Abstract
Improved diagnostics are needed for the detection of Mycobacterium tuberculosis, especially for patients with smear-negative disease. To address this problem, we have screened M. tuberculosis (H37Rv and Erdman strains) genomic expression libraries with pooled sera from patients with extrapulmonary disease and with sera from patients with elevated reactivity with M. tuberculosis lysate. Both serum pools were reactive with clones expressing a recombinant protein referred to here as MTB48. The genomic sequence of the resulting clones was identical to that of the M. tuberculosis H37Rv isolate and showed 99% identity to the Mycobacterium bovis and M. bovis BCG isolate sequences. The genomic location of this sequence is 826 bp upstream of a region containing the esat-6 gene that is deleted in the M. bovis BCG isolate. The mtb48 1,380-bp open reading frame encodes a predicted 47.6-kDa polypeptide with no known function. Southern and Western blot analyses indicate that this sequence is present in a single copy and is conserved in the M. tuberculosis and M. bovis isolates tested but not in other mycobacterial species tested, including Mycobacterium leprae and Mycobacterium avium. In addition, the native protein was detected in the cytoplasm, as was a processed form that was also shed into the medium during culture. Serological analysis of recombinant MTB48 and the M. tuberculosis 38-kDa antigen with a panel of patient and control sera indicates that the inclusion of recombinant MTB48 in a prototype serodiagnostic test increases assay sensitivity for M. tuberculosis infection when it is combined with other known immunodominant antigens, such as the 38-kDa antigen.
Mycobacterium tuberculosis continues to be the major infectious cause of human death in developing countries and has reemerged in industrialized countries (6, 14, 17, 21, 22, 27, 28). This reemergence is due, in part, to infection with the human immunodeficiency virus (HIV), which has led to increased susceptibility to M. tuberculosis and active disease (15, 35), development of new strains of multiple-drug-resistant M. tuberculosis (10, 30), and reduced resources for treatment and surveillance of patients (3, 22). Control of this infection will depend on new antibiotic therapy, improved early detection for both smear-positive and -negative cases, and the development of an effective vaccine. The development of a sensitive rapid serodiagnostic test would complement present methods of diagnosis, including skin testing, DNA amplification, bacterial culture, and radiological imaging and has become a major research goal (4, 11, 12, 13, 14, 16). A rapid and inexpensive assay could reduce the need for the more expensive tests, possibly reducing the cost of diagnosis, and would be of value in an emergency room setting, where early knowledge of a patient's disease status would be helpful. Finally, an inexpensive diagnostic test is needed in developing countries, where more expensive diagnostic tools are not available. To date, many M. tuberculosis antigens, such as the 38-kDa lipoglycoprotein (1), MTC28 (25), MPT32 (31), MTB81 (20), CFP-10 (9), and the 19-kDa (2) and 14-kDa (33) antigens, have been tested for their utilities in the development of a rapid serodiagnostic test (5, 23, 32). However, the sensitivity needed for a useful test has not been achieved due to the heterogeneous human response to M. tuberculosis antigens (23) and to the low seroreactivities of problematic groups, such as HIV-positive patients or those with smear-negative disease (12, 19, 32). Here we describe the cloning of mtb48 by screening a M. tuberculosis genomic expression library with pooled sera from patients with extrapulmonary disease and with sera from patients with elevated reactivity with M. tuberculosis lysate. The mtb48 1,380-bp open reading frame (ORF) encodes a predicted 47.6-kDa polypeptide with no known function. Southern blot and bioinformatic analyses indicate that this sequence is present as a single copy and is highly conserved in the M. tuberculosis and Mycobacterium bovis isolates tested but not in other mycobacterial species tested, including Mycobacterium leprae and Mycobacterium avium. Western blot analysis shows that the native protein is cytoplasmic and not membrane bound. A truncated form of the native protein is found both in the cytoplasm and in the culture filtrate (culture filtrate protein [CFP]), indicating that this form could be shed. Because serological reactivity to MTB48 is complementary to that of other M. tuberculosis antigens, this new antigen adds significantly to the antigen pool that will be needed for a highly sensitive and specific serodiagnostic test.
MATERIALS AND METHODS
Mycobacterial strains.
Sean Skerritt (Seattle Veterans Affairs Hospital, Seattle, Wash.) provided M. tuberculosis strains H37Ra (ATCC 25177), H37Rv, and Erdman (ATCC 35801). M. tuberculosis strain C is a clinical isolate provided by Lee Riley (University of California, Berkeley). Pelleted samples of M. bovis BCG and M. leprae were provided by Paul Tan (Genesis Corp., Auckland, New Zealand). Other species of mycobacteria, including Mycobacterium scrofulaceum (ATCC 19981), Mycobacterium smegmatis (ATCC 19420), Mycobacterium fortuitum (ATCC 6841), Mycobacterium malmoense (ATCC 29571), and Mycobacterium gordonae (ATCC 14470), were obtained from the American Type Culture Collection (ATCC; Manassas, Va.).
Library preparation and serological expression screening.
M. tuberculosis cells were lysed in phenol with a cell disrupter (Mini-Beadbeater; type BX-4; Biospec Products, Bartlesville, Okla.) following the manufacturer's instructions. After the addition of TE buffer (10 mM Tris, 1 mM EDTA [pH 8]), the lysate was extracted two times with phenol, followed by extraction with phenol-chloroform-isoamyl alcohol and chloroform. Twenty micrograms each of M. tuberculosis Erdman and H37Rv strain genomic DNA was sheared with a sonicator (B. Braun Biotech, Inc., Allentown, Pa.) to generate fragments of approximately 0.5 to 5.0 kbp. DNA fragments were blunted with T4 DNA polymerase (Gibco BRL, Grand Island, N.Y.) and ligated to EcoRI adapters (Stratagene, La Jolla, Calif.). Adapted inserts were then phosphorylated with T4 polynucleotide kinase (Stratagene) and size selected with a Sephacryl S-400 HR column (Sigma Chemical Co., St. Louis, Mo.). Insert DNA was ligated to a Lambda ZAP II, EcoRI-digested, calf intestinal alkaline phosphatase-treated vector (Stratagene), and the ligation mixture was packaged with Gigapack II Gold packaging extract (Stratagene).
Immunoreactive proteins were screened from approximately 5 × 105 PFU of recombinant lambda phage from both the Erdman and the H37Rv genomic expression libraries with nitrocellulose filters (Schleicher & Schuell, Keene, N.H.). Reactive plaques were assessed with two pools of Escherichia coli-adsorbed patient sera. The first was a pool of three patient serum samples that were strongly reactive with M. tuberculosis lysate (20), as assessed by enzyme-linked immunosorbent assay (ELISA) (H37Rv library; TbH clones), and the second was a pool of five serum samples from M. tuberculosis-infected patients with extrapulmonary disease (Erdman library; XP clones). Positive plaques were visualized with an alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG), IgA, and IgM (heavy and light chain) secondary antibodies (Zymed Laboratories Inc., South San Francisco, Calif.) developed with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (Gibco BRL). Excision of phagemid was done by the Lambda ZAP II protocol, and the resulting plasmid DNA was sequenced with an ABI 377 automated sequencer (Perkin-Elmer/ABI, Foster City, Calif.) with M13 forward, reverse, and specific internal DNA sequencing primers. Nucleic acid and protein homology searches were performed with software from DNASTAR, Inc. (Madison, Wis.) against the EMBL and GenBank database and the Swiss and PIR and Translated database. Protein analysis was performed with the PSORT program (National Institute for Basic Biology, Okazaki, Japan) and Protean analysis software from DNASTAR, Inc., including the Kyte-Doolittle hydrophilicity plot, Jameson-Wolf antigenicity index, and Emini surface probability plot.
Expression and purification of recombinant antigens.
Four recombinant antigens were generated, including the amino terminus (rTbH4), the carboxy terminus (rXP-1), and the full-length sequence (rMTB48) of MTB48, as well as the mature polypeptide for the M. tuberculosis 38-kDa antigen (r38 kDa). Expression of rTbH4, rXP-1, and r38 kDa was accomplished by amplifying a plasmid insert (TbH4 and XP-1) or M. tuberculosis Erdman genomic DNA (38 kDa) with Pfu polymerase (Stratagene) and clone-specific primers that included a 5′ NdeI restriction site (in italics in the sequences below), an ATG initiation codon (underlined in the sequences below), and a nucleotide sequence coding for 6 histidines (boldface in the sequences below) and a 3′ primer with a stop codon and a HindIII or EcoRI restriction site (italic in the sequences below). Oligonucleotide primers for the amplification of TbH4 included 5′-ATTAGGTCATATGCACCATCACCATCACCATACGCAGTCGCAGACCGTGACGG and 3′-TATAGGAAGCTTCTAATCCTCGGTGTAGAGCGCCTCG. XP-1 was amplified with 5′-CAATTACATATGCATCACCATCACCATCACAACGACGGCGAAGGAACTGTGC and 3′-AACCTGGAATTCGTCCATGCTCACTTCGAC, and the 38-kDa antigen was amplified with 5′-CAATTACATATGCATCACCATCACCATCACTGTGGCTCGAAACCACCGAGC and 3′-GTACGGAATTCGTGGTCAACGAGGCTAGCTGG. Amplification product was digested with the appropriate restriction enzymes, isolated by gel electrophoresis, and ligated into a pET17b plasmid vector (Novagen, Madison, Wis.) previously cut with NdeI and HindIII or EcoRI and dephosphorylated. The ligation mixture was transformed into XL1 Blue MRF′ competent cells (Stratagene), and plasmid DNA was prepared for sequencing (Qiagen Inc., Valencia, Calif.). Recombinant protein was expressed by transforming plasmid DNA into BL21 pLys S competent cells (Novagen) and inducing a single-colony cell culture with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma Chemical Co.). Full-length antigen was amplified with primers 5′-CTAGTTAGTACTCAGTCGCAGACCGTG and 3′-GCAGTGACGAATTCACTTCGACTCC by using 200 ng of total genomic M. tuberculosis Erdman strain DNA and Accuzyme (Intermountain Scientific Corporation, Inc., Kaysville, Utah) polymerase. The PCR product was digested with ScaI and EcoRI and ligated into a pET28 vector modified to include an in-frame methionine and a 6-histidine tag at the amino terminus. The full-length construct was sequenced and transformed into the HMS174 pLys S cell line (Novagen), and a single-colony culture was induced with IPTG. Cultures were disrupted by sonication and recombinant protein was recovered from cell lysate with an Ni-nitrilotriacetic acid agarose matrix (Qiagen Inc.). Purified protein was then passed over a Q anion-exchange column (rXP-1 and rMTB48) (Bio-Rad, Hercules, Calif.), following the manufacturer's instructions, and was finally dialyzed in 10 mM Tris (pH 8.0). Recombinant proteins were quality checked for purity by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), followed by staining with Coomassie blue stain and N-terminal protein sequencing (26), and were quantified by a Micro BCA assay (Pierce, Rockford, Ill.). Recombinants were assayed for endotoxin contamination by the Limulus assay (Bio Whittaker, Walkersville, Md.).
Rabbit antiserum.
Rabbit antiserum to recombinant antigens was prepared by injecting 200 μg of protein in 1 ml of incomplete Freund's adjuvant (IFA; Sigma Chemical Co.) and 100 μg of muramyl dipeptide subcutanously. Six weeks later, the animals were boosted with 100 μg of protein in IFA, followed by a final boost with 50 μg administered intravenously 1 month later. Animals were killed 1 week after the final boost, and sera were stored in aliquots at −20°C.
Study population.
Serum samples were obtained from individuals who had pulmonary tuberculosis alone prior to treatment (culture and/or acid-fast bacillus smear positive) or who had documented coinfections with HIV, as evidenced by a positive HIV type 1- and HIV type 2-specific antibody ELISA. These included samples from Brazil (Roberto Badaro, Federal University of Bahia, Salvador, Brazil), Uganda and South Africa (Milton Tam, Program for Appropriate Technology in Health, Seattle, Wash.), and the Philippines (Babi Tan, American Leprosy Foundation, Cebu City, Philippines) and sera from patients with tuberculosis (Nevin Turgay, Bornova-Izmir, Turkey). Samples from patients with lung cancer and pneumonia (China) were obtained from Robert Cole (ICT Diagnostics, Frenchs Forest, Australia). To further evaluate the specificity of the MTB48 antigen, we obtained sera from individuals who were purified protein derivative (PPD) positive (induration, >10 mm) (culture, clinically, and radiographically negative for tuberculosis) and individuals who were PPD negative (King County Tuberculosis Clinic, Seattle, Wash.). Sera from healthy blood donors (from the United States) were obtained from Boston Biomedica (West Bridgewater, Mass.), and sera from individuals who were HIV seropositive alone were obtained from Robert Ackridge (Fred Hutchinson Cancer Research Institute, Seattle, Wash.).
Southern blot analysis.
Genomic DNA was prepared by standard techniques and digested with PstI (Gibco BRL). One microgram of each digest was run on a 1.0% agarose gel and stained with ethidium bromide to confirm the equivalent loading of each lane prior to overnight transfer to a Nytran nylon membrane (Schleicher & Schuell). Insert DNA isolated by gel electrophoresis was labeled by random hexamer primer extension with [32P]dCTP and hybridized with blots overnight at 65°C. Blots were washed at 65°C twice for 15 min each time with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1× SSC containing 0.1% SDS and exposed to X-ray film (Kodak, XAR-5).
Western blot analysis.
Recombinant antigens and native M. tuberculosis proteins were subjected to SDS-PAGE analysis with 15% polyacrylamide minigels. A total of 200 ng of rMTB48 per lane was used for screening of patient serum by Western blot analysis. Cellular localization of native protein was determined by loading 10 μg of an M. tuberculosis cytoplasm preparation; 2.5 μg each of M. tuberculosis cell membrane fraction, cell wall fraction, and CFPs (provided by John Belisle, Colorado State University, produced through National Institute of Allergy and Infectious Diseases-National Institutes of Health Tuberculosis Research Materials contract N01-AI-25147); and 25 ng of recombinant protein. The proteins were transferred to nitrocellulose (BA-85; Schleicher & Schuell) and blocked for 1 h at room temperature with phosphate-buffered saline (PBS) containing 1% Tween 20. The blots were then washed three times for 10 min each time in PBS containing 0.1% Tween 20 and 0.5 M sodium chloride (wash buffer). Next, the blots were probed for 1 h at room temperature with serum diluted 1:500 in wash buffer, followed by three washes (10 min each time) in wash buffer. The blots were then incubated for 45 min at room temperature with protein A-horseradish peroxidase diluted 1:20,000 in wash buffer and again washed three times (10 min each time) in wash buffer. Finally, the blots were incubated in chemiluminescent substrate (ECL; Amersham Plc, Little Charlton, United Kingdom) for ≈1 min and were then exposed to X-ray film (XAR5) for 10 to 60 s, as required. The integrity of the protein in M. tuberculosis cellular protein fractions was confirmed by probing a Western blot with a rabbit antitubulin antiserum.
ELISA.
Ninety-six-well microtiter plates (Corning Costar, Cambridge, Mass.) were coated overnight at 4°C with recombinant proteins (200 ng/well). The plates were then aspirated and blocked with PBS containing 1% (wt/vol) bovine serum albumin for 2 h at room temperature. This was followed by washing in PBS containing 0.1% Tween 20 (PBST). A dilution of serum (1/100) in PBS containing 0.1% bovine serum albumin was added to the wells, and the plates were incubated for 30 min at room temperature, followed by washing six times with PBST and then incubation with protein A-horseradish peroxidase conjugate (1/20,000 dilution; Sigma Chemical Co.) for 30 min. The plates were then washed six times in PBST and were then incubated with tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) for 15 min. The reaction was stopped by the addition of 1 N sulfuric acid, and the plates were read at 450 nm with an ELISA plate reader (EL311; Biotek Instruments, Hyland Park, Va.). The cutoff for the assays was determined from the mean for the negative population plus 3 standard deviations of the mean. For the data in Table 1, the signal-to-cutoff ratio was determined by dividing the sample ELISA value by the cutoff value.
TABLE 1.
Comparison of the seroreactivities of recombinant proteins TbH4 and XP-1 with full-length MTB48
| Patient identification | ELISA resulta
|
||
|---|---|---|---|
| rXP-1 | rTbH4 | rMTB48 | |
| 383004 | 0.956 | 1.942 | 2.349 |
| 392004 | 7.446 | 4.375 | 5.287 |
| 393004 | 1.515 | 3.994 | 4.379 |
| 395004 | 2.392 | 1.494 | 1.591 |
| 396004 | 0.621 | 1.990 | 2.158 |
| 398004 | 0.902 | 1.737 | 2.241 |
| 407004 | 1.201 | 1.625 | 2.145 |
| 416004 | 0.830 | 1.628 | 1.917 |
| 445004 | 3.642 | 3.387 | 4.076 |
| 454004 | 1.070 | 1.085 | 1.005 |
| 465004 | 0.856 | 2.431 | 2.796 |
| 470004 | 1.595 | 2.795 | 3.455 |
| 476004 | 1.204 | 3.117 | 3.662 |
| 482004 | 2.119 | 4.313 | 4.386 |
| 487004 | 0.626 | 1.795 | 2.080 |
| 489004 | 2.667 | 4.578 | 4.405 |
| 490004 | 0.502 | 1.613 | 1.491 |
| 506004 | 0.452 | 1.444 | 1.524 |
| 514004 | 2.720 | 4.778 | 4.881 |
Data are normalized to a cutoff value (based on the mean plus 3 standard deviations) of 1.0 to allow comparison among groups. Positive results are shown in boldface type.
Nucleotide sequence accession number.
The GenBank accession number for mtb48 is AY029285.
RESULTS
Expression cloning and molecular characterization of mtb48
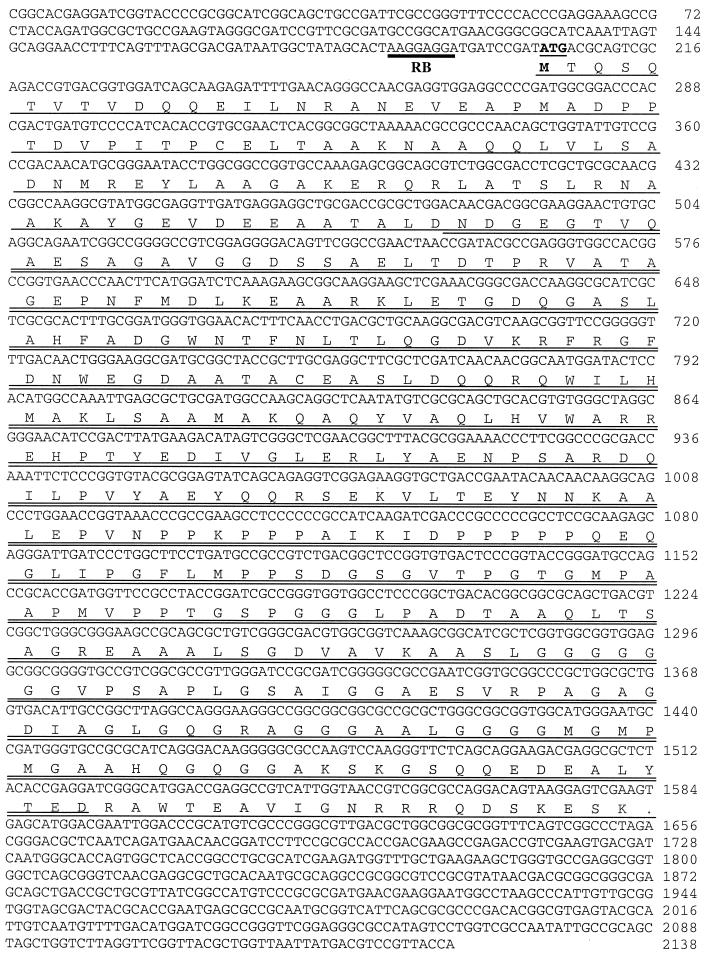
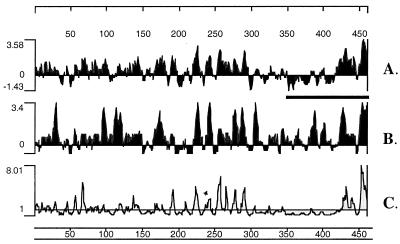
Two genomic expression libraries, constructed with randomly sheared M. tuberculosis Erdman strain and H37Rv strain genomic DNA, were screened with two pools of patient sera. The first pool consisted of sera from five patients with extrapulmonary disease (Erdman library), and the second pool consisted of sera from three patients whose sera were strongly reactive with the M. tuberculosis lysate (H37Rv library). These screenings resulted in the cloning of mtb48 in two sections (clone tbh4 from the H37Rv library and clone xp-1 from the Erdman library) that overlap with 100% identity by 1,041 bp (Fig. 1). Clone tbh4 contains the amino-terminal 1,319 bp, while clone xp-1 contains the carboxy 1,101-bp terminus of a 1,380-bp ORF. The cloned inserts contain a predicted ribosome-binding site (Shine-Dalgarno sequence; Fig. 1) 17 bp upstream from the predicted ATG start codon and an ORF that represents a predicted polypeptide of 47.6 kDa. The predicted polypeptide contains no hydrophobic leader sequence, nor does it contain predicted transmembrane domains, as determined by the PSORT program. However, analysis of hydrophilicity indicates three hydrophobic stretches in the carboxy-terminal half of the polypeptide that could serve as transmembrane regions (Fig. 2).
FIG. 1.
Relationship of full-length MTB48 to partial ORFs from genomic clones tbh4 and xp-1. Partial ORFs for TbH4 (upper line in the underlines) and XP-1 (lower line in the underlines) are underlined, and a double line indicates their overlap. The indicated polypeptide sequences were used to develop constructs for rTbH4, rXP-1, and rMTB48 protein expression. A potential ribosome-binding site (RB) is indicated by a thick bar, and the predicted ATG initiation codon is shown in bold and underlined. The nucleotide sequence number is shown on the right.
FIG. 2.
Protein sequence analysis of MTB48 including hydrophilicity (A), antigenic index (B), and surface probability (C). Analysis was performed with DNA Star protein analysis software (Protean 4.0) by using the Kyte-Doolittle hydrophilicity plot, the Jameson-Wolf antigenic index, and the Emini surface probability plot. A thick bar indicates a region proposed to be cleaved from the native protein to allow release from the cell. Protein amino acid numbers are shown above and below the plots, while the relative peak heights are shown to the left.
Genomic organization of mtb48
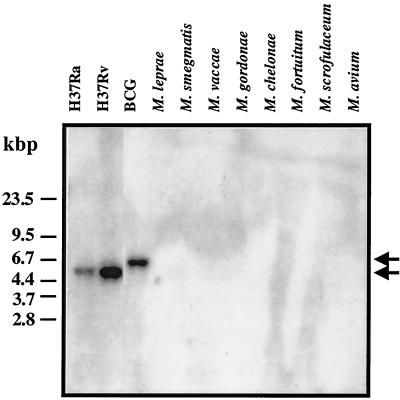
Database searches with the nucleotide and predicted protein sequences for mtb48 resulted in a 100% match with M. tuberculosis H37Rv genomic DNA (GenBank accession number AL022120) and hypothetical protein Rv3881c (GenBank accession number G70803), a 99% match with M. bovis (GenBank accession number U34848) and M. bovis BCG (GenBank accession number U35019) strain DNA, and a 98.7% match with the M. bovis and M. bovis BCG predicted protein sequences. Of interest, a partial genomic sequence (GenBank accession number AF001303) from an Indian M. tuberculosis clinical isolate (isolate NTI 64719) shows at least 98.8 to 99.6% identity to the mtb48 sequence, given the degenerate bases contained in this sequence. Together, this information indicates that the mtb48 sequence is well conserved between species (M. tuberculosis and M. bovis) and between geographically isolated strains from the United States and India. The genomic location of mtb48 is 826 bp upstream from a 9,504-bp sequence (which contains the esat-6 [accession number U34848] gene) that is deleted in the BCG strain of M. bovis (24). Southern blot analysis was performed with genomic DNA from both clinical and environmental Mycobacterium isolates. An mtb48-specific probe hybridized to 5.5-kbp reactive PstI fragments in M. tuberculosis isolates, including strains H37Ra, H37Rv, Erdman (data not shown), and C (data not shown), and to a 6.5-kbp fragment in the M. bovis BCG isolate (Fig. 3). No reactivity was seen with M. leprae, M. smegmatis, Mycobacterium vaccae, M. gordonae, Mycobacterium chelonae, M. fortuitum, M. scrofulaceum, or M. avium. The PstI fragments correspond in size to those of the fragments predicted from the genomic DNA sequence. The difference in fragment size between M. tuberculosis isolates and the M. bovis BCG isolate can be attributed to a deletion event in BCG which removes the PstI site that is found in the M. tuberculosis isolates.
FIG. 3.
Southern blot analysis of mtb48 showing hybridization of a labeled tbh4 nucleotide probe with PstI-digested genomic DNA from the M. tuberculosis and M. bovis BCG isolates tested but not from M. leprae or the environmental mycobacteria tested. Arrows indicate the migration of a labeled 6.5-kbp fragment from the BCG strain of M. bovis (top arrow) and 5.5-kbp DNA fragments from M. tuberculosis isolates (bottom arrow).
Recombinant protein expression and purification.
Four recombinant proteins representing the amino (rTbH4) and caroxy (rXP-1) termini and the full-length sequence (rMTB48) for MTB48 and the mature polypeptide for the M. tuberculosis 38-kDa antigen (r38 kDa) were overexpressed and purified by using a 6-histidine tag and metal affinity chromatography. Recombinant constructs were 1,341, 1,125, 1,404, and 1,080 bp, including the ATG start codon, 6-histidine tag, and stop codon, for rTbH4, rXP-1, rMTB48, and the 38-kDa antigen, respectively, and expressed recombinant proteins were 46.0, 38.6, 48.6, and 36.8 kDa for rTbH4, rXP-1, rMTB48, and the 38-kDa antigen, respectively. All recombinant proteins were tested for purity by N-terminal sequencing, Western blotting, and Coomassie blue staining and were determined to be over 95% pure.
Western blot analysis.
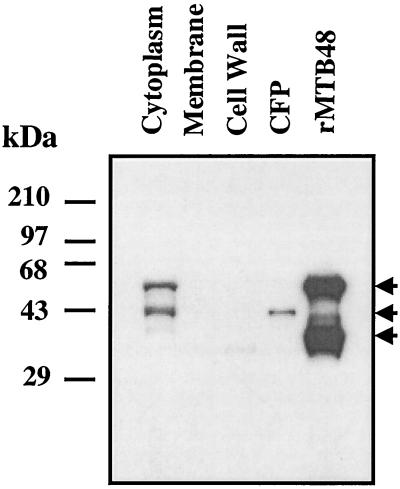
The cellular localization of native protein was determined by probing a Western blot with rabbit rMTB48 antiserum. The blot contained fractionated M. tuberculosis proteins, including cytoplasmic, membrane, and cell wall fractions; CFPs; and rMTB48. Two major bands of approximately equal reactivity at 43 and 55 kDa were seen in the cytoplasmic fraction, while bands with little or no reactivity were observed in the membrane or cell wall fractions. A single band of 43 kDa was seen in the CFP lane, while two bands of 38 and 55 kDa were seen in the recombinant protein lane (Fig. 4). The 43-kDa form found in the CFP fraction appears to be identical in size to the 43-kDa truncated or processed form of MTB48 seen in the cytoplasmic fraction. Both the cytoplasmic native protein and the recombinant protein each show a processed form, with the said forms being of approximately equal molar concentrations, indicating that the two proteins might be processed by the same or a similar process.
FIG. 4.
Western blot analysis indicates that the cellular localization of native MTB48 protein is in the cytoplasm and the culture filtrate (CFP). Ten micrograms of an M. tuberculosis cytoplasm preparation, 2.5 μg each of cell membrane, cell wall, and CFPs, and 25 ng of recombinant MTB48 were separated by SDS-PAGE, transferred to nitrocellulose, and probed with an MTB48 rabbit polyclonal antibody. A 55-kDa band (top arrow) is seen in both the cytoplasm and the recombinant protein lanes, while a 43-kDa band (middle arrow) is seen in both the cytoplasm and the CFP lanes. A second band of reactivity at approximately 38 kDa is shown in the recombinant protein lane (bottom arrow).
ELISA evaluation of recombinant proteins XP-1, TbH4, and MTB48.
Nineteen MTB48-positive patient serum samples were assayed with rXP-1 and rTbH4 to determine the general location of antigenic epitopes on rMTB48 (Table 1). rTbH4 was reactive with 19 of 19 serum samples, while rXP-1 was reactive with 11 of 19 serum samples. These data indicate that approximately 42% of MTB48-positive patient serum samples are reactive only with an amino-terminal epitope(s), while 58% of the serum samples are reactive with a central epitope(s) and, potentially, an additional amino-terminal epitope(s).
ELISA evaluation of sera from patients with tuberculosis and controls.
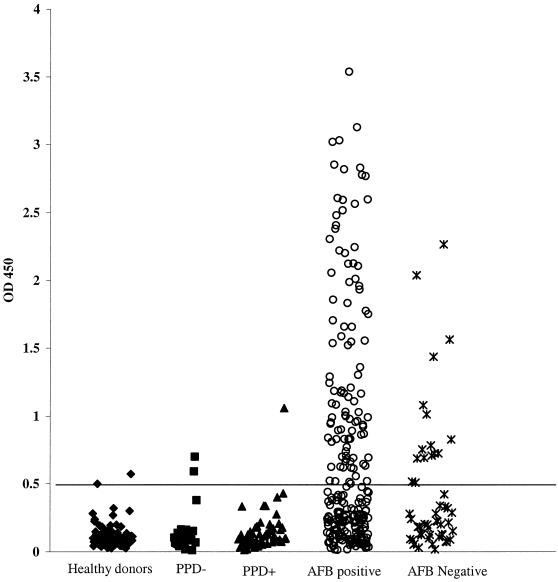
Sera from individuals with tuberculosis in the presence or absence of HIV infection, PPD-positive individuals with no evidence of disease, and PPD-negative individuals were tested by ELISA with tuberculosis lysate, r38 kDa, and rMTB48 (Table 2). The sensitivities of recombinant proteins with serum samples from HIV-infected individuals in Africa with M. tuberculosis coinfections were 44.4 and 66.7% for rMTB48 and r38 kDa, respectively. The sensitivity of rMTB48 and r38 kDa used in combination was 74.1% (a gain of 7.4% over that obtained with r38 kDa only), indicating that the reactivities of the two recombinant antigens were complementary. Overall sensitivities for patients with only M. tuberculosis infection were 29.8 and 43.4% for rMTB48 and r38 kDa, respectively. The sensitivity for the antigens used in combination, however, was 56.3%, indicating a greater contribution (a gain of 12.9%) of rMTB48 with these serum samples. Among the 419 serum samples from patients with only tuberculosis evaluated by ELISA, rMTB48 was reactive with 32.1% of the serum samples from patients with smear-positive tuberculosis and 17.2% of patients with smear-negative disease; and r38 kDa was reactive with 47 and 23.4% of smear-positive and smear-negative patients, respectively. Comparison of the ELISA values for serum samples from M. tuberculosis-infected patients in Brazil and Africa (smear positive) shows a similar sensitivity. rMTB48 detected M. tuberculosis in 32.7% of the Brazilian samples and 30.3% of the African samples, while r38 kDa detected M. tuberculosis in 48.4 and 40.9% of these samples, respectively. Also, the gain in sensitivity with rMTB48 over that with r38 kDa was similar for both: 13.7 versus 12.1%, respectively. The specificities for rMTB48 and r38 kDa, including all control sera, were 97.8 and 96.1%, respectively, while the specificity for rMTB48 and r38 kDa in combination was 94%. No antibody reactivity with rMTB48 was seen for sera from the non-M. tuberculosis-infected control groups, including sera from HIV-positive patients, lung cancer patients, and pneumonia patients. Specificities of 98.2 and 93.1% were determined for MTB48 with class II (PPD positive) and class 0/I (PPD negative) M. tuberculosis-infected sera, respectively. ELISA results for a subset of 248 smear-positive and 52 smear-negative Brazilian M. tuberculosis patient serum samples are displayed graphically in Fig. 5. Taken together, these data indicate that rMTB48 adds to the sensitivity of a 38-kDa antigen-based serological test for M. tuberculosis in a variety of diverse clinical and geographic settings.
TABLE 2.
ELISA reactivities of M. tuberculosis lysate, rMTB48, and r38 kDa with HIV-positive and -negative sera from patients with tuberculosis and controlsa
| Serum group and patient status | Origin | No. of serum samples | HIV status | Smear result | No. of samples positive by ELISA
|
|||
|---|---|---|---|---|---|---|---|---|
| Lysate | MTB48 | 38 kDa | 38 kDa + MTB48 | |||||
| Sera from patients with: | ||||||||
| TB,b HIV, and M. tuberculosis coinfection | Uganda | 17 | + | + | 7 | 8 | 12 | 13 |
| Uganda | 2c | + | − | 1 | 0 | 1 | 1 | |
| South Africa | 8 | + | + | 6 | 4 | 5 | 6 | |
| Total | 27 | 14 | 12 | 18 | 20 | |||
| TB | Brazil | 248 | − | + | 165 | 81 | 120 | 154 |
| 52 | − | − | 24 | 9 | 11 | 16 | ||
| Uganda | 15 | − | + | 10 | 10 | 12 | 12 | |
| South Africa | 51 | − | + | 47 | 10 | 15 | 23 | |
| Turkey | 11 | − | + | 8 | 3 | 9 | 9 | |
| Philippines | 31 | − | + | 24 | 10 | 11 | 17 | |
| 11 | − | − | 7 | 2 | 4 | 5 | ||
| Total | 419 | 285 | 125 | 182 | 236 | |||
| Control sera | ||||||||
| HIV infection | United States | 11 | + | 0 | 0 | 0 | 0 | |
| Class II TB (PPD positive) | Africa | 3 | − | 1 | 0 | 0 | 0 | |
| Europed | 6 | − | 0 | 0 | 0 | 0 | ||
| Southeast Asia | 7 | − | 1 | 0 | 2 | 2 | ||
| United States-Latin America | 41 | − | 4 | 1 | 3 | 4 | ||
| Total | 57 | 6 | 1 | 5 | 6 | |||
| Class 0/I TB (PPD negative) | Africa | 1 | − | 0 | 0 | 0 | 0 | |
| Europed | 3 | − | 2 | 0 | 0 | 0 | ||
| Southeast Asia | 3 | − | 0 | 0 | 0 | 0 | ||
| United States-Latin America | 22 | − | 0 | 2 | 1 | 3 | ||
| Total | 29 | 2 | 2 | 1 | 3 | |||
| Other | ||||||||
| Lung cancer | China | 13 | − | 2 | 0 | 0 | 0 | |
| Pneumonia | China | 18 | − | 5 | 0 | 1 | 1 | |
| Healthy controls | China (Caucasian) | 9 | − | 2 | 0 | 0 | 0 | |
| United States | 95 | − | 1 | 2 | 2 | 4 | ||
| Total | 135 | 10 | 2 | 3 | 5 | |||
Control sera were from HIV-positive, PPD-positive, and PPD-negative individuals, healthy controls, and patients with lung cancer and pneumonia.
TB, tuberculosis.
One individual was positive for tuberculosis by X ray.
Primarily from Russia and its satellite regions where tuberculosis is endemic.
FIG. 5.
Distribution of MTB48 ELISA reactivity among sera from healthy blood donors (n = 95), PPD-positive individuals (n = 57), PPD-negative individuals (n = 29), and Brazilian tuberculosis patients who were smear (acid-fast bacillus [AFB]) positive (n = 248) and negative (n = 52). The assay cutoff value (horizontal bar) was determined from the mean for the healthy donor population plus 3 standard deviations of the mean. OD 450, optical density at 450 nm.
DISCUSSION
The development of a serodiagnostic assay for M. tuberculosis with high degrees of sensitivity and specificity will most likely require a panel of several recombinant antigens or a fusion polyprotein that comprises the critical epitopes. This is due to the highly heterogeneous nature of antigen recognition by infected individuals that results, in part, from diverse genetic backgrounds, exposure to different stage-specific or strain-specific antigens, bacillary loads, patient health, and individual treatments (23). Data presented in this report indicate that MTB48 is an important component of the antigen cocktail that will be required for diagnostic assay development. MTB48, which was cloned from both the Erdman and the H37Rv strains of M. tuberculosis by serological expression screening with M. tuberculosis-infected patient serum, encodes a predicted 47.6-kDa polypeptide (approximately 54 kDa under reducing conditions, possibly due to the high proline content [7.6%]) with no known function and is restricted to virulent and avirulent strains of the M. tuberculosis complex and M. bovis. mtb48 genomic nucleotide sequences from the Erdman (clone xp-1) and H37Rv strains (clone tbh4) were identical, and database searches showed that the mtb48 genomic sequence (Erdman strain) was identical to the H37Rv genomic sequence (GenBank accession number AL022120) and 99% identical to the corresponding M. bovis sequence (GenBank accession number U34848). A partial genomic sequence from an M. tuberculosis clinical isolate from India (GenBank accession number AF001303) also shows approximately 99% identity to mtb48. Taken together, this information indicates that the mtb48 sequence is well conserved between species and between geographically isolated strains from the United States and India. The native MTB48 protein is cytoplasmic and appears to be cleaved near the carboxy terminus to form a 43-kDa protein that is then shed. One cannot rule out the possibility, however, that in the culture filtrate the protein is degraded to the smaller fragment by secreted or shed proteases. The carboxy-terminal segment contains hydrophobic regions that could be removed upon processing, thus allowing secretion or shedding of the antigen. Preliminary epitope mapping shows that of the MTB48-positive patient serum samples tested, 42% recognize an amino-terminal epitope(s), which is missing in rXP-1, while 58% recognize one or more central epitopes, indicating the possibility of several B-cell epitopes along the molecule. Protein analysis (Fig. 2) shows that the amino terminus and central region of the protein are hydrophilic and are predicted to be highly antigenic. Surface probability predictions also indicate clusters of surface-exposed regions in the amino-terminal region and in the midregion of the molecule.
The serodiagnostic potential of rMTB48 compared to that of r38 kDa was evaluated by ELISA. The 38-kDa M. tuberculosis antigen was chosen for comparison because of its relatively high degrees of sensitivity and specificity, as determined in numerous studies (8, 9, 18, 20, 31, 34). Serological sensitivity for the 38-kDa antigen ranges from 16 to 89%, and specificity ranges from 93 to 100% (5, 8, 18, 22, 29, 34), depending largely upon the selection of patient populations in each study. In the present study, overall sensitivity values for rMTB48 and r38 kDa, including those for samples from patients with HIV coinfection and smear-positive and -negative patients, were 30.7 and 44.8%, respectively, with a sensitivity of 57.4% when both antigens were used in combination. Samples from patients with HIV coinfection alone resulted in higher values (44.4, 66.7, and 74.1%, respectively), as did samples from smear-positive patients (32.1, 47, and 60.6%, respectively). Values for samples from smear-negative patients were 17.2, 23.4, and 32.8%, respectively. Thus, MTB48 is a highly conserved antigen and its serological reactivity complements that of the 38-kDa antigen in the detection of M. tuberculosis infection.
Although rMTB48 adds to the overall sensitivity of the 38-kDa antigen, an M. tuberculosis serodiagnostic reagent with high degrees of sensitivity and specificity will depend on the use of several antigens, including r38 kDa and rMTB48. Detection of problematic infections, such as smear-negative infections, depends on a combination of techniques, including serology, detection of clinical symptoms, and radiography. Additional effort must be directed toward the serological detection of smear-negative infections. Although patients with smear-negative infections are less infectious and have a lower mortality rate than patients with smear-positive infections, the number of patients with smear-negative infections is estimated to exceed the number of patients with smear-positive infections, and over half of the patients with smear-negative infections will progress to active disease (7). In addition, coinfections with HIV have disproportionately increased the number of smear-negative cases. Our studies continue to be directed toward the development of a rapid serodiagnostic assay that uses an optimized set of recombinant antigens that will be expressed as one or more fusion polyproteins.
ACKNOWLEDGMENTS
We thank Thomas Vedvick and Darrick Carter for protein sequence data, Dan Hoppe and Joe Parsons for assistance with DNA sequencing, and Stephan Johnson and Rhea Coler for providing genomic DNA for Southern blot analysis. We also thank Jonathan Clapper for performing lipopolysaccharide assays with purified recombinant antigens and John Belisle, who provided fractionated M. tuberculosis cellular proteins (Tuberculosis Research Materials contract N01-AI-25147). We also acknowledge Robert Akridge (Fred Hutchinson Cancer Research Institute) for providing the serum samples from U.S. HIV-positive patients and Charles Nolan at the King County Tuberculosis Clinic for providing PPD-positive and -negative samples.
M.J.L. and D.C.D. contributed equally to the data presented in this report.
This work was partially supported by NIH grants AI-39879 (to R.L.H.) and AI-44373 (to S.G.R.).
REFERENCES
- 1.Andersen A B, Hansen E B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989;57:2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashbridge K R, Prestidge R L, Booth R J, Watson J D. The mapping of an antibody-binding region on the Mycobacterium tuberculosis 19 kilodalton antigen. J Immunol. 1990;144:3137–3142. [PubMed] [Google Scholar]
- 3.Barry S M, Lipman M C, Johnson M A, Prentice H G. Respiratory infections in immunocompromised patients. Curr Opin Pulm Med. 1999;5:168–173. doi: 10.1097/00063198-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann J S, Yuoh G, Fish G, Woods G L. Clinical evaluation of the enhanced Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test for rapid diagnosis of tuberculosis in prison inmates. J Clin Microbiol. 1999;37:1419–1425. doi: 10.1128/jcm.37.5.1419-1425.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan E D, Heifets L, Iseman M D. Immunologic diagnosis of tuberculosis: a review. Tuber Lung Dis. 2000;80:131–140. doi: 10.1054/tuld.2000.0243. [DOI] [PubMed] [Google Scholar]
- 6.Chou T. Emerging infectious diseases and pathogens. Nurs Clin N Am. 1999;34:427–442. [PubMed] [Google Scholar]
- 7.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:97–107. [PubMed] [Google Scholar]
- 8.Demkow U, Zielonka T M, Strzalkowski J, Michalowska-Mitczuk D, Augustynowicz-Kopec E, Bialas-Chromiec B, Kus J, Skopinska-Rozewska E, Zwolska Z. Diagnostic value of IgG antibody levels against 38 kDa mycobacterial antigen. Pneumonol Alergol Pol. 1998;66:509–516. [PubMed] [Google Scholar]
- 9.Dillon D C, Alderson M R, Day C H, Bement T, Campos-Neto A, Skeiky Y A W, Vedvick T, Badaro R, Reed S G, Houghton R. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000;38:3285–3290. doi: 10.1128/jcm.38.9.3285-3290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eltringham I J, Drobniewski F. Multiple drug resistant tuberculosis: aetiology, diagnosis and outcome. Br Med Bull. 1998;54:569–578. doi: 10.1093/oxfordjournals.bmb.a011711. [DOI] [PubMed] [Google Scholar]
- 11.Felix R, Bittner R C. Tuberculosis and radiologic diagnosis 100 years after W.C. Roentgen. Pneumologie. 1995;49:657–662. [PubMed] [Google Scholar]
- 12.Foulds J, O'Brien R. New tools for the diagnosis of tuberculosis: the perspective of developing countries. Int J Tuberc Lung Dis. 1998;2:778–783. [PubMed] [Google Scholar]
- 13.Gillespie S. Molecular techniques for the diagnosis of respiratory bacterial infection. Curr Opin Pulm Med. 1999;5:174–178. doi: 10.1097/00063198-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg A M. The tuberculosis epidemic. Scientific challenges and opportunities. Public Health Rep. 1998;113:128–136. [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn J R. Resurgence of tuberculosis and the impact of HIV infection. Br Med Bull. 1998;54:579–593. doi: 10.1093/oxfordjournals.bmb.a011712. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Bhatia R, Datta K K. Serodiagnosis of tuberculosis. J Commun Dis. 1995;27:208–214. [PubMed] [Google Scholar]
- 17.Hageman J R. Congenital and perinatal tuberculosis: discussion of difficult issues in diagnosis and management. J Perinatol. 1998;18:389–394. [PubMed] [Google Scholar]
- 18.Harboe M, Wiker H G. The 38-kDa protein of Mycobacterium tuberculosis: a review. J Infect Dis. 1992;166:874–884. doi: 10.1093/infdis/166.4.874. [DOI] [PubMed] [Google Scholar]
- 19.Harries A D, Maher D, Nunn P. An approach to the problems of diagnosing and treating adult smear-negative pulmonary tuberculosis in high-HIV-prevalence settings in sub-Saharan Africa. Bull W H O. 1998;76:651–662. [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrickson R C, Douglass J F, Reynolds L D, McNeill P D, Carter D, Reed S G, Houghton R L. Mass spectrometric identification of Mtb81, a novel serological marker for tuberculosis. J Clin Microbiol. 2000;38:2354–2361. doi: 10.1128/jcm.38.6.2354-2361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch C S, Johnson J L, Ellner J J. Pulmonary tuberculosis. Curr Opin Pulm Med. 1999;5:143–150. doi: 10.1097/00063198-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kamholz S L. Resurgence of tuberculosis: the perspective a dozen years later. J Assoc Acad Minor Phys. 1996;7:83–86. [PubMed] [Google Scholar]
- 23.Lyashchenko K, Colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro M L. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66:3936–3940. doi: 10.1128/iai.66.8.3936-3940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manca C, Lyashchenko K, Colangeli R, Gennaro M L. MTC28, a novel 28-kilodalton proline-rich secreted antigen specific for the Mycobacterium tuberculosis complex. Infect Immun. 1997;65:4951–4957. doi: 10.1128/iai.65.12.4951-4957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 27.Migliori G B, Ambrositti M. Epidemiology of tuberculosis in Europe. Monaldi Arch Chest Dis. 1998;53:681–687. [PubMed] [Google Scholar]
- 28.Netto E M, Dye C, Raviglione M C. Progress in global tuberculosis control 1995–1996, with emphasis on 22 high-incidence countries. Global monitoring and surveillance project. Int J Tuberc Lung Dis. 1999;3:310–320. [PubMed] [Google Scholar]
- 29.Pottumarthy S, Wells V C, Morris A J. A comparison of seven tests for serological diagnosis of tuberculosis. J Clin Microbiol. 2000;38:2227–2231. doi: 10.1128/jcm.38.6.2227-2231.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rattan A, Kalia A, Ahmad N. Multidrug-resistant Mycobacterum tuberculosis: molecular perspectives. Emerg Infect Dis. 1998;4:195–209. doi: 10.3201/eid0402.980207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samanich K M, Keen M A, Vissa V D, Harder J D, Spencer J S, Belisle J T, Zolla-Pazner S, Laal S. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 2000;7:662–668. doi: 10.1128/cdli.7.4.662-668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbon A. Development of a serological test for tuberculosis. Problems and potential. Trop Geogr Med. 1994;46:275–279. [PubMed] [Google Scholar]
- 33.Verbon A, Hartskeerl R A, Schuitema A, Kolk A H J, Young D B, Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992;174:1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson R J, Haslov K, Rappuoli R, Giovannoni F, Narayanan P R, Desai C R, Vordermeier H M, Paulsen J, Pasvol G, Ivanyi J, Singh M. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J Clin Microbiol. 1997;35:553–557. doi: 10.1128/jcm.35.3.553-557.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zumla A, Malon P, Henderson J, Grange J M. Impact of HIV infection on tuberculosis. Postgrad Med J. 2000;76:259–268. doi: 10.1136/pmj.76.895.259. [DOI] [PMC free article] [PubMed] [Google Scholar]