Abstract
COVID-19 infection can cause a wide spectrum of symptoms. The audio-vestibular system can also be involved, but there is still debate about this so findings need to be considered carefully. Furthermore, mother to fetus intrauterine transmission of COVID-19 infection in pregnant women is controversial. Few studies are available about the audio-vestibular symptomatology of newborns with intrauterine COVID19 exposure.
Objectives
This study investigates the possible correlation between the COVID19 gestational infection and hearing impairment onset in newborns. The involvement of hearing in COVID19 is verified so the timing and methodology of audiological evaluation of children can be planned.
Methods
Children were subject to newborn hearing screening and audiological evaluation.
Newborn hearing screening is carried out prior to hospital discharge using the Automatic Transient Evoked Otoacoustic Emissions test.
Audiological evaluation is performed within the child age of 4 months by using maternal, pregnancy, and perinatal case history, COVID19 case history, otoscopy, acoustic immittance test, Distortion Product Otoacoustic Emissions test, and the Auditory Brainstem Response test.
Results
63 children were included in the study.
82.5% of these children were subjects of the newborn hearing screening program. The remaining 11 newborns were not subjected to hearing screening due to isolation measures and their audiological evaluation was carried out directly. Only one of 52 screened neonates showed a bilateral REFER test result but hearing threshold was normal at audiological evaluation.
Audiological evaluation showed normal bilateral ABR thresholds in 59/63 children. Four children (6.3% of the total) had ABR threshold alterations but two showed normal threshold at ABR retest performed within 1 month of the first. The other two infants showed monolateral ABR alterations but one of these had a concomitant middle ear effusion. In conclusion, only one child (1.6% of the sample) had an altered ABR. This child had shown one positive SARS-CoV-2 swab in the absence of risk factors for hearing loss.
Conclusion
This study finds no evidence that maternal COVID19 infection is a risk factor in the development of congenital hearing loss in newborns.
Keywords: Hearing loss, Newborn hearing screening, COVID19, SARS-CoV-2, Vertical transmission
1. Introduction
Severe Acute Respiratory Syndrome related CoronaVirus 2 (SARS-CoV-2) first broke out in the city of Wuhan in China at the end of 2019 and subsequently spread throughout the world affecting all age groups [1]. On 30 January 2020 it was declared a global health emergency of international concern and renamed Coronavirus Disease 2019 (COVID-19) [2].
COVID-19 infection can cause a wide spectrum of symptoms ranging from an asymptomatic infection to a severe interstitial pneumonia. Beside pulmonary involvement, it has been demonstrated that COVID19 can also affect the neurological system, with anosmia, hyposmia, hypogeusia, and dysgeusia being the most common symptoms [3]. There is some evidence that even the audio-vestibular system can be involved, as hearing loss, tinnitus, and dizziness were detected in patients with COVID-19 infection, but there is still some debate about this aspect and these findings have to be treated with some caution [4].
Furthermore, mother to fetus intrauterine transmission of COVID-19 infection in pregnant women is controversial. In fact, there are studies that find no evidence of vertical transmission [5], [6] while others suggest it is possible but unlikely, with approximately 3% of neonates born to mothers with COVID-19 reportedly testing positive for SARS-CoV-2 [7], [8], [9]. Finally, other reports suggest that it can be compared to a TORCH infection [10] as the infection rates are similar to those of other pathogens that cause congenital infections [11].
In the case of a neonatal infection, the most common symptoms in decreasing order of probability are: fever, gastrointestinal, respiratory, and neurological manifestations, with lung imaging being abnormal in 64% of cases. A lack of mother–neonate separation from birth is associated with late COVID19 infection, while breastfeeding is not [12].
There are few studies available regarding the audio-vestibular system of newborns with intrauterine COVID19 exposure. Celik et al. [13] found an insufficiency in the medial olivocochlear efferent system according to the TEOAE results, suggesting that cochlear functions should be examined in infants whose mothers have had COVID-19. Alan and Alan [14] demonstrated that testing positive for SARS-CoV-2 PCR in pregnancy is significantly associated with an increased risk of abnormal Newborn Hearing Screening results.
The study presented in this paper evaluates the hearing function of newborns of mothers diagnosed with COVID19 disease detected by nasopharyngeal swab and/or presence of specific immunoglobulin (IgM or IgG).
2. Material and methods
2.1. Study aim
The aim of this study is to verify the auditory function of children born to COVID19 positive mothers.
This study investigates the possible correlation between COVID19 gestational infection and hearing impairment onset in newborns. Hearing involvement of COVID19 will be verified so the timing and methodology of audiological evaluation of children can be planned.
2.2. Study design
This is a prospective observational study.
The study design is monocentric and conducted at the outpatient service of the ENT Department of the “Guglielmo da Saliceto” Hospital in Piacenza, Italy.
This study was registered with the Ethics Committee of the Area Vasta 1 Emilia Nord under number 2020/0073615; date of approval 23/06/2020.
Children born to COVID19-infected mothers were subject to newborn hearing screening and audiological evaluation.
Newborn hearing screening was performed using the Automatic Transient Evoked Otoacoustic Emissions test prior to hospital discharge.
Auditory evaluation was carried out by the child age of 4 months. All patients underwent the following evaluations during the audiological evaluation:
-
•
Maternal, pregnancy, and perinatal case history concerning maternal age, TORCH complex (Toxoplasmosis, Others, Rubella, Cytomegalovirus, Herpes simplex) infections during pregnancy and pathologies, use of drugs, birth weight, gestational age, gender, type of delivery, Apgar score, breastfeeding type, risk factors for hearing loss (according to JCHI 2019) [15], and newborn hearing screening results;
-
•
SARS-CoV-2 case history concerning gestational age of infection, symptomatology, use of drugs, number and result of the nasopharyngeal swabs, and serologic evaluation;
-
•
Otoscopy;
-
•
Acoustic Immittance (Tympanometry and Acoustic Reflex Measures) test;
-
•
Distortion Product Otoacoustic Emissions (DPOAE) test;
-
•
Auditory Brainstem Response (ABR) test.
2.3. Subjects
63 children born to mothers who had contracted COVID19 during pregnancy were included in the study. All children born from 15 February 2020 to 15 February 2021 in the “Guglielmo da Saliceto” Piacenza Hospital were included in the study.
COVID19 mother infection was established with at least one positive nasopharyngeal swab and/or presence of specific immunoglobulin (IgM or IgG) for SARS-CoV-2 infection.
Only children for whom both parents signed the Patient Informed Consent Form and whose mother signed her Patient Informed Consent Form prior to the first assessment were eligible for the clinical investigation.
Children older than 6 months at the time of audiological evaluation were excluded from the study.
2.4. Material
2.4.1. A-TEOAE test
The Automatic Transient Evoked Otoacoustic Emissions (A-TEOAE) test is used in the newborn hearing screening protocol at the “Guglielmo da Saliceto” Hospital. This test is performed in all neonates prior to hospital discharge.
A-TEOAE was carried out using the Madsen AccuScreen device (Natus Medical Incorporated, Denmark). Otoacoustic Emission was elicited by a click of 75 dB(A) ± 5 dB.
Stimuli and responses were recorded using a probe fitted to the ear with individual, disposable ear tips. The device automatically reported the test result for each ear as a PASS or REFER response. A PASS response was approximately the 30/35 dB HL hearing threshold.
2.4.2. Acoustic Immittance test
Acoustic immittance test was carried out using the Tympstar-Pro device (Grason-Stadler, USA).
Tympanometry and Acoustic Reflex Measures were taken at 1000 Hz probe tone frequency in accordance with the recommendation in the JCIH Position Statement (2019).
Tympanograms were classified in accordance to the Liden [16] and Jerger [17] classification system (tympanogram A, B, and C). Acoustic Reflex Measures were classified as Elicited or Non-Elicited.
2.4.3. DPOAE test
The test was carried out in a soundproof room using a MADSEN Capella2 device (Natus Medical Incorporated, Denmark).
The DPOAE were elicited by the simultaneous presentation of two pure tone signals to the ear.
The two signals differed in frequencies (the lower frequency f1 and the higher frequency f2) and intensity. The frequency ratio of f2/f1 was constant at 1.22 for all test frequencies and the stimulus levels were 65 dB SPL for the f1 and 55 dB SPL for f2.
The DPOAE test was applied at frequencies from 1000 to 4000 Hz. DPOAE were classified as Elicited or Non-Elicited for all frequencies tested.
2.4.4. ABR test
ABR test was used to identify the hearing threshold. A threshold less than or equal to 30 dB HL was considered to be normal hearing.
The test was performed in a sound treated booth free from excessive electrical noise using a Viking™ on Nicolet® EDX system (Natus Medical Incorporated, Denmark).
ABR is recorded while neonates are sleeping.
Three disposable electrodes are placed on the upper forehead (Fpz) (positive electrode), on the mastoid of the tested ear (negative electrode), and on a cheek (ground electrode). Electrode impedances were considered normal if the result was less than 5 kΩ between any electrode pair and within 1 kΩ across pairs.
The stimuli used were clicks. The stimulus rate was 21/s and 2000 sweeps were used. 100 Hz high-pass filter and 3000 Hz low-pass filter were applied.
2.5. Statistical analysis
A statistical test comparing the sample proportion of neonates with ABR scores below the threshold of 30 on the Joint Committee on Infant Hearing [15] indications of normal prevalence was used. Specifically, an exact (Clopper-Pearson) method binomial test, suitable for analyzing a small proportion, was carried out. Computations were carried out using the R software [18].
3. Results
3.1. Demographic and clinical characteristics
Sixty-three children were included in the study.
The mean maternal age at time of birth was 32 years (range 21–42 years). During pregnancy TORCH complex was evaluated in 58 mothers (92%) and three certainly had positivity: one for cytomegalovirus, one for toxoplasmosis, and one for syphilis infection. 12 mothers showed various diseases during pregnancy: gestational diabetes in 8 cases; hypothyroidism in 2 cases; hypertension in 2 mothers, 2 with vaginal infection, and only one had thrombophilia. 38% (n = 24) of the mothers took medication during pregnancy. In particular, nonsteroidal anti-inflammatory drugs in 4 cases, antibiotics in 4 cases, levothyroxine sodium in 8 subjects, insulin in 4, an antidepressant in 2, ursodeoxycholic acid in 1, there was 1 case of enoxaparin, and 1 case of an immunomodulator.
Table 1 reports the perinatal case history information regarding type of delivery, gestational age, birth weight, Apgar score, gender, and type of breastfeeding.
Table 1.
Perinatal case-history information.
| Birth weight (g) | mean 3281.5 (range 2270–4250) | |
|---|---|---|
| Gestational age (weeks) | mean 39.3 (range 35–41) | |
| Gender | Female | 36 |
| Male | 27 | |
| Delivery | Cesarean | 18 |
| Vaginal | 45 | |
| Apgar score | 1' | Mean 9.2 (range 6–10) |
| 5' | Mean 9.9 (range 8–10) | |
| Breastfeeding | Maternal | 40 |
| Artificial | 9 | |
| Mixed | 14 | |
Eleven neonates showed problems in perinatal period that did not require additional days of hospitalization. In particular, 4 neonates showed hyperbilirubinemia, 5 respiratory diseases, and had 2 cardiac problems.
3.2. COVID19 infection
Diagnosis of COVID19 maternal infection was based on at least one positive nasopharyngeal swab and/or on the presence of specific immunoglobulin (IgM or IgG) for SARS-CoV-2 infection. 35 mothers had a positive swab only (22 one, 10 two, and 3 more than three swabs), 2 had a positive swab and IgM positivity, 6 had a positive swab and IgG positivity, and 20 IgG positivity only.
63.5% (n = 40) of the mothers contracted COVID19 infection during their third trimester of pregnancy, 4.8% (n = 3) and 7.9% (n = 5) had infection in the second and first trimester, respectively. In 15 cases the period of infection could not be identified with any certainty because these mothers showed only an increase in IgG (Fig. 1 )
Fig. 1.
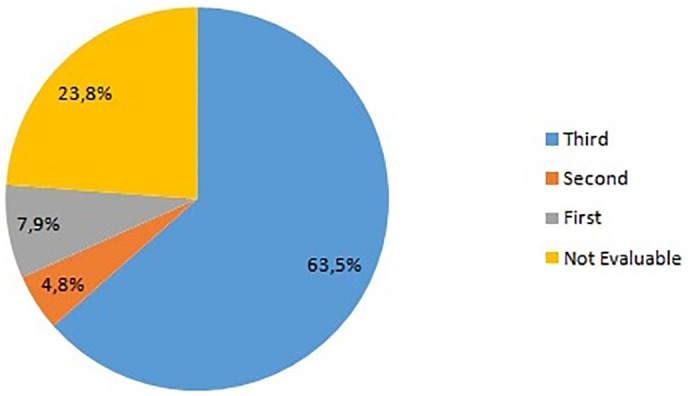
Graphical representation of percentage of pregnancy trimester in which COVID19 infection was diagnosed.
Fig. 2 shows the maternal COVID19 symptoms. 68.2% of the subjects did not show symptomatology and the infection diagnosis was based on at least one positive nasopharyngeal swab and/or on presence of specific immunoglobulin (IgM or IgG) for SARS-CoV-2 infection. In the cases of symptomatic infection, the most frequent symptoms were ageusia, anosmia, and fever followed by cough and pneumonia (Fig. 2).
Fig. 2.
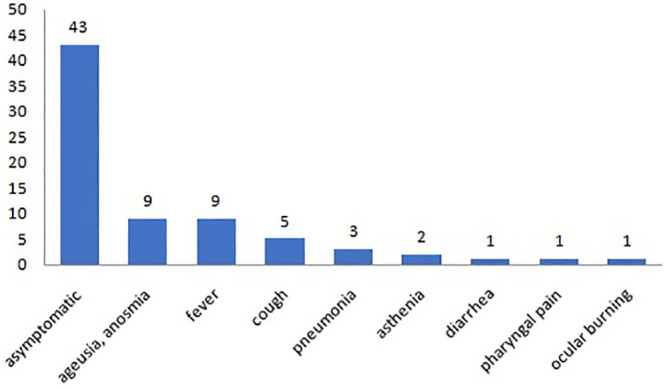
Symptomatology of the affected mothers.
Therapeutic indications for COVID19 infection changed greatly between the start and end of the recruitment of the subjects in this study. The mothers were treated regardless of the symptomatology existing at the beginning of the study whereas in the second and final part of the recruitment only the mothers with specific symptoms were treated with symptomatic drugs. In general, 17 mothers took medication for the infection. The primary medications used, in combination or alone, were: hydroxychloroquine (13), antibiotics (6), nonsteroidal anti-inflammatory drugs (6), and heparin (5).
All neonates were subjected to nasopharyngeal swab at birth. Only two children had one positive swab in the absence of COVID19 symptomatology.
3.3. Newborn hearing screening outcomes
Nine newborns showed risk factors for neonatal hearing loss (risk factors defined in agreement with the Joint Committee on Infant Hearing 2019 [15]). These risk factors were 2 ototoxic drug administration, 3 hearing loss in family history, 3 congenital infections (1 cytomegalovirus, 1 toxoplasmosis, and 1 syphilis), and 1 consanguinity of the parents.
82.5% (n = 52) of the children were subjected to a newborn hearing screening program using the A-TEOAE test prior to hospital discharge. The remaining 11 newborns were not subjected to hearing screening for isolation measures but underwent the audiological evaluation directly
Only one of 52 screened neonates showed a bilateral REFER test result.
3.4. Audiological evaluation
All of the children underwent an audiological evaluation irrespective of the newborn hearing screening results.
Audiological evaluation of the 63 children was performed at mean age of 100.4 days of life (range 44–136).
Acoustic immittance measurements showed: 58 bilateral Type A tympanograms and elicited acoustic reflex, 3 bilateral type B tympanograms and non-elicited acoustic reflex, 1 subject with only right A tympanogram with elicited acoustic reflex (left not executable measurements), and one child showed right B and left A tympanograms with right non-elicited and left elicited acoustic reflex.
The three children with type B tympanograms showed non-elicited DPOAE. Subject with discordant tympanogram in the two sides and subject with non-executable tympanogram showed elicited DPOAE. Bilateral elicited DPOAE was measured in 56 subjects by using bilateral type A tympanograms. Of the remaining two children, DPOAE was non-elicited bilateral in one and non-executable on right side in the other.
Bilateral ABR thresholds were equal to 30 dB HL in 59 children. Two subjects showed a bilateral threshold higher than 30 dB (50–50 and 40–40 dB HL, respectively) but ABR retest, performed within 1 month, was normal. Only two children showed monolateral threshold modification: one case left threshold of 50 dB HL with middle ear (type B tympanogram) and DPOAE alterations, and one case right threshold of 50 dB HL without middle ear or DPOAE alterations. The latter child had shown one positive SARS-CoV-2 swab in the absence of risk factors for hearing loss.
The statistical evaluation, based on evaluation of total number of ABR modification compared to total of live births showed that the null hypothesis of a proportion in line with the reference Joint Committee on Infant Hearing [15] indications is not rejected as the estimated 95% confidence interval (Clopper-Pearson method) ranges from 0.0004 to 0.0880.
The neonate with bilateral REFER result at hearing screening showed normal audiological evaluation with a normal bilateral ABR thresholds (bilateral 30 dB HL) and DPOAE bilateral elicited for all frequencies tested.
Audiological evaluation of the two children with positive swabs showed one case of normal bilateral hearing ABR threshold and one with monolateral threshold modification with type A tympanogram and use of maternal hydroxychloroquine medication.
4. Discussion
Recent studies agree that COVID19 infection is a risk factor in the development of ear problems. Inflammatory processes with excessive production of reactive oxygen species, ototoxicity by antiviral treatments, and hematogenous alterations with hemoglobin attachment and erythrocyte penetration are some of the mechanisms described as being involved in auditory and vestibular alterations [4].
The literature is divided on the possibility of vertical transmission of COVID19, but some studies describe expression of ACE2 and presence of virions in the placenta [19].
Moreover, children have a lower incidence of COVID19 infection and less severe clinical symptoms compared to adults but infection in newborns is also possible [20].
The study presented in this paper evaluated the auditory function of newborns born to COVID19 positive mothers. Only one child (1.9%) showed bilateral REFER results at newborn hearing screening evaluation (prior to hospital discharge).
This data agrees with the Joint Committee on Infant Hearing (2019) [15] indications that describe a reference standard of less than 4% for newborn that do not pass the first step of screening.
On the contrary, the study of Alan and Alan [14] showed that children born to mothers with COVID19 infection had more REFER results compared to the control group. In the Alan and Alan study newborn hearing screening was carried out using automatic ABR test whereas in the study presented in this paper screening was performed using the A-TOAE test. These methodologies evaluate two different aspects of hearing transmission. In particular, otoacustic emission evaluates the functionality of the outer hair cells whereas ABR evaluates cochlear nerve transmission. Recent publications in the literature report that COVID19 infection can affect the peripheral nervous system (PNS) and in particular brainstem cranial nerves and nuclei [21], [22]. COVID19 infection can affect the PNS in two ways: firstly, by direct invasion through downregulation of ACE2 and over-reaction of Renin-Angiotensin-pathway, or secondly, by systemic immune response [22].
Only one paper describes using otoacustic emission to evaluate infants born to mothers with COVID19 infection during pregnancy [13]. In this study three types of otoacustic emission (TEOAE, DPOAE, and controlateral suppression of OAE) were performed on all children. The authors showed that there was a statistically significant difference in TEOAE and controlateral suppression OAE test in patients compared to controls (mothers without infection). In the study presented in this paper the children performed both TEOAE and DPOAE but in two different moments of their life (TEAOE prior to hospital discharge and DPOAE within the child age of 4 months). In contrast to Celik and colleagues, the authors of the present study did not find any alterations in both of the tests. It is not possible to compare the result of the study presented in this paper with those of Celik group because the latter study does not state the age when the infants were evaluated.
In support of this, Dror et al. showed that adults positive for COVID19 infection did not have significant differences between healthy individuals in TEOAE, DPOAE, and ABR tests [23].
Audiological evaluation of the sample studied in the present paper showed four children (6.3% of the total) with ABR threshold alterations but two showed normal threshold at ABR retest within 1 month of the first. The other two infants showed monolateral ABR alterations but one of these had a concomitant middle ear effusion. In conclusion, only one child (1.6% of the sample) had an altered ABR.
The study by Alan and Alan reports a higher incidence of REFER results at ABR test in newborns with positive mothers compared to those without positive mothers (53/118 vs 28/118). This test was performed within 2 weeks of birth. On the contrary, the second ABR test, performed at 4 weeks after birth, did not show difference between groups [14]. In the study presented in this paper the median age of audiological evaluation was approximately 3 months of life. In addition, the four infants with ABR alterations are older than 3 months. Therefore, this data agrees with the results reported by Alan and Alan in their second ABR evaluation. It is possible that COVID19 infection shows auditory neurological involvement only in the first period of life.
In addition, as reported by Muldoon and colleagues, COVID19 infection can be compared with TORCH infections [10]. It is widely reported that uterine TORCH infections have risk factors for hearing loss to develop in children. In particular, the degree of hearing impairment caused by CMV infection is correlated with the viral load and the trimester of mother infection [24].
The COVID19 infection in the sample studied in this paper is in the third trimester in 63.5% of cases. There was no correlation between the four infants with ABR threshold alteration and trimester of infection because three cases were infected in the third trimester and one in the first trimester.
On the contrary, in the study of Alan and Alan 2021 a correlation between trimester of infection and newborn hearing screening results is reported. Children born to mothers who have positive swab in the second trimester had more REFER results compared with the third trimester [14].
It is also reported in the literature that chloroquine and hydroxychloroquine medication have ototoxic effect. In the sample studied in this paper 13 mothers had taken hydroxychloroquine for the COVID19 infection and in two cases children developed ABR modifications but in one case retest ABR was normal. As reported in the study of Alan and Alan [14], the present study could not find a correlation between the use of these drugs and auditory alterations in newborn.
The limited sample size of mothers with COVID19 infection in the first and second trimester restricts the study presented in this paper. For pregnant women infected during their first and second trimesters, further studies focusing on long-term outcomes are needed.
It was not necessary to compare the results presented in this paper with a control group because the prevalence of hearing impairments in the general population is well known in the literature.
5. Conclusion
In the study presented in this paper 6.3% of the infants showed alteration in auditory evaluation and only one of these confirmed the auditory threshold modifications. This data is comparable with global incidence of congenital hearing loss in developed country [25].
In conclusion,.in this study we did not detect a significant correlation between maternal COVID19 infection and congenital hearing loss. More data is needed on early infection since that is typically when there may be possible increased risks of hearing problems.
This study is also a picture of the auditory capacities in the first months of life. Future studies are needed to evaluate the possible long-term auditory sequelae of this global pandemic.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We would like to thank prof. Enrico Fabrizi for the statistical considerations.
We would like, in advance, to thank all children and their families who will take the time to undergo evaluations throughout the study.
Footnotes
Area Vasta = Association of Local Health Authority Districts
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in WuhanChina. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2020. Rolling updates on coronavirus disease (COVID-19)https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-theyhappen [Google Scholar]
- 3.Romána G.C., Spencerc P.S., Reisd J., Buguete A., El Alaoui Farisf M., Katrakg S.M., et al. The neurology of COVID-19 revisited: a proposal from the environmental neurology specialty Group of the World Federation of neurology to implement international neurological registries. J Neurol Sci. 2020;414 doi: 10.1016/j.jns.2020.116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafari Z., Kolb B.E., Mohajerani M.H. Hearing loss, tinnitus, and dizziness in COVID-19: a systematic reviewand meta-analysis. Can J Neurol Sci. 2021;00:1–12. doi: 10.1017/cjn.2021.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z., Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am J Perinatol. 2020;37:1055–1060. doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karimi-Zarchi M., Neamatzadeh H., Alireza Dastgheib S., Abbasi H., Mirjalili S.A., Behforouz A., et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020;39:246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. 2020;11:5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do-Hyun K. Clinical implications of coronavirus disease 2019 in neonates. Clin Exp Pediatr. 2021;64:157–164. doi: 10.3345/cep.2020.01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Oliveira L.V., Costa Azevedo, da Silva C.R., Peixoto Lopes L., Rodrigues Agra I.K. Current evidence of SARS-CoV-2 vertical transmission: an integrative review. Rev Assoc Med Bras. 2020;66(Suppl 2):130–135. doi: 10.1590/1806-9282.66.S2.130. [DOI] [PubMed] [Google Scholar]
- 10.Muldoon K.M., Fowlerb K.B., Peschc M.H., Schleissd M.R. SARS-CoV-2: is it the newest spark in the TORCH? J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11:5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celik T., Simsek A., Koca C.F., Aydin S., Yasar S. Evaluation of cochlear functions in infants exposed to SARS-CoV-2 intrauterine. Am J Otolaryngol. 2021;42 doi: 10.1016/j.amjoto.2021.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alan M.A., Alan C. Hearing screening outcomes in neonates of SARS-CoV-2 positive pregnant women. Int J Ped Otorhinolaryngol. 2021;146 doi: 10.1016/j.ijporl.2021.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jchi Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Earl Hear Detec Intervent. 2019;2019(4):1–44. doi: 10.15142/fptk-b748. [DOI] [Google Scholar]
- 16.Liden G. The scope and application of current audiometric tests. J Laryngol Otol. 1969;83:507–520. doi: 10.1017/s0022215100070651. [DOI] [PubMed] [Google Scholar]
- 17.Jerger J.F. Clinical experience with impedence audiometry. Arch Otolaryngol. 1970;92:311–324. doi: 10.1001/archotol.1970.04310040005002. [DOI] [PubMed] [Google Scholar]
- 18.Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2018. R: a language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 19.Zhang H., Zhang H. Entry, egress and vertical transmission of SARS-CoV-2. J Mol Cell Biol. 2021;13:168–174. doi: 10.1093/jmcb/mjab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rose D.U., Piersigilli F., Ronchetti M.P., Santisi A., Bersani I., Dotta A., et al. The study Group of Neonatal Infectious Diseases of the Italian Society of Neonatology (SIN), novel coronavirus disease (COVID-19) in newborns and infants: what we know so far. It J Ped. 2020;46:56. doi: 10.1186/s13052-020-0820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leven Y., Bösel J. Neurological manifestations of COVID-19 - an approach to categories of pathology. Neurol Res Pract. 2021;3:39. doi: 10.1186/s42466-021-00138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dror A.A., Kassis-Karayanni N., Oved A., Daoud A., Eisenbach N., Mizrachi M., et al. Auditory performance in recovered SARS-COV-2 patients. Otol Neurotol. 2021;42:666–670. doi: 10.1097/MAO.0000000000003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Gomez E., Perez-Carpena P., Flook M., Lopez-Escamez J.A. A systematic review on the Association of Acquired Human Cytomegalovirus Infection with hearing loss. J Clin Med. 2020;9:4011. doi: 10.3390/jcm9124011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal Y., Platz E.A., Niparko J.K. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and nutrition examination survey, 1999–2004. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]


