Abstract
INTRODUCTION:
Bowel movement frequency (BM) is used to titrate lactulose for hepatic encephalopathy (HE). However, stool consistency using the Bristol stool scale (BSS, 0–7) is often ignored.
METHODS:
Pre/post cohorts:
BSS was incorporated into decision-making after training in outpatients with cirrhosis. 2–3BMs/day and BSS 3–4 were considered normal while the rest were considered high or low; concordance between the metrics was evaluated. Medication changes and 6-month admissions were compared between this group (post-BSS) to a comparable previous group (pre-BSS). Concordance and regression analyses for all and HE-related admissions were performed, and comparisons were made for HE-related medication stability.
Longitudinal:
An outpatient group seen twice was analyzed for BSS and BMs.
RESULTS:
Pre/post cohorts:
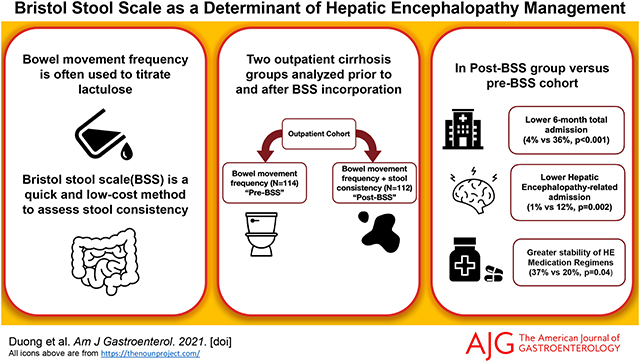
Post-BSS, 112 patients were included with only 46% BSS and BMs concordance and modest BSS/BMs correlation (r=0.27,p=0.005). Compared to a pre-BSS cohort (N=114) there was a lower 6-month total (4% vs.36%,p<0.001) or HE-related admission(1% vs.12%,p=0.002). Regression showed MELD (OR:1.10,p=0.003) and pre/post-BSS (OR:0.04,p<0.001) for all admissions and HE (OR:3.59,p=0.04) and Pre/post (OR:0.16,p=0.02) for HE-related admissions as significant. HE-medication regimens were more stable post-BSS vs pre-BSS (32% vs 20%,p=0.04), which was due to patients with BSS>BMs (p=0.02).
Longitudinal:
33 patients without medication changes or underlying clinical status changes were tested 36±24 days apart. No change in BSS(p=0.73) or BMs (p=0.19) were found.
CONCLUSIONS:
BSS is complementary and additive to bowel movement frequency, can modulate the risk of readmissions and stabilize HE-related therapy changes in outpatients with cirrhosis, and could help personalize HE management.
Keywords: admissions, patient-reported outcomes, bowel movements, opioids, lactulose, adherence
Graphical Abstract
INTRODUCTION:
Hepatic encephalopathy (HE) is a major burden on the patients, families, and healthcare systems through frequent hospitalizations, falls and poor psychosocial outcomes(1). The first-line therapy for HE recurrence prevention and treatment is lactulose(1, 2). While the mechanism of action is unclear, the dogma for lactulose prescription remains achieving at least 2–3 soft bowel movements (BM) daily(1). Lactulose can result in multiple GI adverse events, has a high rate of non-adherence within Western patients and often leads to multiple medication changes but also can precipitate acute kidney injury, hyponatremia, and worsening of hepatic encephalopathy. Thus, caution should be applied when using bowel frequency alone as an endpoint for lactulose therapy.(3). Moreover, this one size fits all policy does not consider baseline bowel movement frequency or consistency and is not associated with objective cognitive performance data(4). Therefore, we need to amalgamate other criteria to potentially reduce admissions and reduce changes in medications. The Bristol stool scale (BSS) is a patient-reported characterization of the bowel movement consistency that has been validated in several conditions but has not been used in patients with cirrhosis(5–7). Our hypothesis is that BSS would complement the BM frequency in modifying the risk for admissions and HE occurrence and stabilize the treatment course in outpatients with cirrhosis.
METHODS:
We performed two separate analyses across outpatients with cirrhosis (Figure 1). For all analyses data were collected regarding daily BSS and BMs after showing patients and caregivers a visual Bristol Stool Scale chart. BSS ranges from 1 through 7 (Supplementary table 1) and 3–4 were considered normal; 5–7 were considered high and 1–2 were considered low. BMs between 2–3/day were considered normal while 0–1/day were low while ≥4BMs/day were considered high. Concordance and discordance between BMs and BSS were analyzed and correlations between BMs and BSS were performed. For example, if BSS is high and BM normal that indicates BSS>BM; on the other hand, if BSS is low and BM normal, that indicates BSS < BM.
Figure 1:
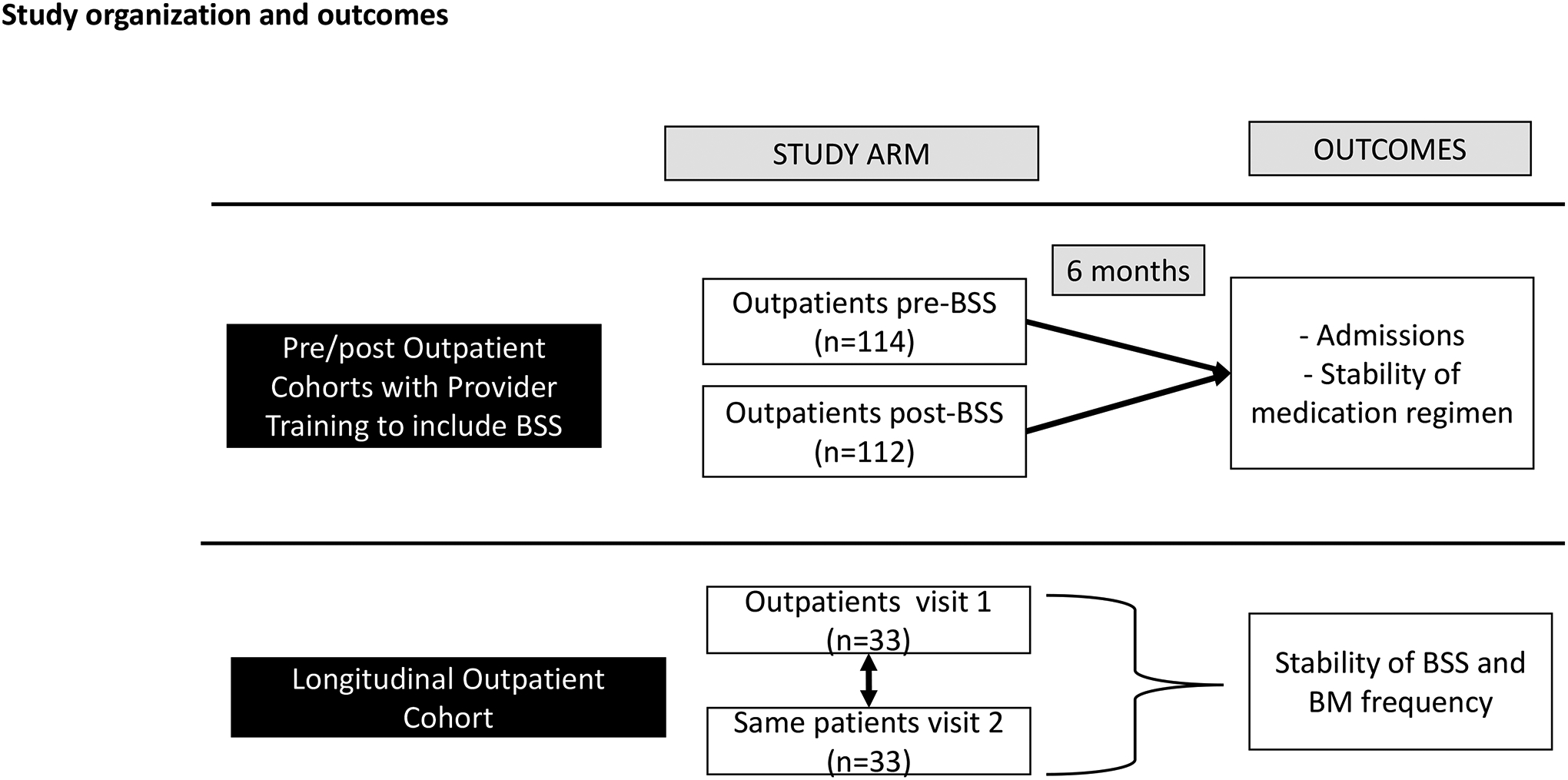
Overview of the study design and outcomes showing the two arms.
Two outpatient groups were studied. The first group comprised of patients with cirrhosis who were seen in the GI and Hepatology clinics at the Richmond Veterans Hospital before implementation of BSS when only BM frequencies were used to develop plans for HE therapies and monitoring as needed as per standard of care. Demographics and disease details, including presence of HE, use and dose of lactulose, rifaximin, opioids, use of fiber, and other laxatives were evaluated, and patients were followed for 6 months for HE-related medication changes or admissions, especially related to HE. We excluded patients with concomitant inflammatory bowel disease, colon resection, those with recent (<3 month) change in opioids, and those with current or recent (within 6 months) C.difficile or other diarrheal infections.
After this, the outpatient hepatology team at the Richmond VAMC (Fellows, Nurse Practitioners and attendings) was trained to assess for BSS for every outpatient, regardless of HE status and lactulose use. This included showing the patients and caregivers a visual of the BSS on the computer and ask them to pick their usual stool form after they had given us their daily BM frequency. The team was asked to incorporate the BSS with the BMs in HE therapy initiation and monitoring, including slowing the rate of lactulose increase in those with BSS that was higher than BMs and considering rifaximin initiation earlier in patients with higher baseline BSS scores. This guidance was only advisory for the clinicians, and no specific standard operating procedure was adopted. This was also to ensure that the clinician(s) could individualize care. Clinicians involved in both time-periods were the same from an attending (JSB, BCD) and NP perspective (ZM, MLG and AM), but the fellows that were supervised by the attendings differed due to the rotations. Similar data as collected for the pre-BSS cohort were recorded. In addition, we performed correlations between BSS and BMs. Again, outcomes and HE-related medication changes (started lactulose, stopped lactulose, reduced/increased lactulose or added rifaximin) over the next 6 months were recorded and compared to the pre-BSS time-period.
We compared outcomes (stability of HE-related medications, all and HE-related admissions over 6 months) between pre and post-BSS groups using unpaired parametric or non-parametric tests as appropriate. Adherence on medications was defined by direct questioning of the patient during return clinical encounters or hospitalizations, filling of the medications using the VA pharmacy and patient contact through phone calls as needed during clinical follow-up. Also, binary logistic regression using backwards elimination was performed for future admissions using all demographic information, cirrhosis severity and medications and pre-BSS vs post-BSS timepoints. Only variables with p<0.10 were included in the multi-variable analysis.
Longitudinal analysis:
Analysis was performed in a separate group of outpatients with cirrhosis who were stable clinically without medication changes and were evaluated twice over 3 months with BSS and BMs. These were assessed for changes over time using paired t-tests.
This was a quality improvement analysis performed across both hospitals.
RESULTS:
Pre/post Outpatient cohorts:
We included 114 patients in the pre-BSS and 112 patients in the post-BSS outpatient cohort (Table 1). Both cohorts were statistically similar with respect to demographics, HE, and medication details.
Table 1:
Comparison of Pre and post-Bristol Stool Scale outpatient cohorts
| Prior Cohort without BSS (N=114) | Cohort with BSS included (N=112) | P value | |
|---|---|---|---|
| Age | 61.0±6.01 | 62.3±12.2 | 0.57 |
| Male sex | 103 (90%) | 98 (88%) | 0.49 |
| MELD score | 12.6±5.6 | 12.3±5.4 | 0.30 |
| Ascites | 52 (46%) | 49 (44%) | 0.77 |
| Prior HE | 50 (44%) | 56 (50%) | 0.36 |
| Lactulose | 50 (44%) | 56 (50%) | 0.36 |
| Lactulose dose (ml) | 24.5±28.6 | 27.8±38.2 | 0.49 |
| Rifaximin | 20 (18%) | 28 (25%) | 0.18 |
| Opioids | 24 (21%) | 18 (16%) | 0.36 |
| Other laxatives | 22 (19%) | 14 (13%) | 0.16 |
| Fiber | 10 (9%) | 14 (13%) | 0.37 |
| Daily bowel movements | 2.1±1.2 | 2.3±1.0 | 0.67 |
| Outcomes | |||
| Stable course of HE medications over 6 months | 22 (20%) | 37 (32%) | 0.04 |
| Future admissions over 6 months | 41 (36%) | 5 (4%) | <0.0001 |
| Future HE-related admission over 6 months | 14 (12%) | 1 (1%) | 0.002 |
BSS: Bristol Stool Scale; HE: hepatic encephalopathy
Analysis within BSS-incorporated cohort:
In the cohort of patients where BSS and BMs were both incorporated after training hepatology staff, only 46% were concordant while 44% had higher BSS than daily BMs (Table 2). There was a modest correlation between the two metrics (Figure 2A). Of the 51 concordant subjects, 46 had both BMs and BSS that were low while only 5 had both high BSS and BM metrics.
Table 2:
Details of the cohort who had Bristol Stool scale data collected
| Cross-sectional outpatients (n=112) | Concordance between BSS and daily BMs | P value (ANOVA, χ2 test) | ||
|---|---|---|---|---|
| BSS<BMs (n=12) | Concordant (n=51) | BSS>BMs (n=49) | ||
| Age (years) | 63.3±9.7 | 62.2±9.3 | 62.2±9.3 | 0.96 |
| Male sex | 9 (75%) | 48 (94%) | 41 (84%) | 0.11 |
| MELD score | 11.6±4.1 | 12.3±5.0 | 12.3±6.1 | 0.90 |
| BSS | 2.6±1.3 | 3.8±0.6 | 5.0±1.0 | <0.0001 |
| Daily BMs | 2.0±1.2 | 2.5±0.8 | 2.2±1.1 | 0.09 |
| Prior HE | 7 (58%) | 32 (63%) | 39 (80%) | 0.92 |
| On rifaximin? | 4 (33%) | 25 (49%) | 20 (41%) | 0.53 |
| On Lactulose? | 5 (42%) | 31 (61%) | 29 (59%) | 0.48 |
| Lactulose dose (ml) | 15.0±19.2 | 23.0±26.9 | 36.1±49.0 | 0.11 |
| Other laxatives | 4 (33%) | 2 (4%) | 8 (16%) | 0.01 |
| Fiber | 1 (8%) | 6 (12%) | 7 (14%) | 0.83 |
| Opioid use | 3 (25%) | 4 (8%) | 5 (10%) | 0.30 |
| Antibiotics | 1 (8%) | 2 (4%) | 2 (4%) | 0.35 |
BSS: Bristol Stool Scale, BM: bowel movements, HE: hepatic encephalopathy
Figure 2:
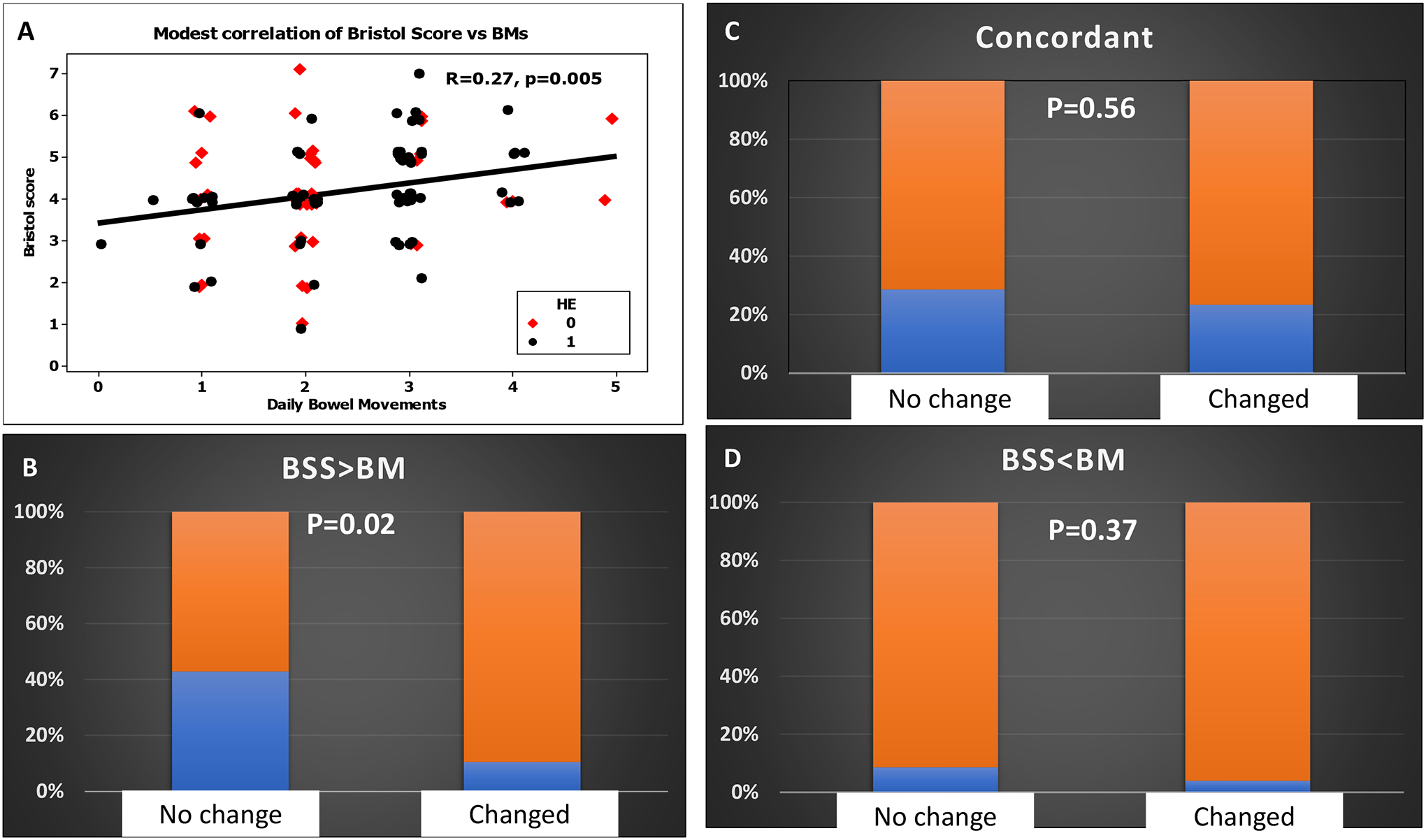
A: Modest correlation of Bristol Stool Scale (BSS) and daily bowel movements (BMs) in outpatients with red diamonds indicating those with prior HE and black circles those without prior HE
B: Proportion of patients with BSS>BMs (blue portion) versus the rest (orange) in those who required HE-related medication changes or not over the next 90 days was statistically significant
C: Proportion of patients with concordant BSS and BMs (blue portion) versus the rest (orange) in those who required HE-related medication changes or not over the next 90 days was statistically similar.
D: Proportion of patients with BSS<BMs (blue portion) versus the rest (orange) in those who required HE-related medication changes or not over the next 90 days was statistically similar.
There were no major differences in disease severity and other medications apart from other laxatives in those who had differences between BSS and daily BMs in outpatients. Patients using rifaximin and lactulose had a higher daily BM rate compared to those who were not. However, BSS was similar regardless of lactulose and rifaximin use. Daily BMs and BSS were not significantly different between those on opioids, laxatives, fiber, and antibiotics compared to those who were not (Table 3).
Table 3:
Comparison of Medications, BSS, and BMs
| Outpatients (n=112) | Daily Bowel Movements | P value | Bristol Stool Scale | P value | ||
|---|---|---|---|---|---|---|
| Without | With | Without | With | |||
| Prior HE | 2.1±0.9 | 2.4±0.9 | 0.07 | 4.1±1.3 | 4.2±1.1 | 0.82 |
| Lactulose yes/no | 2.1±1.1 | 2.5±0.9 | 0.05 | 4.0±1.3 | 4.3±1.1 | 0.27 |
| Rifaximin yes/no | 2.1±1.0 | 2.7±0.9 | 0.001 | 4.1±1.2 | 4.3±1.1 | 0.36 |
| Opioids | 2.4±1.0 | 2.1±1.0 | 0.39 | 4.2±1.2 | 4.3±1.4 | 0.84 |
| Other laxatives | 2.4±0.9 | 2.2±1.5 | 0.69 | 4.2±1.2 | 4.1±1.4 | 0.78 |
| Fiber | 2.3±1.0 | 2.5±1.2 | 0.55 | 4.2±1.2 | 4.1±1.1 | 0.72 |
| Antibiotics | 2.3±1.0 | 3.2±1.1 | 0.14 | 4.2±1.2 | 4.0±2.0 | 0.85 |
Comparison of bowel movements and Bristol Stool scale between those with/without the conditions or those on or not on medications listed on the left column.
Medication changes within 90 days:
A significantly higher rate of patients in BSS group had stable medications related to HE compared to those without BSS incorporated into HE therapy (Table 1). Of the 37 patients in the BSS group that had stable medications, 15 had BSS>BMs, 10 had concordant and 3 had BSS<BMs. When compared to those where HE-related medications were changed, a higher proportion of patients who had BSS>BMs had stable HE-related medications (p=0.02) but the comparisons were statistically similar between BSS<BMs and concordant patients (Figure 2B–D).
Admission rates:
In addition, the prior cohort had a significantly higher 3-month total and HE-related admission rates compared to those where BSS were incorporated. HE-related admissions were seen in 14 patients; the remainder were due to ascites (n=13), GI bleeding (n=9), infection (n=5) and liver unrelated (n=6). None of the five admissions occurred in concordant patients: two with low BSS and high BMs and three with high BSS and low BMs. One admission was because of HE while the remaining were due to ascites (n=2), infection (n=1) and GU bleeding (n=1) in this period.
Regression analyses:
On logistic regression pre vs post era, MELD score, antibiotic use and opioids had p<0.1 on univariable analysis, of which only MELD score (OR 1.10, CI: 1.03–1.18, p=0.003) and pre vs post (OR:0.04, CI: 0.02–0.12) remained significantly associated with admissions. When only HE-related admissions were considered, MELD score, prior HE and pre/post time-periods were significant on univariable analysis, but only prior HE (OR: 3.59, CI: 1.02: 12.7, p=0.04) and Pre/post (OR: 0.16, CI: 0.03–0.75, p=0.02) were significant.
Longitudinal outpatient cohort:
Thirty-three patients (age 60.2±12.9 years, 31 men) were seen 36±24 days apart. Of these 22 had prior HE with all being on lactulose and 17 on rifaximin. Twenty patients had ascites, four had HCC and two had prior SBP. Eight patients were on opioids. MELD score did not change significantly over time (11.8±3.5 versus 12.1±4.8, p=0.24) and none of the medications were changed in the interim. No change in BSS (4.3±1.3 vs 4.4±1.5,p=0.73) or BMs (2.4±0.9 vs 2.7±1.6,p=0.19) were found between the two timepoints (Figure 3).
Figure 3:
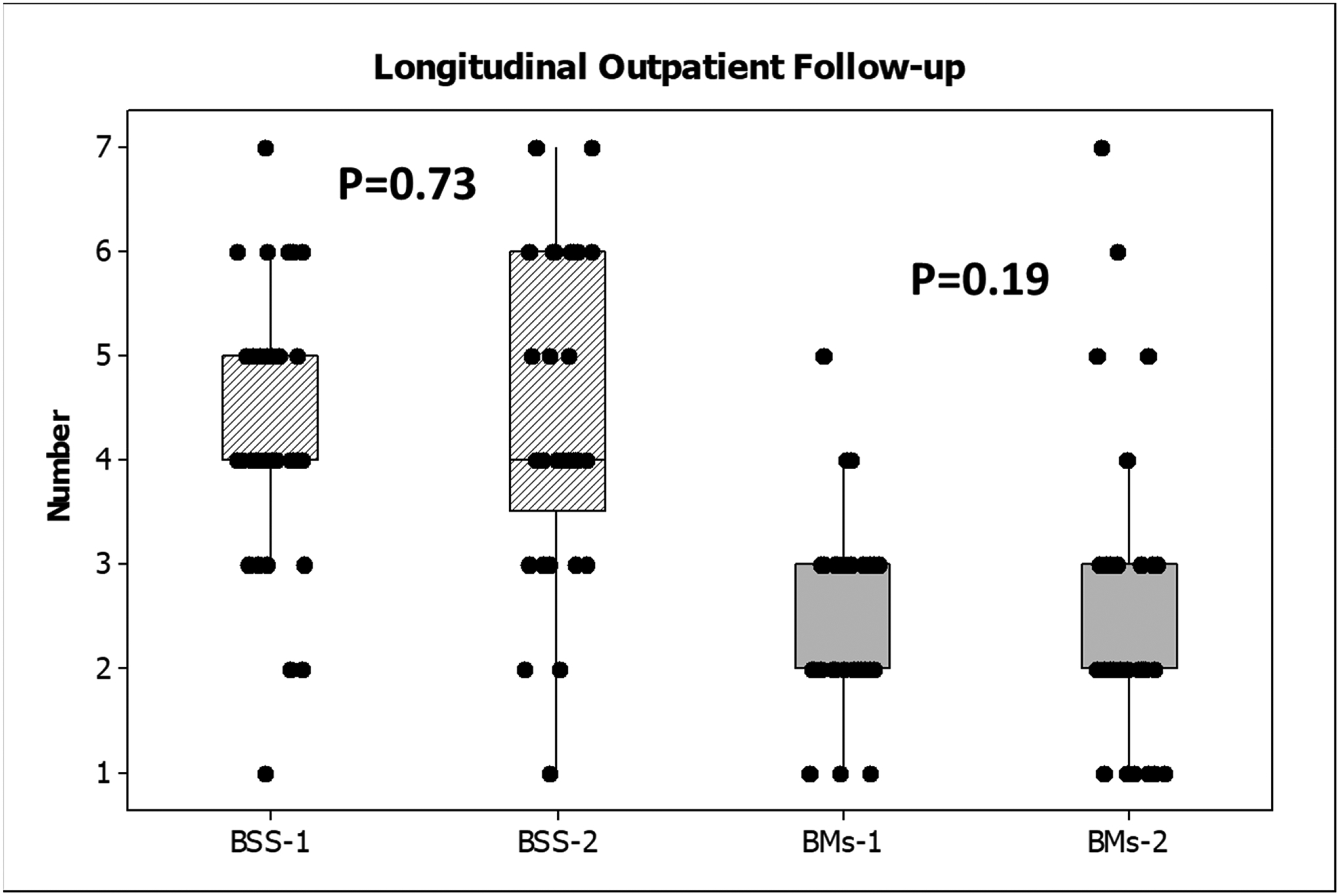
Longitudinal follow-up in outpatients showed no significant difference in Bristol stool scales (hashed bars) or bowel movements (plain gray bars) at visits 1 or 2. Data are presented as median and 95% CI with individual values.
DISCUSSION:
Our results show that the Bristol stool scale (BSS) is complementary and additive to bowel movement (BM) frequency in modulating several important outcomes in outpatients with cirrhosis. BSS and BM frequency are modestly correlated, and almost half of the patients exhibit discordance between these stool consistency as measured by BSS and daily stool frequency.
Patients with cirrhosis, especially those with HE, are prescribed lactulose under the assumption that achieving a set number of BMs implies efficacy(1, 8). However, this dogma is being increasingly challenged with the recent publication from our group determining that regardless of HE or lactulose use, cognitive impairment was not associated with BM frequency(4). The focus on number of BMs and driving doses of lactulose beyond that would be tolerable to most Western patients often leads to readmissions due to non-adherence(3, 9). HE remains the major cause of readmissions, many of which are driven by medication non-adherence to lactulose(10–12). In addition, performing cognitive testing routinely even using simple tests remains beyond the logistic workflow of most practices(13, 14). Therefore, we need other therapeutic targets and modalities that can improve this approach.
The two outpatient cohorts were relatively balanced with respect to most factors that affect BMs and BSS, and the BM frequency and cirrhosis severity was comparable. We included patients regardless of lactulose use and recorded data pertaining to other laxatives, fiber use, antibiotics, and opioids in order to make the results generalizable (15). All of these could impact BMs and BSS and are often used in patients with cirrhosis regardless of HE. The modest correlation and major discordance between daily BMs and BSS are striking. This shows that inquiring only BM frequency only gives an incomplete picture, especially since discordance could have clinical implications such as changes in HE-related medications.
More importantly, we observed a striking reduction in admissions over 6 months, especially related to HE, when BSS was incorporated into the decision-making compared to when only daily BMs were used. The only difference between the two cohorts was the knowledge of the medical teams of the BSS since the medications available, cirrhosis severity and complications and clinical practices were similar during both cohorts. The proximate impact of BSS incorporation was reflected in a greater stability of the HE-related medication regimen compared to the cohort in whom only BMs were used as a biomarker of medication efficacy. This could have tempered the push by the clinicians to increase the lactulose dose based on inputs from both metrics, which could potentially prevent recurrence due to non-adherence or hospitalizations due to dehydration, hypernatremia, acute kidney injury, electrolyte abnormalities because of overuse. This was further confirmed by a greater stability of HE-related medication changes in patients whose BSS>BMs. This demonstrates that higher BSS could counteract the relatively low BMs/day and further increasing lactulose or initiating lactulose in these patients may not add more to the clinical efficacy of medications. Moreover, daily BMs and BSS in patients seen over a month apart were relatively stable that mirrored their disease course. This increases confidence in the stability of this metric in outpatients.
While it is unclear why the BM consistency could have an impact on cirrhosis outcomes other than HE, prior studies have shown that BSS is a major contributor towards stool microbiota change, that could help in cirrhosis-related outcomes(16, 17). Therefore, it could be likely that the reduction in admissions over time could be extended to causes other than HE. While other measures such as acidification, laxative and prebiotic action of lactulose have been considered, the modulation of BSS without necessarily changing BM frequency could result in a potential microbiome-related benefit that could improve the outcomes without the need to push for higher daily BMs and their attendant problems(18, 19). Further studies are needed to examine these changes.
We chose BSS because of the relative familiarity of clinicians with this instrument and the pictorial interface that enhances patient communication with minimal time and effort(20). BSS has been used extensively in intestinal disorders, but we expanded this into the cirrhosis and HE field(6, 7, 20–22). We also wanted to encourage greater patient participation in their care by focusing on BM consistency as well as frequency, which could integrate BSS as a patient-reported outcome that informs the treating teams(23). The results show that within the liver specialty clinics, there was a high uptake of BSS incorporation that portends well for general GI practices who could be more likely to inquire about the BM frequency and consistency in all patients rather than those on lactulose alone compared to liver-focused practices(6, 21).
Our study is limited due to the relatively modest sample sizes from two institutions and a cross-sectional design for the pre/post BSS component. However, the longitudinal analyses add a valuable dimension. We also followed outpatients for six months given the relative rarity of outcomes in outpatients.
We conclude that the Bristol stool scale adds to bowel movement frequency in our characterization of the impact of HE-related therapies in patients with cirrhosis in stable outpatient settings. Bristol stool scale could be used to complement the information provided by bowel movement frequencies in modulating complications of cirrhosis.
Supplementary Material
STUDY HIGHLIGHTS:
WHAT IS KNOWN
Lactulose is first-line therapy for hepatic encephalopathy (HE) and is titrated to bowel movement frequency in clinical practice.
Recent data suggest that bowel movement frequency is not associated with cognitive function in cirrhosis
Current practice based on the daily bowel movement frequency alone could worsen adherence and outcomes in HE through lactulose overuse or underuse.
Bristol stool scale (BSS) is a low-cost, quick, and non-invasive method to assess stool consistency, but it needs validation in patients with cirrhosis.
WHAT IS NEW HERE
When clinic staff were trained to incorporate BSS in addition to daily BMs in their decision making, there was a reduction in HE-related medication changes, as well as all-cause and HE-related admissions compared to a previous outpatient cohort where decision making was based on BM frequency alone.
Daily BM frequency and BSS were modestly correlated and remained stable over time in a separate group re-evaluated without medication changes.
Changes in HE-related medications was higher in those with BSS>BMs indicating an additive impact of BSS in reducing unnecessary medication alterations.
Incorporation of the BSS with BM frequency may help to tailor treatment of HE to be more personalized and reduce medication changes and negative outcomes.
Conflict of interest:
• Guarantor of the article: Jasmohan S Bajaj
• Specific author contributions: NKD, SS, JSB, DP, OS, AF, ZM, MLG, AM collected data, NKD, JSB analyzed the data and produced the first draft, BCD provided critical revision and input, JSB conceptualized the study. All authors approved the final draft.
• Financial support: VA Merit Review 2I0CX00176, R21TR003095 and RO1HS025412 to JSB
• Potential competing interests: None for any author
References:
- 1.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Sharma BC, Agrawal A, et al. Primary prophylaxis of overt hepatic encephalopathy in patients with cirrhosis: an open labeled randomized controlled trial of lactulose versus no lactulose. J Gastroenterol Hepatol 2012;27:1329–35. [DOI] [PubMed] [Google Scholar]
- 3.Rathi S, Fagan A, Wade JB, et al. Patient Acceptance of Lactulose Varies Between Indian and American Cohorts: Implications for Comparing and Designing Global Hepatic Encephalopathy Trials. J Clin Exp Hepatol 2018;8:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duong N, Reuter B, Saraireh H, et al. Bowel Movement Frequency Is Not Linked With Cognitive Function in Cirrhosis. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 6.Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Techniques in Coloproctology 2001;5:163–164. [DOI] [PubMed] [Google Scholar]
- 7.Caroff DA, Edelstein PH, Hamilton K, et al. The Bristol stool scale and its relationship to Clostridium difficile infection. J Clin Microbiol 2014;52:3437–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Lauridsen M, Tapper EB, et al. Important Unresolved Questions in the Management of Hepatic Encephalopathy: An ISHEN Consensus. Am J Gastroenterol 2020;115:989–1002. [DOI] [PubMed] [Google Scholar]
- 9.Kalaitzakis E, Simren M, Olsson R, et al. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol 2006;41:1464–72. [DOI] [PubMed] [Google Scholar]
- 10.Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients With Cirrhosis: A Multistate Population-based Cohort Study. Clin Gastroenterol Hepatol 2016;14:1181–1188 e2. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology 2016;64:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj JS, Sanyal AJ, Bell D, et al. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther 2010;31:1012–7. [DOI] [PubMed] [Google Scholar]
- 13.Campagna F, Montagnese S, Ridola L, et al. The animal naming test: An easy tool for the assessment of hepatic encephalopathy. Hepatology 2017;66:198–208. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj JS, Etemadian A, Hafeezullah M, et al. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology 2007;45:833–4. [DOI] [PubMed] [Google Scholar]
- 15.Moon AM, Jiang Y, Rogal SS, et al. Opioid prescriptions are associated with hepatic encephalopathy in a national cohort of patients with compensated cirrhosis. Aliment Pharmacol Ther 2020;51:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandeputte D, Falony G, Vieira-Silva S, et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016;65:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatton G, Shawcross DL. Is treating the gut microbiome the key to achieving better outcomes in cirrhosis? Expert Rev Gastroenterol Hepatol 2019;13:1–2. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Chauhan A. Use of Lactulose in Hepatic Encephalopathy: Is It Time to Shift Targets? Clin Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 19.Duong NK, Heuman DM, Bajaj JS. Lactulose may not pass the “Acid” test in Hepatic Encephalopathy. Clin Gastroenterol Hepatol 2021. [Google Scholar]
- 20.Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2016;44:693–703. [DOI] [PubMed] [Google Scholar]
- 21.Saad RJ, Rao SS, Koch KL, et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol 2010;105:403–11. [DOI] [PubMed] [Google Scholar]
- 22.Hoekman DR, Lowenberg M, van den Brink GR, et al. A prospective study comparing patient-reported outcomes in Crohn’s disease. Eur J Gastroenterol Hepatol 2020;32:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volk ML, Fisher N, Fontana RJ. Patient knowledge about disease self-management in cirrhosis. Am J Gastroenterol 2013;108:302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


