Abstract
Introduction
Understanding of the natural history of apathy and its impact on patient function is limited. This study examines, in a large, national sample of Alzheimer's disease (AD) patients with long follow‐ups: (1) prevalence, incidence, and persistence of apathy, and (2) impact of apathy on function across dementia severity.
Methods
A longitudinal study of 9823 well‐characterized AD patients in the National Alzheimer's Coordinating Center Uniform Data Set.
Results
Apathy was highly prevalent across disease severity with cumulative prevalence of 48%, 74%, and 82% in Clinical Dementia Rating (CDR) 0.5, 1.0, and 2.0, respectively. Persistence of apathy from clinician judgment varied from visit to visit at earlier disease stages but remained high at moderate dementia. Independent of cognition, persistent apathy was strongly associated with accelerated rate of functional decline.
Discussion
Findings point to important targets for the treatment and management of apathy, include functional outcomes, and study designs that account for variable persistence of the apathy syndrome.
Keywords: apathy, dementia, function, longitudinal studies, mild cognitive impairment
1. INTRODUCTION
Apathy is one of the most common behavioral syndromes in patients with dementia, with significant impact upon the course of the disorder. Apathy has profound consequences for morbidity, mortality, and caregiver burden. It is associated with more impairment in activities of daily living (ADL) than patients’ cognitive status would otherwise suggest and also is associated with increased dementia severity and more rapid disease progression. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Apathetic patients rely on caregivers to initiate activities that they are otherwise capable of doing by themselves and thus have worse quality of life. 6 , 10 Caregivers of patients with apathy report significantly higher levels of distress compared to caregivers of patients without apathy. 11 , 12 Although apathy and depression often co‐occur and have many common symptoms, previous studies have demonstrated apathy as an independent construct in Alzheimer's disease (AD), distinct from depression in prevalence and in its impact on patient outcomes. 7 , 13
Reports on the prevalence of apathy in patients with dementia vary from 19% to 88% across studies. 14 , 15 , 16 , 17 , 18 , 19 A recent meta‐analysis reported pooled prevalence of 48%. 17 Apathy can occur at any time during the course of dementia. Many studies show that prevalence is higher in more severe dementia, although some suggest highest prevalence in moderate stages of disease. 16 , 18 , 19 , 20 Prevalence rates reported in cross‐sectional studies underestimate cumulative prevalence over the course of disease and do not provide insight into whether symptoms are episodic or persist over time. As such, a better understanding of the natural history of apathy with longitudinal follow‐up is needed to aid its assessment and treatment.
Several recent longitudinal studies examined incidence or persistence of apathy. Reports on incidence of apathy ranged from 13.5% over 1 year to as high as 63% in 2 years. 19 , 20 , 21 , 22 , 23 , 24 Reports on persistence of apathy also varied widely, ranging between 10% and 62%. 4 , 19 , 20 , 22 , 23 , 25 , 26 Differences in these results may partially be due to differences in study design, setting, sample characteristics, and apathy measures used. Most of these studies are small, with sample sizes around several hundred. With few exceptions, 9 , 19 lengths of follow‐up have been limited to 1 or 2 years.
Several additional limitations in the existing literature hinder our understanding of the natural history of apathy and its impact on patient outcomes. Although apathy is prevalent in mild cognitive impairment, 7 , 15 , 18 most existing studies have examined apathy in the context of dementia. With a few exceptions, 4 , 14 , 15 , 17 , 20 , 23 studies did not distinguish dementia etiologies. Most studies that examined the effects of apathy on patient outcomes have relied on baseline apathy only, without consideration as to how apathy may occur at a later point during follow‐up. Adjustment for disease severity, co‐morbidities, and other socio‐demographic characteristics was uncommon.
To address many of these issues, we extend a recent cross‐sectional study on the relationships between apathy and function, and aimed to (1) examine prevalence, incidence, and persistence of apathy in a large, national sample of well‐characterized patients with an underlying etiology of AD who had regular assessments over long follow‐up periods, and (2) estimate the impact of apathy on function longitudinally across the spectrum of disease severity. In the current study we focused on patients with underlying AD etiology alone, and will report separately on other etiologies in the future.
RESEARCH IN CONTEXT
Systematic review: Previous studies have demonstrated apathy as an independent construct in Alzheimer's disease (AD), distinct from depression in prevalence and in its impact on patient outcomes. However, understanding of the natural history of apathy in AD over the dementia trajectory and its impact on patient function over time remains limited.
Interpretation: This study demonstrates high prevalence, incidence, and persistence of apathy across the spectrum of dementia severity. Persistent apathy was associated with accelerated rate of decline in function that is independent of cognitive status. Impact of apathy was present even in mildly affected individuals and those with questionable dementia, and not just in patients with moderate and severe dementia.
Future directions: Findings call for assessment of apathy at all stages of cognitive complaint, point to important targets for the treatment and management of apathy, inclusion of functional outcomes, and study designs that account for variable persistence of the apathy syndrome.
HIGHLIGHT
Apathy was highly prevalent across the range of dementia severities with cumulative prevalence of between 48%‐82% in mild to moderate/severe dementia.
Presence of apathy increased over time in those with mild dementia but seemed to plateau in moderate/severe dementia.
Persistence of apathy varied from visit to visit, especially at earlier disease stages.
At moderate dementia, there was steady persistence of apathy.
Independent of cognition, persistent apathy was strongly associated with worse function at baseline, and also with a faster rate of decline.
2. METHODS
2.1. Data source and sample derivation
Data are drawn from the National Alzheimer's Coordinating Center Uniform Data Set (NACC‐UDS). 27 Recruitment, participant evaluation, and diagnostic criteria are detailed elsewhere. 28 Briefly, beginning in September 2005, participants have been followed prospectively from 39 past and present National Institute on Aging–funded Alzheimer's Disease Centers (ADCs) located throughout the United States. 28 All ADCs enroll and follow participants with a standardized protocol and provide data for research through NACC. Participants are followed at ≈12‐month intervals using standard evaluations. Informed consent was provided by all participants and their informants.
Data used in the current study were drawn from all participants enrolled in NACC‐UDS between September 2005 (start date of the UDS) and the December 2019 data freeze (N = 42,022) who had at least one follow‐up visit. Participants who had a primary etiologic diagnosis of AD at baseline, and those who had a primary etiologic diagnosis of AD at more than half of the follow‐up visits were included in the analysis. We further excluded subjects who, at baseline: (1) were younger than 50 (n = 87), (2) had a Clinical Dementia Rating (CDR) = 0 with AD diagnosis (n = 11), and (3) had missing apathy data (n = 31). By year 5, about half of the subjects remained in the study. The analysis included data for up to five UDS visits.
2.2. Measures
2.2.1. Apathy
We identified apathy by using the clinician judgment item within the NACC‐UDS protocol. Study clinicians endorsed that participants currently manifested apathy as a meaningful change in behavior (yes = 1, no = 0). Clinician judgment was based on all available information including clinical assessment, informant report derived from the Neuropsychiatric Inventory (NPI) 29 , 30 apathy subscale, and medical records review. We computed prevalence, incidence, and persistence of clinician‐judged apathy. Prevalence of apathy was defined by (1) presence of apathy: proportion of participants with an endorsement of apathy at a visit; and (2) cumulative prevalence of apathy, defined as the proportion of participants with an endorsement of apathy at least one visit over the entire follow‐up period. Incidence of apathy was defined by (1) incidence between two consecutive visits v and v+1: proportion of participants who did not have an endorsement of apathy at visit v but had an endorsement of apathy at visit v+1, (2) cumulative incidence of apathy: proportion of participants who did not have apathy at baseline, but had an endorsement of apathy at least one visit over the entire follow‐up period. Persistence of apathy between two consecutive visits v and v+1 was defined by proportion of participants who had an endorsement of apathy at visit v and also had an endorsement of apathy at visit v+1.
Based on how often a participant was reported to have apathy throughout the follow‐up period, we categorized participants into four mutually exclusive groups: (1) never apathy across all visits, (2) intermittent apathy (at least one but <50% visits with apathy), (3) persistent apathy (≥ 50% visits with apathy), and (4) always apathy across all visits.
2.2.2. Function
Our main dependent variable is participants’ function, measured using the Functional Assessment Questionnaire (FAQ) reported from interviews with study partners. 31 The FAQ asks whether the participant had any difficulty or needed help with 10 items in the previous 4 weeks on a scale from 0 to 3, corresponding to normal (0), has difficulty but does by oneself (1), requires assistance (2), and dependent (3). Responses to each item were summed to obtain a total FAQ score (range = 0 to 30). Total FAQ score was then divided by the number of tasks attempted to obtain a standardized score. 32 A total of 124 participants (74 CDR = 0.5, 21 CDR = 1, 29 CDR ≥ 2; 1.3% of all participants) who were reported to have not attempted any tasks and had a missing value for all FAQ items were excluded from the analysis.
2.2.3. Dementia severity
Participants were grouped by their baseline severity of dementia as measured by the CDR. 33
2.2.4. Demographic and clinical characteristics
Demographic characteristics included age, sex, race (non‐Hispanic White, non‐Hispanic Black, vs. other), ethnicity (Hispanic/Latino vs. other), years of education, marital status (married/living as married or not), and living alone (yes/no). Participant medical history was obtained by clinician interview and review of medical records as reported to NACC‐UDS. Depressive symptoms were measured using the 15‐item Geriatric Depression Scale (GDS‐15). 34 , 35 Apolipoprotein E (APOE) genotype for participants who are willing to provide samples was reported by the ADCs.
2.3. Statistical analyses
Multivariable analyses were performed using linear mixed models (LMM). Our main independent variable is apathy group (reference group: never apathy throughout the study) and its interactions with time. To estimate the independent effect of apathy on function beyond the effects on function due to dementia severity, baseline CDR (reference group: CDR = 0.5) and their interactions with time were also included. The coefficient on time, measured using UDS visit, estimated overall change in FAQ over time.
Coefficients on apathy group estimated differences in FAQ scores at baseline for each apathy group compared to those who were never apathetic throughout the study. We hypothesized that worse apathy groups would be associated with worse baseline FAQ. The interaction terms between apathy groups and time estimated differences in the rate of change in FAQ over time between apathy groups compared to those with who were never apathetic. A positive coefficient indicated faster decline in FAQ over time in that apathy group compared to those who were never apathetic. Because the model controlled for other covariates including dementia severity, coefficients estimated effects of apathy on function beyond those from dementia severity.
Similarly, coefficients on baseline CDR estimated differences in FAQ scores at baseline for each CDR group, compared to the reference group of individuals with CDR = 0.5. We hypothesized that worse baseline CDR would be associated with worse baseline FAQ. The interaction terms between baseline CDR and time estimated differences in the rate of change in FAQ over time between CDR groups compared to those with baseline CDR = 0.5. A positive coefficient indicated faster decline in FAQ over time in that CDR group compared to those with baseline CDR = 0.5.
Covariates included in the LMM were baseline age; sex; race/ethnicity; years of education; and indicators for history of diabetes, hypertension, and hypercholesterolemia. Models included subjects and ADCs as random intercepts, assuming subjects were nested within each ADC. We tested models that included individual random slopes to allow participants to differ in their overall rate of change over time. Likelihood ratio tests suggested that including a random slope did not improve model fit and was subsequently dropped. Initial models also included interaction terms between CDR and apathy groups. None of the interaction terms were statistically significant and were subsequently dropped. All analyses were performed using Stata 13.0. 36 Statistical significance was set a priori at P < .05.
3. RESULTS
3.1. Baseline sample characteristics
Characteristics of the sample include: average age 74.4 (standard deviation [SD] = 9.1), 47% male, 77% non‐Hispanic White, 11% non‐Hispanic Black, 8.4% Hispanic, average education 14.7 years (SD = 3.6), 17.2% lived alone. Hypertension (48%), hypercholesterolemia (50%), depression in the past 2 years (36%) were common (Table 1). Average Mini‐Mental State Examination (MMSE) was 22.7 (SD = 5.9), GDS was 2.4 (SD = 2.5), FAQ was 11.2 (SD = 9.2). Of those with APOE genotyping, 44% had 0, 43% had 1, and 13% had 2 APOE ε4 allele. Participants were followed for an average of 4 years (SD = 2.2); 28% had 1, 19% had 2, 26.8% had 3 to 4, and 25.9% had 5 or more follow up visits. For CDR, 55.8% of participants (n = 5480) had CDR = 0.5, 32.2% (n = 3163) had CDR = 1, and 12.0% (n = 1280) had CDR = 2 or 3 (8.5% had CDR = 2 (n = 833), and 3.5% had CDR = 3 (n = 347). Except for hypertension, differences in participant characteristics between baseline CDR were all statistically significant (P < .001).
TABLE 1.
Baseline characteristics for all sample and by baseline CDR
| Dementia severity | ||||
|---|---|---|---|---|
| All sample | CDR = 0.5 Questionable/very mild | CDR = 1 Mild | CDR ≥ 2 Moderate/severe | |
| N | 9823 | 5480 | 3163 | 1180 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 74.4 | 73.9 | 74.6 | 76.3 |
| (9.1) | (8.7) | (9.5) | (10.0) | |
| Male (%) | 47.2 | 50.1 | 45.9 | 36.9 |
| Race/ethnicity (%) | ||||
| White | 77.3 | 80.1 | 76.4 | 66.2 |
| Black | 11.2 | 10.8 | 10.9 | 14.2 |
| Hispanic | 8.4 | 5.8 | 9.7 | 16.9 |
| Other | 0.9 | 0.8 | 1.1 | 0.9 |
| Education, years | 14.7 | 15.3 | 14.3 | 13.3 |
| (3.6) | (3.3) | (3.7) | (4.3) | |
| Diabetes (%) | 12.2 | 11.8 | 11.9 | 15.0 |
| Hypertension (%) | 48.4 | 48.2 | 49.0 | 48.2 |
| Hypercholesterolemia (%) | 49.5 | 51.5 | 49.1 | 41.3 |
| Depression in past 2 years (%) | 36.1 | 31.8 | 41.5 | 41.5 |
| Living alone (%) | 17.2 | 21.2 | 13.9 | 7.4 |
| Functional Assessment Questionnaire (FAQ) | 11.2 | 5.3 | 16.1 | 25.6 |
| (9.2) | (5.3) | (6.5) | (5.3) | |
| Geriatric Depression Scale (GDS) | 2.4 | 2.3 | 2.6 | 2.4 |
| (2.5) | (2.4) | (2.6) | (2.6) | |
| Mini‐Mental State Examination (MMSE) | 22.7 | 25.8 | 20.9 | 12.5 |
| (5.9) | (3.2) | (4.5) | (6.6) | |
| Apolipoprotein E (APOE) | ||||
| No ε4 (%) | 43.7 | 45.9 | 41.0 | 40.3 |
| One ε4 (%) | 43.4 | 41.9 | 45.0 | 46.7 |
| Two ε4s (%) | 12.9 | 12.2 | 14.0 | 13.0 |
| APOE missing (%) | 19.1 | 17.7 | 18.2 | 27.8 |
| Total # of follow‐up visits | 4.0 | 4.3 | 3.8 | 3.3 |
| (2.2) | (2.4) | (2.1) | (1.7) | |
| 1 follow‐up visit (%) | 28.1 | 34.0 | 44.1 | 31.9 |
| 2 follow‐up visits (%) | 19.2 | 23.0 | 24.0 | 21.0 |
| 3–4 follow‐up visits (%) | 26.8 | 25.0 | 21.7 | 25.6 |
| 5 or more follow up visits (%) | 25.9 | 18.1 | 10.2 | 21.5 |
Abbreviation: CDR, Clinical Dementia Rating; SD, standard deviation.
Note: Differences between baseline CDR groups all significant at P < .001 except for hypertension (P = .77).
3.2. Prevalence, incidence, and persistence of clinician judgment of apathy over time
3.2.1. Prevalence of apathy
Presence of apathy, that is, proportion of participants with an endorsement of apathy at a visit, increased over time in participants with baseline CDR = 0.5 and 1 (Table 2, Panel A). Among participants with baseline CDR ≥ 2, however, presence of apathy fluctuated around 60% at each visit. Cumulative prevalence of apathy, that is, proportion of participants with an endorsement of apathy in at least one visit over the entire follow‐up period, was 48.0% in those with baseline CDR = 0.5, 73.7% in CDR = 1, and 81.9% in CDR ≥ 2.
TABLE 2.
Prevalence, incidence, and persistence of clinician judged apathy over time
| Baseline CDR | |||
|---|---|---|---|
| CDR = 0.5 Questionable/very mild | CDR = 1 Mild | CDR ≥ 2 Moderate/severe | |
| A. Prevalence (%) | |||
| Presence at a visit | |||
| v1 | 19.6 | 43.9 | 61.1 |
| v2 | 24.3 | 48.8 | 64 |
| v3 | 28.4 | 52.8 | 62.9 |
| v4 | 32.3 | 55.3 | 62.1 |
| v5 | 33.4 | 60.1 | 60.9 |
| Cumulative prevalence | 48 | 73.7 | 81.9 |
| B. Incidence (%) | |||
| Visit to visit incidence | |||
| v1–v2 | 11.9 | 15.7 | 14.2 |
| v2–v3 | 12.6 | 16.4 | 13.3 |
| v3–v4 | 13.4 | 15.8 | 12.7 |
| v4–v5 | 12.3 | 15.4 | 11.9 |
| Cumulative incidence | 32.5 | 37.2 | 28 |
| C. Persistence (%) | |||
| Visit to visit persistence | |||
| v1–v2 | 12.4 | 33.1 | 49.5 |
| v2–v3 | 15.6 | 36 | 48.8 |
| v3–v4 | 18.7 | 39.4 | 48.7 |
| v4–v5 | 20.9 | 44.3 | 48.2 |
| D. Apathy group (%) | |||
| Never apathy (across all visits) | 52 | 26.3 | 18.1 |
| Intermittent apathy (<50% across all visits) | 19.4 | 16 | 10.3 |
| Persistent apathy (≥ 50% across all visits) | 20.8 | 33 | 31 |
| Always apathy (100% across all visits) | 7.8 | 24.6 | 40.6 |
Abbreviation: CDR, Clinical Dementia Rating.
3.2.2. Incidence of apathy
Incidence of apathy between two consecutive visits, that is, proportion of participants who did not have an endorsement of apathy at a visit but had an endorsement of apathy at the next visit, fluctuated between 12% among those with baseline CDR = 0.5, 15% among those with CDR = 1, and 12% among those with CDR ≥ 2 (Table 2, Panel B). Cumulative incidence of apathy, that is, proportion of participants who did not have apathy at baseline, but had an endorsement of apathy in at least one visit over the entire follow‐up period, was 32.5% in those with baseline CDR = 0.5, 37.2% in those with CDR = 1, and 28.9% in those with CDR ≥ 2.
3.2.3. Persistence of apathy
Persistence of apathy between two consecutive visits, that is, proportion of participants who had an endorsement of apathy at two consecutive visits, increased over time in participants with baseline CDR = 0.5 and 1 (Table 2, Panel C). Specifically, among participants with baseline CDR = 0.5, visit‐to‐visit persistence of apathy increased from 12.4% between visits 1 and 2 to 20.9% between visits 4 and 5. Among participants with baseline CDR = 1, visit‐to‐visit persistence of apathy increased from 33.1%% between visits 1 and 2 to 44.3% between visits 4 and 5. Among participants with baseline CDR = 2 or 3, however, visit‐to‐visit persistence of apathy fluctuated around 49%.
Among participants with baseline CDR = 0.5, 19.4% had intermittent apathy, 20.8% had persistent apathy, and 7.8% always had apathy throughout all visits (Table 2, Panel D). The percentages of those within each of these categories among those with baseline CDR = 1 were 16.0%, 33.0%, and 24.6%, and among those with baseline CDR ≥ 2 were 10.3%, 31.0%, and 40.6%, respectively.
3.3. Estimated relationships between apathy, baseline CDR, and function
Adjusted LMM estimation results on the relationships between apathy, CDR, and function over time are shown in Table 3. Figure 1 plots predicted FAQ scores for different apathy groups by baseline CDR over time. In degenerative dementias such as AD, function declines as the dementia severity increases. As expected, FAQ scores worsened by 1.87 points per year for the entire sample (P < .001). Specifically, FAQ scores were 10.5 points higher (worse) in those with CDR = 1 and 19.5 points higher in those with CDR ≥ 2 compared to those with baseline CDR = 0.5 (all P < .001). Rate of decline in function was slower in those with baseline CDR = 1 (b± standard error [SE] = –0.321 ± 0.045) and baseline CDR ≥ 2 (b±SE = –1.746 ± 0.085, both P < .001) compared to participants with baseline CDR = 0.5, suggesting possible stabilization/plateauing. Full results and their interpretations are included in supporting information.
TABLE 3.
Mixed effects regression estimates of the relationships between apathy group, baseline CDR, and functional decline over time
| Variables | Coeff. | Std. Err. | P | [95% conf. interval] |
|---|---|---|---|---|
| Overall rate of worsening over time (by visit) | 1.870 | (0.035) | <.001 | [1.801,1.939] |
| Apathy group (reference: never apathetic in any visit) | ||||
| Intermittent apathy | 0.399 | (0.220) | .070 | [‐0.032,0.830] |
| Persistent apathy | 1.467 | (0.202) | <.001 | [1.070,1.863] |
| Always apathy | 2.519 | (0.253) | <.001 | [2.023,3.015] |
| Relationship between apathy group and rate of decline over time (interactions with time) | ||||
| Intermittent apathy | 0.849 | (0.050) | <.001 | [0.751,0.948] |
| Persistent apathy | 1.253 | (0.051) | <.001 | [1.154,1.353] |
| Always apathy | 1.264 | (0.071) | <.001 | [1.125,1.404] |
| Baseline CDR (reference: CDR = 0.5) | ||||
| CDR = 1 | 10.500 | (0.179) | <.001 | [10.149,10.851] |
| CDR ≥ 2 | 19.458 | (0.308) | <.001 | [18.855,20.062] |
| Relationship between baseline CDR and rate of decline over time (interactions with time) | ||||
| CDR = 1 | ‐0.321 | (0.045) | <.001 | [‐0.409,‐0.233] |
| CDR ≥ 2 | ‐1.746 | (0.085) | <.001 | [‐1.913,‐1.579] |
Notes: Model controlled for age, male, race/ethnic groups, education, indicator for living alone, years of follow up, any APOE ε4 genotype, indicator for missing APOE values, indicators for hypertension, hypercholesterolemia, diabetes, number of medications, Geriatric Depression Scale (GDS), and indicators for ADC sites. Full results and interpretations are included in supporting information.
Abbreviation: APOE, apolipoprotein E; CDR, Clinical Dementia Rating.
FIGURE 1.
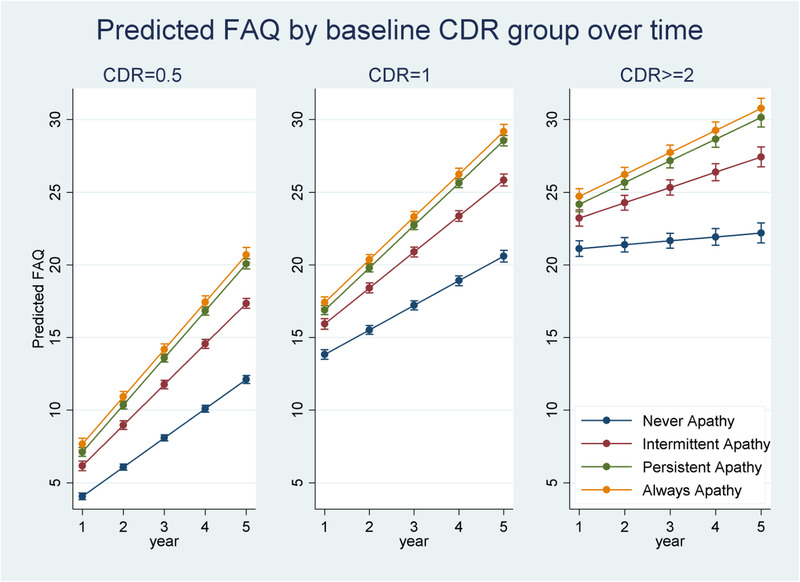
Predicted Functional Assessment Questionnaire (FAQ) at each apathy group by baseline Clinical Dementia Rating (CDR) over time
Beyond the effects of dementia severity and other covariates, more persistent apathy was associated with worse FAQ scores. Specifically, compared to those without apathy, FAQ scores were 1.467 and 2.519 points higher at baseline in those with persistent and always apathy (all P < .001) but participants with intermittent apathy had similar FAQ to those without apathy. Over time, rate of decline in function was faster in those with more persistent apathy. FAQ declined by almost one point per year faster in those with intermittent apathy (b±SE = 0.849 ± 0.050), 1.3 points faster in persistent apathy (b±SE = 1.253 ± 0.051), and 1.3 points faster in those who were always apathetic (b±SE = 1.264 ± 0.071, all P < .001).
4. DISCUSSION
Previous studies have demonstrated apathy as an independent construct in AD, distinct from depression in prevalence and in its impact on patient outcomes. 7 , 13 In this study we examined apathy and its impact over time in a large cohort of extensively characterized patients with an etiologic diagnosis of AD across the spectrum of disease severity, followed yearly for up to 14 years. We constructed parameters for prevalence, incidence, and persistence of apathy at each visit as well as cumulatively over time. The main results are: (1) apathy is highly prevalent across the range of disease severities with cumulative prevalence of 48%, 74%, and 82% in CDR groups of 0.5, 1.0, and 2.0, respectively; (2) at any visit in time, presence of apathy is higher in patients with more severe dementia; (3) presence of apathy increases over time in those with mild dementia, but seems to plateau in those with moderate/severe dementia; (4) there is variability in the persistence of apathy especially at earlier stages of disease, that is, CDR = 0.5 and 1.0; however, at moderate/severe stage dementia (CDR ≥ 2), there seems to be a plateau with fewer new incidents of apathy but steady persistence in those who have already developed it.
More importantly, we examined the independent relationship between persistence of apathy and rate of functional decline over time after controlling for participant demographics and dementia severity. Results show that compared to those without apathy, more persistent apathy is strongly associated with worse function cross‐sectionally and also with faster rate of decline in function longitudinally.
These results have important clinical implications. Specifically, apathy is common across the trajectory of AD course. Its impact is distinct from depression with increased functional decline over time. Further, the impact is present even in mildly affected individuals and those with questionable dementia, and not just in patients with moderate and severe cognitive impairment. Therefore, it is important to assess for apathy at all stages of cognitive complaint. Moreover, the likelihood of developing apathy increases over the duration of the disease. Apathy persists especially as dementia worsens. Functional decline follows persistence of apathy independently of cognitive decline. This pattern of persistence and continued association with poorer functional abilities suggests that apathy may be an important target for treatment with the goal of sustaining function. Finally, raising awareness of apathy as a distinct entity to family and caregivers is important as they may be able to identify modifiable antecedents and effective mitigators that could be the basis of behavioral interventions.
These results also have implications for clinical trial designs. Given that our data show that there is a long trajectory of decline and variability in persistence of the apathy syndrome, trials may need to be designed to ensure meaningful change in apathy could be measured and to focus on functional outcomes with relevant measures. Several trials have demonstrated potential for impacting apathy, cognition, and measures of function. 12 , 37 , 38 , 39 , 40 , 41 These studies have been limited by small numbers of participants, methodological heterogeneity, and brief duration of treatment (2 weeks to 3 months). Longer course of intervention and functional outcomes may have better demonstrated effect. 42 , 43
The study has several limitations. First, although this study is the largest cohort to date of individuals with apathy, it should be noted that the sample is not representative of the general population. For example, compared to the general population with 12 years of education, our sample consists of individuals with almost 3 more years of education who volunteered to participate in research at National Institute on Aging–supported ADCs. Similar to many large national studies such as the Aging, Demographics and Memory Study (ADAMS) in which the sample includes 8.5% non‐Hispanic Blacks and 5.1% Hispanics, 44 and the Alzheimer's Disease Neuroimaging Initiative study, which reported more than 95% of participants were non‐Hispanic White, 45 our sample with 11.2% non‐Hispanic Blacks and 8.4% Hispanics also is substantially less ethnically and racially diverse than the general population. Additionally, the sample also has relatively fewer medical and psychiatric illnesses than the general population of the same age. Second, we defined apathy based on clinical judgment. Although there is currently no standardized definition of apathy, clinician judgments of apathy in the UDS are made based on all clinical information available and may represent the best practices from tertiary medical centers across the United States. In a baseline analysis using the same sample, clinician judgment of presence of apathy was shown to be highly correlated with informant assessment of apathy reported in the NPI‐Q. 7 Over the past several decades there have been efforts to standardize the definition of apathy, develop assessment tools, and operationalize diagnostic criteria. More recently, a task force of international experts developed a set of new diagnostic criteria for apathy in dementia that are awaiting widespread adaptation. 46 , 47
Strengths of this report include the large population of study and the long duration of study; broad range of cognitive impairment severity (from questionable/mild to moderate/severe dementia); extensive clinical characterization of participants including neuropsychological testing, functional assessment, and APOE profiles; and case ascertainment of apathy by ADRC‐based dementia expert clinicians utilizing informant input.
In summary, our report demonstrates high prevalence, incidence, and persistence of apathy across the AD course. These findings, taken together with faster rate of decline in functional capacity due to apathy, highlight the importance of targeting the treatment and management of apathy, include outcome measures that focus on function, and accounting for variable persistence of the apathy syndrome so that meaningful change in apathy and function can be measured.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare. The sponsor played no role in the design, methods, participant recruitment, data collections, analysis, and preparation of the article.
AUTHOR CONTRIBUTIONS
Substantial contributions to conception or design of the work, or the acquisition, analysis, or interpretation of data for the work: Hillel T. Grossman, Mary Sano, Carolyn W. Zhu; drafting of the work: Carolyn W. Zhu; critical revision for important intellectual content: Gregory A. Elder, Judith Neugroschl, Corbett Schimming, Amy Aloysi, Laili Soleimani; final approval of the version to be published: Hillel T. Grossman, Mary Sano, Carolyn W. Zhu, Gregory A. Elder, Judith Neugroschl, Corbett Schimming, Amy Aloysi, Laili Soleimani; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Hillel T. Grossman, Mary Sano, Carolyn W. Zhu, Gregory A. Elder, Judith Neugroschl, Corbett Schimming, Amy Aloysi, Laili Soleimani.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This work was supported by NACC UDS (U01 AG016976) and Alzheimer Disease Research Center at Mount Sinai (U01 P50 AG005138). Hillel T. Grossman, Mary Sano, Gregory A. Elder, Corbett Schimming, Carolyn W. Zhu also are supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Grossman HT, Sano M, Aloysi A, et al. Prevalent, persistent, and impairing: Longitudinal course and impact of apathy in Alzheimer's disease. Alzheimer's Dement. 2021;13:e12169. 10.1002/dad2.12169
Hillel T. Grossman and Carolyn W. Zhu are co‐first authors.
DATA AVAILABILITY STATEMENT
Carolyn W. Zhu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. National Alzheimerr's Coordinating Center Uniform Data Set (NACC‐UDS) used in the current study are available through https://naccdata.org/requesting-data/data-request-process.
REFERENCES
- 1. Boyle PA, Malloy PF, Salloway S, Cahn‐Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214‐221. [PubMed] [Google Scholar]
- 2. Lam LC, Tam CW, Chiu HF, Lui VW. Depression and apathy affect functioning in community active subjects with questionable dementia and mild Alzheimer's disease. Int J Geriatr Psychiatry. 2007;22(5):431‐437. [DOI] [PubMed] [Google Scholar]
- 3. Landes AM, Sperry SD, Strauss ME. Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2005;17(3):342‐349. [DOI] [PubMed] [Google Scholar]
- 4. Lechowski L, Benoit M, Chassagne P, et al. Persistent apathy in Alzheimer's disease as an independent factor of rapid functional decline: the REAL Longitudinal Cohort Study. Int J Geriatr Psychiatry. 2009;24(4):341‐346. [DOI] [PubMed] [Google Scholar]
- 5. Onyike CU, Sheppard JM, Tschanz JT, et al. Epidemiology of apathy in older adults: the Cache County Study. Am J Geriatr Psychiatry. 2007;15(5):365‐375. [DOI] [PubMed] [Google Scholar]
- 6. Yeager CA, Hyer L. Apathy in dementia: relations with depression, functional competence, and quality of life. Psychol Rep. 2008;102(3):718‐722. [DOI] [PubMed] [Google Scholar]
- 7. Zhu CW, Grossman HT, Sano M. Why do they just sit? Apathy as a core symptom of Alzheimer disease. Am J Geriatr Psychiatry. 2019;27(4):395‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77(1):8‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Dalen JW, van Wanrooij LL, Moll van Charante EP, Brayne C, van Gool WA, Richard E. Association of apathy with risk of incident dementia: a systematic review and meta‐analysis. JAMA Psychiatry. 2018;75(10):1012‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez‐Salvador T, Lyketsos CG, Baker A, et al. Quality of life in dementia patients in long‐term care. Int J Geriatr Psychiatry. 2000;15(2):181‐189. [DOI] [PubMed] [Google Scholar]
- 11. Harrison F, Aerts L, Brodaty H. Apathy in dementia: systematic review of recent evidence on pharmacological treatments. Curr Psychiatry Rep. 2016;18(11):103. [DOI] [PubMed] [Google Scholar]
- 12. Theleritis C, Siarkos K, Politis AA, Katirtzoglou E, Politis A. A systematic review of non‐pharmacological treatments for apathy in dementia. Int J Geriatr Psychiatry. 2018;33(2):e177‐e192. [DOI] [PubMed] [Google Scholar]
- 13. Starkstein S, Hayhow B. Apathy in dementia: time to StandUp. Am J Geriatr Psychiatry. 2019;27(4):406‐407. [DOI] [PubMed] [Google Scholar]
- 14. Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157(5):708‐714. [DOI] [PubMed] [Google Scholar]
- 15. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475‐1483. [DOI] [PubMed] [Google Scholar]
- 16. Holtta EH, Laakkonen ML, Laurila JV, Strandberg TE, Tilvis RS, Pitkala KH. Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. J Am Med Dir Assoc. 2012;13(6):541‐545. [DOI] [PubMed] [Google Scholar]
- 17. Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta‐analysis. J Affect Disord. 2016;190:264‐271. [DOI] [PubMed] [Google Scholar]
- 18. Sherman C, Liu CS, Herrmann N, Lanctot KL. Prevalence, neurobiology, and treatments for apathy in prodromal dementia. Int Psychogeriatr. 2018;30(2):177‐184. [DOI] [PubMed] [Google Scholar]
- 19. van der Linde RM, Matthews FE, Dening T, Brayne C. Patterns and persistence of behavioural and psychological symptoms in those with cognitive impairment: the importance of apathy. Int J Geriatr Psychiatry. 2017;32(3):306‐315. [DOI] [PubMed] [Google Scholar]
- 20. Vilalta‐Franch J, Calvo‐Perxas L, Garre‐Olmo J, Turro‐Garriga O, Lopez‐Pousa S. Apathy syndrome in Alzheimer's disease epidemiology: prevalence, incidence, persistence, and risk and mortality factors. J Alzheimers Dis. 2013;33(2):535‐543. [DOI] [PubMed] [Google Scholar]
- 21. Steinberg M, Sheppard JM, Tschanz JT, et al. The incidence of mental and behavioral disturbances in dementia: the cache county study. J Neuropsychiatry Clin Neurosci. 2003;15(3):340‐345. [DOI] [PubMed] [Google Scholar]
- 22. Aalten P, de Vugt ME, Jaspers N, Jolles J, Verhey FR. The course of neuropsychiatric symptoms in dementia. Part I: findings from the two‐year longitudinal Maasbed study. Int J Geriatr Psychiatry. 2005;20(6):523‐530. [DOI] [PubMed] [Google Scholar]
- 23. Wetzels R, Zuidema S, de Jonghe J, Verhey F, Koopmans R. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2‐year period. Am J Geriatr Psychiatry. 2010;18(12):1054‐1065. [DOI] [PubMed] [Google Scholar]
- 24. Manera V, Fabre R, Stella F, et al. A survey on the prevalence of apathy in elderly people referred to specialized memory centers. Int J Geriatr Psychiatry. 2019;34(10):1369‐1377. [DOI] [PubMed] [Google Scholar]
- 25. Steinberg M, Tschanz JT, Corcoran C, et al. The persistence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2004;19(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 26. Helvik AS, Selbaek G, Saltyte Benth J, Roen I, Bergh S. The course of neuropsychiatric symptoms in nursing home residents from admission to 30‐month follow‐up. PLoS One. 2018;13(10):e0206147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249‐258. [DOI] [PubMed] [Google Scholar]
- 28. Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis Assoc Disord. 2006;20(4):210‐216. [DOI] [PubMed] [Google Scholar]
- 29. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233‐239. [DOI] [PubMed] [Google Scholar]
- 30. Cummings JL, McPherson S. Neuropsychiatric assessment of Alzheimer's disease and related dementias. Aging (Milano). 2001;13(3):240‐246. [DOI] [PubMed] [Google Scholar]
- 31. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 32. Spackman DE, Kadiyala S, Neumann PJ, Veenstra DL, Sullivan SD. The validity of dependence as a health outcome measure in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2013;28(3):245‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412‐2414. [DOI] [PubMed] [Google Scholar]
- 34. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 35. Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15‐item geriatric depression scale in functionally impaired, cognitively intact, community‐dwelling elderly primary care patients. J Am Geriatr Soc. 2005;53(9):1570‐1576. [DOI] [PubMed] [Google Scholar]
- 36. Stata Statistical Software: Release 13 [computer program]. Version 11. College Station, Texas: StataCorp LP; 2013. [Google Scholar]
- 37. Ruthirakuhan MT, Herrmann N, Abraham EH, Chan S, Lanctot KL. Pharmacological interventions for apathy in Alzheimer's disease. Cochrane Database Syst Rev. 2018;5:CD012197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sepehry AA, Sarai M, Hsiung GR. Pharmacological therapy for apathy in Alzheimer's disease: a systematic review and meta‐analysis. Can J Neurol Sci. 2017;44(3):267‐275. [DOI] [PubMed] [Google Scholar]
- 39. Boyle PA, Malloy PF. Treating apathy in Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17(1‐2):91‐99. [DOI] [PubMed] [Google Scholar]
- 40. Politis AM, Vozzella S, Mayer LS, Onyike CU, Baker AS, Lyketsos CG. A randomized, controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long‐term care. Int J Geriatr Psychiatry. 2004;19(11):1087‐1094. [DOI] [PubMed] [Google Scholar]
- 41. Theleritis CG, Siarkos KT, Politis AM. Unmet needs in pharmacological treatment of apathy in Alzheimer's disease: a systematic review. Front Pharmacol. 2019;10:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296‐301. [DOI] [PubMed] [Google Scholar]
- 43. Scherer RW, Drye L, Mintzer J, et al. The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial. Trials. 2018;19(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using medicare claims: insights from linked survey and administrative claims data. Alzheimers Dement. 2019;5:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alzheimer's Disease Neuroimaging Initiative (ADNI) Report Index: Demographics. Available at http://adni.loni.usc.edu/wp-content/uploads/2012/08/ADNI_Enroll_Demographics.pdf. Accessed December 10, 2020.
- 46. Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98‐104. [DOI] [PubMed] [Google Scholar]
- 47. Robert P, Lanctot KL, Aguera‐Ortiz L, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry. 2018;54:71‐76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Carolyn W. Zhu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. National Alzheimerr's Coordinating Center Uniform Data Set (NACC‐UDS) used in the current study are available through https://naccdata.org/requesting-data/data-request-process.


