Abstract
Although classically known as an endocrine signal produced by the ovary, 17β-estradiol (E2) is also a neurosteroid produced in neurons and astrocytes in the brain of many different species. In this review, we provide a comprehensive overview of the localization, regulation, sex differences, and physiological/pathological roles of brain-derived E2 (BDE2). Much of what we know regarding the functional roles of BDE2 has come from studies using specific inhibitors of the E2 synthesis enzyme, aromatase, as well as the recent development of conditional forebrain neuron-specific and astrocyte-specific aromatase knockout mouse models. The evidence from these studies support a critical role for neuron-derived E2 (NDE2) in the regulation of synaptic plasticity, memory, socio-sexual behavior, sexual differentiation, reproduction, injury-induced reactive gliosis, and neuroprotection. Furthermore, we review evidence that astrocyte-derived E2 (ADE2) is induced following brain injury/ischemia, and plays a key role in reactive gliosis, neuroprotection, and cognitive preservation. Finally, we conclude by discussing the key controversies and challenges in this area, as well as potential future directions for the field.
Keywords: 17β-Estradiol, Neurosteroid, Aromatase, Neuroprotection, Synaptic plasticity, Memory, Gliosis, Sexual behavior
1. Introduction
Aromatase is a cytochrome P450 enzyme that drives conversion of androgen precursors into estrogens (Fig. 1) (Blakemore and Naftolin, 2016; Simpson et al., 2002). The aromatase-driven catalysis process involves hydroxylation of androgen precursors using three molecules each of NADPH and oxygen to produce one molecule of estrogen (Ryan, 1959). Aromatase is encoded by a single gene, CYP19, which is located on the 21.2 region of chromosome 15 in humans (Simpson et al., 2002). This gene is 123 kb in length, and is expressed in many tissues, including the gonads, bone, breast, adipose, vascular tissue, skin, placenta and brain (Stocco, 2012). Tissue-specific transcripts of aromatase are produced from the alternative use of several first exons that are promoter-specific (Fig. 2) (Bulun et al., 2004; Simpson et al., 1993). Splicing of the untranslated first exons into the coding exons 2 through 10 produces multiple different aromatase transcripts; however, all of the transcripts code for the same protein. Exon 1.f has classically been considered to be the brain-specific variant. However, ovarian-specific exon PII and adipose-specific exons 1.3 and 1.4 are also expressed in the brain of rodents and humans (Prange-Kiel et al., 2016; Yague et al., 2006). It should be mentioned that teleost fish are unique in that they have two aromatase isoforms, CYP19a which encodes aromatase A, and CYP19b, which encodes aromatase B (Tchoudakova and Callard, 1998). CYP19a is expressed in the gonads, while CYP19b is expressed in both the brain and gonads. Although these two genes are structurally different, they have similar catalytic activities and over 20 different regulatory sites in the promoter, including response elements for sex steroid receptors, and several transcription factors that regulate neurogenesis (Piferrer and Blazquez, 2005).
Fig. 1. Simplified Biosynthetic Pathway for Estrogens.
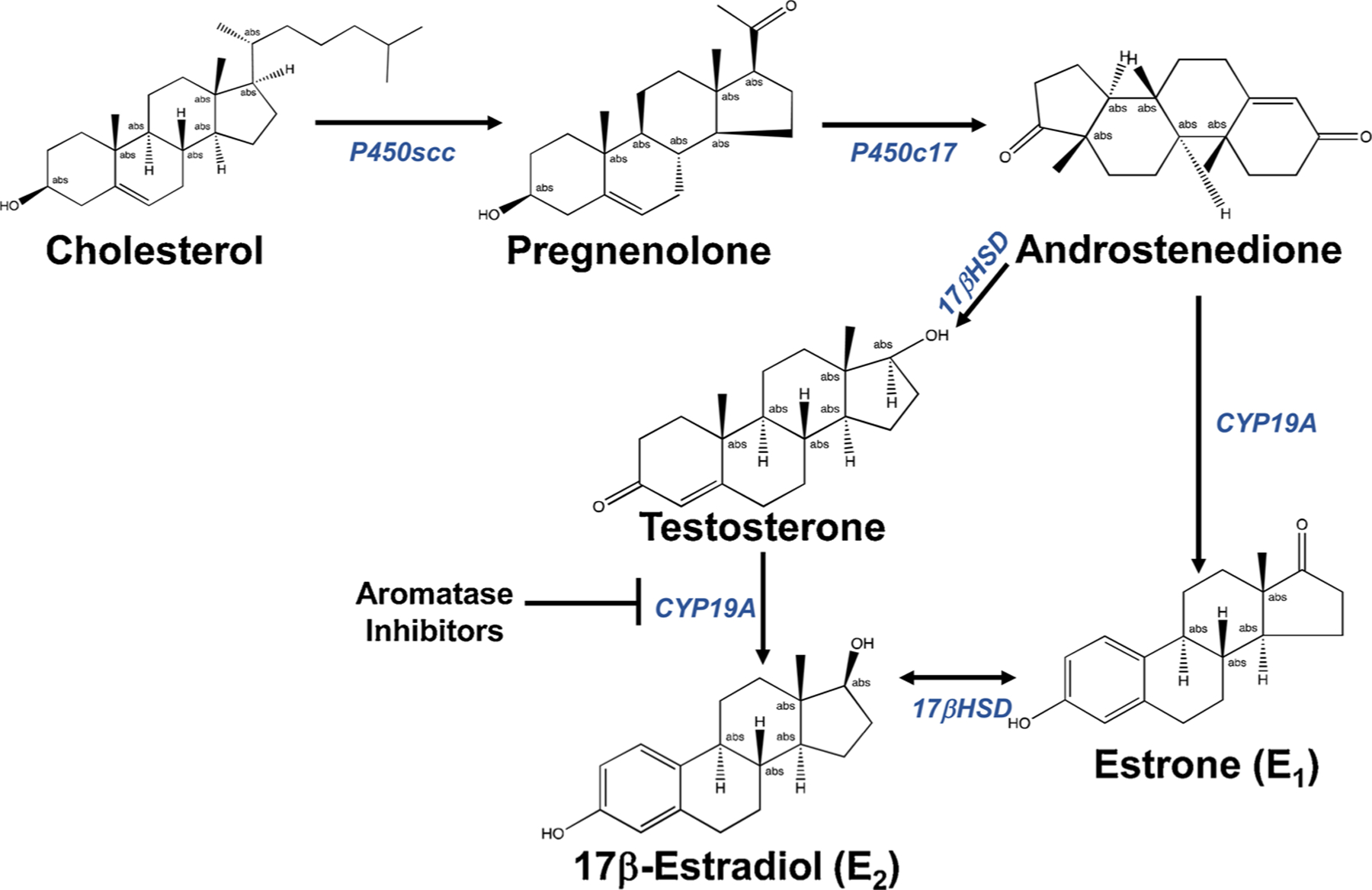
Estrogen synthesis begins with conversion of cholesterol to pregnenolone in mitochondria. Through a series of steps, pregnenolone is converted into androstenedione, which is converted into testosterone and estrone (E1). Testosterone is then converted into 17β-estradiol (E2) through the action of aromatase (CYP19A). As also shown, CYP19A can be inhibited by various aromatase inhibitors for research purposes and for therapies. Chemical structures were generated from the ChemSpider webpage (http://www.chemspider.com).
Fig. 2. Partial Aromatase Gene Structure.
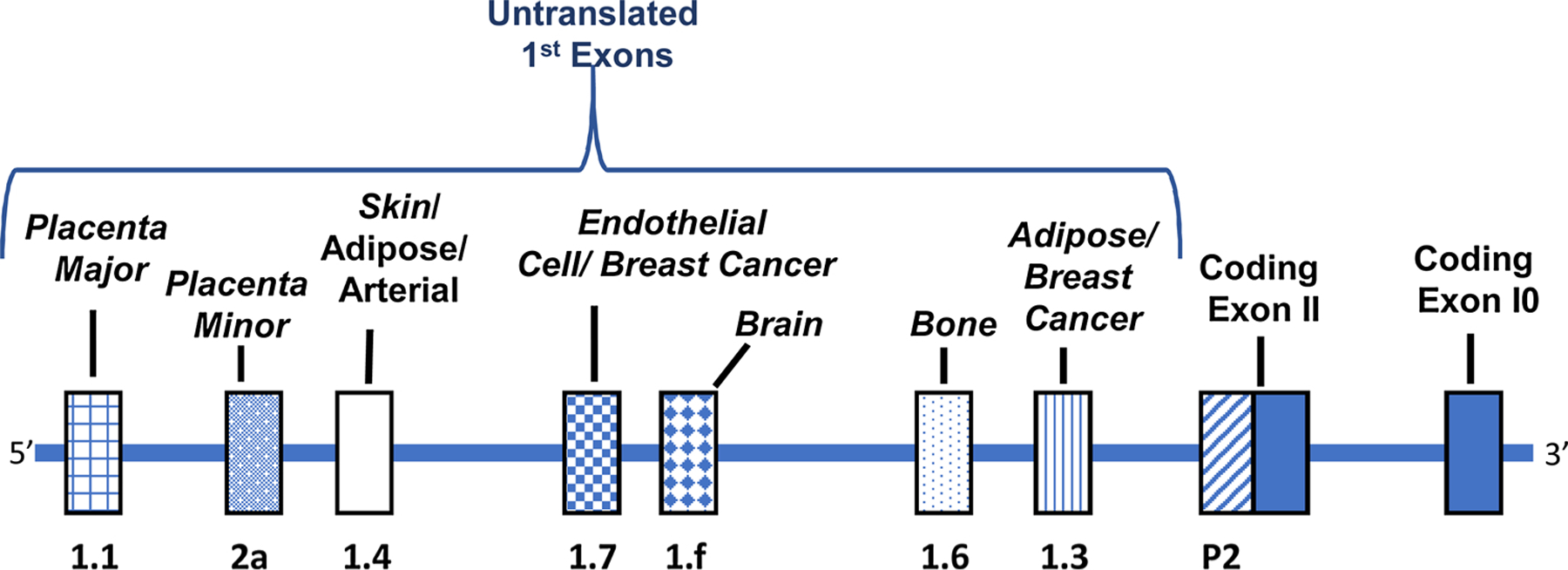
Tissue-specific promoters in untranslated first exons are responsible for tissue-specific transcripts of aromatase. Promoter 1.f is typically considered the brain-specific promoter; however, promoters 1.3 and 1.4 have also been reported to be expressed in the brain.
Estrogens, the product of aromatase activation, are steroid hormones that can act upon estrogen receptors in tissues throughout the body and brain. The most potent and most studied estrogen is 17β-estradiol (E2), while the other estrogens, estrone (E1) and estriol (E3), are considered weak estrogens. E2 has been implicated in the regulation of many diverse physiological and pathological processes, including reproduction, sexual differentiation and behavior, cancer biology, bone physiology, synaptic plasticity, cognitive function, anti-inflammatory actions and neuroprotection (Azcoitia et al., 2018; Boon et al., 2010; Brann et al., 2007; Brocca and Garcia-Segura, 2019; Cortez et al., 2010; Dhandapani and Brann, 2003; Emmanuelle et al., 2021; Khan et al., 2013; Kramar et al., 2013; Saldanha, 2020; Vegeto et al., 2008). While the role of gonadal-derived E2 has been studied extensively, the roles and functions of brain-derived E2 (BDE2) has received less attention and has only recently begun to be fully appreciated. Hence, this review will focus on the localization, regulation and functions of BDE2 in the brain. Much of the work in this area has been conducted in rodents and the songbird. However, where available, we will present and discuss findings in other species including humans and non-human primates. Much of what we know about the roles and functions of BDE2 in the brain has come from studies using pharmacological aromatase inhibitors (see Fig. 3). However, since both neurons and astrocytes can produce E2, using such a cell non-specific pharmacological approach provides challenges in determining the specific role of neuron-derived E2 (NDE2) versus astrocyte-derived E2 (ADE2) in the brain. Whole body global aromatase knockout mice support a role for E2 in anti-inflammation, synaptic plasticity and cognition, and neuroprotection from neurodegenerative disorders (Simpson et al., 2002). However, these studies are poorly suited to distinguish the role and specific contributions of brain-derived versus gonadal-derived aromatase/E2 to these effects. Recent work by our group (Lu et al., 2019, 2020; Wang et al., 2020) using brain cell-specific aromatase knockout animal models has helped address this issue and given important insights on the respective roles and functions of NDE2 versus ADE2 in the brain in both physiological and pathological states. We will review this emerging work, as well as discuss existing controversies, and potential future directions for advancement of knowledge in this important area.
Fig. 3. Primary aromatase inhibitors used to inhibit aromatase activity in the brain in animals and humans.
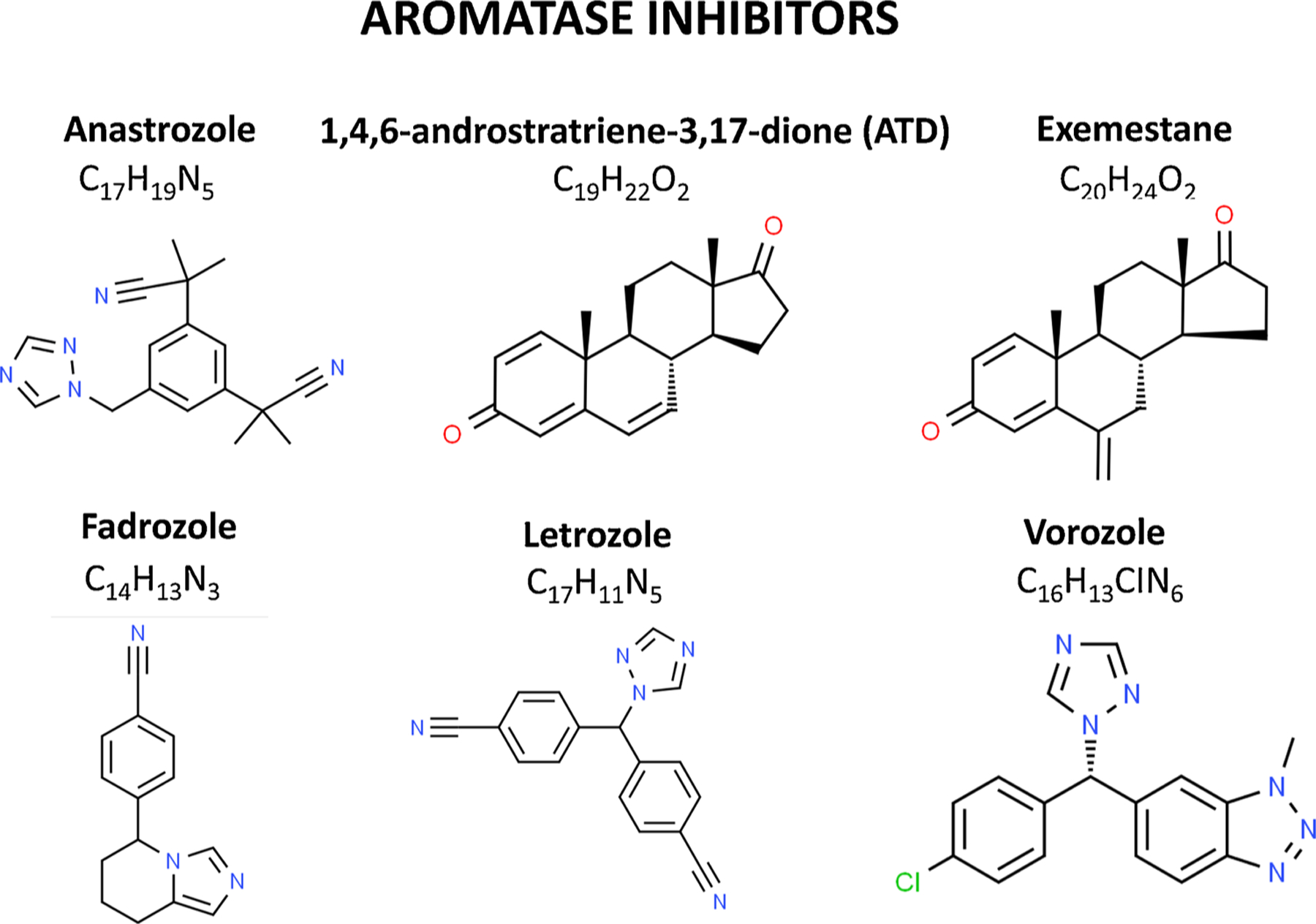
Chemical structures were generated from the ChemSpider webpage (http://www.chemspider.com).
2. Aromatase localization in the brain
2.1. Human
Table 1 shows a summary of brain localization of aromatase in various species. A full description of the localization of aromatase for each species is provided below. The first report of aromatase activity in the brain was made by Naftolin and coworkers when they measured conversion of radiolabeled androgen precursors to estrogens using human fetal brain homogenates and demonstrated the human fetal diencephalon and limbic system possess significant aromatase activity (Naftolin et al., 1971). Subsequent studies using RT-PCR of adult human brain samples revealed highest aromatase mRNA expression in the hypothalamus, amygdala, pons, thalamus, hippocampus, temporal cortex, and frontal cortex (Sasano et al., 1998; Steckelbroeck et al., 1999; Stoffel-Wagner et al., 1999, 1998). Additional work confirmed high expression of aromatase mRNA in the human hippocampus, temporal cortex, and frontal cortex, and found no sex differences in aromatase expression in these brain regions (Stoffel-Wagner et al., 1999). Furthermore, positron emission tomography (PET) imaging using radiolabeled aromatase inhibitors also confirmed widespread aromatase localization in the human brain, with highest concentrations in the thalamus and amygdala, followed by preoptic area (POA), hippocampus, cortex, putamen, cerebellum and white matter (Biegon, 2016). PET imaging further confirmed that there are no significant sex differences in aromatase levels in the human brain except for greater levels in the male left hypothalamus (Takahashi et al., 2018). PET imaging also revealed that regional brain uptake of the radiolabeled aromatase inhibitor, 11C-vorozole did not vary across the menstrual cycle in premenopausal women (Biegon et al., 2015). Immunohistochemical studies to examine the cellular and subcellular localization of aromatase showed widespread aromatase expression in pyramidal neurons in the human temporal cortex and CA1-CA3 regions of the hippocampus, granule cells of dentate gyrus, in a small number of astrocytes, and in some interneurons in the normal and epileptic human brain (Azcoitia et al., 2011; Yague et al., 2010, 2006). Light and electron microscopy ultrastructural studies in the human brain further demonstrated aromatase immunoreactivity throughout the neuronal cell body, including dendrites and axonal processes, and in numerous boutons with synaptic vesicles (Naftolin, 1994; Naftolin et al., 1996). In addition, axon terminals were found to form synapses with immuno-negative and immuno-positive dendrites and neuronal cell bodies (Naftolin, 1994). The synaptic localization of aromatase suggests a possible neuromodulator or neurotransmitter role for neuron-derived estrogen, which will be discussed in a subsequent section.
Table 1.
Summary of Brain Localization of Aromatase.
| Species | Year | Detection Technique | Brain Areas Localized |
|---|---|---|---|
| Human | 1971 | Aromatase Activity Assay (AAA) | • Fetal diencephalon (Naftolin et al., 1971) |
| Rat | 1972 | AAA | • Male and female anterior hypothalamus (HYP) (Naftolin et al., 1972) |
| Turtle | 1977 | AAA | • Strio-amygdaloid complex, HYP (Callard et al., 1977) |
| Rat | 1977 | AAA | • Preoptic area (POA), mediobasal HYP (Selmanoff et al., 1977) |
| Bird, Snake, Shark, Teleost | 1978 | AAA | • Forebrain (Callard et al., 1978) |
| Rat | 1984 | AAA | • Amygdala (AMG), POA, HPC, cerebral cortex, cerebellum, anterior pituitary. In female, no change in estrous cycle or after ovariectomy (Roselli et al., 1984) |
| Rat | 1984 | AAA | • Castration decreased aromatase activity in POA by 60 % (Roselli and Resko, 1984) |
| Monkey | 1986 | AAA | • Highest in HYP, also detected in AMG, hippocampus (HPC), cortex (MacLusky et al., 1986) |
| Songbird | 1990 | AAA | • Nuclei of the song system, Female greater E2 production than male (Vockel et al., 1990) |
| Mouse | 1991 | Immuno-histochemistry (IHC) | • High in medial and tuberal HYP (Balthazart et al., 1991a, b) |
| Quail | 1991 | IHC | • Majority of aromatase-positive neurons in HYP colocalize ERα (Balthazart et al., 1991b) |
| Rat | 1993 | AAA | • Aromatase activity in neurons, low in astrocytes, absent in oligodendrocytes (Negri Cesi et al., 1993) |
| Chicken | 1994 | IHC | • Aromatase high in HYP, HPC (Beyer et al., 1994b) |
| Mouse | 1994 | IHC | • Aromatase immunoreactivity in mouse cultures of hypothalamic and cortical cells, only in neurons (Beyer et al., 1994a) |
| Songbird | 1994 | In situ hybridization (ISH) | • Aromatase mRNA detected in POA, HYP, HPC, neostriatum (Shen et al., 1994) |
| Rat | 1994, 1998 | ISH | • Aromatase mRNA highest in POA, medial preoptic nucleus, bed nucleus of stria terminalis, AMG (Lauber and Lichtensteiger, 1994; Roselli et al., 1998) |
| Human | 1996 | Reverse transcriptase-polymerase chain reaction (RT-PCR) | • Aromatase mRNA highest in pons, thalamus, HYP and HPC (Sasano et al., 1998) |
| Human | 1998 | RT-PCR, AAA | • Aromatase mRNA and activity detected in temporal lobe cortex (Steckelbroeck et al., 1999; Stoffel-Wagner et al., 1998) |
| Quail, Rat, Monkey, Human | 1996 | AAA | • Aromatase activity high in human cerebral cortex vs. subcortical regions (10443682) POA, HYP, limbic areas, dendrites, perikaryal, axons, synaptic vesicles (9053779) |
| Rat, mouse | 1999 | IHC and AAA | • Aromatase immunoreactivity/activity induced in HPC astrocytes after kainic acid or penetrating injury (Garcia-Segura et al., 1999) |
| Human | 1999 | RT-PCR | • Aromatase mRNA expressed in temporal and frontal cortex and HPC, No sex difference (Stoffel-Wagner et al., 1999) |
| Rat | 2003 2004 |
RT-PCR, IHC, electron microscopy (EM), high performance liquid chromatography (HPLC) | • In male rat HPC pyramidal neurons (Shibuya et al., 2003) • Aromatase localized in pre- and post-synaptic sites in male rat HPC, E2 synthesized in HPC and increased by NMDA, E2 very stable in and not significantly metabolized (Hojo et al., 2004) |
| Zebra Finch | 2004 2005 |
IHC, EM | • Aromatase and NMDAR co-expressed in HPC neurons (Saldanha et al., 2004), aromatase is presynaptic (Peterson et al., 2005) |
| Human | 2006 | RT-PCR, IHC | • Aromatase expressed in pyramidal neurons of temporal cortex, small number astrocytes positive, no expression GABAergic interneurons (Yague et al., 2006) |
| Rat | 2006 | RT-PCR, Western blot (WB), IHC, EM, HPLC | • E2 synthesized locally in rat HPC neurons, Basal conc was 1 nM in HPC. Aromatase, P450scc, 3β-HSD, 17β-HSD, STAR all expressed in HPC neurons (Mukai et al., 2006) |
| Monkey | 2008 | IHC | • Aromatase expressed in temporal cortex, HPC pyramidal neurons, dentate gyrus (DG) granule cells, and some interneurons (Yague et al., 2008) |
| Human | 2010 | IHC | • Widespread aromatase expression in HPC pyramidal neurons, DG granule cells, and interneurons in normal and epileptic HPC tissue (Yague et al., 2010) |
| Rat | 2010 | IHC | • Aromatase staining present in synapses and presynaptic terminals of cultured rat cortical neurons (Srivastava et al., 2010) |
| Zebra Finch | 2013 | IHC | • Aromatase expression constitutive in neurons and induced in astrocytes (Saldanha et al., 2013) |
| Rat | 2014 | IHC | • Aromatase expression constitutive in HPC and cortical neurons, induced in astrocytes after cerebral ischemia (Zhang et al., 2014) |
| Rat | 2014 | RT-PCR | • Long-form of aromatase expressed in many brain regions, no sex difference in dorsal HPC expression and no regulation by gonadal/hormone (Tabatadze et al., 2014) |
| Rat | 2016 | Microsome-based AAA using liquid chromatography-mass spectrometry (LC-MS) | • Distribution of aromatase long-form mRNA correlated with AA and was highest in AMG, POA and HPC. (Li et al., 2016) |
| Mouse | 2019 | IHC, WB, High Sensitivity Enzyme-linked immunosorbent assay (ELISA) | • Aromatase expression in HPC and cortex of male and female mouse, E2 levels in HPC and cortex ~1 nM. Genetic deletion of forebrain neuronal aromatase leads to defects in synaptic plasticity, LTP and memory (Lu et al., 2019) |
| Mouse | 2020 | IHC, WB, High Sensitivity ELISA | • Astrocyte aromatase and E2 increased in HPC after cerebral ischemia. Genetic deletion of forebrain neuronal or astrocyte aromatase leads to reduced astrocyte activation and attenuated neuroprotection after cerebral ischemia (Lu et al., 2020; Wang et al., 2020) |
2.2. Non-human primate
Similar to the human, early studies revealed high aromatase activity in the monkey hypothalamus, amygdala, hippocampus and cortex (Flores et al., 1973; MacLusky et al., 1986). Later studies using in situ hybridization confirmed high aromatase mRNA-containing neurons in hypothalamic areas, with highest expression observed in the medial preoptic nucleus, bed nucleus of the stria terminalis and anterior hypothalamus, as well as cortical and medial amygdaloid nucleus, and basal amygdala nucleus – areas important in expression of emotional behaviors and memory processing (Roselli et al., 2001). Studies using RT-PCR also demonstrated significant aromatase expression in the amygdala, mediobasal hypothalamus, hippocampus and prefrontal cortex of the ovariectomized female monkey (Sorwell et al., 2012). At the protein level, immunohistochemical studies confirmed significant aromatase immunoreactive protein localization in the monkey temporal cortex, hippocampal CA1–3 pyramidal neurons, granule neurons of the dentate gyrus, and some interneurons (Yague et al., 2008). Additional studies demonstrated that aromatase localization occurred throughout the neuronal cell body, including dendrites and axons, and in boutons that contained synaptic vesicles (Naftolin et al., 1996).
2.3. Rat
The first studies to localize aromatase in the rat brain used activity assays and focused on the hypothalamus. These studies demonstrated high activity in the male and female hypothalamus (Naftolin et al., 1972), POA and mediobasal hypothalamus (Selmanoff et al., 1977). Subsequent studies using a microsomal based aromatase activity assay and high-performance liquid chromatography (HPLC) measurement of E2 performed a more widespread analysis of the brain and showed the highest aromatase activity and E2 levels in the amygdala, POA and hippocampus of the female rat brain (Li et al., 2016). RT-PCR studies similarly found high expression of aromatase mRNA in the amygdala, bed nucleus of the stria terminalis and POA, followed by the hippocampus and cingulate cortex, with low levels in the brainstem and cerebellum of the adult male and female rat brain (Tabatadze et al., 2014). In keeping with the hippocampus being a site of E2 production, significant mRNA expression for the steroidogenic enzymes necessary for E2 synthesis including aromatase, P450 side chain cleavage (P450scc), P450 17α− hydroxylase (P45017α), 17β-hydroxy steroid dehydrogenase (17β-HSD), 3β-hydroxysteroid dehydrogenase (3β-HSD) was demonstrated in the rat hippocampus (Hojo et al., 2004; Mukai et al., 2006). The basal concentration of E2 was reported to range from 1–8 nM in the male rat hippocampus, and from 0.5 to 2 nM in the rat female hippocampus (Hojo et al., 2004; Mukai et al., 2010, 2006), which is significantly greater than the concentration in the blood. E2 in the hippocampus was reported to be very stable and not significantly converted to other metabolites (Hojo et al., 2004). Further studies of the rat hippocampus revealed that aromatase is localized in neurons basally. For instance, immunohistochemical studies demonstrated significant aromatase localization in pyramidal neurons of the adult male and female rat hippocampal CA1-CA3 regions, and in granule neurons in the dentate gyrus (Hojo et al., 2004; Mukai et al., 2006; Zhang et al., 2014). in vitro studies further confirmed aromatase enzymatic activity in neurons, with no activity observed in astrocytes or oligodendrocytes (Negri Cesi et al., 1992, 1993). Additional studies revealed aromatase was localized in pre- and post-synaptic compartments and the endoplasmic reticulum in the rat hippocampus (Hojo et al., 2004; Mukai et al., 2006; Zhang et al., 2014), and was located at the synapse and in presynaptic terminals in cultured rat cortical neurons (Srivastava et al., 2010). This neuronal and synaptic localization of aromatase is similar to the results observed in the human and non-human primate, as well as in the mouse and bird, as will be discussed below.
2.4. Mouse
Studies in mice are less numerous, but generally consistent with aromatase localization observed in the rat brain. For instance, significant aromatase localization has been reported in the mouse hypothalamus, amygdala, hippocampus and cerebral cortex by immunohistochemistry and RT-PCR (Balthazart et al., 1991a, b; Bender et al., 2017; Beyer et al., 1994a; Ivanova and Beyer, 2000; Lu et al., 2019; Wang et al., 2020). Similar to studies in the rat, aromatase was demonstrated only in neurons of the mouse hippocampus, with no localization observed in astrocytes basally (Lu et al., 2019, 2020; Wang et al., 2020). In vitro studies of cultured mouse neurons and astrocytes confirmed that aromatase was only expressed in neurons and not astrocytes (Beyer et al., 1994a). Mice engineered to express enhanced green fluorescent protein (EGFP) upon aromatase activation showed widespread aromatase expression in the brain, with highest EGFP-positive cell bodies and fibers noted in the amygdala, hypothalamus and bed nucleus of the stria terminalis. In many mouse brain areas, EGFP-positive cells co-expressed estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) or the androgen receptor (Stanic et al., 2014). Collectively, these studies show that similar to observations in monkeys and humans, aromatase is widely distributed in neurons at synaptic locations in both the rat and mouse brain. This suggests that aromatase and BDE2 may regulate synaptic function in the brain. Indeed, there is growing evidence supporting a synaptic role for BDE2, as will be discussed in a subsequent section.
2.5. Bird
One of the earliest reports of brain localization of aromatase in birds was a study that demonstrated significant aromatase activity in the bird forebrain (Callard et al., 1978). Interestingly, this study also confirmed a similar significant aromatase activity level in the forebrain of the snake, sea turtle, opossum, urodele amphibian, teleost and skate (Callard et al., 1978). Immunohistochemical studies in the Japanese quail revealed significant aromatase protein localization in the medial preoptic nucleus, septal region, ventromedial and tuberal hypothalamus, with aromatase and estrogen receptor colocalized in many of the regions (Balthazart et al., 1991b). Aromatase activity was found in synaptosomes, and electron microscopy studies revealed aromatase was present in synaptic boutons in the Japanese quail brain (Balthazart et al., 1991b). Further work confirmed that aromatase is expressed in the POA in many bird species including canaries, house sparrows, zebra finches, ring doves, swifts, grey partridges, barn owls and budgerigars (Metzdorf et al., 1999).
It is important to note that the songbird telencephalon expresses aromatase much more widely and with much higher activity than observed in the quail, which has high aromatase expression localized primarily to the hypothalamus. Thus, the songbird has been particularly useful in determining the role of aromatase and BDE2 in neuroplasticity, memory, and behavior. In situ hybridization documented widespread aromatase localization in the adult zebra finch brain, with highest localization observed in the POA, hypothalamus, hippocampus and neostriatum (Shen et al., 1994). Subsequent work demonstrated aromatase was localized in pre-synaptic boutons in the zebra finch hippocampus and high vocal centre brain areas and that males had more synaptic profiles with aromatase than females (Peterson et al., 2005). In addition, aromatase protein and activity were demonstrated in synaptosomes of the male and female zebra finch anterior and posterior telencephalon, with males exhibiting the highest levels (Rohmann et al., 2007). The aromatase-positive pre-synaptic boutons in the zebra finch brain were found to always innervate aromatase-negative post-synaptic elements (Peterson et al., 2005). Further work demonstrated that aromatase activity was elevated in forebrain synaptic terminals in male zebra finches that were singing for 30 min (Remage-Healey et al., 2009).
2.6. Fish, amphibians and reptiles
As mentioned previously, teleost fish have two aromatase isoforms, CYP19a which encodes aromatase A and is expressed in the gonads, and CYP19b, which encodes aromatase B and is expressed in the brain and gonads (Tchoudakova and Callard, 1998). Another unique characteristic of teleost fish is that aromatase is expressed exclusively in radial glial cells in the brain, and not in neurons (Forlano et al., 2001). While radial glial cells generally disappear in mammals after development (Mori et al., 2005), they remain in the brain of adult fish. In zebrafish, aromatase expression is highest in the telencephalon, POA, thalamus, hypothalamus, optic tectum, and torus semicircularis (Menuet et al., 2005; Pellegrini et al., 2007). Further work demonstrated that aromatase B is upregulated in radial glial cells in the POA and mediobasal hypothalamus by E2 (Menuet et al., 2005). Interestingly, bromodeoxyuridine treatment coupled with IHC revealed that aromatase-positive radial glial cells divide in the zebrafish brain, and over time move away from the ventricles with some differentiating into neurons (Pellegrini et al., 2007). IHC and in situ hybridization showed a similar aromatase localization pattern in the brain of rainbow trout with highest levels in the POA and hypothalamus, ventricles of telencephalon and ventral diencephalon (Menuet et al., 2003). In contrast to teleost fish, aromatase is encoded by a single gene CYP19a1 in amphibians (Iwabuchi et al., 2007). In the amphibian xenopus, aromatase gene expression in the brain occurred from early developmental stages to metamorphosis, with highest expression in the POA and caudal hypothalamus (Urbatzka et al., 2007). It was further found that the aromatase gene is strictly expressed in neurons and not in radial glial cells in the xenopus brain and was not sexually dimorphic (Coumailleau and Kah, 2015). In reptiles, aromatase activity was demonstrated to be high in the forebrain (Callard et al., 1977; Callard et al., 1978). IHC in the red-sided garter snake demonstrated that aromatase is localized in the POA, anterior hypothalamus, nucleus spericus and septum (Krohmer et al., 2002), and inhibition of aromatase activity by administration of an aromatase inhibitor revealed a role for brain aromatase in courtship behavior in the red-sided garter snake (Krohmer, 2020).
3. Aromatase regulation in the brain
Aromatase and BDE2 levels in the brain can be regulated by both transcriptional and post-transcriptional mechanisms, as well as a diverse array of intrinsic and extrinsic factors (Fig. 4). In this section, we will review these key mechanisms and factors that control aromatase expression/activity and E2 production in the brain.
Fig. 4. Summary diagram illustrating multiple processes and factors that have been implicated to regulate brain aromatase.
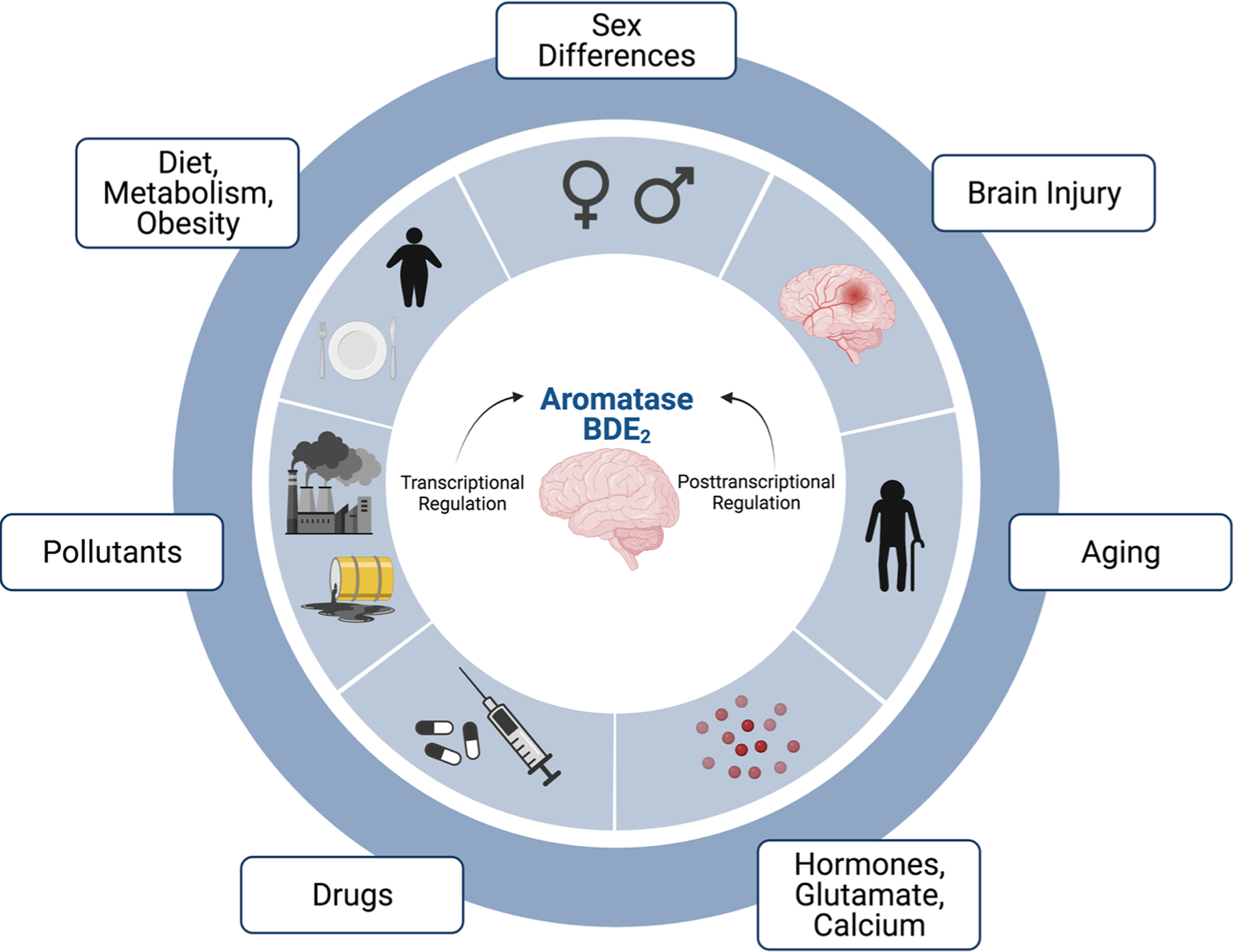
See text for full description and discussion. BDE2 = brain-derived 17β-estradiol. Created with BioRender.com.
3.1. Phosphorylation
There is considerable evidence that phosphorylation is a key mechanism to rapidly regulate aromatase activity and BDE2 production. For instance, early work in quail showed that Ca2+-dependent phosphorylation of aromatase caused a rapid decrease of brain aromatase activity in hypothalamic homogenates and explants (Balthazart et al., 2005, 2001a; Balthazart et al., 2001b). Further work revealed that quail aromatase has 15 predicted consensus phosphorylation sites, and that protein kinase C and protein kinase A are involved in phosphorylation control of aromatase activity (Balthazart et al., 2005). Hayasi and Harada (Hayashi and Harada, 2014) reported that in human JEG-3 cells aromatase is phosphorylated by calcium/calmodulin-dependent protein kinase II (CaMKII) and dephosphorylated by calcineurin, with catalytic activity being reversibly regulated. Additional work revealed that acid phosphatase blocks the inhibiting effects of Ca2+-dependent phosphorylation on aromatase activity in quail (Balthazart et al., 2005). It was also shown that sexual interaction decreases aromatase activity within minutes in male quail medial preoptic nucleus (Cornil, 2018; de Bournonville et al., 2017a, b). The authors proposed that the rapidity of the effect suggests a nongenomic mechanism is involved that may involve glutamate signaling and Ca2+-dependent phosphorylation of aromatase (de Bournonville et al., 2017a). Indeed, glutamate is increased in the medial POA of the male quail during sexual interaction and injection of the glutamate agonist, kainate into the medial preoptic nucleus led to a significant decrease of aromatase activity in male quail (de Bournonville et al., 2017b). Similar to the observation in quail, a rapid decrease of aromatase activity was also observed in the male and female zebra finch hypothalamus, hippocampus and caudomedial nidopallium (NCM) by Ca2+-dependent phosphorylation (Comito et al., 2016). In further support of a role for Ca2+ in regulating brain aromatase, depletion of Ca2+ stores in zebra finch forebrain and rat hippocampal neurons in culture resulted in increased release of E2 (Fester et al., 2016; Remage-Healey et al., 2011) and dephosphorylation of aromatase (Remage-Healey et al., 2011). Intriguingly, E2 treatment increased phosphorylation of aromatase and increased aromatase protein levels in rat hippocampal neurons, suggesting that E2 can regulate aromatase in the rat hippocampus (Remage-Healey et al., 2011).
Subsequent work used liquid chromatography with tandem mass spectrometry analysis to identify phosphorylation sites in human aromatase (Ghosh et al., 2019). The study revealed that human aromatase has as many as 19 phosphorylation sites, of which T462, T162, H475/S478, and Y361 are major and reproducibly detectable (Ghosh et al., 2019). Phosphorylation sites T462, S118 and S478 are highly homologous between species, being present in human, monkey, rat, zebra finch, mouse and chicken. Phosphorylation site Y361 is present in human, monkey, zebra finch, chicken and quail, but not in rat or mouse. Interestingly, phosphorylation of Y361 was shown to enhance aromatase activity, and phosphorylation of S478 in the active site access channel was proposed to also be significant as previous work implicated S478 in catalysis (Ghosh et al., 2009; Kao et al., 2001). An important point to consider is that phosphorylating and dephosphorylating conditions likely work together to regulate aromatase activity. In support of this possibility, work using zebra finch brain homogenates found that acid phosphatase increases aromatase activity in low to moderate phosphorylating conditions, while the opposite effect is observed under high phosphorylating conditions (Hovsepian-Ruby, 2017). In addition, low to moderate levels of acid phosphatase increased aromatase activity and Y361 phosphorylation, while high concentrations strongly inhibited aromatase activity and Y361 phosphorylation (Hovsepian-Ruby, 2017). Another investigative group performed microsecond-long all-atom molecular dynamics simulations to determine how phosphorylation regulates human aromatase catalyzed estrogen synthesis (Ritacco et al., 2019). They proposed that phosphorylation of residue Y361 in aromatase leads to stabilization of its adduct with the CYP450 reductase flavin mononucleotide domain (Ritacco et al., 2019). This may facilitate estrogen biosynthesis by favoring aromatase as it competes with other proteins that require CYP450 s reductase’s electron supply. Finally, a myc-tagged mouse aromatase construct transfected into COS cells or HEK-293T cells was used to further study phosphorylation of mouse aromatase (Miller et al., 2008). The study demonstrated that mutation of S118 to Ala blocked phosphorylation and led to destabilization of aromatase, suggesting that S118 may have an important structural role (Miller et al., 2008). Using a phosphomimetic S118 D mutant, their work provided evidence that S118 phosphorylation decreases aromatase activity (Miller et al., 2008). Taken as a whole, the above studies demonstrate that phosphorylation can either increase or decrease aromatase activity depending on the site(s) phosphorylated. Unfortunately, there are few studies that have examined phosphorylation of brain aromatase in physiological or pathological processes, and thus work to address this deficit is very much needed.
3.2. Glutamate
Glutamate, the major excitatory amino acid transmitter in the brain, has been shown to rapidly regulate neural aromatase activity and BDE2 levels, although species and brain region-specific differences exist. For example, glutamate agonists rapidly decreased aromatase activity in quail hypothalamic explants (Balthazart et al., 2006). The rank order of effectiveness was kainate > AMPA > NMDA. Likewise, glutamate retrodialysis was shown to induce a significant decrease of local E2 levels in the NCM of the zebra finch (Remage-Healey et al., 2008). The glutamate receptor responsible for mediating the effect was not studied, but aromatase and NMDA receptors have been shown to be colocalized in hippocampal neurons of zebra finch (Saldanha et al., 2004), which raises the possibility that NMDAR may mediate the effect. In contrast to the reports in quail and zebra finch, glutamate administration was shown to be stimulatory to BDE2 release in the rat as in vivo kainate administration more than doubled E2 release in the rat hippocampus within two hours (Sato and Woolley, 2016). Similarly, NMDA treatment nearly doubled E2 release in male rat hippocampal slices incubated in vitro (Hojo et al., 2004). The stimulatory effect of NMDA was fully suppressed by treatment with an NMDAR antagonist, MK-801 (Hojo et al., 2004). NMDA also induced production of pregnenolone, an upstream precursor to E2, and this effect was found to be dependent upon NMDAR and Ca2+ influx (Shibuya et al., 2003). Rune’s group found a biphasic effect of NMDA on E2 release from rat hippocampal neurons in culture - a decrease noted at 30 min after NMDA treatment followed by a significant increase at 60 min post-treatment (Fester et al., 2016). Finally, the NMDA receptor agonist, D-aspartate (Errico et al., 2015) increased aromatase mRNA and protein as well as brain E2 levels in the frog, an effect that involved CREB signaling (Burrone et al., 2012; Santillo et al., 2013). Since glutamatergic signaling is a major component of excitatory neurotransmission in the brain, its ability to rapidly regulate BDE2 release could suggest a role for BDE2 in neurotransmission. Indeed, numerous studies have now provided evidence that BDE2 regulates synaptic function, cognition and memory (Brandt et al., 2020; Lu et al., 2019; Mukai et al., 2010, 2006), as will be discussed in a subsequent section.
3.3. Transcriptional regulation
In addition to phosphorylation, there is also abundant evidence that aromatase is regulated at the transcriptional level. For instance, treatment with a hypomethylating agent, 5-aza-2’deoxycytidine caused a 10-fold increase of brain-specific 1.f aromatase promoter transcription and an ~80% increase in aromatase activity in human glioblastoma cells (Tan et al., 2017). There was no effect on the PII aromatase promoter. This finding indicates that methylation can inhibit or restrain aromatase expression and activity in glioblastoma cells. It would be interesting to determine whether methylation can similarly regulate aromatase expression/activity in normal non-transformed neuronal and glia cells in the brain. Regarding the mechanisms underlying transcriptional control of the brain-specific 1.f aromatase promoter, the orphan transcription factor ARP-1 has been shown to bind to the brain-specific exon 1 cis-acting element aro-A1 and induce the 1.f promoter (Honda and Harada, 2020). A functional regulatory role for the interaction was demonstrated via ARP-1 knockdown in mouse neurons, which significantly decreased aromatase induction. Further work by this group revealed that the Lim-homeodomain protein, Lhx2, also appears to be involved in brain-specific 1.f aromatase transcriptional control (Honda et al., 2012). Lhx2 was identified as binding to the mouse brain-specific exon 1 aro-B cis-acting element, and a reporter assay demonstrated Lhx2-dependent aromatase promoter activity was suppressed by siRNA knockdown of Lhx2 expression (Honda et al., 2012). Finally, the human aromatase gene was shown to contain two binding sites for the hormone-dependent transcription factor, retinoic acid-related orphan receptor-alpha (RORA) (Sarachana et al., 2011). Further work indicated a transcriptional regulatory role for RORA as its overexpression in human neuroblastoma cells led to a ~10-fold increase of aromatase expression (Sarachana et al., 2011). The authors further found that estrogen increases RORA expression in the brain, and that expression of RORA and aromatase were both significantly decreased in the frontal cortex of autistic subjects, which the authors postulate may contribute to the disorder (Sarachana et al., 2011).
3.4. Gonadectomy and sex differences
Castration in male rats was found to significantly decrease aromatase mRNA levels in the male rat POA but not in the hippocampus or cingulate cortex (Roselli et al., 1998; Tabatadze et al., 2014). At the enzyme activity level, castration was shown to decrease aromatase activity in the POA (Roselli et al., 1984; Roselli and Resko, 1984), but not in the amygdala or cortex (Roselli et al., 1984). These findings suggest that the testis can regulate brain aromatase and E2 levels, especially in the hypothalamus. The regulatory effect of the testis may be mediated by the gonadal hormone, testosterone, as several studies have shown that testosterone can regulate brain aromatase and E2 levels (Roselli et al., 1984; Roselli and Resko, 1984). Interestingly, additional studies revealed aromatase activity did not change in the female rat POA after ovariectomy or during the estrus cycle (Roselli et al., 1984). Several studies have demonstrated that sex differences exist for aromatase expression in the amygdala and POA of the rat brain. For instance, aromatase mRNA levels in the amygdala were reported to be significantly higher in males as compared to females (Tabatadze et al., 2014). Further work found that E2 levels are significantly higher in the amygdala and POA of male rats as compared to levels in ovariectomized females (Li and Gibbs, 2019). Sex differences were demonstrated in the zebra finch brain with greater aromatase activity observed in the male amygdala and hypothalamus/POA as compared to female (Roselli et al., 1984). The reasons for the sex differences in the zebra finch brain are unclear but they may relate to regulation of key functions controlled by these brain regions, including reproductive behavior, learning and memory, song-recognition, and singing. Sex differences in aromatase expression in the brain could be due to regulation by gonadal hormones and/or sex chromosome complement. Indeed, there is significant evidence of gonadal hormone regulation of aromatase in the brain of various species as described in the next section below. Only a few studies have examined the possibility of sex chromosome complement regulation of aromatase expression and this work was performed in the developing mouse brain (Cisternas et al., 2017, 2015). The investigators used the four core genotypes mouse model in which the sex chromosome complement and gonadal sex are dissociated and found that 16 day old XY mouse had higher aromatase expression in the anterior amygdala and stria terminalis than XX embryos, independent of gonadal sex (Cisternas et al., 2015). Interestingly, E2 or dihydrotestosterone enhanced aromatase expression in cultured amygdaloid neurons from XX embryos but not XY embryos, and this effect was also independent of gonadal sex and appeared to be mediated by ERβ (Cisternas et al., 2017, 2015). It would be interesting to conduct additional studies to determine whether aromatase regulation by sex chromosome complement extends to other brain regions, other ages, and to other species.
In contrast to the sex differences observed in the amygdala, POA and stria terminalis, examination of aromatase mRNA levels and immunoreactive aromatase expression in the hippocampus of the rat did not reveal any sex differences (Fester et al., 2012; Tabatadze et al., 2014), and E2 levels were not significantly different in the intact male hippocampus versus the ovariectomized female rat hippocampus (Li and Gibbs, 2019). In addition, measurement of E2 in the supernatant of cultured female and male hippocampal neurons also revealed no significant sex differences (Fester et al., 2012). However, a sex difference in E2 levels in the hippocampus of the intact male rat versus the intact female proestrus rat has been reported, with hippocampal E2 levels as high as 8 nM reported in the intact male rat versus 1.7 nM in the intact proestrus female rat (Mukai et al., 2010). These contradictory reports on sex differences in hippocampal E2 levels may be due to differences in the study design, including 1) differences due to measuring mRNA and protein as compared to the actual product E2, 2) differences in comparing intact males to ovariectomized females versus comparing intact males to intact females, and 3) differences due to in vitro versus in vivo approaches.
3.5. Hormones
There is growing evidence that brain aromatase is regulated by both steroid and peptide hormones. Testosterone, a substrate for estrogen production, was shown to increase aromatase activity in the POA of castrate male Japanese quail (Balthazart et al., 1990) as well as castrate male and female rat POA, periventricular magnocellular nucleus and posterior medial hypothalamic nucleus (Abdelgadir et al., 1994; Roselli et al., 1984), and in the nuclei of the song system in the castrate male zebra finch (Vockel et al., 1990), and suprachiasmatic nucleus, paraventricular nucleus, ventromedial nucleus and lateral hypothalamus of the castrate male monkey (Roselli and Resko, 1989). The regulation of aromatase activity in the brain by testosterone appears to be exerted at the transcriptional level as testosterone was found to cause an associated increase of aromatase mRNA levels in the brain of several species (Abdelgadir et al., 1994; Harada et al., 1992).
In addition to testosterone, E2 has also been demonstrated to regulate expression of brain aromatase. Using a mouse hypothalamic neuronal cell line, it was shown that ERα interacts with the 1.f promoter and that E2 treatment increased aromatase mRNA, an effect reversed by an ER antagonist or ERα knockdown (Yilmaz et al., 2009). Likewise, E2 increased aromatase mRNA levels in the ovariectomized female rat amygdala (Tabatadze et al., 2014). Similarly, tonic E2 treatment was found to increase aromatase expression in the female mouse hippocampus; however, phasic E2 treatment was shown to actually be inhibitory (Iivonen et al., 2006). Finally, short-term E2 replacement decreased aromatase expression in the monkey hippocampus (Sorwell et al., 2012). Unfortunately, none of these studies examined aromatase activity or E2 levels, so it is unclear whether the aromatase expression results actually contributed to changes in BDE2 levels. Furthermore, it is unclear why some studies found stimulatory effects of E2 while others reported inhibitory effects. It is possible the divergent results could be due to study differences, including species differences, examining different brain locations, utilizing in vitro versus in vivo studies, and/or employing different E2 treatment regimens.
In addition to testosterone and E2, progestins and glucocorticoids have also been implicated to transcriptionally regulate the expression of brain aromatase. For instance, R5020, a synthetic progestin, was shown to increase aromatase promoter 1.f transcription in mouse hypothalamic neuronal cell lines, an effect that was reversed by the progesterone receptor antagonist RU486 and siRNA knockdown of the progesterone receptor (Yilmaz et al., 2011). The physiological importance of this regulatory effect remains unclear. However, progesterone has been reported to act in the hypothalamus to help amplify induction of the gonadotropin releasing hormone (GnRH) surge and luteinizing hormone (LH) surge (Lee et al., 1990; Mahesh and Brann, 1998; Stephens et al., 2015). Interestingly, Terasawa and coworkers (Kenealy et al., 2017; Terasawa, 2018) recently demonstrated a role for BDE2 in GnRH and LH surge induction in ovariectomized monkeys. It would therefore be interesting to perform studies to examine whether progesterone enhances hypothalamic BDE2 in vivo and whether this effect contributes to progesterone’s ability to amplify the GnRH and LH surge. One of the most profound regulations of 1.f promoter transcription reported to date was the finding that the glucocorticoid, dexamethasone can induce up to a 98-fold increase of aromatase mRNA and protein in mouse hypothalamic neuronal cell lines (Brooks et al., 2012). This effect required mediation by the glucocorticoid receptor as it was blocked by a glucocorticoid receptor antagonist and by siRNA knockdown of the glucocorticoid receptor (Brooks et al., 2012). Furthermore, acute stress, which increases the release of corticosterone from the adrenal, was shown to significantly enhance aromatase activity in the POA of quail (Dickens et al., 2013), and to rapidly increase aromatase expression and E2 levels in the paraventricular nucleus of proestrus and ovariectomized female rats (Liu et al., 2011). All of these studies focused on regulatory effects in the hypothalamus. It would also be interesting to examine the hippocampus and amygdala, as well as other brain regions for stress and glucocorticoid regulation of aromatase and BDE2 levels. The POA is known to control gonadotropin secretion, reproduction and sexual behavior, while the paraventricular nucleus is involved in osmoregulation, appetite control, and the body’s response to stress. Thus, the regulation of hypothalamic BDE2 by acute stress and glucocorticoids could be related to one or more of these functions.
In addition to steroid hormones, peptide hormones have also been shown to regulate aromatase activity and BDE2 levels. Of significant interest, the peptide hormone, GnRH was shown to have a biphasic effect upon E2 synthesis in postnatal rat hippocampal slices and hippocampal neurons in culture (Fester et al., 2012; Prange-Kiel et al., 2008). Low doses (10 nM) of GnRH stimulated E2 synthesis while higher doses (500 nM) were inhibitory. The effects of GnRH appeared to be specific as they were blocked by a GnRH receptor antagonist. Another study showed that intracerebroventricular injection of GnRH also increased E2 levels in the hippocampus (Marbouti et al., 2020b). The physiological significance of the GnRH effects on BDE2 are unclear, but they could be related to regulation of synaptic function and memory. In support of this possibility, spine synapse density was shown to be upregulated by GnRH in rat hippocampal neuron cultures, and this effect was blocked by the aromatase inhibitor letrozole (Prange-Kiel et al., 2008). Furthermore, hippocampal administration of GnRH was shown to enhance memory in ovariectomized rats, an effect that was also blocked by central administration of letrozole (Nelson et al., 2016). Finally, intriguingly, another peptide hormone, gonadotropin-inhibitory hormone (GnIH), was recently shown to increase aromatase activity and E2 synthesis in the POA in male quail (Ubuka et al., 2014). The investigators also demonstrated that aromatase cells express GnIH receptor mRNA, and that GnIH fibers form close appositions to aromatase immunoreactive cells in the POA, as well as the bed nucleus of the stria terminalis, mediobasal hypothalamus and periaqueductal grey of the male quail (Ubuka et al., 2014). The authors suggest that GnIH increases BDE2 above its optimal level which may play a role in the ability of GnIH to inhibit aggressive and sexual behavior in males (Ubuka et al., 2014). However, further studies are needed to explore this hypothesis.
3.6. Aging, diet, metabolism and obesity
There is growing evidence that brain aromatase and BDE2 levels are significantly regulated by aging and diet. In the human, PET imaging revealed that normal aging and postmenopausal status were associated with decreased uptake of 11C-vorozole throughout the brain in both men and women, indicating that aging decreases aromatase levels in the human brain (Biegon et al., 2015). In the rat, two studies reported that aromatase expression and activity in the hypothalamus of the rat is unaffected by age (Munetomo et al., 2015; Roselli et al., 1986), while a third study found that the number of immunoreactive aromatase neurons in the POA were decreased in 36-month-old as compared to 6-month-old male quail (Dellovade et al., 1995). Likewise, aromatase expression and E2 levels were found to be decreased in the hippocampus of 19-month-old versus 10-month-old female rats (Chamniansawat and Sawatdiyaphanon, 2018). Aromatase protein expression was also demonstrated to be decreased in the hippocampus of 20-month-old versus 3-month-old female mice (Zhao et al., 2017). While aromatase expression in the monkey hippocampus was unchanged with aging, expression of 17α-hydroxylase and 3β-HSD, key steroidogenic enzymes necessary for E2 synthesis, were significantly decreased in the hippocampus of aged monkeys (Sorwell et al., 2012). As a whole, these studies suggest that aromatase and brain E2 levels may be decreased by aging in certain brain areas, with the hippocampus being most consistently reported to exhibit an age-related decrease of aromatase and E2 levels. Since BDE2 has been implicated in the regulation of many key brain processes, its decrease with aging may contribute, in part, to the age-related decline in key brain processes/functions such as synaptic plasticity, memory and cognition, anti-inflammatory effects, reproductive function, and neuroprotection (Brandt et al., 2020; Duncan and Saldanha, 2020; Garcia-Segura et al., 2003; Lu et al., 2019, 2020; Saldanha, 2020; Terasawa, 2018; Wang et al., 2020).
The effect of diet upon brain aromatase has been little studied, although there has been some work examining the effect of flavonoids and phytoestrogens, as well as hypoglycemia and obesity/body mass index (BMI) on brain aromatase in rodents and humans. Flavonoids are found in many plants and represent the most common polyphenolic compounds in human diet. Red wine is a well-known flavonoid, and in vivo studies revealed a significant stimulatory effect of chronic (8-week) treatment of red wine on aromatase expression and activity in the rat hippocampus (Monteiro et al., 2008). The effect in the hippocampus is unlikely to be due to procyanidins in red wine as a similar 8-week treatment with procyanidins had no effect on hippocampal aromatase expression or activity (Monteiro et al., 2008). Since red wine has been shown in many studies to have anti-oxidant properties and to be neuroprotective (Amodio et al., 2006; Basli et al., 2012), the authors suggested that the neuroprotective effect of red wine may involve mediation by aromatase and BDE2 (Monteiro et al., 2008). However, this intriguing suggestion remains to be tested. In contrast to the effect of flavonoids, phytoestrogens, which are estrogen-like compounds derived from plants, were found to have no effect upon brain aromatase levels in male rats (Lephart et al., 2001; Weber et al., 2001). However, another study found that administration of the phytoestrogen, genistein to ovariectomized rats increased protein synthesis in the brain and this effect was blocked by the aromatase inhibitor, fadrozole (Lyou et al., 2008). The authors suggest that aromatase in the peripheral and in the brain helps mediate the effects of genistein on protein synthesis in the brain (Lyou et al., 2008). PET imaging of the human brain using 11C-vorozole revealed that obesity (BMI) was associated with lower aromatase availability in the amygdala in both male and females, although the mechanism underlying this effect is unclear (Biegon et al., 2020). Interestingly, the investigators found that aromatase availability in the amygdala was positively correlated with personality trait constraint, and they suggested that the brain’s capacity to make E2 may influence the risk of obesity and self-control in men and women (Biegon et al., 2020).
Finally, recent evidence suggests that BDE2 may regulate glucose homeostasis through actions in the ventromedial nucleus (VMN), an important site for control of glucose homeostasis in the body. Quantification of E2 levels in the male rats revealed that acute and chronic hypoglycemia, respectively enhanced or decreased E2 levels in both the rostral and caudal VMN (Bheemanapally et al., 2020). In female rats, acute hypoglycemia increased E2 levels in the rostral VMN but not in the caudal VMN, while chronic hypoglycemia increased E2 in the caudal VMN with no change observed in the rostral VMN. The authors suggest that BDE2 in the VMN may contribute to regulation of glucose homeostasis (Bheemanapally et al., 2020). In possible support of this suggestion, inhibition of BDE2 synthesis in the VMN by letrozole administration attenuated hypoglycemic upregulation of the energy regulator proteins, 5-AMP-activated protein kinase (AMPK) and glucagon in male and female rats (Uddin and Briski, 2021). Furthermore, letrozole treatment was shown to inhibit hypoglycemic-induced glycogen elevation in the VMN, while exerting a stimulatory effect in females (Ibrahim et al., 2020). The authors suggest that BDE2 facilitates hypoglycemic induction of VMN glycogen levels in males, but acts to inhibit glycogen levels in hypoglycemic females (Ibrahim et al., 2020).
3.7. Environmental pollutants
Of significant note, several groups have demonstrated that environmental pollutants can significantly increase expression of brain aromatase and BDE2 release. For example, tributyltin, an environmental pollutant and retinoid X receptor binder, is an organic compound of tin and is a widely used pesticide in marine environments. It can penetrate the blood brain barrier and accumulates in the brain. Interestingly, 48-h treatment with tributyltin (0.1 μM) was shown to increase aromatase expression and led to a 2-fold increase of E2 release from rat hippocampal slices (Munetsuna et al., 2014). Further evidence of a role for retinoid X receptors in regulation of aromatase and BDE2 was demonstrated by studies showing that treatment of rat hippocampal slices with bexarotene, a retinoid X receptor agonist, also increased E2 levels as well as aromatase expression (Ishihara et al., 2019). Bexarotene also attenuated oxygen-glucose deprivation-induced neuronal cell death, and this was suggested to be mediated by BDE2, as it was blocked by letrozole (Ishihara et al., 2019). Intriguingly, the human brain-specific aromatase promoter has six retinoid X receptor half sites, two of which were responsible for the increased aromatase expression by bexarotene (Ishihara et al., 2019). Furthermore, 9-cis-retinoic acid, which can also activate retinoid X receptors, was also shown to induce a 1.7 fold increase of aromatase protein and a 2-fold increase of de novo synthesis of E2 from rat hippocampal slices (Munetsuna et al., 2009). Another well-known environmental toxin, dioxin, has also been shown at doses as low as 1 pM to significantly increase aromatase expression and activity in human glioma cells via an effect that involved extracellular signal regulated kinases (ERK) activation and enhanced CCATT-enhancer binding proteins (C/EBP) binding activity within exon 1.f promoter (Tan et al., 2013). Furthermore, bisphenol A, a plastics monomer and hormonally active pollutant, induced robust brain-specific expression of aromatase in the zebrafish embryo, an effect that involved nuclear estrogen receptors (Chung et al., 2011). The reason why BDE2 is induced by environmental pollutants is unclear. However, environmental pollutants have been implicated to have a detrimental effect upon the brain as they induce oxidative stress, inflammation and apoptosis in the brain (Hassoun et al., 1998; Mitra et al., 2013). Thus, induction of aromatase and BDE2 by environmental pollutants could be a defense mechanism to protect the brain from the oxidative stress and inflammatory damage caused by the environmental pollutants. Further studies are needed to explore this possibility.
3.8. Drugs
Both recreational and medicinal drugs have been implicated to regulate brain aromatase. For instance, studies from multiple species, including humans, suggest that nicotine can inhibit brain aromatase levels. Studies in fetal and neonatal rats and mice found that nicotine administration significantly inhibited forebrain aromatase activity (Bertilsson et al., 1976; von Ziegler et al., 1991). Likewise, PET imaging in female baboons revealed that nicotine administration dose-dependently decreased 11C-vorozole uptake in the brain, with the amygdala and POA showing the largest reductions (Biegon et al., 2012, 2010), indicating that nicotine decreases aromatase levels in the non-human primate brain. Since the doses of nicotine used in the study produced plasma levels similar to those found in smokers, the findings raise the possibility that smoking may inhibit brain aromatase and BDE2 levels, although this remains to be verified. Similar to the effects of nicotine, administration of the barbiturate, phenobarbital (3.5 g/kg for 5 days) in adult male mice was shown to result in a 50 % reduction of brain aromatase activity (Weidenfeld et al., 1983). Brain aromatase activity had returned to control group levels when examined five days after termination of phenobarbital treatment. Chronic (28 day) treatment with the antipsychotic drugs, clozapine and haloperidol, induced an increase in aromatase mRNA levels, but not protein, in male rat brain (Bogus et al., 2019). In contrast, the antipsychotic drug, olanzapine had no effect on aromatase mRNA or protein expression in the brain (Bogus et al., 2019). Since this study did not measure aromatase activity or BDE2 levels, and clozapine and haloperidol regulation did not extend to aromatase protein levels, the significance of these findings are unclear and will require further investigation. Finally, morphine treatment was shown to increase aromatase expression in rat hippocampal neurons and in the brain of male mice and rats (Aloisi et al., 2010; Cui et al., 2011; Khazali and Mahmoudi, 2019), as well as E2 release in rat hippocampal neurons in vitro (Cui et al., 2011). Interestingly, morphine regulation of BDE2 appears to serve a neuroprotective effect as the protective effects of morphine against amyloid toxicity in rat and human neuronal cell cultures was blocked by siRNA knockdown of aromatase (Beyer et al., 1994a).
3.9. Brain injury and inflammation
As mentioned previously, aromatase is generally expressed only in neurons basally, with astrocytes showing little to no expression. However, in 1999 Garcia-Segura’s group was the first to demonstrate that aromatase can be strongly induced in astrocytes in various regions of the male and female rat and mouse brain following a penetrating brain injury or injection of the excitotoxin kainic acid (Garcia-Segura et al., 1999). In kainic acid-injected animals, aromatase-positive astrocytes were observed in all brain regions that had significant neuronal damage, including the hippocampus, pyriform and entorhinal cortex, amygdala, and bed nucleus of the stria terminalis (Garcia-Segura et al., 1999). Interestingly, neuronal aromatase expression was not affected by kainic acid injection. Furthermore, in the penetrating injury model, aromatase-positive astrocytes were observed in all injured brain regions, including the striatum, corpus callosum, cortex, hippocampus, hypothalamus, and thalamus (Garcia-Segura et al., 1999). These findings indicate that aromatase is induced in astrocytes in most areas of the brain following excitotoxic or penetrating brain injury. Furthermore, penetrating brain injury also induced a significant increase of aromatase activity, indicating increased local E2 production (Garcia-Segura et al., 1999). Subsequent studies in the zebra finch confirmed penetrating brain injury upregulated aromatase in astrocytes in the lesion site within 24− 48 h after injury and demonstrated a parallel upregulation of local E2 (Mehos et al., 2016; Peterson et al., 2001). Robust aromatase induction in astrocytes and local E2 elevation has also been observed in the hippocampus at 2–7 days following global cerebral ischemia (GCI) in male and female rats and mice (Cincioglu et al., 2012; Kelicen Ugur et al., 2011; Lu et al., 2020; Wang et al., 2020; Zhang et al., 2014). Focal cerebral ischemia has also been shown to increase aromatase expression in the penumbra/peri-infarct area of the cerebral cortex in rats (Carswell et al., 2005; Zhong et al., 2017).
The mechanisms underlying aromatase induction after brain injury are not entirely clear. However, brain injury is known to induce several pathological mechanisms that have been implicated in the regulation of brain aromatase, such as inflammation, cytokine and prostaglandin production, as well as induction of reactive oxygen species and oxidative stress (Lozano et al., 2015). In support of a potential regulatory role of inflammation and cytokines, it was found that expression of the cytokines, interleukin-1beta (IL-1β) and interleukin-6 (IL-6) are increased several hours prior to the increase of aromatase in astrocytes following a penetrating injury to the brain of male zebra finch (Duncan and Saldanha, 2011). Furthermore, administration of lipopolysaccharide (LPS) or phytohemagglutin to induce inflammation in the brain strongly induced expression of brain aromatase (Duncan and Saldanha, 2011; Sadasivam et al., 2014). Interestingly, LPS-induced acute inflammation was associated with increased brain expression of phosphoenolpyruvate carboxykinase (PEPCK), a key gluconeogenic enzyme (Sadasivam et al., 2014). Further work showed that inhibition of PEPCK by administration of the inhibitor glipizide significantly attenuated the inflammation-induced upregulation of aromatase expression in the brain, as well as the steroidogenic enzymes 3β-HSD, 17β-HSD and steroidogenic acute regulatory protein (STaR) (Sadasivam et al., 2014). The authors suggest that PEPCK may have an important role in regulating brain neurosteroidogenesis mediated by inflammation (Sadasivam et al., 2014). Interestingly, expression of proinflammatory cytokines was increased by inhibition of PEPCK, which the authors suggested could be due to the decreased production of neurosteroids (Sadasivam et al., 2014).
Additional work has implicated prostaglandin E2 (PGE2) as a key factor regulating aromatase and BDE2. PGE2 is increased in the male and female zebra finch brain after a penetrating injury (Pedersen et al., 2017, 2018), and central administration of the prostaglandin inhibitor, indomethacin was shown to reduce brain injury-induced elevation of aromatase and BDE2 in the zebra finch brain (Pedersen et al., 2018). Use of more specific E-prostanoid (EP) receptor antagonists for EP3 and EP4 receptors implicated EP3 receptors in mediating BDE2 release in the injured male zebra finch brain, while EP4 receptors were implicated in injury-induced BDE2 release in females (Pedersen and Saldanha, 2017). Additionally, inflammation and PGE2 have also been demonstrated to increase aromatase activity and BDE2 in the immature rat and human cerebellum, with the PGE2 effect similarly involving EP3 and EP4 mediation (Dean et al., 2012; Wright et al., 2019), which indicates that inflammation and PGE2 regulation of aromatase and BDE2 occurs in other brain areas and in multiple species. Finally, nitrosative stress/oxidative damage to sheep astrocytes or sheep neurons in culture was shown to increase aromatase gene expression and immunoreactive protein levels (Lepore et al., 2009, 2011). This finding raises the possibility that oxidative stress following brain injury may also contribute to enhanced E2 production in the brain. As a whole, the studies indicate that trauma and ischemic injury to the brain causes a robust elevation of aromatase in astrocytes and a corresponding increase in BDE2, possibly through induction of inflammation, cytokines, and prostaglandins. Many of the above studies were performed in birds, with few studies in other species. Thus, future studies using other species are needed. Functionally, the elevation of aromatase and BDE2 after brain injury has been implicated to exert important neuroprotective and anti-inflammatory actions to help protect and repair the injured brain, which will be discussed in detail in a subsequent section (Arevalo et al., 2015; Brocca and Garcia-Segura, 2019; Garcia-Segura et al., 2003, 1999; Wang et al., 2020).
Interestingly, in addition to trauma and ischemic brain injury, there is evidence that aromatase and local E2 are altered in other types of neurodegenerative disorders, although the number of studies is relatively small. For instance, aromatase expression was found to be increased in astrocytes at disease onset in an animal model of amyotrophic lateral sclerosis, followed by decreased expression as the disease progressed (Eisenman, 1988). In addition, examination of brain tissue samples from Alzheimer’s disease (AD) subjects revealed increased aromatase immunoreactivity in the hippocampal CA4 region, but contrastingly aromatase immunoreactivity was decreased in the brains of 5xFADD mice versus wild type controls (Prange-Kiel et al., 2016). Increased aromatase mRNA and immunoreactive protein levels were also demonstrated in the prefrontal cortex of the late-stage human AD brain, with the aromatase increase occurring primarily in astrocytes (Luchetti et al., 2011). Unfortunately, local E2 levels were not determined in these two studies on AD. A subsequent study did measure brain E2 levels in postmortem brain samples from women with AD, as well as aromatase expression and immunoreactivity (Yue et al., 2005). This study found that aromatase expression, aromatase immunoreactivity, and E2 levels were significantly decreased in frontal cortex and cerebellum of AD subjects, as compared to normal controls (Yue et al., 2005). In addition, there was a significant negative correlation between the reduced aromatase expression and amyloid plaque density in the cortex in AD subjects, suggesting that BDE2 may protect the AD brain by regulating plaque formation (Yue et al., 2005). In support of this possibility, APP23/Ar+/− mice, which are transgenic mice that overexpress amyloid precursor protein (APP) and lack aromatase, were found to have increased BACE activity as well as early onset and increased Aβ deposition in the brain (Yue et al., 2005). Finally, seizure activity in a rat model of status epilepticus was found to stimulate de novo synthesis of E2 in the hippocampus (Sato and Woolley, 2016). Furthermore, systemic or intra-hippocampal administration of the AI, fadrozole in gonadectomized rats suppressed kainic acid-induced seizures (Sato and Woolley, 2016). These findings suggest that over-production of hippocampal-derived E2 may have a role in promoting seizures and that aromatase inhibitors may have therapeutic utility for status epilepticus.
4. Role of brain aromatase and BDE2 in sexual differentiation, reproduction and socio-sexual behavior
The above studies demonstrated aromatase localization and BDE2 production in many brain regions in multiple species, and showed regulation occurs by multiple processes and factors, often in a tissue- or cell-specific manner. In the following sections, we will review and discuss the evidence supporting multiple important functional roles implicated for BDE2 in the brain.
4.1. Regulation of sexual differentiation
In many species, including rodents, birds, ruminants and carnivores, neuronal aromatase and BDE2 have been implicated to play a key role in masculinization of the brain (Gorski, 1985), although the evidence for a similar role in other mammalian species such as monkeys and humans is less clear. As discussed previously, aromatase is highly expressed in the hypothalamus and sexual dimorphic regions of the POA (Sasano et al., 1998; Selmanoff et al., 1977). During the perinatal period, aromatase in these and other brain regions acts to convert testosterone to estrogen, which then contributes significantly to sexual differentiation (masculinization) of the brain (McCarthy, 2008). Masculinization of the brain is characterized by expression of male-typical sexual behavior, male-type pattern of gonadotropin secretion, and aggression (Negri-Cesi et al., 2004). In addition, there is a second process of defeminization, in which male ability to exhibit female typical behaviors in adulthood is significantly attenuated (Negri-Cesi et al., 2004). After exposure to testosterone from the developing testes, the mammalian brain develops as male, while in the absence of such exposure it develops as female (Gorski, 1985). In females, the lack of early exposure to testosterone is essential for sexual behavior and expression of the ovulatory surge of gonadotropins (Gorski, 1985; Negri-Cesi et al., 2004).
In support of a key role for aromatase and BDE2 in sexual differentiation of the brain in rodents, early work showed that estrogen administration neonatally in castrate male rats suppressed the female pattern of gonadotropin secretion and behavior, while inducing the male pattern of sexual behavior (Booth, 1977). This finding suggested that sexual differentiation of the brain involves aromatization of testicular androgens to estrogens in the brain. In further support of this suggestion, administration of an aromatase inhibitor perinatally to male rats resulted in reduced development of the sexual dimorphic nucleus in the POA and reduced masculine sexual behavior (Houtsmuller et al., 1994). Injection of an aromatase inhibitor later on day 12 postnatally had no significant effect in male rats, indicating that BDE2 acts in the early postnatal period to induce sexual differentiation (Gonzalez and Leret, 1994). Furthermore, BDE2 actions appear to be mediated by estrogen receptors as postnatal administration of the estrogen receptor antagonist, tamoxifen inhibited sexual differentiation of the brain (Dohler et al., 1984). Further work using estrogen receptor knockout mouse models has suggested that ERα mediates masculinization, while ERβ is critical for the defeminization effects of BDE2 (Kudwa et al., 2006). BDE2 appears to regulate sexual differentiation through multiple processes including regulation of apoptosis, neurite outgrowth, and synaptic patterning in various brain regions (McCarthy, 2008; Tsukahara and Morishita, 2020). Additional work indicates that BDE2 increases excitatory inputs to the POA in order to promote male sexual behavior in adulthood, and there is evidence this effect involves an increase in PGE2 that promotes anchoring of glutamate receptors in dendritic spines (Wright et al., 2010). There is also evidence that BDE2 enhances glutamate release in the hypothalamus as a mechanism to facilitate defeminization (Schwarz et al., 2008).
4.2. Regulation of reproduction
In addition to regulation of sexual differentiation, there is increasing evidence that BDE2 has an important role in adult animals to regulate GnRH and gonadotropin secretion, as well as puberty and socio-sexual behavior. With respect to a role in regulation of gonadotropin secretion, recent work has demonstrated that systemic letrozole administration strongly attenuated the exogenous E2-induced LH surge in ovariectomized monkeys (Kenealy et al., 2017). This effect appears to be due to actions in the hypothalamus to regulate factors that control LH release, as letrozole administration in the median eminence of the hypothalamus of ovariectomized monkeys significantly attenuated the exogenous E2-induced surges of kisspeptin and GnRH, which are the key hypothalamic factors responsible for the LH surge (Kenealy et al., 2013; Terasawa, 2018). Estradiol benzoate administered into the median eminence of ovariectomized monkeys was also found to rapidly stimulate release of pulsatile GnRH and E2 and this effect was blocked by letrozole treatment. The investigators also found that excitation of the mediobasal hypothalamus through electrical stimulation stimulated both GnRH and E2 release, suggesting an activity-dependent regulation of BDE2 in the hypothalamus (Kenealy et al., 2013; Terasawa, 2018). Additional work revealed that direct administration of letrozole into the median eminence of ovariectomized monkeys suppressed spontaneous GnRH release and estradiol benzoate-induced release of GnRH and E2 (Kenealy et al., 2013; Terasawa, 2018). Collectively, these studies provide evidence that BDE2 is involved in regulation of pulsatile GnRH release and is necessary for full expression of the GnRH and LH surge. In addition to these effects, there is also evidence that BDE2 may contribute to negative feedback regulation of gonadotropin secretion. In support of this suggestion, chronic daily letrozole treatment of ovariectomized female monkeys resulted in a suprahypergonadotropic phenotype one month after treatment was initiated (Kraynak et al., 2017). A potential role for BDE2 in negative feedback control in males was also suggested based on the finding that treatment of male monkeys with the aromatase inhibitor, 1,4,6-androstratriene-3,17-dione (ATD) reduced hypothalamic aromatase activity by 80–90 % and resulted in elevation of LH (Ellinwood et al., 1984). Likewise central administration of the aromatase inhibitor fadrozole in male sheep increased LH pulse frequency, suggesting that central aromatization controls pulsatile LH secretion (Sharma et al., 1999).
The above finding that BDE2 may exert negative feedback control over gonadotropin secretion is intriguing and raises the possibility it could participate in restraining GnRH pulsatility until the time of puberty. During the infantile period after birth, there is strong GnRH pulsatility, which becomes quiescent during childhood, and then recommences at the time of puberty. This finding has led to the suggestion that there is a “brake” or inhibitory signal that restrains GnRH pulsatility, which is released at puberty. Intriguingly, Lephart and Ojeda Lephart and Ojeda (1990) found that in both male and female rats, hypothalamic aromatase activity is significantly decreased at the time of puberty. Furthermore, hypothalamic aromatase mRNA and protein levels were also shown to be decreased at the onset of true precocious puberty in female rats (Tian et al., 2004). Additional work revealed that stalk-median eminence levels of E1 and E2 levels are higher at the prepubertal phase in female monkeys than at later early pubertal phase when GnRH release begins to increase at night (Kenealy et al., 2016). This finding suggests that the elevated E1 and E2 are hypothalamic in origin as circulating E1 and E2 are low in prepubertal and early pubertal monkeys. Collectively, these findings raise the possibility that BDE2 may play a role in central inhibition of GnRH before the onset of puberty. This intriguing hypothesis awaits further testing.
4.3. Regulation of socio-sexual behavior
Over the past several decades, there has emerged abundant evidence that BDE2 regulates socio-sexual behavior. A large part of this work has been performed in male quail, although some work has also been performed in other species. Early work in adult male quail found that exogenously administered testosterone must be aromatized to activate copulation (Watson and Adkins-Regan, 1989). In the study, administration of an aromatase inhibitor blocked copulatory behavior in castrate males that had testosterone implants in the POA. Subsequent work demonstrated that systemic administration of the aromatase inhibitor vorozole rapidly inhibited sexual motivation in male quail (Cornil et al., 2006). Additional support for a role of BDE2 in regulation of sexual behavior came from the finding that central administration of vorozole rapidly inhibited sexual motivation but not sexual performance in male quail (Seredynski et al., 2013). Interestingly, central administration of E2 or membrane-impermeable E2 analogs was also able to enhance sexual motivation in estrogen-deplete male quail (Seredynski et al., 2013). This suggests that the effects of BDE2 may involve mediation by membrane estrogen receptors. In support of this suggestion, administration of estrogen receptor antagonists have been shown to decrease sexual motivation (Seredynski et al., 2013). BDE2 may also regulate aggressive behavior in males. In support of this possibility, high aromatase activity in the anterior hypothalamus/POA was shown to be correlated with aggressive behavior in male quail and male song sparrows (Schlinger and Callard, 1989; Soma et al., 2003). Furthermore, administration of an aromatase inhibitor inhibited aggression in reproductively active male quail (Schlinger and Callard, 1990) and in nonbreeding male song sparrows in winter (Soma, 2006), and blocked testosterone-induced aggression in reproductively inactive male quail (Schlinger and Callard, 1990).
Studies in mice also support a role for brain aromatase in sexual behavior. Whole body aromatase knockout in male mice resulted in deficits in motivational and consummatory sexual behavior (Bakker et al., 2002). In addition, a brain-specific aromatase knockout mouse was recently generated by crossing floxed aromatase mice with nestin-cre mice and used to examine the role of BDE2 in male sexual behavior (Brooks et al., 2020). The brain-specific aromatase male mice exhibited a 50 % decrease in number of mounts and intromissions, which could be rescued by testosterone and E2 replacement. The mice had elevated testosterone levels which the authors postulated could be due to a defect in negative feedback upon gonadotropin secretion (Brooks et al., 2020). However, they were unable to demonstrate any significant effect on gonadotropin levels in the knockout mice versus wild type mice.
Finally, there have been contradictory results on whether aromatization is necessary for male sexual behavior in humans. One study reported that in men who received testosterone, treatment with an aromatase inhibitor was associated with significant decreases in sexual desire and erectile function (Finkelstein et al., 2013). In contrast, another study reported that administration of an aromatase inhibitor or an estrogen receptor antagonist had no effect upon sexual function in men (Gooren, 1985). Likewise, it was shown that male sexual function could be maintained without aromatization in healthy men who received the non-aromatizable androgen, 5α-dihydro-testosterone (DHT) (Sartorius et al., 2014). The study found no effect of DHT on 33 measures of sexual function except for a mild decrease in sexual desire. Taken as a whole, the above studies suggest that brain aromatization is necessary for normal sexual behavior in quail and rodents, but the case for a similar role in humans is unclear and requires additional study.
5. Role of brain aromatase and BDE2 in synaptic plasticity and cognitive function
5.1. Synaptic plasticity
BDE2 has been implicated to also play an important role in regulating synaptic plasticity in the brain in a variety of species. Rune and coworkers were the first to report that letrozole treatment of rat hippocampal slices cultured in vitro decreased E2 release and the density of spines and spine-synapses, as well as decreased levels of the presynaptic protein synaptophysin and the postsynaptic protein spinophilin (Kretz et al., 2004). A decrease of spine synapses in rat hippocampal neurons cultured in vitro was also observed following letrozole treatment (Kretz et al., 2004). Furthermore, letrozole treatment in mouse hippocampal slice cultures caused decreases in mitochondrial volume, dendritic spine density and synaptic proteins, and exacerbated Aβ1−42-induced mitochondrial and synaptic plasticity defects (Chang et al., 2013). In vivo letrozole treatment of adult female mice decreased spine synapse density in the hippocampal CA1 region and dentate gyrus molecular layer of the hippocampus; with no significant effect observed in the prefrontal cortex or cerebellum (Bender et al., 2010; Fester et al., 2012; Prange-Kiel et al., 2013). Similar to the in vitro findings, in vivo letrozole treatment also decreased spinophilin and synaptophysin expression levels in the female mouse hippocampus, as well as the glutamate receptor, NMDAR1 (Zhou et al., 2010). Interestingly, Rune and coworkers also reported a sex difference in the letrozole effect on synaptic plasticity, as in vivo peripheral letrozole treatment for 4-weeks decreased hippocampal spine synapses in cycling and ovariectomized female mice (Zhou et al., 2010), as well as cycling female rats, but not in male rats (Fester et al., 2012). In contrast to this report, Zhou et al. found that in vivo letrozole treatment did significantly decrease hippocampal spines, synapses and post-synaptic density (PSD) proteins in male mice (Zhao et al., 2018). They also found decreased PSD thickness and increased actin depolymerization in the letrozole-treated male mice. Regulation of actin polymerization by BDE2 may help explain the changes in spine density observed in the letrozole-treated mice, as the actin cytoskeleton plays an important role in spine formation, elimination and morphology (Harris, 1999; Matus, 2000). It is not clear why the two studies yielded divergent results with regards to the sex-specific effect of letrozole on synaptic plasticity. While both groups used 4-weeks of daily intraperitoneal administration of letrozole, Zhou et al. used a two-fold higher dose of letrozole as compared to Rune’s group (e.g., 80μg/kg BW versus 40μg/kg BW). Interestingly, some studies have found that E2 levels in the male hippocampus are higher than that observed in females (Ooishi et al., 2012). Therefore, a higher dose of letrozole might be required to effectively reduce hippocampal E2 levels in males and observe an inhibitory effect on synaptic plasticity.
Long-term potentiation (LTP) is widely considered a synaptic mechanism for memory (Bliss and Collingridge, 1993; Nicoll, 2017). Over the past decade, a number of groups have examined the role of aromatase and local-derived E2 in LTP in several brain regions. Grassi and coworkers were the first to demonstrate that local-derived E2 is essential for induction of LTP in the brainstem (Grassi et al., 2009). Using male rat brainstem slices, they showed that letrozole had no effect on baseline glutamatergic synaptic responses but prevented LTP produced by high frequency tetanic stimulation (HFS) (Grassi et al., 2009). HFS administered at 5 min after letrozole administration did not induce LTP (Grassi et al., 2009). This finding would appear to rule out relevant storage of E2 and raises the possibility that HFS enhances aromatase activation to rapidly induce E2. In support of this possibility, HFS has been shown to induce E2 release in spinal cord slices, which could be blocked by aromatase inhibitor treatment (Zhang et al., 2012). These findings suggest that activity-dependent regulation of local E2 synthesis and release is a key mechanism mediating HFS-induced LTP.
Subsequent work demonstrated that local-derived E2 similarly mediates LTP in the hippocampus (Grassi et al., 2011). Using male rat hippocampal slices, the investigators found that letrozole reduced the amplitude of LTP by 60 % while not affecting baseline responses. A full LTP response could be rescued by exogenous E2 treatment in letrozole-treated slices (Di Mauro et al., 2017). In additional studies, inhibition of E2 synthesis by letrozole also prevented LTP in medial spiny neurons and cholinergic interneurons in the dorsal striatum of male rats, and this effect could be rescued by exogenous E2 (Tozzi et al., 2015). Letrozole treatment also prevented LTP at the cerebellar parallel fiber-Purkinje cell synapse, which was correlated with impairment of both gain increases and decreases adaption of the vestibular-ocular reflex (Dieni et al., 2018a, b). BDE2 likely acts through estrogen receptors to mediate its synaptic plasticity effect, as administration of an estrogen receptor antagonist also significantly reduced LTP amplitude in hippocampal slices (Grassi et al., 2011). Interestingly, letrozole treatment had no effect upon maintenance of LTP or on pair pulse facilitation, a type of presynaptic plasticity that involves a change in neurotransmitter release probability (Grassi et al., 2011). Further work found that letrozole also had no effect upon induction of long-term depression (LTD) in hippocampal and cerebellar slices, which indicates that BDE2 only regulates induction of LTP and not LTD in the hippocampus and cerebellum (Di Mauro et al., 2017, 2015; Dieni et al., 2018a). Letrozole can lead to an increase of androgens due to inhibition of their conversion to estrogen. This raises the possibility that androgens may mediate the effect of letrozole on LTP. However, the fact that E2 replacement rescued LTP would seem to argue against this possibility. Furthermore, Tozzi et al. (Tozzi et al., 2019) showed that androgen receptor antagonists had no effect on LTP in male rat hippocampal slices. Thus, it appears that activation of estrogen receptors, but not androgen receptors, is critical for inducing LTP in hippocampal pyramidal neurons. The finding of a role of BDE2 in LTP also extends to mice and to females, as 7-day in vivo letrozole treatment reduced LTP amplitude in hippocampal slices from ovariectomized mice by 50 %, and by 20 % in male mice (Vierk et al., 2012). It is not clear why the magnitude of inhibition of LTP in this study in male mice was less than that observed in the above studies for male rats (e.g. 20 % versus 60 % inhibition). It could be due to the different species used, different in vitro versus in vivo letrozole treatment paradigms, and/or different dosing schedules employed in the studies.
Finally, to more specifically explore the roles and function of neuron-derived E2 (NDE2) in the regulation of synaptic plasticity in the brain, our group generated a forebrain-neuron-specific aromatase knock-out (FBN-ARO-KO) mouse model to specifically deplete NDE2 in the forebrain (Lu et al., 2019). Cyp19a1-Cre/LoxP conditional KO mice were created, under the control of the CaMKIIα promoter, which expresses Cre exclusively in forebrain excitatory neurons. Characterization of the mice using PCR, Western blot, IHC and ELISA analysis demonstrated a profound loss of aromatase in hippocampal and cortical neurons with an associated 65–70 % decrease of E2 levels in the hippocampus and cortex of both male and ovariectomized female FBN-ARO-KO mice, as compared to FLOX mice (Lu et al., 2019). Aromatase expression was unchanged in the hindbrain and ovary and there was no change in serum E2 levels in FBN-ARO-KO versus FLOX mice, demonstrating that the knockout was specific for forebrain neurons. Furthermore, the postnatal deletion of the aromatase gene in excitatory forebrain neurons did not result in any apparent defects in brain structure in FBN-ARO-KO mice (Lu et al., 2019).
Interestingly, Golgi analysis revealed that spine density is significantly decreased in the hippocampal CA1 region and cortex of adult male and ovariectomized female FBN-ARO-KO mice, with thin spines showing the greatest decrease in males, and mushroom spines showing the greatest decrease in females (Lu et al., 2019). This finding indicates that NDE2 is an important regulator of spine formation in the cerebral cortex and hippocampal CA1 region in both male and female mice. Examination of synapse number by examining synaptophysin and PSD95 contacts revealed a 33 % decrease of synapse density in the hippocampal CA1 and cortex of male FBN-ARO-KO mice, and a 55–58 % decrease in female FBN-ARO-KO mice (Lu et al., 2019). Thus, this result suggests that NDE2 also regulates forebrain synaptic density in both male and female mice.
We also examined the role of NDE2 in regulation of synaptic transmission and LTP in our FBN-ARO-KO mouse model (Lu et al., 2019). The results revealed a significant defect in the efficiency of synaptic transmission in hippocampal slices of both male and ovariectomized female FBN-ARO-KO mice, which was fully rescued by exogenous E2 (Lu et al., 2019). Examination of LTP in hippocampal slices from FLOX and FBN-ARO-KO mice revealed a 57 % decrease in the amplitude of LTP in male FBN-ARO-KO mice versus a 91 % decrease in female FBN-ARO-KO mice (Lu et al., 2019). As seen in the effect upon synaptic density above and here for LTP, depletion of forebrain E2 in FBN-ARO-KO mice significantly decreased synaptic density and LTP amplitude in both sexes, but a greater percent decrease was observed in ovariectomized females as compared to males. Nevertheless, the effects in both sexes appear functionally significant, as cognitive function was robustly and significantly attenuated in both sexes of FBN-ARO-KO mice (e.g., significantly decreased in ovariectomized females, intact females and intact males) (Lu et al., 2019), as discussed in the section below. It is not entirely clear which comes first following NDE2 depletion, loss of spines and synapses or loss of LTP. It is possible that the loss of spines and synapses could drive a diminishment of LTP in the forebrain. However, in argument against this, Vierk et al. (Vierk et al., 2012) found that LTP impairment preceded the loss of spines and synapses in the hippocampus of letrozole-treated female mice. Furthermore, we found that LTP in hippocampal slices from FBN-ARO-KO mice could be rapidly rescued within minutes by exogenous E2 (Lu et al., 2019), an effect that would seem too fast for induction of spine synapses to have occurred. Interestingly, LTP has been reported to induce spines and synapses in the brain (Toni et al., 1999), further supporting that loss of LTP could drive the loss of spine and synapses following BDE2 depletion. Nevertheless, before definitive conclusions can be reached, more studies are needed to understand the precise temporal dynamics underlying the spine and synapse loss and LTP impairment observed in the FBN-ARO-KO mice.
Mechanistically, both non-genomic and genomic signaling mechanisms may contribute to NDE2 regulation of synaptic plasticity (Lai et al., 2017). In support of a non-genomic mechanism, we found that E2 replacement in vitro could rapidly rescue the decrease in synaptic transmission efficiency and LTP amplitude in hippocampal slices from both male and female FBN-ARO-KO mice (Lu et al., 2019). Furthermore, the E2 rescue appeared to involve rapid mitogen-activated protein kinase (MAPK) signaling as it was blocked by co-administration of the MAPK inhibitor, U0126 (Lu et al., 2019). In further support of a critical role for NDE2 in regulating rapid kinase signaling in the forebrain, both AKT and ERK activation were found to be significantly attenuated in the hippocampus and cortex of both male and female FBN-ARO-KO mice, and this effect could be rescued by exogenous E2 replacement (Lu et al., 2019). Functionally, both AKT and ERK signaling have been implicated to mediate LTP and synaptic plasticity in the brain (Levenga et al., 2017; Mao and Wang, 2016; Sweatt, 2001). Furthermore, exogenous E2 rapidly enhances induction of both signaling pathways in the hippocampus and cortex (Ogiue-Ikeda et al., 2008; Singh, 2001; Singh et al., 1999), and inhibitors to these pathways block exogenous E2-induced LTP in hippocampal slices (Hasegawa et al., 2015). The rapid signaling effects of NDE2 on kinase signaling are proposed to be mediated by estrogen receptors localized at the cell membrane. Indeed, ERα and ERβ, in addition to being localized in the cytoplasm and nucleus, have also been reported to be localized at the cell membrane in neurons (Evinger and Levin, 2005; McEwen et al., 2001; Milner et al., 2005, 2001; Raz et al., 2008), and the newest proposed member of the estrogen receptor family, G-protein coupled estrogen receptor-1 (GPER1), has also been shown to be localized at the membrane in various cell types including neurons (Kumar and Foster, 2020; Raz et al., 2008; Revankar et al., 2005; Waters et al., 2015). Furthermore, all three receptors have been implicated in mediating both the rapid signaling and plasticity effects of E2 in the brain (Fester et al., 2013; Kumar et al., 2015; Kumar and Foster, 2020; Liu et al., 2008; Mukai et al., 2010; Raz et al., 2008; Sellers et al., 2015; Smejkalova and Woolley, 2010; Spencer-Segal et al., 2012). While we did not examine which estrogen receptor mediates NDE2 effects in our studies, future studies are planned to test selective ligands for the three estrogen receptors in our FBN-ARO-KO mice to help determine which receptor(s) mediates rescue of the plasticity and memory defects. Testing of membrane impermeable E2-BSA or E2-dendrimer conjugates to further confirm the role of membrane localized estrogen receptors and cytoplasmic estrogen receptors would also be important. Interestingly, the results of our study did suggest a possible role for NDE2 in differential regulation of estrogen receptor levels in the forebrain, as we found ERβ is up-regulated in the hippocampus and cortex of FBN-ARO-KO mice, while ERα is down-regulated (Lu et al., 2019). Similar to our results in FBN-ARO-KO mice, letrozole treatment was found to also decrease ERα while increasing ERβ in mouse hippocampal slices (Fester et al., 2013). Since neither study examined GPER1 expression, it is not clear whether GPER1 is also regulated by BDE2. Therefore, future studies are needed to address this question. It should be pointed out that since exogenous E2 was able to rescue synaptic plasticity and memory defects in both FBN-ARO-KO mice (Lu et al., 2019) and aromatase inhibitor-treated animals (Tozzi et al., 2015; Tuscher et al., 2016) it suggests that local E2 synthesis may not be necessary for exogenous E2 effects to enhance plasticity and memory.
Finally, we also examined cAMP response element binding protein (CREB) activation and brain-derived neurotropic factor (BDNF) expression in the forebrain of FBN-ARO-KO mice, as both factors are also known to be key regulators of synaptic plasticity and memory (Benito and Barco, 2010; Kida, 2012; Miranda et al., 2019). Interestingly, CREB is a transcription factor activated by phosphorylation that can regulate expression of many genes, including BDNF (Kida, 2012; Shieh et al., 1998). Conversely, BDNF can regulate activation of CREB through tropomyosin receptor kinase (Trk) B receptors (Finkbeiner et al., 1997). Examination of intact male and ovariectomized female FBN-ARO-KO mice revealed a significant decrease in CREB phosphorylation (pCREB) and BDNF expression in the hippocampus and cortex of FBN-ARO-KO mice, as compared to FLOX controls (Lu et al., 2019). This effect appeared to be due to loss of forebrain E2, as reinstatement of E2 levels in the forebrain by exogenous E2 replacement was able to rescue CREB activation and BDNF expression in FBN-ARO-KO mice (Lu et al., 2019). Thus, in addition to regulating rapid kinase signaling, BDE2 appears to be a key regulator of CREB and BDNF in the forebrain, which is proposed to contribute to its plasticity and memory regulatory effects.
Based on our results from the FBN-ARO-KO mice (Lu et al., 2019), a proposed mechanism for NDE2 effects on synaptic plasticity and LTP in the forebrain is illustrated in Fig. 5. As shown in Fig. 5, NDE2 regulation of spines, synapses and LTP is proposed to involve membrane estrogen receptor-mediated rapid PI3K/AKT and MEK/ERK kinase signaling, which is capable of quickly modulating synaptic plasticity. As also shown in Fig. 5, the activated intracellular kinase signaling can in turn phosphorylate the important transcriptional factor, CREB which translocates into the nucleus to facilitate the expression of CREB target genes that regulate synaptic plasticity, including the neurotrophic factor BDNF and the synaptic protein PSD95. Furthermore, BDNF can also regulate synaptic plasticity by coupling to the rapid intracellular kinases. BDNF signaling can also activate cofilin, which is required for F-actin assembly and dendritic spine formation (Briz et al., 2015). Classical nuclear ERα- and ERβ-mediated genomic signaling may also contribute to NDE2 regulation of synaptic plasticity by transactivating estrogen response elements (ERE) in plasticity-related genes and promoting their transcription.
Fig. 5. Schematic illustration of the potential mechanisms underlying neuron-derived E2 (NDE2) regulation of synaptic plasticity.
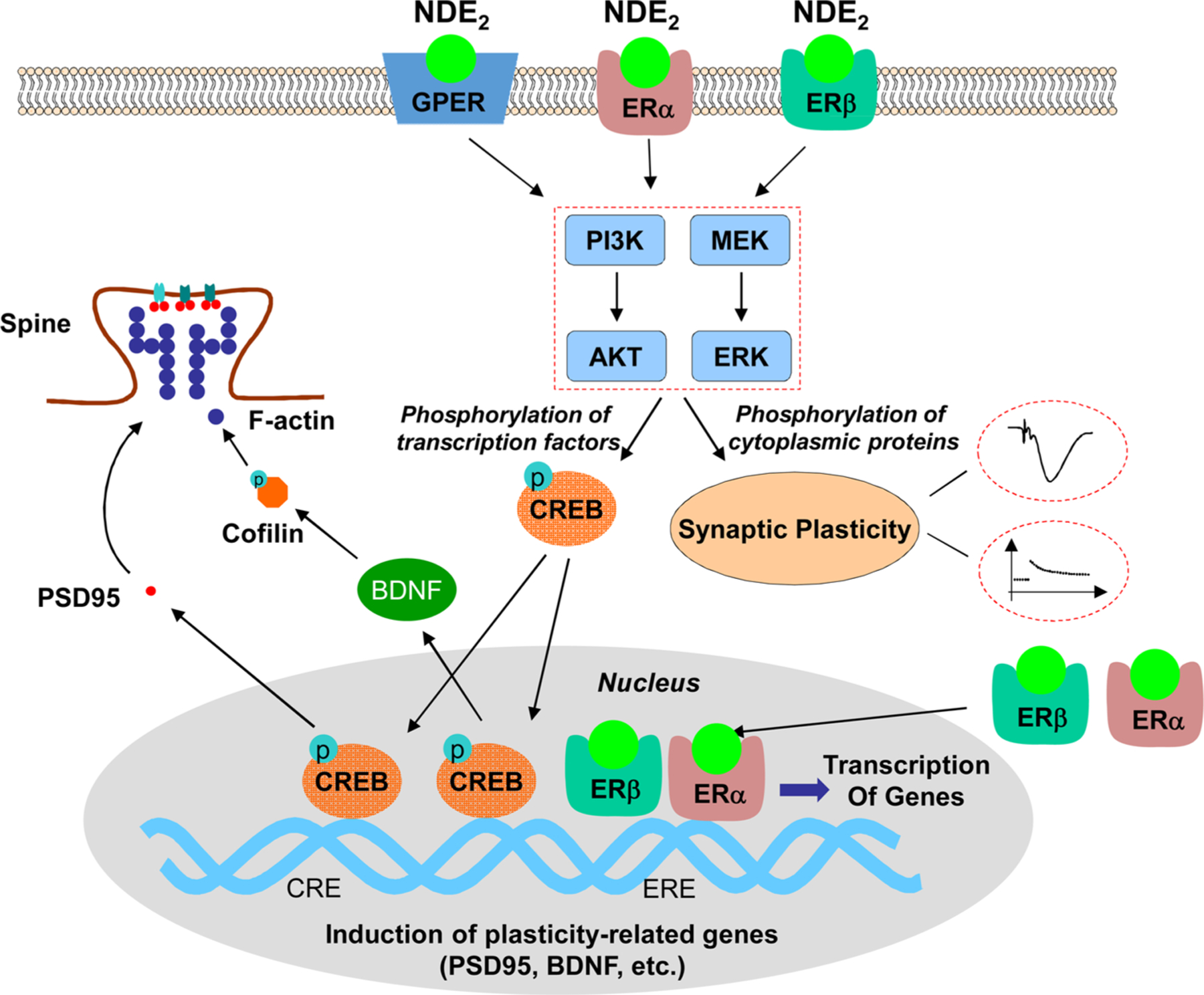
It is proposed that neuron-derived E2 (NDE2) regulates synaptic plasticity via both rapid and genomic signaling mechanisms. 1) Membrane localized estrogen receptors (estrogen receptor-α and β, ERα and ERβ, and G-protein coupled estrogen receptor-1, GPER1) can bind NDE2 and 2) the receptor bound NDE2 then induces rapid PI3K/AKT and MEK/ERK kinase signaling, which is capable of quickly shaping synaptic plasticity. In addition, the activated intracellular kinase signaling also phosphorylates the important transcriptional factor, CREB which further translocates into nucleus to facilitate the expressions of neurotrophic factor BDNF and synaptic protein PSD95. BDNF can also regulate synaptic plasticity by coupling to the rapid intracellular kinases. Moreover, BDNF signaling activates cofilin which is required for F-actin assembly and dendritic spine formation. Intracellular ERα and ERβ act in the genomic signaling pathway by transactivating estrogen response elements (ERE) in regulated genes and promoting genes transcription. CRE = cAMP response element.
5.2. Cognitive function
BDE2 regulation of synaptic plasticity and LTP in the forebrain suggests it may regulate memory. Indeed, there is now a large body of evidence in a variety of species implicating an important role of BDE2 in the regulation of cognitive function. For instance, a number of clinical studies in humans have found that aromatase inhibitor treatment in breast cancer patients is associated with a variety of memory defects, including defects in verbal and visual learning/memory, executive function, and processing speed, which were reversible after cessation of aromatase inhibitor therapy (Bender et al., 2007; Phillips et al., 2011; Rocha-Cadman et al., 2012; Underwood et al., 2018), although there are dissenting studies (Hurria et al., 2014). Furthermore, Bayer and coworkers performed cognitive testing and MRI analysis on postmenopausal women with breast cancer and a control group to examine the effect of aromatase inhibition on cognitive function (Bayer et al., 2015). The study participants underwent cognitive testing twice before the start of letrozole therapy and then again at least 3 months after start of treatment (Bayer et al., 2015). The results revealed that letrozole treatment impaired hippocampal-dependent memory, and MRI analysis revealed this effect was associated with decreased hippocampal activity during encoding (Bayer et al., 2015). Collectively, the above studies provide evidence that BDE2 may be important for cognitive function in humans.
Basic science studies in animals have also confirmed a critical role for BDE2 in cognitive function. One of the first studies to examine this question was a study that used adult castrate male zebra finches (songbirds) with implants of vehicle, an aromatizable androgen (testosterone), a non-aromatizable androgen (5α-DHT), or E2 and measured the effect upon spatial memory (Oberlander et al., 2004). Testosterone- and E2-treated birds, but not 5α-DHT-treated birds, learned the spatial memory task. The investigators concluded that brain aromatization enhances spatial memory in songbirds (Oberlander et al., 2004). Subsequent work utilized aromatase inhibitors to confirm the suggested role of BDE2 in cognitive function. These elegant studies demonstrated that aromatase inhibition in male songbirds disrupts auditory association learning and neural memory for previously heard songs (Macedo-Lima and Remage-Healey, 2020; Yoder et al., 2012). Acute aromatase inhibition was also shown to reduce motivation to sing in male canaries, as well as song acoustic stereotypy, a measure of consistency over song renditions (Alward et al., 2016). Further work showed that local-derived E2 is important for auditory processing in the NCM, a part of the songbird auditory association cortex important for song learning, memorization and perception (Tremere et al., 2009). The study found that aromatase inhibitor infusion in the NCM markedly decreased neuronal firing rates in a dose-dependent manner in birds stimulated with conspecific songs (Tremere et al., 2009). Aromatase inhibition in the NCM was also associated with reduced induction of MAPK-dependent genes, which have been implicated to mediate synaptic plasticity and memory in the songbird (Tremere et al., 2009). In addition to the NCM, there is evidence that local E2 also acts in the hippocampus to regulate spatial memory. For instance, central aromatase inhibition in the male zebra finch attenuated PSD95 levels in the hippocampus and impaired spatial memory in a food-finding task (Bailey et al., 2013, 2017; Bailey and Saldanha, 2015).
A role for BDE2 in cognitive function has also been implicated in rodents. For instance, intracerebroventricular administration of letrozole for 14 days in intact male and female rats decreased hippocampal E2 levels and hippocampal pyramidal neuron firing and caused dose-dependent defects in working memory and novel object recognition memory (Marbouti et al., 2020a). Similarly, letrozole treatment in male and female mice resulted in spatial memory impairment (Liu et al., 2019; Zhao et al., 2018). Furthermore, infusion of letrozole bilaterally into the hippocampus impaired hippocampal memory consolidation in ovarectomized female mice, and this defect could be rescued by exogenous E2 replacement (Tuscher et al., 2016). This finding suggests that BDE2 has a critical role in hippocampal memory consolidation.
To confirm and extend these pharmacological studies, we used the genetic FBN-ARO-KO mouse model to specifically deplete forebrain NDE2 and examined its role in cognitive function (Lu et al., 2019). As shown in Fig. 6, we conducted cognitive behavioral testing on intact male, intact female and ovariectomized female FLOX and FBN-ARO-KO mice using standard behavioral testing paradigms for rodents, including the Barnes maze for testing hippocampal-dependent spatial reference learning and memory, the novel object recognition test for testing hippocampal-dependent spatial recognition memory, the forced swim test for testing for depressive-like behavior, and the fear conditioning test to examine long-term fear memory. The results revealed that the adult intact male, intact female and ovariectomized female FBN-ARO-KO mice all had significant deficits in hippocampal-dependent spatial reference learning and memory, hippocampal-dependent recognition memory, and hippocampal-dependent contextual fear memory (Lu et al., 2019). These differences were not due to differences in locomotor function, as FBN-ARO-KO mice had normal locomotor function and anxiety, as measured by the open field test. Interestingly, ovariectomized female FBN-ARO-KO mice, but not intact male and intact female FBN-ARO-KO mice, exhibited depressive-like behavior in the forced swim test (Lu et al., 2019). The behavior defects in the FBN-ARO-KO mice appear to be due to the loss of forebrain E2 as reinstatement of forebrain E2 levels by exogenous E2 replacement was able to fully rescue the hippocampal-dependent spatial recognition memory and reference memory in ovariectomized female FBN-ARO-KO mice (Lu et al., 2019). We did not examine E2 rescue of fear memory or depressive behavior, and thus future studies are needed to examine this issue.
Fig. 6. Cognitive functions regulated by neuron-derived E2 (NDE2) and the commonly used behavioral tests for rodent studies.
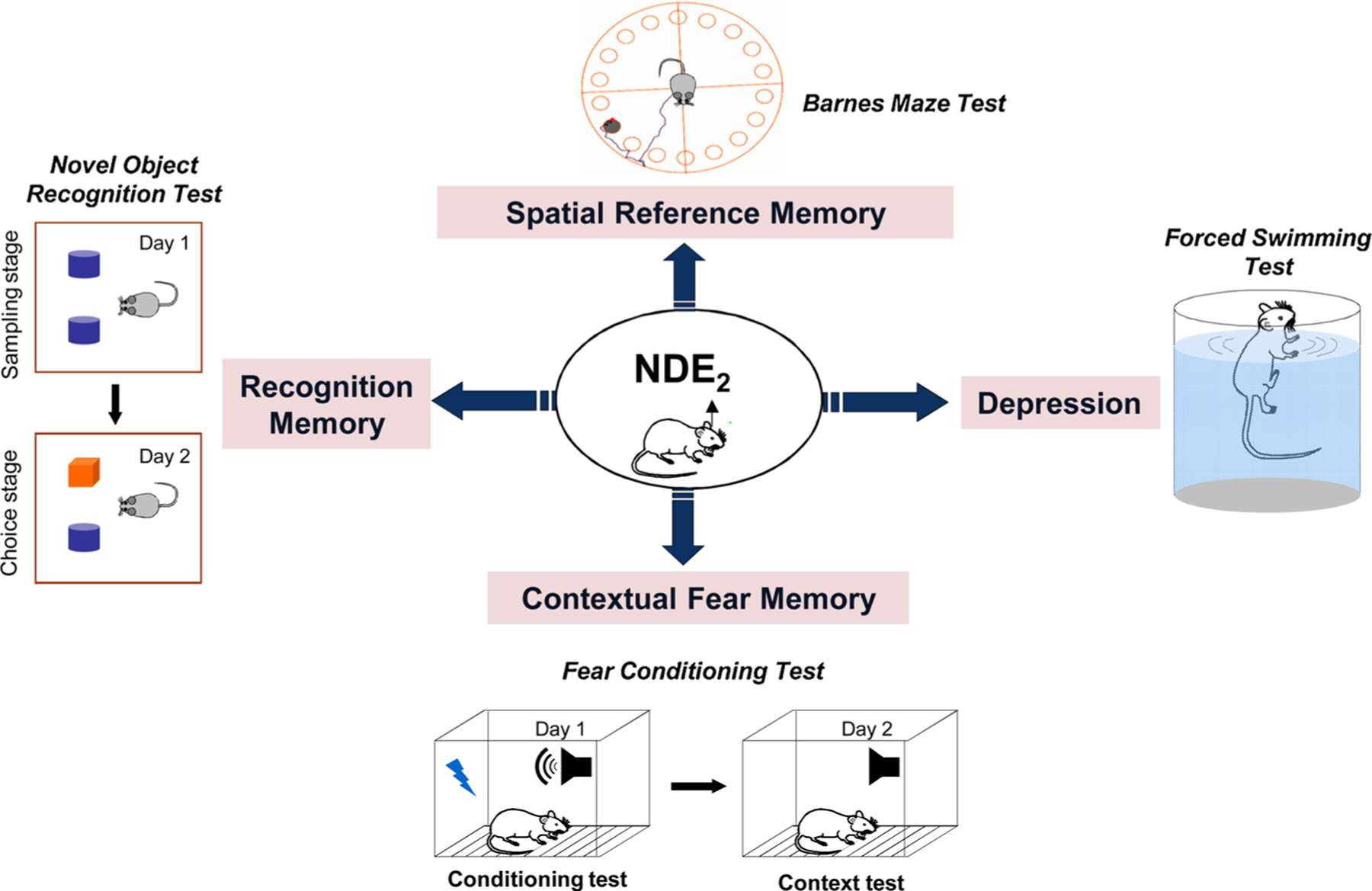
Neuron-derived E2 (NDE2) is critical for hippocampus-dependent spatial reference memory, which is often tested using the Barnes Maze test. Hippocampus-dependent recognition memory is another important cognitive function that is regulated by NDE2 and which can be assessed using the Novel Object Recognition Test (NORT). In addition, NDE2 was also demonstrated to regulate contextual, but not cued fear memory, which can be evaluated using Fearing Conditioning Test. Finally, NDE2 is essential to prevent depressive-like behavior in female mice, which can be tested with the Forced Swimming Test.
Finally, examination of male and ovariectomized female astrocyte-specific aromatase knockout (GFAP-ARO-KO) mice revealed no defects in hippocampal-dependent spatial reference learning and memory, hippocampal-dependent recognition memory or long-term fear memory Wang et al. (2020). This finding indicates that only NDE2 regulates cognitive function in the basal (non-injured) state. This finding is not unexpected as aromatase is low to undetectable in astrocytes basally and is only induced by stress, injury or ischemia. However, as will be discussed in the next section, GFAP-ARO-KO mice do exhibit enhanced cognitive deficits after GCI, which appears to be due to a loss of a neuroprotective function of ADE2 in the GFAP-ARO-KO mice Wang et al. (2020). Taken as a whole, the above studies indicate that NDE2 has a critical role in regulating synaptic plasticity, LTP and memory in a variety of species.
6. Role of brain aromatase and BDE2 in neuroprotection
6.1. Aromatase inhibitor and knockdown studies
As discussed previously, numerous studies have demonstrated that brain injury induces aromatase in astrocytes, which leads to a local increase of BDE2 in the brain. This finding has led to the suggestion that elevation of BDE2 may serve as a protective mechanism in response to brain injury. Azcoitia and coworkers were the first to examine this question in detail (Azcoitia et al., 2001). Based on their previous work showing that aromatase was induced in brain astrocytes after excitotoxin injury (Garcia-Segura et al., 1999), the investigators examined the potential neuroprotective effect of an aromatizable androgen (testosterone) and non-aromatizable androgen (5α-DHT), as well as E2 in the hippocampus. They found that testosterone and E2, but not 5α-DHT, was neuroprotective against the excitotoxin, domoic acid in castrate male rats (Azcoitia et al., 2001). The ability of testosterone to exert neuroprotection was suggested to be due to aromatization to E2 as testosterone’s neuroprotective effect was blocked by administration of the aromatase inhibitor, fadrozole (Azcoitia et al., 2001). Furthermore, a neuroprotective role for aromatase and hippocampal BDE2 was implicated by the finding that systemic or intracerebroventricular administration of fadrozole in intact male rats protected the hippocampus from neuronal damage induced by kainic acid (Azcoitia et al., 2001). In a subsequent study, it was found that the upstream precursors of E2, pregnenolone and dehydroepiandrosterone (DHEA), also protected hippocampal neurons from kainic acid neurotoxicity, and their neuroprotective effect was blocked by fadrozole (Veiga et al., 2003). This finding suggests that the neuroprotective effects of pregnenolone and DHEA are mediated by their conversion to E2. In addition, a neuroprotective role for BDE2 in the hippocampus was further supported by the finding that inhibition of E2 synthesis by letrozole treatment enhanced methyl-mercury-induced neurotoxicity in male rat hippocampal slices in vitro, and this effect could be rescued by E2 replacement (Yamazaki et al., 2013). An additional interesting study examined a potential neuroprotective role of local E2 synthesis in the male rat striatum in the 6-hydroxydopamine-lesioned model of Parkinson’s disease model (McArthur et al., 2007). In this study, central administration of the aromatase inhibitor anastrozole was found to exacerbate 6-hydroxydopamine-induced striatal lesions, suggesting that BDE2 is neuroprotective in the striatum (McArthur et al., 2007). Further work has demonstrated that the neuroprotective effect of aromatase and BDE2 extends to other types of brain injury in addition to excitotoxic injury. For instance, male zebra finches treated with fadrozole and subjected to a penetrating brain injury were found to have a significantly larger lesion size with a greater number of apoptotic nuclei (Wynne and Saldanha, 2004; Wynne et al., 2008). The investigators proposed that E2 from glial cells mediates the neuroprotection after penetrating brain injury. Additional work showed that E2 replacement was able to rescue the defects, which further supports that the loss of local-derived E2 underlies the increased neuronal damage in fadrozole-treated birds (Wynne and Saldanha, 2004; Wynne et al., 2008).
While most studies have examined the neuroprotective effect of aromatase and BDE2 in male animals, a few studies have utilized female animals and demonstrated a similar neuroprotective effect for BDE2 as was observed in males. For instance, early work using a global whole-body knockout of aromatase in female mice demonstrated increased infarct damage in the cortex and striatum of the knockout mice, and the effect in the knockout mice was greater than observed in ovariectomized mice, suggesting that extragonadal E2 has a neuroprotective role (McCullough et al., 2003). Similarly, letrozole treatment significantly increased infarct volume, neuronal damage, apoptosis and cognitive deficits in intact female rats following focal cerebral ischemia (Zhang et al., 2017b). To further confirm the role of BDE2 in females, subsequent studies were conducted in ovariectomized animals so as to remove the potential confound of contributions by gonadal-derived E2. The first studies examined protection of the brain from excitotoxicity and revealed that administration of a low dose of kainic acid, which did not produce neuronal damage in control ovariectomized rats, led to highly significant neuronal damage in the hippocampus of fadrozole-treated ovariectomized female rats (Veiga et al., 2003). In addition, work by our group found that antisense oligonucleotide knockdown of aromatase in the hippocampus of ovariectomized female rats led to significantly enhanced neuronal damage following global cerebral ischemia (GCI), further supporting a neuroprotective role of BDE2 in female animals (Zhang et al., 2014). Furthermore, aromatase knockdown animals had higher microglial activation in the hippocampus following GCI, suggesting that BDE2 exerts an anti-inflammatory effect after cerebral ischemia. In support of an anti-inflammatory role of BDE2 after brain injury, another study found that central fadrozole administration to male and female zebra finches that received a penetrating brain injury caused sustained elevation of tumor necrosis factor-alpha (TNF-α), COX-2 and PGE2 in the injured lobe in both sexes, with females also exhibiting enhanced elevation of IL-1β (Pedersen et al., 2016). Central E2 replacement reversed these effects. These findings suggest that BDE2 may suppress inflammation in part by acting on microglia, which are known to play a key role in inflammation, and which express estrogen receptors and can be regulated by E2 (Villa et al., 2016).
6.2. Conditional knockout studies - role of NDE2 in neuroprotection
Since aromatase is induced in astrocytes after brain injury, it has been inferred that ADE2 mediates neuroprotection of the brain following ischemia or injury. However, since neurons also make E2 constitutively, then NDE2 could also potentially exert a neuroprotective role. In addition, astrocyte and neuronal aromatase are both inhibited by aromatase inhibitors; thus, the inhibitor effects could be due to depletion of either NDE2 or ADE2, or both. Indeed, in vitro studies have found that NDE2 can exert neuroprotection similar to ADE2. For instance, anastrozole treatment in H19–7 hippocampal neurons suppressed E2 production and led to enhanced neuronal cell death following H2O2 treatment (Chamniansawat and Chongthammakun, 2012). Furthermore, morphine treatment of hippocampal neurons was shown to increase release of E2, and this effect was correlated with morphine-induced neuroprotection from Aβ neurotoxicity (Cui et al., 2011). A critical role for hippocampal NDE2 in the morphine-induced neuroprotection was shown by the finding that siRNA knockdown of aromatase blocked morphine neuroprotection from Aβ neurotoxicity. To more specifically determine the role of NDE2 in neuroprotection, we utilized our FBN-ARO-KO mice in which forebrain NDE2 is specifically depleted and performed GCI (Lu et al., 2020). We utilized intact males and ovariectomized females and examined hippocampal neuronal damage, cognitive function, astrocyte reactivity, and astrocyte polarization. The studies revealed that both ovariectomized female and intact male FBN-ARO-KO mice had significantly greater neuronal damage and decreased neuronal structural integrity in the hippocampal CA1 region after GCI, as compared to FLOX controls (Lu et al., 2020). Barnes maze testing revealed that intact male and ovariectomized female FBN-ARO-KO mice both exhibited significantly greater impairment of hippocampal-dependent spatial reference memory after GCI (Lu et al., 2020). These findings indicate that NDE2 exerts a neuroprotective effect in the hippocampus following ischemic brain injury. Global transcriptome analysis coupled with qRT-PCR confirmation further revealed down-regulation of genes involved in reactive astrogliosis, neuroprotection, and neuroinflammation in the hippocampus of FBN-ARO-KO mice as compared to FLOX mice after GCI (Lu et al., 2020). Examination of reactive astrogliosis using immunohistochemistry and Western blot analysis for the astrocyte markers, GFAP or vimentin at 3, 7 and 14 days after GCI revealed that reactive astrogliosis in the hippocampus was significantly compromised in both intact male and ovariectomized female FBN-ARO-KO mice as compared to FLOX mice (Lu et al., 2020). The reduced reactive gliosis may explain the enhanced neuronal damage and worse cognitive outcome following GCI in FBN-ARO-KO mice. In support of this possibility, GFAP−/−Vm−/− mice were found to have compromised reactive astrogliosis and GLT-1-mediated glutamate transport after cerebral ischemia, as well as enhanced neuronal damage (Li et al., 2008). Reactive astrocytes can enhance neuroprotection by increasing uptake of excess glutamate by GLT-1 and releasing neuroprotective factors, such as BDNF, insulin-like growth factor-1 (IGF-1), and even E2, itself (Liu and Chopp, 2016). We thus examined each of these astrocytic neuroprotective factors in FBN-ARO-KO mice after GCI and found that expression of each of these factors (GLT-1, BDNF, IGF-1, aromatase) was significantly decreased in FBN-ARO-KO astrocytes after GCI. Elevation of E2 in the FBN-ARO-KO hippocampus after GCI was also diminished (Lu et al., 2020). These findings indicate that the neuroprotective functions of astrocytes after GCI are significantly impaired by depletion of NDE2, and accordingly, reinstatement of forebrain E2 levels reversed all of the molecular and function defects (Lu et al., 2020).
Our study also assessed how NDE2 regulates reactive astrogliosis. Previous work has shown that fibroblast growth factor-2 (FGF2) is an important factor produced by neurons that functions to suppress reactive astrogliosis (Kang et al., 2014; Zhang et al., 2017a). Interestingly, our RNASeq analysis found that FGF2 was upregulated in the hippocampus of FBN-ARO-KO mice after GCI. Further examination by double immunohistochemistry confirmed that FGF2 was increased in hippocampal neurons in FBN-ARO-KO mice after GCI, and we additionally found increased expression of its major receptor FGFR3 in reactive astrocytes in FBN-ARO-KO mice after GCI, as compared to FLOX mice (Lu et al., 2020). These findings suggest that the attenuated reactive astrogliosis in FBN-ARO-KO mice after GCI may be due to enhanced neuronal FGF2 signaling. This suggestion is supported by our group’s finding that blocking FGF2 signaling by central infusion of a FGFR3-neutralizing antibody rescued reactive astrogliosis after GCI in FBN-ARO-KO mice (Lu et al., 2020). Furthermore, astrocytic expression of BDNF, aromatase and GLT-1 expression were also rescued, which correlated with a significant reduction of neuronal damage after GCI (Lu et al., 2020).
Interestingly, our findings also suggest that NDE2 is important for enhancing polarization of the reactive astrocytes toward an A2 neuroprotective phenotype. Previous transcriptome analysis by the Barres lab (Zamanian et al., 2012) has implicated at least two major types of reactive astrocytes: A1 and A2 astrocytes. A1-type astrocytes are pro-inflammatory and neurotoxic and are induced by inflammatory agents, such as LPS, while A2-type astrocytes are neuroprotective and are induced following cerebral ischemia (Liddelow et al., 2017). We thus examined astrocyte phenotype in FBN-ARO-KO mouse hippocampal astrocytes in our study using qRT-PCR, Western blot analysis and immunohistochemistry for A1 and A2 genes/markers. The results revealed a significant downregulation of A2 astrocyte phenotype in the hippocampus of FBN-ARO-KO mice after GCI (Lu et al., 2020). We did not detect A1 astrocyte induction after GCI, a finding in agreement with the Barres’ group previous results. These results indicate that NDE2 is critical for induction of the neuroprotective A2 reactive astrocyte phenotype following GCI.
Based on our findings in the FBN-ARO-KO mouse described above, we have schematically illustrated a summary of the proposed mechanisms that underlie NDE2 neuroprotection following GCI (see Fig. 7). As shown in Fig. 7, we propose that following GCI, NDE2: 1) suppresses neuronal FGF2 signaling, a negative regulator of reactive astrogliosis, 2) enhances reactive astrogliosis after GCI, 3) enhances the A2 neuroprotective astrocyte phenotype, and 4) increases production of the astrocytic neuroprotective factors, GLT-1, BDNF and IGF-1, as well as ADE2 itself. Collectively, this leads to enhanced neuroprotection and preserved cognitive function following GCI. Furthermore, since NDE2 can be released from neurons, we cannot exclude the possibility that NDE2 also acts on estrogen receptors on neighboring neurons as an additional mechanism to facilitate neuroprotection.
Fig. 7. Proposed mechanisms underlying neuron-derived E2 (NDE2) neuroprotection in the ischemic brain.
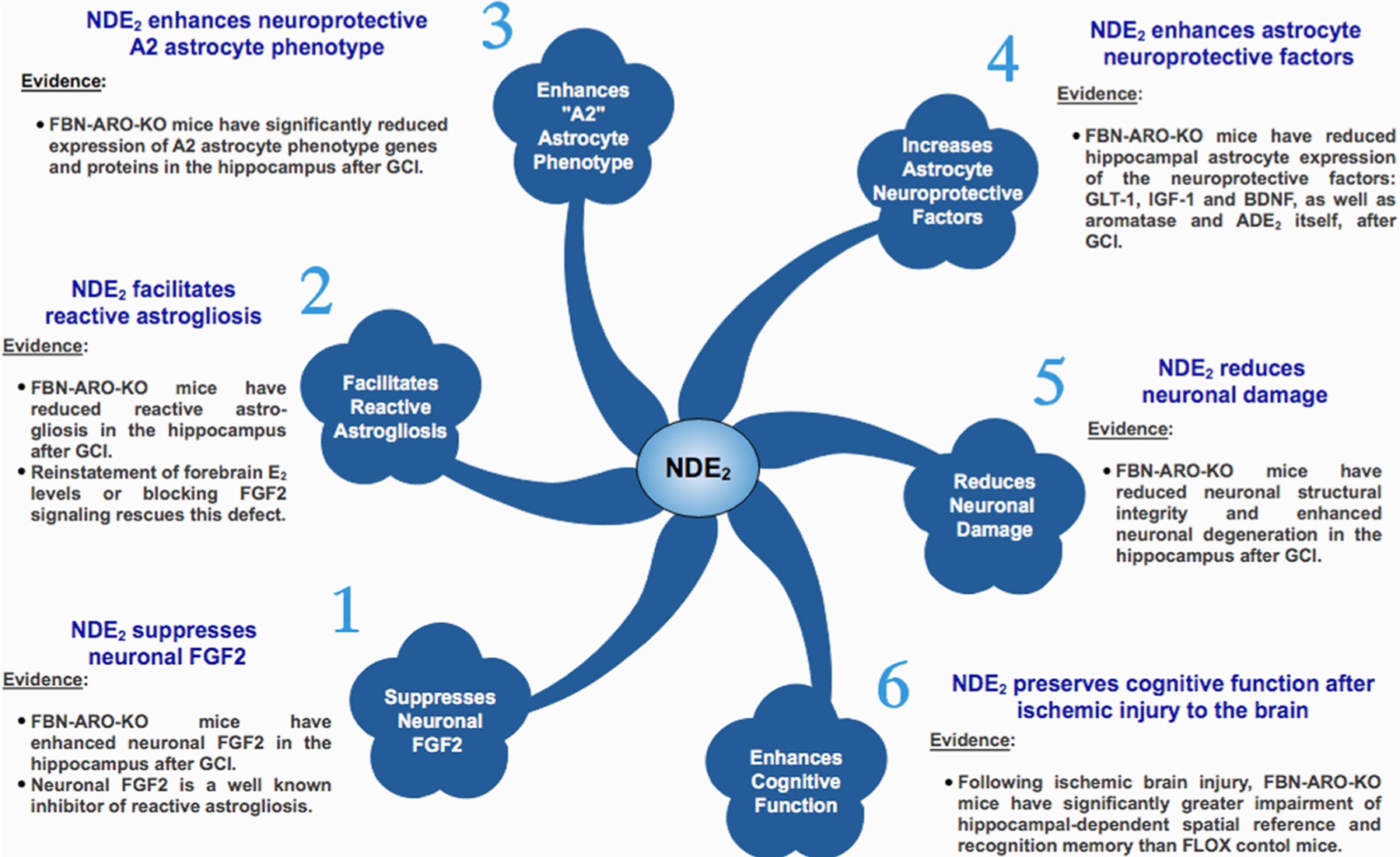
See text for full description. FBN-ARO-KO: forebrain neuron-specific aromatase knockout.
6.3. Conditional knockout studies - role of ADE2 in neuroprotection
To further determine the role of ADE2 in neuroprotection and neuroinflammation in ischemic brain injury, we utilized our GFAP-ARO-KO mice that have aromatase and E2 specifically depleted in astrocytes (Wang et al., 2020). GFAP-ARO-KO mice were viable and fertile, with normal gross brain structure, normal aromatase expression in neurons, normal basal forebrain E2 levels, normal astrocyte morphology, intensity and distribution, and normal cognitive function basally (Wang et al., 2020). The lack of a phenotype basally is not unexpected, as aromatase is not expressed basally in astrocytes and is only induced following stress, brain injury or ischemia. Note that the knockout of aromatase was specific for astrocytes, as neuronal aromatase and basal forebrain E2 levels were unchanged in the GFAP-ARO-KO mice. In contrast, after GCI, we found that intact male, intact female and ovariectomized female GFAP-ARO-KO mice: 1) failed to exhibit the normal elevation of astrocyte aromatase and hippocampal E2 levels after ischemia; 2) exhibited a profound reduction of ischemia-induced reactive astrogliosis; 3) had increased hippocampal neuronal damage, 4) exhibited enhanced microglia activation and reduced expression of microglial homeostatic genes, and 5) had enhanced cognitive deficits after GCI (Wang et al., 2020). RNA-Seq analysis further revealed that the normal upregulation of the A2 panel of reactive astrocyte genes was significantly attenuated in the ischemic GFAP-ARO-KO mouse hippocampus, suggesting that ADE2 is critical for induction of the A2 astrocyte phenotype after ischemic brain injury (Wang et al., 2020).
Our study also examined the molecular pathways and mechanisms that might underlie ADE2 regulation of reactive astrogliosis after GCI. Along these lines, RNA-seq analysis revealed that the IL-6/Janus kinase (JAK)/signal transducer and activator of transcription3 (STAT3) signaling pathway was negatively correlated with GFAP-ARO-KO after GCI (Wang et al., 2020). This is intriguing as the JAK-STAT3 pathway plays a critical role in mediating reactive astrogliosis after ischemia and injury (Ceyzeriat et al., 2016; Liddelow and Barres, 2017). Our study also found that STAT3 activation in the hippocampus after GCI is significantly suppressed in GFAP-ARO-KO astrocytes (Wang et al., 2020), which may explain the reduced reactive astrogliosis in our GFAP-ARO-KO mice after GCI. In support of this possibility, previous work demonstrated a failure of reactive astrogliosis in mice with conditional deletion of STAT3 following spinal cord injury (Okada et al., 2006), and this effect was associated with increased lesion volume and attenuated functional recovery. Interestingly, we also found that induction of leukemia inhibitory factor (LIF), a member of the IL-6 cytokine family and an upstream regulator of STAT3 (Murakami et al., 2019), was strongly attenuated in GFAP-ARO-KO astrocytes, as compared with FLOX astrocytes after GCI (Wang et al., 2020). Finally, E2 replacement fully rescued the defects in JAK-STAT3 signaling and reactive astrogliosis and was able to reverse the enhanced neuronal damage and microglial activation in GFAP-ARO-KO mice after GCI (Wang et al., 2020). Fig. 8 summarizes the mechanisms that are proposed to underlie ADE2 neuroprotection after GCI. Based on the findings discussed above, we propose that ADE2 has several key roles following GCI, including 1) being critical for reactive astrogliosis in the hippocampal CA1 region, 2) inducing JAK-STAT3 signaling in astrocytes, 3) facilitating induction of the A2 panel of reactive astrocyte genes, 4) suppressing microglia activation, and 5) reducing neuronal damage and preserving hippocampal-dependent cognitive functions.
Fig. 8. Proposed mechanisms underlying astrocyte-derived E2 (ADE2) neuroprotection in the ischemic brain.
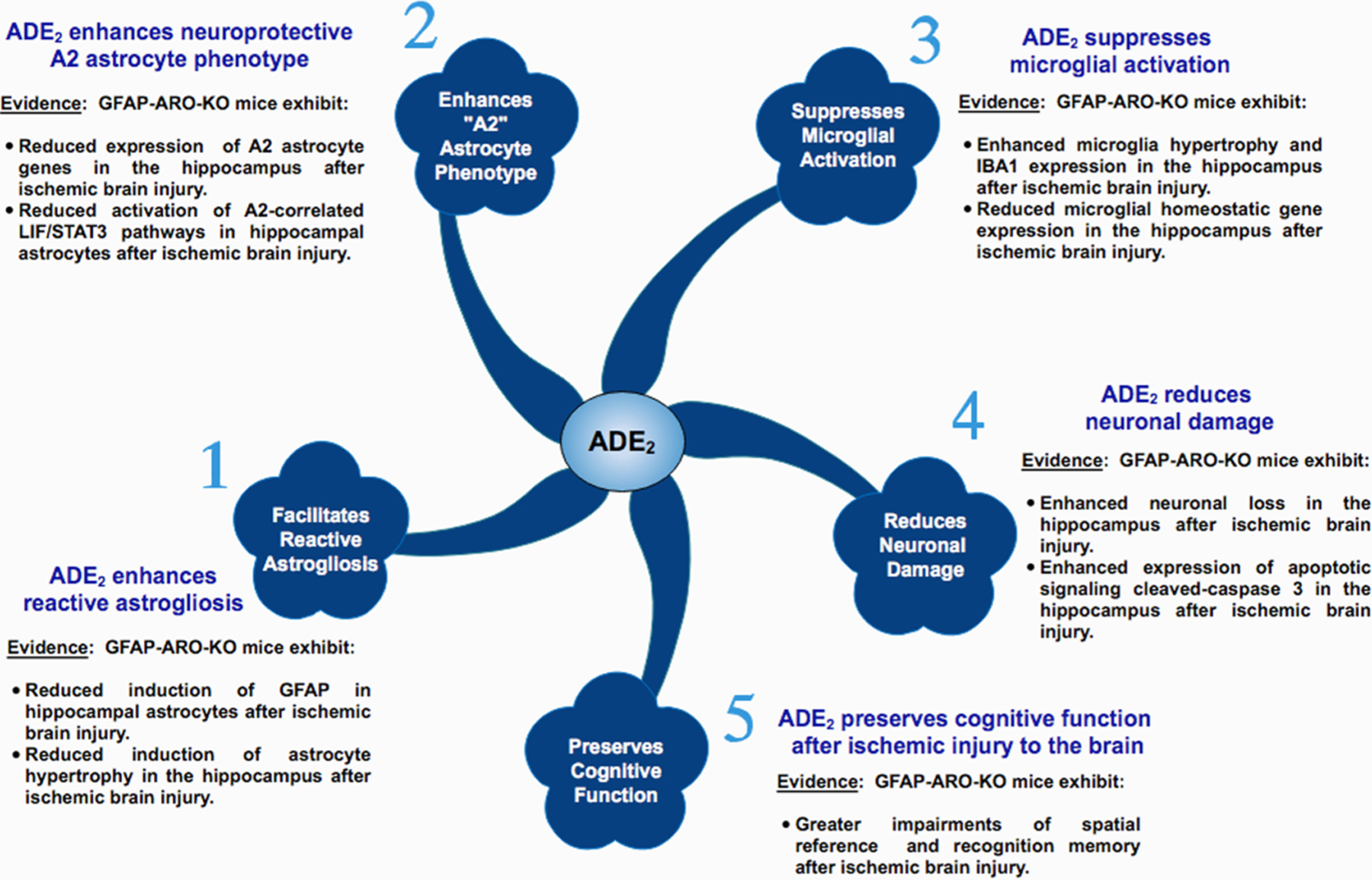
See text for full description. GFAP-ARO-KO: astrocyte-specific aromatase knockout.
7. Future directions and conclusions
Despite the remarkable progress described above in elucidating BDE2 functions in the brain, there remain many unanswered questions that require further studies. Below, we have summarized key questions and future directions that could help advance this important research area and provide clarity to areas where controversy currently exists.
7.1. Development of new animal models
An important caveat of the FBN-ARO-KO mouse studies is that deletion of aromatase was specifically targeted in forebrain excitatory glutamatergic neurons. While these constitute the major excitatory neurons in the brain, other types of neurons also express aromatase, including inhibitory neurons (Hoyk et al., 2014; Yague et al., 2010, 2008). To address the role of NDE2 in these neurons, future studies are needed to develop genetic models that ablate aromatase specifically in these other neuronal types. Furthermore, in addition to the forebrain, aromatase is also expressed in the midbrain and hindbrain. Therefore, future studies are needed to develop genetic models for targeted deletion in these brain areas to help elucidate the function of BDE2 in these brain areas.
An intriguing finding of our studies was that even though neuronal aromatase and NDE2 appear uncompromised in the GFAP-ARO-KO mice, NDE2 was unable to compensate for the loss of ADE2. One explanation for this could be that the effects of NDE2 and ADE2 are additive and loss of one or the other sources of local E2 will yield a similar phenotype. To address this question, we have generated double aromatase knockout mice by crossing the FBN-ARO-KO and GFAP-ARO-KO mice and will use these mice in future studies to determine if the double knockout mice have greater defects than the single knockout mice after GCI. Alternatively, NDE2 neuroprotective effects may require ADE2 mediation. In potential support of this possibility, NDE2 was shown to regulate reactive astrogliosis and ADE2 generation by suppressing neuronal FGF2 signaling, which serves as an inhibitory “brake” to astrocyte activation following ischemia (Lu et al., 2020).
It should also be mentioned that BDE2 effects on astrocyte activation may be injury- or context-specific. For instance, contrary to the reduced reactive astrogliosis observed in our knockout mice after GCI, aromatase inhibitor treatment in male zebra finches with a penetrating wound injury was associated with increased reactive gliosis around the wound area (Wynne et al., 2008). This discrepancy could be due to differences in the injury model (ischemic versus traumatic injury), differences in approaches (chronic knockout versus an acute pharmacological knockdown of aromatase), or to species differences. To rule out that the differences were due to different approaches, we examined the effect of pharmacological inhibition of aromatase by letrozole in our GCI model. The results revealed that letrozole treatment in wild type male mice yielded a similar reduced reactive astrogliosis in the hippocampus after GCI as observed in the GFAP-ARO-KO mice (Wang et al., 2020). Thus, the difference in results does not appear to be due to different approaches, but rather appears to be due to different injury models and/or species differences. To provide greater clarity on this issue, in the future, we plan to use our knockout mice to study the role of NDE2 and ADE2 following different types of brain injury, including penetrating brain injury, traumatic brain injury, and excitotoxic injury. Furthermore, we have also generated inducible conditional astrocyte-specific knockout mice using tamoxifen-inducible Gfap-cre/ERT2 and Aldh1l1-cre/ERT2 mouse lines that we plan to utilize as an additional tool in the future to further interrogate and enhance our understanding of the roles and actions of ADE2 in brain function.
7.2. Sex differences
There are conflicting reports on sex differences in BDE2 actions in the brain especially regarding effects on synaptic plasticity. Some studies found evidence of a role in females but not in males, while others indicate a significant role in both sexes. Thus, future studies on BDE2 actions and functions in the brain should include both sexes and do direct comparisons where possible so as to better clarify sex differences in BDE2 effects and actions in the brain. We also plan to more thoroughly interrogate for sex differences using our conditional aromatase knockout models. While we observed similar gross phenotypes in both sexes in our knockout mice, we did not do comprehensive direct comparisons of the two sexes that are needed to reveal specific sex differences in effects, magnitude, and/or temporal patterns of the processes under study.
7.3. Additional clinical studies
While animal studies have shown that aromatase is upregulated in astrocytes in the brain after stroke and trauma, it is unclear whether a similar upregulation occurs in humans. Future studies using post-mortem human brain samples could examine this question. Since NDE2 has been implicated to regulate memory and cognition, approaches that increase NDE2 in the brain could potentially have efficacy at memory improvement/enhancement in humans. Intriguingly, one such approach to increase NDE2 could be exercise, as exercise has been reported to enhance E2 levels in the hippocampus in ovariectomized rats, and this effect was correlated with enhanced cognitive function (Kaidah et al., 2016). Thus, it would be interesting to determine whether exercise can similarly upregulate aromatase/E2 in the brain in humans. It would also be intriguing to determine whether exercise enhancement of cognition may involve NDE2 mediation, especially since NDE2 has been shown to regulate BDNF, spine and synapse density, and LTP – all key regulators and/or processes that underlie memory. Studies are already underway in our laboratory to address this interesting question.
7.4. Microglial regulation
Few studies have examined BDE2 for regulatory effects upon microglia. This is surprising as microglia are well known to possess estrogen receptors and exogenously administered E2 can regulate microglia function. Our study using GFAP-ARO-KO mice did provide evidence that ADE2 regulates microglial activation and microglial homeostatic gene expression in the hippocampus after GCI (Wang et al., 2020). However, follow up studies are needed to further explore the functional consequences of such regulation, the mechanisms underlying them, whether they extend to other types of brain injuries and neurodegenerative disorders, and whether NDE2 can exert similar regulation of microglial activation and function.
In conclusion, the findings described in this review provide substantial evidence of aromatase expression and local E2 production in many different brain areas of almost all species studied to date. E2 is produced in neurons basally, while production in astrocytes is induced by stress, brain injury or ischemia. A strength of this body of work is that many different techniques were used in studying the localization of aromatase and E2 production in the brain, and many different approaches were used to confirm the roles of BDE2 in the brain, including aromatase inhibitor and knockdown studies, as well as global aromatase and brain cell-specific aromatase knockout animal models. A role for NDE2 is implicated in a diverse spectrum of functions in the brain, including regulation of sexual differentiation, reproduction, socio-sexual behavior, synaptic plasticity, LTP, memory and cognition, auditory processing, injury-induced reactive gliosis and astrocyte phenotype, neuroprotection, and anti-inflammatory effects. Following its induction after brain injury or ischemia, ADE2 has been implicated in the regulation of reactive gliosis, astrocyte phenotype, neuroprotection, cognitive preservation, and anti-inflammatory effects. Collectively, these findings demonstrate a critical role for BDE2 in many different brain functions in both physiological and pathological conditions.
Acknowledgements
The authors research described in this review research was supported by Research Grant NS088058 from the National Institutes of Neurological Disorders and Stroke, National Institutes of Health.
Abbreviations:
- 3β-HSD
3β -hydroxysteroid dehydrogenase
- 17β-HSD
17β -hydroxysteroid dehydrogenase
- AD
Alzheimer’s disease
- ADE2
astrocyte-derived estradiol/estrogen
- AMG
amygdala
- APP
amyloid precursor protein
- ATD
1,4,6-androstratriene-3,17-dione
- BDE2
brain-derived estradiol/estrogen
- BDNF
brain-derived neurotropic factor
- CaMKII
calcium/calmodulin-dependent protein kinase II
- c/EBP
enhanced CCATT-enhancer binding proteins
- CREB
cAMP response element binding protein
- DHT
5 α –dihydrotestosterone
- E2
17β-estradiol
- EGFP
enhanced green fluorescent protein
- EP3/4
E-prostanoid receptor 3/4
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- ERK
extracellular signal regulated kinase
- FBN-ARO-KO
forebrain neuronal aromatase knockout
- GCI
global cerebral ischemia
- GFAP
glial fibrillary acidic protein
- GFAP-ARO-KO
astrocyte aromatase knockout
- GnIH
gonadotropin inhibitory hormone
- GnRH
gonadotropin releasing hormone
- HFS
high frequency tetanic stimulation
- HPC
hippocampus
- HPLC
high performance liquid chromatography
- HYP
hypothalamus
- IHC
immunohistochemistry
- OVX
ovariectomized
- LIF
leukemia inhibitory factor
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- LH
luteinizing hormone
- LPS
lipopolysaccharide
- LTD
long term depression
- LTP
long term potentiation
- NCM
caudomedial nidopallium
- NDE2
neuron-derived estradiol/estrogen
- P45017α
P450 17 α-hydroxylase
- P450scc
P450 side chain cleavage
- PEPCK
phosphoenolpyruvate carboxykinase
- PET
positron emission tomography
- PGE2
prostaglandin E2
- POA
preoptic area
- PSD
post-synaptic density
- PSD95
postsynaptic density 95
- RORA
retinoic acid-related orphan receptor-alpha
- RT-PCR
reverse transcriptase-polymerase chain reaction
- STaR
steroidogenic acute regulatory protein
- VMN
ventromedial nucleus
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE, 1994. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology 135, 395–401. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I, Fiorenzani P, Maddalena M, Rossi A, Tomei V, Sorda G, Danielli B, Rovini M, Cappelli A, Anzini M, Giordano A, 2010. Aromatase and 5-alpha reductase gene expression: modulation by pain and morphine treatment in male rats. Mol. Pain 6, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, de Bournonville C, Chan TT, Balthazart J, Cornil CA, Ball GF, 2016. Aromatase inhibition rapidly affects in a reversible manner distinct features of birdsong. Sci. Rep 6, 32344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio R, Esposito E, De Ruvo C, Bellavia V, Amodio E, Carruba G, 2006. Red wine extract prevents neuronal apoptosis in vitro and reduces mortality of transgenic mice. Ann. N. Y. Acad. Sci 1089, 88–97. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Azcoitia I, Garcia-Segura LM, 2015. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci 16, 17–29. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM, 2001. Brain aromatase is neuroprotective. J. Neurobiol 47, 318–329. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM, 2011. Estradiol synthesis within the human brain. Neuroscience 191, 139–147. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Arevalo MA, Garcia-Segura LM, 2018. Neural-derived estradiol regulates brain plasticity. J. Chem. Neuroanat 89, 53–59. [DOI] [PubMed] [Google Scholar]
- Bailey DJ, Saldanha CJ, 2015. The importance of neural aromatization in the acquisition, recall, and integration of song and spatial memories in passerines. Horm. Behav 74, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Ma C, Soma KK, Saldanha CJ, 2013. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology 154, 4707–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Makeyeva YV, Paitel ER, Pedersen AL, Hon AT, Gunderson JA, Saldanha CJ, 2017. Hippocampal aromatization modulates spatial memory and characteristics of the synaptic membrane in the male zebra finch. Endocrinology 158, 852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J, 2002. Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm. Behav 42, 158–171. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Hendrick JC, 1990. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol. Behav 47, 83–94. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Harada N, 1991a. Distribution of aromatase-immunoreactive cells in the mouse forebrain. Cell Tissue Res 263, 71–79. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Harada N, 1991b. Neuroanatomical specificity in the co-localization of aromatase and estrogen receptors. J. Neurobiol 22, 143–157. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF, 2001a. Phosphorylation processes mediate rapid changes of brain aromatase activity. J. Steroid Biochem. Mol. Biol 79, 261–277. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF, 2001b. Rapid and reversible inhibition of brain aromatase activity. J. Neuroendocrinol 13, 63–73. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF, 2005. Interactions between kinases and phosphatases in the rapid control of brain aromatase. J. Neuroendocrinol 17, 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF, 2006. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology 147, 359–366. [DOI] [PubMed] [Google Scholar]
- Basli A, Soulet S, Chaher N, Merillon JM, Chibane M, Monti JP, Richard T, 2012. Wine polyphenols: potential agents in neuroprotection. Oxid. Med. Cell. Longev, 805762, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer J, Rune G, Schultz H, Tobia MJ, Mebes I, Katzler O, Sommer T, 2015. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology 56, 213–225. [DOI] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Brufsky AM, Ryan CM, Vogel VG, Rastogi P, Cohen SM, Casillo FE, Berga SL, 2007. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause 14, 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Zhou L, Wilkars W, Fester L, Lanowski JS, Paysen D, Konig A, Rune GM, 2010. Roles of 17ss-estradiol involve regulation of reelin expression and synaptogenesis in the dentate gyrus. Cereb. Cortex 20, 2985–2995. [DOI] [PubMed] [Google Scholar]
- Bender RA, Zhou L, Vierk R, Brandt N, Keller A, Gee CE, Schafer MK, Rune GM, 2017. Sex-dependent regulation of aromatase-mediated synaptic plasticity in the basolateral amygdala. J. Neurosci 37, 1532–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito E, Barco A, 2010. CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33, 230–240. [DOI] [PubMed] [Google Scholar]
- Bertilsson L, Suria A, Costa E, 1976. Gamma-aminobutyric acid in rat superior cervical ganglion. Nature 260, 540–541. [DOI] [PubMed] [Google Scholar]
- Beyer C, Green SJ, Barker PJ, Huskisson NS, Hutchison JB, 1994a. Aromatase-immunoreactivity is localised specifically in neurones in the developing mouse hypothalamus and cortex. Brain Res 638, 203–210. [DOI] [PubMed] [Google Scholar]
- Beyer C, Tramonte R, Hutchison RE, Sharp PJ, Barker PJ, Huskisson NS, Hutchison JB, 1994b. Aromatase-immunoreactive neurons in the adult female chicken brain detected using a specific antibody. Brain Res. Bull 33, 583–588. [DOI] [PubMed] [Google Scholar]
- Bheemanapally K, Ibrahim MMH, Briski KP, 2020. Ultra-high-performance liquid chromatography-electrospray ionization-mass spectrometry for high-neuroanatomical resolution quantification of brain estradiol concentrations. J. Pharm. Biomed. Anal 191, 113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, 2016. In vivo visualization of aromatase in animals and humans. Front. Neuroendocrinol 40, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Kim SW, Logan J, Hooker JM, Muench L, Fowler JS, 2010. Nicotine blocks brain estrogen synthase (aromatase): in vivo positron emission tomography studies in female baboons. Biol. Psychiatry 67, 774–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Alia-Klein N, Fowler JS, 2012. Potential contribution of aromatase inhibition to the effects of nicotine and related compounds on the brain. Front. Pharmacol 3, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Alexoff DL, Kim SW, Logan J, Pareto D, Schlyer D, Wang GJ, Fowler JS, 2015. Aromatase imaging with [N-methyl-11C]vorozole PET in healthy men and women. J. Nucl. Med 56, 580–585. [DOI] [PubMed] [Google Scholar]
- Biegon A, Alia-Klein N, Alexoff DL, Fowler JS, Kim SW, Logan J, Pareto D, Preston-Campbell R, Wang GJ, Hildebrandt T, 2020. Relationship of estrogen synthesis capacity in the brain with obesity and self-control in men and women. Proc. Natl. Acad. Sci. U. S. A 117, 22962–22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore J, Naftolin F, 2016. Aromatase: contributions to physiology and disease in women and men. Physiology (Bethesda) 31, 258–269. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. [DOI] [PubMed] [Google Scholar]
- Bogus K, Palasz A, Suszka-Switek A, Worthington JJ, Krzystanek M, Wiaderkiewicz R, 2019. Chronic antipsychotic treatment modulates aromatase (CYP19A1) expression in the male rat brain. J. Mol. Neurosci 68, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon WC, Chow JD, Simpson ER, 2010. The multiple roles of estrogens and the enzyme aromatase. Prog. Brain Res 181, 209–232. [DOI] [PubMed] [Google Scholar]
- Booth JE, 1977. Sexual behaviour of neonatally castrated rats injected during infancy with oestrogen and dihydrotestosterone. J. Endocrinol 72, 135–141. [DOI] [PubMed] [Google Scholar]
- Brandt N, Fester L, Rune GM, 2020. Neural sex steroids and hippocampal synaptic plasticity. Vitam. Horm 114, 125–143. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM, 2007. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz V, Zhu G, Wang Y, Liu Y, Avetisyan M, Bi X, Baudry M, 2015. Activity-dependent rapid local RhoA synthesis is required for hippocampal synaptic plasticity. J. Neurosci 35, 2269–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocca ME, Garcia-Segura LM, 2019. Non-reproductive functions of aromatase in the central nervous system under physiological and pathological conditions. Cell. Mol. Neurobiol 39, 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC, Zhao H, Yilmaz MB, Coon VJ, Bulun SE, 2012. Glucocorticoid-induction of hypothalamic aromatase via its brain-specific promoter. Mol. Cell. Endocrinol 362, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DC, Coon VJ, Ercan CM, Xu X, Dong H, Levine JE, Bulun SE, Zhao H, 2020. Brain aromatase and the regulation of sexual activity in male mice. Endocrinology 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Takayama K, Suzuki T, Sasano H, Yilmaz B, Sebastian S, 2004. Organization of the human aromatase p450 (CYP19) gene. Semin. Reprod. Med 22, 5–9. [DOI] [PubMed] [Google Scholar]
- Burrone L, Santillo A, Pinelli C, Baccari GC, Di Fiore MM, 2012. Induced synthesis of P450 aromatase and 17beta-estradiol by D-aspartate in frog brain. J. Exp. Biol 215, 3559–3565. [DOI] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ, 1977. Identification of aromatase in the reptilian brain. Endocrinology 100, 1214–1218. [DOI] [PubMed] [Google Scholar]
- Callard GV, Petro Z, Ryan KJ, 1978. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology 103, 2283–2290. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM, 2005. Brain aromatase expression after experimental stroke: topography and time course. J. Steroid Biochem. Mol. Biol 96, 89–91. [DOI] [PubMed] [Google Scholar]
- Ceyzeriat K, Abjean L, Carrillo-de Sauvage MA, Ben Haim L, Escartin C, 2016. The complex STATes of astrocyte reactivity: How are they controlled by the JAK-STAT3 pathway? Neuroscience 330, 205–218. [DOI] [PubMed] [Google Scholar]
- Chamniansawat S, Chongthammakun S, 2012. A priming role of local estrogen on exogenous estrogen-mediated synaptic plasticity and neuroprotection. Exp. Mol. Med 44, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamniansawat S, Sawatdiyaphanon C, 2018. Age-related memory impairment associated with decreased endogenous estradiol in the Hippocampus of female rats. Int. J. Toxicol 37, 207–215. [DOI] [PubMed] [Google Scholar]
- Chang PK, Boridy S, McKinney RA, Maysinger D, 2013. Letrozole potentiates mitochondrial and dendritic spine impairments induced by beta amyloid. J. Aging Res, 538979, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Genco MC, Megrelis L, Ruderman JV, 2011. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci. U. S. A 108, 17732–17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cincioglu M, Kismali G, Ugur SA, Kelicen-Ugur P, 2012. Indinavir inhibits the expression of cytoplasmic aromatase and nuclear SREBP in the hippocampus of reperfusion injury-induced ischemic rats. J. Steroid Biochem. Mol. Biol 130, 81–89. [DOI] [PubMed] [Google Scholar]
- Cisternas CD, Tome K, Caeiro XE, Dadam FM, Garcia-Segura LM, Cambiasso MJ, 2015. Sex chromosome complement determines sex differences in aromatase expression and regulation in the stria terminalis and anterior amygdala of the developing mouse brain. Mol. Cell. Endocrinol 414, 99–110. [DOI] [PubMed] [Google Scholar]
- Cisternas CD, Cabrera Zapata LE, Arevalo MA, Garcia-Segura LM, Cambiasso MJ, 2017. Regulation of aromatase expression in the anterior amygdala of the developing mouse brain depends on ERbeta and sex chromosome complement. Sci. Rep 7, 5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comito D, Pradhan DS, Karleen BJ, Schlinger BA, 2016. Region-specific rapid regulation of aromatase activity in zebra finch brain. J. Neurochem 136, 1177–1185. [DOI] [PubMed] [Google Scholar]
- Cornil CA, 2018. On the role of brain aromatase in females: why are estrogens produced locally when they are available systemically? J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol 204, 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J, 2006. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm. Behav 49, 45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez V, Mann M, Brann DW, Vadlamudi RK, 2010. Extranuclear signaling by estrogen: role in breast cancer progression and metastasis. Minerva Ginecol 62, 573–583. [PMC free article] [PubMed] [Google Scholar]
- Coumailleau P, Kah O, 2015. Expression of the cyp19a1 gene in the adult brain of Xenopus is neuronal and not sexually dimorphic. Gen. Comp. Endocrinol 221, 203–212. [DOI] [PubMed] [Google Scholar]
- Cui J, Wang Y, Dong Q, Wu S, Xiao X, Hu J, Chai Z, Zhang Y, 2011. Morphine protects against intracellular amyloid toxicity by inducing estradiol release and upregulation of Hsp70. J. Neurosci 31, 16227–16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bournonville C, Ball GF, Balthazart J, Cornil CA, 2017a. Rapid changes in brain aromatase activity in the female quail brain following expression of sexual behaviour. J. Neuroendocrinol 29. [DOI] [PubMed] [Google Scholar]
- de Bournonville C, Smolders I, Van Eeckhaut A, Ball GF, Balthazart J, Cornil CA, 2017b. Glutamate released in the preoptic area during sexual behavior controls local estrogen synthesis in male quail. Psychoneuroendocrinology 79, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean SL, Wright CL, Hoffman JF, Wang M, Alger BE, McCarthy MM, 2012. Prostaglandin E2 stimulates estradiol synthesis in the cerebellum postnatally with associated effects on Purkinje neuron dendritic arbor and electrophysiological properties. Endocrinology 153, 5415–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade TL, Rissman EF, Thompson N, Harada N, Ottinger MA, 1995. Co-localization of aromatase enzyme and estrogen receptor immunoreactivity in the preoptic area during reproductive aging. Brain Res 674, 181–187. [DOI] [PubMed] [Google Scholar]
- Dhandapani K, Brann D, 2003. Neuroprotective effects of estrogen and tamoxifen in vitro: a facilitative role for glia? Endocrine 21, 59–66. [DOI] [PubMed] [Google Scholar]
- Di Mauro M, Tozzi A, Calabresi P, Pettorossi VE, Grassi S, 2015. Neo-synthesis of estrogenic or androgenic neurosteroids determine whether long-term potentiation or depression is induced in hippocampus of male rat. Front. Cell. Neurosci 9, 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mauro M, Tozzi A, Calabresi P, Pettorossi VE, Grassi S, 2017. Different synaptic stimulation patterns influence the local androgenic and estrogenic neurosteroid availability triggering hippocampal synaptic plasticity in the male rat. Eur. J. Neurosci 45, 499–509. [DOI] [PubMed] [Google Scholar]
- Dickens MJ, Cornil CA, Balthazart J, 2013. Neurochemical control of rapid stress-induced changes in brain aromatase activity. J. Neuroendocrinol 25, 329–339. [DOI] [PubMed] [Google Scholar]
- Dieni CV, Ferraresi A, Sullivan JA, Grassi S, Pettorossi VE, Panichi R, 2018a. Acute inhibition of estradiol synthesis impacts vestibulo-ocular reflex adaptation and cerebellar long-term potentiation in male rats. Brain Struct. Funct 223, 837–850. [DOI] [PubMed] [Google Scholar]
- Dieni CV, Sullivan JA, Faralli M, Contemori S, Biscarini A, Pettorossi VE, Panichi R, 2018b. 17 beta-estradiol synthesis modulates cerebellar dependent motor memory formation in adult male rats. Neurobiol. Learn. Mem 155, 276–286. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA, 1984. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology 38, 297–301. [DOI] [PubMed] [Google Scholar]
- Duncan KA, Saldanha CJ, 2011. Neuroinflammation induces glial aromatase expression in the uninjured songbird brain. J. Neuroinflammation 8, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KA, Saldanha CJ, 2020. Central aromatization: a dramatic and responsive defense against threat and trauma to the vertebrate brain. Front. Neuroendocrinol 56, 100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman LM, 1988. Histochemical localization of 5’-nucleotidase in the reeler mutant mouse. Neurosci. Lett 94, 70–75. [DOI] [PubMed] [Google Scholar]
- Ellinwood WE, Hess DL, Roselli CE, Spies HG, Resko JA, 1984. Inhibition of aromatization stimulates luteinizing hormone and testosterone secretion in adult male rhesus monkeys. J. Clin. Endocrinol. Metab 59, 1088–1096. [DOI] [PubMed] [Google Scholar]
- Emmanuelle NE, Marie-Cecile V, Florence T, Jean-Francois A, Francoise L, Coralie F, Alexia V, 2021. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico F, Mothet JP, Usiello A, 2015. D-aspartate: an endogenous NMDA receptor agonist enriched in the developing brain with potential involvement in schizophrenia. J. Pharm. Biomed. Anal 116, 7–17. [DOI] [PubMed] [Google Scholar]
- Evinger AJ 3rd, Levin ER, 2005. Requirements for estrogen receptor alpha membrane localization and function. Steroids 70, 361–363. [DOI] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM, 2012. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J. Steroid Biochem. Mol. Biol 131, 24–29. [DOI] [PubMed] [Google Scholar]
- Fester L, Labitzke J, Hinz R, Behem C, Horling K, Bernhard T, Bader MI, Vollmer G, Rune GM, 2013. Estradiol responsiveness of synaptopodin in hippocampal neurons is mediated by estrogen receptor beta. J. Steroid Biochem. Mol. Biol 138, 455–461. [DOI] [PubMed] [Google Scholar]
- Fester L, Brandt N, Windhorst S, Prols F, Blaute C, Rune GM, 2016. Control of aromatase in hippocampal neurons. J. Steroid Biochem. Mol. Biol 160, 9–14. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME, 1997. CREB: a major mediator of neuronal neurotrophin responses. Neuron 19, 1031–1047. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ, 2013. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med 369, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores F, Naftolin F, Ryan KJ, 1973. Aromatization of androstenedione and testosterone by rhesus monkey hypothalamus and limbic system. Neuroendocrinology 11, 177–182. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH, 2001. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J. Neurosci 21, 8943–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB, 1999. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 89, 567–578. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I, 2003. Aromatase: a neuroprotective enzyme. Prog. Neurobiol 71, 31–41. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Griswold J, Erman M, Pangborn W, 2009. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Egbuta C, Kanyo JE, Lam TT, 2019. Phosphorylation of human placental aromatase CYP19A1. Biochem. J 476, 3313–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Leret ML, 1994. Injection of an aromatase inhibitor after the critical period of sexual differentiation. Pharmacol. Biochem. Behav 47, 183–186. [DOI] [PubMed] [Google Scholar]
- Gooren LJ, 1985. Human male sexual functions do not require aromatization of testosterone: a study using tamoxifen, testolactone, and dihydrotestosterone. Arch. Sex. Behav 14, 539–548. [DOI] [PubMed] [Google Scholar]
- Gorski RA, 1985. Sexual dimorphisms of the brain. J. Anim. Sci 61 (Suppl. 3), 38–61. [DOI] [PubMed] [Google Scholar]
- Grassi S, Frondaroli A, Dieni C, Scarduzio M, Pettorossi VE, 2009. Long-term potentiation in the rat medial vestibular nuclei depends on locally synthesized 17beta-estradiol. J. Neurosci 29, 10779–10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Tozzi A, Costa C, Tantucci M, Colcelli E, Scarduzio M, Calabresi P, Pettorossi VE, 2011. Neural 17beta-estradiol facilitates long-term potentiation in the hippocampal CA1 region. Neuroscience 192, 67–73. [DOI] [PubMed] [Google Scholar]
- Harada N, Yamada K, Foidart A, Balthazart J, 1992. Regulation of aromatase cytochrome P-450 (estrogen synthetase) transcripts in the quail brain by testosterone. Brain Res. Mol. Brain Res 15, 19–26. [DOI] [PubMed] [Google Scholar]
- Harris KM, 1999. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol 9, 343–348. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung BC, Yamazaki T, Kawato S, 2015. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: involvement of kinase networks. Brain Res 1621, 147–161. [DOI] [PubMed] [Google Scholar]
- Hassoun EA, Wilt SC, Devito MJ, Van Birgelen A, Alsharif NZ, Birnbaum LS, Stohs SJ, 1998. Induction of oxidative stress in brain tissues of mice after subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci 42, 23–27. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Harada N, 2014. Post-translational dual regulation of cytochrome P450 aromatase at the catalytic and protein levels by phosphorylation/dephosphorylation. FEBS J 281, 4830–4840. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S, 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U. S. A 101, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda SI, Harada N, 2020. ARP-1 regulates the transcriptional activity of the aromatase gene in the mouse brain. Front. Endocrinol. (Lausanne) 11, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Kozako T, Shimeno H, Soeda S, Harada N, 2012. LIM-homeodomain transcription factor, Lhx2, is involved in transcriptional control of brain-specific promoter/exon 1f of the mouse aromatase gene. J. Neuroendocrinol 24, 1367–1374. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK, 1994. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol. Behav 56, 535–541. [DOI] [PubMed] [Google Scholar]
- Hovsepian-Ruby L, 2017. Phosphorylation of Aromatase in the Zebra Finch Brain University of California, Los Angeles. [Google Scholar]
- Hoyk Z, Csakvari E, Gyenes A, Siklos L, Harada N, Parducz A, 2014. Aromatase and estrogen receptor beta expression in the rat olfactory bulb: neuroestrogen action in the first relay station of the olfactory pathway? Acta Neurobiol. Exp. (Wars) 74, 1–14. [DOI] [PubMed] [Google Scholar]
- Hurria A, Patel SK, Mortimer J, Luu T, Somlo G, Katheria V, Ramani R, Hansen K, Feng T, Chuang C, Geist CL, Silverman DH, 2014. The effect of aromatase inhibition on the cognitive function of older patients with breast cancer. Clin. Breast Cancer 14, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MMH, Uddin MM, Bheemanapally K, Briski KP, 2020. Sex-dimorphic aromatase regulation of ventromedial hypothalamic nucleus glycogen content in euglycemic and insulin-induced hypoglycemic rats. Neurosci. Lett 737, 135284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivonen S, Heikkinen T, Puolivali J, Helisalmi S, Hiltunen M, Soininen H, Tanila H, 2006. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen alpha and beta mRNA levels in ovariectomized female mice. Neuroscience 137, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Sakurai H, Oguro A, Tsuji M, Vogel CFA, Yamazaki T, 2019. Retinoid X receptor-mediated neuroprotection via CYP19 upregulation and subsequent increases in estradiol synthesis. J. Steroid Biochem. Mol. Biol 193, 105421. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Beyer C, 2000. Ontogenetic expression and sex differences of aromatase and estrogen receptor-alpha/beta mRNA in the mouse hippocampus. Cell Tissue Res 300, 231–237. [DOI] [PubMed] [Google Scholar]
- Iwabuchi J, Wako S, Tanaka T, Ishikawa A, Yoshida Y, Miyata S, 2007. Analysis of the p450 aromatase gene expression in the Xenopus brain and gonad. J. Steroid Biochem. Mol. Biol 107, 149–155. [DOI] [PubMed] [Google Scholar]
- Kaidah S, Soejono SK, Partadiredja G, 2016. Exercise improves hippocampal estrogen and spatial memory of ovariectomized rats. Bratisl. Lek. Listy 117, 94–99. [DOI] [PubMed] [Google Scholar]
- Kang W, Balordi F, Su N, Chen L, Fishell G, Hebert JM, 2014. Astrocyte activation is suppressed in both normal and injured brain by FGF signaling. Proc. Natl. Acad. Sci. U. S. A 111, E2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Korzekwa KR, Laughton CA, Chen S, 2001. Evaluation of the mechanism of aromatase cytochrome P450. A site-directed mutagenesis study. Eur. J. Biochem 268, 243–251. [DOI] [PubMed] [Google Scholar]
- Kelicen Ugur P, Lule S, Cincioglu M, Pekiner C, Gursoy-Ozdemir Y, 2011. Megestrol acetate inhibits the expression of cytoplasmic aromatase through nuclear C/EBPbeta in reperfusion injury-induced ischemic rat hippocampus. Eur. J. Pharmacol 654, 217–225. [DOI] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E, 2013. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J. Neurosci 33, 19051–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Kapoor A, Terasawa E, 2016. Neuroestradiol in the stalk median eminence of female rhesus macaques decreases in association with puberty onset. Endocrinology 157, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Garcia JP, Kohlenberg LK, Terasawa E, 2017. Obligatory role of hypothalamic neuroestradiol during the estrogen-induced LH surge in female ovariectomized rhesus monkeys. Proc. Natl. Acad. Sci. U. S. A 114, 13804–13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, Dhandapani KM, Zhang QG, Brann DW, 2013. Estrogen regulation of spine density and excitatory synapses in rat prefrontal and somatosensory cerebral cortex. Steroids 78, 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazali H, Mahmoudi F, 2019. Morphine and kisspeptin influences on 5-alpha reductase and aromatase gene expression in adult male rats. Iran. J. Basic Med. Sci 22, 1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, 2012. A functional role for CREB as a positive regulator of memory formation and LTP. Exp. Neurobiol 21, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Babayan AH, Gall CM, Lynch G, 2013. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience 239, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak M, Flowers MT, Shapiro RA, Kapoor A, Levine JE, Abbott DH, 2017. Extraovarian gonadotropin negative feedback revealed by aromatase inhibition in female marmoset monkeys. Am. J. Physiol. Endocrinol. Metab 313, E507–E514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM, 2004. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci 24, 5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohmer RW, 2020. Courtship in the male red-sided garter snake is dependent on neural aromatase activity during winter dormancy. J. Exp. Zool. A Ecol. Integr. Physiol 333, 275–283. [DOI] [PubMed] [Google Scholar]
- Krohmer RW, Bieganski GJ, Baleckaitis DD, Harada N, Balthazart J, 2002. Distribution of aromatase immunoreactivity in the forebrain of red-sided garter snakes at the beginning of the winter dormancy. J. Chem. Neuroanat 23, 59–71. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF, 2006. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience 138, 921–928. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC, 2020. G protein-coupled estrogen receptor: rapid effects on hippocampal-dependent spatial memory and synaptic plasticity. Front. Endocrinol. (Lausanne) 11, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bean LA, Rani A, Jackson T, Foster TC, 2015. Contribution of estrogen receptor subtypes, ERalpha, ERbeta, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice. Hippocampus 25, 1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YJ, Yu D, Zhang JH, Chen GJ, 2017. Cooperation of genomic and rapid nongenomic actions of estrogens in synaptic plasticity. Mol. Neurobiol 54, 4113–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber ME, Lichtensteiger W, 1994. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology 135, 1661–1668. [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE, 1990. Progesterone enhances the surge of luteinizing hormone by increasing the activation of luteinizing hormone-releasing hormone neurons. Endocrinology 127, 2604–2606. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Ojeda SR, 1990. Hypothalamic aromatase activity in male and female rats during juvenile peripubertal development. Neuroendocrinology 51, 385–393. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Adlercreutz H, Lund TD, 2001. Dietary soy phytoestrogen effects on brain structure and aromatase in Long-Evans rats. Neuroreport 12, 3451–3455. [DOI] [PubMed] [Google Scholar]
- Lepore G, Gadau S, Mura A, Zedda M, Farina V, 2009. Aromatase immunoreactivity in fetal ovine neuronal cell cultures exposed to oxidative injury. Eur. J. Histochem 53, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore G, Gadau S, Peruffo A, Mura A, Mura E, Floris A, Balzano F, Zedda M, Farina V, 2011. Aromatase expression in cultured fetal sheep astrocytes after nitrosative/oxidative damage. Cell Tissue Res 344, 407–413. [DOI] [PubMed] [Google Scholar]
- Levenga J, Wong H, Milstead RA, Keller BN, LaPlante LE, Hoeffer CA, 2017. AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gibbs RB, 2019. Detection of estradiol in rat brain tissues: contribution of local versus systemic production. Psychoneuroendocrinology 102, 84–94. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M, 2008. Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow Metab 28, 468–481. [DOI] [PubMed] [Google Scholar]
- Li J, Oberly PJ, Poloyac SM, Gibbs RB, 2016. A microsomal based method to detect aromatase activity in different brain regions of the rat using ultra performance liquid chromatography-mass spectrometry. J. Steroid Biochem. Mol. Biol 163, 113–120. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Barres BA, 2017. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chopp M, 2016. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol 144, 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ, 2008. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci 11, 334–343. [DOI] [PubMed] [Google Scholar]
- Liu J, Hu P, Qi XR, Meng FT, Kalsbeek A, Zhou JN, 2011. Acute restraint stress increases intrahypothalamic oestradiol concentrations in conjunction with increased hypothalamic oestrogen receptor beta and aromatase mRNA expression in female rats. J. Neuroendocrinol 23, 435–443. [DOI] [PubMed] [Google Scholar]
- Liu M, Xing F, Bian C, Zhao Y, Zhao J, Liu Y, Zhang J, 2019. Letrozole induces worse hippocampal synaptic and dendritic changes and spatial memory impairment than ovariectomy in adult female mice. Neurosci. Lett 706, 61–67. [DOI] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV, 2015. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat 11, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sareddy GR, Wang J, Wang R, Li Y, Dong Y, Zhang Q, Liu J, O’Connor JC, Xu J, Vadlamudi RK, Brann DW, 2019. Neuron-derived estrogen regulates synaptic plasticity and memory. J. Neurosci 39, 2792–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sareddy GR, Wang J, Zhang Q, Tang FL, Pratap UP, Tekmal RR, Vadlamudi RK, Brann DW, 2020. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. J. Neurosci 40, 7355–7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti S, Bossers K, Van de Bilt S, Agrapart V, Morales RR, Frajese GV, Swaab DF, 2011. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer’s disease. Neurobiol. Aging 32, 1964–1976. [DOI] [PubMed] [Google Scholar]
- Lyou S, Kawano S, Yamada T, Okuyama S, Terashima T, Hayase K, Yokogoshi H, 2008. Role of estrogen receptors and aromatase on brain protein synthesis rates in ovariectomized female rats fed genistein. Nutr. Neurosci 11, 155–160. [DOI] [PubMed] [Google Scholar]
- Macedo-Lima M, Remage-Healey L, 2020. Auditory learning in an operant task with social reinforcement is dependent on neuroestrogen synthesis in the male songbird auditory cortex. Horm. Behav 121, 104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, Goldman-Rakic PS, 1986. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc. Natl. Acad. Sci. U. S. A 83, 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW, 1998. Regulation of the preovulatory gonadotropin surge by endogenous steroids. Steroids 63, 616–629. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang JQ, 2016. Synaptically localized mitogen-activated protein kinases: local substrates and regulation. Mol. Neurobiol 53, 6309–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbouti L, Zahmatkesh M, Riahi E, Sadr SS, 2020a. Inhibition of brain 17beta-estradiol synthesis by letrozole induces cognitive decline in male and female rats. Neurobiol. Learn. Mem 175, 107300. [DOI] [PubMed] [Google Scholar]
- Marbouti L, Zahmatkesh M, Riahi E, Shafiee Sabet M, 2020b. GnRH protective effects against amyloid beta-induced cognitive decline: a potential role of the 17beta-estradiol. Mol. Cell. Endocrinol 518, 110985. [DOI] [PubMed] [Google Scholar]
- Matus A, 2000. Actin-based plasticity in dendritic spines. Science 290, 754–758. [DOI] [PubMed] [Google Scholar]
- McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE, 2007. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J. Neurochem 100, 678–692. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, 2008. Estradiol and the developing brain. Physiol. Rev 88, 91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD, 2003. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J. Neurosci 23, 8701–8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA, 2001. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. U. S. A 98, 7093–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehos CJ, Nelson LH, Saldanha CJ, 2016. A quantification of the injury-induced changes in central aromatase, oestrogenic milieu and steroid receptor expression in the Zebra finch. J. Neuroendocrinol 28, 12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O, 2003. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: comparison with estrogen receptor alpha. J. Comp. Neurol 462, 180–193. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, Kah O, 2005. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol 485, 304–320. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L, 1999. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol 407, 115–129. [PubMed] [Google Scholar]
- Miller TW, Shin I, Kagawa N, Evans DB, Waterman MR, Arteaga CL, 2008. Aromatase is phosphorylated in situ at serine-118. J. Steroid Biochem. Mol. Biol 112, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE, 2001. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol 429, 355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE, 2005. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol 491, 81–95. [DOI] [PubMed] [Google Scholar]
- Miranda M, Morici JF, Zanoni MB, Bekinschtein P, 2019. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci 13, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Gera R, Siddiqui WA, Khandelwal S, 2013. Tributyltin induces oxidative damage, inflammation and apoptosis via disturbance in blood-brain barrier and metal homeostasis in cerebral cortex of rat brain: an in vivo and in vitro study. Toxicology 310, 39–52. [DOI] [PubMed] [Google Scholar]
- Monteiro R, Faria A, Mateus N, Calhau C, Azevedo I, 2008. Red wine interferes with oestrogen signalling in rat hippocampus. J. Steroid Biochem. Mol. Biol 111, 74–79. [DOI] [PubMed] [Google Scholar]
- Mori T, Buffo A, Gotz M, 2005. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr. Top. Dev. Biol 69, 67–99. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S, 2006. Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology 84, 255–263. [DOI] [PubMed] [Google Scholar]
- Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanaka Y, Ogiue-Ikeda M, 2010. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim. Biophys. Acta 1800, 1030–1044. [DOI] [PubMed] [Google Scholar]
- Munetomo A, Hojo Y, Higo S, Kato A, Yoshida K, Shirasawa T, Shimizu T, Barron A, Kimoto T, Kawato S, 2015. Aging-induced changes in sex-steroidogenic enzymes and sex-steroid receptors in the cortex, hypothalamus and cerebellum. J. Physiol. Sci 65, 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munetsuna E, Hojo Y, Hattori M, Ishii H, Kawato S, Ishida A, Kominami SA, Yamazaki T, 2009. Retinoic acid stimulates 17beta-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology 150, 4260–4269. [DOI] [PubMed] [Google Scholar]
- Munetsuna E, Hattori M, Yamazaki T, 2014. Stimulation of estradiol biosynthesis by tributyltin in rat hippocampal slices. Endocr. Res 39, 168–172. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kamimura D, Hirano T, 2019. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity 50, 812–831. [DOI] [PubMed] [Google Scholar]
- Naftolin F, 1994. Brain aromatization of androgens. J. Reprod. Med 39, 257–261. [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z, 1971. Aromatization of androstenedione by limbic system tissue from human foetuses. J. Endocrinol 51, 795–796. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z, 1972. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology 90, 295–298. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J, 1996. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63, 149–155. [DOI] [PubMed] [Google Scholar]
- Negri Cesi P, Melcangi RC, Celotti F, Martini L, 1992. Aromatase activity in cultured brain cells: difference between neurons and glia. Brain Res 589, 327–332. [DOI] [PubMed] [Google Scholar]
- Negri Cesi P, Melcangi RC, Celotti F, Martini L, 1993. Distribution of aromatase activity in cultured neurons and glia cells. J. Steroid Biochem. Mol. Biol 44, 637–639. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Celotti F, Motta M, 2004. Sexual differentiation of the brain: role of testosterone and its active metabolites. J. Endocrinol. Invest 27, 120–127. [PubMed] [Google Scholar]
- Nelson BS, Black KL, Daniel JM, 2016. Circulating estradiol regulates brain-derived estradiol via actions at GnRH receptors to impact memory in ovariectomized rats. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, 2017. A brief history of long-term potentiation. Neuron 93, 281–290. [DOI] [PubMed] [Google Scholar]
- Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ, 2004. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm. Behav 45, 250–258. [DOI] [PubMed] [Google Scholar]
- Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto T, Kawato S, 2008. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res. Rev 57, 363–375. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H, 2006. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med 12, 829–834. [DOI] [PubMed] [Google Scholar]
- Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H, 2012. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J. Steroid Biochem. Mol. Biol 131, 37–51. [DOI] [PubMed] [Google Scholar]
- Pedersen AL, Saldanha CJ, 2017. Reciprocal interactions between prostaglandin E2- and estradiol-dependent signaling pathways in the injured zebra finch brain. J. Neuroinflammation 14, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AL, Nelson LH, Saldanha CJ, 2016. Centrally synthesized estradiol is a potent anti-inflammatory in the injured Zebra finch brain. Endocrinology 157, 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AL, Brownrout JL, Saldanha CJ, 2017. Central administration of indomethacin mitigates the injury-induced upregulation of aromatase expression and estradiol content in the zebra finch brain. Endocrinology 158, 2585–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AL, Brownrout JL, Saldanha CJ, 2018. Neuroinflammation and neurosteroidogenesis: reciprocal modulation during injury to the adult zebra finch brain. Physiol. Behav 187, 51–56. [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Mouriec K, Anglade I, Menuet A, Le Page Y, Gueguen MM, Marmignon MH, Brion F, Pakdel F, Kah O, 2007. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol 501, 150–167. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Saldanha CJ, Schlinger BA, 2001. Rapid upregulation of aromatase mRNA and protein following neural injury in the zebra finch (Taeniopygia guttata). J. Neuroendocrinol 13, 317–323. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ, 2005. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc. Biol. Sci 272, 2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Aldridge J, Ribi K, Sun Z, Thompson A, Harvey V, Thurlimann B, Cardoso F, Pagani O, Coates AS, Goldhirsch A, Price KN, Gelber RD, Bernhard J, 2011. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1–98 trial. Breast Cancer Res. Treat 126, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piferrer F, Blazquez M, 2005. Aromatase distribution and regulation in fish. Fish Physiol. Biochem 31, 215–226. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM, 2008. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J. Cell Biol 180, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Schmutterer T, Fester L, Zhou L, Imholz P, Brandt N, Vierk R, Jarry H, Rune GM, 2013. Endocrine regulation of estrogen synthesis in the hippocampus? Prog. Histochem. Cytochem 48, 49–64. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Dudzinski DA, Prols F, Glatzel M, Matschke J, Rune GM, 2016. Aromatase expression in the Hippocampus of AD patients and 5xFAD mice. Neural Plast, 9802086, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW, 2008. Rapid estrogen signaling in the brain. Neurosignals 16, 140–153. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA, 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci 11, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Oyama RK, Schlinger BA, 2009. Elevated aromatase activity in forebrain synaptic terminals during song. J. Neuroendocrinol 21, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong S, Maidment NT, Schlinger BA, 2011. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J. Neurosci 31, 10034–10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER, 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630. [DOI] [PubMed] [Google Scholar]
- Ritacco I, Spinello A, Ippoliti E, Magistrato A, 2019. Post-translational regulation of CYP450s metabolism as revealed by all-atoms simulations of the aromatase enzyme. J. Chem. Inf. Model 59, 2930–2940. [DOI] [PubMed] [Google Scholar]
- Rocha-Cadman X, Massie MJ, Du Hamel K, 2012. Aromatase inhibitors and mood disturbances. Palliat. Support. Care 10, 225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ, 2007. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev. Neurobiol 67, 1–9. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA, 1984. Androgens regulate brain aromatase activity in adult male rats through a receptor mechanism. Endocrinology 114, 2183–2189. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA, 1989. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol. Reprod 40, 929–934. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Ellinwood WE, Resko JA, 1984. Regulation of brain aromatase activity in rats. Endocrinology 114, 192–200. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Kaler LW, Resko JA, 1986. Hypothalamic aromatase activity in young and old male rats. Neurobiol. Aging 7, 121–125. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA, 1998. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol. Reprod 58, 79–87. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Klosterman S, Resko JA, 2001. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J. Comp. Neurol 439, 208–223. [DOI] [PubMed] [Google Scholar]
- Ryan KJ, 1959. Biological aromatization of steroids. J. Biol. Chem 234, 268–272. [PubMed] [Google Scholar]
- Sadasivam M, Ramatchandirin B, Balakrishnan S, Selvaraj K, Prahalathan C, 2014. The role of phosphoenolpyruvate carboxykinase in neuronal steroidogenesis under acute inflammation. Gene 552, 249–254. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, 2020. Estrogen as a neuroprotectant in both sexes: stories from the bird brain. Front. Neurol 11, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL, 2004. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J. Comp. Neurol 469, 522–534. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Burstein SR, Duncan KA, 2013. Induced synthesis of oestrogens by glia in the songbird brain. J. Neuroendocrinol 25, 1032–1038. [DOI] [PubMed] [Google Scholar]
- Santillo A, Pinelli C, Burrone L, Chieffi Baccari G, Di Fiore MM, 2013. D-Aspartic acid implication in the modulation of frog brain sex steroid levels. Gen. Comp. Endocrinol 181, 72–76. [DOI] [PubMed] [Google Scholar]
- Sarachana T, Xu M, Wu RC, Hu VW, 2011. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS One 6, e17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius GA, Ly LP, Handelsman DJ, 2014. Male sexual function can be maintained without aromatization: randomized placebo-controlled trial of dihydrotestosterone (DHT) in healthy, older men for 24 months. J. Sex. Med 11, 2562–2570. [DOI] [PubMed] [Google Scholar]
- Sasano H, Takashashi K, Satoh F, Nagura H, Harada N, 1998. Aromatase in the human central nervous system. Clin. Endocrinol. (Oxf.) 48, 325–329. [DOI] [PubMed] [Google Scholar]
- Sato SM, Woolley CS, 2016. Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV, 1989. Aromatase activity in quail brain: correlation with aggressiveness. Endocrinology 124, 437–443. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV, 1990. Aromatization mediates aggressive behavior in quail. Gen. Comp. Endocrinol 79, 39–53. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM, 2008. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron 58, 584–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Raval P, Srivastava DP, 2015. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front. Neuroendocrinol 36, 72–89. [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK, 1977. Aromatization and 5alpha-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology 101, 841–848. [DOI] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Christophe VJ, Ball GF, Cornil CA, 2013. Neuroestrogens rapidly regulate sexual motivation but not performance. J. Neurosci 33, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TP, Blache D, Blackberry MA, Martin GB, 1999. Role of peripheral and central aromatization in the control of gonadotrophin secretion in the male sheep. Reprod. Fertil. Dev 11, 293–302. [DOI] [PubMed] [Google Scholar]
- Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT, 1994. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Brain Res. Mol. Brain Res 24, 227–237. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, Enami T, Suzuki K, Tanabe N, Ishii H, Mukai H, Takahashi T, Hattori TA, Kawato S, 2003. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim. Biophys. Acta 1619, 301–316. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A, 1998. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron 20, 727–740. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Corbin CJ, Mendelson CR, 1993. Tissue-specific promoters regulate aromatase cytochrome P450 expression. J. Steroid Biochem. Mol. Biol 44, 321–330. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M, 2002. Aromatase–a brief overview. Annu. Rev. Physiol 64, 93–127. [DOI] [PubMed] [Google Scholar]
- Singh M, 2001. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine 14, 407–415. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G Jr., Guan X, Warren M, Toran-Allerand CD, 1999. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J. Neurosci 19, 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS, 2010. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J. Neurosci 30, 16137–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, 2006. Testosterone and aggression: berthold, birds and beyond. J. Neuroendocrinol 18, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ, 2003. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. J. Neurobiol 56, 209–221. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF, 2012. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol. Aging 33 (1487) e1481–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S, 2012. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 202, 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P, 2010. Estrogen receptor ss activity modulates synaptic signaling and structure. J. Neurosci 30, 13454–13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC, 2014. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors alpha and beta, and androgen receptors. PLoS One 9, e90451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelbroeck S, Heidrich DD, Stoffel-Wagner B, Hans VH, Schramm J, Bidlingmaier F, Klingmuller D, 1999. Characterization of aromatase cytochrome P450 activity in the human temporal lobe. J. Clin. Endocrinol. Metab 84, 2795–2801. [DOI] [PubMed] [Google Scholar]
- Stephens SB, Tolson KP, Rouse ML Jr., Poling MC, Hashimoto-Partyka MK, Mellon PL, Kauffman AS, 2015. Absent progesterone signaling in Kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology 156, 3091–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco C, 2012. Tissue physiology and pathology of aromatase. Steroids 77, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Steckelbroeck S, Schwaab R, Schramm J, Bidlingmaier F, Klingmuller D, 1998. Expression of CYP19 (aromatase) mRNA in the human temporal lobe. Biochem. Biophys. Res. Commun 244, 768–771. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D, 1999. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J. Steroid Biochem. Mol. Biol 70, 237–241. [DOI] [PubMed] [Google Scholar]
- Sweatt JD, 2001. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem 76, 1–10. [DOI] [PubMed] [Google Scholar]
- Tabatadze N, Sato SM, Woolley CS, 2014. Quantitative analysis of long-form aromatase mRNA in the male and female rat brain. PLoS One 9, e100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Hosoya T, Onoe K, Takashima T, Tanaka M, Ishii A, Nakatomi Y, Tazawa S, Takahashi K, Doi H, Wada Y, Watanabe Y, 2018. Association between aromatase in human brains and personality traits. Sci. Rep 8, 16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Wong TY, Wang Y, Huang J, Leung LK, 2013. CYP19 expression is induced by 2,3,7,8-tetrachloro-dibenzo-para-dioxin in human glioma cells. Mol. Cell. Endocrinol 375, 106–112. [DOI] [PubMed] [Google Scholar]
- Tan W, Zhu Z, Ye L, Leung LK, 2017. Methylation dictates PI.f-specific CYP19 transcription in human glial cells. Mol. Cell. Endocrinol 452, 131–137. [DOI] [PubMed] [Google Scholar]
- Tchoudakova A, Callard GV, 1998. Identification of multiple CYP19 genes encoding different cytochrome P450 aromatase isozymes in brain and ovary. Endocrinology 139, 2179–2189. [DOI] [PubMed] [Google Scholar]
- Terasawa E, 2018. Neuroestradiol in regulation of GnRH release. Horm. Behav 104, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian ZZ, Zhao H, Chen BY, 2004. Decreased hypothalamic aromatization in female rats of true precocious puberty. Neurosci. Lett 366, 92–96. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D, 1999. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampa C, Di Mauro M, Mazzocchetti P, Costa C, Di Filippo M, Grassi S, Pettorossi VE, Calabresi P, 2015. Endogenous 17beta-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front. Cell. Neurosci 9, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, Durante V, Manca P, Di Mauro M, Blasi J, Grassi S, Calabresi P, Kawato S, Pettorossi VE, 2019. Bidirectional synaptic plasticity is driven by sex neurosteroids targeting estrogen and androgen receptors in hippocampal CA1 pyramidal neurons. Front. Cell. Neurosci 13, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R, 2009. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J. Neurosci 29, 5949–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Morishita M, 2020. Sexually dimorphic formation of the preoptic area and the bed nucleus of the stria terminalis by neuroestrogens. Front. Neurosci 14, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM, 2016. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm. Behav 83, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Haraguchi S, Tobari Y, Narihiro M, Ishikawa K, Hayashi T, Harada N, Tsutsui K, 2014. Hypothalamic inhibition of socio-sexual behaviour by increasing neuroestrogen synthesis. Nat. Commun 5, 3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MM, Briski KP, 2021. Neuroestradiol regulation of ventromedial hypothalamic nucleus 5’-AMP-activated protein kinase activity and counterregulatory hormone secretion in hypoglycemic male versus female rats. AIMS Neurosci 8, 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood EA, Rochon PA, Moineddin R, Lee PE, Wu W, Pritchard KI, Tierney MC, 2018. Cognitive sequelae of endocrine therapy in women treated for breast cancer: a meta-analysis. Breast Cancer Res. Treat 168, 299–310. [DOI] [PubMed] [Google Scholar]
- Urbatzka R, Lutz I, Kloas W, 2007. Aromatase, steroid-5-alpha-reductase type 1 and type 2 mRNA expression in gonads and in brain of Xenopus laevis during ontogeny. Gen. Comp. Endocrinol 153, 280–288. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Benedusi V, Maggi A, 2008. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrinol 29, 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga S, Garcia-Segura LM, Azcoitia I, 2003. Neuroprotection by the steroids pregnenolone and dehydroepiandrosterone is mediated by the enzyme aromatase. J. Neurobiol 56, 398–406. [DOI] [PubMed] [Google Scholar]
- Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM, 2012. Aromatase inhibition abolishes LTP generation in female but not in male mice. J. Neurosci 32, 8116–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Vegeto E, Poletti A, Maggi A, 2016. Estrogens, neuroinflammation, and neurodegeneration. Endocr. Rev 37, 372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J, 1990. Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. J. Neurobiol 21, 808–825. [DOI] [PubMed] [Google Scholar]
- von Ziegler NI, Schlumpf M, Lichtensteiger W, 1991. Prenatal nicotine exposure selectively affects perinatal forebrain aromatase activity and fetal adrenal function in male rats. Brain Res. Dev. Brain Res 62, 23–31. [DOI] [PubMed] [Google Scholar]
- Wang J, Sareddy GR, Lu Y, Pratap UP, Tang F, Greene KM, Meyre PL, Tekmal RR, Vadlamudi RK, Brann DW, 2020. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J. Neurosci 40, 9751–9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA, 2015. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J. Neurosci 35, 2384–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JT, Adkins-Regan E, 1989. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm. Behav 23, 432–447. [DOI] [PubMed] [Google Scholar]
- Weber KS, Setchell KD, Lephart ED, 2001. Maternal and perinatal brain aromatase: effects of dietary soy phytoestrogens. Brain Res. Dev. Brain Res 126, 217–221. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Siegel RA, Yanai J, 1983. Effect of phenobarbital on in vitro aromatization of testosterone to estradiol by adult male mice brain. J. Steroid Biochem 18, 201–203. [DOI] [PubMed] [Google Scholar]
- Wright CL, Schwarz JS, Dean SL, McCarthy MM, 2010. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol. Metab 21, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Hoffman JH, McCarthy MM, 2019. Evidence that inflammation promotes estradiol synthesis in human cerebellum during early childhood. Transl. Psychiatry 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne RD, Saldanha CJ, 2004. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J. Neuroendocrinol 16, 676–683. [DOI] [PubMed] [Google Scholar]
- Wynne RD, Walters BJ, Bailey DJ, Saldanha CJ, 2008. Inhibition of injury-induced glial aromatase reveals a wave of secondary degeneration in the songbird brain. Glia 56, 97–105. [DOI] [PubMed] [Google Scholar]
- Yague JG, Munoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I, 2006. Aromatase expression in the human temporal cortex. Neuroscience 138, 389–401. [DOI] [PubMed] [Google Scholar]
- Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, Morrison JH, 2008. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res 1209, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A, 2010. Aromatase expression in the normal and epileptic human hippocampus. Brain Res 1315, 41–52. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yamamoto M, Ishihara Y, Komatsu S, Munetsuna E, Onizaki M, Ishida A, Kawato S, Mukuda T, 2013. De novo synthesized estradiol protects against methylmercury-induced neurotoxicity in cultured rat hippocampal slices. PLoS One 8, e55559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz MB, Wolfe A, Cheng YH, Glidewell-Kenney C, Jameson JL, Bulun SE, 2009. Aromatase promoter I.f is regulated by estrogen receptor alpha (ESR1) in mouse hypothalamic neuronal cell lines. Biol. Reprod 81, 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz MB, Wolfe A, Zhao H, Brooks DC, Bulun SE, 2011. Aromatase promoter I. f is regulated by progesterone receptor in mouse hypothalamic neuronal cell lines. J. Mol. Endocrinol 47, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Lu K, Vicario DS, 2012. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport 23, 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R, 2005. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc. Natl. Acad. Sci. U. S. A 102, 19198–19203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA, 2012. Genomic analysis of reactive astrogliosis. J. Neurosci 32, 6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiao X, Zhang XM, Zhao ZQ, Zhang YQ, 2012. Estrogen facilitates spinal cord synaptic transmission via membrane-bound estrogen receptors: implications for pain hypersensitivity. J. Biol. Chem 287, 33268–33281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Tang H, Dong Y, Chan A, Sareddy GR, Vadlamudi RK, Brann DW, 2014. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol. Cell. Endocrinol 389, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu S, Liang KY, Li K, Zou ZP, Yang CL, Tan K, Cao X, Jiang Y, Gao TM, Bai XC, 2017a. Neuronal mTORC1 is required for maintaining the nonreactive state of astrocytes. J. Biol. Chem 292, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZL, Qin P, Liu Y, Zhang LX, Guo H, Deng YL, Yizhao L, Hou YS, Wang LY, Miao Y, Ma YL, Hou WG, 2017b. Alleviation of ischaemia-reperfusion injury by endogenous estrogen involves maintaining Bcl-2 expression via the ERalpha signalling pathway. Brain Res 1661, 15–23. [DOI] [PubMed] [Google Scholar]
- Zhao Y, He L, Zhang Y, Zhao J, Liu Z, Xing F, Liu M, Feng Z, Li W, Zhang J, 2017. Estrogen receptor alpha and beta regulate actin polymerization and spatial memory through an SRC-1/mTORC2-dependent pathway in the hippocampus of female mice. J. Steroid Biochem. Mol. Biol 174, 96–113. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bian C, Liu M, Zhao Y, Sun T, Xing F, Zhang J, 2018. Orchiectomy and letrozole differentially regulate synaptic plasticity and spatial memory in a manner that is mediated by SRC-1 in the hippocampus of male mice. J. Steroid Biochem. Mol. Biol 178, 354–368. [DOI] [PubMed] [Google Scholar]
- Zhong YH, Dhawan J, Kovoor JA, Sullivan J, Zhang WX, Choi D, Biegon A, 2017. Aromatase and neuroinflammation in rat focal brain ischemia. J. Steroid Biochem. Mol. Biol 174, 225–233. [DOI] [PubMed] [Google Scholar]
- Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM, 2010. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 151, 1153–1160. [DOI] [PubMed] [Google Scholar]


