sneezing, coughing, dyspnea, rhinorrhea as well as fatigue and fever, approximately 2 to 4 days following infection (10). The most common clinical signs of SARS-CoV-2 infection include low-to-high fever, non-productive cough, myalgia, dyspnea, fatigue, standard or decreased leukocyte counts, and confirmed evidence of pneumonia on chest radiography; less common symptoms of SARS-CoV-2 infection include headache, abdominal pain, dizziness, nausea, vomiting and diarrhea (11).
However, some patients may rapidly progress to developing symptoms of severe dyspnea or hypoxemia, associated with ARDS (12–16). Patients with ARDS require mechanical ventilatory support and suffer high mortality rate as a result of shock, septicemia, and multiple organ dysfunction syndrome (10). The proportion of patients with COVID-19 admitted to hospital who are diagnosed with ARDS using oxygenation criteria ranges between 20.0% (17) and 67.0% (13), and reaches 100% in mechanically ventilated patients (18).
Risk factors for the clinical outcomes of COVID-19 pneumonia have not been well described. Hu X.S. et al. investigated the risk factors associated with the development of ARDS in patients with COVID-19 using univariate logistic analysis (19). The researchers determined that age, comorbidities, dyspnea, dry/ moist rales, lung consolidative/mixed opacities, and lymphocytosis, D-dimer, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase (LDH), C-reactive protein (CRP) and procalcitonin levels were associated with ARDS. Logistic multivariate analysis defined dyspnea, dry/moist rales, and high lactate dehydrogenase activity as independent risk factors. Thus, compared to the non-ARDS group, the group of patients with ARDS were significantly older, had more comorbidities, dyspnea, higher D-dimer and CRP levels, higher LDH activity.
In another study, risk factors associated with the development of ARDS and progression from ARDS to death in COVID-19 pneumonia patients were found to be older age, neutrophilia, and coagulation dysfunction (20).
Mortality rate in SARS-CoV-2-associated ARDS.
Studies suggest that COVID-19 caused ARDS has worse outcomes than ARDS from other causes. For instance, Bellani et al. showed that ICU and hospital mortality from typical ARDS was 35.3% (95% confidence interval (CI), 33.3–37.2%) and 40.0% (95% CI, 38.1–42.1%), respectively (21). According to Wu et al. analysis, COVID-19-related ARDS ICU mortality ranged between 26.0% and 61.5%, and in patients who received mechanical ventilation, the mortality reached between 65.7% to 94.0% (20). A mortality rate comparison across 289 critical care units of England, Wales and Northern Ireland between a cohort of critical care COVID-19 patients and a historic cohort of patients with non-COVID-19 viral pneumonias showed that among 10,834 patients with COVID-19 70.1% were male, with median age 60 years. Of these, 36.9% had a ratio of arterial oxygen partial pressure (PaO 2 ) to fractional inspired oxygen (FiO 2 ) of ≤13.3 kPa (≤100 mmHg) consistent with severe ARDS and 72% received invasive ventilation (22). Acute hospital mortality was higher than that for the 5,782 critical care patients non-COVID-19 viral pneumonias (42.0% vs. 24.7%).
Arulkumaran et al. studied whether patients with COVID-19 pneumonia who died following invasive mechanical ventilation (IMV) had a more advanced disease compared to survivors (23). Of the 47 mechanically ventilated COVID-19 patients included in the study, 26 (57.0%) patients died in hospital. On IMV initiation, 61.0% of patients fulfilled the Berlin criteria for severe ARDS. This contrasts with 36.9% in the first 24 hours of ICU admission in study of the other researchers (22). Despite this higher proportion, the mortality rate was comparable for patients mechanically ventilated within the first 24 hours.
Tang X. et al. compared the severity of respiratory failure and mortality rate of patients with ARDS infected with either COVID-19 (n=73) or influenza A virus subtype H1N1 (H1N1) (n=75) and found that severity of the failure was not equal between the two cohorts (24). The PaO 2 /FIO 2 levels in patients with COVID-19 were higher than those in patients with H1N1, meaning that these patients received initial respiratory support via noninvasive methods, which ultimately produced higher failure rates. The mortality of COVID-19 patients with ARDS was 28.8% (25). While patients with H1N1 exhibited significantly lower oxygenation than patients with COVID-19, there was no difference in the mortality rate between the two groups.
Yang X. et al. report opposite findings when comparing mortality rate in SARS-CoV-2-associated ARDS and H1N1-associated ARDS (26). Their retrospective observational study compared mortality rate in 73 adult patients with SARS-CoV-2-associated ARDS to that in 68 patients with 2009 H1N1-associated ARDS (27); both groups were treated with extracorporeal membrane oxygenation (ECMO). The SARS-CoV-2 patients had a median age of 62 (range 33–78) years; 42 (63.6%) were males. ECMO was initiated following severe respiratory failure on mechanical ventilation with a median PaO 2 /FiO 2 of 71.9 mmHg and a median partial pressure of carbon dioxide (PCO 2 ) of 62 mmHg on arterial blood test. The median duration from symptom onset to IMV and to ECMO initiation was, respectively, 19 and 23 days. Before and after ECMO initiation, 58.9 and 69.9%, respectively, of the patients received prone position. Since ECMO initiation, the 30-day mortality and 60-day mortality was 63.0 and 80.8%, respectively. In 2009 H1N1 patients, the median PaO 2 /FiO 2 was 56 mmHg, while 20% of patients received prone position ventilation before ECMO initiation and the mortality was 21%. Thus, even though the PaO 2 /FiO 2 level in the patients with H1N1-associated ARDS was similar to that of patients with SARS-CoV-2-associated ARDS, and even with a considerable increase in the proportion of patients receiving prone position ventilation, the mortality was almost quadrupled in the latter cohort.
In COVID-19-associated ARDS, death is a result of respiratory failure (53%), respiratory failure combined with cardiac failure (33.0%), myocardial damage and circulatory failure (7.0%); the rest of cases are death from an unknown cause (20, 28). An analysis of lung tissue from 41 COVID-19 patients that died showed diffuse alveolar damage (DAD) in different patterns and proportions (29).
Notably, older patients that died after shorter periods of hospital stay (up to 8 days) had a significantly larger proportion of the intermediate, fibro-proliferative pattern and less of the exudative pattern than younger patients that died within the same period. Similar lung pathology results in patients that had died because of SARS-CoV-2 infection were reported in other studies: most of the patients presented with different stages of DAD and a high frequency of macro- and microvascular thrombosis, producing a clinical picture of ARDS (30, 31). Duarte-Neto A.N. et al. observed pulmonary thrombotic events in different segments of the pulmonary circulation system, from capillaries to the large arterial branches in COVID-19 patients (32).
Classical ARDS: pathophysiology and diagnostic criteria.
Since it was first described in 1967 by Ashbaugh D. G. et al. (33), the ARDS has been recognized as a major clinical problem worldwide, carrying a high morbidity and mortality burden (34, 35). It is a destructive lung injury during an uncontrolled inflammatory process that causes severe alveolar damage and capillary basement membrane leakage, which leads to progressive respiratory failure (16). Some researchers consider ARDS not a disease, but a clinically defined condition with acute respiratory failure occurring de novo because of clearly determined pulmonary and non-pulmonary insults (36). Examples of pulmonary causes for ARDS may include, but are not limited to, fat embolism, viral pneumonia, smoke inhalation, polymer fume fever, disseminated intravascular coagulopathy; while non-pulmonary insults could be related to pancreatitis, trauma, sepsis, hypovolemic shock, transfusion reaction, cardiopulmonary bypass, abdominal compartment syndrome, etc. (37).
The diagnosis of classical ARDS is based on 2011 Berlincriteria (25),whichincludetiming,oxygenation, positive end-expiratory pressure (PEEP) requirement, chest imaging, and origin of edema (Table 1).
Table 1.
Berlin criteria for ARDS
| Characteristics | ARDS Berlin definition |
|---|---|
| Timing | Within 7 days of a known clinical insult or new or worsening respiratory symptoms |
| Oxygenation | Mild: PaO2/FiO2 >200 mmHg, but ≤300 mmHg; |
| Moderate: PaO2 /FiO2 >100 mmHg, but ≤200 mmHg; | |
| Severe: PaO2 /FiO2 ≤100 mmHg | |
| PEEP requirement | Minimum 5 cm H2O PEEP required by invasive mechanical ventilation |
| Chest imaging | Bilateral opacities not fully explained by effusions, lobar/lung collapse or nodules by chest radiograph or CT |
| Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload (need objective assessment, such as echocardiography, to exclude hydrostatic edema if no risk factor present) |
PEEP: positive end-expiratory pressure; PaO 2 : arterial oxygen tension; FiO 2 : inspiratory oxygen fraction; CT: computed to-mography.
Three phases can be differentiated in the course of classical ARDS: acute (exudative), subacute and chronic. The acute phase of ARDS lasts the first 7 days and is characterized by the development of hypoxemia, interstitial and alveolar edema with accumulation of neutrophils, macrophages and erythrocytes in the alveoli, destruction of the alveolar epithelium caused by endothelial and epithelial lesions, and formation of hyaline membranes. Development of pulmonary edema is a primary sign of ARDS. In the next 7 days, during the subacute phase, edema gradually lessens; alveolar structures are restored as a result of the type II alveolar epithelial cells proliferation. In the chronic stage (14 days after the initiation), neutrophil infiltrates develop with an increase in mononuclear cells and alveolar macrophages in the alveoli along with persistent restoration of the alveolar epithelium.
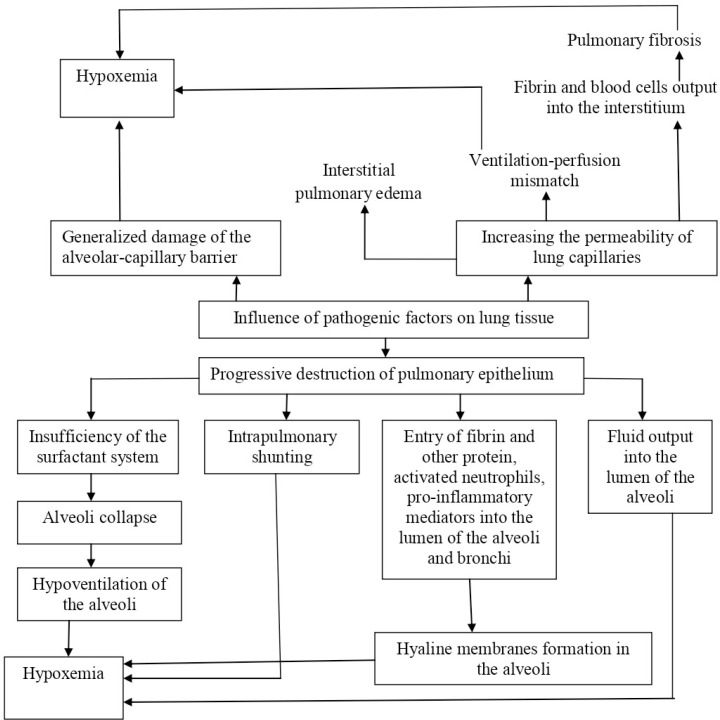
A complex interaction of numerous factors contributes to ARDS development, including the release of pro-inflammatory cytokines which recruit neutrophils into the lungs, where they are activated and release toxic mediators damaging the capillary endothelium and alveolar epithelium, leading to alveolar edema (38, 39). This, in time, impairs gas exchange, decreases lung compliance and increases pulmonary blood pressure (40). Progressive dysfunction of pulmonary hemodynamics develops as a result (Fig. 1).
Fig. 1.
Pathophysiology of ARDS
Respiratory failure in ARDS in most patients is of multifactorial origin and a consequence of arterial hypoxemia resulting from filling of the alveoli with protein-rich fluid, decreased lung compliance (stretching) caused by interstitial and alveolar edema with surfactant dysfunction, followed by dead space increase caused by the damage to or destruction of the microvascular bed (41, 42). Altered fluid balance in the lungs results from increased permeability of the alveolar-capillary barrier and the release of blood plasma into the alveolar space with extensive stasis in the arterioles, venules and capillaries (43). Damage to the epithelium, especially the type II alveolar epithelium which is involved in surfactant production and is a precursor to type I squamous epithelium (Clara cells), is an important mechanism of ARDS progression. Destruction of the type II cells and reduced surfactant production with subsequent alveolar collapse contributes to the development of alveolar edema, which further exacerbates surfactant dysfunction (44).
Increased capillary permeability is a characteristic feature of ARDS. Damage to the capillary endothelium and alveolar epithelium in conjunction with impaired fluid movement through the alveolar space results in the accumulation of protein-rich fluid within the alveoli. Pulmonary edema resulting from increased capillary permeability is recognized as the main feature of the early stages of ARDS (45). This overhydrating of lung tissue, one of the forms of pulmonary edema, markedly differs from cardiogenic pulmonary edema since the pulmonary capillaries possess normal values of hydrostatic pressure. Since the hydrostatic pressure in the pulmonary capillaries is unchanged, the main pathogenic mechanism of pulmonary edema in ARDS is an abnormal increase in the permeability of the alveolar-capillary membrane, direct damage by inhaled substances or aspirated gastric acid, or indirect effect of activated and aggregated blood cells (in sepsis and/or endotoxemia) (46). This results in diffuse alveolar damage with the release of pro-inflammatory cytokines, such as interleukin-1, interleukin-6, tumor necrosis factor α and others. Neutrophils accumulated in the lungs are activated by cytokines, releasing toxic mediators such as active oxygen metabolites, nitrogen monoxide compounds, and proteases (47).
Central to the pathophysiology of ARDS is the presence of fibrin-rich exudates (hyaline membranes), developing as a result of activation of coagulation and inhibition of fibrinolysis which disrupt normal function of the air-blood barrier (48). Formation of the hyaline membrane in the alveoli is followed by interstitial widening, by edema and then fibroblast proliferation (28). Up-regulation of procoagulant activity in the alveolar compartment has been proposed as the driving force for intra-alveolar fibrin deposition (49). Concentrations of D-dimer, a blood protein fragment resulting from clot degradation, are significantly increased in the edema fluid of patients with ARDS (50).
While chronic alcohol abuse and active or passive cigarette smoke have been associated with an increased incidence of classical ARDS (36), no association with environmental pollution has been established (34). Vitamin D deficiency may increase the risk of ARDS (51). This substance is involved in modulating networks of innate and adaptive immune function (52); its deficiency is also linked to an increased risk of other respiratory disorders, including pneumonia (53) and sepsis (54).
Differences and similarities between COVID-19-associated ARDS and classical ARDS.
Several studies suggest that COVID-19-associated ARDS might be drastically different from a classical ARDS, since while the patients with the former exhibit significant hypoxemia, they also have quite compliant lungs compared to patients with ARDS unrelated to COVID-19. Because of it, typical protective ventilatory settings might not be indicated in patients with COVID-19-related ARDS (55, 56).
The typical chest CT findings of COVID-19 show bilateral ground-glass shadow with a peripheral lung distribution (57). Although there is consolidation and exudation, it is not a classical ARDS image. Lung compliance can be relatively normal in some COVID-19-associated ARDS patients. This is observably inconsistent with ARDS caused by other factors (12).
Grasselli et al. compared physiological and morphological features of invasively ventilated COVID-19 patients (n=297) to those previously described for classical ARDS (n=960) (58). Median static compliance of the respiratory system was 28% higher in COVID-19 patients (41 mL/cm H 2 O [interquartile range (IQR) 33–52]) than in those with classical ARDS (32 mL/cm H 2 O [IQR 25–43], p<0.0001). Only 6% of patients with COVID-19-associated ARDS had compliances greater than the 95th percentile of the patients with classical ARDS. Thus, the authors reach conclusion that patients with COVID-19-associated ARDS have respiratory mechanics that largely match those of classical ARDS. Researchers also found more than two times increase in the 28-day mortality in a subgroup of patients that had a combination of very high D-dimer concentrations and low static compliance compared to the patients who had increases of either D-dimer concentration or static compliance individually.
In contrast, Tsolaki et al. points out that while median static respiratory system compliance was significantly higher in patients with COVID-19-associated ARDS compared to those with classical ARDS, the observed 28% difference would have been even more pronounced had the groups been better matched (the classical ARDS group had a significantly lower percentage of patients with severe ARDS compared with the COVID-19-associated ARDS group) (59). In classical ARDS, static compliance correlated to ARDS severity in an almost linear way: as hypoxemia worsened, static compliance worsened as well; however, in COVID-19-associated ARDS, static compliance remained unchanged, despite worsening oxygenation. Therefore, the extent of hypoxemia might be affected by additional factors unrelated to alveolar flooding or collapse, the two main pathophysiology features in classical ARDS.
A study of specific features of COVID-19-related ARDS, including injury site of COVID-19, specificity of clinical features, timing of onset and severity based on oxygenation index, points out that early exudative stage of classic ARDS presents diffuse alveolar damage with destruction of epithelial and endothelial cells, while the most common respiratory symptom of COVID-19 is dry cough (59.4–82%), with reduced sputum production (12). In regards to specificity of clinical features, the researchers point out that clinical symptoms were inconsistent with the severity of laboratory and imaging findings (12, 57).
The Berlin Criteria state that onset of ARDS should be within 7 days of a known clinical insult or new or worsening respiratory symptoms (Table 1). The reported onset of COVID-19-associated ARDS was found to be longer in several studies. Huang C. et al. (60) reported 41 cases of COVID-19 in which the median time from onset of symptoms to ARDS was 9.0 days (8.0–14.0). Subsequently, Wang D. et al. (17) reported 138 cases of COVID-19 in which the median time from the first symptom to ARDS was 8.0 days (6.0–12.0), and Zhou et al. (61) reported 12.0 days (8.0–15.0).
The Berlin Criteria define three ARDS stages based on oxygenation index (Table 1). Even now, the clinical features of COVID-19-associated ARDS are not yet clear. In standard treatment protocol for COVID-19 (National Health Commission of China, 2020) COVID-19-associated ARDS was divided into three categories based on oxygenation index (PaO2/ FiO2) on PEEP ≥ 5 cmH2O: mild (200 mmHg ≤ PaO 2 /FiO 2 <300 mmHg), mild-moderate (150 mmHg ≤ PaO2/FiO2 < 200 mmHg), and moderate-severe (PaO 2 /FiO 2 < 150 mmHg) (12).
Recently, some investigators hypothesized that COVID-19 disease is characterized by an increased pulmonary blood flow with intrapulmonary right to left shunt at any stage of the disease, introducing the acronym “AVDS” (Acute Vascular Distress Syndrome) (62, 63). Infection of endothelial cells by SARS-Cov-2 and endotheliitis (64) can be explained by the presence of ACE-2 receptors on vascular endothelial cells (65). All these findings suggest a specific pulmonary vascular disorder induced by SARS-CoV-2, pointing out to an AVDS rather than an atypical ARDS.
Gattinoni and colleagues also suggest that ARDS related to COVID-19 is not a classical ARDS, because the patients have a better respiratory system compliance that is unrelated to the amount of shunt. In a case series of 16 mechanically ventilated patients with COVID-19, they described severe hypoxemia despite relatively normal lung compliance, an unusual finding in patients with severe ARDS (56). In eight patients, blood gases and CT scans revealed a large shunt fraction despite relatively small amounts of gasless tissue, suggesting hyperperfusion of poorly ventilated lung regions. Because the lungs appeared relatively open, the authors recommend a lower PEEP treatment approach, as well as avoiding prone positioning, especially because of potentially limited human resources during the pandemic.
In a second report, Gattinoni L. et al. highlighted the non-uniformity of patients with COVID-19-associated ARDS and proposed two primary phenotypes: type L (low values of elastance, pulmonary ventilation/perfusion ratio, lung weight, and recruitability) and type H (high values of elastance, right-to-left shunt, lung weight, and recruitability), with the latter being more consistent with what they describe as typical severe ARDS (66). The authors suggest that most patients present early with type L, and then some transition to type H, potentially due to the synergistic effects of worsening COVID-19 pneumonia and patient self-inflicted lung injury. The team produced a follow-up report elaborating these key points (55).
Notably, treatment recommendations based on these conceptual physiological models (55, 56, 66) are opposed to long-standing evidence-based interventions such as low tidal volume ventilation and prone positioning, which alarmed some researchers. For instance, Fan E. et al. suggest that reported phenotypic heterogeneity in patients with COVID-19-associated ARDS, while interesting, could be over interpreted or inappropriately applied in the ICU, potentially leading to detrimental ventilatory management strategies (67). Similarly, Bos answering the question whether patients with COVID-19- associated ARDS are inherently different from “typical” ARDS, noted that COVID-19-associated ARDS is an etiological sub-phenotype of ARDS with specific characteristics: frequent DAD, a higher than expected respiratory system compliance, low PaO 2 /FiO 2 values, frequent non-focal morphology, and potentially intensive systemic inflammation. He advises maintaining the highest standard of clinical practice and resisting the temptation to introduce alternative treatments that might result in harm (35).
Haudebourg et al. compared respiratory mechanics and lung recruitability of 30 patients with COVID-19– associated ARDS vs 30 non–COVID-19–associated ARDS (68). Researchers found that respiratory mechanics of patients with COVID-19–associated ARDS was heterogenous and as a global picture not much different from that of their non–COVID-19 counterparts; patients with COVID-19–associated ARDS had a higher recruitment-to-inflation (R/I) ratio suggesting a higher recruitability. It should be noted that in patients with COVID-19–associated ARDS, the R/I ratio was significantly correlated with the PaO 2 /FIO 2 ratio but not with the respiratory system compliance.
Ziehr et al. characterized COVID-19 respiratory failure in 66 patients managed with mechanical ventilation and established ARDS protocols (69). Upon initiation of mechanical ventilation, the patients had a median PaO 2 :FIO 2 of 182, dead-space fraction of 0.45, and compliance of 35 ml/cm H 2 O – findings that are consistent with data of patients with classic ARDS (25). The COVID-19 patients exhibited a spectrum of impaired gas exchange and respiratory system mechanics, and very few patients had near normal compliance.
In recent years, the pulmonary critical care community accepted that ARDS can be split into sub-phenotypes, which respond to interventions differently (70). Heterogeneity can be noted in 1) the causes of lung injury, 2) physiological changes, 3) morphology of affected lung parenchyma, and 4) biological response. Post hoc analyses of randomized clinical trials indicate that patients with systemic hyperinflammation might respond differently to higher end-expiratory pressure, restrictive fluid management, or immunomodulation with simvastatin, while the patients with a non-focal lung morphology benefit more from recruitment than prone positioning (71, 72). However, even though there is a strong evidence for these ARDS sub-phenotypes, these personalized approaches can only be implemented into clinical practice after they are validated in prospective clinical trials (36). The additional evidence for the existence of sub-phenotypes and customized treatment options for COVID-19-associated ARDS phenotypes remains urgently needed.
CONCLUSION
The prognosis of COVID-19 is hard to predict, both now and in the near future, as many non-specific symptoms arise, and many unknowns, including genetic predisposition markers, still exist. Disease is hard to control due to asymptomatic carriers, and so far, in our opinion, the only viable option for control remains complete vaccination of not only at-risk group, but all human population. COVID-19-associated ARDS is an expectable serious complication of COVID-19 that necessitates early recognition and comprehensive management. The notable mechanisms of COVID-19-associated ARDS include severe pulmonary infiltration/edema and inflammation, leading to impaired alveolar homeostasis, alteration of pulmonary physiology resulting in pulmonary fibrosis, endothelial inflammation and vascular thrombosis. Despite some distinct differences between COVID-19-associated ARDS and classical ARDS as defined by Berlin criteria, general treatment principles, such as lung-protective ventilation and rehabilitation concepts should be applied whenever possible. At the same time, ventilatory settings for COVID-19-associated ARDS require to be adapted in individual cases, depending on respiratory mechanics, recruitability and presentation timing.
REFERENCES
- 1.Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020; 20: 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roshanshad A, Kamalipour A, Ashraf MA, Roshanshad R, Jafari S, Nazemi P, et al. The efficacy of remdesivir in coronavirus disease 2019 (COVID-19): a systematic review. Iran J Microbiol 2020; 12: 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belice T, Demir I. The gender differences as a risk factor in diabetic patients with COVID-19. Iran J Microbiol 2020; 12: 625–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, Mcgoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 5.Kyrychko YN, Blyuss KB, Brovchenko I. Mathematical modelling of the dynamics and containment of COVID-19 in Ukraine. Sci Rep 2020; 10: 19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamyshnyi A, Krynytska I, Matskevych V, Marushchak M, Lushchak O. Arterial hypertension as a risk comorbidity associated with COVID-19 pathology. Int J Hypertens 2020; 2020: 8019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burki TK. Coronavirus in China. Lancet Respir Med 2020; 8: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfortmueller CA, Spinetti T, Urman RD, Luedi MM, Schefold JC. COVID-19-associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment - a narrative review. Best Pract Res Clin Anaesthesiol 2021; 35: 351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajjar LA, Costa IBSDS, Rizk SI, Biselli B, Gomes BR, Bittar CS, et al. Intensive care management of patients with COVID-19: a practical approach. Ann Intensive Care 2021; 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibodeau R, Jafroodifar A, Quraeshi S, Lisi M. SARS-CoV-2 infection leading to ischemic and hemorrhagic brain lesions and acute respiratory distress syndrome. Radiol Case Rep 2021; 16: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty C, Sharma AR, Sharma G, Bhattacharya M, Lee SS. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci 2020; 24: 4016–4026. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care 2020; 24: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med 2020; 382: 872–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther 2021; 12: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state. JAMA 2020; 323: 1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu XS, Hu CH, Zhong P, Wen YJ, Chen XY. Risk factors associated with acute respiratory distress syndrome in COVID-19 patients outside Wuhan: a double-center retrospective cohort study of 197 cases in Hunan, China. World J Clin Cases 2021; 9: 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 22.Richards-Belle A, Orzechowska I, Gould DW, Thomas K, Doidge JC, Mouncey PR, et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med 2020; 46: 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arulkumaran N, Green J, Khan A, Bonnici T, Longobardo A, Singer M. Influence of respiratory and inflammatory parameters preceding intubation on survival of patients with COVID-19 ARDS-A single centre retrospective analysis. J Crit Care 2021; 62: 289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest 2020; 158: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Hu M, Yu Y, Zhang X, Fang M, Lian Y, et al. Extracorporeal membrane oxygenation for SARS-CoV-2 acute respiratory distress syndrome: a retrospective study from Hubei, China. Front Med (Lausanne) 2021; 7: 611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009; 302: 1888–1895. [DOI] [PubMed] [Google Scholar]
- 28.Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 2020; 213: 54–56e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauad T, Duarte-Neto AN, da Silva LFF, de Oliveira EP, de Brito JM, do Nascimento ECT, et al. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir Res 2021; 22: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Michele S, Sun Y, Yilmaz MM, Katsyv I, Salvatore M, Dzierba AL, et al. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol 2020; 154: 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabrese F, Pezzuto F, Fortarezza F, Hofman P, Kern I, Panizo A, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch 2020;477: 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro-Filho J, et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 2020; 77: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers 2019; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev 2017; 26: 160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bos LDJ. COVID-19-related acute respiratory distress syndrome: not so atypical. Am J Respir Crit Care Med 2020; 202: 622–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med 2011; 183: 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA 2018; 319: 698–710. [DOI] [PubMed] [Google Scholar]
- 38.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011; 6: 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Lopez A, Albaiceta GM. Repair after acute lung injury: molecular mechanisms and therapeutic opportunities. Crit Care 2012; 16: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawal G, Yadav S, Kumar R. Acute respiratory distress syndrome: an update and review. J Transl Int Med 2018; 6: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006; 27: 337–349. [DOI] [PubMed] [Google Scholar]
- 42.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 2010; 137: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol 2015; 194: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002; 346: 1281–1286. [DOI] [PubMed] [Google Scholar]
- 45.Koegelenberg CF, Irusen EM, Cooper R, Diacon AH, Taljaard JJ, Mowlana A, et al. High mortality from respiratory failure secondary to swine-origin influenza A (H1N1) in South Africa. QJM 2010; 103: 319–325. [DOI] [PubMed] [Google Scholar]
- 46.Pierrakos C, Karanikolas M, Scolletta S, Karamouzos V, Velissaris D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res 2012; 4: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol 2009; 40: 519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sebag SC, Bastarache JA, Ware LB. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol 2011; 12: 1481–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 2020; 100: 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003; 285(1): L20–28. [DOI] [PubMed] [Google Scholar]
- 51.Dancer RC, Parekh D, Lax S, D’souza V, Zheng S, Bassford CR, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015; 70: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012; 76: 315–325. [DOI] [PubMed] [Google Scholar]
- 53.Quraishi SA, Bittner EA, Christopher KB, Camargo CA, Jr. Vitamin D status and community-acquired pneumonia: results from the third national health and nutrition examination survey. PLoS One 2013; 8(11): e81120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol 2015; 15: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 2020; 323: 2329–2330. [DOI] [PubMed] [Google Scholar]
- 56.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201: 1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 2020; 295: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 2020; 8: 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsolaki V, Zakynthinos GE, Mantzarlis K, Vazgiourakis V, Makris D. Pathophysiology of COVID-19-associated acute respiratory distress syndrome. Lancet Respir Med 2021; 9(1): e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahjoub Y, Rodenstein DO, Jounieaux V. Severe covid-19 disease: rather AVDS than ARDS? Crit Care 2020; 24: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jounieaux V, Basille D, Abou-Arab O, Guillaumont MP, Andrejak C, Mahjoub Y. Pure SARS-CoV-2 related AVDS (Acute Vascular Distress Syndrome). BMC Infect Dis 2021; 21: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marushchak M, Maksiv K, Krynytska I. ACE gene I/D polymorphism and arterial hypertension in patients with COPD. Pneumologia 2019; 68: 114–119. [Google Scholar]
- 66.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46: 1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020; 8: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haudebourg AF, Perier F, Tuffet S, de Prost N, Razazi K, Mekontso Dessap A, et al. Respiratory mechanics of COVID-19-versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 202: 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med 2020; 201: 1560–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med 2016; 194: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care 2019; 25: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med 2019; 7: 870–880. [DOI] [PubMed] [Google Scholar]