Abstract
Introduction
Shoulder pathology may be symptomatic or asymptomatic depending on the patient. We report the first case of a COVID-19 vaccination administration precipitating symptomatic calcific tendinitis from pre-existing, asymptomatic calcific tendinitis.
Case presentation
A 50-year-old Thai male began experiencing left shoulder pain about 3 hours following a COVID-19 vaccination. He waited at home for the pain to improve, and when it did not improve in about 3 days he decided to see a doctor at the orthopedics clinic. He was sent for ultrasonography of his shoulder, which revealed calcific tendinitis of the subscapularis tendon.
Discussion
A SIRVA is normally considered if post-vaccination shoulder pain has not improved within a few days following a vaccination in a patient without shoulder pain prior to the vaccination. In our patient, a COVID-19 vaccination precipitated asymptomatic calcific tendinitis to symptomatic calcific tendinitis.
Conclusion
Previously asymptomatic shoulder pathologies can be precipitated to symptomatic by a COVID-19 vaccination.
Keywords: COVID-19, Injection, Pain, Shoulder, Vaccine
Highlights
-
•
A SIRVA is normally considered if post-vaccination shoulder pain has not improved within a few days following a vaccination.
-
•
The COVID-19 vaccination can precipitate the patient's formerly asymptomatic calcific tendinitis to symptomatic calcific tendinitis.
-
•
Before diagnosis SIRVA, the serious condition such as septic arthritis of shoulder should be ruled out.
1. Introduction
A patient may experience side effects after a vaccine administration, either locally and/or systematically. Local symptoms are mainly things such as pain, redness, warmth, swelling, itching and/or bruising, while systematic symptoms involve things such as skin rash, gastrointestinal side effects (nausea, vomiting and/or diarrhea), headache, fever, malaise, chills, and joint or muscle pain [1,2]. If these clinical symptoms continue longer than few days, a shoulder injury related to vaccine administration (SIRVA) should be considered [[3], [4], [5], [6], [7]]. A SIRVA is usually the result of an incorrect vaccine injection technique, usually from one (or more) of three things: using the wrong landmark or incorrect needle direction and/or depth of needle penetration. Prior to this case, only a few SIRVA cases following a COVID-19 vaccination have been reported [[8], [9], [10], [11]], and most of them were traced to an incorrect vaccine administration technique. In this study, we report a case of symptomatic calcific tendinitis precipitated by a COVID-19 vaccination following the SCARE guideline [12].
2. Case presentation
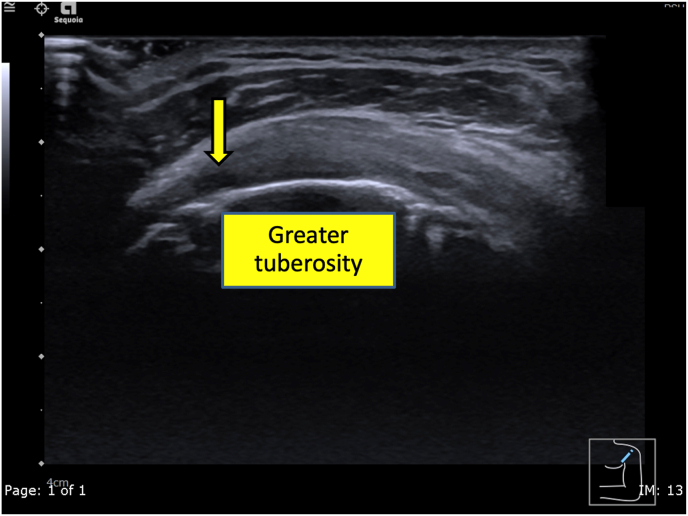
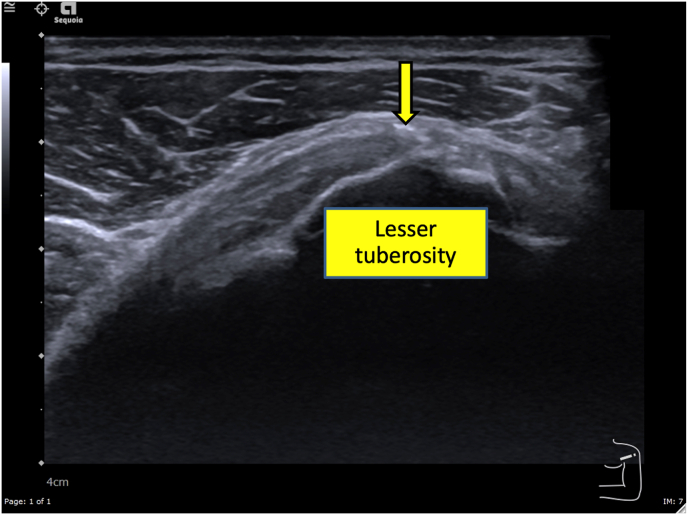
A 50-year-old Thai male without underlying disease, abnormal family history or genetic information, or pre-existing shoulder pain received a 1st dose of the Oxford-AstraZeneca COVID-19 vaccine on 15 June 2021, and a 2nd dose of the same vaccine on 07 September 2021 in the southern part of Thailand. The 2nd dose was given by a practitioner nurse using a 1.5-inch, 25-gauge needle in an injection site based on the landmark of 3 finger breadths below the midlateral border of the acromial process, with the needle direction was perpendicular to the skin at the injection site. Three hours after receiving the second dose, he began to feel moderate shoulder pain when moving the injection shoulder in any direction. The pain persisted, and at 3 days post-injection and he finally decided he should see a doctor. At the orthopedic clinic, a physical examination showed tenderness at the deltoid area and moderate pain in all directions of shoulder motion. Ultrasonography of the left shoulder showed swelling of the supraspinatus tendon (Fig. 1) and calcific tendinitis of the left subscapularis tendon (Fig. 2). He was treated with oral prednisolone (30 mg/day) for 10 days and his pain gradually improved over the next few weeks.
Fig. 1.
An ultrasonographic short axis view of the supraspinatus tendon in the modified Crass position of the patient showing an ill-defined hypoechoic area (yellow arrow) mainly at the anterior fiber of the mildly swollen supraspinatus tendon. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
A transverse ultrasonographic image over the lesser tuberosity of the left shoulder with the patient in the external rotation position showing an area of linear calcification near the footprint of the subscapularis tendon, diagnosed as calcific tendinitis.
3. Discussion
A SIRVA is normally considered if post-vaccination shoulder pain has not improved within a few days following a vaccination in a patient without shoulder pain prior to the vaccination [13,14]. In our patient, a COVID-19 vaccination precipitated asymptomatic calcific tendinitis to symptomatic calcific tendinitis.
Prior to the mass vaccinations beginning in early 2021 to deal with the COVID pandemic, SIRVAs were most common following an influenza vaccination [[3], [4], [5], [6],[13], [14], [15]]. Since the initiation of this mass vaccination program in the world. There were some cases of SIRVA have been reported, all to date blamed on an incorrect landmark and/or needle direction of injection [9,11]. In Thailand the mass vaccination program began in April 2021, and since then we have had three cases of SIRVA in our institution, the largest tertiary care facility in southern Thailand. All of our SIRVAs were traced to incorrect vaccine administration techniques, two from an incorrect needle direction [9,10], and one following an incorrect location due to use of an incorrect vaccination landmark [8].
Calcific tendinitis can be diagnosed by patient history, physical examination, and/or imaging studies. Not all patients with calcific tendinitis have the clinical symptom of shoulder pain, and incidences of asymptomatic calcific tendinitis have been reported from 2.7% to 20% [16]. In our case, the injection technique was found to be correct, so other causes were considered, and following ultrasonography we found a linear calcification near the footprint of the subscapularis tendon, which immediately led to the probable diagnosis of the COVID-19 vaccination had precipitating the patient's formerly asymptomatic calcific tendinitis to symptomatic calcific tendinitis. The main symptom of this patient was also compatible with the diagnosis of calcific tendinitis because the symptoms were a sudden pain and limited range of motion of left shoulder after a COVID-19 vaccine injection [16]. This case is quite similar to an earlier case report of combined subacromial-subdeltoid bursitis and supraspinatus tear after a COVID-19 vaccination, which was determined to have precipitated an existing but asymptomatic rotator cuff tear to a symptomatic rotator cuff tear [17].
4. Conclusions
Previously asymptomatic shoulder pathologies can be precipitated to symptomatic by a covid vaccination.
Ethical approval
The present study was waived by the Prince of Songkla University Institutional Review Board, Faculty of Medicine, Songklanagarind Hospital, Prince of Songkla University (IRB number REC 64-595-11-1).
Sources of funding
No funding was involved regarding this case report.
Author contribution
Chaiwat Chuaychoosakoon —Preparation of case report, Literature review, Writing the paper.
Prapakorn Klabklay —Preparation of case report, Literature review, Writing the paper.
Pathawin Kanyakool —Preparation of case report, Literature review, Writing the paper.
Pattira Boonsri —Preparation of case report, Literature review, Writing the paper.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Registration of research studies
This case report is not first in man.
Guarantor
Chaiwat Chuaychoosakoon, M.D.
Declaration of competing interest
No conflicts of interest.
Acknowledgements
The authors sincerely thank the patient for allowing us to report this case and David Patterson of the International Affairs Office of the Faculty of Medicine, Prince of Songkla University for English proofreading.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103347.
Contributor Information
Prapakorn Klabklay, Email: pglabgly@yahoo.com.
Pattira Boonsri, Email: bpattira@medicine.psu.ac.th.
Pathawin Kanyakool, Email: rak-micky@hotmail.com.
Chaiwat Chuaychoosakoon, Email: psu.chaiwat@gmail.com, chaiwat.c@psu.ac.th.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., Hu C., Selvachandran S., Antonelli M., Murray B., Canas L.S., Molteni E., Graham M.S., Modat M., Joshi A.D., Mangino M., Hammers A., Goodman A.L., Chan A.T., Wolf J., Steves C.J., Valdes A.M., Ourselin S., Spector T.D. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/s1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temiz S.A., Abdelmaksoud A., Dursun R., Durmaz K., Sadoughifar R., Hasan A. Pityriasis rosea following SARS-CoV-2 vaccination: a case series. J. Cosmet. Dermatol. 2021;20:3080–3084. doi: 10.1111/jocd.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins M., Rupp D., Goebel L.J. Post-influenza vaccine subdeltoid bursitis. Cureus. 2020;12:10–13. doi: 10.7759/cureus.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook I.F. Subdeltoid/subacromial bursitis associated with influenza vaccination. Hum. Vaccines Immunother. 2014;10:605–606. doi: 10.4161/hv.27232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright A., Patel R., Motamedi D. Influenza vaccine-related subacromial/subdeltoid bursitis: a case report. J. Radiol. Case Rep. 2019;13:24–31. doi: 10.3941/jrcr.v13i6.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Littrell L.A., Leslie D.F., Bierle D.M., Wenger D.E. Progressive monoarticular inflammatory arthritis following influenza vaccination. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5:204–209. doi: 10.1016/j.mayocpiqo.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szari S., Belgard A., Adams K., Freiler J. Shoulder injury related to vaccine administration: a rare reaction. Fed. Pract. 2019;36:380–384. [PMC free article] [PubMed] [Google Scholar]
- 8.Boonsri P., Chuaychoosakoon C. Combined subacromial-subdeltoid bursitis and supraspinatus tear following a COVID-19 vaccination: a case report. Ann. Med. Surg. 2021;69:102819. doi: 10.1016/j.amsu.2021.102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantarelli Rodrigues T., Hidalgo P.F., Skaf A.Y., Serfaty A. Skeletal Radiol; 2021. Subacromial-subdeltoid Bursitis Following COVID-19 Vaccination: a Case of Shoulder Injury Related to Vaccine Administration (SIRVA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuaychoosakoon C., Parinyakhup W., Tanutit P., Maliwankul K., Klabklay P. Shoulder injury related to Sinovac COVID-19 vaccine: a case report. Ann. Med. Surg. 2021;68:102622. doi: 10.1016/j.amsu.2021.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honarmand A.R., Mackey J., Hayeri R. Shoulder injury related to vaccine administration (SIRVA) following mRNA COVID-19 vaccination: report of 2 cases of subacromial-subdeltoid bursitis. Radiol. Case Reports. 2021;16:3631–3634. doi: 10.1016/j.radcr.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Hesse E.M., Atanasoff S., Hibbs B.F., Adegoke O.J., Ng C., Marquez P., Osborn M., Su J.R., Moro P.L., Shimabukuro T., Nair N. Shoulder injury related to vaccine administration (SIRVA): petitioner claims to the national vaccine injury compensation program, 2010–2016. Vaccine. 2020;38:1076–1083. doi: 10.1016/j.vaccine.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahbaz M., Blanc P.D., Domeracki S.J., Guntur S. Shoulder injury related to vaccine administration (SIRVA): an occupational case report. Workplace Health & Saf. 2019;67:501–505. doi: 10.1177/2165079919875161. [DOI] [PubMed] [Google Scholar]
- 15.Bodor M., Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25:585–587. doi: 10.1016/j.vaccine.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Kim M.-S., Kim I.-W., Lee S., Shin S.-J. Diagnosis and treatment of calcific tendinitis of the shoulder. Clin. Shoulder Elb. 2020;23:210–216. doi: 10.5397/cise.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly P., Macleod I., Macfarlane R., Windley J., Emery R.J.H. Dead men and radiologists don't lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann. R. Coll. Surg. Engl. 2006;88:116–121. doi: 10.1308/003588406X94968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.