Abstract
Background:
A novel paediatric disease, multi-system inflammatory syndrome in children, has emerged during the 2019 coronavirus disease pandemic.
Objectives:
To describe the short-term evolution of cardiac complications and associated risk factors in patients with multi-system inflammatory syndrome in children.
Methods:
Retrospective single-centre study of confirmed multi-system inflammatory syndrome in children treated from 29 March, 2020 to 1 September, 2020. Cardiac complications during the acute phase were defined as decreased systolic function, coronary artery abnormalities, pericardial effusion, or mitral and/or tricuspid valve regurgitation. Patients with or without cardiac complications were compared with chi-square, Fisher’s exact, and Wilcoxon rank sum.
Results:
Thirty-nine children with median (interquartile range) age 7.8 (3.6–12.7) years were included. Nineteen (49%) patients developed cardiac complications including systolic dysfunction (33%), valvular regurgitation (31%), coronary artery abnormalities (18%), and pericardial effusion (5%). At the time of the most recent follow-up, at a median (interquartile range) of 49 (26–61) days, cardiac complications resolved in 16/19 (84%) patients. Two patients had persistent mild systolic dysfunction and one patient had persistent coronary artery abnormality. Children with cardiac complications were more likely to have higher N-terminal B-type natriuretic peptide (p = 0.01), higher white blood cell count (p = 0.01), higher neutrophil count (p = 0.02), severe lymphopenia (p = 0.05), use of milrinone (p = 0.03), and intensive care requirement (p = 0.04).
Conclusion:
Patients with multi-system inflammatory syndrome in children had a high rate of cardiac complications in the acute phase, with associated inflammatory markers. Although cardiac complications resolved in 84% of patients, further long-term studies are needed to assess if the cardiac abnormalities (transient or persistent) are associated with major cardiac events.
Keywords: Paediatric multisystem inflammatory syndrome temporally associated with 2019 coronavirus disease, multi-system inflammatory syndrome in children, 2019 coronavirus disease pandemic, paediatric cardiology, myocarditis, coronary artery
The 2019 coronavirus disease pandemic has been anything but predictable.1 Early in the pandemic, due to the Bayesian thinking, there was a concern for missed or delayed diagnosis of other diseases including Kawasaki disease.2 To our dismay, a novel paediatric disease termed multi-system inflammatory syndrome in children emerged with some patients having features resembling Kawasaki disease.3–11 Earlier reports from Europe and New York described high rates of cardiac complications including extracorporeal membrane oxygenation utilisation and death.12–17 Washington, District of Columbia metropolitan epicentre, had a 2019 coronavirus disease surge later than Europe or New York and thus multi-system inflammatory syndrome in children at our centre appeared later allowing for prompt protocolisation and standardisation of care.18 In this article, we report cardiac findings of multi-system inflammatory syndrome in childrenfrom a single large tertiary paediatric cardiac centre in Washington, District of Columbia. Our aims were to describe the type and frequency of cardiac complications in patients with multi-system inflammatory syndrome in children, the short-term progression and to assess demographics, clinical and laboratory findings, and therapies associated with cardiac complications in patients with multi-system inflammatory syndrome in children.
Materials and methods
This was a retrospective, observational study of all patients admitted to Children’s National Hospital from 29 March, 2020 to 1 September, 2020 who met the Centers for Disease Control and Prevention multi-system inflammatory syndrome in children case definition.19 Patient demographics, clinical features, laboratory values, diagnostic investigations (including echocardiograms), and therapies were extracted from the electronic medical records. To assess cardiac complication progression, a retrospective review of electronic medical records was carried out. Review of cardiac findings follow-up is current as of 8 February, 2021.
Shortly after the emergence of the (multi-system inflammatory syndrome in children) illness, a multi-disciplinary committee (Children’s National Hospital multi-system inflammatory syndrome in children task force) was created with representation from allergy/immunology, cardiology, critical care, emergency medicine, clinical pharmacy, gastroenterology, haematology, hospitalist medicine, infectious disease, laboratory medicine, rheumatology, and neurology. At the onset, the task force met three times per week for planning purposes and eventually this was scaled down to once a week. During the meeting, all potential cases and plan of care were reviewed. The multi-system inflammatory syndrome in children task force developed institutional guidelines for diagnosis and management including initiating prompt intravenous immunoglobulin as the first-line therapy, establishing a multi-system inflammatory syndrome in children order set, formulating a parent handout and staffing a common email to answer queries by Children’s National Hospital Faculty and staff as well as community paediatricians and nurse practitioners. The treatment algorithm followed by our institution is unique in its use of Anakinra as an adjunct or second-line therapy rather than corticosteroids partly due to local experience with viral myocarditis. This decision was also informed by systematic meta-analyses and Cochrane review of viral myocarditis treatment that did not support routine use of corticosteroids.20,21 In addition, earlier reports cautioned against the use of corticosteroids in patients with 2019 coronavirus disease.22 As evidence evolved, the algorithms were updated including corticosteroids as a third-line agent.23,24 Furthermore, Anakinra was considered a good choice due to experience using it in systemic juvenile idiopathic arthritis, macrophage activation syndrome, haemophagocytic lymphohistiocytosis, Kawasaki disease, sepsis trials, and adult with 2019 coronavirus disease.25 It has a short half-life that enables early initiation in sick patients when other infectious diagnoses have not been completely ruled out.
The multi-system inflammatory syndrome in children task force held a daily huddle, which was held on a virtual online platform, with the primary care team to better coordinate care and communication across all the specialists. The huddle was organised by the primary hospitalist medicine team taking care of active patients with multi-system inflammatory syndrome in children and attended by all consulting services. Discussions on second-line immune modulation therapy, weaning of therapies, timing of follow-up echocardiogram and any other radiology studies, and discharge planning were discussed for each patient.24 Members of the multi-system inflammatory syndrome in children task force developed a standardised discharge process that included followup in-person with cardiology and via telemedicine with rheumatology and haematology. In addition, the cardiology evaluation during and after hospital discharge was standardised.
Cardiac complications during the acute phase were defined as the presence of at least one of the following: decreased ventricular systolic function if ejection fraction was <55% or shortening fraction <28% (this was further classified into mild ventricular systolic dysfunction for ejection fraction between 40 and 54%, moderate for ejection fraction between 30 and 39%, and severe for ejection fraction <30%);26,27 coronary artery abnormalities defined as (a) dilation (z score 2–2.4), (b) small aneurysm (z score 2.5–4.9);28,29 more than trivial pericardial effusion; and atrioventricular valve regurgitation defined as more than trivial mitral valve regurgitation and/or more than mild tricuspid valve regurgitation.
At this centre, for patients with multi-system inflammatory syndrome in children, the left main coronary, left anterior descending coronary, and right coronary arteries were routinely measured and z score calculated.29 The team has benefited from participating in a multicentre quality improvement project through the American College of Cardiology to ensure measurement and calculation of z scores in children with Kawasaki disease.30 Ejection fraction was calculated using the Simpson’s rule.31 When assessing valvular regurgitation, the team followed the American Society of Echocardiography guidelines.32 The echocardiogram laboratory has a system in place to assess the proximal isovelocity surface area and the effective regurgitant orifice area.32
Statistical analysis
The cohort was divided into two groups based on presence or absence of cardiac complications. The analysis focused on a comparison of these two groups across demographics, clinical features including fulfilling Kawasaki disease diagnostic criteria, laboratory findings, therapy received, and clinical outcomes. All data were summarised using descriptive statistics of the median and interquartile range and frequency/crosstabs depending on data type. Continuous variables were analysed using the nonparametric Wilcoxon rank sum test as normality was not assumed. Categorical variables were analysed using the chi-square and Fisher’s exact test. A p value ≤ 0.05 was considered statistically significant. All analyses were performed using STATA version 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp. LLC.).
Children’s National Hospital Institutional Review Board approved this study with a waiver of consent granted. Author A.S.H. had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Thirty-nine children met the Centers for Disease Control and Prevention case definition of multi-system inflammatory syndrome in Children and were included in this study. The median (interquartile range) age was 7.8 (3.6–12.7) years. Eighteen (46%) were female. An underlying medical diagnosis was noted in 13 (33%) patients (Table 1). Thirty-one (79%) required admission to the critical care unit. None of the children required the use of extracorporeal membrane oxygenation and there were no deaths. All patients were discharged home with a median (interquartile range) length of stay of 12 (8–14) days. Twenty (51%) were non-Hispanic Black, 17 (44%) were Hispanic, and 0 were non-Hispanic White. Concurrently, 5262 patients with non-multi-system inflammatory syndrome in children were admitted to Children’s National Hospital during the same period (29 March, 2020–21 September, 2020); 1652 (31.4%) were Non-Hispanic Black, 1136 (21.6%) were Hispanic, and 1387 (26.4%) were non-Hispanic White (p < 0.01).
Table 1.
Comparison of demographics, patient characteristics, and presenting symptoms stratified by presence of cardiac complications.
| Variable of interest | All cases (n = 39) | Cardiac complications present (n = 19) | Cardiac complications absent (n = 20) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | p-value | |
| Age in years (median and IQR) | 7.8 (3.612.7) | 8.8 (3.7–13.7) | 6.7 (4.1–10.9) | 0.25 |
| Specific age bands | ||||
| <1 | 0 (0%) | 0 (0%) | 0 (0%) | 0.59 |
| 1–<5 | 13 (33%) | 7 (37%) | 6 (30%) | |
| 5– <10 | 13 (33%) | 5 (26%) | 8 (40%) | |
| 10– <15 | 11 (28%) | 5 (26%) | 6 (30%) | |
| 15+ | 2 (5%) | 2 (11%) | 0 (0%) | |
| Sex (% female) | 18 (46%) | 7 (37%) | 11 (55%) | 0.26 |
| Race/ethnicity | ||||
| Non-Hispanic Black | 20 (51%) | 11 (58%) | 9 (45%) | 0.18 |
| Non-Hispanic White | 0 (0%) | 0 (0%) | 0 (0%) | |
| Hispanic | 17 (44%) | 6 (32%) | 11 (55%) | |
| Other/unknown | 2 (5%) | 2 (11%) | 0 (0%) | |
| Insurance | ||||
| Private | 5 (13%) | 2 (11%) | 3 (15%) | 1 |
| Public | 32 (82%) | 16 (84%) | 16 (80%) | |
| Unknown or self-pay | 2 (5%) | 1 (5%) | 1 (5%) | |
| Any underlying condition | 13 (33%) | 8 (42%) | 5 (25%) | 0.32 |
| Symptoms at presentation | ||||
| Fever | 39 (100%) | 19 (100%) | 20 (100%) | |
| Any respiratory (cough, SOB, rhinorrhea, congestion) | 16 (41%) | 9 (47%) | 7 (35%) | 0.52 |
| Abdominal pain | 32 (82%) | 15 (80%) | 17 (85%) | 0.7 |
| Rash | 17 (44%) | 9 (47%) | 8 (40%) | 0.75 |
| Conjunctival injection | 24 (62%) | 15 (79%) | 9 (45%) | 0.05 |
| Peripheral extremity changes or swelling or erythema | 8 (21%) | 4 (21%) | 4 (20%) | 1 |
| Mucous membrane changes (strawberry tongue and lip erythema/cracking) | 25 (64%) | 15 (79%) | 10 (50%) | 0.1 |
| Kawasaki disease | ||||
| Meets Kawasaki disease diagnostic criteria (complete and incomplete) | 18 (46%) | 12 (63%) | 6 (30%) | 0.06 |
| Meets complete Kawasaki disease criteria | 6 (33%) n = 18 | 3 (25%) n = 12 | 3 (50%) n = 6 | 0.34 |
IQR = interquartile range.
Statistically significant differences are marked by bold rows.
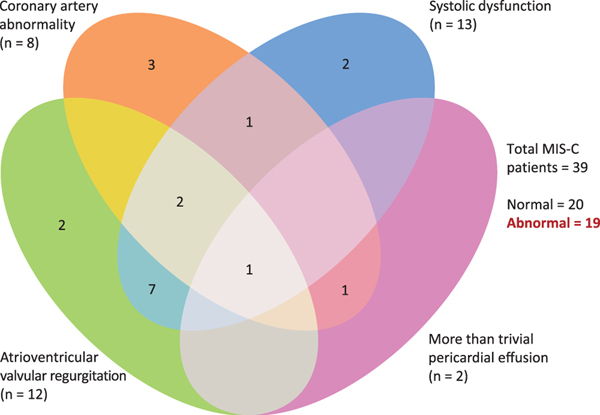
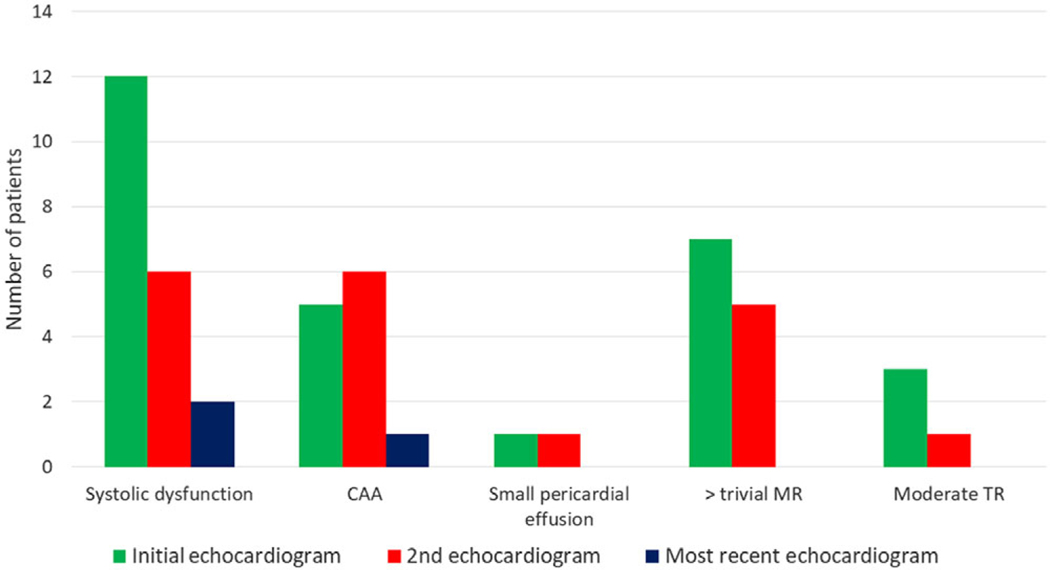
Nineteen (49%) patients developed cardiac complications during their hospitalisation. Mild systolic dysfunction (ejection fraction of 47 ± 4.7) was seen in 13 (33%) patients, atrioventricular valve regurgitation was seen in 12 (31%) patients (worst grade was mild mitral valve regurgitation (n = 5), moderate mitral valve regurgitation (n = 4), moderate tricuspid valve regurgitation (n = 2) and one patient had a combination of mild mitral valve regurgitation and moderate tricuspid valve regurgitation), coronary artery abnormalities were noted in 8 (21%) patients (isolated left main coronary artery dilation (n = 3), isolated left anterior descending coronary artery dilation (n = 2) and dual coronary artery involvement (n = 3) as outlined below), and small pericardial effusion was seen in 2 (5%) patients. Figure 1 shows the distribution of cardiac complications. Figure 2 displays the progression of cardiac complications. One patient had a small left main coronary artery aneurysm (z score +2.9) and left anterior descending coronary artery dilation (z score +2.1) that subsequently resolved. Another patient developed a small left main coronary artery aneurysm (z score +2.6) and left anterior descending coronary artery dilation (z score +2.1) on the second echocardiogram that later resolved. A third patient developed small left main coronary artery and right coronary artery aneurysms (z scores +2.8 and +2.6, respectively) that decreased in size and were noted as only dilation (z score +2.3) at last follow-up after a median (interquartile range) of 54 (31.5–55.5) days.
Figure 1.
Distribution of cardiac complications in multi-system inflammatory syndrome in children.
Figure 2.
Progression of cardiac complications. CAA = coronary artery abnormality; MR = mitral valve regurgitation; TR = tricuspid valve regurgitation.
At the time of the most recent follow-up, at a median (interquartile range) of 49 (26–61) days, cardiac complications resolved in 16/19 (84%) patients. Two patients had persistent mild systolic dysfunction and one patient had persistent coronary artery abnormality (dilation, z score +2.3). Pericardial effusion, mitral valve regurgitation, and tricuspid valve regurgitation resolved in all patients (Fig 2).
As shown in Table 1, conjunctival injection was more likely to be seen among children who developed cardiac complications (15 (79%) versus 9 (45%), p = 0.05). All other demographics, patient characteristics, and presenting symptoms were not different between the groups including fulfilling Kawasaki disease diagnostic criteria.
The maximal N-terminal B-type natriuretic peptide (pg/ml) was higher in children with cardiac complications compared to those without (14,396 (8814–20,158) versus 3205.5 (1466–10,625), p = 0.01). Initial white blood cell counts (K/mcl) were higher in patients with multi-system inflammatory syndrome in children who developed cardiac complications compared to those who did not (11.32 (9.08–17.59) versus 7.225 (5.34–8.81), p = 0.01). A similar finding was seen with initial neutrophils (K/mcl) (74.5 (68–80) versus 63 (38–71), p = 0.02). Minimal lymphocytes (K/mcl) were lower in children with cardiac complications compared to those without (4 (3–13) versus 10 (8–13), p = 0.05). All other laboratory values were not statistically different between the groups including percentage of children who were severe acute respiratory syndrome coronavirus-2 polymerase chain reaction positive and troponin level (Table 2).
Table 2.
Comparison of laboratory results among multi-system inflammatory syndrome in children cohort stratified by presence of cardiac complications.
| Variable of interest | All cases (n = 39) | Cardiac complications present (n = 19) | Cardiac complications absent (n = 20) | p-value |
|---|---|---|---|---|
| Medians and IQR | Medians and IQR | Medians and IQR | ||
| SARS-CoV-2 (% PCR positive) (n (%)) | 20 (51%) | 9 (47%) | 11 (55%) | 0.75 |
| SARS-CoV-2 (% antibody positive) (n (%)) | 36 (97%) (n = 37) | 17 (100%) (n = 17) | 19 (95%) | 0.54 |
| Creatinine - initial (mg/dl) | 0.49 (0.40–0.77) | 0.59 (0.42–1.20) | 0.48 (0.39–0.55) | 0.15 |
| Creatinine - max (mg/dl) | 0.54 (0.43–0.83) | 0.63 (0.43–1.20) | 0.52 (0.44–0.64) | 0.13 |
| BUN - initial (mg/dl) | 11 (8–15) | 13 (8–21) | 9.5 (7.5–11.5) | 0.09 |
| BUN - max (mg/dl) | 17 (14–22) | 19 (15–25) | 15.5 (13–18.5) | 0.09 |
| CRP - initial (mg/dl) | 14.15 (10.37–18.73) | 13.4 (8.99–19.12) | 14.92 (11.49–17.60) | 0.76 |
| CRP - max (mg/dl) | 17.29 (12.52–22.20) | 16.85 (11.40–23.02) | 17.60 (14.90–21.65) | 0.63 |
| LDH - initial (unit/L) | 287.5 (234–349) (n = 38) | 292 (256–349) (n = 18) | 287.5 (228–342) | 0.54 |
| LDH - max (unit/L) | 362.5 (310–491) (n = 38) | 383 (331–636) (n = 18) | 343 (304–459.5) | 0.18 |
| Ferritin - initial (ng/L) | 314.5 (242–566) (n = 38) | 432 (249–598) (n = 18) | 313.5 (225.5–496.5) | 0.29 |
| Ferritin - max (ng/L) | 528 (340–855) (n = 38) | 688 (414–937) (n = 18) | 407.5 (321.5–618.5) | 0.07 |
| Fibrinogen - initial (mg/dl) | 495 (397–601) | 511 (426–565) | 476.5 (396.0–622.5) | 0.75 |
| Fibrinogen - max (mg/dl) | 518 (426–645) | 551 (485–683) | 477 (403.0–622.5) | 0.12 |
| Hemoglobin - initial (gm/dl) | 11.5 (10.3–12.6) | 11.8 (10.4–13.3) | 11.2 (10.15–12.1) | 0.17 |
| Hemoglobin - min (gm/dl) | 8.4 (7.3–9.2) | 8.4 (7.2–9.2) | 8.3 (7.4–9.15) | 0.99 |
| WBC - initial (K/mcl) | 9.08 (5.88–15.34) | 11.32 (9.08–17.59) | 7.225 (5.34–8.81) | 0.01 |
| WBC - min (K/mcl) | 4.74 (4.16–5.58) | 4.7 (4.43–5.55) | 4.9 (3.565–5.615) | 0.79 |
| WBC - max (K/mcl) | 15.34 (10.57–20.75) | 16.78 (11.47–23.95) | 13.07 (9.525–18.065) | 0.17 |
| Manual lymphocyte - initial (K/mcl) | 11 (4–23) (n = 35) | 11 (3–19) (n = 18) | 13 (8–25) (n = 17) | 0.24 |
| Manual lymphocyte - min (K/mcl) | 8 (3–13) (n = 35) | 4(3–13) (n = 18) | 10 (8–13) (n = 17) | 0.05 |
| Manual Lymphocyte - max (K/mcl) | 48 (36–69) (n = 35) | 48.5 (36–62) (n = 18) | 47 (37–69) (n = 17) | 0.83 |
| Segmented neutrophil - initial (K/mcl) | 71 (59–79) (n = 35) | 74.5 (68–80) (n = 18) | 63 (38–71) (n = 17) | 0.02 |
| Segmented neutrophil - min (K/mcl) | 34 (18–52) (n = 35) | 35 (24–44) (n = 18) | 34 (17–52) (n = 17) | 0.29 |
| Segmented neutrophil - max (K/mcl) | 80 (71–87) (n = 35) | 83 (72–89) (n = 18) | 76 (64–82) (n = 17) | 0.17 |
| Eosinophil - initial (K/mcl) | 2 (1–3) (n = 34) | 2 (1–3) (n = 18) | 2 (1–3) (n = 16) | 0.89 |
| Eosinophil - max (K/mcl) | 4 (3–8) (n = 34) | 4 (3.0–8.0) (n = 18) | 3.5 (2.5–8.0) (n = 16) | 0.94 |
| Platelet count - initial (K/mcl) | 162 (126–236) | 164 (151–272) | 153 (102–234) | 0.23 |
| Platelet count - min (K/mcl) | 147 (99–187) | 151 (127–187) | 127.5 (76–183) | 0.19 |
| Platelet count - max (K/mcl) | 612 (509–700) | 547 (495–698) | 616 (521.5–710) | 0.55 |
| ALT - initial (unit/L) | 29 (17–53) | 25 (17–43) | 37 (18–74) | 0.27 |
| ALT - max (unit/L) | 46 (28–77) | 46 (27–69) | 48 (31–106) | 0.87 |
| AST - initial (unit/L) | 39 (21–60) | 37 (27–57) | 42.5 (17–74) | 0.88 |
| AST - max (unit/L) | 60 (43–102) | 60 (43–105) | 60 (44.5–84.5) | 0.86 |
| PTT activated - initial (second) | 35.75 (33.5–40.2) (n = 38) | 35.75 (33.6–38.5) (n = 18) | 36.75 (33.1–42.2) | 0.49 |
| PTT activated - max (second) | 44.3 (38.9–48.3) (n = 38) | 44.35 (38.7–51.9) (n = 18) | 44.2 (39.35–47.75) | 0.98 |
| Prothrombin time - initial (second) | 15.65 (14.7–17) (n = 38) | 15.95 (15–16.6) (n = 18) | 15.65 (14.3–17.05) (n = 20) | 0.619 |
| Prothrombin time - max (second) | 17 (15.8–18.1) (n = 38) | 17 (15.8–20) (n = 18) | 16.95 (15.8–17.95) (n = 20) | 0.629 |
| Prothrombin time INR - initial | 1.235 (1.14–1.37) (n = 38) | 1.265 (1.18–1.33) (n = 18) | 1.235 (1.11–1.375) | 0.61 |
| Prothrombin time INR - max | 1.37 (1.25–1.48) (n = 38) | 1.37 (1.25–1.67) (n = 18) | 1.365 (1.25–1.46) | 0.63 |
| N-terminal B-type natriuretic peptide - initial (pg/ml) | 1695 (627–4498) (n = 38) | 2292.5 (598–4814) (n = 18) | 1692.5 (788–3565) | 0.99 |
| N-terminal B-type natriuretic peptide - max (pg/ml) | 9347 (2426–17,886) (n = 38) | 14,396 (8814–20,158) (n = 18) | 3205.5 (1466–10,624.5) | 0.01 |
| Troponin I - initial (ng/ml) | 0.08 (0.04–0.13) (n = 33) | 0.06 (0.04–0.19) (n = 17) | 0.085 (0.035–0.12) (n = 16) | 0.75 |
| Troponin I - max (ng/ml) | 0.16 (0.09–0.66) (n = 33) | 0.27 (0.1–1.04) (n = 17) | 0.145 (0.09–0.435) (n = 16) | 0.41 |
| D-Dimer - initial (ug/ml) | 3.17 (1.97–5.72) | 3.45 (1.99–5.96) | 3.00 (1.93–4.39) | 0.39 |
| D-Dimer - max (ug/ml) | 4.02 (3.10–7.12) | 4.88 (3.10–7.79) | 4.01 (2.89–6.66) | 0.69 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; CRP = C-reactive protein; INR = international normalised ratio; IQR = interquartile range; LDH = lactate dehydrogenase; Max = maximal; Min = minimal; PCR = polymerase chain reaction; PTT = partial thromboplastin time; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; WBC = white blood cells.
Statistically significant differences are marked by bold rows.
Milrinone therapy was utilised more in children who developed cardiac complications compared to those who had no cardiac complications (8 (42%) versus 2 (10%), p = 0.03). There were no other differences in vasoactive support, immunotherapy including intravenous immunoglobulin and immunosuppressive agents, anticoagulation or aspirin therapy among the groups (Table 3). Thirty-one patients (79.5%) required an intensive care unit admission. The reason for intensive care unit admissions included combined advanced respiratory and inotropic support (n = 6), isolated advanced respiratory support (n = 10), isolated inotropic support (n = 10), and finally five patients had clinical deterioration requiring multiple fluid intravenous boluses but did not require advanced respiratory therapies or inotropic support. Patients with multi-system inflammatory syndrome in children who developed cardiac complications were more likely to be admitted to the intensive care unit (18 (95%) versus 13 (65%), p = 0.04) (Table 4).
Table 3.
Comparison of medication use among multi-system inflammatory syndrome in children cohort stratified by presence of cardiac complications.
| Variable of interest | All cases (n = 39) | Cardiac complications present (n = 19) | Cardiac complications absent (n = 20) | p-value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Any vasoactive | 21 (54%) | 12 (63%) | 9 (45%) | 0.34 |
| Dopamine | 1 (3%) | 0 | 1 (5%) | 1.00 |
| Epinephrine | 21 (54%) | 12 (63%) | 9 (45%) | 0.34 |
| Milrinone | 10 (26%) * | 8 (42%) | 2 (10%) | 0.03 |
| Norepinephrine | 6 (15%) | 5 (26%) | 1 (5%) | 0.09 |
| Immune therapy | ||||
| Intravenous immunoglobulin | 38 (97%) | 18 (95%)** | 20 (100%) | 0.49 |
| Anakinra | 29 (74%) | 16 (84%) | 13 (65%) | 0.27 |
| Any steroid | 10 (26%) | 7 (37%) | 3 (15%) | 0.16 |
| Dexamethasone | 1 (3%) | 0 | 1 (5%) | 1.00 |
| Hydrocortisone | 9 (23%) | 6 (32%) | 3 (15%) | 0.27 |
| Prednisone | 1 (3%) | 1 (5%) | 0 | 0.49 |
| Any anticoagulant (enoxaparin or rivaroxaban) | 35 (90%) | 18 (95%) | 17 (85%) | 0.61 |
| Aspirin | 38 (97%) | 18 (95%) | 20 (100%) | 0.49 |
The indication for milrinone therapy included myocardial systolic dysfunction (n = 6), shock (n = 3), and low blood pressure (n = 1).
One patient was diagnosed retrospectively with multi-system inflammatory syndrome.The patient presented in shock with multi-system involvement and received hydrocortisone prior to the first report of multi-system inflammatory syndrome in children in United Kingdom in April 2020. Therefore, the patient did not receive intravenous immunoglobulin (IVIG) as per our protocol. The patient had mild systolic dysfunction that resolved by time of last follow up. No coronary artery abnormality was seen.
Statistically significant differences are marked by bold rows.
Table 4.
Clinical support and outcomes among multi-system inflammatory syndrome in children cohort stratified by presence of cardiac complications.
| Variable of interest | All cases (n = 39) | Cardiac complications present (n = 19) | Cardiac complications absent (n = 20) | p-value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| ICU admission | 31 (79%) | 18 (95%) | 13 (65%) | 0.04 |
| Respiratory ventilator support | 4 (12.9%) | 2 (15.4%) | 2 (10%) | 1.00 |
| ECMO | 0 | 0 | 0 | |
| Mortality | 0 | 0 | 0 | |
| ICU LOS (median and IQR) | 4 (2–6) | 4.5 (2–6) | 2 (2–5) | 0.3 |
| Hospital LOS (median and IQR) | 12 (8–14) | 12.5 (12–17) | 11 (8–14) | 0.2 |
ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay.
Statistically significant differences are marked by bold rows.
Discussion
We report the frequency and type of cardiac complications in patients who presented with multi-system inflammatory syndrome in children at a tertiary paediatric centre. These occurred in 49% of the multi-system inflammatory syndrome in children cohort but most resolved by the time of the most recent cardiac follow-up evaluation, at a median of 49 days. Patients with multi-system inflammatory syndrome in children who developed cardiac complications were more likely to present with conjunctival injection, higher maximum N-terminal B-type natriuretic peptide, higher initial white blood cell counts, higher initial neutrophil counts, and lower minimal lymphocyte counts. They were also more likely to have received milrinone and be admitted to the intensive care unit.
The strengths of this study include the uniform care of these patients that were managed under a protocol that endorses prompt immunotherapy, namely intravenous immunoglobulin as a first-line treatment, providing insight into cardiac complications in a cohort of patients followed by one centre. As we had the benefit of learning from other centres that faced earlier cases of multi-system inflammatory syndrome in children, our centre was able to develop protocols early in the multi-system inflammatory syndrome in children surge. In addition, this study provides short-term outcomes for cardiac complications in patients with multi-system inflammatory syndrome in children. Finally, to our knowledge, this is one of the earlier papers to assess risk factors associated with development of cardiac complications in patients with confirmed multi-system inflammatory syndrome in children.
In our cohort, the most common cardiac complication seen was systolic dysfunction, followed by atrioventricular valve regurgitation and coronary artery abnormalities. This is similar to previous studies reporting cohorts of patients with multi-system inflammatory syndrome in children in Europe and eastern United States of America.33,34 Similar to our finding, Belhadjer et al and Feldstein et al showed resolution of cardiac complications in the majority of patients by the time of most recent follow-up.33,35 Further work is still needed to assess if transient or persistent cardiac complications in patients with multi-system inflammatory syndrome in children is associated with long-term development of cardiomyopathy.
Children with cardiac complications were more likely to present with conjunctival injection. While an interesting finding, it needs further study to clarify the possible relationship. Coronary artery abnormalities were the third most common cardiac finding among our cohort of patients with multi-system inflammatory syndrome in children at a rate of 21% even though 95% of our patients received timely intravenous immunoglobulin. This rate is five times the rate reported in appropriately treated children with classical Kawasaki disease (excluding Kawasaki disease shock syndrome) in the intravenous immunoglobulin era (4%).28 The underlying cause and the pathophysiology of multi-system inflammatory syndrome in children is still to be identified. A debate of how similar or dissimilar multi-system inflammatory syndrome in children is to Kawasaki disease continues to exist.3,36,37 The higher rate of coronary artery abnormalities in our cohort of multi-system inflammatory syndrome in children, despite appropriate and timely intravenous immunoglobulin therapy suggests a more aggressive inflammatory disease with multi-system inflammatory syndrome in children. Of note, the race/ethnicity in our cohort revealed predominantly minority children of either non-Hispanic Black or Hispanic, which is different than what our centre’s general admissions were during the same period or traditionally reported in children with Kawasaki disease.3,38,39 Further research is needed to assess the role of genetic and environmental factors in the pathophysiology of multi-system inflammatory syndrome in children.
Children with cardiac complications also presented with higher maximal N-terminal B-type natriuretic peptide. B-type natriuretic peptide has been shown to have predictive value in identifying severity of heart failure in children.40 Further larger prospective studies are needed to assess if there is a B-type natriuretic peptide threshold that can predict cardiac complications in patients with multi-system inflammatory syndrome in children.
Higher initial white blood cell counts, higher initial neutrophils, and lower lymphocytes were more common among our patients with cardiac complications. Several blood counts have also been shown previously to correlate with cardiac disease. Cuinet et al found that patients with cardiogenic shock have early neutrophilia, correlating with shock severity. More importantly, those with the most severe shock had reduced lymphocytes.41 Further larger prospective studies are needed to assess if white blood cell counts and its subtypes have prognostic prediction for severity of cardiac disease in patients with multi-system inflammatory syndrome in children.
Finally, children with cardiac complications were more likely to have received milrinone and be admitted to the intensive care unit. This is not surprising as the most common cardiac complication was systolic dysfunction. Milrinone therapy is commonly used by intensivists caring for children with cardiac disease.42,43 In a survey of intensivists caring for cardiac patients, 97% of respondents used milrinone routinely.42 While more children with cardiac complications were admitted to the intensive care unit, the rate among those with no cardiac disease was also substantial.
Limitations
This was a single-centre retrospective study. In this report, we did not include rhythm assessment or electrocardiogram findings. As the sample size was small (n = 39), a multivariate analysis was not conducted since the outcome was a binary variable (cardiac complications (yes/no)). While further long-term study is needed to assess progression and long-term impact, we included short-term follow-up echocardiogram data in this report.
Conclusions
Our patients with multi-system inflammatory syndrome in children had a high rate of cardiac complications in the acute phase which exceeded rates historically observed in the setting of Kawasaki disease. In this small cohort, we identified factors associated with development of cardiac complications. Although cardiac complications resolved in 84% of patients, further long-term studies are needed to assess if the cardiac abnormalities (transient or persistent) are associated with major cardiac events.
Acknowledgments
Acknowledgements. The authors wish to thank Angela Doty and Lindsay Attaway for their editorial assistance; Emily Ansusinha and Jacob Cheng, PhD, MS, PStat for data gathering; and John Barber, MS and Jiaxiang Gai, MS for statistical analysis and generating tables. The authors are grateful for Yue-Hin Loke, Michael Bell, Meghan Delaney and the rest of the Children’s National Hospital multi-system inflammatory syndrome in children task force and the entire Children’s National Heart Institute team members for their devotion during the 2019 coronavirus disease pandemic.
Financial support. All authors have reported that they have no relationships relevant to the contents of this paper to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr Harahsheh is supported by a Sub-agreement from the Johns Hopkins University with funds provided by Grant No. R61HD105591 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the Office of the Director, National Institute of Health (OD). Dr DeBiasi receives partial salary support from the National Institute of Child Health and Human Development- National Institute of Health Grant No. R61 HD105618. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the Office of the Director, National Institute of Health (OD), or the Johns Hopkins University.
Footnotes
Ethical standards. Children’s National Hospital Institutional Review Board approved this study with a waiver of consent granted.
Conflicts of interest. None.
References
- 1.Harahsheh AS, Selekman RE, Simpson JN, et al. Children’s hospital ambulatory response to the 2019 novel coronavirus disease (COVID-19) pandemic. J Ambul Care Manag 2021; 44: 184–196. [DOI] [PubMed] [Google Scholar]
- 2.Harahsheh AS, Dahdah N, Newburger JW, et al. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr 2020; 222: 261–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loke YH, Berul CI, Harahsheh AS. Multisystem inflammatory syndrome in children: is there a linkage to Kawasaki disease? Trends Cardiovasc Med 2020; 30: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark BC, Sanchez-de-Toledo J, Bautista-Rodriguez C, et al. Cardiac abnormalities seen in pediatric patients during the SARS-CoV2 pandemic: an international experience. J Am Heart Assoc 2020; 9: e018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Haq N, Asmar BI, Deza Leon MP, et al. SARS-CoV-2-associated multisystem inflammatory syndrome in children: clinical manifestations and the role of infliximab treatment. Eur J Pediatr 2021; 180: 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias MD, McCrindle BW, Larios G, et al. Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the international Kawasaki disease registry. CJC Open 2020; 2: 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 2021; 180: 307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsaied T, Tremoulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation 2021; 143: 78–88. [DOI] [PubMed] [Google Scholar]
- 9.Harahsheh AS, Dahdah N, Newburger JW, et al. Reply. J Pediatr 2020; 224: 184.e1–185.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royal College of Paediatric and Child Health. Paediatric multisystem inflammatory syndrome temporally associated with COVID-19. 2020. Retrieved May 10, 2020, from https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatricmultisystem-%20inflammatory%20syndrome-20200501.pd
- 11.Jonat B, Gorelik M, Boneparth A, et al. Multisystem inflammatory syndrome in children associated with coronavirus disease 2019 in a children’s hospital in New York City: patient characteristics and an institutional protocol for evaluation, management, and follow-up. Pediatr Crit Care Med 2021; 22: e178–e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics 2021; 147: e2020024554. [DOI] [PubMed] [Google Scholar]
- 14.Valverde I, Singh Y, Sanchez-de-Toledo J, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation 2021; 143: 21–32. [DOI] [PubMed] [Google Scholar]
- 15.Kaushik S, Aydin SI, Derespina KR, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr 2020; 224: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children – United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeBiasi RL, Song X, Delaney M, et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J Pediatr 2020; 223: 199.e1–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Multisystem Inflammatory Syndrome in Children (MIS-C). Associated with Coronavirus Disease 2019 (COVID-19). 2020. Retrieved May 15, 2020, from https://emergency.cdc.gov/han/2020/han00432.asp.
- 20.Li Y, Yu Y, Chen S, Liao Y, Du J. Corticosteroids and intravenous immunoglobulin in pediatric myocarditis: a meta-analysis. Front Pediatr 2019; 7: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldeira D, Lopes LR, Vaz-Carneiro A, Costa J. Cochrane corner: corticosteroids for viral myocarditis. Rev Port Cardiol 2015; 34: 65–67. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Clinical Management of COVID-19: Interim Guidance, 2020. Retrieved June 15, 2021, from https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf.
- 23.van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and metaanalysis on clinical outcomes. Crit Care 2020; 24: 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBiasi R, Harahsheh A, Srinivasalu H, et al. Multisystem inflammatory syndrome of children: sub-phenotypes, risk factors, biomarkers, cytokine profiling and viral sequencing. J Pediatr 2021; 25: S0022–3476(21)005187. doi: 10.1016/j.jpeds.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Maniscalco V, Abu-Rumeileh S, Mastrolia MV, et al. The off-label use of anakinra in pediatric systemic autoinflammatory diseases. Ther Adv Musculoskelet Dis 2020; 12: 1759720X20959575. doi: 10.1177/1759720X20959575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantor PF, Lougheed J, Dancea A, et al. Presentation, diagnosis, and medical management of heart failure in children: Canadian cardiovascular society guidelines. Can J Cardiol 2013; 29: 1535–1552. [DOI] [PubMed] [Google Scholar]
- 27.Margossian R, Schwartz ML, Prakash A, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol 2009; 104: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135: e927–e999. [DOI] [PubMed] [Google Scholar]
- 29.Olivieri L, Arling B, Friberg M, Sable C. Coronary artery z score regression equations and calculators derived from a large heterogeneous population of children undergoing echocardiography. J Am Soc Echocardiogr 2009; 22: 159–164. [DOI] [PubMed] [Google Scholar]
- 30.Teitel DF, Newburger JW, Sutton N, et al. Development and utility of quality metrics for ambulatory pediatric cardiology in Kawasaki disease. Clin Pediatr 2020; 59: 245–251. [DOI] [PubMed] [Google Scholar]
- 31.Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010; 23: 465–495. [DOI] [PubMed] [Google Scholar]
- 32.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 33.Belhadjer Z, Meot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142: 429–436. [DOI] [PubMed] [Google Scholar]
- 34.Matsubara D, Kauffman HL, Wang Y, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol 2020; 76: 1947–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of us children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325: 1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulman ST. Pediatric coronavirus disease-2019-associated multisystem inflammatory syndrome. J Pediatric Infect Dis Soc 2020; 9: 285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamrani L, Manlhiot C, Elias MD, et al. Kawasaki disease shock syndrome versus classical Kawasaki disease, a meta-analysis and comparison with SARS-CoV-2 multisystem inflammatory syndrome. Can J Cardiol 2021: S0828–282X(21)00290–7. doi: 10.1016/j.cjca.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura Y, Yashiro M, Uehara R, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2007–2008 nationwide survey. J Epidemiol 2010; 20: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997–2007. Pediatr Infect Dis J 2010; 29: 483–488. [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto M, Manabe H, Nakau K, et al. The role of N-terminal pro-B-type natriuretic peptide in the diagnosis of congestive heart failure in children. Correlation with the heart failure score and comparison with B-type natriuretic peptide. Circ J 2010; 74: 998–1005. [DOI] [PubMed] [Google Scholar]
- 41.Cuinet J, Garbagnati A, Rusca M, et al. Cardiogenic shock elicits acute inflammation, delayed eosinophilia, and depletion of immune cells in most severe cases. Sci Rep 2020; 10: 7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roeleveld PP, de Klerk JCA. The perspective of the intensivist on inotropes and postoperative care following pediatric heart surgery: an international survey and systematic review of the literature. World J Pediatr Congenit Heart Surg 2018; 9: 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003; 107: 996–1002. [DOI] [PubMed] [Google Scholar]