Abstract
Introduction:
Dietary modification is common in patients with digestive diseases to improve symptoms; however, food avoidance can become problematic. Avoidant restrictive food intake disorder (ARFID) is characterized as failure to meet one’s nutritional needs due to sensory hypersensitivity, lack of interest in eating, or fear of aversive consequences from eating and is associated with negative medical and psychosocial outcomes. This is the first study to characterize ARFID behaviors in adults with achalasia (ACH), celiac sprue (CS), eosinophilic esophagitis (EoE), and inflammatory bowel disease (IBD).
Methods:
In this cross-sectional study, 289 adults aged 18+ completed self-report measures evaluating use of dietary treatment, ARFID symptoms, and psychosocial outcomes. Primary analyses investigated the occurrence of ARFID in patients with achalasia, celiac, EoE, and IBD. Secondary analyses explored the associations between ARFID symptoms and clinical and psychosocial outcomes.
Results:
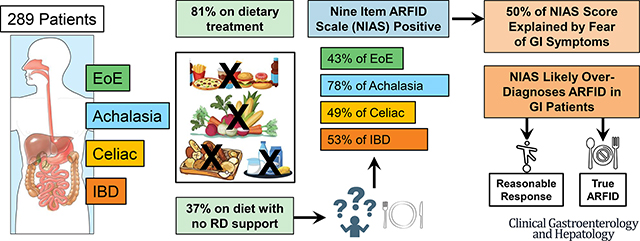
53.7% of the total sample met diagnostic criteria for ARFID based on the NIAS, with 78.4% of patients with achalasia meeting criteria. Patients on a physician-directed diet demonstrated greater fear of GI symptoms (p=.025), less interest in food (p=.046), and a higher total NIAS score (p=.045). For patients using dietary therapy, those who had met with a dietitian reported higher NIAS scores (p=.039). Food avoidance/restriction was associated with increased anxiety and depression, and diminished health-related quality of life.
Conclusion:
It is likely rates of ARFID, as measured by the NIAS, are inflated in these four patient groups. These findings highlight the need for updated assessments of ARFID in patients with complex digestive diseases.
Keywords: ARFID, digestive disease, diet
Graphical Abstract
Introduction
Gastrointestinal diseases classified as “organic” are those in which there are observable physiological changes in the digestive tract, which can sometimes occur in response to food. For example, in someone with celiac disease, exposure to gluten causes damage to the intestinal villi, resulting in symptoms of diarrhea, bloating, nausea/vomiting, and fatigue. Similarly, in eosinophilic esophagitis (EoE), exposure to dietary and environmental triggers produces an immune-mediated reaction in the esophagus leading to histological and structural changes. The role of diet in inflammatory bowel disease (IBD) is being revisited, with recent studies implicating pro-inflammatory foods (e.g., red meat, refined grains) in changing the gut microbiome and modulating intestinal inflammation.1 While specific foods are not implicated in the etiology of achalasia, a major motor disorder characterized by esophageal aperistalsis, the mechanical changes to the esophagus and lower esophageal sphincter make certain textures more difficult to swallow.2 Thus, dietary modification is commonly used among patients with gastrointestinal conditions as a symptom management strategy.
While many patients experience symptom reduction from elimination of food triggers, they are typically just one factor driving the symptom experience, and it is likely they could continue to have symptoms even with strict adherence to dietary recommendations. Additionally, patients may need to engage in a trial-and-error process of food elimination and reintroduction to identify food triggers. For example, in EoE, physicians may recommend the four-food or six-food elimination diet while patients learn their unique food triggers. For some patients, this can lead to generalized food avoidance and further restriction in an attempt to minimize symptoms, sometimes with little benefit. Similarly, up to 90% of patients with IBD report food avoidance to manage abdominal pain, bowel frequency, and diarrhea, especially during periods of active disease.3 However, patients with IBD may also avoid foods while in remission, sometimes out of the belief that certain foods may contribute to disease relapse.4–6 Though dietary modifications can often contribute to improvements in quality of life, there are cases when food avoidance becomes problematic and even dangerous for patients. Clinicians must then assess whether the extent of food avoidance or restriction exceeds what is expected given the medical condition.
Included in the Diagnostic and Statistics Manual-5th Edition (DSM-5), Avoidant/Restrictive Food Intake Disorder (ARFID) has gained increasing attention in adult gastroenterology populations. Unlike other eating disorders, food avoidance or restriction in ARFID is driven by aversion to the sensory characteristics of food (picky eating), lack of interest in eating (appetite), or concern about the aversive consequences of eating (fear), including an increase in GI symptoms. Food avoidance in ARFID is associated with negative medical or psychosocial consequences, including significant weight loss or nutritional deficiency, dependence on enteral feeding or oral nutritional supplements, or psychosocial impairment. The symptoms of ARFID overlap significantly with those present in many gastrointestinal conditions, including early satiety, loss of appetite, and fear of gastrointestinal symptoms in response to eating, which can make it particularly difficult to diagnose in patients with pre-existing digestive conditions. Patients with ARFID in the gastroenterology setting will describe limiting themselves to a small number of “safe” foods, often consumed in very small quantities. They may eat in private to avoid judgment from others for their eating habits and experience significant anxiety about food reintroduction.
Previous studies in ARFID in gastroenterology patients have focused on its prevalence within the general patient population or in functional/motility conditions such as irritable bowel syndrome (IBS) or gastroparesis. Existing studies have estimated the prevalence of ARFID in gastroenterology patients to be between 10–20%.7–10 Up to 40% of participants presenting for gastrointestinal motility evaluations had symptoms of ARFID in one study, though only 23% of these patients endorsed the medical or psychosocial consequences associated with eating behaviors that might suggest a more likely diagnosis of ARFID, including BMI < 16, nutritional deficiencies, or social isolation while eating.11 Differences in prevalence rates of ARFID may also reflect methodological differences: retrospective analyses have used chart review to identify ARFID symptoms whereas prospective analyses have used the Nine Item ARFID Screen (NIAS) as a screening measure for ARFID.12
Developed by Zickgraf and Ellis,12 the NIAS is the only existing screening measure for ARFID that can be administered in a brief format. Other assessment tools used in non-gastroenterology samples include the Pica, ARFID, and Rumination Disorder Interview (PARDI), though this has not been used in gastroenterology samples and would be cumbersome to use in clinical practice.13 As the NIAS assesses symptoms that may be present at baseline in patients with gastrointestinal symptoms, Murray et al used a set of modifying questions with the NIAS to assess the medical and psychosocial consequences of dietary restriction to distinguish between “likely” and “probable” ARFID in patients with gastroparesis.11
There is a need to explore the incidence of ARFID among organic gastrointestinal conditions for which dietary modification is a critical aspect of disease management to differentiate between normative and problematic avoidance. Thus, in this study we aim to investigate the occurrence of ARFID in patients with achalasia, celiac sprue, EoE, and IBD as measured by the NIAS. This is the first study to characterize ARFID behaviors in these specific patient cohorts. A secondary aim is to explore the associations between ARFID symptoms and clinical and psychosocial outcomes.
Methods
Patients ages 18 and up were recruited via an outpatient gastroenterology practice, a research dedicated database (researchmatch.org), and social media (Twitter). Data was collected anonymously online in a study-specific RedCap database. Screening questions were first presented to increase the likelihood of diagnostic accuracy due to the self-report nature of the data. Those passing the screeners were presented a series of questionnaires:
Demographic Information:
Age, gender, race, ethnicity, marital status, highest level of educational attainment.
Clinical Information:
Diagnosis, age at diagnosis, use of dietary treatments (MD prescribed or self-directed), use of prescribed medications, utilization of a dietitian including if the dietitian specialized in GI disease, procedures (endoscopy, colonoscopy) in past year, outpatient visits for their GI illness in past year, enteral nutrition status, other treatments (open ended).
Nine Item ARFID Screen (NIAS):
A nine item, self-report measure that asks patients to rate avoidant or restrictive eating behaviors across a 6-point Likert scale.12 Responses to the NIAS produce a total score and subscale scores for each of the three domains (picky, appetite, and fear). Individuals scoring above 23 points on the total scale are considered to meet criteria for ARFID. However, the use of subscale scores may be valuable in measuring ARFID in gastroenterology samples, where scores higher than 12 on an individual subscale may be indicative of ARFID.11 Therefore, presence of ARFID in this sample is defined as scores greater than 23 on the total scale or greater than 12 on an individual subscale, unless otherwise specified.
NIH-PROMIS Global-10:
The PROMIS Global-10 measures of functioning and HRQoL for a wide variety of chronic diseases and conditions. Specifically, it evaluates self-reported physical function, pain, fatigue, emotional distress, social health and general perceptions of health. A total score was computed for each subscale.
Statistical Analysis
Data were exported from RedCap into SPSS (v27 for Macintosh) for statistical analyses. Total scores were computed for the NIAS and each of the 3 subscales, and the PROMIS HRQoL scales. Initial tests for normal distribution were performed (Skewness/Kurtosis +/− 2.0) to determine the need for non-parametric tests. Continuous variables are presented as Mean (SD) with ranges, and categorical are presented as percentage (frequency). We evaluated the factor structure of the NIAS via Principal Components Factor Analysis (PCFA) with varimax rotation in this sample since it has not been validated for GI patients. An Eigenvalue of 1.0 was set to identify sub-scales with associated Scree plot.
NIAS total score and each subscale score were dichotomized (Yes=1, No=0) for the person meeting the cutoff criteria for a positive ARFID score. Between group differences for two-level categorical variables (e.g., marital status, ethnicity, using a dietary treatment) on the NIAS were evaluated using independent samples t-Tests; for three or more level categorical variables (e.g., diagnosis, dietitian support, gender, race, education) univariate one-way ANOVA with Tukey post-hoc testing was used. Pearson’s correlations assessed relationships between NIAS scores and HRQoL constructs. Statistical significance was set at P < .05 for all analyses.
Results
Characteristics of Study Sample
Demographic characteristics of the sample are displayed in Table 1. Across all four groups, our sample was predominantly White, non-Hispanic, and female. There were no differences between genders (p= .298), race (p= .197), ethnicity (p= .413), or education (p= .238) for presence of ARFID. People who are married are less likely to have ARFID (p = .026).
Table 1.
Demographic Characteristics of Study Sample
| Achalasia N=40 |
Celiac N=76 |
IBD N=120 |
EoE N=53 |
P | |
|---|---|---|---|---|---|
|
| |||||
| Age (in years) | 48.16 (14.2) | 41.59 (15.1) | 44.13 (14.5) | 41.64 (12.3) | .094 |
| 20 – 78 | 22 – 71 | 18 – 82 | 18 – 68 | ||
|
| |||||
| Gender | .053 | ||||
| Male | 20.0% (8) | 5.3% (4) | 19.2% (23) | 11.3% (6) | |
| Female | 75.0% (30) | 92.1% (70) | 78.3% (94) | 77.4% (41) | |
| Transgender | - | 0% | 1.7% (2) | - | |
| Other | - | 2.6% (2) | - | - | |
| Did not say | 5.0% (2) | - | 0.8% (1) | 11.3% (6) | |
|
| |||||
| Race | .768 | ||||
| White | 80.0% (32) | 93.4% (71) | 90.8% (109) | 81.1% (43) | |
| Black/African American | 5.0% (2) | - | 0.8% (1) | 1.9% (1) | |
| Latino/a | 2.0% (2) | 1.3% (1) | 1.7% (2) | 1.9% (1) | |
| Native Pacific Islander or Native American | - | - | 0.8% (1) | - | |
| Asian | - | 1.3% (1) | 1.7% (2) | - | |
| Other | 5.0% (2) | 3.9% (3) | 3.3% (4) | 3.8% (2) | |
| Did not say | 5.0% (2) | - | 0.8% (1) | 11.3% (6) | |
|
| |||||
| Ethnicity | .955 | ||||
| Non-Hispanic | 87.5% (35) | 96.1% (73) | 95.8% (115) | 84.9% (45) | |
| Hispanic | 5.0% (2) | 3.9% (3) | 3.3% (4) | 3.8% (2) | |
| Did not say | 7.5% (3) | - | 0.8% (1) | 11.3% (6) | |
|
| |||||
| Married/Co-Habitation | 67.5% (27) | 53.9% (41) | 71.4% (85) | 68.1% (32) | .071 |
|
| |||||
| Educational Attainment | .554 | ||||
| High School | 2.5% (1) | 6.6% (5) | 5.0% (6) | 5.7% (3) | |
| Some College | 12.5% (5) | 9.2% (7) | 14.2% (17) | 18.9% (10) | |
| Trade School | 5.0% (2) | 1.3% (1) | 5.8% (7) | 3.8% (2) | |
| College Graduate | 45.0% (18) | 40.8% (31) | 35.0% (42) | 39.6% (21) | |
| Post-Graduate Degree | 30.0% (12) | 40.8% (31) | 38.3% (46) | 20.8% (11) | |
| Did not say | 5.0% (2) | 1.3% (1) | 1.7% (2) | 11.3% (6) | |
|
| |||||
| Age at Diagnosis | 41.89 (16.3) | 32.17 (14.7) | 29.50 (14.5) | 34.34 (13.1) | <.001 |
| 7 – 77 | 2 – 66 | 5 – 61 | 1 – 62 | ||
|
| |||||
| Using Physician-Directed Diet | 22.5% (9) | 53.9% (41) | 12.5% (15) | 30.2% (16) | <.001 |
|
| |||||
| Using Self-Directed Diet | 57.5% (23) | 59.2% (45) | 50.0% (60) | 49.1% (26) | .521 |
|
| |||||
| Enteral Nutrition | |||||
| Partial | - | 2.9% (2) | 3.9% (3) | 1.9% (1) | .782 |
| Full | - | 1.5% (1) | 1.3% (1) | - | .702 |
|
| |||||
| Dietitian (RD) Support | .113 | ||||
| No | 60.0% (24) | 52.6% (40) | 50.8% (61) | 50.9% (27) | |
| Yes, GI specializing | 10.0% (4) | 28.9% (22) | 24.2% (29) | 9.4% (5) | |
| Yes, general RD | 25.0% (10) | 18.4% (14) | 23.2% (29) | 28.3% (15) | |
| Did not say | 5.0% (2) | - | 0.8% (1) | 11.3% (6) | |
Clinical characteristics of the sample, including disease and treatment related information, are displayed in Table 2. Approximately half of patients with celiac were using a physician-prescribed diet, which was a significantly greater proportion than the other groups (p < .001). Conversely, roughly half of all patients in the sample were using a self-directed diet with no differences between groups (p=.521). Only 50% to 60% of patients received dietitian support to manage their nutrition or dietary changes. The use of Partial Enteral Nutritional (EN) or Full EN was low, with less than 5% endorsing its use.
Table 2.
Percentage of Patients Meeting Established Cutoff for Positive ARFID Screen
| All N=289 |
Achalasia N=40 |
Celiac N=76 |
IBD N=120 |
EoE N=53 |
P | |
|---|---|---|---|---|---|---|
| NIAS > 23 | 48.9% | 75.7% | 42.1% | 51.3% | 31.0% | < .001 |
| Picky Eating > 12 | 15.8% | 10.8% | 17.1% | 19.7% | 7.1% | .216 |
| Lack of Interest > 12 | 17.6% | 32.4% | 13.2% | 19.7% | 7.1% | .017 |
| Fear GI Symptoms > 12 | 37.1% | 70.3% | 25.0% | 35.9% | 33.3% | < .001 |
| ARFID+ | 53.7% | 78.4% | 48.7% | 53.0% | 42.9% | .008 |
Notes: ARFID+ = NIAS > 23 or any subscale > 12
Performance of the NIAS
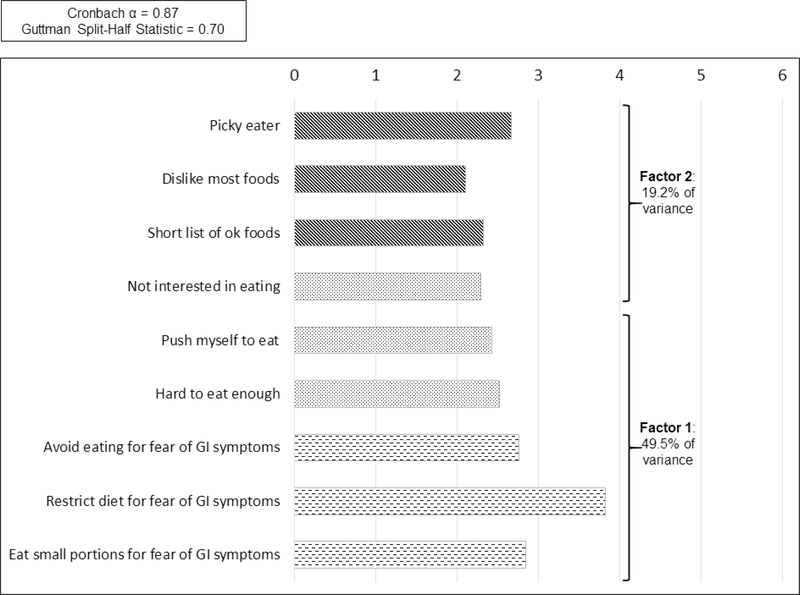
Results of the PCFA revealed a two-factor structure of the NIAS in this sample (Figure 1) versus the 3-factor structure previously reported in its validation studies. Half of the variance in the total NIAS score related to the three original items of the fear of GI symptoms subscale, and two items from the lack of interest in food subscale. The remaining item from the lack of interest subscale loaded with the three items from the picky eater scale. Inter-item correlations ranged from 0.119 (Items 2 and 9, p=.051) and 0.726 (Items 5 and 6, p<.001) with most being in the small to medium correlational size. Average scores by NIAS item support that the fear of GI symptoms items, specifically restricting one’s diet, are the main contributors to overall NIAS score in these patients (Figure 2). Even though the NIAS factor structure differed in this cohort, we used standard subscales and their respective cutoffs in order to compare results with prior publications.
Figure 1.
Principle Components Factor Analysis of Nine Item ARFID Scale with Mean Score for Each Item
Figure 2.
Mean Scores for Total NIAS and Subscales by Diagnosis Group
Figure 1. Factor analysis demonstrating the two-factor structure of the Nine Item ARFID Screen (NIAS) in this sample.
Figure 2. Average scores by NIAS item for each disease group.
Prevalence of ARFID
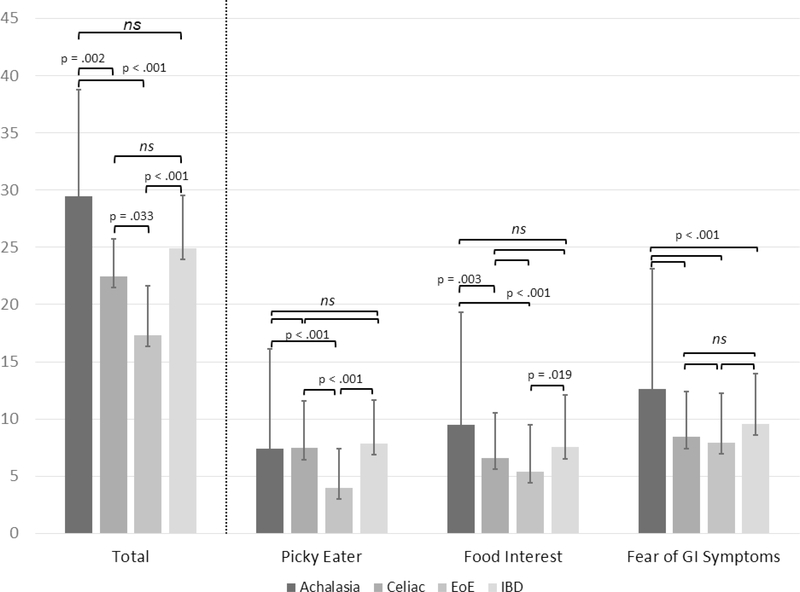
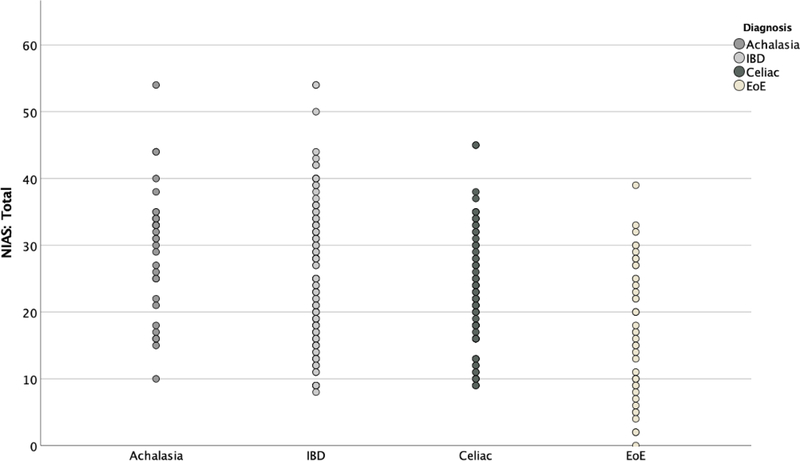
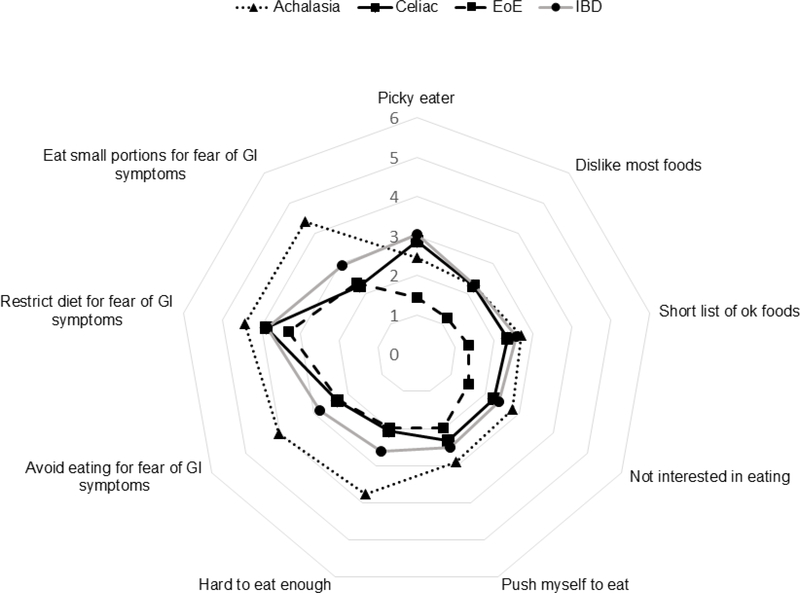
The proportion of patients scoring above clinical cutoff scores on the NIAS are presented in Table 2. 53.7% of the total sample reported symptoms of ARFID (total score >23 or individual subscale score > 12). Strikingly, almost 80% of patients with achalasia met the NIAS criteria for ARFID. Patients with celiac, IBD, and EoE also scored considerably higher than previously published rates in other GI diseases, but 20–30% lower than those with achalasia (p<.001). The distribution of NIAS scores varied across GI diagnosis (Figure 4). Subscale analyses show the greatest proportion of the NIAS score relates to fear of GI symptoms, with patients with achalasia again scoring substantially higher than the other 3 groups (Figure 3). Lack of interest in food also occurred in greater proportion among achalasia patients than the other groups (p= .017); however only 32% of achalasia patients met this criterion compared to 70% fearing GI symptoms. The lowest percentage of patients met the cutoff for picky eating (7% to 20%), and did not differ between the four groups (p= .216).
Figure 4.
Scatterplot of Mean Total NIAS Score by Diagnosis Group
Figure 3.
Radar Plot of Mean Score per NIAS Question for Each Diagnosis Group
Figure 3. Subscale analysis demonstrating factors contributing to total NIAS score.
Figure 4. Scatterplot demonstrating individual total NIAS scores based on diagnosis.
Relationship between ARFID and HRQoL
No differences existed between diagnostic groups for HRQoL or psychological distress. Patients with more ARFID symptoms reported significantly more anxiety and depression, and poorer HRQoL. The largest relationships existed between ARFID and poorer social functioning (r = -0.46), suggesting patients with ARFID are more likely to withdraw socially and feel less able to engage with others.
Dietary Treatment and ARFID
Consistent with ARFID scale elevations, patients using a self-directed diet reported significantly more fear of GI symptoms than those using a physician-directed diet (Mean = 10.18, SD = 4.37 vs. Mean = 8.48, SD = 4.47, p=.002); while picky eating (p=.894), lack of interest (p=.922) and total NIAS score (p=.172) did not differ based on dietary treatment. Patients on a physician-directed diet demonstrated differences from those on a self-directed diet on all NIAS scales except for picky eating (p=.580). Fear of GI symptoms was again higher for those on a physician directed diet (Mean = 10.33, SD = 4.48 vs. Mean = 9.01, SD = 4.45, p=.025). However, patients on a physician-prescribed diet also showed less interest in food (p= .046) and a higher total NIAS score (p=.045) than those on a self-directed diet.
Dietitian Consultation and ARFID
No differences existed between patients who had met with a dietitian or not, or between the type of dietitian consulted (GI specialist versus general) for total NIAS score (p=.064). This was also seen in the NIAS subscale scores with no differences for fear of GI symptoms (p=.165), picky eater (p=.139), or lack of interest in food (p=.189). However, when examining only patients who were using a dietary treatment (physician or self-directed, N=200), meeting with a dietitian was associated with significant differences in total NIAS score. Specifically, patients who were using a dietary therapy and who had met with a dietitian reported higher NIAS total scores than those on a diet who had not met with a dietitian (Mean= 26.43, SD = 9.58 vs. Mean= 22.59, SD= 10.22, p=.039). Subscales on the NIAS did not significantly differ between patients using dietary treatment who had met with a dietitian and those who had not (Fear of GI symptoms p=.249, picky eating p=.065, lack of interest in food p=.195), suggesting no single construct of ARFID contributed to these differences and this finding may be due to Type I error.
Discussion
This is the first study to evaluate the prevalence of ARFID in patients with achalasia, celiac disease, EoE, and IBD. Our data reflect that using the gold standard measure, the NIAS, prevalence rates of ARFID are high among patients with these organic gastrointestinal conditions. In fact, taken at face value, our results would suggest that 75% of patients with achalasia meet diagnostic criteria for ARFID. However, it is more likely the NIAS is overestimating prevalence rates of ARFID within this population. Symptoms of gastrointestinal disease can overlap with those assessed by the NIAS, including early satiety and postprandial fullness, and it is common for patients with gastrointestinal symptoms to experience fear related to the possibility of experiencing symptoms. Factor analysis of the NIAS in this sample identified only two subscales versus three reported for its validation studies, with items related to fear of GI symptoms contributing to more of the variance in NIAS score than picky eating or lack of interest in food. As such, using the NIAS may over-inflate ARFID diagnoses in adults with chronic GI disease, underscoring a need to develop a screening measure that better differentiates ARFID in patients with gastrointestinal symptoms.
While the NIAS may not be able to differentiate ARFID within patients with gastrointestinal disease, our findings demonstrate symptoms of food avoidance and restriction are associated with negative psychosocial outcomes for patients, including increased anxiety, depression, fatigue, and pain interference, as well as decreased social and physical functioning. In our sample, following a diet and having met with a dietitian were associated with a higher likelihood of having ARFID based on fear of food. We may hypothesize that those with more severe food restriction were referred to a dietitian over the course of treatment. As the data gathered in this study was cross-sectional, we are unable to draw conclusions about whether excessive dietary restriction preceded the referral to a dietitian or if it was a consequence of it as the patient attempted to implement dietary recommendations. However, one study found that nearly 90% of patients who met criteria for ARFID were at some point prescribed the low FODMAP diet and over 70% of these patients were prescribed dietary intervention while already meeting criteria for ARFID or being actively underweight and/or losing weight.9 Additional study on the role of dietary intervention in patients with ARFID is warranted.
Although diet and eating behaviors are topics of interest within IBD, EoE, and celiac research, they have not yet been studied in achalasia. However, the results of this study suggest that patients with achalasia significantly modify their diet and/or eating behaviors to accommodate their symptoms. While some of these modifications may certainly be adaptive and assist patients in managing their condition, they also have significant impacts on food-related quality of life, with modifications perhaps beyond what is necessary. These findings highlight the need for increased research on diet and eating behaviors in achalasia to inform treatment recommendations and disease management strategies.
When assessing food avoidance in gastrointestinal disease, it is important to understand the broader context of diet quality and the impact of food avoidance on functioning. In the only IBD study on nutrition and dietary exclusion, the average daily intake of calcium, vitamin A, and zinc was significantly lower in the food exclusion group than in the non-exclusion group.14 This is likely attributable to decreased consumption of raw fruits and vegetables, which are often reported to exacerbate symptoms in IBD.1, 15 However, for adolescents with ARFID (without gastrointestinal symptoms), dietary intakes were significantly lower in vegetables and protein, higher in added sugars and total carbohydrates, and lower in vitamins K and B12 compared to healthy controls.16 As dietary intake does not always correlate with serum micronutrient levels,17 additional study on the characteristics of dietary intake and associated serum micronutrient levels in patients with ARFID is warranted. In addition, research on the differences in nutritional status between people who exclude foods and those who do not may be helpful in characterizing adaptive from problematic avoidance. Although malnutrition resulting from ARFID can exacerbate GI complaints, it can also have significant effects on a patient’s overall health, including increased risk of mortality and diffuse medical complications. Thus, it is critical for providers to assess and treat ARFID in patients with GI conditions to prevent risk of further medical comorbidities.
This study has some limitations. Notably, ARFID symptoms were assessed using a self-report measure and not corroborated with clinician assessment. In addition, although the total sample was large, achalasia and eosinophilic esophagitis (EoE) were less well represented. Thus, additional study of the prevalence rates among these conditions would be valuable to replicate our findings. Disease activity in IBD was not measured in this study to distinguish rates of ARFID during a disease flare compared to in remission. Given food avoidance in IBD tends to be greater during periods of increased disease activity, it is likely the prevalence rates of ARFID according to the NIAS might fluctuate based on whether the patient is in remission. Lastly, as our sample was predominantly Caucasian women, our findings may not be applicable to other demographic groups.
Clinical Implications
This study highlights the importance of routine assessment of eating behaviors in patients with chronic digestive conditions. Although the role of diet has been a more prominent focus in conditions like IBD, this study underscores a pressing need to understand dietary modification in chronic esophageal conditions such as achalasia. Based on the results of this study, which likely demonstrated the tendency of the NIAS to over-inflate pathological food avoidance in patients with organic digestive conditions, we do not recommend it be used universally as an indicator of problematic food avoidance. However, it could instead be a starting point for discussion with patients around food-related anxiety and diet modifications used to manage symptoms. For patients endorsing items on the NIAS, it is worthwhile for providers to inquire about the change in eating behaviors and what factors may be driving the behavior. Although these behaviors could be driven by food related anxiety, they could also reflect a worsening of symptoms and may suggest additional workup and/or intervention is warranted. However, patients demonstrating significant food-related avoidance or dietary restriction may benefit from referral to a dietitian and/or psychologist with specialized gastroenterology training to address these concerns.
Conclusion
The NIAS may inform whether a patient is engaging in avoidant or restrictive eating; however, it is unable to differentiate whether the degree of food avoidance/restriction is beyond what would be expected in the course of gastrointestinal disease. Although it may facilitate conversations about eating behaviors between providers and patients, we caution providers to not be overly reliant on the NIAS as a means of screening for ARFID in patients with gastrointestinal conditions, as it is likely to pathologize what may otherwise be adaptive eating behavior. Thus, one of the next priorities in ARFID diagnosis and treatment is developing a screening measure that more accurately differentiates normative food avoidance from pathological food avoidance in patients with gastrointestinal conditions.
Study Highlights:
WHAT IS KNOWN:
Many patients with organic gastrointestinal conditions modify their diet to manage symptoms
Food avoidance can be associated with negative medical and psychosocial outcomes
WHAT IS NEW HERE:
The NIAS may over-inflate rates of ARFID in patients with organic gastrointestinal conditions
Patients with achalasia exhibit a high level of food avoidance and restriction
Food avoidance is associated with decreased quality of life
What you need to know:
Background:
Many patients with digestive disease modify their diet to manage symptoms, sometimes resulting in Avoidant/Restrictive Food Intake Disorder (ARFID). However, its prevalence is not known in patients with organic conditions.
Findings:
According to the Nine Item ARFID Screen, rates of ARFID appear high in patients with achalasia, eosinophilic esophagitis, celiac, and inflammatory bowel disease. ARFID was associated with negative psychosocial outcomes.
Implications for Patient Care:
The NIAS likely overestimates the rates of ARFID in patients with organic gastrointestinal conditions. Thus, it should be only a starting point for discussing food avoidance and restriction.
Declaration of Funding Source:
Tiffany Taft is supported by an NIDDK Grant (No. P01DK117824/DK/NIDDK).
Footnotes
Conflicts of Interest: Tiffany Taft has 100% ownership in Oak Park Behavioral Medicine LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levine A, Rhodes JM, Lindsay JO, et al. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clinical Gastroenterology and Hepatology 2020;18:1381–1392. [DOI] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh A, Kinneally J, Robertson T, et al. Food avoidance in outpatients with Inflammatory Bowel Disease - Who, what and why. Clin Nutr ESPEN 2019;31:10–16. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AB, Lee D, Long MD, et al. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci 2013;58:1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova MJ, Chaparro M, Molina B, et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients With Inflammatory Bowel Disease. J Crohns Colitis 2017;11:1430–1439. [DOI] [PubMed] [Google Scholar]
- 6.Limdi JK, Aggarwal D, McLaughlin JT. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 2016;22:164–70. [DOI] [PubMed] [Google Scholar]
- 7.Murray HB, Bailey AP, Keshishian AC, et al. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clin Gastroenterol Hepatol 2020;18:1995–2002.e1. [DOI] [PubMed] [Google Scholar]
- 8.Harer K, Baker J, Reister N, et al. Avoidant/Restrictive Food Intake Disorder in the Adult Gastroenterology Population: An Under-Recognized Diagnosis?: 417. The American journal of gastroenterology 2018;113:S247–S248. [Google Scholar]
- 9.Harer KN, Jagielski CH, Riehl ME, et al. 272 – Avoidant/Restrictive Food Intake Disorder Among Adult Gastroenterology Behavioral Health Patients: Demographic and Clinical Characteristics. Gastroenterology (New York, N.Y. 1943) 2019;156:S-53–S-53. [Google Scholar]
- 10.Zia JK, Riddle M, DeCou CR, et al. Prevalence of Eating Disorders, Especially DSM-5's Avoidant Restrictive Food Intake Disorder, in Patients with Functional Gastrointestinal Disorders: A Cross-Sectional Online Survey. Gastroenterology (New York, N.Y. 1943) 2017;152:S715–S716. [Google Scholar]
- 11.Burton Murray H, Jehangir A, Silvernale CJ, et al. Avoidant/restrictive food intake disorder symptoms are frequent in patients presenting for symptoms of gastroparesis. Neurogastroenterol Motil 2020;32:e13931. [DOI] [PubMed] [Google Scholar]
- 12.Zickgraf HF, Ellis JM. Initial validation of the Nine Item Avoidant/Restrictive Food Intake disorder screen (NIAS): A measure of three restrictive eating patterns. Appetite 2018;123:32–42. [DOI] [PubMed] [Google Scholar]
- 13.Bryant-Waugh R, Micali N, Cooke L, et al. Development of the Pica, ARFID, and Rumination Disorder Interview, a multi-informant, semi-structured interview of feeding disorders across the lifespan: A pilot study for ages 10–22. The International journal of eating disorders 2019;52:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim HS, Kim SK, Hong SJ. Food Elimination Diet and Nutritional Deficiency in Patients with Inflammatory Bowel Disease. Clin Nutr Res 2018;7:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton H, Pedley KC, Stewart RJC, et al. Inflammatory Bowel Disease: Are Symptoms and Diet Linked? Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harshman SG, Wons O, Rogers MS, et al. A Diet High in Processed Foods, Total Carbohydrates and Added Sugars, and Low in Vegetables and Protein Is Characteristic of Youth with Avoidant/Restrictive Food Intake Disorder. Nutrients 2019;11:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr 2007;31:311–9. [DOI] [PubMed] [Google Scholar]