Abstract
AIM
To study sex differences in attention-deficit/hyperactivity disorder (ADHD) symptoms, we explored whether X chromosome absence or excess is independently associated with deficits in attention and hyperactivity, executive function, and processing speed.
METHOD
We assessed 116 children (ages 3y 10mo–11y 11mo, mean 8y 5mo, SD 1y 11mo) with a variable number of sex chromosomes: 36 females with Turner syndrome (45, X0), 20 males with Klinefelter syndrome (47, XXY), 37 typically developing females (XX), and 23 typically developing males (XY).
RESULTS
X chromosome absence was associated with increased attention problems, hyperactivity, and deficits in inhibitory control, compared with female children with XX (all p<0.003). Conversely, X chromosome excess was associated with weakness in working memory (p=0.018) and approached significance for attention problems (p=0.071) but not with hyperactivity, or weakness in inhibitory control relative to male children with XY. Using nonparametric effect size to quantify the clinical effect revealed that X chromosome absence affected attention, hyperactivity, executive function, and processing speed (all r>0.4), while X excess affected in-laboratory as well as parent-reported working memory (all r>0.4).
INTERPRETATION
Our observations provide compelling evidence that the absence or excess of an X chromosome distinctly affects cognition and behaviors associated with ADHD.
Sexual dimorphism in neurodevelopmental disorders, such as autism spectrum disorder and attention-deficit/hyperactivity disorder (ADHD), is widely recognized. For ADHD, differences between males and females in prevalence, course, comorbidities, and clinical manifestations are hallmarks of this diagnosis. Concerning clinical manifestations, female children with ADHD are more likely than males to be diagnosed with the predominantly inattentive type of ADHD,1 while symptom severity is higher in males than females.2 The complex array of genetic, hormonal, and social differences associated with ADHD obscures the biological factors affecting sexual dimorphism in this disorder.3 Not surprisingly, traditional methods comparing males and females with idiopathic ADHD versus groups of typically developing peers have yielded limited information about the relative contribution of biological factors to observed behavioral differences between the sexes.4 The study of sex chromosome aneuploidies (SCAs), which are associated with changes in sex chromosome number and constitution, has emerged as a promising strategy for elucidating genetic, hormonal, and inflammatory substrates of sexual dimorphism in the manifestation of ADHD symptoms.3,5 This approach of studying children with SCAs is aimed at simplifying the investigation of complex behaviors such as attention and hyperactivity in humans.
Two common SCAs are Klinefelter syndrome (most commonly a 47, XXY karyotype; 1 in 500 male live births)6 and Turner syndrome (most commonly 45, X0; 1 in 2000 female live births).7 In typically developing females (XX), one of two X chromosomes undergoes inactivation, yet about 15% of inactivated X chromosome genes escape inactivation, resulting in expression of two gene copies; only one gene copy is present in female children with Turner syndrome. Genes on the additional X chromosome in Klinefelter syndrome (47, XXY) also escape inactivation, resulting in increased X chromosome gene expression in male children with Klinefelter syndrome compared with typically developing peers, along with Y chromosome gene expression.
Turner syndrome and Klinefelter syndrome present inverse profiles of strengths and weaknesses in many cognitive dimensions in the context of overall ‘normal’ intelligence.8–10 While female children with Turner syndrome present with relative weakness in visuospatial abilities and strengths in verbal skills, males with Klinefelter syndrome usually present with relative strength in visuospatial abilities and weaknesses in verbal skills. Nevertheless, both SCAs have been associated with weaknesses in social cognition11–14 and executive function,9,15–17 and ADHD symptoms.16,18–20 While several studies show that females with Turner syndrome present with weaknesses in processing speed,21 data about Klinefelter syndrome for males are less conclusive. Some studies show no differences compared with typically developing peers.22 Others find weaknesses in processing speed, with some evidence for more impairment in verbal rather than non-verbal information processing.15
With respect to ADHD symptoms, 25% of female children and adolescents with Turner syndrome present with an extensive set of related executive function weaknesses23,24 and meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for ADHD;25 while we reported in our previous study15 that half of a distinct cohort of female children with Turner syndrome displayed significant levels of attention problems and hyperactivity. Using a national registry, 4.9% of individuals with Klinefelter syndrome had registered or received treatment for a diagnosis of ADHD.26 In contrast, children with Klinefelter syndrome had a 63% rate of ADHD diagnosis using the Kiddie-Sads-Present and Lifetime Version interview.27 Use of the Conners’ Parent Rating Scale-Revised Long Version indicates that 42% of male children with Klinefelter syndrome have significantly elevated rates of ADHD compared with typically developing peers.28 Similarly, using the DSM-IV, 36% of males with Klinefelter syndrome meet diagnostic criteria for ADHD, with predominantly inattentive symptoms.29
Given previous reports of significantly elevated risks for ADHD symptomatology in Turner syndrome and Klinefelter syndrome, we hypothesized that both SCAs would be associated with an overall increase in ADHD symptoms in the new cohort described here. However, on the basis of our previous results outlining elevated levels of hyperactivity in female children with Turner syndrome compared with those with non-syndromic ADHD,16 we further predicted that differences in X chromosome number would be associated with distinct ADHD symptom profiles for Turner syndrome and Klinefelter syndrome. Specifically, we hypothesized that Turner syndrome (45, X0) would be associated with a ‘male ADHD profile’ (i.e. increased attention problems, hyperactivity, and executive function weaknesses), while Klinefelter syndrome (47, XXY) would be associated with a ‘female ADHD profile’ (i.e. predominantly increased attention problems, along with a corresponding pattern of executive function weaknesses).
To test our hypotheses, we compared female children with Turner syndrome and male children with Klinefelter syndrome versus their respective typically developing sex-matched peers on a comprehensive assessment of ADHDsymptoms and associated cognitive measures of executive function. Also, we contrasted each SCA with the opposite-sex typically developing comparison groups to provide additional information about the effects of sex chromosome number and constitution (specifically X chromosome number) on cognition and behavior. To the best of our knowledge, such a direct assessment comparing ADHD profiles among females with Turner syndrome, males with Klinefelter syndrome, and typically developing peers has not been conducted to date.
METHOD
Participants
One-hundred and sixteen participants (ages 3y 10mo–11y 11mo, mean 8y 5mo, SD 1y 11mo) were included in this study: 36 females with Turner syndrome and 20 males with Klinefelter syndrome were recruited through pediatric endocrinologists, medical geneticists, the national Turner Syndrome Society network, the Association for X and Y Chromosome Variations, and the Center for Interdisciplinary Brain Sciences Research website. Thirty-seven typically developing female and 23 male controls were recruited through local parent organizations, advertisements, and from siblings of participants with Turner syndrome or Klinefelter syndrome. More information about the participants can be found in Appendix S1 (online supporting information).
This study was approved by the local Institutional Review Board of the Stanford University School of Medicine, and informed written consent was obtained from a legal guardian for all participants, as well as written assent from participants over 7 years of age.
Study design
The four groups constituting this study were compared on behavioral measures of attention and hyperactivity using the Behavior Assessment System for Children, Second Edition, specifically hyperactivity and attention problems, and on cognitive–behavioral measures of executive function. Cognitive and behavioral measures of executive function included the Behavior Rating Inventory of Executive Function, specifically inhibit, shift, emotional control, working memory, and plan/organize; the Developmental NEuroPSYchological Assessment, version 2 (NEPSY-2), specifically auditory attention, response inhibition, naming, inhibition, and switching; and the age appropriate Wechsler Intelligence scale (Wechsler Intelligence Scale for Children, Fourth Edition or Wechsler Preschool and Primary Scale of Intelligence, Third Edition working memory (digit span and letter–number sequencing) and processing speed (coding and symbol search) subscales. Details on measures can be found in Appendix S1.
Data analysis
For all analyses, we used R software for statistical computing version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). We used non-parametric tests given the non-normal distribution of the data (particularly for the male groups). Group ages and full-scale IQ scores were compared with a Kruskal–Wallis test. Fisher’s exact test was used to compare pubertal development,30 represented by Tanner scores for pubic hair and female breast/male genitalia development. Owing to the low frequencies of participants with a Tanner score greater than 2, we combined participants in Tanner stages 3 (n=7) and 4 (n=2) into one group (Table 1).
Table 1:
Demographics
| Turner syndrome |
Klinefelter syndrome |
Typically developing females |
Typically developing males |
|||||
| n | 36 |
20a |
37 |
23 |
||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||
|
| ||||||||
| Age, y:mo | 8:5 (1:11) | 8:5 (2:2) | 8:10 (1:7) | 8:0 (2:2) | ||||
| Age range, y:mo | 3:10–10:10 | 4:9–11:2 | 4:11–11:8 | 3:11–11:11 | ||||
| FSIQ | 93.2 (12.7) | 96.5 (11.2) | 111.0 (10.1) | 111.3 (8.4) | ||||
| PSI/PIQ | 91.8 (14.7) | 103.6 (13.6) | 112.8 (11.6) | 113.1 (11.2) | ||||
| VCI/VIQ | 105.4 (13.9) | 97.2 (12.1) | 111.6 (13.1) | 116.0 (14.4) | ||||
| PSI/PSQ | 82.4 (15.2) | 92.1 (10.4) | 100.2 (11.2) | 97.0 (12.0) | ||||
| WMI | 90.0 (10.5) | 89.1 (14.0) | 103.7 (10.1) | 102.0 (9.0) | ||||
| Attention problems, n (%) | 16 (42.1) | 11 (55) | 5 (13) | 2 (8.6) | ||||
| Hyperactivity, n (%) Medications, n (%) | 16 (42.1) | 8 (40) | 2 (5) | 2 (8.6) | ||||
| Growth hormone | 31 (81.6) | — | — | — | ||||
| Sex hormones | 1 (0.025mg estrogen patch) | 1 Oxandrolone | — | — | ||||
| Stimulants | — | — | — | — | ||||
|
| ||||||||
| Pubic hair | Breasts | Pubic hair | Male genitalia | Pubic hair | Breasts | Pubic hair | Male genitalia | |
| Tanner stage | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
|
| ||||||||
| 1 | 35 (92.1) | 34 (89.5) | 19 (100) | 16 (84.2) | 26 (72.2) | 26 (72.2) | 21 (95.5) | 19 (86.4) |
| 2 | 1 (2.6) | 4 (10.5) | 0 | 2 (10.5) | 7 (19.4) | 9 (25) | 1 (4.5) | 1 (4.5) |
| 3 | 2 (5.3) | 0 | 0 | 1 (5.3) | 2 (5.6) | 0 | 0 | 2 (9.1) |
| 4 | 0 | 0 | 0 | 0 | 1 (2.8) | 1 (2.8) | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Of the cohort with Klinefelter syndrome, two participants were diagnosed prenatally, seven postnatally, and for 11 participants these data were not available. Full-scale IQ (FSIQ) from the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-111) or Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV); PRI/PIQ, composite scores for the Perceptual Comprehension Index from the WISC-IV or performance IQ from the WPPSI-III; VCI/VIQ, Verbal Comprehension Index from the WISC-IV or verbal IQ from the WPPSI-III; WMI, Working Memory Index from the WISC-IV; PSI/PSQ, the Processing Speed Index from the WISC-IV or Processing Speed Quotient from the WPPSI-III.
We used a Kruskal–Wallis test to compare attention problems, hyperactivity, and cognitive measures of executive function between groups. For measurements with significant Kruskal–Wallis test results, a Wilcoxon rank-sum test (Mann-Whitney U test) was used to conduct post hoc analyses comparing the study group pairs. Behavior Rating Inventory of Executive Function T-scores are already adjusted for sex; thus, for this measurement, we only compared same-sex group pairs (i.e. Turner syndrome vs typically developing females, and Klinefelter syndrome vs typically developing males).
Holm’s adjustment30 was used to control for multiple comparisons for each cognitive–behavioral outcome category (instrument), namely Behavior Assessment System for Children, Second Edition, Behavior Rating Inventory of Executive Function, NEPSY, and IQ subtests.
Finally, to measure the clinical effect of sex chromosomes on cognition and behavior, we used between-subject, nonparametric effect sizes r using the rank-biserial correlation (specifically, we used the r-function effectsize::rank_biserial).31 This rank is calculated from the Wilcoxon rank-sum test: a non-parametric statistical test used to compare two nonpaired groups. The rank-biserial correlation is calculated using average ranks from two sets of data and sample size in each group. To interpret the calculated value, one can draw on the interpretation of the classical Pearson’s correlation coefficient (r), hence the strength of the relationship.31
RESULTS
Demographics
The study groups did not differ significantly in age (Kruskal–Wallis =1.19, p=0.76; Table 1), or pubertal development (Fisher’s exact test, p=0.22; Table 1). As expected,11,12,32 full-scale IQ differed significantly between groups (Kruskal–Wallis =43.2, p<0.001), with both SCAs receiving comparably lower full-scale IQ scores than sex-matched typically developing peers. However, it should be noted that all four groups demonstrated a mean full-scale IQ within the range of average intelligence (Table 1 and Fig. S1 [online supporting information]).
Behavioral measures of ADHD symptoms
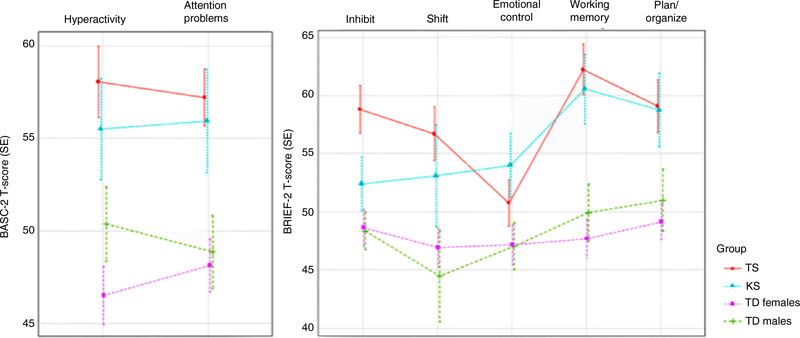
The four study groups differed in behavioral measures of attention problems and hyperactivity (Table 2 and Fig. 1). Post hoc analysis revealed that females with Turner syndrome had elevated parent-reported attention problems and hyperactivity compared with typically developing females. Overall, males with Klinefelter syndrome had elevated parent-reported attention problems but not hyperactivity compared with typically developing males. Using a cut-off of 60 and above in the Behavior Assessment System for Children, Second Edition assessment (scores above the ‘at-risk’ level for the measured behavior), we observed that 42% of females with Turner syndrome were reported by their parents to have attention problems and hyperactivity. Fifty-five percent of males with Klinefelter syndrome were parent-reported as having attention problems while 40% had hyperactivity (Table 1).
Table 2:
Summary statistics
| Kruskal-Wallisx χ2 (p) | TS vs TD females, p | TS vs TD males, p | KS vs TD females, p | KS vs TD males, p | |
|---|---|---|---|---|---|
|
| |||||
| Attention problems | 21.43 (<0.001) | <0.001 | <0.01 | <0.01 | 0.07 |
| Hyperactivity | 17.98 (<0.001) | <0.001 | <0.01 | <0.05 | 0.26 |
| Inhibit | — | <0.05 | — | — | 0.22 |
| Shift | — | <0.05 | — | — | 0.08 |
| Emotional control | — | 0.25 | — | — | 0.06 |
| Working memory | — | <0.001 | — | — | <0.05 |
| Plan/organize | — | <0.05 | — | — | 0.07 |
| Auditory attention | 7.88 (<0.05) | <0.05 | <0.05 | 0.22 | 0.15 |
| Response inhibition | 11.14 (<0.05) | <0.01 | <0.05 | 0.07 | 0.24 |
| Naming | 0.19 (0.34) | 0.19 | 0.99 | <0.05 | 0.28 |
| Inhibition | 17.93 (<0.001) | <0.001 | <0.01 | 0.13 | 0.19 |
| Switching | 2.78 (0.43) | 0.14 | 0.26 | 0.47 | 0.61 |
| Digit span | 21.57 (<0.001) | <0.001 | <0.001 | <0.01 | 0.05 |
| Letter-number sequencing | 26.67 (<0.001) | <0.001 | <0.01 | <0.001 | <0.001 |
| Coding | 20.89 (<0.001) | <0.001 | <0.05 | <0.05 | 0.25 |
| Symbol search | 23.71 (<0.001) | <0.001 | <0.001 | 0.67 | 0.55 |
TS, female children with Turner syndrome; KS, male children with Klinefelter syndrome; TD, typically developing.
Figure 1:
Behavioral symptoms of attention-deficit/hyperactivity disorder (ADHD) and executive functions in female children with Turner syndrome (TS), male children with Klinefelter syndrome (KS), typically developing (TD) females, and typically developing males. Behavioral symptoms of ADHD were measured using the attention problems and hyperactivity scales of the Behavior Assessment System for Children, Second Edition (BASC-2). Executive function profile was measured using the attention and executive function scales/subtests of the Behavior Rating Inventory of Executive Function (BRIEF) scales: inhibit, shift, emotional control, working memory, and plan/organize. SE, standard error.
Executive function: parent-reported and in-person laboratory evaluation
In general, we observed that females with Turner syndrome scored higher (i.e. were more impaired) than typically developing females on most of the parent-reported executive function metrics. Males with Klinefelter syndrome scored higher only on working memory compared with typically developing males (Table 2 and Fig. 1).
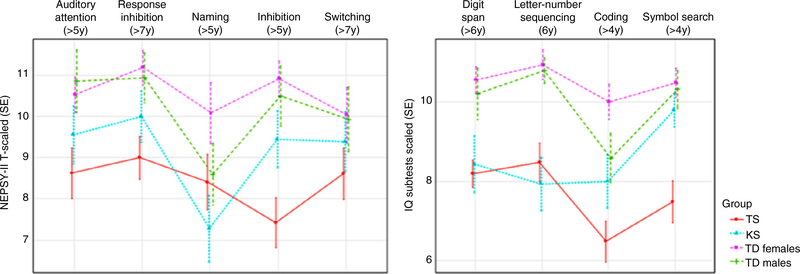
The four study groups differed significantly in cognitive tasks of attention and inhibition (Table 2 and Fig. 2). Post hoc analyses revealed that females with Turner syndrome performed worse on attention and inhibition tasks than typically developing comparison groups.
Figure 2:
Detailed cognitive profile for female children with Turner syndrome (TS), male children with Klinefelter syndrome (KS), typically developing (TD) females, and typically developing males using the Developmental NEuroPSYchological Assessment (NEPSY) measure of attention and executive function: auditory attention, response set, naming, inhibition, and switching. Subtests of the Wechsler Preschool and Primary Scale of Intelligence Third Edition (for children <6y) or Wechsler Intelligence Scale for Children, Fourth Edition (for children ≥6y): digit span, letter–number sequencing, coding, and symbol search. SE, standard error.
Notably, across all groups, 24 participants were not within the required age range (>7y) for the response inhibition and switching subtests of the NEPSY-2. However, the percentage of participants who did not complete this task did not differ between groups (=5.91, p=0.12; see Appendix S1 for the complete number of participants who completed each subtest of the NEPSY-2, and other tests administered).
We further evaluated processing speed across the entire age range (3y 10mo–11y 11mo) using processing speed subtests from the IQ measures. We observed differences between study groups for coding and symbol search (Table 2 and Fig. 2); post hoc analyses revealed that females with Turner syndrome scored lower than typically developing females. Conversely, scores from males with Klinefelter syndrome were generally comparable to those of typically developing males on tasks measuring processing speed (coding and symbol search) although were lower compared with typically developing females for coding (p=0.026).
Finally, SCA groups differed from comparison groups on working memory subtests from the IQ measures (Table 2 and Fig 2). Scores for females with Turner syndrome and males with Klinefelter syndrome were lower than those of the typically developing comparison groups of both sexes for the digit span and letter–number sequencing subtests.
The effects of X chromosome number on cognition and behavior
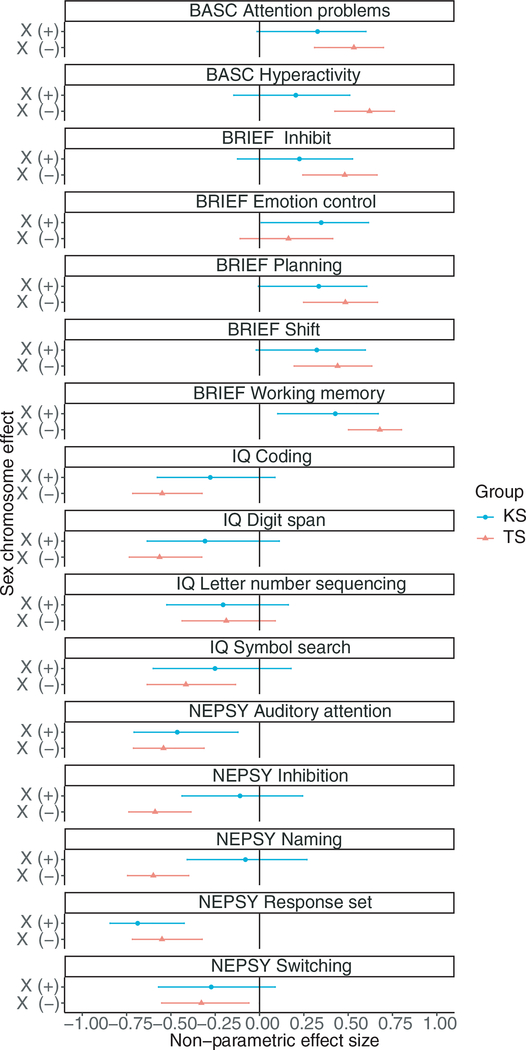
To measure the clinical effect of sex chromosome variation on ADHD symptoms and executive function, we calculated effect sizes and confidence intervals for all measures significantly different between the groups (Figs 1 and 2). We observed significant effects (confidence interval did not cross 0) of Turner syndrome and Klinefelter syndrome on parent-reported as well as in-laboratory measures of working memory (parent-reported working memory, digit span letter–number sequencing) compared with same-sex typically developing groups (Fig. 3). In contrast, Turner syndrome but not Klinefelter syndrome had a significant effect on attention and hyperactivity (parent-reported attention problems and hyperactivity, auditory attention) and broad-ranging problems with executive functions including inhibition (parent-reported inhibit and inhibition), planning (parent-reported plan/organize), shifting (parent-reported shift and response set), processing speed, and working memory (coding, symbol search, digit span, and letter–number sequencing). These results suggest that while children with SCAs have ADHD symptoms and associated weaknesses in executive function compared with typically developing peers, each SCA has a distinct profile of ADHD symptoms and related executive function weaknesses.
Figure 3:
Study design and the measured effects of sex chromosome variation on attention-deficit/hyperactivity disorder (ADHD) phenotype and executive function. We compared females with Turner syndrome (TS) and males with Klinefelter syndrome (KS) with sex-matched typically developing peers. Comparing males with Klinefelter syndrome (XXY) versus typically developing males (XY), theoretically measuring the effect of an X chromosome addition. Comparing females with Turner syndrome (45, X0) versus typically developing females (XX), theoretically measuring effect of missing an X chromosome. Effect size (rank-biserial correlation, r): x-axis, non-parametric effect size (confidence interval); y-axis, Turner syndrome versus typically developing females (XX) X(-), Klinefelter syndrome (XXY) versus typically developing males (XY) X(+). A vertical line was added at r=0 to mark confidence interval significance.47 BASC, Behavior Assessment System for Children; BRIEF, Behavior Rating Inventory of Executive Function; NEPSY, Developmental NEuroPSYchological Assessment.
DISCUSSION
Our findings show that females with Turner syndrome and males with Klinefelter syndrome display increased ADHD symptoms and weaknesses in executive function relative to typically developing comparison groups. Further, we found distinct behavioral and cognitive profiles in these genetic conditions. Compared with groups of typically developing females and males, females with Turner syndrome display elevated levels of attention problems, hyperactivity, and weaknesses in inhibition skills. Males with Klinefelter syndrome show attention problems and working memory weaknesses compared with groups of typically developing females and males. Using effect sizes, we further confirm the clinical significance of our results, demonstrating significant effect sizes for the group with Turner syndrome for attention problems, hyperactivity, and weaknesses in auditory attention, inhibition skills, and processing speed. For both SCAs we found significant effect sizes for parent-reported working memory and in-laboratory measures of working memory compared with same-sex comparison groups (Fig. 3).
Our findings are mostly in line with previous studies of ADHD symptoms15,22,25–28 and executive function8,9,15,2 in individuals with Turner syndrome or Klinefelter syndrome. For females with Turner syndrome, an increase in attention problems and hyperactivity has been well docu-mented.15,22 The observed pattern of ADHD symptoms (i.e. increased attention problems without hyperactivity) among males with Klinefelter syndrome is also in line with previous studies in young males (<10y) with Klinefelter syndrome.7,30 The results of the present study expand the previous framework of ADHD in each of the syndromes by finding a distinctive profile of ADHD symptoms in Turner syndrome and Klinefelter syndrome when tested simultaneously.
Similarly, we observed a distinct executive function profile in Turner syndrome and Klinefelter syndrome. In Turner syndrome, affected domains included working memory, processing speed, and inhibition abilities. These results are consistent with earlier studies reporting weaknesses in these executive function domains using the NEPSY,12,16 Behavior Rating Inventory of Executive Function,23 and Rey Figure Organizational score,33 as well as tasks such as the Contingency Naming Task34 and the Stroop Test35 measuring inhibition skills. In contrast, in Klinefelter syndrome, affected domains included parent-measured and in-laboratory working memory, but not in processing speed or inhibition skills (Fig. 3). The deficits in inhibition in Turner syndrome are aligned with the broader literature of ADHD in females, showing a link between performance in response inhibition tasks and hyperactive–impulsive symptoms.36 The deficits in parent-reported working memory measures are aligned with the cognitive measures of working memory (assessed using the digit span and letter–number sequencing subtests) in both groups. Yet, it should be noted that these measures also tend to be lower in children with reading impairments, a common finding in Klinefelter syndrome.37,38 Thus, for Klinefelter syndrome, we cannot conclude whether these findings are specifically secondary to learning impairments or attention problems. Overall, the observation of inhibition deficits in Turner syndrome (45, X0) but not in Klinefelter syndrome (47, XXY) suggest a protective effect of the X chromosome in this domain.
The number of X chromosomes is thought to affect neurodevelopment through various mechanisms including genes that escape X inactivation, the pattern of random X inactivation in females, parental X imprinting, and epigenetic effects.39 Furthermore, the number and constitution of sex chromosomes may also have a more widespread effect on genome-wide gene expression.40 X chromosome genes that escape inactivation and lack a Y chromosome homologue are potential candidates for sexual dimorphism41 because of the resulting variable gene dose in males and in females. The loss of genes that escape inactivation in Turner syndrome is suggested as a possible explanation for the susceptibility of females with Turner syndrome to developing ADHD symptoms and executive function weaknesses.15 A recent study41 examining gene expression in SCA confirmed the haploinsufficiency of genes that escape inactivation in Turner syndrome. Raznahan et al.41 found that inactivated X-linked genes are overexpressed in Turner syndrome yet undergo further silencing with mounting numbers of X chromosomes (XXY or XXX or XXYY). Therefore, it is predicted that males with Klinefelter syndrome who have two X chromosomes are theoretically more likely to phenotypically mimic females with idiopathic ADHD. The results of the present study support this hypothesis. The distinct ADHD symptoms and executive function profile found in the focal SCAs provide a potential biological explanation for the male predominance in early neurodevelopmental disorders, including ADHD,4 and the relatively high rates of ADHD symptoms of attention problems and hyperactivity in females with Turner syndrome.
Caution should be exercised when interpreting results of this study because of several limitations. Our groups of typically developing males and those with Klinefelter syndrome were smaller than the typically developing female and Turner syndrome groups, probably contributing to the result that many of the comparisons of males with Klinefelter syndrome versus typically developing peers yielded only a near-significant trend. Analyses of effect size allowed us partly to overcome this limitation.42 For the group with Klinefelter syndrome, we included limited data on the timing of diagnosis (Table 1). Unlike Turner syndrome, which is associated with a physical phenotype that leads to syndrome detection, children with Klinefelter syndrome might not present an apparent phenotype indicating the condition. Previous work has shown that the degree of impairment faced by young people with sex chromosome trisomies, including Klinefelter syndrome, is correlated with the timing of diagnosis (either pre- or postnatal).43 Another limitation of this study is that although comparisons in prepubertal individuals with SCAs provide a rather lucid examination of the effects of changes in sex chromosome number and constitution, changes in hormonal profile (especially in Turner syndrome) probably also affect neurodevelopment.44 On processing speed measures, one should consider that the Wechsler Intelligence Scale for Children, Fourth Edition processing speed index has a large motor component—particularly the coding subtest. Thus, the motor difficulties that are known to be associated with Turner syndrome45 and Klinefelter syndrome18 could be contributing to the reduced processing speed observed in Turner syndrome. Finally, given the relatively small sample size of our male groups and our choice of using non-parametric testing, we did not include IQ as a covariate in our group comparisons. This approach potentially limits our ability to test whether group differences in ADHD symptoms and executive function are derived from SCA genotype only or also by overall cognitive abilities.
In conclusion, we herein expand the framework for examining the effect of X chromosome number on ADHD symptoms and associated cognitive constructs using two SCAs, enabling unique contrast of X chromosome absences versus excess. Our results stress the association between X chromosome number and sexually dimorphic presentation of attention, hyperactivity, and executive function, and inform clinical management in these common SCAs. We suggest that specific treatments and outcome measures targeting symptoms of hyperactivity and inhibition skills should be used in clinical management of ADHD symptoms in Turner syndrome. For Klinefelter syndrome, observations from the present study and others18 suggest that male children have an increased tendency for attention problems without hyperactivity, and therefore are more likely to be underdiagnosed and undertreated (similarly to female children with idiopathic ADHD46). Thus, increased vigilance for symptoms of attention problems in Klinefelter syndrome may affect long-term outcomes in individuals with this common genetic condition.
Supplementary Material
Appendix S1: Participant information.
Figure S1: Cognitive profile for female children with Turner syndrome, male children with Klinefelter syndrome, typically developing females, and typically developing males.
What this paper adds.
X chromosome number has a distinctive effect on attention-deficit/hyperactivity disorder symptoms.
X chromosome absence is associated with increased attention problems, hyperactivity, and weakness in executive functions.
X chromosome excess is associated with weaknesses in attention and working memory.
Clinical management for children with Turner syndrome should focuson attention problems, hyperactivity, and weak inhibition skills.
Clinical management for children with Klinefelter syndrome should focuson attention problems and impaired working memory.
ACKNOWLEDGEMENTS
The Turner Syndrome Society, the Turner Syndrome Foundation, and the Association for X and Y Chromosome Variations (AXYS) made this work possible. We sincerely thank all of the families who volunteered to participate. This work was supported by grants from The National Institute of Mental Health (MH099630), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD049653 and HD092847) to Dr Reiss (PI). Funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD090209) and the Fuisz family provided support to Dr. Green (PI). Funding from The National Institute of Mental Health (MH097120) provided support to Dr Hong (PI). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. Dr Reiss is an unpaid medical advisor for the Turner Syndrome Society and Turner Syndrome Foundation. Dr Green is an unpaid medical advisor for the Rasopathies Network. Drs Green, Flash, Jo, Klabunde, Hong, and Reiss and Mss Shrestha and Shankar report no biomedical financial interests or potential conflicts of interest.
ABBREVIATIONS
- NEPSY
Developmental NEuroPSYchological Assessment
- SCA
Sex chromosome aneuploidy
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, et al. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry 2002; 159:36–42. [DOI] [PubMed] [Google Scholar]
- 2.Arnett AB, Pennington BF, Willcutt EG, DeFries JC, Olson RK. Sex differences in ADHD symptom severity. J Child Psychol Psychiatry 2015; 56: 632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan B Sex differences in neurodevelopmental disorders. Dev Med Child Neurol 2021; 63: 492. [DOI] [PubMed] [Google Scholar]
- 4.Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2010; 33: 357–73. [DOI] [PubMed] [Google Scholar]
- 5.Green T, Flash S, Reiss AL. Sex differences in psychiatric disorders: what we can learn from sex chromosome aneuploidies. Neuropsychopharmacology 2019; 44: 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giltay JC, Maiburg MC. Klinefelter syndrome: clinical and molecular aspects. Exp Rev Mol Diagn 2010; 10: 765–76. [DOI] [PubMed] [Google Scholar]
- 7.Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol 2017; 177:G1–70. [DOI] [PubMed] [Google Scholar]
- 8.Hong DS, Reiss AL. Cognition and behavior in Turner syndrome: a brief review. Pediatr Endocrinol Rev 2012; 9 (Suppl 2): 710–2. [PMC free article] [PubMed] [Google Scholar]
- 9.Boada R, Janusz J, Hutaff-Lee C, Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev Disabil Res Rev 2009; 15: 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geschwind DH, Boone KB, Miller BL, Swerdloff RS. Neurobehavioral phenotype of Klinefelter syndrome. Ment Retard Dev Disabil Res Rev 2000; 6: 107–16. [DOI] [PubMed] [Google Scholar]
- 11.Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, et al. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics 2012; 129: 769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepage JF, Dunkin B, Hong DS, Reiss AL. Contribution of executive functions to visuospatial difficulties in prepubertal girls with Turner syndrome. Dev Neuropsychol 2011; 36: 988–1002. [DOI] [PubMed] [Google Scholar]
- 13.Tartaglia NR, Wilson R, Miller JS, Rafalko J, Cordeiro L, Davis S, et al. Autism spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. J Dev Behav Pediatr 2017; 38: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross J, Zinn A, McCauley E. Neurodevelopmental and psychosocial aspects of Turner syndrome. Ment Retard Dev Disabil Res Rev 2000; 6: 135–41. [DOI] [PubMed] [Google Scholar]
- 15.Boone K, Swerdloff RS, Miller BL, Geschwind DH, Razani J, Lee A, et al. Neuropsychological profiles of adults with Klinefelter syndrome. J Int Neuropsychol Soc 2001; 7: 446–56. [DOI] [PubMed] [Google Scholar]
- 16.Green T, Bade Shrestha S, Chromik LC, Rutledge K, Pennington BF, Hong DS, Reiss AL. Elucidating X chromosome influences on attention deficit hyperactivity disorder and executive function. J Psychiatr Res 2015; 68: 217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong DS, Reiss AL. Cognitive and neurological aspects of sex chromosome aneuploidies. Lancet Neurol 2014; 13: 306–18. [DOI] [PubMed] [Google Scholar]
- 18.Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MPD, Kushner H, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A 2008; 146: 708–19. [DOI] [PubMed] [Google Scholar]
- 19.Cordeiro L, Tartaglia N, Roeltgen D, Ross J. Social deficits in male children and adolescents with sex chromosome aneuploidy: a comparison of XXY, XYY, and XXYY syndromes. Res Dev Disabil 2012; 33: 1254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon TJ, Takarae Y, DeBoer T, McDonald-McGinn DM, Zackai EH, Ross JL. Overlapping numerical cognition impairments in children with chromosome 22q11.2 deletion or Turner syndromes. Neuropsychologia 2008; 46:82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong DS, Reiss AL. Cognition and behavior in Turner syndrome: a brief review. Pediatr Endocrinol Rev 2012; 9 (Suppl 2): 710–2. [PMC free article] [PubMed] [Google Scholar]
- 22.van Rijn S, Swaab H. Executive dysfunction and the relation with behavioral problems in children with 47, XXY and 47,XXX. Genes Brain Behav 2015; 14: 200–8. [DOI] [PubMed] [Google Scholar]
- 23.Lepage JF, Dunkin B, Hong DS, Reiss AL. Impact of cognitive profile on social functioning in prepubescent females with Turner syndrome. Child Neuropsychol 2013; 19: 161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong D, Kent JS, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev 2009; 15: 270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell HF, Wallis D, Mazzocco MMM, Moshang T, Zackai E, Zinn AR, et al. Increased prevalence of ADHD in Turner syndrome with no evidence of imprinting effects. J Pediatr Psychol 2006; 31: 945–55. [DOI] [PubMed] [Google Scholar]
- 26.Cederlöf M, Ohlsson Gotby A, Larsson H, Serlachius E, Boman M, Långström N, et al. Klinefelter syndrome and risk of psychosis, autism and ADHD. J Psychiatr Res 2014; 48: 128–30. [DOI] [PubMed] [Google Scholar]
- 27.Bruining H, Swaab H, Kas M, van Engeland H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics 2009; 123: e865–70. [DOI] [PubMed] [Google Scholar]
- 28.Ross JL, Tartaglia N, Merry DE, Dalva M, Zinn AR. Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes Brain Behav 2015; 14: 137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tartaglia N, Cordeiro L, Howell S, Wilson R, Janusz J. The spectrum of the behavioral phenotype in boys and adolescents 47, XXY (Klinefelter syndrome). Pediatr Endocrinol Rev 2010; 8(Suppl 1): 151–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Kerby DS. The simple difference formula: an approach to teaching nonparametric correlation. Comp Psychol 2014; 3: 11.IT.3.1. [Google Scholar]
- 31.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6:65–70. [Google Scholar]
- 32.Green T, Fierro KC, Raman MM, Foland-Ross L, Hong DS, Reiss AL. Sex differences in amygdala shape: insights from Turner syndrome. Human Brain Map 2016; 37: 1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross JL, Stefanatos GA, Kushner H, Zinn A, Bondy C, Roeltgen D. Persistent cognitive deficits in adult women with Turner syndrome. Neurology 2002; 58: 218–25. [DOI] [PubMed] [Google Scholar]
- 34.Kirk JW, Mazzocco MM, Kover ST. Assessing executive dysfunction in girls with fragile X or Turner syndrome using the Contingency Naming Test (CNT). Dev Neuropsychol 2005; 28: 755–77. [DOI] [PubMed] [Google Scholar]
- 35.Temple CM, Carney R. Reading skills in children with Turner’s syndrome: an analysis of hyperplexia. Cortex 1996; 32: 335–45. [DOI] [PubMed] [Google Scholar]
- 36.Miller M, Loya F, Hinshaw SP. Executive functions in girls with and without childhood ADHD: developmental trajectories and associations with symptom change. J Child Psychol Psychiatry 2013; 54: 1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bender BG, Linden M, Robinson A. Reading Disabilities, Genetic and Neurological Influences. Dordrecht/Boston/London: Springer Science & Business Media, 1991: 127–39. [Google Scholar]
- 38.Pennington BF, Bender B, Puck M, Salbenblatt J, Robinson A. Learning disabilities in children with sex chromosome anomalies. Child Dev 1982; 53: 1182–92. [PubMed] [Google Scholar]
- 39.Arnold AP. A general theory of sexual differentiation. J Neurosci Res 2017; 95: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raznahan A, Parikshak NN, Chandran V, Blumenthal JD, Clasen LS, Alexander-Bloch AF, et al. Sex-chromosome dosage effects on gene expression in humans. Proc Natl Acad Sci USA 2018; 115: 7398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Printzlau F, Wolstencroft J, Skuse DH. Cognitive, behavioral, and neural consequences of sex chromosome aneuploidy. J Neurosci Res 2017; 95: 311–9. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J Statistical Power Analysis for the Behavioral Sciences. Mahwah, NJ: Routledge, 2013. [Google Scholar]
- 43.Bishop DVM, Brookman-Byrne A, Gratton N, Gray E, Holt G, Morgan L, et al. Language phenotypes in children with sex chromosome trisomies. Wellcome Open Res 2018; 3: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modi DN, Sane S, Bhartiya D. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod 2003; 9: 219–25. [DOI] [PubMed] [Google Scholar]
- 45.Hutaff-Lee C, Bennett E, Howell S, Tartaglia N. Clinical developmental, neuropsychological, and social–emotional features of Turner syndrome. Am J Med Genet C 2019; 181:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mowlem FD, Rosenqvist MA, Martin J, Lichtenstein P, Asherson P, Larsson H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur Child Adolesc Psychiatry 2019; 28: 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 2007; 82: 591–605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Participant information.
Figure S1: Cognitive profile for female children with Turner syndrome, male children with Klinefelter syndrome, typically developing females, and typically developing males.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.