Abstract
A recent clinical trial found no effect of chronic intranasal oxytocin on social behaviour in children with autism spectrum disorders. The result is not surprising, as oxytocin facilitates social learning but does not directly cause prosocial behaviour. In future trials, oxytocin should be paired with behavioural therapy to enhance learning and improve social behaviour.
A phase II clinical trial that tested the ability of intranasal oxytocin to improve social behaviour in children with autism spectrum disorders (ASD) recently made headlines for unambiguously demonstrating no clinical effect1. However, many of us in the oxytocin research community were unsurprised by these negative results. In fact, the results are consistent with what we have been advocating for years: oxytocin enhances social salience rather than social behaviour, and for oxytocin administration to be therapeutic and influence behaviour, it must be paired with an appropriate social context2–6.
Published in The New England Journal of Medicine, the randomized, double-blind, parallel-group, placebo-controlled study by Sikich et al. was rigorous, robust, and thorough in both design and execution1. Children and adolescents with ASD aged 3–17 years received twice-daily target doses of 24–40 international units (IU) of intranasal oxytocin or placebo for a maximum of 24 weeks. Social behaviour was assessed by the primary outcome — a clinical questionnaire completed by parents or guardians at 4-week intervals — and secondary outcomes that included additional social behavioural assessments and an IQ test. Subtler neurocognitive effects of the sort often reported in the preclinical literature were not assessed, but such effects would not be clinically meaningful without appreciable behavioural changes. Data were collected from 277 of the 290 participants enrolled across seven academic sites, a sample size more than adequate to detect an effect of clinically meaningful size. However, oxytocin administration had no effect on any of the outcome measures. As ASD is highly heterogeneous, it remains possible that chronic oxytocin could be beneficial for a small phenotypic subset of individuals despite this effect being undetectable in large, diverse populations. Nevertheless, these results align with those of a clinical trial from Japan, published in 2020, and provide strong evidence that children should not be prescribed chronic intranasal oxytocin independently of context7.
Although chronic, context-independent pharmacological intervention is often effective in other organ systems — for example, the use of angiotensin-converting enzyme (ACE) inhibitors to lower blood pressure — this type of approach is likely to be less effective for addressing complex psychiatric issues such as social behavioural deficits2,6. Indeed, oxytocin does not directly cause prosocial behaviour in the mechanistic sense in which ACE inhibitors directly lower blood pressure. Instead, oxytocin changes the way in which incoming social stimuli are perceived and processed, which can influence behaviour and social learning in various ways8.
Oxytocin increases the salience of social stimuli and fine-tunes neural processes so that an organism can better attend and respond to those stimuli9. The drug enhances the signal-to-noise ratio of social information in brain regions that process incoming sensory signals and facilitates the flow of that information to areas involved in affective and cognitive processes, reward learning and memory8,9. The behavioural effects of this increased social salience depend largely on context and the type of social information the organism receives. This effect of context is one reason the literature has shown ostensibly contradictory behavioural effects of oxytoxin: the hormone can enhance trust or suspicion, affiliation or aggression, sexual arousal or learning and memory. Given the context-dependent nature of oxytocin’s effects, the fact that Sikich et al.1 found no effect of context-independent oxytocin administration is unsurprising.
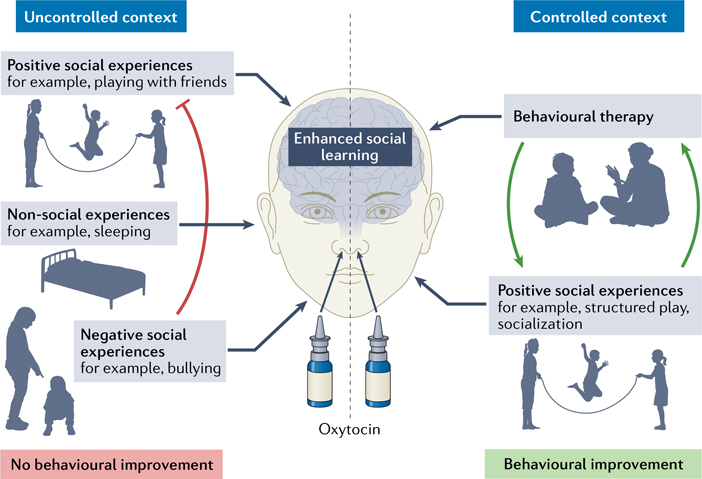
So how should we approach the use of oxytocin to treat the social deficits of ASD? Evidence that ASD is caused by decreased oxytocin signalling is lacking, and now we have compelling evidence that chronic administration of oxytocin is not beneficial. However, oxytocin is a powerful neuromodulator: it makes the brain more attentive to social stimuli, promotes synaptic plasticity, and facilitates social learning by modulating neural circuits8,9. Therefore, oxytocin administration might open a brief window of time for the brain to learn new social information and behaviours. We believe that oxytocin should be used as a tool to prime the brain for receiving, processing and learning social information during cognitive and behavioural therapy interventions4,6 (FIG. 1).
Fig. 1 |. The effects of oxytocin depend on social context.
Oxytocin enhances the salience of social information and primes the brain for social learning. Administering oxytocin without controlling social context is unlikely to yield clinically beneficial results, and learning from negative experiences might counteract learning from positive experiences (red line). To improve social behaviour, oxytocin administration should be paired with opportunities for constructive social learning, such as behavioural therapy sessions and positive social experiences, which can positively reinforce learned social behaviours (green arrows).
Cognitive and behavioural therapies can effectively address the social deficits of ASD, but up to 40 h of therapy is required per week. Administration of oxytocin immediately before therapy might enhance the efficacy of therapy and reduce the number of hours required to achieve results. Oxytocin might also be beneficial when administered before positive social experiences, with the aim of promoting social engagement and reinforcing skills learned in therapy. In addition, oxytocin-assisted therapy might be most effective during sensitive periods very early in life when the brain can more easily learn social skills through experience, similar to the way that language is learnt10. Future research must elucidate the interaction between oxytocin administration and social context so that interventions can be designed to activate the appropriate neural circuitry and influence clinically meaningful behaviours6,8.
Even in a therapeutic context, intranasal oxytocin is a suboptimal method for enhancing oxytocin signalling. Oxytocin has a short half-life, its ability to penetrate the blood–brain barrier is hotly contested, and its ability to diffuse intracerebrally to reach relevant brain areas has been questioned2,4,6. These limitations are likely to contribute to the relatively small effect sizes observed in previous studies of intranasal oxytocin — studies that use radiolabelled imaging probes to examine the dynamics of oxytocin in the human brain are greatly needed. Nevertheless, no other clinical intervention intended to increase the signalling of a neurochemical involves administering the neurochemical itself. Instead, the compounds that are administered increase the neurochemical’s synthesis (for example, levodopa for dopamine), synaptic release (for example, amphetamines for dopamine and norepinephrine), time in synapses (for example, acetyl-cholinesterase inhibitors for acetylcholine or SSRIs for serotonin) or effect on receptors (for example, benzodiazepines and barbiturates for GABA). We also use compounds that bind directly to the target receptor (for example, opioids). Therefore, effective clinical oxytocin intervention might require a second generation of therapeutics — compounds that cross the blood–brain barrier and either enhance endogenous oxytocin signalling or bind specifically to oxytocin receptors3,4,6
Although the data presented by Sikich et al.1 clearly demonstrate that chronic intranasal oxytocin administration independent of context is not beneficial for children with ASD, they do not provide a reason to abandon translational oxytocin research. On the contrary, these data are a clarion call for investigators to re-evaluate flawed assumptions, re-examine oxytocin neurophysiology and redesign interventional approaches. Oxytocin is a powerful tool, but chronic supplementation is not the correct application. Enhancing oxytocin signalling remains a promising therapeutic possibility that must continue to be pursued with this newfound knowledge. Future clinical studies must explore the potential of pairing oxytocin with therapeutic contexts, and we must redouble translational efforts to design second-generation pharmacotherapies that enhance endogenous oxytocin signalling.
Acknowledgements
This work was supported by NIH grants P50MH100023 and R01MH112788 to L.J.Y. and NIH P51OD011132 to Yerkes National Primate Research Center.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Sikich L et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N. Engl. J. Med 385, 1462–1473 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young LJ When too much of a good thing is bad: chronic oxytocin, development, and social impairments. Biol. Psychiatry 74, 160–161 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Young LJ Oxytocin, social cognition and psychiatry. Neuropsychopharmacology 40, 243–244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young LJ & Barrett CE Can oxytocin treat autism? Science 347, 825–826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamay-Tsoory SG & Abu-Akel A The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Ford CL & Young LJ Translational opportunities for circuit-based social neuroscience: advancing 21st century psychiatry. Curr. Opin. Neurobiol 68, 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamasue H et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol. Psychiatry 25, 1849–1858 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Froemke RC & Young LJ Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci 44, 359–381 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walum H & Young LJ The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci 19, 643–654 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMayo MM, Young LJ, Hickie IB, Song YJC & Guastella AJ Circuits for social learning: a unified model and application to autism spectrum disorder. Neurosci. Biobehav. Rev 107, 388–398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]