Abstract
Introduction:
A major cause of injury and the second cause of death worldwide is stroke. Among several infectious agents considered as the risk factor of stroke, some pathogens demonstrated stronger robust associations with stroke. Proposing an accurate correlation between infectious microorganisms and stroke provides valuable information for early intervention and control of the infections.
Methods:
In this study, we searched the literature using the Web of Science, PMC/Medline via PubMed, and Scopus databases up to July 2018 without time and language restrictions. After quality assessment, 16 articles were included in the study. The whole data extraction process was independently conducted by two reviewers.
Results:
Based on the results of the studies, viruses, such as Hepatitis C virus (HCV), Hepatitis B virus (HBV), Human Immunodeficiency Virus (HIV), Herpes Simplex Virus Type-1, 2 (HSV-1, 2), Varicella-Zoster Virus (VZV or Chickenpox), and West Nile virus (WNV) seem to be common causes of ischemic stroke. Moreover, the association of other microbial categories, such as Streptococcus mutans (in bacteria), Toxocara spp. and Toxoplasma gondii (in parasites), and Rhizopus sp. (in fungi) with stroke was reported.
Conclusion:
Considering the adverse role of the above-mentioned microorganisms, it is necessary to implement some preventive measures for stroke treatment.
Keywords: Stroke, Infection, Viruses, Bacteria, Fungi
Highlights
Viruses seem to be common causes of ischemic stroke. Moreover, the association of other microbial categories, such as bacteria, parasites, and fungi, with stroke was reported.
Plain Language Summary
By promoting atherosclerosis, inflammation, and local thrombosis, common bacterial and viral infections between the categories of microorganisms may increase susceptibility to stroke. In this regard, among the supposed risk factors for stroke, infectious agents are considered. Overall, our data add to the growing body of evidence linking different microorganism categories, particularly viruses to stroke. In the other hand, considering the adverse role of the microorganisms is necessary to implement some preventive measures for stroke treatment.
1. Introduction
In adults, one of the major causes of disability and the second cause of death is stroke, globally. More than 85% of stroke-induced deaths worldwide concern countries with low socioeconomic status. Not all modifiable risk factors for stroke, however, are recognized (O’Donnell et al., 2010). In this regard, among the supposed risk factors for stroke, infectious agents are considered. Some diseases should be avoided and the occurrence of their disease could be minimized to cope with conventional risk factors for stroke (Fugate et al., 2014). There are two types of stroke, including ischemic and hemorrhagic (Sirven & Malamut, 2008). In most relevant investigations, ischemic stroke indicated a stronger association with systemic infection (Grau, Urbanek, & Palm, 2010), compared to hemorrhagic stroke (O’Donnell MJ et al., 2010; Fugate et al., 2014). Microorganisms, such as bacteria, viruses, fungi, and parasites are responsible for infectious diseases (Murray, 2017; Saberi, Akhondzadeh & Kazemi, 2018; Saberi, Roudbary, Ghayeghran, Kazemi & Hosseininezhad, 2018). By promoting atherosclerosis, inflammation, and local thrombosis, common bacterial and viral infections between the categories of microorganisms may increase susceptibility to stroke (Manousakis, Jensen, Chacon, Sattin, & Levine, 2009).
Herpesviruses are a family of persistent viruses, i.e., widespread; by inducing various inflammatory effects, they can periodically reactivate from latency to cause significant morbidity (Forbes et al. 2017). More than 130 herpesviruses are recognized (Brown & Newcomb, 2011). Nine of these viruses infect humans, as follows: Herpes Simplex Viruses 1 and 2 (HSV-1 & HSV-2, also known as HHV1 & HHV2, respectively, which can cause orolabial herpes & genital herpes), Varicella-Zoster Virus (VZV, also called HHV-3 & referred to as the cause of chickenpox & shingles), Epstein-Barr Virus (EBV or HHV-4, which leads to several diseases, including mononucleosis & some cancers), Human Herpesvirus 6A and 6B (HHV-6A & HHV-6B), Human Cytomegalovirus (HCMV or HHV-5), Human Herpesvirus 7 (HHV-7), and Kaposi’s Sarcoma-Associated Herpesvirus (KSHV, also known as HHV-8) (Carter J & Saunders V, 2007). West Nile Virus (WNV) is a neurotropic flavivirus, i.e., associated with other significant human pathogens, including dengue viruses, yellow fever viruses, and Japanese encephalitis viruses, and has been a major cause of viral encephalitis, globally (Samuel & Diamond, 2006). In the differential diagnosis of unexplained meningitis or encephalitis, WNV infection should be included because a high index of clinical concern occurs about the outcomes of relevant laboratory tests (Kemmerly, 2003).
Chronic infections, i.e., currently discussed as risk factors for stroke are mostly periodontitis and infections with Streptococcus mutans (Inenaga et al., 2018), Helicobacter pylori (Palm, Urbanek, & Grau, 2009; Ashtari, Shayegannejad, Saberi, Karkheyran, & Khosravi, 2008), and Chlamydia pneumoniae (Palm F et al., 2009; Ashtari, Shayegannejad, Saberi, Karkheyran, & Khosravi, 2007). Some have indicated that the risk of stroke may be associated with infection (Huang, Kang, & Zhao, 2013; Abdallah et al., 2018). We performed this systematic review to identify the most common microorganisms related to stroke.
2. Methods
Search strategy
In conjunction with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, this study was performed (Moher, Liberati, Tetzlaff, Altman, & The PRISMA Group; 2009). We also searched for international databases such as Medline/PMC (via PubMed), Scopus, and Web of Science. There was not any limitation in the date of articles (except until July 2018), language, and type of documents.
Table 1 defines the search strategy. Using the OR logic, the search words with identical meanings were combined and were coupled using the AND logic.
Table 1.
Search strategy applied in the PubMed, Scopus, and Web of Science databases
| Database | Syntax |
|---|---|
| PubMed | “(stroke[Title/Abstract]) AND (bacteri*[Title/Abstract] OR viral[Title/Abstract] OR virus[Title/Abstract] OR fung*[Title/Abstract] OR parasit*[Title/Abstract])” |
| Scopus | “(TITLE-ABS-KEY (stroke) AND TITLE-ABS-KEY (bacteria*) OR TITLE-ABS-KEY (viral OR virus) OR TITLE-ABS-KEY (fung*) OR TITLE-ABS-KEY (parasite*))” |
| “TOPIC: (stroke) AND TOPIC: (bacteria* OR viral OR virus OR fung* OR parasite*) | |
| Web of Science | TITLE: (stroke) AND TITLE: (bacteria* OR viral OR virus OR fung* OR parasite*)” |
In our systematic analysis, an article was omitted if it was:
A study on animal models
A study on vectors in therapy
An article studying post-stroke infection in patients such as surveying nosocomial infection in patients
An article studying one of the strokes along with other neurological diseases such as septic embolism, meningitis, stress, and endocarditis
A systematic review and meta-analysis
Quality assessment
To evaluate the content of papers, we used the Criteria for Reporting of Diagnostic Accuracy (STARD). The criteria for the consistency of completeness and reliability of the documentation of diagnostic precision studies were included (Scales Jr, Dahm, Sultan, Campbell-Scherer, & Devereaux, 2008). The methodological consistency (risk of bias) of the studies according to the Downs & Black criterion was independently evaluated by two reviewers (Downs & Black, 1998). By consensus, any differences were settled and reviewed by a third reviewer.
Data extraction
First, to assess the initial eligibility, we reviewed the titles and abstracts of the retrieved papers; if necessary, we analyzed the complete articles in-depth to select them for the study. Disagreements between authors in data extraction were settled by consensus.
The following details were extracted from the papers selected: first author’s name, year of publication, location of the study, type of study, number of cases participated, age of participants, case group, control group, stroke classification in case group, diagnosis test used for microorganism, infection type, and main results concerning the correlation between infection and stroke in patients.
3. Results
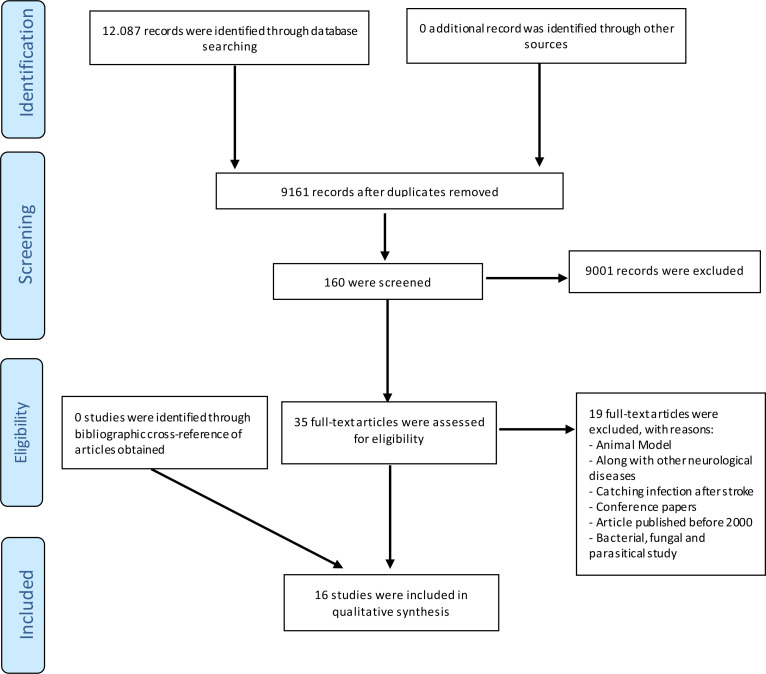
After eliminating the duplicate articles and reviewing the titles and abstracts, 9161 articles remained. Thirty-Five full texts were assessed for eligibility after removing 9001 unrelated records. Figure 1 demonstrates the search strategy based on the PRISMA Flow Diagram. Table 2 provides the overview of selected papers.
Figure 1.
Literature search and analysis collection flow diagram
Table 2.
The main features of studies investigating the role of infectious agents in strokes
| Author, Year, County | Study Type | Number of Participants | Age (year) | Case Group | Control Group | Stroke Classification in the Case Group | Diagnosis Test for Microorganism | Infection Agent | Main Results |
|---|---|---|---|---|---|---|---|---|---|
| Narayan et al. 2002, USA | Case report | 1 | A 13-year-old girl | - | - | Hemorrhagic (n=1) | None (patient had a known history of AIDS) | HIV | - A 13-year-old girl with a confirmed history of AIDS and a low CD-4 count had left basal ganglion stroke and full left middle cerebral artery (MCA) occlusion with fusiform dilatation of the left internal carotid artery proximal to and extending through the left A1 portion of the ophthalmic artery. The alleged etiology of vasculopa thy is HIV-associated. |
| Nogueras & Rodriguez. 2002, Spain | Observation | 1 | A 31-year-old man | - | - | Ischemic (n=1) | PCR to reveal the presence of HIV-integrated DNA in the tissues | HIV | -A 31-year-old, HIV infected man with multi-infarction in the brain and recurrent stroke - Histologic studies showed HIV-related angiitis of CNS. |
| Moriuchi et al. 2000, Japan | Case Report | 1 | A 12-year-old girl | - | - | Ischemic (n=1) | -PCR to amplify VZV-specific DNA sequence in her CSF sample/- specific intrathecal antibody | VZV (or HHV-3 or Chick enpox) | - In both immunocom petent and immuno compromised hosts, varicella-zoster virus (VZV) vasculopathy in the (CNS) is the cause of stroke. This disease has been associated with both varicella (primary infection) and herpes zoster (reactivation). |
| Amlie-Lefond & Gilden, 2016 USA | Review | - | - | - | - | - | - VZV-specific antigen in cerebral arteries | - VZV DNA in vitreous fluid VZV (or HHV-3 or Chicken pox) | - VZV vasculopathy is a catalyst for stroke in children and adults. It affects large vessels in infants, while it includes large and tiny arteries in adults. Also, VZV causes giant cell arteritis immunopathology. - In infants, vaccination effectively prevents varicella. |
| Powell et al. 2015, Mexico | Case Report | 1 | A 51-year-old man | - | - | Ischemic (n=1) | ELISA to determine VZV immunoglobulin G (IgG) ratio in his CSF sample serum PCR to amplify VZV-specific DNA sequence in his CSF sample | VZV (or HHV-3 or Chicken pox) | - The immunocompromised case was treated with high dose intravenous methyl prednisolone and 2 doses of 1 g of rituximab. (due to pauci-immune glomerulo nephritis) -Dermatomal herpes zoster followed by a stroke with a good outcome by intrave nous acyclovir. |
| Arase et al. 2014, Japan | Cohort | 7,635 | >30 to <80 | 4.646 | - | Hemorrhagic (n=24) Non hemorrhagic (n=4.621) | ELISA PCR to HCV-genotyping COBAS TaqMan HCV test to determine HCV-RNA | HCV | HCV clearance reduces intracerebral hemorrhagic stroke. -The HCV clearance prevents the aggravation of prothrombin deficit and platelets diminution. |
| Liao et al. 2012, Taiwan | Cohort | 20,470 | 20–70 | 4,094 | 16,376 | None | None | HCV | - HR of stroke is 1.27 (95% CI: 1.14 to 1.41) with hepatitis C infection compared with non-infected patients. - Chronic infection with hepatitis C increases the risk of stroke. |
| Sung et al. 2007, Korea | Cohort | 521.421 | 30– 64 men | 13 902 HBsAg-seropositive men with normal LFT and normal clinical history; 203 294 HBsAg seronegative men with abnormal LFT (196 192), a clinical history of liver dysfunction, (1353), or both (5749); and 28 772 HBsAg-seropositive men with abnormal LFT (22 096), a history of liver dysfunction (631), or both (6045). | 275 453 HBsAg-seronegative men with normal LFT and no clinical history of liver dysfunction | Ischemic (n= 4223) Hemorrhagic (n= 2523) |
Reverse hemagglutination ELISA | HBV | - A decreased risk of ischaemic stroke and an increased risk of hemorrhagic stroke was correlated with seropositivity of HBs Ag. - This tends to be secondary to liver dysfunction associated with viral hepatitis B infection, not due to proinflammatory effects triggered by atherothrombosis. |
| Tseng et al. 2016, Taiwan | Cohort | 1,000,000 | 22,303 | 89,212 | Ischemic (n= 1258) | None | HBV | - HBV reduces the risk of acute ischemic stroke. | |
| Elkind et al. 2016, New York | Original | 326 | 3.1–14.3 | 326 | 115 | Ischemic (n=187) | ELISA IgM/IgG antibodies to herpes simplex viruses (HSV) 1 and 2 | Herpes viruses (HSV-1,2-) | - Past infection with HSV did not increase the risk of stroke – Acute HSV infection doubled the odds of child hood AIS. |
| Sas et al. 2009, The Netherlands | Case Report | 1 | A 3-year-old girl | - | - | Ischemic (n=1) | PCR to amplify HSV-1 DNA sequence in her CSF and serum sample |
HSV-1 | - A unusual cause of pediatric arterial ischemic stroke is herpes simplex virus encephalitis. - A healthy 3-year-old gir with ischemic stroke due to HSV 1 encephalitis was confirmed by PCR and anti-HSV-1 IgG serocon version. |
| Terlizzi et al. 2013, Italy | Case Report | 1 | A 10-year-old girl | - | - | Ischemic (n=1) | ELISA (Ig M and Ig G to HSV1)PCR | HSV-1 | - A 10-year-old child with ischemic stroke due to HSV1 infection was confirmed by Ig M to HSV1 in serum and seroconversion to Ig G. CSF PCR was negative. |
| Alexander et al. 2006, USA | Case Report | 1 | A 9-year-old gir | - | - | Ischemic (n=1) | ELISA | WNV | - In areas where West Nile virus transmission is present, West Nile virus is in the differential diagnosis of stroke etiology. |
HIV: Human Immunodeficiency Virus; CSF: Cerebrospinal Fluid; VZV: Varicella-zoster Virus; MTB: Mycobacterium Tuberculosis; TP: Treponema Pallidum; HHV-3: Human Herpesvirus Type 3; WNV: West Nile Virus; CMV: Cytomegalovirus; EBV: Epstein Barr Virus; AIS: Arterial Ischemic Stroke; HCV: Hepatitis C Virus; HBV: Hepatitis B Virus; HAV: Hepatitis A Virus; HBSAg: Hepatitis B Virus Surface Antigen; HSV-1: Herpes Simplex Virus Type-1; ELISA: Enzyme-linked Immunosorbent Assay; EIA: Enzyme Immunoassay; RIA: Radioimmunoassay; CES: Cardioembolic Stroke; NVAF: Nonvalvular Atrial Fibrillation.
DiscussionThis systematic review comprehensively presented studies of the risk of subsequent stroke in infections and their reactivation. A greater understanding of this relationship contributes to educating strategies for the prevention of public health strokes.
Human Immunodeficiency Virus (HIV)
Cerebral stroke is a rare complication of HIV infection (Tipping, de Villiers, Wainwright, Candy, & Bryer, 2007). Numerous case report studies highlighted a different case of stroke that HIV was found to be the stroke etiology (Manwani, Stretz, & Sansing, 2016), especially in children or young patients. The laboratory examination of HIV is therefore mandatory in pediatric strokes, especially in the high-risk population (Narayan, Samuels, & Barrow, 2002; Nogueras C et al., 2002). The cause of stroke in such patients is HIV-associated vasculopathy (Tipping et al., 2007). The HIV associated-vasculopathy subtypes included non-atherosclerotic vasculopathy, accelerated atherosclerosis, and HIV-associated vasculitis (Benjamin et al., 2017). Two other proposed vasculopathy mechanisms are the inflammation of the vessel wall by an opportunistic infection (Benjamin et al., 2017) and rarely the primary angiitis of the CNS in HIV-infected patients, as the leading causes of stroke (Nogueras et al., 2002).
In a study by Tipping in South Africa on the stroke, 6.1% of stroke patients were HIV infected, 96% were cerebral infarction, and the others were intracerebral hemorrhage (4%). HIV infection was proposed as a stroke risk factor (OR=6.4, 95%CI: 3.1–13.2). In 20 % of patients, HIV-associated vasculopathy was reported. Moreover, 11% of them, revealed extra-cranially (11%) as total or significant carotid occlusion. The autopsy of one patient with occlusion of the extracranial internal carotid artery by thrombus demonstrated the fibrosis of atrial adventitia with neovascularisation and lymphoplasmacytic infiltration with positive for neutrophil elastase, CD3, CD8, and CD68 and negative for CD20 and P24.
Intracranial vasculopathy was characterized by severe intracranial vascular degenerative ectasia with superimposed thrombosis in HIV-positive patients. Interestingly, the CD4 count was maintained relative to intracranial vasculopathy in patients with extracranial vasculopathy (Tipping et al., 2007).
Smith et al. conducted a study in the UK on adult ischemic stroke patients (64 HIV-infected & 107 HIV-uninfected); accordingly, they argued that the most common etiology was HIV-associated vasculopathy (38%), followed by opportunistic infections (25%). Furthermore, accelerated atherosclerotic vasculopathy patients were not as immunosuppressed as other subtypes of HIV-related vasculopathy (Smith, 2017). In a large number (25%) of patients, stroke was observed by the mechanism of the Immune Reconstitution-Like Syndrome (IRIS) after Antiretroviral Therapy (ART) (Benjamin et al., 2017). After an immunodeficient condition, IRIS occurs during immune system recovery. It is associated with a rapid decrease in HIV viral load (of 2 logs), a low nadir, then an increased CD4+ count following the introduction of ART, and anemia (Haddow et al., 2009; Johnson & Nath, 2014). The mechanism of infiltration of active T cells may explain this phenomenon (Johnson & Nath, 2014).
Herpes Simplex Virus (HSV)
An unusual cause of pediatric arterial ischemic stroke is HSV encephalitis (Askalan R et al., 2001). Elkind et al. assessed the role of HSV in children Acute Ischemic Stroke (AIS) and by the serological study found that acute herpesvirus infection especially HSV-1 doubled the odds of childhood AIS; even if the infection is sub-clinical. However, based on their results, past infection was not associated with stroke (Elkind et al., 2016). Furthermore, there is some limited case report in this field, of which we report two cases below. A 3-year-old girl with bilateral occipital ischemic stroke, due to HSV-1 encephalitis, was identified by Sas et al. HSV-PCR in serum and cerebrospinal fluid and anti-HSV-1 immunoglobulin G seroconversion, occurring within 5 days of admission were confirmed. Both results were consistent with primary infection with HSV-1 (Sas et al., 2009). Terlizzi et al. also described another arterial ischemic stroke after primary HSV-1 infection in a 10-year-old girl. Ig-M antibodies to HSV1 presented relatively high specificity and positive predictive value. Seroconversion to immunoglobulin G antibodies occurred 16 days after admission, which makes the correlation stronger. CSFPCR for HSV-1 was negative (Terlizzi et al., 2014). Elbers et al. described that if the CSF test is performed in 3–14 days after symptoms onset, the negative HSV-PCR does not rule out the acute infection (Elbers et al., 2007).
Varicella-Zoster Virus (VZV)
A common cause of stroke is VZV. Varicella is associated with approximately one-third of arterial ischaemic strokes in the pediatric population (Askalan et al., 2001), i.e., a neurotropic alpha-herpesvirus. Chickenpox is the main infection in childhood. To our understanding, during which, viruses in neural ganglia, including dorsal root ganglia, cranial nerve, and autonomic ganglia, become latent (Mahalingam et al., 1990). Herpes zoster (shingles) reactivates the virus and, less commonly, meningoencephalitis, myelitis, numerous severe eye disorders, and VZV vasculopathy can occur when immunity to VZV mediated by cells decreases with immunosuppression or increasing age. All these complications may occur in the absence of a rash (Amlie-Lefond & Gilden, 2016). Interestingly, in zoster patients receiving oral anti-viral therapy, it has been suggested that the risk of stroke decreases relative to that of untreated zoster patients (Nagel, Jones, & Wyborny, 2017). VZV-Associated stroke can be followed primarily by varicella infection, Herpes Zoster (HZO), or other forms of herpes zoster in immunocompromised and immunocompetent individuals. In this respect, Moriuchi et al. explained various VZV-associated stroke syndromes and vasculitis of the CNS in the literature (Moriuchi & Rodriguez, 2000).
The study of the different databases revealed an increase in stroke rate after zoster in younger adults and after ophthalmic zoster, especially early after zoster. The records of the Taiwanese National Health Research Institute demonstrated a 30% increased risk of stroke within 1 year after zoster (Kang, Ho, Chen, & Lin, 2009), which increases by 4.5-fold with ophthalmic zoster (Lin et al., 2010). The Danish National Registry reported a 126 % increased risk of stroke within 2 weeks after zoster, a 17 % increased risk from 2 weeks to 1 year after zoster, and a 5 % increase in the risk of stroke after the first year (Sreenivasan et al., 2013). Data from the general practice database of the UK Health Improvement Network showed a 1.15-fold increase in the risk of Transient Ischemic Attacks (TIAs), especially in patients under 40 years of age. The risk of stroke and TIAs increased by 1.74 and 2.42 folds, respectively, in the category of patients under 40 years of age (Breuer, Pacou, Gautier, & Brown, 2014). Furthermore, the UK Clinical Practice Research Datalink assessment indicated that over time, the risk of stroke after zoster decreased, with a statistically significant age-adjusted incidence of 1.63 at 1–4 weeks, 1.42 at 5–12 weeks, and 1.23 at 13–26 weeks after zoster, and no decrease at later times (Langan, Minassian, Smeeth, & Thomas, 2014). A Swedish register-based longitudinal study found in all age groups Within 1 year of Zoster, a 1.34-fold elevated risk of stroke (Sundstrom et al., 2015). In the UK report, in patients under the age of 40 years, the risk of stroke enhanced 10.3 fold within 1 year of zoster (Breuer et al., 2014). Another UK research found that within 2 weeks of zoster, the risk of stroke increased 2.4-fold (Minassian, Thomas, Smeeth, Douglas, Brauer, & Langan, 2015). The risk of stroke increased 1.53 fold in the first US population-based study within 3 months after zoster (Yawn, Wollan, Nagel, & Gilden, 2016).
Several pathophysiologies were proposed for the role of VZV in producing stroke, as follows:
Nonspecific inflammation and transient thrombophilia, especially a deficiency in protein S (Fu, Nguyen, & Sanossian, 1994).
Triggering the immunopathology of giant cell arteritis (Amlie-Lefond & Gilden, 2016).
VZV vasculopathy (the predominant pathophysiology), which causes 2 important trends: a large vessel (uni-focal) and a small vessel (multifocal) (Gilden et al., 2002). In this regard, large vessel involvement more commonly affects the immunocompetent with obvious neurological deficit, whereas small vessel involvement more commonly affects the immunocompromised individuals with nonspecific Central Nervous System (CNS) involvement. A case was reported by Powell et al. in this category (Table 2) (Powell, Patel, Franco-Paredes, & Lopez, 2015).
Pathophysiology of VZV Vasculopathy: In pathophysiology, children and adults are equivalent. As intracerebral arteries and veins receive a rich supply of trigeminal afferent fibers, when the virus reactivates during zoster; especially after ophthalmic zoster, it spreads transactionally to intracerebral arteries from trigeminal or other cranial nerve ganglia. VZV is the only human virus that has been demonstrated to replicate in cerebral arteries. VZV infects and induces granulomatous arteritis at all levels of the cerebral arteries.
Pathology of VZV vasculopathy: It inflicts granulomatous arteritis, as previously described. Cowdry A inclusion bodies, multinucleated giant cells, herpes virions, VZV DNA, and VZV antigen are revealed by its pathological and virological examination, suggesting VZV productive arterial infection.
Virologic Confirmation of VZV Vasculopathy: The presence of VZV vasculopathy is confirmed by positive VZV PCR (30%) or anti-VZV antibody (anti-VZV IgG antibody) (93%) in CSF. VZV vasculopathy does not rule out the absence of cells in the CSF. It could be due to an early CSF review during the first weeks after a stroke (3–14 days) (Amlie-Lefond & Gilden, 2016).
Hepatitis C Virus (HCV)
Certainly, the correlation between HCV infection and atherosclerosis is controversial (White, Ratziu, & El-Serag, 2008). However, the mechanisms that can explain its role in stroke are atherogenesis and atherosclerosis in carotid and aortic (Ishizaka et al., 2002). In human carotid plaques, HCV RNA was observed, and there is clear evidence of an association between atherosclerosis and HCV infection (Boddi et al., 2010). In the carotid wall, this may be due to a persistent inflammatory mechanism (Ishizaka et al., 2002).
In a study conducted by Liao et al. on a longitudinal population-based cohort, the Taiwan National Health Insurance Research Database reported 4,094 newly diagnosed adults with HCV and 16376 matched adults without HCV in 2002–2004. Stroke cases from 2002–2008 were reported. For individuals with HCV and without HCV, the combined risk of stroke was equal to 2.5% and 1.9%, respectively (P<0.0001), with modified Hazard Ratios (HRs) of stroke being 1.27 for HCV patients (95%CI: 1.14–1.41) (Liao et al., 2012).
The role of HCV clearance in reducing ischemic stroke development remains unassessed. Notably, it was treated for a hemorrhagic intracerebral stroke. The hemorrhagic propensity increases due to prothrombin deficiency and platelet diminution in liver dysfunction, and HCV treatment results in a decrease in hemorrhagic stroke (Arase et al., 2014). For further information in this regard, it is suggested to assess the role of HCV treatment on the development of thrombotic and atherosclerotic events.
Hepatitis B Virus (HBV)
In some studies, HBsAg seropositivity was associated with an increased risk of hemorrhagic stroke and a decreased risk of ischemic stroke, i.e., interesting and contradictory to the other microorganisms examined (Tong, Wang, Xu, Yang, & Xiong, 2005). Tseng et al. stated that, even with rising age, HBV decreases the risk of ischemic stroke relative to the general population (Tseng, Muo, Hsu, & Kao, 2016).
Additionally, the risk of ischemic and hemorrhagic stroke was calculated by Sung et al. with multivariable-adjusted HRs (95%CIs) of 0.79 (0.68, 0.90) and 1.33 (1.15, 1.52), respectively. With healthy liver function, the risk of stroke does not improve with HBsAg seropositivity; however, the risk of hemorrhagic stroke and ischaemic stroke rises with liver failure relative to HBsAg-seronegative males. This association, therefore, depends on the liver function, which has no pro-inflammatory effect on the patient’s coagulation condition (Sung, Song, Choi, Ebrahim, & Davey Smith, 2007). Contrarily, Pearce et al. analyzed serological evidence of the previous infection based on HBV immunoglobulin G seropositivity among 13,904 National Health and Nutrition Review Survey III (NHANES III) respondents; consequently, they found a link between HBV seropositivity and stroke among 20–59 year-olds (Pearce, Bracher, Jones, & Kruszon-Moran, 2018) without the separation of the ischemic from a hemorrhagic stroke that can affect the results.
West Nile Virus (WNV)
WNV is transmitted to humans through blood transfusions, infected mosquitoes, organ transplantation, and even trans-utero and probably through breastfeeding (Hayes & O’Leary, 2004). The neurologic signs of WNV infection are a spectrum of meningoencephalitis and WNV poliomyelitis-like syndrome (Sejvar, 2004) and motor neuropathy (Daroff, 2015). However, the isolated vasculitis of CNS due to this infection, which resulted in an ischemic stroke, was reported. The young girl with a left middle cerebral artery stroke was recorded by Alexander et al. Serum and CSF acute and subacute convalescent WNV antibodies (IgG and IgM) were greater than the healthy range. Cross-Reactivity with other flaviviruses was ruled out in the state reference laboratory by parallel monoclonal antibody testing. The presence of WNV-IgM antibody in CSF fluid strongly indicates WNV infection, based on the Centers for Disease Control and Prevention (CDC) criteria. The stroke in this child is therefore most likely due to cerebral vasculitis that is secondary to infection with WNV (Alexander, Lasky, & Graf, 2006).
According to our findings, the frequency of other microorganisms, such as fungi, bacteria, and parasites, was much lower than viruses in patients with stroke. Therefore, we have only explained one or two common cases in each category, i.e., reported in more studies.
Toxoplasma gondii, Toxocara spp.
While a high cumulative burden of viral and bacterial pathogens can increase the risk of stroke, the contribution of parasitic infections has rarely been studied concerning the cumulative burden of the pathogen and risk of stroke (Pearce et al., 2018). In this regard, few studies considered the role of parasites in studies of the pathogen burden and the impact on the outcome of stroke, even though a parasite infection, like T. Gondii is widespread in the United States, as is Toxocara spp. (Won, Kruszon-Moran, Schantz, & Jones, 2008). A correlation between serological evidence of prior T. gondii and Toxocara infection with stroke was reported by Pearce and associates (Pearce et al., 2018).
Rhizopus sp.
Although invasive CNS fungal infections are rare, the associated morbidity and mortality, especially among immunosuppressed individuals, can be very high (Panackal & Williamson, 2015).
Several genera belonging to the Moraceae family, such as Rhizopus, Mucor, and Absidia, cause Zygomycosis (Mucormycosis). In 95% of instances, Rhizopus is the offending organism (Rhizopus arrhizus and Rhizopus oryzae). It is often difficult to recover Zygomycosis fungi from clinical samples; however, they can be easily cultivated on routine mycological media, as the hyphal elements get weakened during biopsy procedure or laboratory processing and are thus made non-viable. For the diagnosis of zygomycosis, no standard rapid serological method is available (Shankar, et al., 2007). Fu et al. (2015) indicated that clinicians should consider invasive sinusitis as a rare cause of stroke in diabetics. The prognosis is very poor once the subarachnoid space and the basal arteries of the brain have been invaded. Early identification and care are the keys to improving performance, and it is important to evaluate the neuroimaging sinuses in all cases of stroke.
Streptococcus Mutans (SM)
Specific types of SM known as dental caries pathogens possess a Collagen-Binding Protein (CBP) among human oral bacteria, which is determined by the corresponding gene. They demonstrate inhibition of platelet aggregation and activation of Matrix Metalloproteinase-9 (MMP-9). Inenaga et al. found ischemic and hemorrhagic stroke rates by saliva sampling and culture obtained from 429 stroke patients (Inenaga et al., 2018). Nakano et al. documented that CBP-positive SM is a potential risk factor for hemorrhagic stroke, not only through the inhibition of platelet aggregation but also in injured arteries by activated MMP-9 (Nakano et al., 2011).
5. Conclusion
Overall, these data add to the growing body of evidence linking different microorganism categories, particularly viruses to stroke. Further research is required, however, to understand the pathogenesis mechanisms of microorganisms, as a risk factor for stroke in patients and to determine the effect of therapy on risk.
Suggestions: We require a meta-analysis to better clarify this systematic review; To be more precise, it is recommended to use more specialized keywords and specifically search with one microorganism’s name; Studies included may have significantly different methodologies that might limit our ability to draw reliable conclusions from the existing evidence base.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the 2013 version of the Declaration of Helsinki.
Acknowledgments
The authors would like to thank the Deputy of Research and Technology, Guilan University of Medical Sciences, Rasht.
Footnotes
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors’ contributions
Conceptualization and supervision: Alia Saberi, Shahin Akhondzadeh; Methodology: Samaneh Kazemi, Samira Kazemi; Investigation: Alia Saberi, Samaneh Kazemi, Samira Kazemii; Writing the original draft, review, and editing: All authors.
Conflict of interest
The authors declared no conflicts of interest.
References
- Abdallah A., Chang J. L., O’Carroll C. B., Musubire A., Chow F. C., Wilson A. L., et al. (2018). Stroke in human immunodeficiency virus-infected individuals in Sub-Saharan Africa (SSA): A systematic review. Journal of Stroke and Cerebrovascular Diseases, 27(7), 1828–36. [DOI: 10.1016/j.jstrokecerebrovasdis.2018.02.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. J., Lasky A. S., Graf W. D. (2006). Stroke associated with central nervous system vasculitis after West Nile virus infection. Journal of Child Neurology, 21(7), 623–5. [DOI: 10.1177/08830738060210071301] [DOI] [PubMed] [Google Scholar]
- Amlie-Lefond C., Gilden D. (2016). Varicella zoster virus: A common cause of stroke in children and adults. Journal of Stroke and Cerebrovascular Diseases, 25(7), 1561–9. [DOI: 10.1016/j.jstrokecerebrovasdis.2016.03.052] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase Y., Kobayashi M., Kawamura Y., Suzuki F., Suzuki Y., Akuta N., et al. (2014). Impact of virus clearance for the development of hemorrhagic stroke in chronic hepatitis C. Journal of Medical Virology, 86(1), 169–75. [DOI: 10.1002/jmv.23777] [DOI] [PubMed] [Google Scholar]
- Ashtari F., Shayegannejad V., Saberi A., Rabiee E. (2008). Relationship between helicobacter pylori immunoglobulin G antibody and thrombotic ischemic stroke. Acta Medica Iranica, 46(4), 303–6. https://acta.tums.ac.ir/index.php/acta/article/view/3488 [Google Scholar]
- Ashtari F., Shayegannejad V., Saberi A., Karkheyran F., Khosravi E. (2007). [Chlamydia pneumoniae infection and thrombotic ischemic stroke (Persian)]. Journal of Isfahan Medical School, 24(83), 8–13. http://jims.mui.ac.ir/index.php/jims/article/view/31 [Google Scholar]
- Askalan R., Laughlin S., Mayank S., Chan A., MacGregor D., Andrew M., et al. (2001). Chickenpox and stroke in childhood: A study of frequency and causation. Stroke, 32(6), 1257–62. [DOI: 10.1161/01.STR.32.6.1257] [DOI] [PubMed] [Google Scholar]
- Benjamin L. A., Allain T. J., Mzinganjira H., Connor M. D., Smith C., Lucas S., et al. (2017). The role of human immunodeficiency virus-associated vasculopathy in the etiology of stroke. The Journal of Infectious Diseases, 216(5), 545–53. [DOI: 10.1093/infdis/jix340] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddi M., Abbate R., Chellini B., Giusti B., Giannini C., Pratesi G., et al. (2010). Hepatitis C virus RNA localization in human carotid plaques. Journal of Clinical Virology, 47(1), 72–5. [DOI: 10.1016/j.jcv.2009.10.005] [DOI] [PubMed] [Google Scholar]
- Breuer J., Pacou M., Gautier A., Brown M. M. (2014). Herpes zoster as a risk factor for stroke and TIA: A retrospective cohort study in the UK. Neurology, 83(2), e27–33. [DOI: 10.1212/WNL.0000000000000584] [DOI] [PubMed] [Google Scholar]
- Brown J. C., Newcomb W. W. (2011). Herpesvirus capsid assembly: Insights from structural analysis. Current Opinion in Virology, 1(2), 142–9. [DOI: 10.1016/j.coviro.2011.06.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J., Saunders V. (2007). Virology: Principles and applications. Chichester: John Wiley & Sons. https://books.google.com/books?id=EKRgZCdr-74C&dq [Google Scholar]
- Downs S. H., Black N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomised studies of health care interventions. Journal of Epidemiology & Community Health, 52(6), 377–84. [DOI: 10.1136/jech.52.6.377] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers J. M., Bitnun A., Richardson S. E., Ford-Jones E. L., Tellier R., Wald R. M., et al. (2007). A 12-year prospective study of childhood herpes simplex encephalitis: Is there a broader spectrum of disease? Pediatrics, 119(2), e399–407. [DOI: 10.1542/peds.2006-1494] [DOI] [PubMed] [Google Scholar]
- Elkind M. S. V., Hills N. K., Glaser C. A., Lo W. D., Amlie-Lefond C., Dlamini N., et al. (2016). Herpesvirus infections and childhood arterial ischemic stroke: Results of the VIPS study. Circulation, 133(8), 732–41. [DOI: 10.1161/CIRCULATIONAHA.115.018595] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes H. J., Benjamin L., Breuer J., Brown M. M., Langan S. M., Minassian C., et al. (2017). The association between human herpesvirus infections and stroke: A systematic review protocol. BMJ Open, 7(5), e016427. [DOI: 10.1136/bmjopen-2017-016427] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. A., Nguyen P. L., Sanossian N. (2015). Basilar artery territory stroke secondary to invasive fungal sphenoid sinusitis: A case report and review of the literature. Case Reports in Neurology, 7(1), 51–8. [DOI: 10.1159/000380761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugate J. E., Lyons J. L., Thakur K. T., Smith B. R., Hedley-Whyte E. T., Mateen F. J. (2014). Infectious causes of stroke. The Lancet Infectious Diseases, 14(9), 869–80. [DOI: 10.1016/S1473-3099(14)70755-8] [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Mahalingam R., Cohrs R. J., Kleinschmidt-De-Masters B. K., Forghani B. (2002). The protean manifestations of varicella-zoster virus vasculopathy. Journal of Neurovirology, 8(Suppl 2), 75–9. [DOI: 10.1080/13550280290167902] [DOI] [PubMed] [Google Scholar]
- Grau A. J., Urbanek Ch., Palm F. (2010). Common infections and the risk of stroke. Nature Reviews Neurology, 6(12), 681–94. [DOI: 10.1038/nrneurol.2010.163] [DOI] [PubMed] [Google Scholar]
- Haddow L. J., Easterbrook P. J., Mosam A., Khanyile N. G., Parboosing R., Moodley P., et al. (2009). Defining immune reconstitution inflammatory syndrome: Evaluation of expert opinion versus 2 case definitions in a South African cohort. Clinical Infectious Diseases, 49(9), 1424–32. [DOI: 10.1086/630208] [DOI] [PubMed] [Google Scholar]
- Hayes E. B., O’Leary D. R. (2004). West Nile virus infection: A pediatric perspective. Pediatrics, 113(5), 1375–81. [DOI: 10.1542/peds.113.5.1375] [DOI] [PubMed] [Google Scholar]
- Huang H., Kang R., Zhao Zh. (2013). Hepatitis C virus infection and risk of stroke: A systematic review and meta-analysis. PLoS One, 8(11), e81305. [DOI: 10.1371/journal.pone.0081305] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inenaga Ch., Hokamura K., Nakano K., Nomura R., Naka Sh., Ohashi T., et al. (2018). A potential new risk factor for stroke: Streptococcus mutans with collagen-binding protein. World Neurosurgery, 113, e77–81. [DOI: 10.1016/j.wneu.2018.01.158] [DOI] [PubMed] [Google Scholar]
- Ishizaka N., Ishizaka Y., Takahashi E., Tooda E. I., Hashimoto H., Ryozo Nagai R., et al. (2002). Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. The Lancet, 359(9301), 133–5. [DOI: 10.1016/S0140-6736(02)07339-7] [DOI] [PubMed] [Google Scholar]
- Johnson T. P., Nath A. (2014). New insights into immune reconstitution inflammatory syndrome of the central nervous system. Current Opinion in HIV and AIDS, 9(6), 572–8. [DOI: 10.1097/COH.0000000000000107] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. H., Ho J. D., Chen Y. H., Lin H. C. (2009). Increased risk of stroke after a herpes zoster attack: A population-based follow-up study. Stroke, 40(11), 3443–8. [DOI: 10.1161/STROKEAHA.109.562017] [DOI] [PubMed] [Google Scholar]
- Daroff R. Bradley’s neurology in clinical practice. Amsterdam: Elsevier; 2015. https://www.elsevier.com/books/bradleys-neurology-in-clinical-practice-2-volume-set/daroff/978-0-323-28783-8 [Google Scholar]
- Kemmerly S. A. (2003). Diagnosis and treatment of West Nile infections. The Ochsner Journal, 5(3), 16–7. [PMC free article] [PubMed] [Google Scholar]
- Langan S. M., Minassian C., Smeeth L., Thomas S. L. (2014). Risk of stroke following herpes zoster: A self-controlled case-series study. Clinical Infectious Diseases, 58(11), 1497–503. [DOI: 10.1093/cid/ciu098] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. C., Su T. C., Sung F. C., Chou W. H., Chen T. L. (2012). Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One, 7(2), e31527. [DOI: 10.1371/journal.pone.0031527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. C., Chien C. W., Ho J. D. (2010). Herpes zoster ophthalmicus and the risk of stroke: A population-based follow-up study. Neurology, 74(10), 792–7. [DOI: 10.1212/WNL.0b013e3181d31e5c] [DOI] [PubMed] [Google Scholar]
- Mahalingam R., Wellish M., Wolf W., Dueland A. N., Cohrs R., Vafai A., et al. (1990). Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. The New England Journal of Medicine, 323(10), 627–31. [DOI: 10.1056/NEJM199009063231002] [DOI] [PubMed] [Google Scholar]
- Manousakis G., Jensen M. B., Chacon M. R., Sattin J. A., Levine R. L. (2009). The interface between stroke and infectious disease: Infectious diseases leading to stroke and infections complicating stroke. Current Neurology and Neuroscience Reports, 9(1), 28. [DOI: 10.1007/s11910-009-0005-x] [DOI] [PubMed] [Google Scholar]
- Manwani B., Stretz Ch., Sansing L. H. (2016). Stroke as the initial manifestation of the human immunodeficiency virus. Stroke, 47(4), e60–2. [DOI: 10.1161/STROKEAHA.115.011840] [DOI] [PubMed] [Google Scholar]
- Minassian C., Thomas S. L., Smeeth L., Douglas I., Brauer R., Langan S. M. (2015). Acute cardiovascular events after herpes zoster: A self-controlled case series analysis in vaccinated and unvaccinated older residents of the United States. PLoS Medicine, 12(12), e1001919. [DOI: 10.1371/journal.pmed.1001919] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62(10), 1006–12. [DOI: 10.1016/j.jclinepi.2009.06.005] [DOI] [PubMed] [Google Scholar]
- Moriuchi H., Rodriguez W. (2000). Role of varicella-zoster virus in stroke syndromes. The Pediatric Infectious Disease Journal, 19(7), 648–53. [DOI: 10.1097/00006454-200007000-00014] [DOI] [PubMed] [Google Scholar]
- Murray P. (2017). Basic medical microbiology. 1st Ed. Amsterdam: Elsevier. https://www.elsevier.com/books/basic-medical-microbiology/murray/978-0-323-47676-8 [Google Scholar]
- Nagel M. A., Jones D., Wyborny A. (2017). Varicella zoster virus vasculopathy: The expanding clinical spectrum and pathogenesis. Journal of Neuroimmunology, 308, 112–7. [DOI: 10.1016/j.jneuroim.2017.03.014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K., Hokamura K., Taniguchi N., Wada K., Kudo Ch., Nomura R., et al. (2011). The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nature Communications, 2, 485. [DOI: 10.1038/ncomms1491] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P., Samuels O. B., Barrow D. L. (2002). Stroke and pediatric human immunodeficiency virus infection: Case report and review of the literature. Pediatric Neurosurgery, 37(3), 158–63. [DOI: 10.1159/000064395] [DOI] [PubMed] [Google Scholar]
- Nguyên P., Munzer M., Reynaud J., Richard O., Pouzol P., François P. (1994). Varicella and thrombotic complications associated with transient protein C and protein S deficiencies in children. European Journal of Pediatrics, 153(9), 646–9. [DOI: 10.1007/BF02190684] [DOI] [PubMed] [Google Scholar]
- Nogueras C., Sala M., Sasal M., Viñas J., Garcia N., Bella M. R., et al. (2002). Recurrent stroke as a manifestation of primary angiitis of the central nervous system in a patient infected with human immunodeficiency virus. Archives of Neurology, 59(3), 468–73. [DOI: 10.1001/archneur.59.3.468] [DOI] [PubMed] [Google Scholar]
- O’Donnell M. J., Xavier D., Liu L., Zhang H., Chin L., Rao-Melacini P., et al. (2010). Risk factors for ischemic and intracerebral hemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. The Lancet, 376(9735), 112–23. [DOI: 10.1016/S0140-6736(10)60834-3] [DOI] [PubMed] [Google Scholar]
- Palm F., Urbanek Ch., Grau A. (2009). Infection, its treatment and the risk for stroke. Current Vascular Pharmacology, 7(2), 146–52. [DOI: 10.2174/157016109787455707] [DOI] [PubMed] [Google Scholar]
- Panackal A. A., Williamson P. R. (2015). Fungal infections of the central nervous system. Continuum, 21(6 Neuroinfectious Disease), 1662–78. [DOI: 10.1212/CON.0000000000000241] [DOI] [PubMed] [Google Scholar]
- Pearce B. D., Bracher A., Jones J. L., Kruszon-Moran D. (2018). Viral and parasitic pathogen burden and the association with stroke in a population-based cohort. International Journal of Stroke, 13(5), 481–95. [DOI: 10.1177/1747493017729269] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. R., II, Patel Sh., Franco-Paredes C., Lopez F. A. (2015). Varicella-zoster virus vasculopathy: The growing association between herpes zoster and strokes. The American Journal of the Medical Sciences, 350(3), 243–5. [DOI: 10.1097/MAJ.0000000000000327] [DOI] [PubMed] [Google Scholar]
- Saberi A., Akhondzadeh Sh., Kazemi S. (2018). Infectious agents and different course of multiple sclerosis: A systematic review. Acta Neurologica Belgica, 118(3), 361–77. [DOI: 10.1007/s13760-018-0976-y] [DOI] [PubMed] [Google Scholar]
- Saberi A., Roudbary S. A., Ghayeghran A. R., Kazemi S., Hosseininezhad M. (2018). Diagnosis of meningitis caused by pathogenic microorganisms using magnetic resonance imaging: A systematic review. Basic and Clinical Neuroscience, 9(2), 73–86. [DOI: 10.29252/nirp.bcn.9.2.73] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M. A., Diamond M. S. (2006). Pathogenesis of West Nile Virus infection: A balance between virulence, innate and adaptive immunity, and viral evasion. Journal of Virology, 80(19), 9349–60. [DOI: 10.1128/JVI.01122-06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas A. M. G., Niks E. H., Lequin M. H., Catsman-Berrevoets C. E., de Wit M. C. Y. (2009). Herpes simplex virus type-1 encephalitis and occipital ischemic stroke. Pediatric Neurology, 41(4), 294–6. [DOI: 10.1016/j.pediatrneurol.2009.04.021] [DOI] [PubMed] [Google Scholar]
- Scales C. D., Dahm Ph., Sultan Sh., Campbell-Scherer D., Devereaux P. J. (2008). How to use an article about a diagnostic test. Journal of Urology, 180(2), 469–76. [DOI: 10.1016/j.juro.2008.04.026] [DOI] [PubMed] [Google Scholar]
- Sejvar J. J. (2004). West Nile virus and “poliomyelitis”. Neurology, 63(2), 206–7. [DOI: 10.1212/01.WNL.0000130361.62281.69] [DOI] [PubMed] [Google Scholar]
- Shankar S. K., Mahadevan A., Sundaram C., Sarkar Ch., Chacko G., Lanjewar D. N. (2007). Pathobiology of fungal infections of the central nervous system with special reference to the Indian scenario. Neurology India, 55(3), 198–215. [DOI: 10.4103/0028-3886.35680] [DOI] [PubMed] [Google Scholar]
- Sirven J. I., Malamut B. L. Eds.. (2008). Clinical neurology of the older adult. 2nd Ed. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Smith B. (2017). Toward understanding the when and why of human immunodeficiency virus-associated stroke. The Journal of Infectious Diseases, 216(5), 509–10. [DOI: 10.1093/infdis/jix343] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan N., Basit S., Wohlfahrt J., Pasternak B., Munch T. N., Nielsen L. P., et al. (2013). The short- and long-term risk of stroke after herpes zoster - a nationwide population-based cohort study. PLoS One, 8(7), e69156. [DOI: 10.1371/journal.pone.0069156] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström K., Weibull C. E., Söderberg-Löfdal K., Bergström T., Sparén P., Arnheim-Dahlström L. (2015). Incidence of herpes zoster and associated events including stroke--a population-based cohort study. BMC Infectious Diseases, 15, 488. [DOI: 10.1186/s12879-015-1170-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J., Song Y. M., Choi Y. H., Ebrahim Sh., Davey Smith G. (2007). Hepatitis B virus seropositivity and the risk of stroke and myocardial infarction. Stroke, 38(5), 1436–41. [DOI: 10.1161/STROKEAHA.106.466268] [DOI] [PubMed] [Google Scholar]
- Terlizzi V., Improta F., Di Fraia T., Sanguigno E., D’Amico A., Buono S., et al. (2014). Primary herpes virus infection and ischemic stroke in childhood: A new association? Journal of Clinical Neuroscience, 21(9), 1656–8. [DOI: 10.1016/j.jocn.2013.12.023] [DOI] [PubMed] [Google Scholar]
- Thomas S. L., Minassian C., Ganesan V., Langan S. M., Smeeth L. (2014). Chickenpox and risk of stroke: A self-controlled case series analysis. Clinical Infectious Diseases, 58(1), 61–8. [DOI: 10.1093/cid/cit659] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping B., de Villiers L., Wainwright H., Candy S., Bryer A. (2007). Stroke in patients with human immunodeficiency virus infection. Journal of Neurology, Neurosurgery & Psychiatry, 78(12), 1320–4. [DOI: 10.1136/jnnp.2007.116103] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong D. Y., Wang X. H., Xu C. F., Yang Y. Z., Xiong S. D. (2005). Hepatitis B virus infection and coronary atherosclerosis: Results from a population with relatively high prevalence of hepatitis B virus. World Journal of Gastroenterology, 11(9), 1292–6. [DOI: 10.3748/wjg.v11.i9.1292] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. H., Muo C. H., Hsu C. Y., Kao C. H. (2016). Association of hepatitis B virus infection with decreased ischemic stroke. Acta Neurologica Scandinavica, 134(5), 339–45. [DOI: 10.1111/ane.12548] [DOI] [PubMed] [Google Scholar]
- Wang Y., Xiong J., Chen X., Niu M., Chen X., Guan Y., et al. (2017). Hepatitis B virus infection and decreased risk of stroke: A meta-analysis. Oncotarget, 8(35), 59658–65. [DOI: 10.18632/oncotarget.19609] [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. L., Ratziu V., El-Serag H. B. (2008). Hepatitis C infection and risk of diabetes: A systematic review and meta-analysis. Journal of Hepatology, 49(5), 831–44. [DOI: 10.1016/j.jhep.2008.08.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won K. Y., Kruszon-Moran D., Schantz P. M., Jones J. L. (2008). National seroprevalence and risk factors for zoonotic Toxocara spp. infection. The American Journal of Tropical Medicine and Hygiene, 79(4), 552–7. [DOI: 10.4269/ajtmh.2008.79.552] [DOI] [PubMed] [Google Scholar]
- Yawn B. P., Wollan P. C., Nagel M. A., Gilden D. (2016). Risk of stroke and myocardial infarction after herpes zoster in older adults in a US community population. Mayo Clinic Proceedings, 91(1), 33–44. [DOI: 10.1016/j.mayocp.2015.09.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zis P., Stritsou P., Angelidakis P., Tavernarakis A. (2016). Herpes simplex virus type 2 encephalitis as a cause of ischemic stroke: Case report and systematic review of the literature. Journal of Stroke and Cerebrovascular Diseases, 25(2), 335–9. [DOI: 10.1016/j.jstrokecerebrovasdis.2015.10.002] [DOI] [PubMed] [Google Scholar]