Abstract
The sensitivity and specificity of seven methods (agar dilution, broth microdilution, Etest at 0.5 and 2.0 McFarland (McF) inocula, two agar screening methods, and population studies [PS]) were evaluated in a double-blind study involving 284 methicillin-resistant Staphylococcus aureus (MRSA) strains and 45 Staphylococcus strains with reduced susceptibilities to vancomycin (SRSV). The results were compared to the population analysis profile-area under the curve ratio method (PAP-AUC ratio compared to that of Mu3) as described by Wootton et al. The agar screening method using brain heart infusion agar (6 μg of vancomycin per ml) gave a sensitivity of 22% and a specificity of 97%. A similar method using Mueller-Hinton agar (5 μg of vancomycin per ml) gave a sensitivity of 20% and a specificity of 99%. The PS method detected 34 false positives (12%) and gave a sensitivity of 71% and a specificity of 88%. Etest using 0.5 and 2.0 McF inocula gave sensitivities and specificities of 82 and 93% and of 96 and 97%, respectively. The best Etest interpretative criteria for the 2.0 McF inoculum was ≥8 mg of vancomycin per liter and ≥8 μg teicoplanin per ml or ≥12 μg of teicoplanin per ml. The direct colony suspension inoculum for this method was found to be equally accurate in detecting (hetero-)glycopeptide-intermediate S. aureus compared to the overnight broth inoculum preparation method. Agar dilution and broth microdilution using the NCCLS breakpoint criteria for vancomycin gave sensitivities and specificities of 20 and 100% and of 11 and 100%, respectively. Using the Etest with a 2.0 McF inoculum, six different media were assessed against a selection of SRSV (n = 48) and MRSA (n = 12). Brain heart infusion agar yielded the highest sensitivity and specificity values: 88 and 88%, respectively.
Recently, a clinical isolate of Staphylococcus aureus (Mu50) for which the vancomycin MIC was 8 μg/ml was reported from Japan (10). The breakpoint of vancomycin for S. aureus as published by the British Society for Antimicrobial Chemotherapy is 4 μg/ml, and thus Mu50 is classified as resistant (5). For the NCCLS, there is a susceptible breakpoint of ≤4 μg/ml and a resistant breakpoint of ≥32 μg/ml; therefore, Mu50 is classified as intermediate (10, 16). Currently, cases of infection with vancomycin-intermediate strains still appear to be rare, with isolated reports mainly originating from France and the United States (6, 18, 19, 20).
A second type of vancomycin-intermediate resistance has been reported from Japan that appears to be much more common (10). These strains display a vancomycin MIC below the breakpoint but possess bacterial populations (ca. 1/106) which can grow in the presence of >4 μg of vancomycin per ml. Strains expressing these features are termed hetero-vancomycin-intermediate S. aureus (hVISA). These bacteria are also resistant to teicoplanin and thus are termed by some groups as glycopeptide-intermediate S. aureus (GISA) or hetero-GISA (hGISA). Clearly, the terminology used depends on which method is used to classify this type of resistance but, for the sake of simplicity, both GISA and hGISA can be referred to as Staphylococcus strains with reduced susceptibility to vancomycin (SRSV). Staphylococci displaying the heterogeneous phenotype have been isolated from various locations including the United States, Japan, and Europe (3, 8, 9, 10, 15, 19, 20, 21; Z. Gulay, T. Atay, M. Kucukguven, and N. Yulug, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother. [ICAAC], abstr. C-136, 1998).
It has been suggested that heteroresistance has been responsible for the failure of vancomycin therapy; however, clear clinical evidence for this is lacking. Before the prevalence and clinical relevance of hGISA can be assessed, a reliable method for their detection must be established. Several methods have been proposed, including the gradient plate, population studies (PS), and population analysis profiles (PAPs) (9, 13; M. Woottoon, R. A. Howe, T. R. Walsh, and A. P. MacGowan, Abstr. 38th ICAAC, abstr. C-139, 1998). A pilot study from a hospital in the United Kingdom examining 100 MRSA strains demonstrated that when the PAP was used as a reference method, PS gave a false-positive rate of 7% (22). Thus, before epidemiological data should be reported, attention must be given to the method of detection. Inoculum density and preparation, media, and incubation period, as well as the method of resistance detection, are all criteria that must be properly evaluated with a large population study. We describe here such an undertaking, in which 329 MRSA strains and other Staphylococcus strains were screened in a “double-blind study” comparing various methodologies that can be incorporated into routine laboratories and their ability to successfully detect SRSV as judged by population analysis profiles. This study also explores the relationship between SRSV and the hGISA phenotype.
MATERIALS AND METHODS
The bacterial strains used were Mu50 (GISA) and Mu3 (archetype hGISA) (9) and two MRSA strains, Smh26 and Smh2, from the Bristol Centre for Antimicrobial Research and Evaluation, Bristol, United Kingdom, displaying differing degrees of insusceptibility to vancomycin. In addition, 2 GISA strains, 2 GISE (glycopeptide-intermediate Staphylococcus epidermidis; strain collection from AB Biodisk, Solna, Sweden) strains, 2 clinical isolates from a patient with vancomycin therapy failure (A. Wanger, personal communication), and 49 clinical isolates of MRSA from the global SENTRY surveillance program (University of Iowa College of Medicine). SENTRY strains 281 to 294 were isolates from Brazil (n = 4), Chile (n = 2), Argentina (n = 5), Texas (n = 2), and Missouri (n = 1). SENTRY strains 295 to 329 included isolates from France (n = 6), Turkey (n = 1), Portugal (n = 5), Italy (n = 2), Poland (n = 2), Australia (n = 2), Hong Kong (n = 6), Singapore (n = 6), South Africa (n = 2), and Taiwan (n = 2). More than 100 MRSA strains (including some clinically significant MRSE strains) collected at Southmead Hospital between 1985 and 1997 were randomly mixed, with the other isolates often being duplicated. The number of SRSV, as determined by PAP-AUC ratios (below), totaled 45. All strains, except for the SENTRY collection, were often duplicated (blind repeats) to bring the total number of test strains to 329. Reference strains of S. aureus ATCC 29213 and ATCC 43300 were also included. All methods described here, apart from the PAP-AUC method, were carried out in duplicate.
PS.
The simplified population analysis method was carried out in a similar manner to that as described by Hiramatsu et al. (10). Specific inocula were plated out onto brain heart infusion (BHI) agar containing vancomycin (4 μg/ml). Growth at 24 h was deemed to be of the GISA phenotype, and growth occurring at between 24 and 48 h was deemed an hGISA strain (10). Isolates expressing these phenotypes were confirmed by subculturing onto BHI containing 8 μg of vancomycin per ml. The subculturing normally carried out for this method was not undertaken since it was deemed an inadequate procedure for routine laboratory testing (10).
PAP-AUC ratio.
Modified PAPs were performed as previously described by Wootton et al. (22; Wootton et al., 38th ICAAC). After 24 h of incubation in Tryptone Soyer broth (Oxoid, Basingstoke, Hampshire, United Kingdom), a neat culture and dilutions of 1/108 and 1/105 were spiral plated (Don Whitley spiral platers) onto BHI agar (Oxoid) plates containing 0.5, 1, 2, 2.5, 4, and 8 μg of vancomycin per ml. Colonies were counted after 48 h of incubation in air at 35°C. The numbers of CFU/milliliter was plotted against the vancomycin concentration by using GraphPad Prism (San Diego, Calif.). The modified PAPs were inspected visually, and the data were analyzed differently from previous reports (10). Using the log graph of viable count versus vancomycin concentration, the AUC was calculated for each strain. Every test sample run was accompanied by using the Mu3, Mu50, and ATCC Staphylococcus strains as controls. A ratio was then calculated by dividing the AUC of the test strain by the AUC of Mu3.
PAP-AUC criteria for the determination of vancomycin resistance, glycopeptide-intermediate Staphylococcus spp. (GIS), and hetero-GIS (hGIS) are as previously described (22) and are based on multiple tests with both Mu3 and Mu50: ≤0.90 = MRSA, 0.90 to 1.3 = hGIS, and ≥1.3 = GIS.
Vancomycin agar screen.
The method undertaken was exactly that described by Tenover et al. (20). All plates were inoculated by preparing, in sterile water, a suspension grown overnight from a blood agar plate equivalent to a 0.5 McFarland standard. A total of 10 μl of the suspension was dropped onto BHI agar containing vancomycin (6 μg/ml). Plates were incubated for 48 h, and growth was observed after both 24 and 48 h.
Modified vancomycin agar screen.
The method undertaken was exactly as described by Hubert et al. (14). All plates were inoculated by preparing, in sterile water, a suspension grown overnight from a blood agar plate equivalent to a 0.5 McFarland standard. Then, 10 μl of the suspension was dropped onto Mueller-Hinton agar (MHA) containing vancomycin (5 μg/ml). Plates were incubated for 48 h at 35°C, and growth was observed after both 24 and 48 h.
Broth microdilution.
The procedure undertaken was precisely as previously described in the NCCLS M7-A5 approved standard (16). Colonies were taken from overnight blood agar plates, and sterile saline was inoculated to make a 0.5 McFarland suspension inoculum. Cation-adjusted Mueller-Hinton broth (Oxoid) was inoculated with the suspension inoculum, grown at 35°C units, and read after 18 h of incubation. For both this method and the agar dilution, S. aureus strains ATCC 29213 and Mu50 were used as controls.
Agar dilution.
The procedure undertaken was precisely as previously described in the NCCLS M7-A5 approved standard (16). The inoculum was prepared as described for broth microdilution and further diluted such that the 104 CFU spots were delivered to the MHA (Oxoid) surface. The plates were incubated at 35°C units and read after 18 h of incubation.
Etest.
The Etest MICs were determined by using two inoculum densities, 0.5 and 2.0 McFarland values (A. Bolmström, Å. Karlsson, and P. Wong, unpublished results). Strains were grown overnight on blood agar plates. Randomly selected single colonies were inoculated into fresh Mueller-Hinton broth and grown overnight. The turbidity of the overnight broth was adjusted to 0.5 and 2.0 McFarland standards using fresh broth. Then, 200 μl of this suspension was pipetted onto a 90-mm BHI agar (BBL; Becton Dickinson, Cockeysville, Md.) plate and streaked out evenly with a swab. This method provided a consistent inoculum that was found to give more reproducible results when testing for this particular type of resistance than simply by allowing the swab to soak up the liquid inoculum. For detecting this type of resistance by this method, BBL was considered the medium of choice from previous studies (unpublished results). After being dried for approximately 10 min, Etest strips (AB BIODISK) for vancomycin (0.016 to 256 μg/ml) and teicoplanin (0.016 to 256 μg/ml) were applied. The direct colony suspension method was also performed on a subset of strains for which the overnight colonies were homogenized in Mueller-Hinton broth to an inoculum turbidity of either a 0.5 or a 2.0 McFarland standard. All plates were incubated at 35°C for 24 and 48 h and read three times by different people.
The detection of SRSV by using different media was also evaluated with the Etest. BHI (BBL), the Diagnostic Sensitivity Test agar (DST; Oxoid), Isosensitest agar (ISO; Oxoid), Isosensitest with 5% sheep's blood (ISOB; Oxoid), MHA (Oxoid), and Nutrient Agar (NA; Oxoid) were each tested examined to determine whether detectable differences occurred between medium preparations. Media were obtained from either Oxoid or BBL (Becton Dickinson).
Specificity and sensitivity.
The performance of each method in detecting SRSV (hGISA or GISA) was evaluated by comparison with the PAP-AUC ratio. Each method was assessed for its specificity and sensitivity in discriminating SRSV from MRSA strains (7). The specificity is based on the number of correct negative results, i.e., the true number of MRSA strains that were correctly identified. The sensitivity is based on the number of SRSV that were correctly identified.
RESULTS
Prior to this particular study, the reproducibility of the PAP-AUC was assessed by numerous repeated tests carried out over a period of several months. For Mu3 (n = 14) and Mu50 (n = 15), the PAP-AUC had a calculated mean of 0.997 ± a standard deviation (SD) of 0.09 (9.0%) and 1.615 ± 0.19 (11.7%). This method has shown itself to be more robust compared to the PS since it is likely to just detect resistance rather than select for it (13, 22; Wootton et al., 38th ICAAC).
The sensitivities and specificities for the different methods used in this study are shown in Table 1. The PS method correctly identified 32 vancomycin-resistant Staphylococcus strains (VRS) but gave 34 false positives (10.3%) and 13 false negatives, thus giving a sensitivity of 71% and a specificity of 88%. The false-negative result is due to the lack of detection of strains displaying the heterogeneous phenotype that have originated from the United States and the United Kingdom. The false-positive results had no correlation to the increased vancomycin or teicoplanin MICs found by any of the other methods, except for two strains where Etest (2.0 McFarland) MICs for teicoplanin were ≥12 μg/ml (data not shown). Of the new isolates displaying the VRS phenotype arising from the study (Texas and SENTRY program isolates), the PS method correctly identified (as judged by a PAP-AUC ratio of >0.90) 16 of 20 strains (Table 2). The PAP-AUC ratio of these isolates ranged from 0.98 to 3.01 (data not shown).
TABLE 1.
Sensitivity and specificity values for the different methods in discriminating hGIS and GIS from MRSA
| Method | Correct no. of GIS and/or hGIS identified (n = 45) | No. of false positives | No. of false negatives | % Sensitivity | % Specificity |
|---|---|---|---|---|---|
| PS | 32 | 34 | 13 | 71 | 88 |
| Broth dilution-vancomycin | 5 | 0 | 40 | 11 | 100 |
| Broth dilution-teicoplanin | 18 | 1 | 27 | 40 | >99 |
| Agar dilution-vancomycin | 9 | 0 | 36 | 20 | 100 |
| Agar dilution-teicoplanin | 21 | 0 | 24 | 47 | 100 |
| Etest, 0.5 McFarland | 37 | 20 | 8 | 82 | 93 |
| Etest, 2.0 McFarland | 43 | 7 | 2 | 96 | 97 |
| BHI screening method | 10 | 8 | 35 | 22 | 97 |
| Mueller-Hinton screening method | 9 | 3 | 36 | 20 | 99 |
TABLE 2.
PAP-AUC ratio, PS, and MIC values of new strains identified in the present studya
| Isolate | PAP-AUC ratio | PS identification | No. of strains resistant or intermediate to:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vancomycin
|
Teicoplanin
|
|||||||||
| Etest (0.5)b | Etest (2.0) | AD | BD | Etest (0.5) | Etest (2.0) | AD | BD | |||
| 23-3400A | 1.2 | MRSA | 4 | 8 | 4 | 1 | 8 | 8 | 2 | 2 |
| MDR25 | 1.07 | hGISA | 4 | 12 | 4 | 1 | 12 | 12 | 4 | 4 |
| MDR38 | 1.08 | MRSA | 4 | 8 | 2 | 1 | 4 | 8 | 2 | 1 |
| MDR51 | 1.01 | hGISA | 4 | 4 | 4 | 1 | 8 | 12 | 8 | 4 |
| MDR58 | 1.15 | MRSA | 4 | 4 | 4 | 1 | 6 | 12 | 4 | 2 |
| MDR61 | 1.08 | hGISA | 6 | 8 | 4 | 1 | 12 | 16 | 4 | 2 |
| MDR63 | 0.96 | MRSA | 4 | 4 | 4 | 2 | 4 | 6 | 4 | 4 |
| MDR65 | 3.01 | GISA | 4 | 12 | 4 | 2 | 12 | 12 | 4 | 4 |
| MDR79 | 1.21 | GISA | 2 | 4 | 4 | 2 | 6 | 12 | 8 | 4 |
| MDR99 | 1.33 | GISA | 4 | 8 | 4 | 2 | 12 | 12 | 4 | 4 |
| MDR101 | 1.32 | GISA | 4 | 6 | 4 | 2 | 12 | 12 | 8 | 4 |
| MDR104 | 1.25 | GISA | 4 | 8 | 4 | 2 | 6 | 8 | 4 | 4 |
| MDR106 | 1.08 | hGISA | 3 | 6 | 2 | 1 | 6 | 8 | 4 | 4 |
| MDR152 | 1.14 | hGISA | 4 | 8 | 4 | 2 | 4 | 12 | 8 | 4 |
Boldface type signifies where the method correctly identified an SRSV as judged by the PAP-AUC ratio. AD, agar dilution; BD, broth dilution. McFarland standard values are given in parentheses.
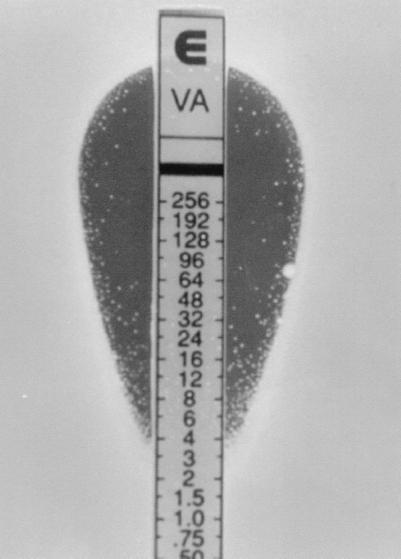
Etest using both the 0.5 and the 2.0 McFarland inocula gave both a high sensitivity and a high specificity (Table 1). The Etest interpretative criteria for the 2.0 McFarland inocula to detect SRSV were ≥8 μg of vancomycin per ml plus ≥8 μg of teicoplanin per ml or ≥12 μg of teicoplanin per ml. Etest values of 6 μg/ml were not converted to the next upper twofold value. Based on these criteria, Etest correctly detected 43 hGIS and/or GIS strains, giving only 7 false positives and 2 false negatives, at a sensitivity of 96% and a specificity of 97%. The Etest teicoplanin interpretative criteria of ≥12 μg/ml detected a further 9 of the 45 positives that would have been missed by the criteria of vancomycin and teicoplanin of ≥8 μg/ml only. The results read at 24 h (unpublished) did not differ greatly from those at 48 h and did not drastically alter the specificity or sensitivity of the test. However, the microcolonies associated with heterogeneously resistant populations were clearer and easier to read when incubated for 48 h (Fig. 1).
FIG. 1.
Typical Etest result when using the macromethod of Mu50 possessing a PAP-AUC of 1.51. The microcolonies appear only after the plates have been incubated for 48 h. In this particular instance and under these conditions, cells can grow at up to a vancomycin concentration of 48 μg/ml.
The best cutoff values to detect SRSV using the 0.5 McFarland Etest method was vancomycin at ≥4 μg/ml plus teicoplanin at ≥6 μg/ml. Using these criteria, the sensitivity and specificity were 82 and 93%, respectively, the discrepancy arising from the large number of false positives (19). Both the 0.5 and the 2.0 McFarland inocula successfully detected GISA strain Mu50, hGISA strains Mu3 and Smh26, and GISE strain 2218, with minor errors for hGISA strain Smh2 and GISE strain 18518.
Agar dilution using the NCCLS breakpoint criteria for vancomycin (I = 8 to 16, R ≥ 32 μg/ml) and teicoplanin (I = 16, R ≥ 32 μg/ml) (16) detected only 9 of 45 and 21 of 45 SRSV strains, respectively (Table 1). Of these, the majority of were strains possessing a PAP-AUC ratio of ≥1.50 rather than strains conferring the heteroresistant phenotype (PAP-AUC ratio of 0.90 to 1.29). For vancomycin, NCCLS broth microdilution detected only 5 of 45 hGIS or GIS and had a sensitivity of 11% and a specificity of 100%. The teicoplanin results for the broth microdilution faired better, with a sensitivity of 40% and a specificity of >99%. If a flagging system were to be used for strains for which the MIC was ≥4 mg/liter; highlighting potentially resistant strains, particularly for the agar dilution method, then 28 of 45 hGISA or GISA would have been correctly identified (results not shown). These data confirm the NCCLS and Centers for Disease Control (CDC) guidelines that strains possessing a vancomycin MIC of ≥4 μg/ml should be assessed more closely. Of the confirmed GISA and GISE isolates tested, their vancomycin resistance was detected at 100% by agar dilution but only at ca. 60% by microdilution (results not shown). New VRS isolates arising from the SENTRY program and the results of the various methods are summarized in Table 2.
The screening methods previously reported as a first-line detection system were also evaluated in this study. Surprisingly, both methods mainly detected stains demonstrating the homogeneous type of resistance, as shown by the PAP-AUC ratios. The sensitivities and specificities for these methods using BHI and Mueller-Hinton media were 22 and 97% and 20 and 99%, respectively. These methods were repeated in duplicate, and only once did a discrepancy occur. This one result was repeated, and the third result was recorded in the data set. The poor level of sensitivity of this method is largely due to the high number of false negatives, mainly for hGISA.
The results suggest that the routine method of choice in screening for SRSV is the Etest using the higher inoculum. However, this was performed on BHI, a medium not generally used for susceptibility testing. Therefore, a selection of VRS (hGISA, n = 37; GISA, n = 11; and MRSA, n = 12) was used to test different media with the Etest. The Etest interpretative criteria determined for BHI were also used for the other media. Compared to the PAP-AUC results, BHI gave the highest sensitivity and specificity: 88 and 88%, respectively. NA was found to have a reasonable sensitivity and specificity (77 and 67%), followed by DST medium (54 and 63%), ISOB (46 and 57%), MHA (44 and 55%) and, finally, ISO (42 and 53%). The data indicate that BHI is the medium of choice in discriminating SRSV from MRSA when using the Etest at the “macro” inoculum (i.e., a 2 McFarland density).
All of the methods showed high reproducibility when the results for the duplicated strains were compared against each other (data not shown). Both agar screening methods also showed good reproducibility, with only one strain giving ambiguous results when tested in duplicate. There were no major errors associated with the PAP-AUC method, since all duplicate results repeatedly fell into the same MRSA, hGISA, and GISA categories. PAP-AUC results for the duplicate strains showed a maximum variation of <10%, excluding the one strain that was 16% but did not affect the classification of this strain into the above categories. When duplicated results were compared for the simple population method (10), four strains tested as both MRSA and hVISA, and one strain tested as both hVISA and VISA.
DISCUSSION
From the PAP-AUC ratios determined for isolates displaying the vancomycin-resistant phenotype, cutoff values were given for the GISA and hGISA phenotypes based on Mu3 and Mu50. This method has been specifically designed to detect “resistance” rather than to select for it, circumventing the high number of false positives found with other population methods. The cut of 0.90 for VRS is based not only on the profile continually presented by Mu3, possibly the best characterized of all the hGISA, but also by the distribution of all strains examined in this study. The PAP-AUC method has been shown to be highly reproducible with an SD of <10%, which has also been used to assess the criteria for the 0.90 cutoff value. Examination of the organisms' PAP-AUC ratios presented a distinct demarcation line between those stains possessing a PAP-AUC of <0.9 and those with a PAP-AUC ratio of >1.0. In fact, only two strains gave a PAP-AUC of between 0.8 and 0.9 (0.81 and 0.83) and were not positive by any other method.
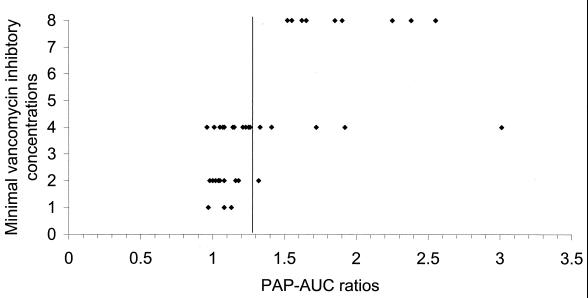
Occasionally, strains would give a PAP-AUC ratio similar to that for Mu50 but have a vancomycin MIC of ≤4 μg/ml. The MIC (using agar dilution) plotted against the PAP-AUC demonstrated that there is no obvious correlation between the two methods (Fig. 2). The serial dilution of the MIC methodology simply may not be sensitive enough to detect finite changes in the staphylococcal response to vancomycin that may be detected by detailed population profiles.
FIG. 2.
Correlation of agar dilution vancomycin MIC values compared to PAP-AUCs. The demarcation line shown delineates hGIS and GIS based on repeated PAP-AUC ratios from strain Mu50. Strains possessing a PAP-AUC ratio of >1.3 have populations as resistant as Mu50 and, by this method, have been characterized as GIS. In some instances, strains with a significant PAP-AUC ratio had a vancomycin MIC of only 4 μg/ml.
The emergence of S. aureus with reduced susceptibility to vancomycin arising from MRSA is of utmost concern. It is particularly worrisome if there are heteroresistant strains that appear to be susceptible by conventional testing but can express resistance at a low frequency. These strains, which are currently difficult to detect in most clinical laboratories, may potentially give rise to strains homogeneously resistant to vancomycin in vivo. Furthermore, it has been recently been shown that such isolates adhere to artificial surfaces 5- to 20-fold more than does MRSA, providing a further problem for its management in nosocomial environments (T. R. Walsh, M. Wootton, R. A. Howe, P. M. Bennett, and A. P. MacGowan, Abstr. 39th ICAAC, abstr. C-44, 1999). The debate as to whether hVRSA are responsible for clinical failures can only be resolved when meaningful surveillance studies can be undertaken to statistically correlate the results with glycopeptide consumption and clinical outcome. However, there exists the possibility that the epidemiology of hGISA is mimicking that seen in the heteroresistance to methicillin which was studied some years ago (1, 2). Thus, it would be prudent to critically review some of the commonly used methods to detect the heterogeneously glycopeptide-resistant phenotypes.
The results from this double-blind multicenter study clearly demonstrate that the PS method differs significantly from PAP-AUC ratios in discriminating SRSV from MRSA. This, in part, may explain the high incidence of SRSV from surveillance studies performed by the PS method (8, 10). The incidence figures have generally ranged from 8 to 10%, numbers that coincide with the level of false positives (10.3%) found in this study. Surveillance studies using the PAP-AUC ratio have so far detected SRSV at <1% (22). The method proposed for the detection of hGISA by Hiramatsu et al. is similar to the method used by Daum et al. to select S. aureus resistant to vancomycin, and thus it remains unclear whether this method detects vancomycin resistance or selects for it (4, 10).
Standardized reference methods for susceptibility testing, namely, NCCLS broth microdilution and agar dilution, also performed suboptimally in detecting hGISA. This is probably due to the small inoculum and relatively poorer support of growth on MHA. Since the resistance phenotype is only present in ca. 1 of 106 cells, the resistant cells are simply not present in large enough numbers in the starting inoculum of these methods. It has been recently suggested that the flagging of staphylococcus strains with a vancomycin MIC of ≥4 mg/liter should be undertaken and sent to an appropriate reference laboratory (16). Interestingly, in this study, such criteria would have detected 28 (many hGIS) of the 45 positives with agar dilution but fewer with broth microdilution. The data from this study support the initiatives of the CDC and the NCCLS. Given the inoculum used in standard antimicrobial testing methods, it is perhaps unreasonable to expect these methods to detect such small resistant subpopulations, i.e., hGISA. The Etest, in contrast to reference methods, could be effectively adapted to a higher inoculum and a richer medium such as BHI to optimize the detection of hGISA. The Etest breakpoints of vancomycin at ≥8 μg/ml and teicoplanin at ≥8 μg/ml or teicoplanin at ≥12 μg/ml performed surprisingly well and circumvented the “inoculum problem” that tends to occur with staphylococcus-glycopeptide interactions. The predefined, stable gradient concept was found to be largely “inoculum tolerant” in maintaining the categorical results of susceptible strains despite the high inoculum used. Only 7 of 329 (2.1%) MRSA strains were falsely identified as SRSV. The Etest with an inoculum of 0.5 performed well using the criteria of vancomycin at ≥4 mg/liter and teicoplanin at ≥6 mg/liter. However, this was shown to detect too many false positives, largely due to the low vancomycin cutoff level. Raising this level would significantly reduced the number of hGISA and GISA detected; therefore, this method does not appear to be as sensitive as the macromethod using the higher inoculum.
This study indicates that the use of the Etest under the macromethod conditions described elsewhere (4) provides a reliable and sensitive method for the detection of subtle variations in glycopeptide resistance, including heteroresistance, without incurring too many false positives. However, it is also recommended that all SRSV-positive results by Etest be confirmed with PAP-AUC ratios and, as far as possible, by the clinical outcome of therapy.
ACKNOWLEDGMENTS
We thank Ron Jones and Michael Pfaller (University of Iowa, College of Medicine, Anti-Infectives Research Center Iowa City, Iowa) of the SENTRY surveillance program for the donation of strains.
REFERENCES
- 1.Ayliffe G A J. The progressive intercontinetal spread of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1997;24(Suppl. 1):S74–S79. doi: 10.1093/clinids/24.supplement_1.s74. [DOI] [PubMed] [Google Scholar]
- 2.Berger-Bachi B, Strassle A, Kayser F H. Characterisation of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986;5:697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- 3.Bierbaum G, Fuchs K, Lenz W, Szekat C, Sahl H G. Presence of Staphylococcus aureus with reduced susceptibility to vancomycin in Germany. Eur J Clin Microbiol Infect Dis. 1999;18:691–696. doi: 10.1007/s100960050380. [DOI] [PubMed] [Google Scholar]
- 4.Boyle-Vavara S, Berke S K, Lee J C, Daum R S. Reversion of the glycopeptide resistance phenotype in Staphylococcus aureus clinical isolates. Antimicrob Agent Chemother. 2000;44:272–277. doi: 10.1128/aac.44.2.272-277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.British Society of Antimicrobial Chemotherapy. BSAC News, Spring 1998. London, England: British Society of Antimicrobial Chemotherapy; 1998. Standard disc sensitivity testing method; p. 9. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States. Morb Mortal Wkly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 7.Fleiss J L. An introduction to applied probability. In: Fleiss J L, editor. Statistical methods for rates and proportions, 2nd ed. Wiley Series in Probability and Statistics. New York, N.Y: John Wiley & Sons, Inc.; 1981. pp. 1–8. [Google Scholar]
- 8.Geisel R, Schmitz F J, Thomas L, Berns G, Zetsche O, Ulrich B. Emergence of heterogeneous intermediate vancomycin resistance in Staphylococcus aureus isolates in the Dusseldorf area. J Antimicrob Chemother. 1999;43:846–848. doi: 10.1093/jac/43.6.846. [DOI] [PubMed] [Google Scholar]
- 9.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 11.Howe R A, Bowker K B, Walsh T R, Feest T G, MacGowan A P. Vancomycin-resistant Staphylococcus aureus. Lancet. 1998;351:602. doi: 10.1016/S0140-6736(05)78597-4. [DOI] [PubMed] [Google Scholar]
- 12.Howe R A, Wootton M, Bennett P M, MacGowan A P, Walsh T R. Interactions between methicillin and vancomycin in Staphylococcus aureus displaying different phenotypes of vancomycin susceptibility: a new method to discriminate hetero-vancomycin resistance. J Clin Microbiol. 1999;37:3068–3071. doi: 10.1128/jcm.37.9.3068-3071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe R A, Wootton M, Walsh T R, Bennett P M, MacGowan A P. Heterogeneous resistance to vancomycin in Staphylococcus aureus. J Antimicrob Chemother. 2000;45:130–131. doi: 10.1093/jac/45.1.130. [DOI] [PubMed] [Google Scholar]
- 14.Hubert S K, Mohammed J M, Fridkin S K, Gaynes R P, MacGowan J E, Tenover F C. Glycopeptide intermediate Staphylococcus aureus: evaluation of a novel screening method and results of a survey of selected U.S. hospitals. J Clin Microbiol. 1999;37:3590–3593. doi: 10.1128/jcm.37.11.3590-3593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchese A, Balistreri G, Toneli E, Debbia E A, Schito G C. Heterogeneous vancomycin resistance in methicillin-resistant Staphylococcus aureus strains isolated in a large Italian hospital. J Clin Microbiol. 2000;38:866–869. doi: 10.1128/jcm.38.2.866-869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7–A5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 17.Pfeltz R F, Singh V K, Schmidt J L, Batten M A, Baranyk C S, Nadak M J, Jayaswal R K, Wilkinson B J. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental background. Antimicrob Agents Chemother. 2000;44:294–303. doi: 10.1128/aac.44.2.294-303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploy M C, Grelaud C, Martin C, de Lumley L, Denis F. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet. 1998;351:1212. doi: 10.1016/s0140-6736(05)79166-2. [DOI] [PubMed] [Google Scholar]
- 19.Smith T L, Pearson M L, Wilcox K R, Cosme Cruz P H, Lancaster M V, Robinson-Dunn B, Tenover F C, Zervos M J, Band J D, White E, Jarvis W R. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 20.Tenover F C, Lancaster M, Hill B, Steward C, Socker S, Hancock G, O'Hara C, Clark N, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong S S, Ho P L, Woo P C, Yuen K Y. Bacteraemia caused by staphylococci with inducible vancomycin resistance. Clin Infect Dis. 1999;29:760–770. doi: 10.1086/520429. [DOI] [PubMed] [Google Scholar]
- 22.Wootton M, Howe R A, Hillman R, Walsh T R, Bennett P M, MacGowan A P. A modified population analysis profile method to detect Staphylococcus aureus with decreased susceptibilities to vancomycin in a UK hospital. J Antimicrob Chemother. 2001;47:399–404. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]