Abstract
Introduction:
Depression and anxiety are the most common psychiatric disorders. These conditions widely occur in industrial societies and severely affect individuals’ lives. N-Acetylcysteine (NAC) is a mucolytic compound with antioxidant and anti-inflammatory effects. This study aimed to investigate the potential therapeutic effects of NAC on chronic noise-induced depression- and anxiety-like behaviors in mice.
Methods:
Fifty male BALB/c mice were randomly divided into 5 groups, as follows: control, noise90 dB, noise110 dB, noise 90+NAC, and noise 110+NAC groups. Animals in the noise groups were exposed to 90 dB 2 h/day and 110 dB 2 h/day for 30 days. The NAC groups received NAC (325 mg/kg P.O.) 20 min after being exposed to noise. To evaluate depressive- and anxiety-like behaviors, the examined mice were subjected to the Open Field Test (OFT), Sucrose Preference Test (SPT), Tail Suspension Test (TST), and Elevated Plus Maze (EPM) tasks. At the end of the behavioral tests, the study animals were sacrificed. Accordingly, the levels of Malondialdehyde (MDA), Total Antioxidant Capacity (TAC), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPx) were determined in the Hippocampus (HIP) and the Prefrontal Cortex (PFC).
Results:
The obtained results suggested that noise exposure would induce anxiety- and depressive-like behaviors, being reversed by NAC administration. Moreover, chronic administration of NAC significantly increased antioxidant enzyme activities and reduced lipid peroxidation (MDA levels) in the PFC and HIP of noise-exposed mice.
Conclusion:
Our findings revealed that administrating NAC would reduce the adverse effects of noise on the brain and would exert anti-depressant and anxiolytic effects.
Keywords: Noise stress, Depression, Anxiety, N-Acetylcysteine, Oxidative stress
Highlights
Depression and anxiety are the most common psychiatric disorders.
Long exposure to environmental stressors may increase the risk of psychiatric conditions.
Oxidative stress damage is a major contributor to noise stress-induced anxiety and depression.
Our findings revealed that administrating NAC would reduce the adverse effects of noise on the brain.
Plain Language Summary
Depression and anxiety are the most common psychiatric disorders, which occur in industrial societies. It severely affects individuals’ lives. NAC is a mucolytic compound with antioxidant and anti-inflammatory effects. We investigated the potential therapeutic effects of NAC on chronic noise-induced depression- and anxiety-like behaviors. Oxidative stress damage is a major contributor to noise stress-induced anxiety and depression. Therefore, therapeutic strategies enhancing the enzymatic antioxidant activities. Our findings revealed that administrating NAC would reduce the adverse effects of noise on the brain and would exert anti-depressant and anxiolytic effects.
1. Introduction
Prolonged exposure to environmental stress-ors may increase the risk of psychiatric conditions, such as depression and anxiety with negative impacts on the quality of life in industrial societies (Urán, Cáceres, & Guelman, 2010; Wang et al., 2016). In general, exposure to annoying sounds, i.e., higher than human tolerance threshold (Beutel et al., 2016) not only affects the auditory system, but also is associated with disturbances in structures of the central nervous system, hormonal axes, behavior, and cognitive abilities (Bielefeld et al., 2007; Urán et al., 2010). Noise stress is an unavoidable part of daily life, i.e., produced by urban traffic, air crafts, household appliances, and factories. Evidence suggests that noise exposure promotes structural and biochemical changes in the brain, leading to biopsychological or behavioral changes in humans and animals (Manikandan et al., 2006; Rylander, 2004).
At molecular levels, mechanisms, including oxidative stress, neuroinflammation, as well as the neurodegeneration of neuronal cells in the Hippocampus (HIP) and Pre-frontal Cortex (PFC) contribute to the appearance of emotional deficits, like depression and anxiety (Salim, Chugh, & Asghar, 2012; Young, Bruno, & Pomara, 2014).
Imbalance occurring between the generation and elimination of the reactive molecules with free-radical properties results in oxidative stress; subsequently, it imposes substantial damage to components, such as Deoxyribonucleic Acid (DNA), proteins, and lipids (Mahmoodzadeh, Mazani, & Rezagholizadeh, 2017; Salim, 2014).
Considering the brain’s propensity to consume high oxygen levels as well as its high unsaturated fatty acids content and limited antioxidant capacity, a slight increase in free radicals levels results in significant changes in neuronal activity (Srinivasan, Wankhar, Rathinasamy, & Rajan, 2015; Wang et al., 2016).
Oxidative stress damage is a major contributor to noise stress-induced anxiety and depression. Therefore, therapeutic strategies enhancing the enzymatic antioxidant activities, such as Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), and Catalase (CAT) or diminishing lipid peroxidation and the production of Reactive Oxygen Species (ROS), or scavenging free radicals in the brain may play a prophylactic role on depression and anxiety (Li et al., 2000; Maria Michel, Pulschen, & Thome, 2012; Xu, Richardson, & Li, 2003). N-Acetylcysteine (NAC), as a precursor of L-cysteine and reduced glutathione, is a vital cellular antioxidant (Żukowski et al., 2018) with anti-inflammatory and anti-apoptotic (Zafarullah, Li, Sylvester, & Ahmad, 2003) effects. Interestingly, its low molecular weight enables it to readily cross the blood-brain barrier and improve numerous psychiatric disorders, such as autism, schizophrenia, Alzheimer’s disease, and myoclonic epilepsy (Bavarsad Shahripour, Harrigan, & Alexandrov, 2014; Garg, Singh, Singh, & Rizvi, 2018; Tardiolo, Bramanti, & Mazzon, 2018). The current study aimed to investigate the effects of NAC on behavioral changes and oxidative status in the HIP and PFC of the mice exposed to two different noise intensities (90 dB & 110 dB).
2. Methods
This study was performed on 50 adult male BALB/c mice, weighing 28-30 g. The required animals were obtained from the animal center of Tabriz University of Medical Sciences. The animals were housed 10 mice per cage in a standard condition, temperature 22 °C, 12:12h light/dark cycle, and free access to food and water during the study. All procedures of the project were per the NIH Animal Care and Use Committee Guidelines and were approved by the Ethics Committee of AJA University of Medical Science (Code: IR.AJAUMS.Rec.1397.003).
The study mice were randomly assigned into 5 groups (10 mice/group) as follows: control, noise 90 dB, noise 110 dB, noise 90+NAC, and noise 110+NAC. The control group animals were not exposed to noise while noise groups were exposed to the 90 dB and 110 dB 2 h/day for 30 days. Moreover, NAC 325 mg/kg was administered by gastric gavage 20 min after noise exposure for 30 days to the mice in the noise 90+NAC and noise 110+NAC groups. Besides, the control, noise 90 dB, and noise 110 dB groups received distilled water for 30 days. The selected dose for NAC was per previously published data on rodents (Bielefeld et al., 2007; Coleman, Huang, Liu, Kopke, & Jackson, 2010; Wu, Hsu, Cheng, & Guo, 2010).
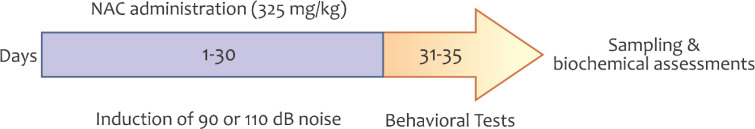
The examined animals were exposed to sound pressure level white noise 90dB or 110 dB 2 h/day for 30 days. A computer software (NCH Tone Generator 3.26) was used to produce white noise and delivered to a loudspeaker, placed at a 30-cm distance from the explored animal’s cage. The noise intensity was adjusted by a sound level meter (Smart Tools co., Ltd., Japan) (Figure 1).
Figure 1.
The timeline of the study
Behavioral assessments were as follows:
The Elevated Plus Maze (EPM) apparatus was used to assess anxiety-like behavior. Briefly, the plus-shaped apparatus consisted of two open arms (30 × 5 cm) and two closed arms (30 × 5 × 15 cm), and a central platform (5 × 5 cm), i.e., elevated 50 cm above the floor. The mouse was gently placed in the center of the apparatus facing an open arm. Moreover, its activities were recorded for 6 min and the time spent in the Open Arms (OAT), as well as the number of Open Arms Entries (OAE), were calculated (Salehpour, Mahmoudi, & Eyvazzadeh, 2018).
Open Field Test (OFT): This test is commonly used to assess spontaneous motor activity and anxiety (Habr, Bernardi, Conceição, Freitas, & Felicio, 2011). For OFT, a square black open field arena (33 × 33 × 33 cm) was used. The mouse was placed in the center of the apparatus and its activity was videotaped. The total traveled distance, center distance, and velocity were recorded and analyzed using a video tracking program (Etho Vision™, Noldus, Netherlands) (Tanaka, Young, Halberstadt, Masten, & Geyer, 2012).
Tail Suspension Test (TST): This test is widely used to investigate depressive-like behaviors in mice (Cryan, Mombereau, & Vassout, 2005; Mahmoudi, Farhoudi, Talebi, Sabermarouf, & Sadigh-Eteghad, 2015). In this test, each mouse was suspended by fixing the tail from a holder using adhesive tape, 50 cm above the floor for 6 min. Besides, the immobility time was calculated over the last 4 min of the test.
Sucrose Preference Test (SPT): The SPT is based on the animal’s natural preference for sweets. The SPT was used to assess noise stress-induced anhedonia (Cline et al., 2015). For conducting the SPT, the mouse was exposed to two pre-weighed bottles, one containing 2% sucrose solution and the other containing water. The study animals were trained for 3 days, and on day 4, the amounts of sucrose and water consumption were measured (Sáenz, Villagra, & Trías, 2006). Sucrose Preference (SP) was calculated using the following equation: SP = [sucrose solution consumption (mL)/total fluid consumption (ml)] × 100.
For biochemical evaluation, after completing the behavioral tests, the study mice were deeply anesthetized using ketamine and xylazine mixture (80 mg/kg and 8 mg/kg, respectively). After decapitation, the brain tissue was carefully removed, and the HIP and PFC were quickly separated on ice and then stored at −80°C.
The frozen HIP and PFC tissue samples were homogenized in the 1.15% KCl solution and centrifuged at 7000 rpm (4°C) for 10 min. The acquired supernatant was collected for determining the lipid peroxidation (MDA level) and antioxidant enzymes activities, including SOD and GPx, as well as TAC levels. Total protein concentration in the tissue was measured by the Bradford, in which Bovine Serum Albumin (BSA) was applied as a protein standard (Bradford, 1976).
The activity of Superoxide Dismutase (SOD) in the HIP and PFC was measured using a Randox kit (Randox Laboratories Ltd, Crumlin, United Kingdom) according to the manufacturer’s instructions (Pourmemar et al., 2017). Briefly, the reaction of free radicals, i.e., produced by xanthine and xanthine oxidase and (2-(4-iodophenyl)-3-(4-nitrophenyl)-5 phenyltetrazolium chloride (I.N.T) produces colored molecules, i.e., inhibited by SOD. The absorbance was measured at 505 nm at 37 °C and the results were expressed as U/mg protein. The activity of GPx in the HIP and PFC tissues was assessed using RANSEL (Randox Laboratories Ltd, Crumlin, United Kingdom) kit according to the Paglia and Valentine method (Paglia & Valentine, 1967). In brief, glutathione is initially oxidized by GPx, then oxidized glutathione is converted to reduced glutathione by glutathione reductase in the presence of NADPH. A decrease in NADPH indicates the activity of GPx in the homogenate. The absorbance was read at 340 nm at 37 °C, and GPx activity was expressed as U/mg protein.
The Total Antioxidant Capacity (TAC) was determined by the ability of the tissue to reduce Fe 3+ to Fe2+ in the presence of 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ). TAC was determined by a Randox total antioxidant status kit (Randox Laboratories Ltd, Crumlin, United Kingdom) according to the 2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid] (ABTS •+) method. The suppression degree of the blue-green color of the ABTS •+ by antioxidants was determined at 600 nm and expressed as mmol/L (Benzie & Strain, 1996). For measuring the hippocampal and cortical Malondialdehyde (MDA) levels, a product of lipid peroxidation, the Thiobarbituric Acid Reactive Substances (TBARS) was implemented. Briefly, 200 μL of the supernatant was mixed with 2 ml orthophosphoric acid (1%) and 1 ml Thiobarbituric Acid (TBA) (0.67%) and incubated at the boiling point for 45 min. The pink color generated by the reaction of MDA and TBA was extracted by n-butanol, and its absorption was read at 532 nm. The relevant results were presented as nmol/mg protein (Uchiyama & Mihara, 1978). The obtained data were expressed as Mean±SEM. The collected results were analyzed by oneway Analysis of Variance (ANOVA), followed by Tukey’s posthoc test using Graph Pad Prism 6.01 (Graph Pad Software Inc., La Jolla, CA, USA). P<0.05 was considered statistically significant.
3. Results
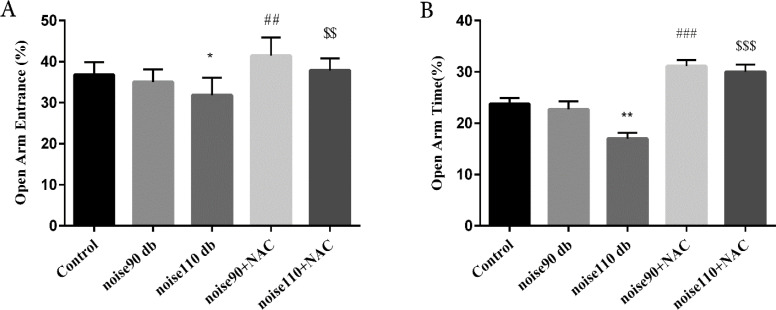
The EPM test results revealed that exposure to noise 110 dB significantly decreased OAE% (P<0.05) and OAT% (P<0.01), compared to the control mice. Exposure to noise 90 dB provided no significant effect on OAE% and OAT%. Moreover, treatment with NAC (325 mg/kg) for 30 days increased OAE% (noise90+NAC: P<0.01; noise110+NAC: P<0.001) and OAT% (noise90+NAC: P<0.01; noise110+NAC: P<0.001) (Figure 2).
Figure 2.
The effects of NAC (325 mg/kg) on the percentage
A: Open arms entries (OAE%); and B: Open arms time (OAT%) in the Elevated Plus-Maze (EPM) test in BALB/c mice exposed to noise stress.
The data are presented as Mean±SEM (n=10 animals/group). *P<0.05 and **P<0.01 vs. control group, ## P<0.01 and ### P<0.001 vs. noise 90 dB group, and $$ P<0.01 and $$$ P<0.001 vs. noise 110 dB group.
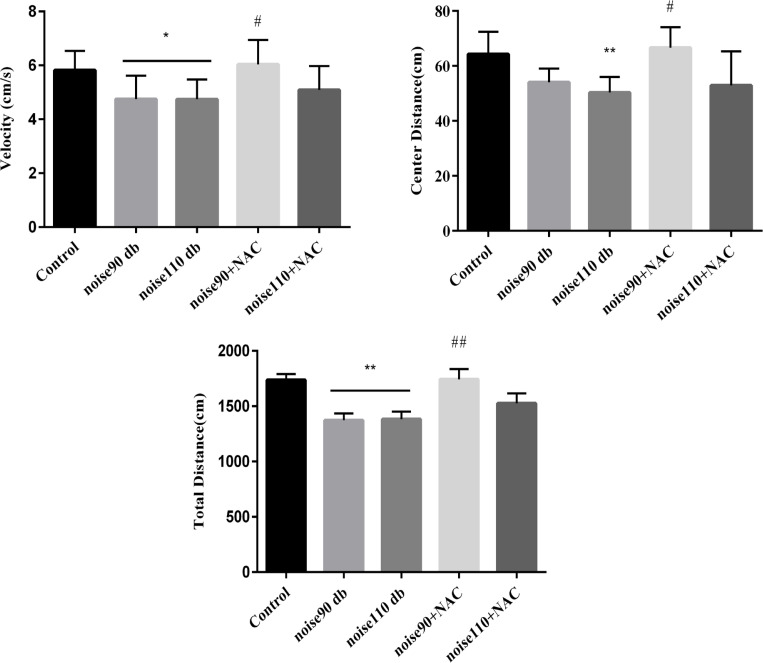
Normal mice intrinsically have exploratory behaviors. As Figure 3 shows, noise exposure significantly decreased total traveled distance (Figure 3-A), center distance (Figure 3-B), and velocity (Figure 3-C), compared to the control group. However, NAC treatment in the noise 90+NAC group significantly increased total distance (P<0.01), center distance (P<0.05), and velocity (P<0.05), compared to the noise 90 dB group. Moreover, the NAC regimen could not affect the aforementioned parameters, compared to the mice, exposed to noise 110.
Figure 3.
The effects of NAC (325 mg/kg)
A: Total distance; B: Center distance; and C: Velocity in the OFT in BALB/c mice exposed to noise stress.
Data are presented as Mean±SEM (n=10 animals/group). *P<0.05 and **P<0.01 vs. control group and # P<0.05 and ## P<0.01 vs. noise 90 dB group.
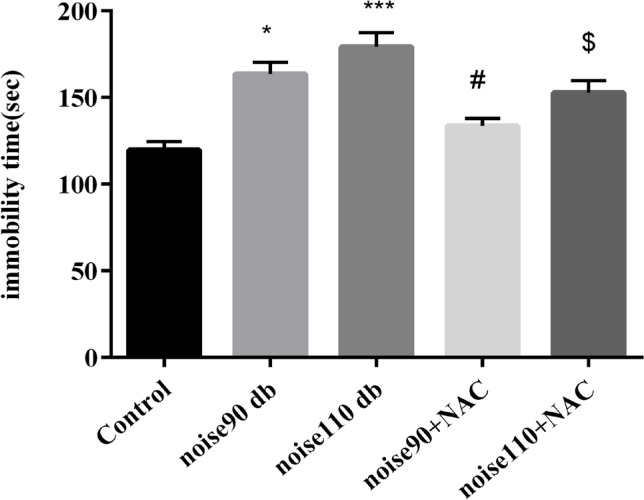
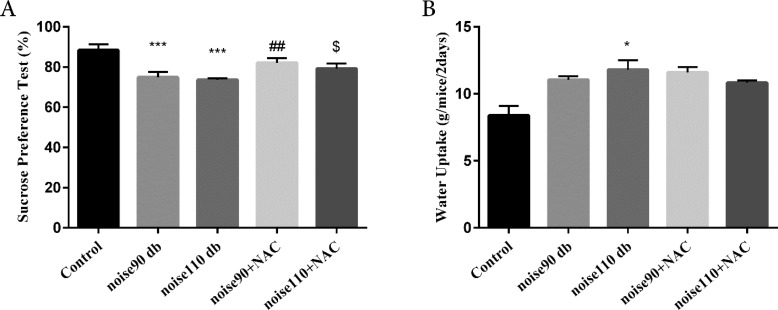
The TST findings indicated that exposure to both noise intensities significantly (P<0.05 for noise 90 dB group & P<0.001 for noise 110 dB group) increased immobility time, compared to the control mice. However, administrating NAC significantly decreased immobility time in the noise-exposed groups (P<0.05 for 90 dB and 110 dB intensities) (Figure 4). The SPT results demonstrated that noise 90 dB and 110 dB groups had significantly (P<0.001 for both groups) lower preference for sucrose solution than the control group, indicating a depressive-like behavior. However, NAC treatment significantly increased SP in the 90 dB and 110 dB noise-exposed groups (P<0.01 for noise 90 dB group & P<0.05 for noise 110 dB group) (Figure 5-A). For further investigation, water intake, i.e., an indicator for anxiety behaviors, was measured. The relevant results suggested that only exposure to 110 dB noise increased the water consumption of mice, compared to the control animals (P<0.05) (Figure 5-B). NAC treatment had no significant effect on the water consumption in the noise stress-exposed groups.
Figure 4.
The effects of NAC (325 mg/kg) on immobility time in the TST in BALB/c mice exposed to noise stress
Data are presented as Mean±SEM (n=10 animals/group). *P<0.05 and ***P<0.001 vs. control group, # P<0.05 vs. noise 90 dB group, and $ P<0.05 vs. noise 110 dB group.
Figure 5.
The effects of NAC (325 mg/kg)
A: Sucrose Preference Test (SPT); and B: Water uptake in BALB/c mice exposed to noise stress.
Data are presented as Mean±SEM (n=10 animals/group). *P<0.05 and ***P<0.001 vs. control group, ## P<0.01 vs. noise 90 dB group, and $ P<0.05 vs. noise 110 dB group.
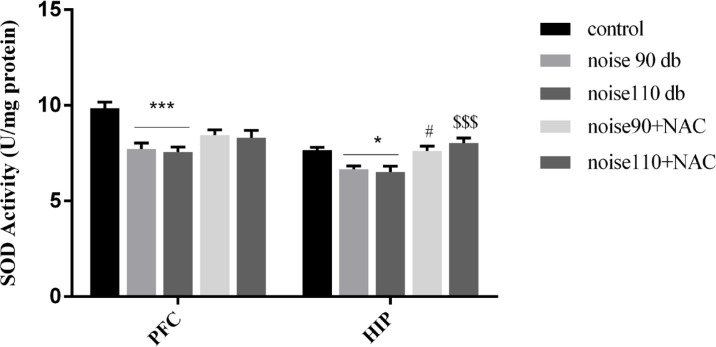
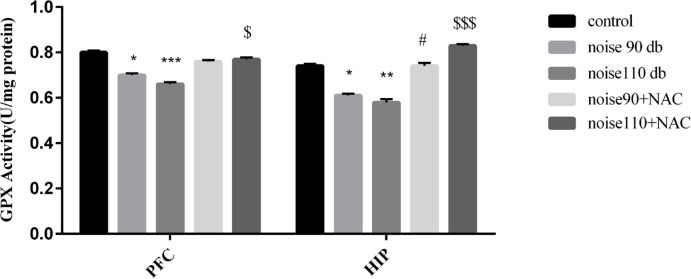
As shown in Figure 6, the activity of SOD was significantly reduced in both HIP (right panel) and PFC (left panel) of noise 90 dB and noise 110 dB groups, compared to the control mice. Although treatment with NAC did not significantly change the activity of the SOD in the PFC, there were significant increases in the activity of the SOD in the HIP of noise 90+NAC (P<0.05) and noise 110+NAC (P<0.001) groups. As per Figure 7, noise exposure significantly reduced GPx activity in the PFC and HIP of noise 90 dB (P<0.05 for both areas) and noise 110 dB (P<0.001 for PFC & P<0.01 for HIP) groups. However, treatment with NAC significantly increased GPx activity in the PFC of the noise110+NAC group (P<0.05) and the HIP of the noise 90+NAC (P<0.05) and noise110+NAC (P<0.001) groups.
Figure 6.
The effects of NAC (325 mg/kg) on the Superoxide Dismutase (SOD) activity in the Prefrontal (PFC) and Hippocampus (HIP) of BALB/c mice exposed to noise stress
Data are presented as Mean±SEM (n=10 animals/group). *P<0.05 and ***P<0.001 vs. control group; # P<0.05 vs. noise 90 dB group, and $$$ P<0.001 vs. noise 110 dB group.
Figure 7.
The effects of NAC (325 mg/kg) on Glutathione Peroxidase (GPx) activity in the Prefrontal Cortex (PFC) and Hippocampus (HIP) areas of BALB/c mice exposed to noise stress
Data are presented as Mean±SEM (n=10 animals/group). *P<0.05, **P<0.01, and ***P<0.001 vs. control group, # P<0.05 vs. noise 90 dB group, and $ P<0.05 and $$$ P<0.001 vs. noise 110 dB group.
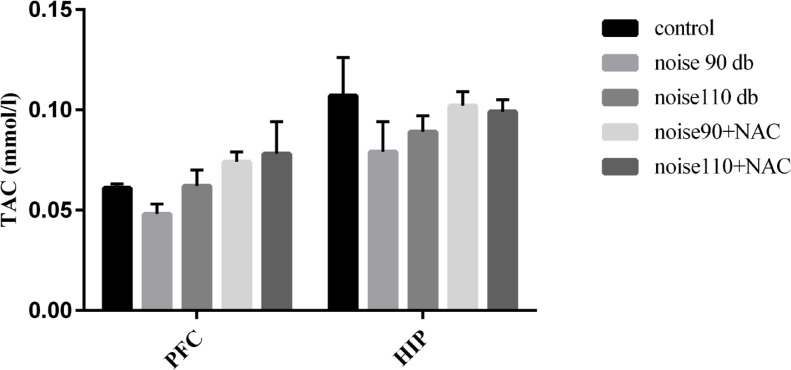
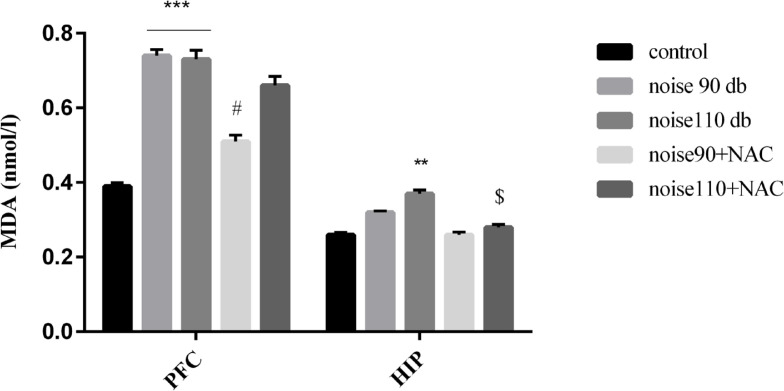
Although TAC levels were decreased in the PFC and HIP areas of the noise-exposed groups, there was no significant difference among different research groups. Moreover, NAC treatment presented no significant effect on the TAC levels in the noise stress-exposed groups (Figure 8). The collected results signified that noise exposure significantly increased lipid peroxidation and consequently MDA levels, in the PFC (P<0.001) of the mice in the noise 90 dB and noise 110 dB groups, and HIP (P<0.01) of the mice noise 110 dB group. However, treatment with NAC significantly decreased lipid peroxidation in the PFC of the noise 90 dB (P<0.05) and HIP of the noise 110 dB group (P<0.05) (Figure 9).
Figure 8.
The effects of NAC (325 mg/kg) on TAC in the PFC and HIP of BALB/c mice exposed to noise stress Data are presented as Mean±SEM (n=10 animals/group).
Figure 9.
The effects of NAC (325 mg/kg) MDA levels, as an index of lipid peroxidation, in the PFC and HIP of BALB/c mice exposed to noise stress
Data are presented as Mean±SEM (n=10 animals/group). **P<0.01 and ***P<0.001 vs. control group, # P<0.05 vs. noise 90 dB group, and $ P<0.05 vs. noise 110 dB group.
4. Discussion
The current study findings demonstrated that noise stress exposure-induced anxiety-like behaviors, assessed in the EPM and OFT task. Moreover, noise-exposed mice exhibited more depressive-like behaviors, indicated by increased immobility time in the TST and decreased sucrose preference, compared to the control animals. Noise can trigger several health problems, such as cardiovascular diseases, sleep disturbance, and negative emotions, such as irritability, distress, and exhaustion. Moreover, evidence suggests that environmental noise substantially contributes to mood disturbances, such as depression and anxiety (Beutel et al., 2016; Haines, Stansfeld, Job, Berglund, & Head, 2001).
In line with our results, noise stress and loud noise were reported to induce depressive-like behaviors in rodents. Naqvi et al. reported that exposure to 100 dB noise 4 h/day for 15 days increased the immobility time in the TST (Naqvi, Haider, Perveen, & Haleem, 2012). Moreover, Salehpour et al. demonstrated that acute (2 h a day) or chronic (2 h/day for 12 weeks) noise stress exposures at levels of 90 dB and 110 dB induce depressive-like behaviors in mice (Salehpour et al., 2018). We also found that noise-exposed animals exhibited anhedonia, as a core symptom of depressive disorders, indicated by low sucrose preference. Similarly, Dong et al. argued that dental noise exposure resulted in sucrose preference deficits in mice (Dong et al., 2016). Furthermore, we found that exposure to noise 90 dB and 110 dB significantly decreased %OAT and %OAE in the EPM and decreased center crossing and total traveled distance in the OFT, indicating the development of anxiety-like behaviors. In line with our results, previous preclinical and clinical reports stated that unpleasant noise exposure could lead to anxiety-like behaviors (Beutel et al., 2016; Naqvi et al., 2012; Wankhar, Devi, & Ashok, 2014).
Evidence reveals that noise can modulate neurotransmitter levels, namely dopamine, and serotonin, and decrease neurogenesis in different regions of the brain (Dong et al., 2016; Ravindran, Devi, Samson, & Senthilvelan, 2005). Decreased levels of serotonin or dopamine are associated with depressive-like symptoms, such as hopelessness and anhedonia (Bressan & Crippa, 2005; Elhwuegi, 2004). Besides, noise stress can affect the Hypothalamic-Pituitary-Adrenal (HPA) axis. Accordingly, this condition causes the dysregulation of the HPA axis and an increase in the blood glucocorticoids, which eventually results in anxiety-like behaviors (Burow, Day, & Campeau, 2005; Spreng, 2000). Therefore, noise exposure seemed to have induced depressive- and anxiety-like behaviors through these mechanisms (Michaud et al., 2003; Spreng, 2000). However, NAC treatment markedly attenuated these behavioral changes in noise-exposed mice. Fernandes et al. also reported that NAC ameliorates depressive symptoms in depressed patients, compared to the placebo (Fernandes, Dean, Dodd, Malhi, & Berk, 2016). Additionally, a study reported that NAC administration for 7 days could reverse anxiety-like behaviors and oxidative stress following the chronic unpredictable stress in zebrafish (Mocelin et al., 2019).
Previous studies reflected that oxidative stress and excessive production of free radicals substantially contribute to the pathogenesis of depression and anxiety (Maes, Galecki, Chang, & Berk, 2011; Smaga et al., 2015), and agents with antioxidant effects can improve anxiety and depressive symptoms (Lee et al., 2013). In this study, the results of the biochemical assessment indicated that noise exposure increased lipid peroxidation and diminished enzymatic antioxidant defense in the PFC and HIP. Similar to our results, previous studies suggested that noise exposure enhances free radicals production in different parts of the brain, including the HIP and PFC (Wang et al., 2016). Manikandan et al. argued that the MDA levels and SOD activity were increased 1, 15, and 30 days after noise exposure (100 dB 4h/day), and catalase and GPX activities were enhanced on days 1 and 15; however, decreased on the 30 days in the noise stress group (Manikandan et al., 2006). Moreover, chronic exposure to noise stress at 90 dB or 110 dB levels (2 h/day for 3 months) markedly increased the brain MDA levels while decreased SOD and GPx activities and TAC levels. However, acute exposure (2 h) increased brain MDA levels and enhanced SOD and GPx activities and TAC levels (Salehpour, Mahmoudi, Farajdokht, & Eyvazzadeh, 2018). However, chronic NAC could markedly diminish MDA levels and enhanced SOD and GPx activities in the PFC and HIP, i.e., accompanied by improved anxiety- and depressive-like behaviors in the noise-exposed mice. The antioxidant effects of NAC were determined in previous studies (Mao, Goswami, Kalen, Goswami, & Sarsour, 2016; Massaad, Washington, Pautler, & Klann, 2009; Nagata et al., 2007). Given that NAC is an important precursor in the production of glutathione, NAC might inhibit the harmful effects of free radicals on the brain structures by producing glutathione (Berk et al., 2008). Further investigations are required to confirm the findings of this study and detailed data about the effects of NAC in this field.
5. Conclusion
The current study data revealed that NAC treatment would attenuate noise exposure-induced anxiety- and depressive-like behaviors; this was possibly achieved by decreasing lipid peroxidation and improving the antioxidant defense system in HIP and PFC of the mice.
Ethical Considerations
Compliance with ethical guidelines
All project procedures were per the NIH Animal Care and Use Committee Guidelines and were approved by the Ethics Committee of AJA University of Medical Science (Code: IR.AJAUMS.REC.1397.003).
Footnotes
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors’ contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflicts of interest.
References
- Bavarsad Shahripour R., Harrigan M. R., Alexandrov A. V. (2014). N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain and Behavior, 4(2), 108–22. [DOI: 10.1002/brb3.208] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie I. F. F., Strain J. J. (1996). The ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 239(1), 70–6. [DOI: 10.1006/abio.1996.0292] [DOI] [PubMed] [Google Scholar]
- Berk M., Copolov D., Dean O., Lu K., Jeavons S., Schapkaitz I., et al. (2008). N-acetyl cysteine as a glutathione precursor for schizophrenia-a double-blind, randomized, placebo-controlled trial. Biological Psychiatry, 64(5), 361–8. [DOI: 10.1016/j.biopsych.2008.03.004] [DOI] [PubMed] [Google Scholar]
- Beutel M. E., Jünger C., Klein E. M., Wild Ph., Lackner K., Blettner M., et al. (2016). Noise annoyance is associated with depression and anxiety in the general population- the contribution of aircraft noise. Plos One, 11(5), e0155357. [DOI: 10.1371/journal.pone.0155357] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld E. C., Kopke R. D., Jackson R. L., Coleman J. K. M., Liu J., Henderson D. (2007). Noise protection with N-acetyl-lcysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Oto-Laryngologica, 127(9), 914–9. [DOI: 10.1080/00016480601110188] [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–54. [DOI: 10.1006/abio.1976.9999] [DOI] [PubMed] [Google Scholar]
- Bressan R. A., Crippa J. A. (2005). The role of dopamine in reward and pleasure behaviour-review of data from preclinical research. Acta Psychiatrica Scandinavica, 111(s427), 14–21. [DOI: 10.1111/j.1600-0447.2005.00540.x] [DOI] [PubMed] [Google Scholar]
- Burow A., Day H. E. W., Campeau S. (2005). A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Research, 1062(1–2), 63–73. [DOI: 10.1016/j.brainres.2005.09.031] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline B. H., Anthony D. C., Lysko A., Dolgov O., Anokhin K., Schroeter C., et al. (2015). Lasting downregulation of the lipid peroxidation enzymes in the prefrontal cortex of mice susceptible to stress-induced anhedonia. Behavioural Brain Research, 276, 118–29. [DOI: 10.1016/j.bbr.2014.04.037] [DOI] [PubMed] [Google Scholar]
- Coleman J., Huang X., Liu J., Kopke R., Jackson R. (2010). Dosing study on the effectiveness of salicylate/N-acetylcysteine for prevention of noise-induced hearing loss. Noise & Health, 12(48), 159–65. [DOI: 10.4103/1463-1741.64972] [DOI] [PubMed] [Google Scholar]
- Cryan J. F., Mombereau C., Vassout A. (2005). The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neuro-science & Biobehavioral Reviews, 29(4–5), 571–625. [DOI: 10.1016/j.neubiorev.2005.03.009] [DOI] [PubMed] [Google Scholar]
- Dong Y., Zhou Y., Chu X., Chen Sh., Chen L., Yang B., et al. (2016). Dental noise exposed mice display depressive-like phenotypes. Molecular Brain, 9, 50. [DOI: 10.1186/s13041-016-0229-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhwuegi A. S. (2004). Central monoamines and their role in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 28(3), 435–51. [DOI: 10.1016/j.pnpbp.2003.11.018] [DOI] [PubMed] [Google Scholar]
- Fernandes B. S., Dean O. M., Dodd S., Malhi G. S., Berk M. (2016). N-Acetylcysteine in depressive symptoms and functionality: A systematic review and meta-analysis. The Journal of Clinical Psychiatry, 77(4), e457–66. [DOI: 10.4088/JCP.15r09984] [DOI] [PubMed] [Google Scholar]
- Garg G., Singh S., Singh A. K., Rizvi S. I. (2018). N-acetyl-L-cysteine attenuates oxidative damage and neurodegeneration in rat brain during aging. Canadian Journal of Physiology and Pharmacology, 96(12), 1189–96. [DOI: 10.1139/cjpp-2018-0209] [DOI] [PubMed] [Google Scholar]
- Habr S. F., Bernardi M. M., Conceição I. M., Freitas T. A., Felicio L. F. (2011). Open field behavior and intra-nucleus accumbens dopamine release in vivo in virgin and lactating rats. Psychology & Neuroscience, 4(1), 115–21. [DOI: 10.3922/j.psns.2011.1.013] [DOI] [Google Scholar]
- Haines M. M., Stansfeld S. A., Job R. F. S., Berglund B., Head J. (2001). Chronic aircraft noise exposure, stress responses, mental health and cognitive performance in school children. Psychological Medicine, 31(2), 265–77. [DOI: 10.1017/S0033291701003282] [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Lee S. J., Han Ch., Patkar A. A., Masand P. S., Pae C. U. (2013). Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 46, 224–35. [DOI: 10.1016/j.pnpbp.2012.09.008] [DOI] [PubMed] [Google Scholar]
- Li X. M., Chlan-Fourney J., Juorio A. V., Bennett V. L., Shrikhande S., Bowen R. C. (2000). Antidepressants upregulate messenger RNA levels of the neuroprotective enzyme superoxide dismutase (SOD1). Journal of Psychiatry and Neuroscience, 25(1), 43–7. [PMC free article] [PubMed] [Google Scholar]
- Maes M., Galecki P., Chang Y. S., Berk M. (2011). A review on the Oxidative And Nitrosative Stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(3), 676–92. [DOI: 10.1016/j.pnpbp.2010.05.004] [DOI] [PubMed] [Google Scholar]
- Mahmoodzadeh Y., Mazani M., Rezagholizadeh L. (2017). Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicology Reports, 4, 455–62. [DOI: 10.1016/j.toxrep.2017.08.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi J., Farhoudi M., Talebi M., Sabermarouf B., Sadigh-Eteghad S. (2015). Antidepressant-like effect of modafinil in mice: Evidence for the involvement of the dopaminergic neurotrans-mission. Pharmacological Reports, 67(3), 478–84. [DOI: 10.1016/j.pharep.2014.11.005] [DOI] [PubMed] [Google Scholar]
- Manikandan S., Padma M. K., Srikumar R., Parthasarathy N. J., Muthuvel A., Devi R. S. (2006). Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial pre-frontal cortex. Neuroscience Letters, 399(1–2), 17–22. [DOI: 10.1016/j.neulet.2006.01.037] [DOI] [PubMed] [Google Scholar]
- Mao G., Goswami M., Kalen A. L., Goswami P. C., Sarsour E. H. (2016). N-acetyl-L-cysteine increases MnSOD activity and enhances the recruitment of quiescent human fibroblasts to the proliferation cycle during wound healing. Molecular Biology Reports, 43(1), 31–9. [DOI: 10.1007/s11033-015-3935-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria Michel T., Pulschen D., Thome J. (2012). The role of oxidative stress in depressive disorders. Current Pharmaceutical Design, 18(36), 5890–9. [DOI: 10.2174/138161212803523554] [DOI] [PubMed] [Google Scholar]
- Massaad C. A., Washington T. M., Pautler R. G., Klann E. (2009). Overexpression of SOD-2 reduces hippocampal super-oxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences, 106(32), 13576–81. [DOI: 10.1073/pnas.0902714106] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud D. S., McLean J., Keith S. E., Ferrarotto C., Hayley Sh., Khan S. A., et al. (2003). Differential impact of audiogenic stress-ors on Lewis and Fischer rats: Behavioral, neurochemical, and endocrine variations. Neuropsychopharmacology, 28(6), 1068–81. [DOI: 10.1038/sj.npp.1300149] [DOI] [PubMed] [Google Scholar]
- Mocelin R., Marcon M., D’ambros S., Mattos J., Sachett A., Siebel A. M., et al. (2019). N-acetylcysteine reverses anxiety and oxidative damage induced by unpredictable chronic stress in zebrafish. Molecular Neurobiology, 56(2), 1188–95. [DOI: 10.1007/s12035-018-1165-y] [DOI] [PubMed] [Google Scholar]
- Nagata K., Iwasaki Y., Yamada T., Yuba T., Kono K., Hosogi Sh., et al. (2007). Overexpression of manganese su-peroxide dismutase by N-acetylcysteine in hyperoxic lung injury. Respiratory Medicine, 101(4), 800–7. [DOI: 10.1016/j.rmed.2006.07.017] [DOI] [PubMed] [Google Scholar]
- Naqvi F., Haider S., Perveen T., Haleem D. J. (2012). Sub-chronic exposure to noise affects locomotor activity and produces anxiogenic and depressive like behavior in rats. Pharmacological Reports, 64(1), 64–9. [DOI: 10.1016/S1734-1140(12)70731-4] [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. (1967). Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine, 70(1), 158–69. [PubMed] [Google Scholar]
- Pourmemar E., Majdi A., Haramshahi M., Talebi M., Karimi P., Sadigh-Eteghad S. (2017). Intranasal cerebrolysin attenuates learning and memory impairments in D-galactose-induced senescence in mice. Experimental Gerontology, 87(Pt A), 16–22. [DOI: 10.1016/j.exger.2016.11.011] [DOI] [PubMed] [Google Scholar]
- Ravindran R., Devi R. S., Samson J., Senthilvelan M. (2005). Noise-stress-induced brain neurotransmitter changes and the effect of Ocimum sanctum (Linn) treatment in albino rats. Journal of Pharmacological Sciences, 98(4), 354–60. [DOI: 10.1254/jphs.FP0050127] [DOI] [PubMed] [Google Scholar]
- Rylander R. (2004). Physiological aspects of noise-induced stress and annoyance. Journal of Sound and Vibration, 277(3), 471–8. [DOI: 10.1016/j.jsv.2004.03.008] [DOI] [Google Scholar]
- Sáenz J. C. B., Villagra O. R., Trías J. F. (2006). Factor analysis of forced swimming test, sucrose preference test and open field test on enriched, social and isolated reared rats. Behavioural Brain Research, 169(1), 57–65. [DOI: 10.1016/j.bbr.2005.12.001] [DOI] [PubMed] [Google Scholar]
- Salehpour F., Mahmoudi J., Eyvazzadeh N. (2018). Effects of acute and chronic noise stress on depressive- and anxiety-like behaviors in mice. Journal of Experimental and Clinical Neuro-sciences, 5(1), 1–6. [DOI: 10.13183/jecns.v5i1.73] [DOI] [Google Scholar]
- Salehpour F., Mahmoudi J., Farajdokht F., Eyvazzadeh N. (2018). Noise stress impairs social interaction in adult male mice: Role of oxidative stress and neuroendocrine markers. Crescent Journal of Medical and Biological Sciences, 5(4), 272–8. http://www.cjmb.org/text.php?id=273 [Google Scholar]
- Salim S. (2014). Oxidative stress and psychological disorders. Current Neuropharmacology, 12(2), 140–7. [DOI: 10.2174/1570159X11666131120230309] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S., Chugh G., Asghar M. (2012). Chapter one - Inflammation in anxiety. In Donev R. (Ed.), Inflammation in neuropsychiatric disorders. Advances in protein chemistry and structural biology (pp. 1–25). Vol. 88. Oxford: Academic Press. [DOI: 10.1016/B978-0-12-398314-5.00001-5] [DOI] [PubMed] [Google Scholar]
- Smaga I., Niedzielska E., Gawlik M., Moniczewski A., Krzek J., Przegaliński E., et al. (2015). Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacological Reports, 67(3), 569–80. [DOI: 10.1016/j.pharep.2014.12.015] [DOI] [PubMed] [Google Scholar]
- Spreng M. (2000). Possible health effects of noise induced cortisol increase. Noise & Health, 2(7), 59–64. [PubMed] [Google Scholar]
- Srinivasan S., Wankhar W., Rathinasamy Sh., Rajan R. (2015). Neuroprotective effects of Indigofera tinctoria on noise stress affected Wistar albino rat brain. Journal of Applied Pharmaceutical Science, 5(6), 58–65. [DOI: 10.7324/JAPS.2015.50609] [DOI] [Google Scholar]
- Tanaka Sh., Young J. W., Halberstadt A. L., Masten V. L., Geyer M. A. (2012). Four factors underlying mouse behavior in an open field. Behavioural Brain Research, 233(1), 55–61. [DOI: 10.1016/j.bbr.2012.04.045] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiolo G., Bramanti P., Mazzon E. (2018). Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules, 23(12), 3305. [DOI: 10.3390/molecules23123305] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M., Mihara M. (1978). Determination of malonalde-hyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry, 86(1), 271–8. [DOI: 10.1016/0003-2697(78)90342-1] [DOI] [PubMed] [Google Scholar]
- Uran S. L., Caceres L. G., Guelman L. R. (2010). Effects of loud noise on hippocampal and cerebellar-related behaviors.: Role of oxidative state. Brain Research, 1361, 102–14. [DOI: 10.1016/j.brainres.2010.09.022] [DOI] [PubMed] [Google Scholar]
- Wang Sh., Yu Y., Feng Y., Zou F., Zhang X., Huang J., et al. (2016). Protective effect of the orientin on noise-induced cognitive impairments in mice. Behavioural Brain Research, 296, 290–300. [DOI: 10.1016/j.bbr.2015.09.024] [DOI] [PubMed] [Google Scholar]
- Wankhar D., Devi R. S., Ashok I. (2014). Emblica officinalis outcome on noise stress induced behavioural changes in Wistar albino rats. Biomedicine & Preventive Nutrition, 4(2), 219–24. [DOI: 10.1016/j.bionut.2013.12.011] [DOI] [Google Scholar]
- Wu H. P., Hsu C. J., Cheng T. J., Guo Y. L. (2010). N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hearing Research, 267(1–2), 71–7. [DOI: 10.1016/j.heares.2010.03.082] [DOI] [PubMed] [Google Scholar]
- Xu H., Richardson J. S., Li X. M. (2003). Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology, 28(1), 53–62. [DOI: 10.1038/sj.npp.1300009] [DOI] [PubMed] [Google Scholar]
- Young J. J., Bruno D., Pomara N. (2014). A review of the relationship between proinflammatory cytokines and major depressive disorder. Journal of Affective Disorders, 169, 15–20. [DOI: 10.1016/j.jad.2014.07.032] [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Li W. Q., Sylvester J., Ahmad M. (2003). Molecular mechanisms of N-acetylcysteine actions. Cellular and Molecular Life Sciences CMLS, 60(1), 6–20. [DOI: 10.1007/s000180300001] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żukowski P., Maciejczyk M., Matczuk J., Kurek K., Waszkiel D., Żendzian-Piotrowska M., et al. (2018). Effect of N-acetylcysteine on antioxidant defense, oxidative modification, and salivary gland function in a rat model of insulin resistance. Oxidative Medicine and Cellular Longevity, 2018, 6581970. [DOI: 10.1155/2018/6581970] [DOI] [PMC free article] [PubMed] [Google Scholar]