Abstract
Background:
Injuries to the articular cartilage in the knee are common in jumping athletes, particularly high-level basketball players. Unfortunately, these are often diagnosed at a late stage of the disease process, after tissue loss has already occurred.
Purpose/Hypothesis:
To evaluate longitudinal changes in knee articular cartilage and knee function in National Collegiate Athletic Association (NCAA) basketball players and their evolution over the competitive season and off-season.
Study Type:
Longitudinal, multisite cohort study.
Population:
Thirty-two NCAA Division 1 athletes: 22 basketball players and 10 swimmers.
Field Strength/Sequence:
Bilateral magnetic resonance imaging (MRI) using a combined T1ρ and T2 magnetization-prepared angle-modulated portioned k-space spoiled gradient-echo snapshots (MAPSS) sequence at 3T.
Assessment:
We calculated T2 and T1ρ relaxation times to compare compositional cartilage changes between three timepoints: preseason 1, postseason 1, and preseason 2. Knee Osteoarthritis Outcome Scores (KOOS) were used to assess knee health.
Statistical Tests:
One-way variance model hypothesis test, general linear model, and chi-squared test.
Results:
In the femoral articular cartilage of all athletes, we saw a global decrease in T2 and T1ρ relaxation times during the competitive season (all P < 0.05) and an increase in T2 and T1ρ relaxation times during the off-season (all P < 0.05). In the basketball players’ femoral cartilage, the anterior and central compartments respectively had the highest T2 and T1ρ relaxation times following the competitive season and off-season. The basketball players had significantly lower KOOS measures in every domain compared with the swimmers: Pain (P < 0.05), Symptoms (P < 0.05), Function in Daily Living (P < 0.05), Function in Sport/Recreation (P < 0.05), and Quality of Life (P < 0.05).
Conclusion:
Our results indicate that T2 and T1ρ MRI can detect significant seasonal changes in the articular cartilage of basketball players and that there are regional differences in the articular cartilage that are indicative of basketball-specific stress on the femoral cartilage. This study demonstrates the potential of quantitative MRI to monitor global and regional cartilage health in athletes at risk of developing cartilage problems.
Level of Evidence:
2
Technical Efficacy Stage:
2
Participation in certain high-level sports can lead to chronic knee injuries.1 Basketball is a high-impact sport that requires sport-specific movements (jumping, cutting, pivoting) that put extra stress on players’ knees. In basketball players, the patellofemoral area of their knee is specifically susceptible to injury, with patellofemoral injuries identified as the most common cause of missed games in the National Basketball Association (NBA).2 An increased prevalence of abnormal imaging findings has also been identified in the knees of basketball players using conventional morphologic magnetic resonance imaging (MRI).3-6 However, morphologic MRI is mostly sensitive to global, late-stage degeneration and significant knee abnormalities.5 Conversely, quantitative MRI (qMRI) provides a noninvasive method to study matrix depletion in knee articular cartilage,7 which is a known risk of degenerative disease progression.8 Despite the rich literature on late-stage morphological changes in basketball players using MRI, the compositional and structural changes associated with early cartilage degeneration that occur prior to the visible morphological changes have not been as well studied. The characterization of early cartilage matrix degeneration may allow for interventions that can reverse negative changes before degenerative morphological joint changes are visible on conventional MRI.
To quantify early compositional and structural changes in the articular knee cartilage of basketball players, T2 and T1ρ relaxation time mapping can be used.8 Increases in T2 and T1ρ relaxation times are considered reflective of tissue changes in articular cartilage, with prolongation considered representative of collagen damage9 and loss of gly-cosaminoglycan content.8 Prolongation of both these metrics are unfavorable changes that can be early signs of degeneration. Past studies have shown that T2 and T1ρ qMRI can be used to quantify changes in the articular knee cartilage of athletes, including marathon runners compared to age and gender-matched controls,10 basketball players compared to swimmers,11 and basketball players at a pre- and postseason timepoints.12 However, the biological meaning of qMRI metrics in young athletes is still unclear, and the role of T2 and T1ρ for injury management and the monitoring of the subsequent recovery process is yet to be established. In addition, the effect of the competitive season versus the off-season on the knees of basketball players and if one season has more of a negative effect are unclear. The off-season is used typically for injury recovery, strength training, and improving sport-specific skills, but it is unknown whether recovery in basketball players actually occurs during this time.
Knee Osteoarthritis Outcome Scores (KOOS) is a tool that quantifies knee functionality that has been shown to have internal consistency, test–retest reliability, and construct validity in young and old adults with knee injuries.13 Furthermore, it has been shown to be a reliable and valid instrument in measuring knee health differences in previously injured and uninjured athletes.14,15
The overall aim of this study was to present a framework for using qMRI and KOOS to respectively quantify compositional and structural changes in knee cartilage and knee functionality in order to investigate changes in basketball players during their competitive season and off-season and to compare them with swimmers. Specifically, we aimed to: (1) compare the changes in knee articular cartilage using qMRI (T2 and T1ρ), (2) compare knee functionality using KOOS, and (3) follow the athletes over multiple seasons to detect the onset of longitudinal change and possible recovery.
Materials and Methods
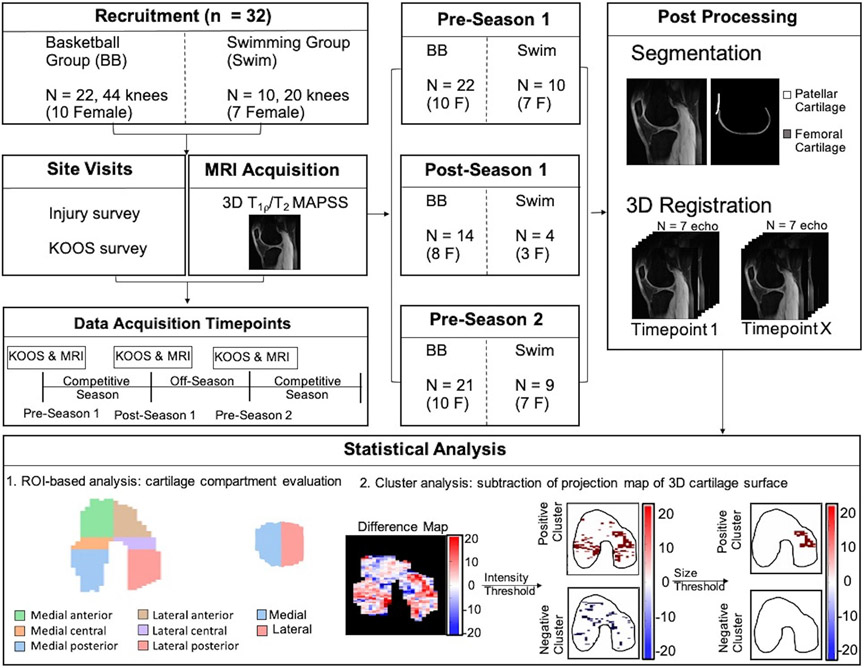
This multicenter longitudinal study was conducted per the rules and procedures approved by the Ethics Committee and Institutional Review Boards of the three participating sites: Stanford University, University of California San Francisco, and Hospital for Special Surgeries. All participants provided informed written consent before participation in the study. A schematic overview of the study methodology is shown in Fig. 1. This study used qMRI to characterize knee health of National Collegiate Athletic Association (NCAA) Division 1 (D1) basketball players and swimmers. Swimmers were chosen as the control population due to their representation in literature as athletes with a lower prevalence of injuries compared to basketball players and other athletes.16 This study included two cohorts of NCAA D1 athletes (from two D1 programs): 22 basketball players (10 female, ages 18–20, body mass index [BMI] = 23.1 ± 2.0, 44 knees) and 10 swimmers (7 female, ages 18–20, BMI = 23.3 ± 1.5, 20 knees). All swimmers had a BMI within the healthy range (BMI of 18.5–24.9). Five of the basketball players (23%) were within an overweight BMI range (BMI of 25.0–30), and the other 17 (77%) were within a healthy BMI range. Within the basketball cohort, 10 of the 22 players had a past surgically or nonsurgically treated knee injury to either their bone or tendon. Of those injuries, 70% were basketball-related injuries. Upon enrollment in the study, all participants were first- or second-year college students on a D1 basketball or swim team. Participants were questioned about their overall knee health and past history of participation in competitive sports. Basketball players with prior anterior cruciate ligament (ACL) injury were included if they were fully recovered and playing again. Athletes were withdrawn from the study if they discontinued training or retired from play. Athletes with conditions precluding them from receiving an MRI scan were excluded. Swimmers with a prior knee injury, knee pain, surgery, or past participation in competitive jumping sports (basketball/volleyball) were excluded. The exclusion of swimmers with previous injuries was to remove possible confounding factors in this low-impact control population. Any knee injuries they may have had are assumed to be from nonswimming activities based on the low prevalence of swimming-related knee injuries that occur in swimmers.17 All MRI scans were reviewed by a musculoskeletal radiologist, and incidental findings of high clinical significance were conveyed to the athlete’s physician for follow-up. Images were also provided at the request of the team physician and reviewed by an experienced musculoskeletal radiologist.
Figure 1:
Schematic overview of the study methodology.
Site visits occurred respectively for the preseason and postseason between the months of September–October and May–June. Three timepoints were included in this study: preseason 1, postseason 1, and preseason 2. The timepoints occurred between October 2017 and November 2019.
Questionnaires
During each visit, participant knee health was measured using the KOOS questionnaire.18 KOOS consists of the following subsections: Symptoms, Pain, Function in Daily Living (ADL), Function in Sport and Recreation (Sport/Rec), and Quality of Life (QOL). These scores were compared between athlete populations and with age-matched reference values found in literature.19 At the preseason 1 visit, athletes were asked about past surgically or nonsurgically treated injuries. The trainers estimated the type and volume of different exercises, including strength training, conditioning, and on-court/skill (basketball) and pool (swimmers) time during the competitive season and off-season. During the competitive season, athletes self-reported missed practices for the number of days and specific hours of practice that they missed.
MRI Protocol
The athletes underwent an MRI at each timepoint using a 3.0 T scanner (MR750 GE Healthcare, Waukesha, WI) with an 18-channel Transmit/Receive knee coil (Quality Electrodynamics, Mayfield Village, OH). All athletes were scanned on the same 3.0T scanner at Stanford University. Every subject was given time to rest and unload their knees prior to their MRI scan and was scanned a minimum of an hour following practice to limit any temporary practice-related loading effects on their knee cartilage. Both knees were scanned consecutively, with the imaged knee in the magnet isocenter. The MRI protocol included a three-dimensional (3D) sagittal combined T1ρ and T2 magnetization-prepared angle-modulated portioned k-space spoiled gradient-echo snapshots (MAPSS) sequence.20,21 MAPSS was acquired in an oblique sagittal scan plane with the following acquisition parameters: 256 × 256 matrix, 14 or 16 cm field of view, 4-mm slice thickness, a variable slice number for full knee coverage (~30), repetition time = 6.5 seconds, spin lock frequency for T1ρ measurements = 500 Hz, flip angle = 60°, and a 10-minute scan time. The MAPSS sequence included an interchangeable preparation, in which either T1ρ or T2 preparation could be run for every echo. T2-weighted scans were acquired using a T2 preparation consisting of an Malcolm-Levitt (MLEV) train of nonselective composite pulses, while T1ρ-weighted images were acquired using a B0- and B1-compensated spin lock module. For the first Spin-lock Time (TSL) = 0 and first Echo Time (TE) = 0, T1ρ- and T2-weighted images share the first image. T2 and T1ρ relaxation time maps were computed at various echo/spin lock (TE/TSL) times, which respectively included for T2 and T1ρ: 0.0, 12.9, 25.7, and 51.3 ms and 0.0, 10, 40, and 80 ms. The different T2 and T1ρ-weighted images were respectively used to calculate T2 and T1ρ using a monoexponential fit.
Region of Interest (ROI)-Based Analysis
The femoral cartilage was manually segmented using the first echo of the MAPSS sequence (ITK-SNAP, 3.8.0) at baseline (preseason 1) for each subject. 3D scans acquired at postseason 1 and preseason 2 timepoints were registered to corresponding baseline (preseason 1) scans with a rigid transformation centered on the femoral cartilage ROI using Elastix.22 The patellar cartilage was manually segmented at each time point using the first echo of the MAPSS sequence because the small patellar cartilage volume relative to the voxel size increased the risk of a partial volume effect during registration. Using cylinder fitting of the segmented femoral articular cartilage and angular binning along with the segmentation’s center of mass, we created two-dimensional (2D) projection maps of the femoral cartilage,23 in which regions were automatically divided into the lateral and medial condyles and then into the anterior, central, and posterior compartments within each condyle (Fig 1, Panel ‘Statistical Analysis). Patellar cartilage regions were automatically divided into lateral and medial regions. For analysis of the MAPSS images, these masks were applied directly to select T2 and T1ρ relaxation times (in ms) in the femoral and patellar cartilage, respectively. The mean and standard deviation for T2 and T1ρ relaxation times were calculated for each subregion of the given tissue. For both the femoral and patellar cartilage, differences in average T2 and T1ρ relaxation times were calculated over three time intervals: competitive season (postseason 1 – preseason 1), off-season (preseason 2 – postseason 1), and 1 year (preseason 2 – preseason 1).
Quantitative T2 and T1ρ maps for femoral cartilage were projected into the 2D plane for visualization using methods described in a previous study,23 which used radial projections of 1° increments from the center of the 3D image. Quantitative values of voxels within each projection bin were averaged. For patellar cartilage, the T2 and T1ρ values were averaged in the anterior to posterior direction to obtain 2D projection map visualizations.
Cluster Analysis
A clustering method23 was utilized to identify longitudinal differences in 2D T1ρ and T2 relaxation projection maps in the basketball players. Within the cluster analysis, we identified clusters of contiguous pixels with a size greater than 15 mm2 where T1ρ and T2 increases were higher than two standard deviations above the changes seen in our comparison population of swimmers. Differences between projection maps at (postseason 1 – preseason 1), (preseason 2 – postseason 1) and (pre-season 2 – pre-season 1) intervals were clustered based on intensity and size thresholds calculated for the basketball players using the swimmers’ (our low-impact control group) average changes and standard deviations in T1ρ and T2 relaxation times. The intensity and size thresholds were, respectively, 3.42 ms and 15 mm2 for T2 and 5.13 ms and 15 mm2 for T1ρ. For the femoral cartilage, the coregistered projection maps were subtracted from each other to obtain the difference values. For the patellar cartilage, separate segmentations for each timepoint were created, and only pixels that were present in both maps were used for a pixel-pixel subtraction due to the slightly different shaped segmentations.
Phantom Repeatability
To evaluate the repeatability of quantitative metrics in a single scanner over multiple timepoints, we imaged a custom phantom (GE Healthcare), which consisted of falcon tubes with various amounts of agarose to vary relaxation times. The phantom included six labeled tubes with three different agarose solutions [2%, 3%, 4%], and each solution was placed in two different tubes. To evaluate intrascanner variability, the phantom was imaged before the start of the athlete scanning period at each of the three timepoints. Imaging was performed using the MAPSS sequence. T2 and T1ρ relaxation time maps were computed using a monoexponential fit of signal data acquired at various TE/TSL times. For the phantom analysis, T2 and T1ρ relaxation times were extracted using Horos Software (v3.3.5, Annapolis, MD).
MAPSS Repeatability
To assess the reproducibility of the MAPSS sequence, we scanned six healthy volunteers twice, consecutively, at one site (three female, ages 24–31). Between the two scans, subjects stood and walked 3 meters. Femoral cartilage was manually segmented on the first scan, and the second scan was then registered to the first using the same method described in the ROI analysis section.
Statistical Analysis
A one-way variance model hypothesis test with a Tukey post-hoc test, Bonferroni correction, and α = 0.05 was used to detect differences of T1ρ or T2 within one timepoint, and a general linear model was used across different timepoints. A chi-squared statistical test was used for categorical variable comparisons. Statistical analysis was conducted using Minitab (Minitab LLC, Pennsylvania, 19.2), and a P value of < 0.05 was considered statistically significant. Intrascanner reproducibility of T2 and T1ρ measurements for each phantom solution was assessed using the coefficient of variance (CV). The repeatability of T2 and T1ρ was assessed with CV in the posterior, central, and anterior regions of the femoral cartilage in Scans 1 and 2. A comparison between the repeatability of T2 and T1ρ and the differences in T2 and T1ρ relaxation times during the competitive season and off-season for the basketball players and swimmers was assessed using a one-way variance model hypothesis test.
Results
Cohort Distributions and Questionnaire Results
At the preseason 1 timepoint, the basketball players had significantly lower KOOS (P < 0.05) in all subscales compared to the swimmers: Pain, Symptoms, ADL, Sport/Rec, and QOL. There were insignificant changes in KOOS between preseason 1 and preseason 2 in both cohorts (Basketball Players: Pain: P = 0.155, Symptoms: P = 0.443, ADL: P = 0.202, Sport/Rec: P = 0.265, QOL: P = 0.231 and Swimmers: Pain: P = 0.484, Symptoms: P = 0.527, ADL: P = 0.123, Sport/Rec: P = 0.635, QOL: P = 0.170) (Fig. 2). The average KOOS subsection scores, at preseason 1 and preseason 2, for the basketball players were lower than the average scores for normative reference values for 18–25-year-olds, while the swimmers had higher average scores.
Figure 2:
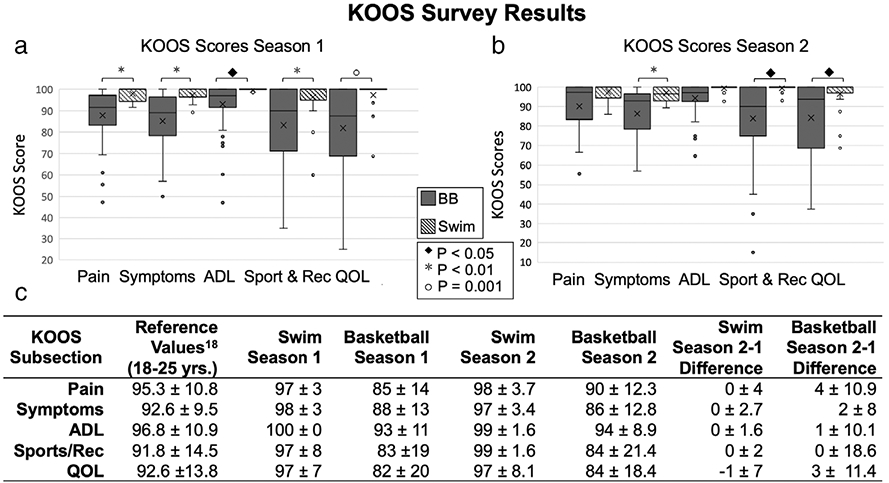
(a, b) Summary of KOOS season 1 and season 2 subsections and (c) reference values.18 In the graphs, circles represent outliers, and the whiskers indicate the 10th and 90th percentiles.
With missed practices, basketball players were significantly more likely to be held out of practice for any reason, not exclusive to injury, than swimmers (P < 0.05). In addition, the percentage of basketball players held out of >1 practice (86%) during the competitive season was significantly higher than for the swimmers (33%) (P < 0.05). The reported workout breakdown for the basketball players by percentage of total practice hours for the competitive season was 12% (2/17 hours) strength and 88% (15/17 hours) of on-court/skill time. For the spring off-season, it was 31% (2.5/8 hours) strength, 19% (1.5/8 hours) conditioning, and 50% (4/8 hours) on-court/skill time. Finally, for the summer off-season, it was 25% (2/8 hours) strength, 25% (2/8 hours) conditioning, and 50% (4/8 hours) on-court/skill time. For both the competitive season and off-season, swimmers swam 77% (20/26 hours) and had dryland (running/weight lifting) workouts for 23% (6/26 hours) of practice time.
Quantitative MRI Analysis Summary
There were 32 athletes (22 basketball) imaged at preseason 1, 18 athletes (14 basketball) imaged at postseason 1, and 30 athletes (21 basketball) imaged at preseason 2 (Fig. 1). Athletes were only included in an analysis between two timepoints if they were scanned at both timepoints. Depending on participant availability at each timepoint, there was a differing number of participants in each comparison. Due to suboptimal image quality, one basketball dataset (one knee) for the preseason 2 timepoint was excluded.
A summary of preseason 1, postseason 1, and preseason 2 T2 and T1ρ relaxation times for patellar and femoral cartilage is presented in Fig. 3. For each preseason and postseason timepoint and difference comparisons, all means, standard deviations, and statistical comparisons can be found in Table 1.
Figure 3:
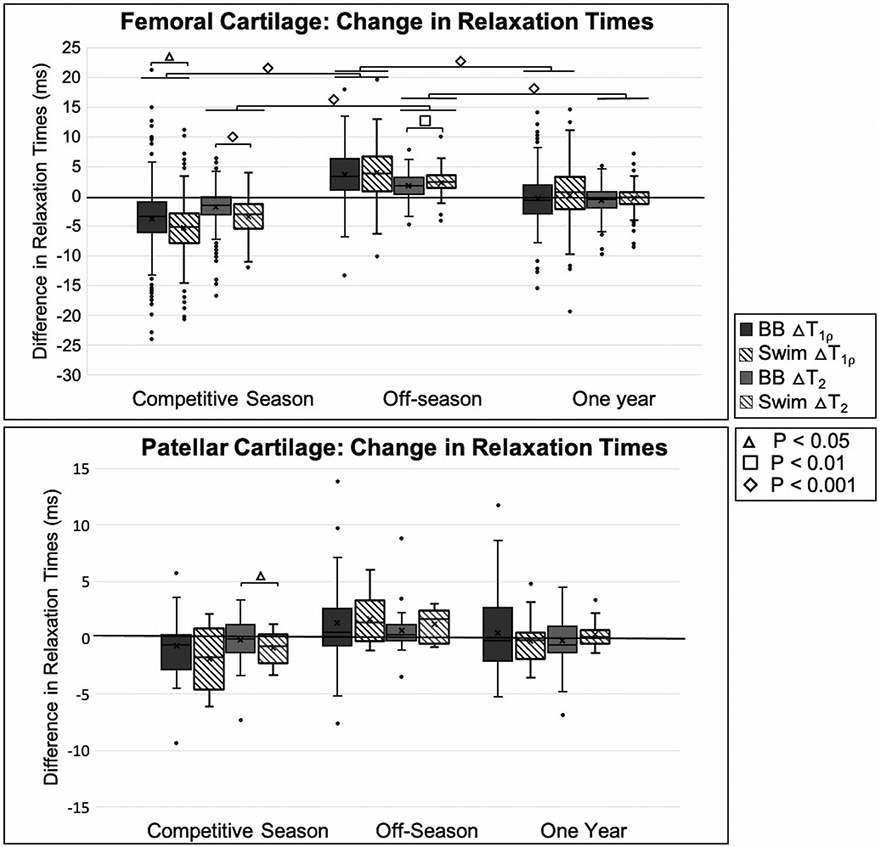
Summary of femoral cartilage difference results. Competitive season represents the difference in quantitative magnetic resonance imaging (qMRI) metrics for (postseason 1 – preseason 1), off-season represents the difference in qMRI metrics for (preseason 2 – postseason 1), and 1 year represents the difference in qMRI metrics for (preseason 2 – preseason 1). The horizontal line above basketball players and swimmers in T2 and T1ρ relaxation times represents significant differences within each athlete group at different timepoints. In both graphs, circles represent outliers, and the whiskers indicate the 10th and 90th percentiles.
Table 1.
Means and Standard Deviations at Every Timepoint and Difference Comparison for Basketball Players and Swimmers
| [a] Femoral Cartilage | Timepoint 1 | Timepoint 2 | P-Value |
|---|---|---|---|
| BB | |||
| Preseason 1 vs. Postseason 1 |
T2: 33.1 ± 1.6 T1p: 43.4 ± 2.1 |
T2: 30.7 ± 4.0 T1p: 39.0 ± 5.8 |
T2: <0.001 T1p: <0.001 |
| Postseason 1 vs. Preseason 2 |
T2: 30.7 ± 4.0 T1p: 39.0 ± 5.8 |
T2: 32.5 ± 1.7 T1p: 43.0 ± 2.9 |
T2: <0.001 T1p: <0.001 |
| Swim | |||
| Preseason 1 vs. Postseason 1 |
T2: 32.9 ± 1.7 T1p: 44.5 ± 2.5 |
T2: 29.5 ± 3.2 T1p: 39.2 ± 5.5 |
T2: <0.001 T1p: <0.001 |
| Postseason 1 vs. Preseason 2 |
T2: 29.5 ± 3.2 T1p: 39.2 ± 5.5 |
T2: 32.2 ± 1.6 T1p: 43.7 ± 3.7 |
T2: <0.001 T1p: <0.001 |
| [b] Basketball Players | Right Knee | Left Knee | P-Value |
| Femoral cartilage | |||
| Preseason 1 |
T2: 32.5 ± 4.4 T1p: 42.1 ± 5.3 |
T2: 32.6 ± 4.4 T1p: 42.6 ± 5.6 |
T2: 0.805 T1p: 0.054 |
| Postseason 1 |
T2: 29.8 ± 3.7 T1p: 37.6 ± 5.0 |
T2: 30.5 ± 3.9 T1p: 38.8 ± 5.7 |
T2: 0.106 T1p: <0.05 |
| Preseason 2 |
T2: 31.7 ± 3.7 T1p: 41.8 ± 5.4 |
T2: 32.1 ± 3.8 T1p: 41.7 ± 5.4 |
T2: 0.356 T1p: 0.869 |
| Patellar Cartilage | |||
| Preseason 1 |
T2: 32.0 ± 2.2 T1p: 41.4 ± 2.7 |
T2: 32.8 ± 2.7 T1p: 42.9 ± 4.2 |
T2: 0.140 T1p: 0.054 |
| Postseason 1 |
T2: 31.8 ± 2.0 T1p: 40.5 ± 3.5 |
T2: 32.4 ± 2.9 T1p: 42.4 ± 4.3 |
T2: 0.307 T1p: <0.05 |
| Preseason 2 |
T2: 32.4 ± 2.3 T1p: 41.6 ± 3.9 |
T2: 33.0 ± 2.6 T1p: 43.9 ± 4.9 |
T2: 0.238 T1p: <0.05 |
| [c] | Basketball Average | Swim Average | P-Value |
| Femoral Cartilage | |||
| Preseason 1 |
T2: 33.1 ± 1.6 T1p: 43.4 ± 2.1 |
T2: 32.9 ± 1.7 T1p: 44.5 ± 2.5 |
T2: 0.581 T1p: 0.078 |
| Difference: Post 1 – Pre 1 |
T2: −1.9 ± 2.9 T1p: −3.8 ± 4.9 |
T2: −3.4 ± 3.0 T1p: −5.3 ± 5.1 |
T2: <0.001 T1p: 0.002 |
| Postseason 1 |
T2: 30.7 ± 4.0 T1p: 39.0 ± 5.8 |
T2: 29.5 ± 3.2 T1p: 39.2 ± 5.5 |
T2: <0.001 T1p: 0.725 |
| Difference: Pre 2 – Post 1 |
T2: 1.6 ± 3.1 T1p: 3.5 ± 4.5 |
T2: 2.4 ± 2.7 T1p: 4.0 ± 5.9 |
T2: 0.015 T1p: 0.348 |
| Preseason 2 |
T2: 32.5 ± 1.7 T1p: 43.0 ± 2.9 |
T2: 32.2 ± 1.6 T1p: 43.7 ± 3.7 |
T2: 0.501 T1p: 0.404 |
| Difference: Pre 2 – Pre 1 |
T2: −0.7 ± 1.6 T1p: −0.5 ± 2.7 |
T2: −0.3 ± 1.4 T1p: 0.2 ± 3.9 |
T2: 0.326 T1p: 0.457 |
| Patellar Cartilage | |||
| Preseason 1 |
T2: 32.2 ± 2.2 T1p: 41.6 ± 3.2 |
T2: 32.1 ± 1.6 T1p: 43.1 ± 2.5 |
T2: 0.917 T1p: 0.004 |
| Difference: Post 1 – Pre 1 |
T2: −0.2 ± 2.4 T1p: −0.8 ± 3.5 |
T2: −1.0 ± 1.8 T1p: −1.9 ± 3.2 |
T2: 0.159 T1p: 0.177 |
| Postseason 1 |
T2: 32.2 ± 2.4 T1p: 41.4 ± 3.7 |
T2: 31.4 ± 1.5 T1p: 42.0 ± 2.7 |
T2: 0.105 T1p: 0.458 |
| Difference: Pre 2 – Post 1 |
T2: 0.6 ± 2.4 T1p: −1.3 ± 5.1 |
T2: 1.1 ± 1.8 T1p: 1.5 ± 2.8 |
T2: 0.395 T1p: 0.864 |
| Preseason 2 |
T2: 32.0 ± 2.4 T1p: 42.0 ± 4.2 |
T2: 32.4 ± 1.4 T1p: 42.8 ± 2.5 |
T2: 0.280 T1p: 0.187 |
| Difference: Pre 2 – Pre 1 |
T2: −0.1 ± 2.1 T1p: 0.4 ± 3.9 |
T2: 0.3 ± 1.7 T1p: −0.3 ± 2.8 |
T2: 0.200 T1p: 0.282 |
Bolded P-values represent significance using an α = 0.05.
T2 and T1ρ Relaxation Time: Basketball Competitive Season
There was a significant decrease in T2 and T1ρ relaxation times in the femoral cartilage from preseason 1 to postseason 1 (all P < 0.05). In the femoral cartilage at preseason 1, the anterior and central regions had significantly higher T1ρ relaxation times than the posterior region (anterior, central: 43.82 ± 4.63 ms, 43.73 ± 6.74 ms vs. posterior: 42.59 ± 3.77 ms, P < 0.05), and the anterior had significantly higher T2 relaxation times than the central and posterior regions (45.01 ± 6.88 ms vs. central, posterior: 42.45 ± 7.29 ms, 41.44 ± 3.54 ms, P < 0.05). After the competitive season, the anterior region had significantly higher T2 and T1ρ relaxation times (T2: 31.72 ± 2.67 ms, P < 0.05 and T1ρ: 40.77 ± 5.07 ms, P < 0.05) compared to the central and posterior regions (T2: 28.61 ± 4.17, 30.11 ± 2.75 and T1ρ: 37.07 ± 6.27 ms, 36.75 ± 3.36 ms). Comparing the femoral cartilage in the right and left knees, there was no significant difference for preseason 1 with regard to T1ρ relaxation times (P = 0.424), but at postseason 1, the left knee had significantly higher T1ρ relaxation times (38.78 ± 5.65 vs. 37.61 ± 4.97 ms, P < 0.05). In the patellar cartilage, there was no significant difference between the knees at preseason 1 for T1ρ relaxation times (P = 0.182), but at postseason 1, the left knee had significantly higher T1ρ relaxation times (42.34 ± 4.19 vs. 41.09 ± 3.27 ms, P < 0.05).
T2 and T1ρ Relaxation Time: Basketball Off-Season
In the off-season, basketball players showed a significant increase in T1ρ and T2 relaxation times (both P < 0.05). In the femoral cartilage, during the off-season, basketball players showed the largest increase in T2 and T1ρ relaxation times in the central region (T2: 2.22 ± 3.26 ms and T1ρ: 4.52 ± 6.26 ms, both P < 0.05) compared to the posterior and anterior regions (T2: 1.67 ± 3.65 ms, 1.00 ± 2.01 ms and T1ρ: 3.66 ± 2.87 ms, 2.35 ± 3.36 ms).
At postseason 1 and preseason 2, the left knee’s patellar cartilage had significantly higher T1ρ relaxation times (42.40 ± 4.27 ms, 43.91 ± 4.86 ms, both P < 0.05) than the right knee (40.47 ± 3.50 ms, 41.59 ± 3.88 ms).
ROI Analysis: Basketball Players Versus Swimmers
At baseline, there was no significant difference in T2 and T1ρ relaxation times between basketball players and swimmers in the femoral cartilage (P = 0.581, P = 0.078), but in the patellar cartilage, swimmers had significantly higher average T1ρ relaxation times (43.14 ± 2.49 ms vs. 41.63 ± 3.22 ms, P < 0.05). At the postseason 1 timepoint, the basketball players had significantly higher T2 relaxation times in the femoral cartilage compared to the swimmers (30.74 ± 3.96 ms vs. 29.48 ± 3.21 ms, P < 0.05). Global femoral cartilage relaxation time reduction among swimmers was significantly greater than among basketball players during the competitive season (T2: −3.44 ± 2.99 ms vs. −1.85 ± 2.87 ms, P < 0.05, T1ρ: −5.32 ± 5.12 ms vs. −3.84 ± 4.88 ms, P < 0.05). This difference was the largest in the anterior compartment of the femoral cartilage for T1ρ (P < 0.05) and T2 (P < 0.05) and in the patellar cartilage for T2(P < 0.05).
In the off-season, both basketball players and swimmers showed a significant increase in T1ρ and T2 relaxation times (all P < 0.05), and the swimmers had a significantly higher increase in T2 relaxation times (2.35 ± 2.70 ms vs. 1.63 ± 3.10 ms, P < 0.05). There was no significant difference in T1ρ and T2 relaxation times in the patellar cartilage between the athlete cohorts following the off-season (P = 0.501, P = 0.404).
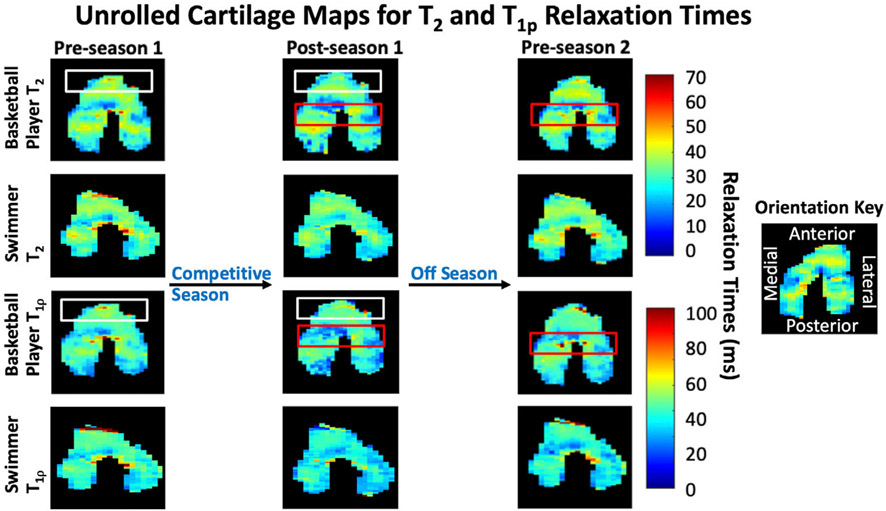
T1ρ relaxation time changes during the competitive season and off-season for basketball players and swimmers were significantly greater than T2 relaxation time changes (all P < 0.05). A 2D projection map of the femoral cartilage for a representative basketball player and swimmer is shown in Fig. 4.
Figure 4:
Two-dimensional projection maps of the three-dimensional femoral cartilage surface for a representative basketball player and swimmer. For the basketball players, the white box outlines the anterior region where the most significant change occurred in the competitive season, and the red box outlines the central region where the most significant increase occurred during the off-season.
Differences between preseason 1 versus preseason 2 showed an overall decrease in T2 relaxation times in the femoral cartilage for the basketball players (P < 0.05) and swimmers P < 0.05) and no significant change in T1ρ relaxation times (P = 0.476, P = 0.458). Regionally, in the basketball players, T1ρ relaxation times increased significantly in the anterior region compared to the central and posterior regions (P < 0.05), while T2 relaxation times in the anterior region increased significantly compared to only the posterior region (P < 0.05).
There was no detectable difference between previously injured and noninjured basketball players in T2 and T1ρ during the competitive season, off-season, or over the whole year (competitive season: P = 0.072, P = 0.684, off-season: P = 0.954, P = 0.755, whole year: P = 0.546, P = 0.577).
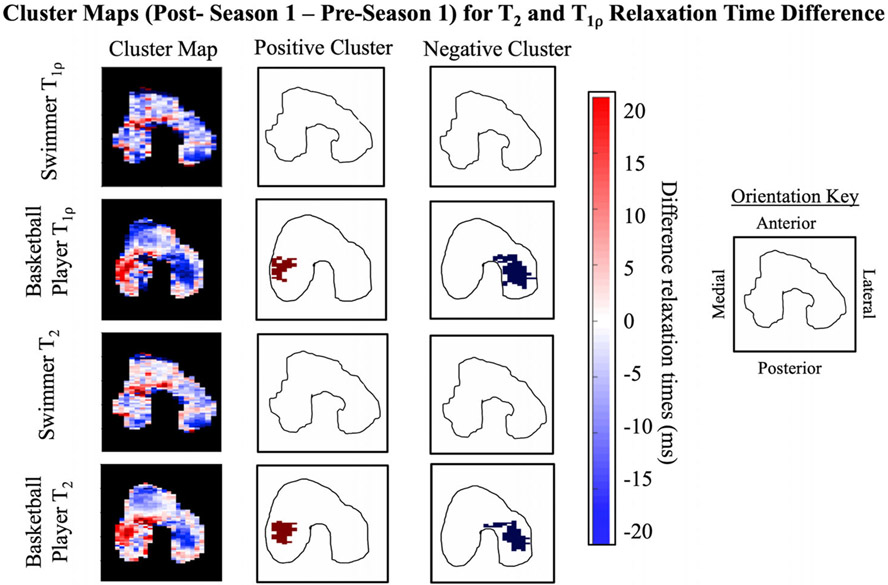
Cluster Analysis: Basketball Players Versus Swimmers
Following the competitive season, the percentage of cluster area in the femoral cartilage with significant positive clusters was significantly higher in the basketball players compared to the swimmers for T2 and T1ρ (both P < 0.05). 2D projection maps show the positive and negative clusters present in a representative basketball player and swimmer for T2 and T1ρ relaxation times in Fig. 5.
Figure 5:
Cluster analysis maps from a representative basketball player and swimmer. Positive and negative cluster maps shown are after the intensity and size thresholds had been applied. The cluster analysis of the femoral cartilage shows a basketball player with significant positive clusters in the medial central compartment and significant negative clusters in the lateral central compartment compared to a swimmer with no significant positive or negative clusters.
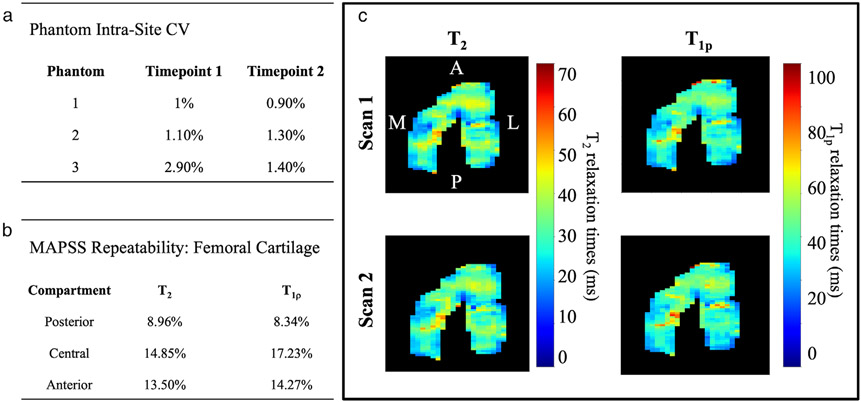
Phantom and MAPSS Repeatability
Intrascanner repeatability with the phantom across the three timepoints was high with CVs of 1–2.9% for T1ρ and 0.9–1.4% for T2 (Fig. 6a). The MAPSS repeatability analysis showed a moderate CV for the posterior, central, and anterior regions for T1ρ of 8–15% and T2 of 8–18% (Fig. 6b). T2 and T1ρ relaxation time maps in the femoral cartilage of a representative subject are shown in Fig. 6c. T2 and T1ρ relaxation time changes during the competitive season and off-season for the basketball players and swimmers exceeded the average scan–rescan variability (all P < 0.05).
Figure 6:
(a) A summary of coefficient of variance (CV) for phantom repeatability analysis across two timepoints for T2 and T1ρ relaxations. (b) A summary of CV for MAPSS repeatability analysis in two back-to-back scans. (c) A representative subject from the MAPSS repeatability analysis.
Discussion
This longitudinal study used multiparametric qMRI mapping to quantify changes occurring in the femoral and patellar cartilage over a basketball player’s competitive season and off-season. We demonstrated, through an ROI-based analysis technique, that compositional and structural changes in the articular cartilage of basketball players showed significant decreases in T2 and T1ρ relaxation times, associated with a positive effect on cartilage microstructure during their competitive season, and an increase in T2 and T1ρ relaxation times, associated with a negative effect on cartilage microstructure, during their off-season. Both T1ρ and T2 relaxation times are known to be affected by hydration levels in the cartilage, and an increase in the relaxation times is respectively associated with collagen matrix degeneration or loss of cartilage proteoglycan content.8 Our quantitative results also showed a higher range of differences in the T1ρ relaxation times compared to the T2 relaxation times and larger regional differences with T1ρ relaxation times in the femoral cartilage of the basketball players. The smaller changes in T2 relaxation times are consistent with early degenerative changes which show that the collagen matrix is not affected as much as proteoglycan loss; these changes are respectively correlated with T2 and T1ρ relaxation times. Ideally, future studies would use T2 and T1ρ MRI sequences as noninvasive means of assessing cartilage to avoid overtraining and joint overload, but if only one method can be used, this study and others involving marathon runners10, 24 have shown that T1ρ may be more sensitive to early degenerative changes.
Differences in KOOS Between Basketball Players, Swimmers, and Age-Matched Controls
The questionnaire data served as a measure of knee symptoms/pain and functional knee impairment. The KOOS results suggested that basketball players have worse knee functionality than an average 18–25-year-old, while swimmers have better knee functionality.
T2 and T1ρ Changes in the Competitive Season and Off-Season
The significant differences in the anterior femoral cartilage and patellar cartilage of the basketball player are congruent with previous literature, which shows that, in professional basketball players, patellofemoral injuries are the most common cause of missed games2 and that, in asymptomatic college basketball player’s knees, the majority of abnormalities are found in the patellofemoral joint.6 The higher T2 and T1ρ relaxation times in the anterior region of the femoral cartilage compared to the central and posterior regions at postseason 1 but not at preseason 1 or preseason 2 indicates that the higher time spent playing basketball during the competitive season could be causing increased stress on the anterior portion of the knee. This could be due to the increased mechanical loading of the patellar tendon in the knee during the landing of a jump,25 which occurs in basketball with rebounds, blocks, shots, interceptions, etc. Changes during the off-season indicate that this increase was temporary and that recovery in the anterior cartilage occurred during the off-season. In addition, the basketball players had significantly higher T2 relaxation times in their femoral cartilage at postseason 1 compared to the swimmers, but this difference was not present between the athlete cohorts at preseason 1. This difference could be due to the loading differences during the competitive season in the knee joint of basketball players and swimmers, who respectively experience loading of high magnitude during running exercises and low-magnitude loading during water exercises. This may suggest that differences in the pattern of progression of degenerative joint diseases can be a function of sport based on the type of knee loading that occurs, similar to the findings by Peers et al.11
The increase in T1ρ and T2 relaxation times for both athlete cohorts in the off-season could suggest that the off-season’s workouts put more stress on their joints. Moderate exercise has been seen to improve joint symptoms and function compared to a nonintervention control group,26 but further study would be needed to see if that same effect is present in athlete populations and at what point exercise becomes detrimental to cartilage health. The differing percentages of on-court/skill and strength/conditioning in the competitive season and off-season and respective decrease and increase of T1ρ and T2 relaxation times suggest that different types of exercise could have a negative or positive effect on knee articular cartilage.
Based on the quantitative difference results, the difference over a whole year was smaller than the changes seen separately during the competitive season and off-season. This is in line with the different training typically performed by athletes during competitive season and off-season. Therefore, our results suggest that, within athlete populations, the effects of the competitive season and the off-season should be considered separately when a complete assessment of cartilage health and recovery is desired.
Cluster Analysis
The inclusion of the cluster analysis in the articular cartilage analysis was used to quantify focal longitudinal changes. During the competitive season, we saw more significant positive clusters in the basketball players compared to the swimmers, indicating that they had more specific areas of increasing focal change in their femoral cartilage, while the swimmer’s change was generally seen across the entire femoral cartilage. With basketball players, it seems that a cluster analysis could be most useful when looking at specific subjects with focal areas of change. The representative subject for the cluster analysis showed significant positive clusters on one side of the central compartment of their femoral cartilage when comparing preseason 1 to postseason 1 scans. This may indicate that this individual is placing more load on one side of their knee, but these findings warrant further investigation.
MAPSS Repeatability
The scan-to-scan variation in qMRI metrics both, in phantoms and healthy volunteers at each site, during the course of the study showed high to moderate repeatability of T1ρ and T2. Therefore, we are confident of the quantitative accuracy of our results. The MAPSS scan-to-scan variation was also lower than the changes we saw during the competitive season and off-season in the basketball players and swimmers, indicating that the identified changes were not driven by scan–rescan variability.
Limitations
Our study included a small number of subjects, and the majority of the swimmers was female. In addition, the seasons were defined only with the college basketball season, but in reality, the swimmers have a different competitive season timeframe than the basketball players. Due to the challenging nature of scanning time-limited student-athletes, it was difficult to scan athletes immediately preceding/following their season, and due to their busy schedules, certain subjects missed timepoints, which might have affected our analysis and statistical power between different measures. Our qMRI data were acquired at a relatively coarse resolution, which precluded laminar differences and may have resulted in some partial volume effects in the femoral cartilage. However, reproducibility of measures was high, suggesting that the longitudinal analysis was unaffected. In addition, for increased statistical power, we grouped together previously injured and noninjured basketball players, which is a possible confounding variable. However, we observed no significant difference in relaxation times based on injury history, and thus, we believe this potential confounding variable does not introduce bias in the study.
The total scan time for MAPSS of 10 minutes could be difficult for patients in pain to tolerate. Our study is a feasibility and proof-of-concept study, and in future studies, MRI acquisition could be accelerated through the use of compressed sensing27 and artificial intelligence techniques, such as super-resolution28 and supervised deep learning,28 so that it is more suitable for patients.
Conclusion
Our results showed that T2 and T1ρ MRI can detect significant seasonal changes in the articular cartilage of basketball players. The average decrease in T2 and T1ρ relaxation times following the competitive season and the average increase after the off-season indicated that sports could impact a player’s articular cartilage over a relatively short period. The difference in significantly affected regions in the basketball players indicated that the competitive season and off-season put increased stress on different compartments of a player’s femoral cartilage. We also saw, based on KOOS, that basketball players have worse knee health than swimmers and an age-matched nonathlete cohort. However, at this time, the clinical (KOOS) and qMRI (T2 and T1ρ) results observed in this study do not allow for individual athlete assessment and predictions. The observed group effects we saw do, however, indicate the usefulness of a future study that focuses on individual effects over the course of multiple competitive seasons. Future research will focus on monitoring the athletic populations over two more seasons (total of three full NCAA seasons) to obtain better insight of the natural history of biochemical and microstructural changes in joint tissue. Overall, identifying that microstructural changes in articular cartilage, as seen on MRI, may be reversible or may contribute to ongoing damage indicates how MRI can identify early degenerative changes and recovery. Identification of early changes further indicates how MRI can play a role in managing training load and rest during the competitive season and off-season. This management can help to prevent permanent structural damage in high-level athletes.
Acknowledgments
Contract grant sponsor: NBA and GE Healthcare Orthopedics and Sports Medicine Collaboration.
References
- 1.Driban JB, Hootman JM, Sitler MR, Harris KP, Cattano NM. Is participation in certain sports associated with knee osteoarthritis? A systematic review. J Athl Train 2017;52(6):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drakos MC, Domb B, Starkey C, Callahan L, Allen AA. Injury in the National Basketball Association: A 17-year overview. Sports Health 2010;2(4):284–290. 10.1177/1941738109357303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan LD, Schurhoff MR, Selesnick H, Thorpe M, Uribe JW. Magnetic resonance imaging of the knee in asymptomatic professional basketball players. Arthrosc J Arthrosc Relat Surg 2005;21(5):557–561. 10.1016/j.arthro.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Pappas GP, Vogelsong MA, Staroswiecki E, Gold GE, Safran MR. Magnetic resonance imaging of asymptomatic knees in collegiate basketball players: The effect of one season of play. Clin J Sport Med 2016; 26(6):483–489. 10.1097/JSM.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walczak BE, McCulloch PC, Kang RW, Zelazny A, Tedeschi F, Cole BJ. Abnormal findings on knee magnetic resonance imaging in asymptomatic NBA players. J Knee Surg 2008;21(1):27–33. [DOI] [PubMed] [Google Scholar]
- 6.Major NM, Helms CA. MR imaging of the knee: Findings in asymptomatic collegiate basketball players. Am J Roentgenol 2002;179(3):641–644. 10.2214/ajr.179.3.1790641. [DOI] [PubMed] [Google Scholar]
- 7.Disler DG, Recht MP, McCauley TR. MR imaging of articular cartilage. Skeletal Radiol 2000;29(7):367–377. 10.1007/s002560000222. [DOI] [PubMed] [Google Scholar]
- 8.Choi JA, Gold GE. MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am 2011;19(2):249–282. 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhadtaq HA, Xia Y, Moody JB, Matyas JR. Detecting structural changes in early experimental osteoarthritis of tibial cartilage by microscopic magnetic resonance imaging and polarised light microscopy. Ann Rheum Dis 2004;63(6):709–717. 10.1136/ard.2003.011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luke AC, Stehling C, Stahl R, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: Does long-distance running lead to cartilage damage? Am J Sports Med 2010;38(11):2273–2280. 10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- 11.Peers SC, Maerz T, Baker EA, et al. T1ρ magnetic resonance imaging for detection of early cartilage changes in knees of asymptomatic collegiate female impact and nonimpact athletes. Clin J Sport Med 2014;24(3):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelsong MA, Pappas G, Staroswiecki E, et al. Are sports good for your knees? An MRI evaluation of the effects of basketball on knee health in Division I College Athletes. 19th Annual Meeting of the International Society for Magnetic Resonance in Medicine in Montreal, Canada. May, 2011. Proc Intl Soc Mag Res Med 2011;19:507. [Google Scholar]
- 13.Collins NJ, Prinsen CAC, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee injury and osteoarthritis outcome score (KOOS): Systematic review and meta-analysis of measurement properties. Osteoarthr Cartil 2016;24(8):1317–1329. 10.1016/j.joca.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Roi GS, Monticone M, Salvoni M, Sassi R, Alberti G. Self-reported knee symptoms assessed by KOOS questionnaire in downhill runners (skyrunners). PLoS One 2015;10(4):1–12. 10.1371/journal.pone.0126382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and osteoarthritis outcome score (KOOS); reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthr Cartil 2011;19(4):406–410. 10.1016/j.joca.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Brant JA, Johnson B, Brou L, Comstock RD, Vu T. Rates and patterns of lower extremity sports injuries in all gender-comparable US high school sports. Orthop J Sport Med 2019;7(10):1–7. 10.1177/2325967119873059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanigan C, Fields SK, Comstock RD. Epidemiology of knee injuries among US high school. Am Coll Sport Med 2014;45(3):462–469. 10.1249/MSS.0b013e318277acca.EPIDEMIOLOGY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 19.Williamson T, Sikka R, Tompkins M, Nelson BJ. Use of the knee injury and osteoarthritis outcome score in a healthy United States population. Am J Sports Med 2016;44(2):440–446. 10.1177/0363546515616812. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Han ET, Busse RF, Majumdar S. In vivoT1ρ mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med 2008; 59(2):298–307. 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Wyatt C, Rivoire J, et al. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: Repeatability and diurnal variation. J Magn Reson Imaging 2014;39:1287–1293. 10.1002/jmri.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein S, Staring M, Murphy K, Viergever M, Pluim J. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 2010;29(1):196–205. 10.1109/tmi.2009.2035616. [DOI] [PubMed] [Google Scholar]
- 23.Monu UD, Jordan CD, Samuelson BL, Hargreaves BA, Gold GE, McWalter EJ. Cluster analysis of quantitative MRI T2 and T1ρ relaxation times of cartilage identifies differences between healthy and ACL-injured individuals at 3T. Osteoarthr Cartil 2017;25(4):513–520. 10.1016/j.joca.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stehling C, Luke A, Stahl R, et al. Meniscal T1rho and T2 measured with 3.0 T MRI increases directly after running a marathon. Skeletal Radiol 2011;40:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards S, Steele JR, Cook JL, Purdam CR, McGhee DE, Munro BJ. Characterizing patellar tendon loading during the landing phases of a stop-jump task. Scand J Med Sci Sports 2012;22(1):2–11. 10.1111/j.1600-0838.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 26.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycos-aminoglycan content in knee cartilage: A four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum 2005;52(11):3507–3514. 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 27.Lustig MV, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58: 1182–1195. [DOI] [PubMed] [Google Scholar]
- 28.Johnson PM, Recht MP, Knoll F. Improving the speed of MRI with artificial intelligence. Semin Musculoskelet Radiol 2020;24(1):12–20. 10.1055/s-0039-3400265. [DOI] [PMC free article] [PubMed] [Google Scholar]