Abstract
In spite of rapid advances in understanding of signaling networks associated with the incidence and therapeutic-sensitivity, breast cancer (BC) still remains the most commonly diagnosed and prevalent cancer in women. Emergence of resistance to hormonal interventions in estrogen-receptor (ER) positive BC coupled to loss of ER expression and activation of ER-independent growth factor, heat-shock, MYC and WNT pathways along with distinct mechanisms of therapeutic-resistance in HER2 over-expressing and triple-negative subtypes of BC collectively necessitates deeper profiling of the mechanistic networks regulated by potential lead anticancer compounds intended for further development to target BC. A significant part of the search for novel lead anticancer compounds for BC has focused on phytochemicals including flavonoids found in citrus fruits, which have shown promising anticancer activity. Based on the initial studies which revealed the anticancer effect of 2HF in BC, we employed an advanced TMT 10plex labeled proteomic approach to characterize the changes in non-phosphorylated and phosphorylated proteomic profile of ER+ MCF7, triple-negative MDA-MB231 and HER2+ SKBR3 BC cells, and MCF10A normal breast epithelial cells. 2HF induced significant changes in the proteins responsible for BC incidence, metastases and therapeutic sensitivity in BC cells.
Keywords: Breast Cancer, 2’-Hydroxyflavanone, estrogen receptor, TMT 10plex, Mass Spectrometry
Introduction
Breast cancer (BC) is a challenging cancer widely diagnosed in women [1]. The statistics from World Cancer Report indicate that BC is the most commonly diagnosed cancer in women throughout the world [2]. In USA, 1 in 8 women have the risk of developing BC in their life-time [3]. Endogenous estrogens and life-style induced carcinogenic stimuli like smoking and alcohol influence the incidence, progression and therapeutic response of breast tumors [4]. The expression of estrogen receptor (ER), progesterone receptor (PR) and epidermal growth factor receptor 2 (ERBB2/HER2) are established drivers of tumor progression and determinants of therapeutic response in BC [5]. Activation of ER independent downstream cascades like PI3K/AKT/mTOR pathway, activation of MYC and HSP90, over expression of HER2 and loss of tumor suppressor PTEN have been implicated in both BC metastases and therapy resistance [6–8]. In spite of rapid advances in therapy, effective preventive strategies and novel therapeutic interventions to control the metastatic progression and recurrence risk of BC remain a strong need and hence constitute a contemporary focus of developmental cancer therapeutics.
Many of the phytochemicals including citrus flavonoids have been studied and evaluated for their anticancer effects in multiple cancers [9, 10]. Our previous studies revealed that citrus flavonoid 2’-hydroxyflavanone (2HF) can decrease the survival of both ER+ MCF7 and triple-negative MDA-MB231 BC cells [11]. 2HF has also shown promising efficacy in VHL-mutant renal cell carcinoma [12]. 2HF has been shown to inhibit the progression of lung cancer by down-regulation of matrix metalloproteinase (MMP)-2 and urokinase-type plasminogen activator (u-PA) [13]. 2HF has also been shown to inhibit the progression of castration-resistant prostate cancer by regulating AKT/STAT3 signaling [14]. 2HF targets osteosarcomas by up regulating tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and death receptor 5 (DR5) [15]. In this regard, we further conducted analyses of the proteomic profile of 2HF treated and control ER+ MCF7, triple-negative MDA-MB231 and HER2+ SKBR3 BC cells, and MCF10A normal breast epithelial cells. Our study provided a comprehensive and integrated evidence for the potential of 2HF in targeting all the clinically relevant subtypes of BC.
Material and methods
Reagents:
TMT 10plex™ isobaric label reagent set 1× 5 mg, catalog number: 90406, High-Select™ TiO2 Phosphopeptide Enrichment Kit, catalog number: A32993, High-Select™ Fe-NTA Phosphopeptide Enrichment Kit, catalog number: A32992 (Thermo Scientific) and Pierce™ High pH Reversed-Phase Peptide Fractionation Kit (PN: 84868), Pierce™ Quantitative Colorimetric Peptide Assay (PN: 23275), Waters Oasis cartridge HLB 3cc (60mg) Extraction Cartridges (PN WAT094226), iodoacetamide (IAA, Sigma, I6125), 2,2,2-trifluoroethanol (TFE, Fluka-91690), tris(2-carboxyethyl)phosphine (TCEP, Thermo-20490), Trypsin-LysC, triethylammonium bicarbonate (TEAB, Thermo-90114), Trypsin/LysC mix MS grade (Promega-V5073), trichloro acetic acid (TCA, Fisher-BP555-250), formic acid (Fisher-A117-50), trifluoroacetric acid (TFA, Fisher-A116).
Cell lines and cultures:
Human breast untransformed (MCF10a) and cancer (MCF7, SKBR3, and MDA-MB231) cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). All cells were cultured at 37 °C in a humidified atmosphere of 5 % CO2 in the appropriate medium: DMEM/F12 with 15 mM Hepes buffer, 5% horse serum, 10 μg/ml insulin, 20 ng/ml EGF, 100 ng/ml cholera toxin, 0.5 μg/ml hydrocortisone (MCF10a) and DMEM (MCF7, SKBR3, and MDA-MB231) medium, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) solution. A large number of stocks were cryo-preserved at the time of acquisition and all studies were performed on cell cultures that are no more than 10 passages from the time of cryopreservation. The authentication of cell lines was done by analyzing fifteen different human short tandem repeat (STR) at Genomic Core, Beckman Research Institute of City of Hope, Duarte, CA, to test for interspecies contamination. All the cells were also tested for Mycoplasma once every 3 months using a standard commercially available PCR kit or the MycoAlert Mycoplasma Detection Kit (Lonza).
Cell Lysis, digestion, TMT-labeling and phosphopeptide enrichment:
Cells were treated with 50 μM 2HF for 24 h, washed with PBS, centrifuged and the resulting cell pellet was lysed in 100 mM TEAB containing lysis buffer supplemented with protease and phosphatase inhibitor cocktail. The equivalent of 1 mg (350 μL) were precipitated by adding 20% TCA and the resulting pellets were denatured by suspending in 50 μL of 200 mM TEAB and 50 μL TFE, reduced with TCEP at 56 °C for one hour then alkylated with IAA for one hour, in the dark. The samples were diluted with 50 mM TEAB to a TFE concentration below 5%, then digested overnight at 37 °C with trypsin (added in a 1/80 ratio). The digestion was stopped by adding 10 μL of formic acid. The digested samples were processed according to Waters and Thermo Scientific guidelines for each of the kits used, as follows: digested samples were SPE purified using Waters Oasis HLB cartridges, then quantitated by Pierce quantitative colorimetric assay and labeled with TMT 10plex™ isobaric labeling kit [16]. After labeling, the samples were combined (each sample in a replicate set was labeled with a different label), resulting in three labeled replicate samples. These were then processed for phosphopeptide enrichment, using sequentially the TiO2 and then Fe-NTA phosphopeptide enrichment kits. The labeled peptides were purified by Waters Oasis HLB cartridge and quantitated again using the Pierce colorimetric peptide assay before taking the same amount of each TMT- labeled sample and fractionating the mixture of peptides in eight fractions, using the High pH Fractionation kit. Each of the fractions was evaporated to dryness in a SpeedVac then reconstituted in 50 μL 0.1% TFA and stored at − 80 °C before LCMS analysis.
LC-MS/MS analysis:
The peptides were loaded for 2 min at 30 μL/min in buffer A (1% formic acid in water) on a 300 μm × 5 mm Thermo trapping column, then separated on a Thermo EasySpray column 75 μm × 500 mm at 300 nL/min, maintained at 45 °C, using a Thermo-Dionex Ultimate 3000 nano UHPLC and a 2h method as follows: 3–7% buffer B (0.1% formic acid in 80% acetonitrile) and 97% buffer A (0.1% formic acid in water), 8 min, 7% B to 32% B, at 100 min and 50% B at 105 min, then 95% B from 106 to107 min, then reverted to 3% B at 109 min. All solvents and solvent modifiers were Thermo Optima LC/MS grade. The eluting peptides were analyzed in an Orbitrap Lumos fitted with a EasySpray nanospray source with the following parameters: Spray voltage: 2300 V, ion transfer tube temperature: 275 °C, with internal mass calibration (Easy-IC) enabled and a 3s cycle time during which the following MS experiments were performed: MS1 was acquired in positive ion-mode in orbitrap at 120K resolution, scan range (m/z): 400–1500, max injection time: 50 ms, AGC target: 400000, 1 microscan. RF lens (%): 30, with MIPS, charge states 2–7, and Dynamic exclusion ± 10 ppm, for 90s after 1 MS2 scan. For phosphopeptide enriched samples, MS2 was acquired in orbitrap at 30K resolution, filter precursor selection range - mass range (m/z): 400–1200, maximum injection time (ms): 60, AGC target: 50000, with a quadrupole isolation window of 0.7 Da, first mass (m/z): 120, activation type: CID, collision energy (%): 35, activation time (ms):10, activation Q: 0.25 with multistage activation with a Neutral Loss Mass of 97.9673. For the phosphopeptide depleted samples, MS2 used identical parameters except spectra were acquired in the ion trap with rapid scan speed, multistage activation was not used, and the AGC target was 104 ions. MS3 was acquired in orbitrap at 60K resolution, scan range (m/z): 100–500, maximum injection time (ms): 118, AGC target 50000, isolation mode: quadrupole, isolation window (Da): 2, multi-notch isolation: True, MS2 isolation window (Da): 2, number of notches: 10, activation type: HCD, collision energy (%): 65.
Data analysis:
The MS/MS spectra were extracted and searched using Thermo Proteome Discoverer 2.2/Protein Metrics - Byonic 2.15.7 against the NCBI Homo Sapiens Reference Sequences database (55985 sequences 11/2014), with the following parameters: Trypsin (full), two maximum missed cleavages, 6 < peptide length < 144, precursor mass tolerance: 10 ppm, fragment mass tolerance: 0.02 Da for phosphopeptide enriched and 0.6 Da for depleted samples, target FDR: 0.01 on both peptide and protein level, based on reverse sequence decoy database, only spectra scored “high” confidence in Proteome Discoverer were accepted. Fixed modifications: carbamidomethyl (C), TMT 10plex (K and peptide N-terminus), variable modifications: methionine oxidation and protein N-terminus acetylation. The appropriate correction factors for the isotope purity were used for the quantification method from the TMT batch certificate. For the phosphoprotein analysis, a variable phosphorylation was also considered (S, T, Y) in addition to the modifications mentioned above. The TMT reporter ions from MS3 were integrated with a tolerance of 20 ppm, integration method: most confident centroid, activation type: HCD. The significance of fold changes in differentially regulated nonphosphorylated proteins was further analyzed by Ingenuity Pathway Analyses (IPA; Ingenuity Systems, CA). The statistically significant protein expression fold differences from 2HF and control samples for each of the cell lines were thoroughly analyzed using IPA. The differences in canonical pathways, upstream regulators and networks were analyzed using IPA along with further search for latest findings on key nodes of signaling regulators.
Immunoblotting:
Supernatant proteins from control and 2HF treated cell lysates were resolved by SDS-PAGE and transferred onto polyvinylidene fluoride membrane. Change in the level of desired protein was determined by densitometric scanning of the immuno-reactive bands. Equal loading of proteins was confirmed by stripping and re-probing the membranes with β-actin antibodies.
Statistical Analysis:
All data were evaluated with a two-tailed unpaired student’s t test are expressed as the mean ± SD. The statistical significance of differences between control and treatment groups was determined by ANOVA followed by multiple comparison tests. The p value < 0.05 was considered significant. Activation or inhibition differences were considered statistically significant when z score was either above 2 or below −2.
Results
The ER+ MCF7, triple-negative MDA-MB231 and HER2+ SKBR3 BC cells, and MCF10A normal breast epithelial cells were treated with 50 μM 2HF for 24 h followed by protein extraction, TMT labeling and phosphopeptide enrichment. The TMT 10-plex labeling and phosphopeptide enrichment workflow is presented in Fig.1. The TMT 10plex labeled samples were grouped into two fractions for nonphosphorylated and phosphorylated peptide analyses. Following LC-MS/MS analyses, the MS/MS spectra were searched using Thermo Proteome Discoverer 2.2/Protein Metrics - Byonic 2.15.7 against the NCBI Homo Sapiens Reference Sequences database as described in the Experimental methods section . In total, 5384 nonphosphorylated protein groups (67533 peptide groups, 201881 PSMs out of 482725 MSMS spectra) and 3459 phosphorylated protein groups (19248 peptide groups, 51546 PSMs out of 252499 MSMS spectra) were quantified in Proteome Discoverer 2.2 using TMT 10plex reporter ion intensity. The lists of differentially expressed nonphosphorylated and phosphorylated proteins are presented in Supplementary Tables 1 and 2 respectively.
Figure 1.
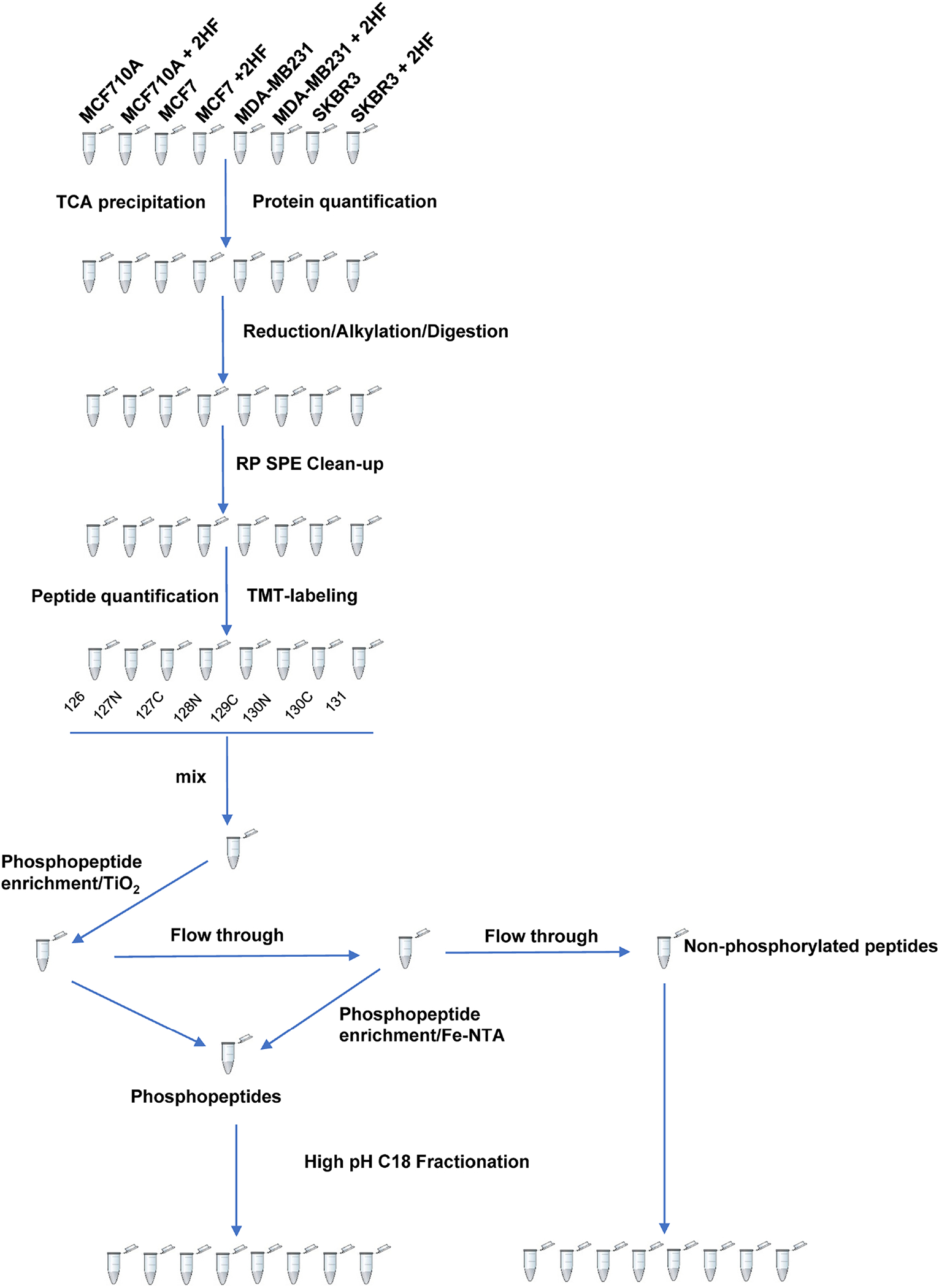
TMT 10-plex labeling, phosphopeptide enrichment and fractionation workflow.
Significance of 2HF induced protein changes to BC pathogenesis, progression and therapy:
The 2HF treatment resulted in a number of proteins in BC cells (Supplementary Tables 1 and 2). We did not observe significant changes in the proteomic profile of MCF10A normal breast epithelial cells as relevant to BC across all the replicates tested. The ER+ MCF7 BC cells had several significant protein changes following 2HF treatment. The PCNA-associated factor (PAF) regulates the physiological function of PCNA and promotes tumor cell proliferation [17]. PAF particularly mediates the switch of replicative DNA synthesis to translation DNA synthesis (TLS), which enables the tumors to proliferate in spite of DNA damaging lesions [18]. PAF is over-expressed in multiple cancers including breast tumors [17, 19]. Over-expression of PAF mediates hyperplastic changes in breast epithelium while PAF also acts as a WNT signaling enhancer to promote cancer stem cell state in breast tumors [20]. 2HF treatment led to down-regulation of PAF1 in all the treated MCF7, MDA-MB231 and SKBR3 BC cells as identified and quantified by average reporter ion counts and Western blot analyses (Fig. 2 and Supplemental Fig. S1). The kruppel-like factor16 (KLF16), which couples histone acetylase and deacetylase mediated regulation of gene transcription, is over-expressed in tumors where it promotes proliferation by up-regulating CDK4 and down-regulating p21 expression [21, 22]. We observed a down-regulation of KLF16 following 2HF treatment in MCF7 and MDA-MB231 BC cells with no significant changes in MCF10A normal breast epithelial and SKBR3 BC cells (Supplementary Table 1). The COUP transcription factor 1 (COUP-TF1/NR2F1) mediates the ERK dependent estrogen receptor alpha (ERα) gene transcription in an estradiol (E2)-independent manner [23, 24]. The over-expression of COUP-TF1 in MCF7 cells also mediates BC cell proliferation in an E2-dependent manner thereby denoting a role for COUP-TF1 in both the presence and absence of E2 [25]. We observed a down-regulation of COUP-TF1 levels in ER+ MCF7 BC cells following 2HF treatment. The study by Svotelis et al identified that histone H2A.Z promotes the ERα mediated gene transcription and proliferation by stabilizing the nucleosomes along the DNA axis during translation and by facilitating estrogen responsive enhancer function during gene transcription [26]. The same study revealed that the ectopic expression of H2A.Z can also increase BC proliferation even in the presence of low levels of E2 [26]. We observed that 2HF treatment down-regulates H2A.Z in ER+ MCF7 BC cells.
Figure 2. Quantification of PCNA Associated Factor 1 (PAF1) by Mass Spectrometry and Western blot:
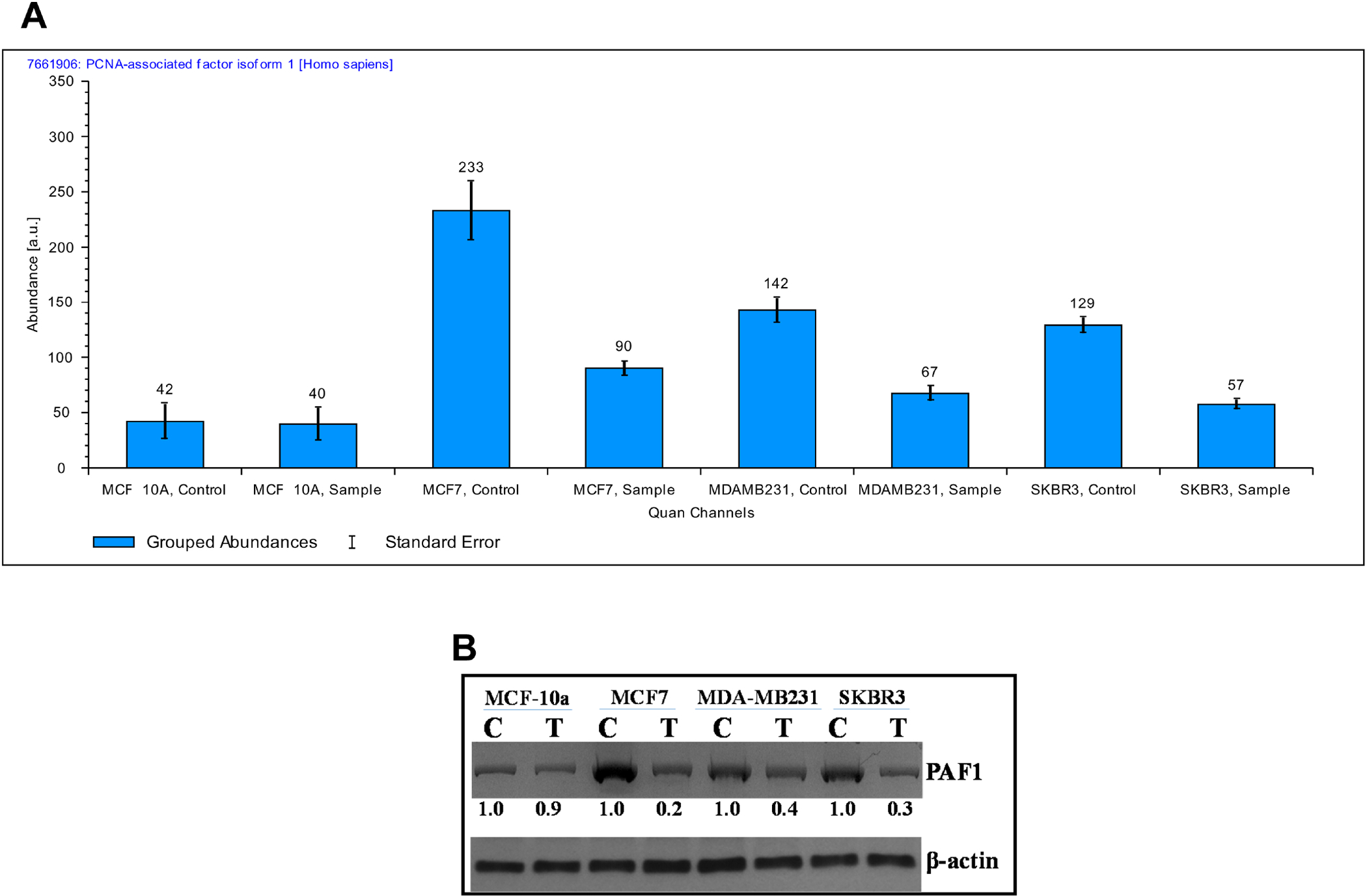
(A) Bar diagrams showing the average reporter ion counts for PAF1 following MS/MS analyses. (B) Aliquots of crude membrane extracts (40 μg) from control and 2HF (50μM, 24h) treated MCF10a, MCF7, MDA-MB231, and SKBR3 cells were applied to SDS-PAGE and subjected to Western blot analyses against anti-PAF1 IgG. Numbers below the blots represent the fold change in the levels of proteins as compared to control as determined by scanning densitometry. β-actin was used as an internal control. C, control; T, 50 μM 2HF treated.
In addition to being a key metabolic protein involved in nucleotide synthesis and thus being a critical regulator of tumor proliferation, the ribonucleotide reductase M2 subunit (RRM2) is increased in a BC [27]. The sequence coverage and a representative RRM2 peptide identified are presented in Supplemental Fig. S2. RRM2 has also been implicated in NFκB activation and MMP-9 expression that in turn promote tumor cell invasiveness [28]. A combined metabolic and gene-expression analyses identified RRM2 as a marker of tumor progression and a critical determinant of good vs. poor survivors in BC [29]. RRM2 expression was particularly associated with tamoxifen resistance in BC [30]. 2HF treatment led to down-regulation of RRM2 in all the treated BC cells without any effect on MCF10A normal breast epithelial cells. The mitochondrial oxidative phosphorylation is up-regulated in BC and Cytochrome C Oxidase Copper chaperone (COX17) was characterized as an up-regulated gene associated with increased oxidative phosphorylation in BC [31]. 2HF treatment led to down-regulation of COX17 protein in MDA-MB231 and SKBR3 BC cells with modest effect on MCF7 BC cells. The Ubiquitin-conjugating enzyme E2C (UBE2C) is associated with malignant progression of BC and also serves as a marker of micro-calcifications seen in early breast tumors [32]. The UBE2C expression is positively associated with tumor size, histological grade, clinical stage, lymph node metastases, HER2 expression and Ki67 positivity in BC [33]. UBE2C has negative correlation with ER expression thereby denoting it as a marker for ER− BC [33]. The 2HF treatment led to down-regulation of UBE2C in triple-negative MDA-MB231 and HER2+ SKBR3 BC cells without significant effect in ER+ MCF7 BC cells (Supplementary Table 1).
The expression of Hemeoxygenase 1 (HO-1) is known to inhibit the invasion and migration of BC cells [34]. We observed a striking upregulation of HO-1 following 2HF treatment in all treated cells with a predominant effect in HER2+ SKBR3 BC cells as identified and quantified by average reporter ion counts and Western blot analyses (Fig. 3 and Supplemental Fig. S3). The Basal Cell Adhesion Molecule (BCAM) encodes a luthern blood group glycoprotein that serves as a laminin5 receptor and is involved in cellular adhesion, cytoskeletal organization and metastases driven by growth factor aberrations, including EGFR mutations [35]. 2HF led to down-regulation of BCAM in HER2+ SKBR3 BC cells. Glycine decarboxylase (GLDC), a critical enzyme associated with glycine metabolism, is highly expressed in HER2+ BC [36]. GLDC expression is also increased in both primary and metastatic BC and associated with poor overall survival in particularly patients with brain metastatic BC [37]. The 2HF treatment led to a significant down-regulation of GLDC particularly in HER2+ SKBR3 BC cells without any significant effect in ER+ MCF7, triple-negative MDA-MB231 BC cells. Inflammatory BC (IBC) represents an aggressive form of BC with a high degree of local and metastatic progression [38]. HER2 over expression is associated with increased brain and liver metastases in IBC and addition of HER2 monoclonal antibody was shown to improve the survival in IBC [39, 40]. The MARCKS protein, which regulates filopodium and lamellopodium formation and couples protein kinase C and calmodulin signaling, is over-expressed in BC and positively associated with HER2 expression and metastases [41, 42]. 2HF treatment led to down-regulation of MARCKS related protein particularly in HER2+ SKBR3 BC cells without much effect in other tested BC cells. HER2 signaling has been known to positively stimulate the rate of synthesis of hypoxia inducible factor 1 (HIF1) and further mediate angiogenesis and tumor progression [43]. The transport of cytosolic HIF1-α in to nucleus for gene-expression effects is mediated by importin 4 and 7 [44]. 2HF treatment down-regulated importin 4 in HER2+ SKBR3 BC cells (Supplementary Table 1).
Figure 3. Quantification of Heme Oxygenase 1 (HO1) by Mass Spectrometry and Western blot:
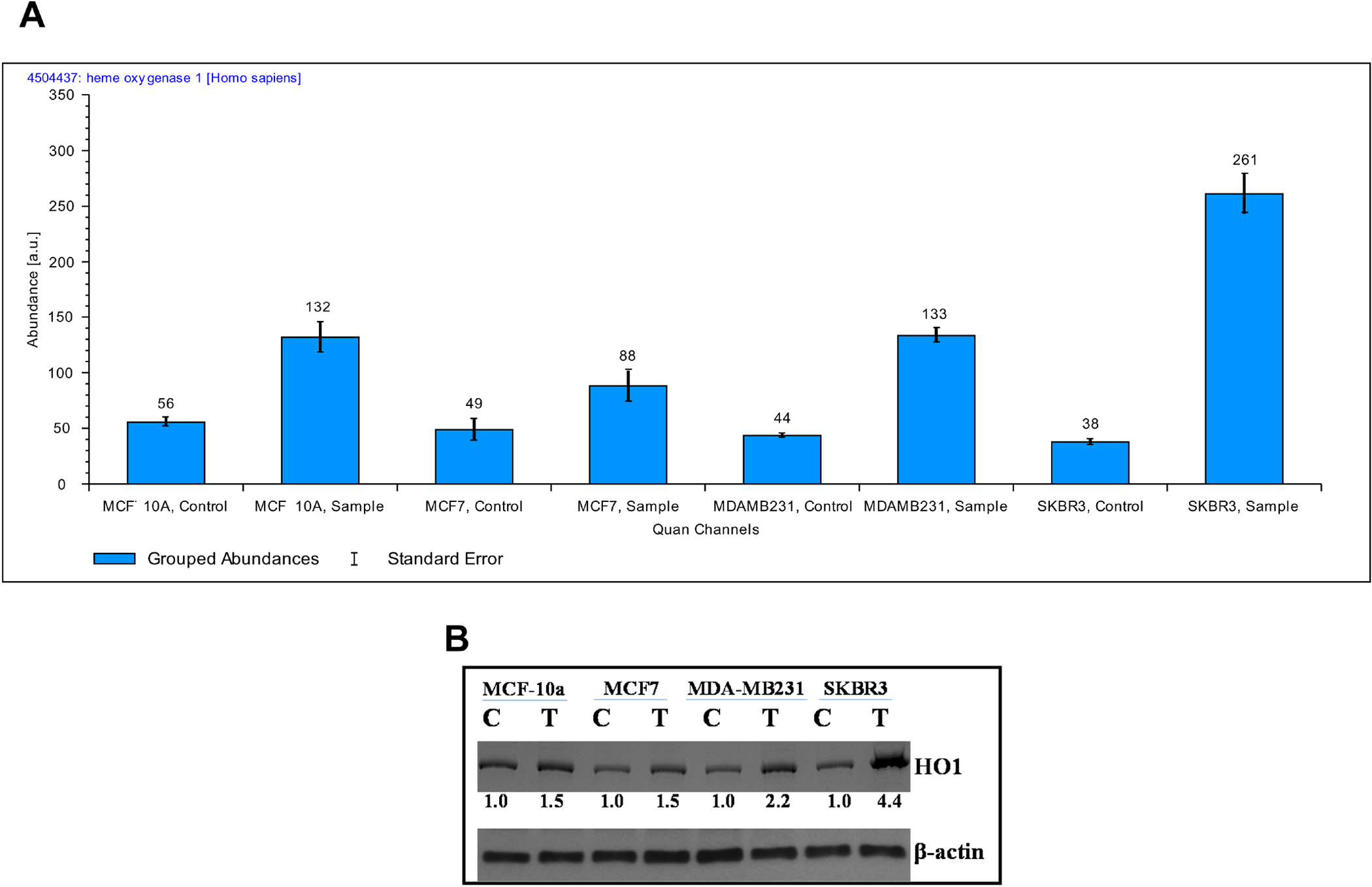
(A) Bar diagrams showing the average reporter ion counts for HO1 following MS/MS analyses. (B) Aliquots of crude membrane extracts (40 μg) from control and 2HF (50μM, 24h) treated MCF10a, MCF7, MDA-MB231, and SKBR3 cells were applied to SDS-PAGE and subjected to Western blot analyses against anti-HO1 IgG. Numbers below the blots represent the fold change in the levels of proteins as compared to control as determined by scanning densitometry. β-actin was used as an internal control. C, control; T, 50 μM 2HF treated.
The uracil-DNA glycosylase isoform 2 (UNG2) is a critical protein involved in base excision repair (BER) [45]. The UNG2 phosphorylation by GSK3 leads to resistance to floxuridine and promotes tumor cell survival [46]. 2HF treatment led to decrease in the levels of pUNG2 in MCF7 BC cells. The retinoblastoma-like protein 1 (RBL1) is a protein similar to retinoblastoma (Rb) protein, induces G2/M phase arrest following radiation in MCF7 cells and is known to be a putative tumor suppressor that is activated by dephosphorylation [47, 48]. The 2HF treatment decreased the levels of pRBL1 isoform A in MCF7 BC cells (Supplementary Table 2). Syndecan-1 is an integral membrane heparin sulphate proteoglycan, activated by phosphorylation on cytoplasmic tyrosine residues and is known to control cell invasion and migration [49, 50]. Syndecan-1 is known to regulate the proliferative effects of IL-6 mediated STAT3 signaling in BC stem cells [51]. The syndecan-1 status also positively correlates with high histological tumor grade in BC [52]. 2HF treatment down-regulated the p-syndecan-1 in MCF7 BC cells without significant effects on MDA-MB231 and SKBR3 BC cells. The transcription factor AP4 controls epithelial mesenchymal transition (EMT) via SNAIL and induces stem cell properties [53]. 2HF decreased the levels of pTFAP4 in MCF7 and MDA-MB231 BC cells without significant effect in SKBR3 BC and MCF10A normal breast epithelial cells. The DnaJ homolog subfamily member C2 (DNAJC2) belongs to the family of M-phase phosphoproteins (MPP) that act as a molecular chaperone for nascent polypeptide chains during ribosomal protein synthesis [54, 55]. 2HF treatment caused down regulation of p-DNAJC2 in MDA-MB231 BC cells. Neural cell adhesion molecule L1 (L1CAM) is a target gene of Wnt signaling [56]. The expression of L1CAM is associated with lymph node involvement, high tumor grade, HER2 expression, ER-negative status, shorter disease-free and overall survival in BC [57]. The phosphorylation of eukaryotic translation initiation factor 4E-binding protein 2 (4EBP2) is known to be mediated by PI3K/ mTOR signaling, and dephosphorylation of 4EBP2 can mediate cytotoxicity of kinase inhibitors in tumors [58, 59]. There was a strong down-regulation of pL1CAM and p4EBP2 following 2HF treatment in HER2+ SKBR3 BC cells. The endoplasmic reticulum stress mediates the activity of many anticancer drugs [60]. The protein Lunapark interacts with ubiquitin ligase complex and localized to endoplasmic reticulum following stress [61]. 2HF caused down-regulation of pLunapark in MCF7 BC cells. The ubiquitin fusion degradation protein 1 (UFD1) mediates endoplasmic reticulum stress response with cell cycle control [62]. The sequence coverage and representative UFD1 phosphopeptide identified are presented in Supplemental Fig. S4. 2HF treatment led to strong up regulation of pUFD1 isoform A in MCF7, MDA-MB231 and SKBR3 BC cells (Supplementary Table 2).
Ingenuity Pathway Analyses (IPA) of differentially regulated pathways and networks:
We conducted the IPA to determine the differential regulation of cellular pathways and networks. The differentially regulated top canonical pathways are presented in Table 1. The Supplementary Tables 3–6 provide information on changes in the upstream regulators following 2HF treatment in MCF10A normal breast epithelial and MCF7, MDA-MB231 and SKBR3 BC cells. 2HF treatment led to inhibition of estrogen signaling and down-regulation of estrogen target proteins DNA methyl transferase 1 (DNMT1), RRM2, and topoisomerase 2A (TOP2A) in ER+ MCF7 BC cells (Fig 4A, z score: - 2.202) [63, 64]. The activation of PI3K/AKT/mTOR pathway serves as a major mechanism of resistance to hormonal therapy in BC [65]. The inhibition of mTOR has been shown to overcome endocrine resistance even in the presence of low levels of PTEN in BC [66]. 2HF treatment led to inhibition of mTOR network in BC (Fig 4B). The Ubiquitin-Conjugating Enzyme (UBE2C) and malate dehydrogenase 2 (MDH2) which are induced by mTOR signaling were down regulated while PP2CA, a critical sensitizer of endocrine therapy, was up regulated in ER+ MCF7 BC cells following 2HF treatment (Fig 4B, z score: - 2.333) [67, 68]. The expression of retinoic acid receptor (RARA) is increased in ER+ BC [69]. The RARA cooperates with and potentiates the E2 induced gene transcription in ER+ BC [70]. The mitogen-activated protein kinase 1 (MAPK1) and nuclear receptor co-activator 5(NCOA5) positively regulated by RARA were downregulated while C18orf25, associated with ubiquitin dependent catabolic process, was upregulated following 2HF treatment (Fig 4C, z score: - 2.449) [71, 72]. The 2HF treatment also led to inhibition of the network of small molecule biochemistry focused on ribosomal transcription of proteins in MCF7 BC cells (Supplemental Fig S5). In addition, the cell cycle network was inhibited following 2HF treatment in MCF7 BC cells (Supplemental Fig S6).
Table 1:
Top Canonical Pathways Regulated by 2HF Treatment in BC cells
| Top Canonical Pathways | |
|---|---|
| Name | p-value |
| MCF7 (2HF vs. Control) | |
| Protein Ubiquitination Pathway | 3.34E-14 |
| EIF2 signaling | 2.08E-06 |
| tRNA charging | 4.69E-06 |
| NRF2-mediated oxidative stress response | 4.02E-05 |
| Regulation of cellular mechanics by Calpain Protease | 6.15E-05 |
| MDA-MB231 (2HF vs. Control) | |
| EIF2 signaling | 5.82E-11 |
| tRNA charging | 1.88E-08 |
| mTOR signaling | 5.53E-07 |
| Regulation of eIF4 and p70S6K signaling | 7.27E-07 |
| Aryl hydrocarbon receptor signaling | 1.24E-06 |
| SKBR3 (2HF vs. Control) | |
| Protein Ubiquitination Pathway | 1.73E-15 |
| EIF2 signaling | 5.68E-10 |
| Regulation of eIF4 and p70S6K signaling | 6.13E-07 |
| Huntington Disease Signaling | 2.10E-06 |
| Sirtuin Signaling Pathway | 6.73E-06 |
Figure 4. Effect of 2HF regulated protein expression changes in ER+ MCF7 BC cells.
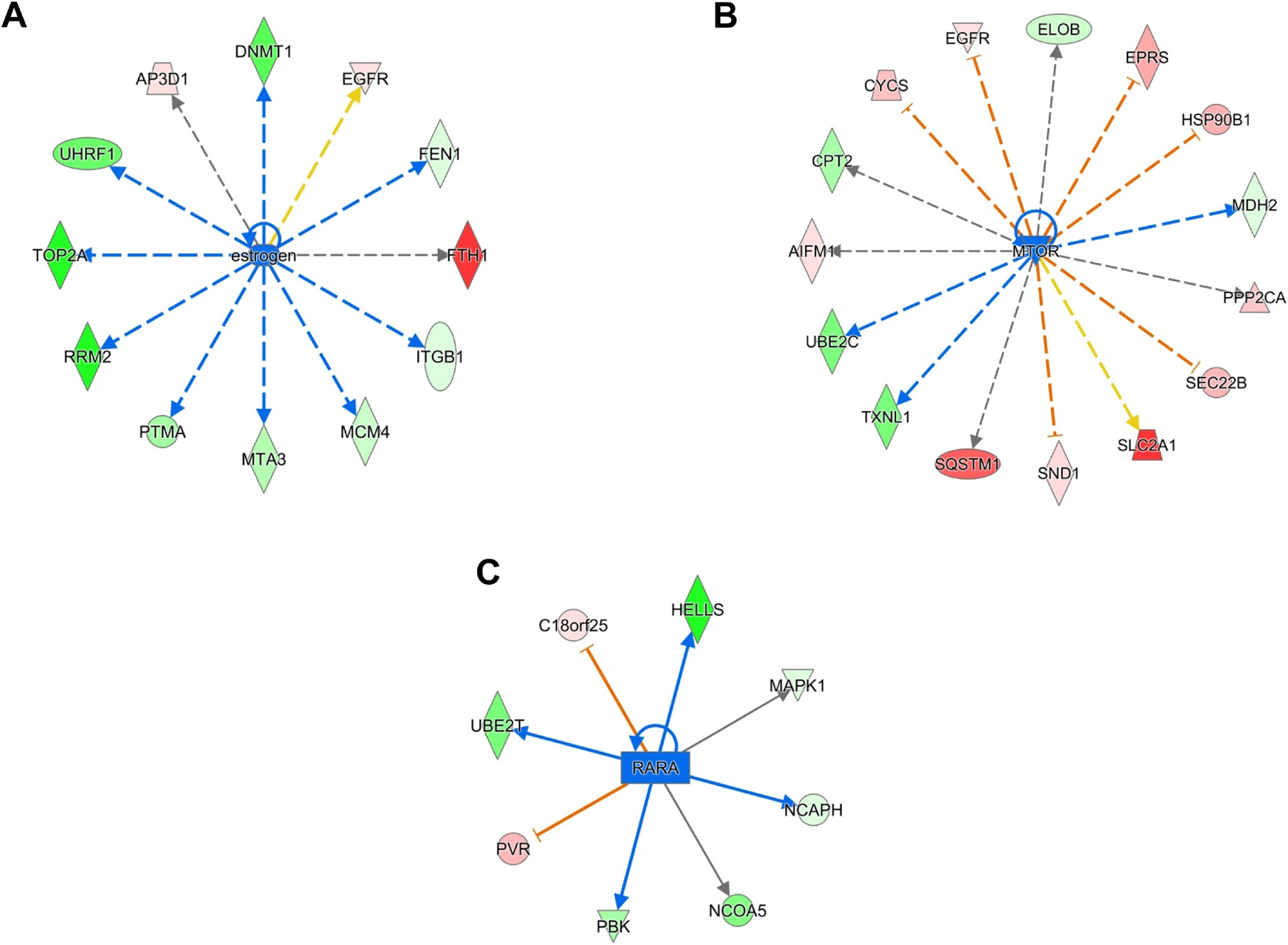
A, Ingenuity Pathway Analyses (IPA) revealing the differentially expressed estrogen network proteins. B, IPA revealing the differentially expressed mTOR network proteins. C, IPA revealing the differentially expressed RARA network proteins.
The loss of expression of phosphatase and tensin homolog deleted from chromosome 10 (PTEN) is associated with larger tumor size, higher TNM stage, lymph node metastasis, aggressive triple-negative phenotype, poor overall and disease free survival in BC [73]. The proteins AKT1, CDC20, and CDK4, whose levels are inhibited by PTEN signaling, were decreased while NDRG2 and IDH2, whose levels are increased by PTEN, were increased following PTEN network activation in MDA-MB231 BC cells (Fig 5A, z score: - 2.438) [74, 75]. Though traditionally associated with neuroblastomas, over-expression of MYCN is also associated with poor prognosis in BC [76]. A study focused on assessing the MCYN and cMyc overlapping targets revealed that the core transcriptional targets common to both regulate the basal-type BCs, a group with highest MYC expression among the BC subtypes [77]. In a study, Bertucci and co-workers characterized that 71% of triple-negative BCs were basal-like and 77% of basal-like BCs were triple-negative thereby showing a significant association between the two classes of BC [78]. 2HF treatment led to inhibition of MYCN signaling in triple-negative MDA-MB231 BC cells (Fig 5B, z score: - 3.584). The expression of HSP90 is increased and associated with aggressive and therapeutically refractory progression of breast tumors while HSP90 inhibitors have shown promising efficacy in triple-negative BC [79–81]. 2HF down-regulated HSP90 network and targets including AKT1, CDK4 and CDK2 in triple-negative MDA-MB231 BC cells (Fig 5C, z score: - 2.193) [82, 83]. The 2HF treatment also inhibited the oncoprotein MITF signaling along with down regulating associated proteins including NCAPD2, RFC5 and UBE2C in triple-negative MDA-MB231 BC cells (Supplemental Fig S7) [84]. FGF7 is an interleukin-1β induced paracrine growth factor secreted by tumor fibroblasts known to mediate BC proliferation [85]. FGF7 also induces loss of progesterone receptor (PR) thereby contributing to triple-negative state in BC [86]. 2HF treatment led to inhibition of FGF7 signaling along with down regulation of associated proteins including CDK2, CDK4, LPCAT1 and SDC1 in triple-negative MDA-MB231 BC cells (Supplemental Fig S8) [87, 88].
Figure 5. Effect of 2HF regulated protein expression changes in triple-negative MDA-MB231 BC cells.
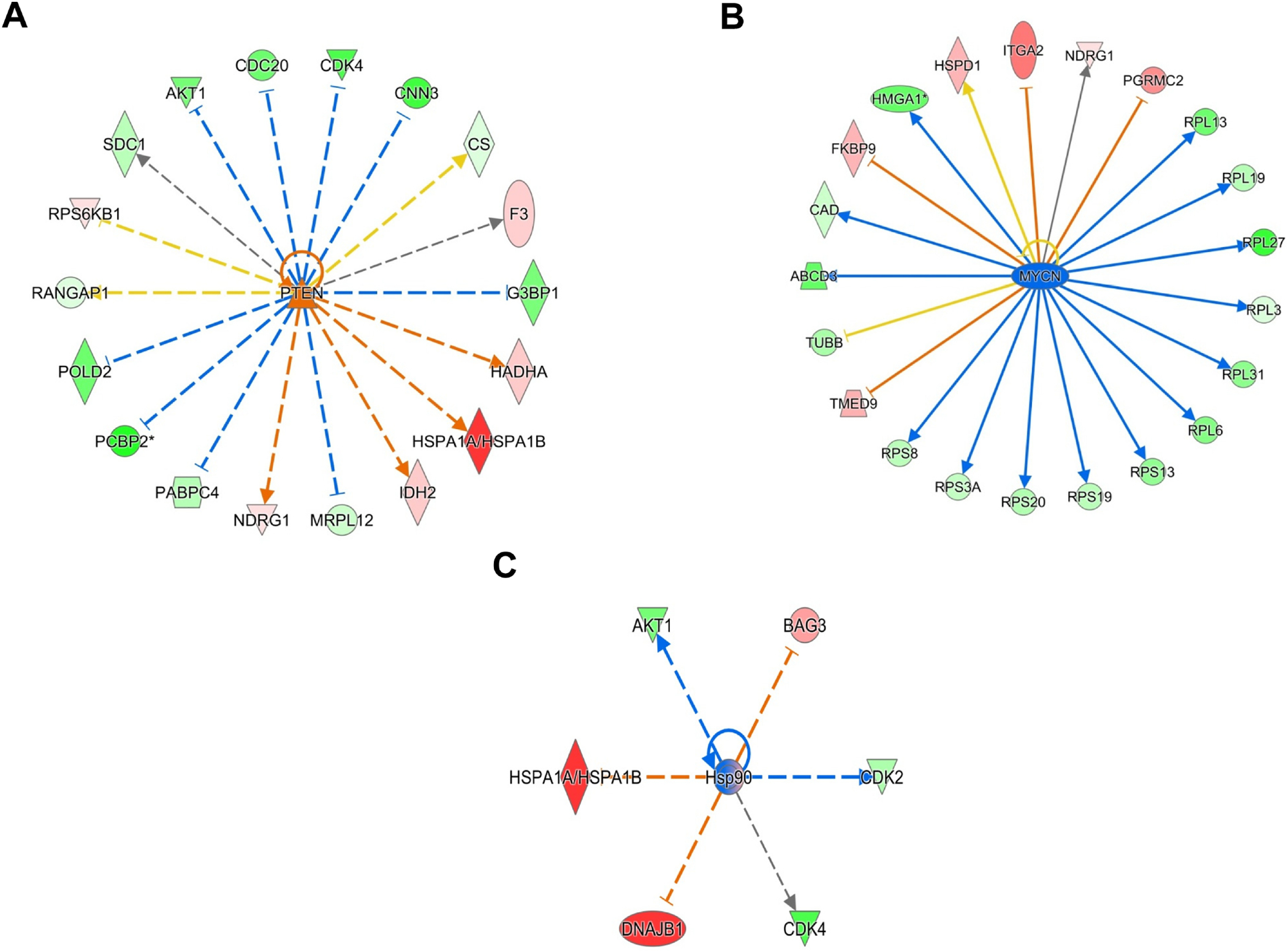
A, Ingenuity Pathway Analyses (IPA) revealing the differentially expressed PTEN network proteins. B, IPA revealing the differentially expressed MYCN network proteins. C, IPA revealing the differentially expressed HSP90 network proteins.
The p70S6k is a critical downstream effector in HER2/mTOR signaling pathway [89]. We observed an inhibition of p70S6k network and its targets including CDK4 and EEF2 in HER2+ SKBR3 BC cells (Fig 6A, z score: - 2.0). The PI3K-AKT is the major axis of HER2 signaling in HER2 over expressing BCs which further activates HSF1 and regulates the transcription of HSP90 targets [90]. 2HF treatment inhibited the HSP90 network and associated proteins including CDK4, RPS6 and AKT1 in HER2+ SKBR3 BC cells (Fig 6B, z score: - 2.211) [91]. The tumor suppressor let-7 serves as a marker of poor differentiation in epithelial cancers with higher levels being observed in the initial epithelial phenotype stages as compared to advanced, dedifferentiated, and mesenchymal phenotype tumors of epithelial origin [92]. HER2 expression leads to repression of let-7 in BC cells [93]. 2HF treatment activated tumor suppressor let-7 signaling along with down-regulating proteins repressed by let-7 including RRM1/2, PKM and MCK4/6/7 (Fig 6C, z score: - 2.433) [94].
Figure 6. Effect of 2HF regulated protein expression changes in HER2+ SKBR3 BC cells.
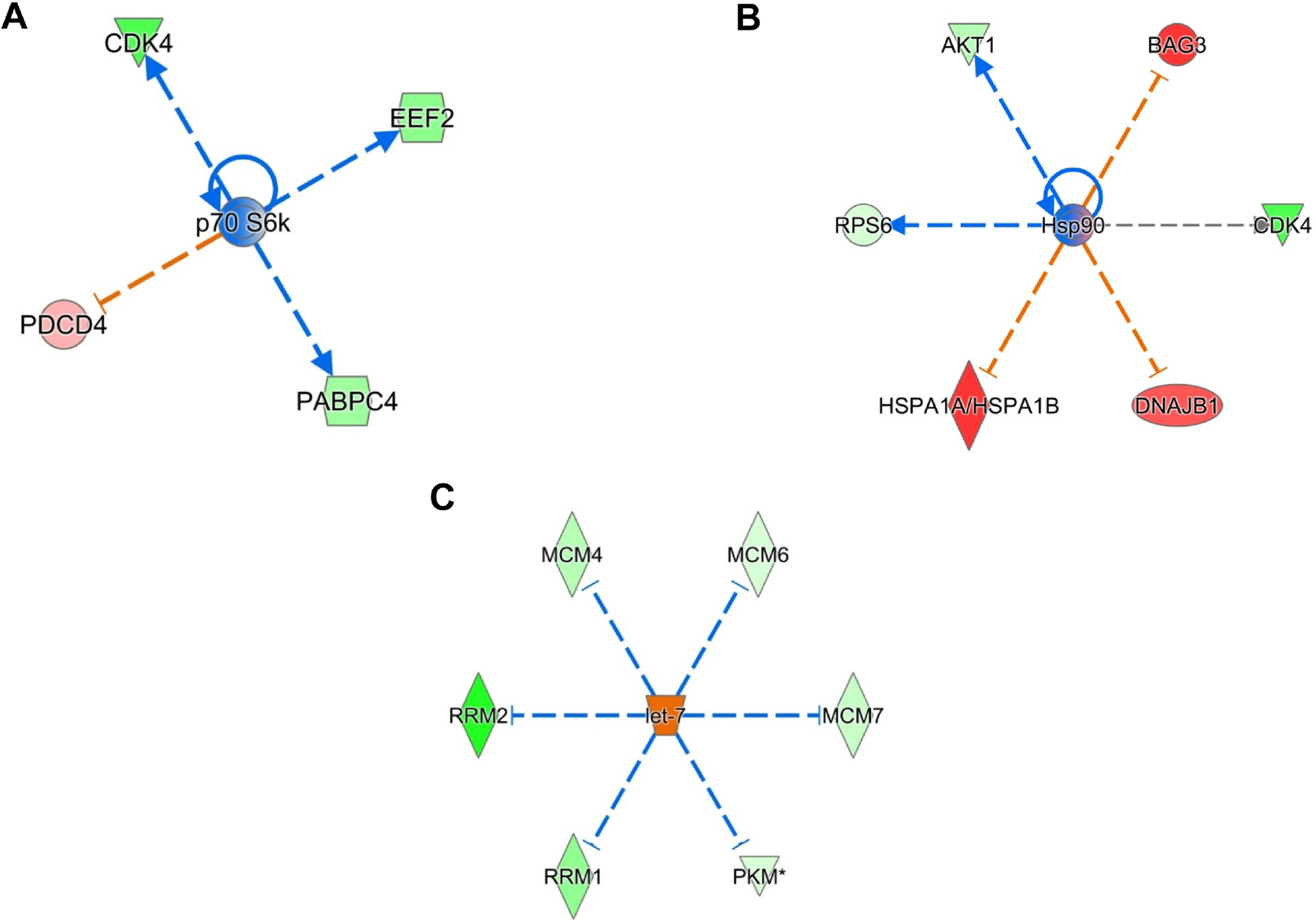
A, Ingenuity Pathway Analyses (IPA) revealing the differentially expressed p70S6k network proteins. B, IPA revealing the differentially expressed HSP90 network proteins. C, IPA revealing the differentially expressed let-7 network proteins.
The small molecule 2HF effectively regulated many critical proteins and networks of importance to BC pathogenesis, progression and therapy. The regulation of estrogen, RARA, PTEN, MYC, HSP90 and p70S6K signaling by 2HF as revealed by IPA of differentially expressed proteins that were detected and quantified by TMT 10plex mass spectrometry was a characteristic finding that underscores the significance of 2HF for BC interventions. Further Western blot analyses of protein lysates from 2HF treated and control cells revealed down-regulation of oncoproteins HSP90, DNMT1 and MYC along with upregulation of tumor suppressor PTEN in ER+ MCF7, triple-negative MDA-MB231 and HER2+ SKBR3 BC cells (Fig 7).
Figure 7. Effect of 2HF (50 μM for 24h) on HSP90, DNMT1, PTEN, and cMyc expression in breast cancer cells:
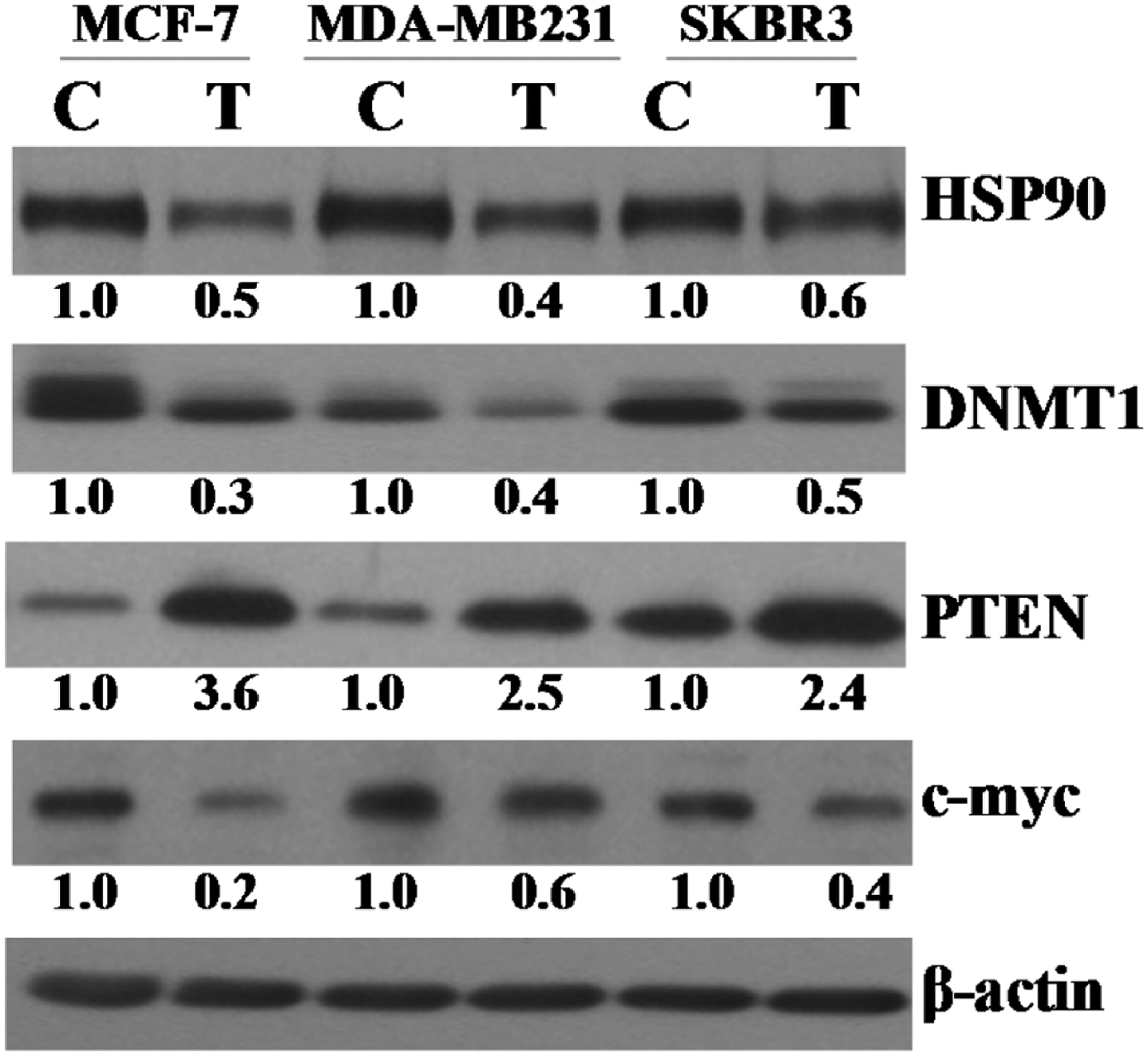
Aliquots of crude membrane extracts (40 μg) from control and 2HF (50μM, 24h) treated MCF7, MDA-MB231, and SKBR3 cells were applied to SDS-PAGE and subjected to Western blot analyses against HSP90, DNMT1, PTEN, and cMyc antibodies. Numbers below the blots represent the fold change in the levels of proteins as compared to control as determined by scanning densitometry. β-actin was used as an internal control. C, control; T, 50 μM 2HF treated.
2HF regulated a number of cellular pathways in BC cells (Table 1 and Fig. 8). The top canonical pathways differentially regulated by 2HF were EIF2 signaling in all the tested BC cells, and protein ubiquitination pathway in ER+ MCF7 and HER2+ SKBR3 BC cells. Among the other signaling cascades regulated by 2HF, many were related to cell-matrix signaling, invasion and inflammation related pathways. Paxillin is a critical mediator of cell-matrix signaling and in particular, mediates the estrogen signaling via FAK/N-WASP-ARP2/3 complex thereby contributing to estrogen mediated cell motility and invasion in BC cells [95]. The hepatocyte growth factor (HGF) is associated with increased invasiveness via MAPK pathway activation in BC cells [96, 97]. The insulin growth factor 1 (IGF1) is associated with BC proliferation via Akt and MAPK pathway activation [98]. The interleukin 3 (IL3) is associated with inflammation and oncogenesis while IL3 blockage has been associated with mechanisms of anticancer agents like IL3 receptor antibodies [99]. 2HF treatment led to down-regulation of paxillin, HGF, oncogenic phospholipase C and p70S6K signaling in MCF7 and SKBR3 BC cells along with inhibiting IGF1 and IL3 signaling in SKBR3 cells (Fig. 8).
Figure 8. Heatmap showing the effect of 2HF (50 μM for 24h) on critical cellular canonical pathways:

The effect of 2HF on cellular canonical pathways was analyzed by IPA. The heatmap shows the activated (red) and inhibited (blue) canonical pathways following 2HF treatment in MCF10A normal epithelial and MCF7, MDA-MB231 and SKBR3 breast cancer cells.
Discussion
Novel anticancer small molecules capable of inhibiting signaling networks of direct relevance to BC prevention and therapy have been a focus of research since decades. The loss of ER in combination with over-expression and/activity of PI3K/AKT/mTOR signaling is a known factor that contributes to endocrine resistance in hormone positive BC [100]. In spite of the development of targeted inhibitors for growth factors, combinatorial therapeutics focused on anthracyclines, taxanes, CDK4/6 inhibitors, and hormonal therapies including anti-estrogens and aromatase inhibitors, the quest for an ideal molecule capable of multi-network inhibition, without any significant toxic effects on normal cells, has gained momentum in developmental cancer therapeutics [4, 7–9]. Based on the promising anticancer spectrum of 2HF in BC, this study focused on elucidating the changes in proteomic profile of ER+ MCF7, triple-negative MDA-MB231 and HER2+ SKBR3 BC cells using TMT 10plex proteomics method [11, 16].
2HF treatment led to regulation of several critical signaling proteins and nodes of fundamental relevance BC initiation, progression and therapeutic response. One of the interesting observations was the ability of 2HF to target multiple signaling nodes that have mutual cross-talk in regulating survival and therapeutic resistance in each of the respective subtypes of BC cells. First, the inhibition of estrogen, RARA and mTOR networks which are known to interact in regulating the BC response to hormonal therapy was one of the striking findings. Second, the inhibition of MYC signaling in triple-negative MDA-MB231 BC cells along with activation of PTEN tumor suppressor network was a finding of translational significance given the high degree of expression and activity of MYC in basal and triple-negative breast tumors [77]. Third, the inhibition of p70S6K and activation of let-7, a determinant of tumor differentiation and a tumor suppressor that is normally repressed by HER2, in HER2+ SKBR3 BC cells following 2HF treatment represents another significant finding of this study [92, 93]. The regulation of cancer invasion and proliferation associated pathways mediated by EIF2, paxillin, HGF, IGF-1 and IL3 signaling provides additional mechanistic basis for the translational potential of 2HF in BC. In addition, identification of additional targets of 2HF will help us understand the molecular mechanisms of its anticancer properties. Taken together, the chemotherapeutic and chemopreventive potential of 2HF, as shown by its ability to specifically target the cancerous cells, represents a novel strategy to combat BC.
Conclusions
Naturally occurring compounds have grabbed increased attention for the prevention of early stage of carcinogenesis and neoplastic progression before the occurrence of invasive malignant diseases; therefore, many of these compounds have been regarded as chemoprevention agent. Flavonoids, a group of plant metabolites, provide health benefits through cell signalling pathways and antioxidant effects, are found in a variety of fruits and vegetables. Out of eight flavanones including flavanone, 2-OH flavanone, 4-OH flavanone, 6-OH flavanone, 7-OH flavanone, naringenin, nargin, and taxifolin used, results of the cell viability assay indicate that 2-OH flavanone (2HF) showed the most potent cytotoxic effect towards various tumor cells (101). 2HF causes significant cytotoxicity to the cancer cells and also inhibits angiogenesis, which is the key survival mechanism for BC growth and metastasis (11).
Ingenuity Pathway Analyses (IPA) of differentially expressed proteins revealed that 2HF treatment leads to inhibition of estrogen, mTOR and RARA signaling in ER+ MCF7 BC cells, activation of tumor suppressor PTEN, and inhibition of MYCN and HSP90 signaling in triple-negative MDA-MB231 BC cells, and activation of let-7 tumor suppressor, and inhibition of p70S6K and HSP90 signaling in HER2+ SKBR3 BC cells. In summary, this study characterized the changes in the proteomic profile of major subtypes of BC cells along with characterizing the critical and translationally significant regulation of major upstream regulators and networks that collectively provide strong rationale for further development and testing 2HF based interventions for BC prevention and therapy.
Future perspectives
It is now well recognized that bioactive polyphenols present in edible plants can interfere with cancer initiation, promotion, and progression, acting as chemopreventive agents (102, 103). In this regard, the ability of 2HF to suppress the in-vitro angiogenesis and VEGF expression in BC subtypes, without affecting normal cells, represents the much-desired ability of an ideal chemopreventive drug to specifically guard against the breast carcinoma (11, 104). Present study warrants further clinical studies that are needed to standardize the doses, routes of administration, organ specificity and bioavailability in humans. The current investigations also indicate that the use of these bioactive natural compounds regularly in the diet can serve as preventive as well as therapeutic strategies for cancers of multiple organs and origins. Based on these observations, we conclude that cell proliferation and apoptosis are valid biomarkers to assess 2HF response in future clinical trials, and flavanones explain the inverse association between fruits and vegetables consumption and BC risk.
Supplementary Material
Significance.
Effective management of breast cancer, the most common cancer in the women worldwide, remains a major clinical-translational challenge. ERα and HER2 status have long served as the predictors of breast tumor behavior and response to therapy. Novel anticancer small molecules capable of inhibiting signaling networks of direct relevance to breast cancer prevention and therapy have been a focus of research since decades. The loss of ERα in combination with over expression and/activity of PI3K/AKT/mTOR signaling is a known factor that contributes to endocrine resistance in hormone positive breast cancer. In spite of the development of targeted inhibitors for growth factors, combinatorial therapeutics focused on anthracyclines, taxanes, CDK4/6 inhibitors, and hormonal therapies including anti-estrogens and aromatase inhibitors, the quest for an ideal molecule capable of multi-network inhibition, without any significant toxic effects on normal cells, has gained momentum in developmental cancer therapeutics. Based on the promising anticancer spectrum of 2’-hydroxyflavanone (2HF) in breast cancer, this study focused on elucidating the changes in proteomic profile of control and 2HF treated ER+ MCF7, triple-negative MDA-MB231 and HER2+ SKBR3 breast cancer cells using TMT 10Plex proteomics method. Our results further revealed that 2HF regulates critical signaling nodes including estrogen network, mTOR, HSP90, let-7 and p70S6K signaling which are of direct relevance to breast cancer prevention and therapy. In summary, present study characterized the regulation of proteomic profile by 2HF in all the three major subtypes of breast cancer along with elucidating the inhibition of critical upstream regulators and networks associated with therapeutic response in breast cancer.
Highlights.
2HF inhibits estrogen, RARA and mTOR networks in breast cancer
2HF inhibits MYC signaling in triple-negative breast cancer
2HF inhibits p70S6K and activates let-7
2HF regulates critical cellular canonical pathways in breast cancer
Acknowledgments
This work was supported in part by the Department of Defense grant (W81XWH-16-1-0641). Funding from the Beckman Research Institute of the City of Hope is also acknowledged. We sincerely thank Ravi Salgia, MD, PhD, Professor and Chair, Department of Medical Oncology at City of Hope for providing research space and support.
The abbreviations used are
- AKT
protein kinase B (PKB), also known as Akt
- BC
breast cancer
- BCAM
basal cell adhesion molecule
- CDK
cyclin dependent kinase
- COUP-TF1
COUP transcription factor 1
- COX17
cytochrome C oxidase copper chaperone
- DNAJC2
DnaJ homolog subfamily member C2
- DNMT1
DNA methyl transferase 1
- E2F1
E2F transcription factor 1
- 4EBP2
eukaryotic translation initiation factor 4E-binding protein 2
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial mesenchymal transition
- ER
estrogen receptor
- ERBB2
Erb-B2 receptor tyrosine kinase 2 or HER2
- ERK
extracellular signal-regulated kinase
- GLDC
Glycine decarboxylase
- GSK3
glycogen synthase 3 kinase
- H2A.Z
histone H2 A.Z
- HER2
receptor tyrosine-protein kinase erbB-2
- 2HF
2’-hydroxyflavanone
- HIF1
hypoxia inducible factor 1
- HO-1
hemeoxygenase 1
- HSP90
heat shock protein 90
- IBC
inflammatory breast cancer
- IPA
Ingenuity pathway analyses
- KLF16
kruppel-like factor16
- L1CAM
neural cell adhesion molecule L1
- MAPK1
mitogen-activated protein kinase 1
- MARCKS
myristoylated alanine rich protein kinase C substrate
- MMP
matrix metallo proteinase
- mTOR
mammalian target of rapamycin
- PAF
PCNA-associated factor
- PI3K
phosphoinositide-3 kinase
- PR
progesterone Receptor
- PTEN
phosphatase and tensin homolog deleted from chromosome 10
- RARA
retinoic Acid Receptor
- RBL1
retinoblastoma-like protein 1
- RRM2
ribonucleotide reductase M2 subunit
- SDS-PAGE
sodium-dodecyl sulfate polyacrylamide gel electrophoresis
- TMT
tandem mass tag
- TNF
tumor necrosis factor
- TOP2A
topoisomerase 2A
- TRAIL
TNF-related apoptosis-inducing ligand
- UBE2C
ubiquitin-conjugating enzyme E2C
- UFD1
ubiquitin fusion degradation protein 1
- UNG2
uracil-DNA glycosylase isoform 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Data Availability:
The data supporting the findings of this study are available within the article and its Supplementary Files. All other relevant source data are is available from the corresponding authors upon request.
References
- [1].Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). (2017) SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2016 SEER data submission, posted to the SEER web site [Google Scholar]
- [2].Stewart BW, Wild CP, eds. (2014) World cancer report. IARC. 2014 [Google Scholar]
- [3].Siegel RL, Miller KD, Ahmedin J (2017) Cancer statistics. CA Cancer J Clin. 67:7–30 [DOI] [PubMed] [Google Scholar]
- [4].Dalasanur Nagaprashantha L, Adhikari R, Singhal J, Chikara S, Awasthi S, Horne D, and Singhal SS (2018) Translational opportunities for broad-spectrum natural phytochemicals and targeted agent combinations in breast cancer. Int. J. Cancer 142, 658–670 [DOI] [PubMed] [Google Scholar]
- [5].Brenton JD, Carey LA, Ahmed AA, and Caldas C (2005) Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J. Clin. Oncol 23, 7350–7360 [DOI] [PubMed] [Google Scholar]
- [6].Whitesell L, Santagata S, Mendillo ML, Lin NU, Proia DA, and Lindquist S (2014) HSP90 empowers evolution of resistance to hormonal therapy in human breast cancer models. Proc. Natl. Acad. Sci. U. S. A 111, 18297–18302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Davis NM, Sokolosky M, Stadelman K, Abrams SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D’Assoro A, Drobot L, Rakus D, Gizak A, Laidler P, Dulinska-Litewka J, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Montalto G, Cervello M, Fitzgerald TL, Demidenko Z, Martelli AM, Cocco L, Steelman LS, and McCubrey JA (2014) Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: Possibilities for therapeutic intervention. Oncotarget 5, 4603–4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].LoRusso PM (2016) Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J. Clin. Oncol 34, 3803–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].So FV, Guthrie N, Chambers AF, Moussa M, and Carroll KK (1996) Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr. Cancer 26, 167–181 [DOI] [PubMed] [Google Scholar]
- [10].Guthrie N and Carroll KK (1998) Inhibition of mammary cancer by citrus flavonoids. Adv. Exp. Med. Biol 439, 227–236 [DOI] [PubMed] [Google Scholar]
- [11].Singhal J, Nagaprashantha L, Chikara S, Awasthi S, Horne D, and Singhal SS (2017) 2’-Hydroxyflavanone: a novel strategy for targeting breast cancer. Oncotarget. 8, 75025–75037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nagaprashantha LD, Vatsyayan R, Singhal J, Lelsani P, Prokai L, Awasthi S, and Singhal SS (2011) 2’-hydroxyflavanone inhibits proliferation, tumor vascularization and promotes normal differentiation in VHL-mutant renal cell carcinoma. Carcinogenesis 32, 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsiao YC, Kuo WH, Chen PN, Chang HR, Lin TH, Yang WE, Hsieh YS, and Chu SC (2007) Flavanone and 2’-OH flavanone inhibit metastasis of lung cancer cells via down-regulation of proteinases activities and MAPK pathway. Chem. Biol. Interact 167, 193–206 [DOI] [PubMed] [Google Scholar]
- [14].Wu K, Ning Z, Zhou J, Wang B, Fan J, Zhu J, Gao Y, Wang X, Hsieh JT, and He D (2014) 2’-hydroxyflavanone inhibits prostate tumor growth through inactivation of AKT/STAT3 signaling and induction of cell apoptosis. Oncol. Rep 32, 131–138 [DOI] [PubMed] [Google Scholar]
- [15].Lu KH, Chen PN, Lue KH, Lai MT, Lin MS, Hsieh YS, and Chu SC (2014) 2’-hydroxyflavanone induces apoptosis of human osteosarcoma 143 B cells by activating the extrinsic TRAIL- and intrinsic mitochondria-mediated pathways. Nutr. Cancer 66, 625–635 [DOI] [PubMed] [Google Scholar]
- [16].Huang FK, Zhang G, Lawlor K, Nazarian A, Philip J, Tempst P, Dephoure N, and Neubert TA (2017) Deep coverage of global protein expression and phosphorylation in breast tumor cell lines using TMT 10-plex isobaric labeling. J. Proteome Res 16, 1121–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yu P, Huang B, Shen M, Lau C, Chan E, Michel J, Xiong Y, Payan DG, and Luo Y (2001) p15(PAF), a novel PCNA associated factor with increased expression in tumor tissues. Oncogene 20, 484–489 [DOI] [PubMed] [Google Scholar]
- [18].De Biasio A, de Opakua AI, Mortuza GB, Molina R, Cordeiro TN, Castillo F, Villate M, Merino N, Delgado S, Gil-Carton D, Luque I, Diercks T, Bernado P, Montoya G, and Blanco FJ (2015) Structure of p15(PAF)-PCNA complex and implications for clamp sliding during DNA replication and repair. Nat. Commun 6, 6439. [DOI] [PubMed] [Google Scholar]
- [19].Kais Z, Barsky SH, Mathsyaraja H, Zha A, Ransburgh DJ, He G, Pilarski RT, Shapiro CL, Huang K, and Parvin JD (2011) KIAA0101 interacts with BRCA1 and regulates centrosome number. Mol. Cancer Res 9, 1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang X, Jung YS, Jun S, Lee S, Wang W, Schneider A, Sun Oh Y, Lin SH, Park BJ, Chen J, Keyomarsi K, and Park JI (2016) PAF-wnt signaling-induced cell plasticity is required for maintenance of breast cancer cell stemness. Nat. Commun 7, 10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Daftary GS, Lomberk GA, Buttar NS, Allen TW, Grzenda A, Zhang J, Zheng Y, Mathison AJ, Gada RP, Calvo E, Iovanna JL, Billadeau DD, Prendergast FG, and Urrutia R (2012) Detailed structural-functional analysis of the kruppel-like factor 16 (KLF16) transcription factor reveals novel mechanisms for silencing Sp/KLF sites involved in metabolism and endocrinology. J. Biol. Chem 287, 7010–7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ma P, Sun CQ, Wang YF, Pan YT, Chen QN, Liu WT, Liu J, Zhao CH, Shu YQ, and Li W (2017) KLF16 promotes proliferation in gastric cancer cells via regulating p21 and CDK4. Am. J. Transl. Res 9, 3027–3036 [PMC free article] [PubMed] [Google Scholar]
- [23].Deblois G and Giguere V (2003) Ligand-independent coactivation of ERalpha AF-1 by steroid receptor RNA activator (SRA) via MAPK activation. J. Steroid Biochem. Mol. Biol 85, 123–131 [DOI] [PubMed] [Google Scholar]
- [24].Metivier R, Gay FA, Hubner MR, Flouriot G, Salbert G, Gannon F, Kah O, and Pakdel F (2002) Formation of an hER alpha-COUP-TFI complex enhances hER alpha AF-1 through Ser118 phosphorylation by MAPK. EMBO J. 21, 3443–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Le Dily F, Metivier R, Gueguen MM, Le Peron C, Flouriot G, Tas P, and Pakdel F (2008) COUP-TFI modulates estrogen signaling and influences proliferation, survival and migration of breast cancer cells. Breast Cancer Res. Treat 110, 69–83 [DOI] [PubMed] [Google Scholar]
- [26].Svotelis A, Gevry N, Grondin G, and Gaudreau L (2010) H2A.Z overexpression promotes cellular proliferation of breast cancer cells. Cell Cycle 9, 364–370 [DOI] [PubMed] [Google Scholar]
- [27].Zhang H, Liu X, Warden CD, Huang Y, Loera S, Xue L, Zhang S, Chu P, Zheng S, and Yen Y (2014) Prognostic and therapeutic significance of ribonucleotide reductase small subunit M2 in estrogen-negative breast cancers. BMC Cancer 14, 664–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Duxbury MS and Whang EE (2007) RRM2 induces NF-kappaB-dependent MMP-9 activation and enhances cellular invasiveness. Biochem. Biophys. Res. Commun 354, 190–196 [DOI] [PubMed] [Google Scholar]
- [29].Putluri N, Maity S, Kommagani R, Creighton CJ, Putluri V, Chen F, Nanda S, Bhowmik SK, Terunuma A, Dorsey T, Nardone A, Fu X, Shaw C, Sarkar TR, Schiff R, Lydon JP, O’Malley BW, Ambs S, Das GM, Michailidis G, and Sreekumar A (2014) Pathway-centric integrative analysis identifies RRM2 as a prognostic marker in breast cancer associated with poor survival and tamoxifen resistance. Neoplasia 16, 390–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shah KN, Wilson EA, Malla R, Elford HL, and Faridi JS (2015) Targeting ribonucleotide reductase M2 and NF-kappaB activation with didox to circumvent tamoxifen resistance in breast cancer. Mol. Cancer Ther 14, 2411–2421 [DOI] [PubMed] [Google Scholar]
- [31].Whitaker-Menezes D, Martinez-Outschoorn UE, Flomenberg N, Birbe RC, Witkiewicz AK, Howell A, Pavlides S, Tsirigos A, Ertel A, Pestell RG, Broda P, Minetti C, Lisanti MP, and Sotgia F (2011) Hyperactivation of oxidative mitochondrial metabolism in epithelial cancer cells in situ: Visualizing the therapeutic effects of metformin in tumor tissue. Cell Cycle 10, 4047–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chou CP, Huang NC, Jhuang SJ, Pan HB, Peng NJ, Cheng JT, Chen CF, Chen JJ, and Chang TH (2014) Ubiquitin-conjugating enzyme UBE2C is highly expressed in breast microcalcification lesions. PLoS One 9, e93934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mo CH, Gao L, Zhu XF, Wei KL, Zeng JJ, Chen G, and Feng ZB (2017) The clinicopathological significance of UBE2C in breast cancer: A study based on immunohistochemistry, microarray and RNA-sequencing data. Cancer Cell Int 17, 83-017-0455-1. eCollection 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin CW, Shen SC, Hou WC, Yang LY, and Chen YC (2008) Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol. Cancer Ther 7, 1195–1206 [DOI] [PubMed] [Google Scholar]
- [35].Bartolini A, Cardaci S, Lamba S, Oddo D, Marchio C, Cassoni P, Amoreo CA, Corti G, Testori A, Bussolino F, Pasqualini R, Arap W, Cora D, Di Nicolantonio F, and Marchio S (2016) BCAM and LAMA5 mediate the recognition between tumor cells and the endothelium in the metastatic spreading of KRAS-mutant colorectal cancer. Clin. Cancer Res 22, 4923–4933 [DOI] [PubMed] [Google Scholar]
- [36].Kim SK, Jung WH, and Koo JS (2014) Differential expression of enzymes associated with serine/glycine metabolism in different breast cancer subtypes. PLoS One 9, e101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kim HM, Jung WH, and Koo JS (2014) Site-specific metabolic phenotypes in metastatic breast cancer. J. Transl. Med 12, 354-014-0354-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].van Uden DJ, van Laarhoven HW, Westenberg AH, de Wilt JH, and Blanken-Peeters CF (2015) Inflammatory breast cancer: An overview. Crit. Rev. Oncol. Hematol 93, 116–126 [DOI] [PubMed] [Google Scholar]
- [39].Li J, Xia Y, Wu Q, Zhu S, Chen C, Yang W, Wei W, and Sun S (2017) Outcomes of patients with inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes: A population-based study from the SEER program. Oncotarget 8, 49370–49379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Monneur A, Bertucci F, Viens P, and Goncalves A (2014) Systemic treatments of inflammatory breast cancer: An overview. Bull. Cancer 101, 1080–1088 [DOI] [PubMed] [Google Scholar]
- [41].Iioka H, Ueno N, and Kinoshita N (2004) Essential role of MARCKS in cortical actin dynamics during gastrulation movements. J. Cell Biol 164, 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Manai M, Thomassin-Piana J, Gamoudi A, Finetti P, Lopez M, Eghozzi R, Ayadi S, Lamine OB, Manai M, Rahal K, Charafe-Jauffret E, Jacquemier J, Viens P, Birnbaum D, Boussen H, Chaffanet M, and Bertucci F (2017) MARCKS protein overexpression in inflammatory breast cancer. Oncotarget 8, 6246–6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Laughner E, Taghavi P, Chiles K, Mahon PC, and Semenza GL (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol 21, 3995–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chachami G, Paraskeva E, Mingot JM, Braliou GG, Gorlich D, and Simos G (2009) Transport of hypoxia-inducible factor HIF-1alpha into the nucleus involves importins 4 and 7. Biochem. Biophys. Res. Commun 390, 235–240 [DOI] [PubMed] [Google Scholar]
- [45].Pettersen HS, Sundheim O, Gilljam KM, Slupphaug G, Krokan HE, and Kavli B (2007) Uracil-DNA glycosylases SMUG1 and UNG2 coordinate the initial steps of base excision repair by distinct mechanisms. Nucleic Acids Res. 35, 3879–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Baehr CA, Huntoon CJ, Hoang SM, Jerde CR, and Karnitz LM (2016) Glycogen synthase kinase 3 (GSK-3)-mediated phosphorylation of uracil N-glycosylase 2 (UNG2) facilitates the repair of floxuridine-induced DNA lesions and promotes cell survival. J. Biol. Chem 291, 26875–26885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kurimchak A, Haines DS, Garriga J, Wu S, De Luca F, Sweredoski MJ, Deshaies RJ, Hess S, and Grana X (2013) Activation of p107 by fibroblast growth factor, which is essential for chondrocyte cell cycle exit, is mediated by the protein phosphatase 2A/B55alpha holoenzyme. Mol. Cell. Biol 33, 3330–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pan D, Chen Y, Du Y, Ren Z, Li X, and Hu B (2017) Methylation of promoter of RBL1 enhances the radioresistance of three dimensional cultured carcinoma cells. Oncotarget 8, 4422–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ott VL and Rapraeger AC (1998) Tyrosine phosphorylation of syndecan-1 and -4 cytoplasmic domains in adherent B82 fibroblasts. J. Biol. Chem 273, 35291–35298 [DOI] [PubMed] [Google Scholar]
- [50].Matsumoto Y, Zhang Q, Akita K, Nakada H, Hamamura K, Tsuchida A, Okajima T, Furukawa K, Urano T, and Furukawa K (2013) Trimeric tn antigen on syndecan 1 produced by ppGalNAc-T13 enhances cancer metastasis via a complex formation with integrin alpha5beta1 and matrix metalloproteinase 9. J. Biol. Chem 288, 24264–24276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ibrahim SA, Hassan H, Vilardo L, Kumar SK, Kumar AV, Kelsch R, Schneider C, Kiesel L, Eich HT, Zucchi I, Reinbold R, Greve B, and Gotte M (2013) Syndecan-1 (CD138) modulates triple-negative breast cancer stem cell properties via regulation of LRP-6 and IL-6-mediated STAT3 signaling. PLoS One 8, e85737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lendorf ME, Manon-Jensen T, Kronqvist P, Multhaupt HA, and Couchman JR (2011) Syndecan-1 and syndecan-4 are independent indicators in breast carcinoma. J. Histochem. Cytochem 59, 615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jackstadt R, Roh S, Neumann J, Jung P, Hoffmann R, Horst D, Berens C, Bornkamm GW, Kirchner T, Menssen A, and Hermeking H (2013) AP4 is a mediator of epithelial-mesenchymal transition and metastasis in colorectal cancer. J. Exp. Med 210, 1331–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jung P, Menssen A, Mayr D, and Hermeking H (2008) AP4 encodes a c-MYC-inducible repressor of p21. Proc. Natl. Acad. Sci. U. S. A 105, 15046–15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jaiswal H, Conz C, Otto H, Wolfle T, Fitzke E, Mayer MP, and Rospert S (2011) The chaperone network connected to human ribosome-associated complex. Mol. Cell. Biol 31, 1160–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shkurnikov MY, Knyazev EN, Wicklein D, Schumacher U, Samatov TR, and Tonevitskii AG (2016) Role of L1CAM in the regulation of the canonical wnt pathway and class I MAGE genes. Bull. Exp. Biol. Med 160, 807–810 [DOI] [PubMed] [Google Scholar]
- [57].Schroder C, Schumacher U, Fogel M, Feuerhake F, Muller V, Wirtz RM, Altevogt P, Krenkel S, Janicke F, and Milde-Langosch K (2009) Expression and prognostic value of L1CAM in breast cancer. Oncol. Rep 22, 1109–1117 [DOI] [PubMed] [Google Scholar]
- [58].Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, and Ruggero D (2012) The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bi C, Zhang X, Lu T, Zhang X, Wang X, Meng B, Zhang H, Wang P, Vose JM, Chan WC, McKeithan TW, and Fu K (2017) Inhibition of 4EBP phosphorylation mediates the cytotoxic effect of mechanistic target of rapamycin kinase inhibitors in aggressive B-cell lymphomas. Haematologica 102, 755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Farooqi AA, Li KT, Fayyaz S, Chang YT, Ismail M, Liaw CC, Yuan SS, Tang JY, and Chang HW (2015) Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour Biol. 36, 5743–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhao Y, Zhang T, Huo H, Ye Y, and Liu Y (2016) Lunapark is a component of a ubiquitin ligase complex localized to the endoplasmic reticulum three-way junctions. J. Biol. Chem 291, 18252–18262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen M, Gutierrez GJ, and Ronai ZA (2011) Ubiquitin-recognition protein Ufd1 couples the endoplasmic reticulum (ER) stress response to cell cycle control. Proc. Natl. Acad. Sci. U. S. A 108, 9119–9124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shi JF, Li XJ, Si XX, Li AD, Ding HJ, Han X, and Sun YJ (2012) ERalpha positively regulated DNMT1 expression by binding to the gene promoter region in human breast cancer MCF-7 cells. Biochem. Biophys. Res. Commun 427, 47–53 [DOI] [PubMed] [Google Scholar]
- [64].Buterin T, Koch C, and Naegeli H (2006) Convergent transcriptional profiles induced by endogenous estrogen and distinct xenoestrogens in breast cancer cells. Carcinogenesis 27, 1567–1578 [DOI] [PubMed] [Google Scholar]
- [65].Bostner J, Karlsson E, Pandiyan MJ, Westman H, Skoog L, Fornander T, Nordenskjold B, and Stal O (2013) Activation of akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res. Treat 137, 397–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fu X, Creighton CJ, Biswal NC, Kumar V, Shea M, Herrera S, Contreras A, Gutierrez C, Wang T, Nanda S, Giuliano M, Morrison G, Nardone A, Karlin KL, Westbrook TF, Heiser LM, Anur P, Spellman P, Guichard SM, Smith PD, Davies BR, Klinowska T, Lee AV, Mills GB, Rimawi MF, Hilsenbeck SG, Gray JW, Joshi A, Osborne CK, and Schiff R (2014) Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-targeting mammalian target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase. Breast Cancer Res. 16, 430-014-0430-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Heinonen H, Nieminen A, Saarela M, Kallioniemi A, Klefstrom J, Hautaniemi S, and Monni O (2008) Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer. BMC Genomics 9, 348-2164-9-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhao H, Li D, Zhang B, Qi Y, Diao Y, Zhen Y, and Shu X (2017) PP2A as the main node of therapeutic strategies and resistance reversal in triple-negative breast cancer. Molecules 22, 10.3390/molecules22122277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Han QX, Allegretto EA, Shao ZM, Kute TE, Ordonez J, Aisner SC, Rishi AK, and Fontana JA (1997) Elevated expression of retinoic acid receptor-alpha (RAR alpha) in estrogen-receptor-positive breast carcinomas as detected by immunohistochemistry. Diagn. Mol. Pathol 6, 42–48 [DOI] [PubMed] [Google Scholar]
- [70].Ross-Innes CS, Stark R, Holmes KA, Schmidt D, Spyrou C, Russell R, Massie CE, Vowler SL, Eldridge M, and Carroll JS (2010) Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 24, 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tetel MJ (2009) Nuclear receptor coactivators: Essential players for steroid hormone action in the brain and in behaviour. J. Neuroendocrinol 21, 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Coyle KM, Maxwell S, Thomas ML, and Marcato P (2017) Profiling of the transcriptional response to all-trans retinoic acid in breast cancer cells reveals RARE-independent mechanisms of gene expression. Sci. Rep 7, 16684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Li S, Shen Y, Wang M, Yang J, Lv M, Li P, Chen Z, and Yang J (2017) Loss of PTEN expression in breast cancer: Association with clinicopathological characteristics and prognosis. Oncotarget 8, 32043–32054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Steinbach N (2017) PTEN affects gene expression and histone modifications and plays a role in the regulation of transcription Columbia University Academic Commons, 10.7916/D8GH9W9R. [DOI] [Google Scholar]
- [75].Abounader R, Reznik T, Colantuoni C, Martinez-Murillo F, Rosen EM, and Laterra J (2004) Regulation of c-met-dependent gene expression by PTEN. Oncogene 23, 9173–9182 [DOI] [PubMed] [Google Scholar]
- [76].Mizukami Y, Nonomura A, Takizawa T, Noguchi M, Michigishi T, Nakamura S, and Ishizaki T (1995) N-myc protein expression in human breast carcinoma: Prognostic implications. Anticancer Res. 15, 2899–2905 [PubMed] [Google Scholar]
- [77].Chandriani S, Frengen E, Cowling VH, Pendergrass SA, Perou CM, Whitfield ML, and Cole MD (2009) A core MYC gene expression signature is prominent in basal-like breast cancer but only partially overlaps the core serum response. PLoS One 4, e6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, and Birnbaum D (2008) How basal are triple-negative breast cancers? Int. J. Cancer 123, 236–240 [DOI] [PubMed] [Google Scholar]
- [79].Proia DA, Zhang C, Sequeira M, Jimenez JP, He S, Spector N, Shapiro GI, Tolaney S, Nagai M, Acquaviva J, Smith DL, Sang J, Bates RC, and El-Hariry I (2014) Preclinical activity profile and therapeutic efficacy of the HSP90 inhibitor ganetespib in triple-negative breast cancer. Clin. Cancer Res 20, 413–424 [DOI] [PubMed] [Google Scholar]
- [80].Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, and Kluger HM (2007) High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 67, 2932–2937 [DOI] [PubMed] [Google Scholar]
- [81].Beliakoff J and Whitesell L (2004) Hsp90: An emerging target for breast cancer therapy. Anticancer Drugs 15, 651–662 [DOI] [PubMed] [Google Scholar]
- [82].Burrows F, Zhang H, and Kamal A (2004) Hsp90 activation and cell cycle regulation. Cell Cycle 3, 1530–1536 [DOI] [PubMed] [Google Scholar]
- [83].Fu J, Koul D, Yao J, Wang S, Yuan Y, Colman H, Sulman EP, Lang FF, and Yung WK (2013) Novel HSP90 inhibitor NVP-HSP990 targets cell-cycle regulators to ablate Olig2-positive glioma tumor-initiating cells. Cancer Res. 73, 3062–3074 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [84].Hartman ML and Czyz M (2015) MITF in melanoma: Mechanisms behind its expression and activity. Cell Mol. Life Sci 72, 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Palmieri C, Roberts-Clark D, Assadi-Sabet A, Coope RC, O’Hare M, Sunters A, Hanby A, Slade MJ, Gomm JJ, Lam EW, and Coombes RC (2003) Fibroblast growth factor 7, secreted by breast fibroblasts, is an interleukin-1beta-induced paracrine growth factor for human breast cells. J. Endocrinol 177, 65–81 [DOI] [PubMed] [Google Scholar]
- [86].Piasecka D, Kitowska K, Czaplinska D, Mieczkowski K, Mieszkowska M, Turczyk L, Skladanowski AC, Zaczek AJ, Biernat W, Kordek R, Romanska HM, and Sadej R (2016) Fibroblast growth factor signalling induces loss of progesterone receptor in breast cancer cells. Oncotarget 7, 86011–86025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Niu J, Chang Z, Peng B, Xia Q, Lu W, Huang P, Tsao MS, and Chiao PJ (2007) Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J. Biol. Chem 282, 6001–6011 [DOI] [PubMed] [Google Scholar]
- [88].Korc M and Friesel RE (2009) The role of fibroblast growth factors in tumor growth. Curr. Cancer Drug Targets 9, 639–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Grammer TC, Cheatham L, Chou MM, and Blenis J (1996) The p70S6K signalling pathway: A novel signalling system involved in growth regulation. Cancer Surv. 27, 271–292 [PubMed] [Google Scholar]
- [90].Schulz R, Streller F, Scheel AH, Ruschoff J, Reinert MC, Dobbelstein M, Marchenko ND, and Moll UM (2014) HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer. Cell Death Dis. 5, e980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Pashtan I, Tsutsumi S, Wang S, Xu W, and Neckers L (2008) Targeting Hsp90 prevents escape of breast cancer cells from tyrosine kinase inhibition. Cell Cycle 7, 2936–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, Jewell DA, Feig C, Lengyel E, and Peter ME (2007) Let-7 expression defines two differentiation stages of cancer. Proc. Natl. Acad. Sci. U. S. A 104, 11400–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu D, Deng Q, Sun L, Wang T, Yang Z, Chen H, Guo L, Liu Y, Ma Y, Guo N, and Shi M (2015) A Her2-let-7-beta2-AR circuit affects prognosis in patients with Her2-positive breast cancer. BMC Cancer 15, 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sun X, Liu J, Xu C, Tang SC, and Ren H (2016) The insights of let-7 miRNAs in oncogenesis and stem cell potency. J. Cell. Mol. Med 20, 1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shortrede JE, Uzair ID, Neira FJ, Flamini MI, and Sanchez AM (2016) Paxillin, a novel controller in the signaling of estrogen to FAK/N-WASP/Arp2/3 complex in breast cancer cells. Mol. Cell. Endocrinol 430, 56–67. [DOI] [PubMed] [Google Scholar]
- [96].Kuang W, Deng Q, Deng C, Li W, Shu S, and Zhou M (2017) Hepatocyte growth factor induces breast cancer cell invasion via the PI3K/Akt and p38 MAPK signaling pathways to up-regulate the expression of COX2. Am. J. Transl. Res 9, 3816–3826. [PMC free article] [PubMed] [Google Scholar]
- [97].Leung E, Xue A, Wang Y, Rougerie P, Sharma VP, Eddy R, Cox D, and Condeelis J (2017) Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway. Oncogene. 36, 2680–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Christopoulos PF, Msaouel P, and Koutsilieris M (2015) The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 14, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Broughton SE, Hercus TR, Hardy MP, McClure BJ, Nero TL, Dottore M, Huynh H, Braley H, Barry EF, Kan WL, Dhagat U, Scotney P, Hartman D, Busfield SJ, Owczarek CM, Nash AD, Wilson NJ, Parker MW, and Lopez AF (2014) Dual mechanism of interleukin-3 receptor blockade by an anti-cancer antibody. Cell Rep. 8, 410–419. [DOI] [PubMed] [Google Scholar]
- [100].Nicolini A, Ferrari P, Kotlarova L, Rossi G, and Biava PM (2015) The PI3K-AKt-mTOR pathway and new tools to prevent acquired hormone resistance in breast cancer. Curr. Pharm. Biotechnol 16, 804–815 [DOI] [PubMed] [Google Scholar]
- [101].Shin SY, Kim JH, Lee JH, Lim Y, and Lee YH (2012) 2’-Hydroxyflavanone induces apoptosis through Egr-1 involving expression of Bax, p21, and NAG-1 in colon cancer cells. Mol. Nutr. Food Res 56, 761–774. [DOI] [PubMed] [Google Scholar]
- [102].Vainio H and Weiderpass E (2006) Fruit and vegetables in cancer prevention. Nutr. Cancer 54, 111–142. [DOI] [PubMed] [Google Scholar]
- [103].Amin AR, Kucuk O, Khuri FR, and Shin DM (2009) Perspectives for cancer prevention with natural compounds. J. Clin. Oncol 16, 2712–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Singhal J, Chikara S, Horne D, Salgia R, Awasthi S, and Singhal SS (2018) 2’-Hydroxyflavanone inhibits in vitro and in vivo growth of breast cancer cells by targeting RLIP76. Mol. Carcinogenesis (in-press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Files. All other relevant source data are is available from the corresponding authors upon request.


