Abstract
Astrocytes, a major type of glial cell, are known to play key supportive roles in brain function, contributing to ion and neurotransmitter homeostasis, maintaining the blood-brain barrier and providing trophic and metabolic support for neurons. Besides these support functions, astrocytes are emerging as important elements in brain physiology through signaling exchange with neurons at tripartite synapses. Astrocytes express a wide variety of neurotransmitter transporters and receptors that allow them to sense and respond to synaptic activity. Principal among them are the G-protein-coupled receptors (GPCRs) in astrocytes because their activation by synaptically released neurotransmitters leads to mobilization of intracellular calcium. In turn, activated astrocytes release neuroactive substances called gliotransmitters, such as glutamate, GABA, and ATP/adenosine that lead to synaptic regulation through activation of neuronal GPCRs. In this review we will present and discuss recent evidence demonstrating the critical roles played by GPCRs in the bidirectional astrocyte-neuron signaling, and their crucial involvement in the astrocyte-mediated regulation of synaptic transmission and plasticity.
Introduction
Presynaptic action potentials trigger the release of chemical substances (neurotransmitters) into the synaptic cleft to elicit a multitude of postsynaptic responses that ultimately affect the excitability of the postsynaptic cell. This intercellular signaling that occurs at synapses, originally thought to encompass only presynaptic and postsynaptic neurons, forms the basis of all complex brain functions. However this view upheld until the 1980s was perhaps too simplistic. Now it is clear that glial cells, known as astrocytes, are also active players in synaptic transmission. Astrocytes are as numerous as neurons and have important house-keeping functions such as buffering of excess potassium and neurotransmitters in the extracellular space, providing essential nutrients to neurons and providing structural support around synapses (Sidoryk-Wegrzynowicz et al., 2011; Sofroniew and Vinters, 2010; Vasile et al., 2017). However astrocytes also contain the molecular machinery to respond to neurotransmitters released by neurons and more importantly, to release neuroactive substances termed as gliotransmitters to influence nearby neurons (Araque et al., 2014; Halassa et al., 2007; Perea and Araque, 2010; Perea et al., 2009; Santello et al., 2012; Savtchouk and Volterra, 2018). Such bidirectional communication between astrocytes and neurons was epitomized in the concept of a tripartite synapse in which presynaptic, postsynaptic elements, and intervening astrocyte processes interact and signal to each other (Araque et al., 1999; Halassa et al., 2007; Perea et al., 2009). The tripartite synapse has now been demonstrated in diverse brain regions and in multiple organisms including humans (Allen, 2014, 2019; Guerra-Gomes et al., 2017; Martin-Fernandez et al., 2017; Navarrete et al., 2013; Santello et al., 2019). Central to this view of astrocytes as active contributors in brain information processing are the astrocytic receptors that bind and respond to neurotransmitters and the neuronal receptors that are activated or inhibited by gliotransmitters. These receptors are mainly of the G-protein coupled receptor (GPCR) class which are seven helix transmembrane proteins that initiate intracellular signaling via coupling to G-proteins (Ross, 1989; Vassilatis et al., 2003). In this review we discuss the main types of GPCRs found in astrocytes and neurons that partake in this bidirectional glia-neuronal communication. Our emphasis are on the studies using either in situ or in vivo preparations. Other excellent reviews related to this topic can be found elsewhere (Araque et al., 2014; Papouin et al., 2017; Santello et al., 2012; Savtchouk and Volterra, 2018; Zorec et al., 2012).
1. Neuron to astrocyte communication via GPCRs: general principles
1.1. GPCRs in astrocytes transduce neuronal signals into Ca2+ responses
It has become clear that although astrocytes are electrically unexcitable cells, they are highly responsive to neurotransmitters secreted by neurons. Such responses in astrocytes are manifested by intracellular Ca2+ fluctuations in their processes and soma. Generally, GPCR activation in astrocytes promotes increases of astrocytic Ca2+ levels (Guerra-Gomes et al., 2017; Khakh and McCarthy, 2015; Shigetomi et al., 2016; Zorec et al., 2012) although in some instances Ca2+ decreases have been reported (Jennings et al., 2017; Xin et al., 2019). These astrocytic responses were reported in a wide variety of brain regions both in vitro and in vivo preparations as discussed below. Fluorescent Ca2+ indicators such as fluo-3 or fura-2 were first deployed to monitor astrocytic Ca2+ fluctuations at rest conditions and following GPCR activation (Bernstein et al., 1998; Perea and Araque, 2005; Yagodin et al., 1995). Robust Ca2+ signals evoked by GPCR activation were spatially associated with the astrocytic cell body and sometimes to the thick branches as well. Astrocytic Ca2+ responses is linked to the activation of the phospholipase C (PLC)/inositol 1,4,5-trisphosphate (IP3) pathway as follows: upon GPCR activation, PLC hydrolyzes the membrane lipid phosphatidylinositol 4,5-bisphosphate to generate diacylglycerol (DAG) and IP3, leading to IP3 receptor (IP3R) activation and Ca2+ release from the endoplasmic reticulum (ER) (Agulhon et al., 2008)(Figure 1). Accordingly, the genetic knockout of the primary IP3R in astrocytes, the IP3R2 (Holtzclaw et al., 2002; Petravicz et al., 2008; Sharp et al., 1999), largely ablates Ca2+ responses in astrocytes. However, in situ astrocytes have a highly complex morphology with numerous fine processes which were not technically accessible for Ca2+ imaging in these early studies. In the past few years, remarkable advances in multiphoton microscopy and development of novel genetically encoded Ca2+ indicators (GECI) have finally allowed the crossing of several technical thresholds and begun to generate high-resolution, quantitative and comprehensive data sets Ca2+ signals in astrocytes (Poskanzer and Yuste, 2016; Shigetomi et al., 2013; Stobart et al., 2018; Ye et al., 2017). Indeed, evidence from these studies showed a high level of Ca2+ activity in astrocytic processes. Ca2+ signals in astrocytes in discrete regions of their processes, termed microdomains, are often uncorrelated with events in the soma (Bindocci et al., 2017; Otsu et al., 2015). These results suggest that astrocyte Ca2+ dynamics are highly heterogeneous and more complex than previously assumed and that activity in microdomains may be subject to local independent modulation (Volterra et al., 2014). Moreover, these microdomains spontaneous signals may originated form Ca2+ sources other than by release from IP3R2 as they remain largely unaffected in the IP3R2 knockout mouse (Stobart et al., 2018). Thus, Ca2+ sources in astrocytes may involve other pathways such as mitochondrial release (Agarwal et al., 2017) or opening of TRPC1 channels (Malarkey et al., 2008). Nevertheless, there is broad agreement that GPCR activation leads to an enhanced probability of Ca2+ signals in astrocytes in their microdomains and later, at higher levels of activation, these Ca2+ transients are propagated to the soma (Araque et al., 2014; Volterra et al., 2014).
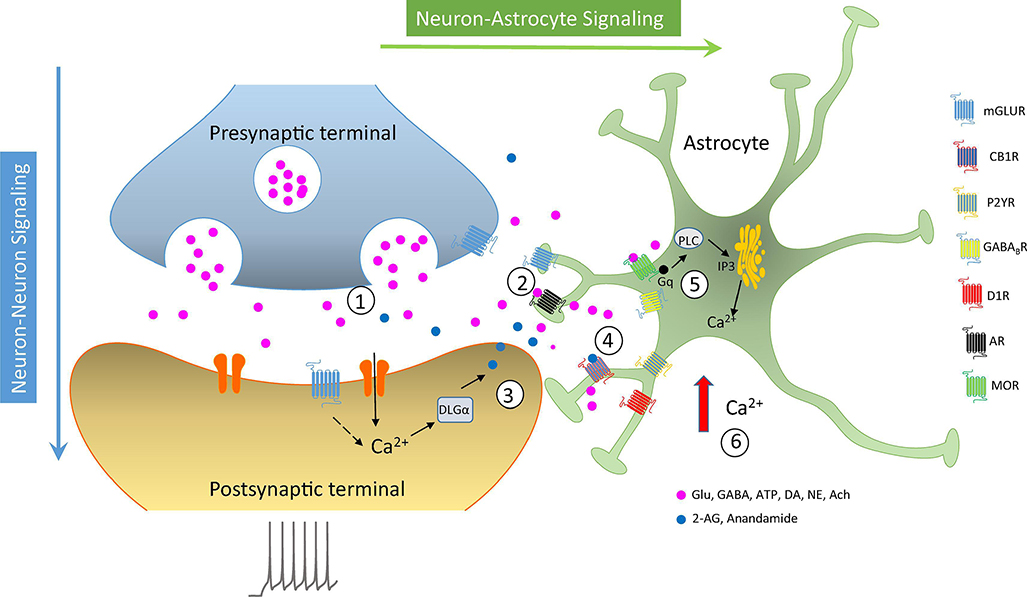
FIGURE 1: Neuron-Astrocyte Signaling at the Tripartite Synapse.
Presynaptic neurons release neurotransmitters (1) that bind to GPCR in postsynaptic cells and astrocytes (2). Activation of GPCRs in astrocytes elicits the activation of PLC cascade (5) resulting in elevation of intracellular Ca2+ (6). Increases in postsynaptic Ca2+ also leads to release of ECBs (3) that activate CBIRs (4). mGluR, metabotropic glutamate receptor; CB1R, cannabinoid receptor type 1; P2YR, purinergic receptor type 2; GABABR, GABA receptor type B; D1R; dopamine receptor type 1; AR, adenosine receptor; MOR, p-type opioid receptor; DLGa, diacylglycerol lipase type a; PLC, phospholipase C.
1.2. GPCRs are efficient signal transducers in astrocytes even at low levels of expression
We can conjecture that GPCRs in astrocytes are particularly well suited for translating neurotransmitter signals to Ca2+ responses for various reasons (Araque et al., 2014). First, the multi-step amplifying signaling cascade afforded by the G-protein signaling (Ross, 1989) allows the detection of neurotransmitter by astrocytes even at very low levels of expression. Hence it is not surprising that expression of various GPCRs in astrocytes is relatively low in comparison to their neuronal counterparts and escaped detection using common immunocytochemical or in situ hybridization techniques. The endocannabinoid receptor CB1R, for instance, are abundantly expressed in neurons while in astrocytes they seem to be expressed in such low levels that its functional significance remained in doubt until recently (Busquets-Garcia et al., 2018; Navarrete and Araque, 2008; Navarrete et al., 2014) Second, in comparison to ionotropic receptors, GPCRs have a much higher affinity for their ligand, favoring astrocytic responses at tripartite synapses. While ionotropic receptors are usually activated in micromolar range, many GPRCs are activated at nanomolar concentrations. The purinergic signaling in astrocytes illustrates this point. Astrocytes in many brain regions are thought to express both ionotropic P2X receptors and metabotropic P2Y receptors (Abbracchio and Ceruti, 2006). However, P2Y receptors are activated at much lower concentrations then P2X receptors (Del Puerto et al., 2013). Therefore, Ca2+ increases in astrocytes elicited by synaptically released ATP is mostly associated with P2Y receptors (Shigetomi et al., 2018). Third, GPCRs in astrocytes may respond to neurotransmitters with extended time course due to their relatively slow desensitization properties. Temporal integration of GPCR responses afforded by slow desensitization again allows receptor signaling even at low levels of expression. This may be the case of mGluR5 in astrocytes to which glutamate signal to intracellular effectors showed little decreased responses even after 15 min agonist exposure (Balazs et al., 1997). Fourth, GPCRs are not focally expressed in astrocytic membranes rather they are expressed broadly, allowing the detection and integration of neurotransmitter signals of multiple synaptic contacts in a single astrocyte. For instance, for GABAB receptors in hippocampal astrocytes, immunoreactivity is found in glial cell bodies and in astrocyte processes surrounding and adjacent to the synapses (Charles et al., 2003). This broad membrane expression of GPCRs in astrocytes allows the efficient temporal integration of GPCR-mediated signals even if these receptors are expressed at relatively low levels (Araque et al., 2014).
1.3. GPCR expression in astrocytes: regional and local heterogeneity
Describing cell types on the basis of their morphology has been a common practice in neurobiology. Astrocytes residing in the gray matter or in the white matter have long been morphologically distinguished as protoplasmic and fibrous astrocytes, respectively (Peters et al., 1976). In addition, some heterogeneity in regard to expression of Glial Acidic Fibrillary Protein (GFAP) or ion channels in astrocytes were noted (Miller, 2018). More recently, with the advent of genetically labeling techniques of astrocytes and molecular profiling, an unexpected level of heterogeneity among astrocytes has been revealed (Chai et al., 2017). RNA-sequencing and proteomics in distinct brain regions portrays a complex picture of local and age-dependent heterogeneity among astrocytes (Chai et al., 2017; Cuevas-Diaz Duran et al., 2019; John Lin et al., 2017; Morel et al., 2017). The implication of such molecular diversity among astrocytes is that astrocyte responses to GPCR activation may differ among brain regions (inter-regional) or even within a brain region (intraregional). This is notably apparent in the dorsal striatum, in which two distinct subpopulations of astrocytes intermingle, each communicating selectively with a distinct subpopulation of medium spinal neurons (D1 or D2 type) (Martin et al., 2015).
1.4. Both inhibitory and excitatory neurotransmitters increase astrocyte Ca2+ activity
Specificity of neurotransmitter actions via GPCRs in neurons relies on activation of unambiguous G-protein pathways (Weis and Kobilka, 2018). G-proteins are heterotrimers (encompassing a&Y subunits) generally classified into four families, Gs, Gi/o, Gq, and G12/13 based on the functional similarity of the Ga subunit (Cabrera-Vera et al., 2003; Ross, 1989; Weis and Kobilka, 2018). In neurons, Ga subunits of Gs and Gq classes in general, have an excitatory action in Ca2+ and electrical activity through activation of adenylyl cyclase and the phospholipase C pathway (Dunlap et al., 1987; Hille, 1992; Huang and Thathiah, 2015). On the other hand, Gi/o pathways in neurons are known to decrease Ca2+ signaling and electrical excitability (Hille, 1992; Huang and Thathiah, 2015). This is exemplified by the multitude of recent studies using DREADDs (designer receptors exclusively activated by designer drugs) in neurons (Roth, 2016). To date, the reported actions on many neuronal types are by either silencing (e.g., Gi-based DREADDs) or activation (e.g., Gq -based DREADDs) of neuronal electrical and Ca2+ activity. However, the opposing actions of Gq and Gi/o pathways and their cognate GPCRs in cellular excitability are less clear in astrocytes. Both Gq and Gi/o coupled receptors when activated by endogenous neurotransmitters seem to evoke increases in Ca2+ activity in astrocytes (Covelo and Araque, 2018; D’Ascenzo et al., 2007; Durkee et al., 2019; Navarrete and Araque, 2008). Moreover both Gq and Gi/o protein-coupled DREADDs promote Ca2+ increases in astrocytes (Durkee et al., 2019). In summary, the dominant effect of GPCR activation via multiple G-protein signaling pathways in astrocytes is elevation of Ca2+ levels.
2. GPCRs in astrocytes activated by neurotransmitters
2.1. Neurotransmitter Adenosine triphosphate (ATP)
Purinergic neurotransmission involving ATP as the signaling molecule is found in many synapses in the central and peripheral nervous systems (Abbracchio et al., 2009). Purinergic signaling are mediated by two families of GPCRs: P1 receptors that bind to adenosine and P2 receptors that are sensitive to ATP and adenosine diphosphate (ADP)(Abbracchio et al., 2009). ATP released as neurotransmitter or neuromodulator by neurons can act directly in astrocytic purinergic receptors as ATP or as a breakdown down product as ADP, AMP, and adenosine (Del Puerto et al., 2013; Fields and Burnstock, 2006). Breakdown of ATP occurs via extracellular ectonucleotidases. A plethora of different subtypes of P1 receptors have been described in astrocytes although A1R an A2ARs have received the most attention (Boison et al., 2010). In regard to P2 receptors in astrocytes, there are reports for both ionotropic P2X and the metabotropic P2Y receptors (Abbracchio and Ceruti, 2006). The presence of multiple purinergic receptors at the same astrocyte may lead to an abundance of effects, the common denominator being an increase in intracellular Ca2+. P2Y receptors which are coupled to Gq proteins, the activation of which stimulates phospholipase C and subsequent release of Ca2+ from intracellular stores and activation of protein kinase C in response to inositol 1,4,5-trisphosphate and diacylglycerol production, respectively (Erb et al., 2006; Erb and Weisman, 2012; Weisman et al., 2012). In the acutely isolated optic nerve, P2X and P2Y agonists raised intracellular astrocyte Ca2+. However, P2Y receptors were activated at nanomolar concentrations, whereas P2X purinoreceptors were only activated above 10 μM (James and Butt, 2001). In hippocampal slices, exogenous application of ATP or electrical stimulation of neuronal afferents lead to intracellular Ca2+ increases of astrocytes mediated by P2Y1 receptors (Bowser and Khakh, 2004; Di Castro et al., 2011; Shigetomi et al., 2008). Astrocytes in the hippocampus via P2Y1 receptors show localized Ca2+ events that are sensitive to spontaneous synaptic events (Di Castro et al., 2011). In the olfactory bulb, purinergic signaling via A2A and P2Y1 receptors in astrocytes evoke Ca2+ responses in astrocytes (Doengi et al., 2008). In the nucleus accumbens, P2Y1 receptor-mediated Ca2+ transients are also present (Molnar et al., 2011). In contrast to the well documented actions of purinergic receptor activation on Ca2+ signaling in astrocytes, the physiological consequences of this signaling remain less clear. The intercellular glia-to-glia signaling over long distances via ATP release to evoke intercellular calcium waves has been observed in retinal explants (Newman, 2001) but it is unclear whether such intercellular Ca2+ waves occur in vivo. Activation of P2Y1 receptors in astrocytes has been linked to actions in either the postsynaptic or presynaptic neuron in the tripartite synapse. For example, P2Y1 receptor activation of astrocytes in the spinal promotes the release of glutamate from astrocytes and subsequent activation of postsynaptic extrasynaptic NMDARs to generate the so called slow inward currents or SICs (Table 1)(Bardoni et al., 2010). SICs, which have been detected in several brain areas, increase neuronal excitability. P2Y1 receptor induced release of glutamate from astrocytes in the hippocampus also have been linked to activation of presynaptic NMDARs to cause an increase if excitatory receptor activity (Santello et al., 2011). Other actions of P2Y1 receptor activation in astrocytes has been the activation of neuronal mGluRs or P2X receptoes to promote increases of neuronal excitability or modulation of synaptic activity (Table 1).
TABLE 1:
GPCR astrocyte to neuronal signaling in diverse brain areas and actions in neurons.
| GPCR in astrocytes | Brain Region | Gliotransmitter | Receptor in the Neuron | Action in the Neuron | Reference |
|---|---|---|---|---|---|
| Alpha-1 Adrenergic | Hypothalamic paraventricular nucleus | ATP | AMPA | Increase of EPSC amplitude | (Gordon, Baimoukhametova et al. 2005) |
| Alpha-1 Adrenergic | Cortex | ATP | ATP | LTP modulation | (Pankratov and Lalo 2015) |
| CB1 | Cortex | ATP and D-serine | P2X | LTP modulation | (Rasooli-Nejad, Palygin et al. 2014) |
| CB1 | Hippocampus | Glutamate | mGlu | Heterosynaptic potentiation | (Navarrete and Araque 2010) |
| CB1 | Hippocampus | Glutamate | NMDA | LTD | (Han, Kesner et al. 2012) |
| CB1 | Cortex | Glutamate | NMDA | LTD | (Min and Nevian 2012) |
| CB1 | Hippocampus | Glutamate | mGluR | Synaptic Potentiation | (Covelo and Araque 2018) |
| CB1 | Striatum | Glutamate | mGLuR1/5 | Heterosynaptic potentiaon | (Martin, Bajo-Graneras et al. 2015) |
| CB1 | Hippocampus | D-serine | NMDA | LTP modulation | (Robin, Oliveira da Cruz et al. 2018) |
| CB1 | Amygdala | ATP | A1 | Synaptic Inhibition | (Martin-Fernandez, Jamison et al. 2017) |
| CB1 | Neocortex | ATP | P2X | LTP modulation | (Rasooli-Nejad, Palygin et al. 2014) |
| D1 | Nucleus Accumbens | Dopamine | ATP/adenosine | EPSC amplitude depression | (Corkrum et al., 2020) |
| GABAB | Hippocampus | Glutamate | AMPA/NMDA | Frequency increase of miniature IPSCs | (Kang, Jiang et al. 1998) |
| GABAB | Hippocampus | Glutamate | mGlu | Transient Heterosynaptic Depression | (Andersson, Blomstrand et al. 2007) |
| GABAB | Medial nucleus of the trapezoid body | D-serine | NMDA | Slow Inward Currents | (Reyes-Haro, Muller et al. 2010) |
| GABAB | Hippocampus | ATP | A1 | Heterosynaptic depression of EPSCs | (Serrano, Haddjeri et al. 2006) |
| GABAB | Striatum | thrombospondin-1 | Enhance synaptic transmission and excitability | (Nagai, Rajbhandari et al. 2019) | |
| GABAB | Hippocampus | ATP | A1 | Enhancement of synaptic inhibition | (Matos, Bosson et al. 2018) |
| GABAB | Hippocampus | ATP | A1 | Synaptic Depression | (Covelo and Araque 2018) |
| GABAB | Hippocampus | Glutamate | mGlu | Synaptic Potentiation | (Perea, Gomez et al. 2016) |
| GABAB | Hippocampus | Glutamate | mGlu | Synaptic Potentiation | (Covelo and Araque 2018) |
| mACh | Hippocampus | Glutamate | NMDA | Slow Inward Currents | (Perea and Araque 2005) |
| mACh | Hippocampus | Glutamate | mGlu | LTP | (Navarrete, Perea et al. 2012) |
| mACh | Cortex | Glutamate | NMDA | Slow Inward Currents | (Chen, Sugihara et al. 2012) |
| mACh | Cortex | D-serine | NMDA | Modulation of LTP | (Takata, Mishima et al. 2011) |
| Group I mGlu | Hippocampus | Glutamate | NMDA | Slow Inward Currents | (Fellin, Pascual et al. 2004) |
| mGlu | Hippocampus | Glutamate | NMDA | Slow Inward Currents | (Sasaki, Kuga et al. 2011) |
| mGlu | Ventro basal thalamus | Glutamate | NMDA | Slow Inward Currents | (Pirttimaki, Hall et al. 2011) |
| mGlu | Hypothalamic paraventricular nucleus | ATP | P2X | Increase of EPSC amplitude | (Gordon, Iremonger et al. 2009) |
| mGlu | Hippocampus and Cortex | Glutamate | NMDA | Slow Inward Currents | (Gomez-Gonzalo, Martin-Fernandez et al. 2017) |
| mGluR2 | Thalamus | Glutamate | mGlu | Synaptic inhibition | (Copeland, Wall et al. 2017) |
| mGluR3 | Hippocampus | eCB | CB1 | Transient Heterosynaptic Depression | (Smith, Bekar et al. 2019) |
| mGluR5 | Cortex | Glutamate | NMDA | Slow Inward Currents | (Ding, Fellin et al. 2007) |
| mGluR5 | Hippocampus | ATP | A2A | Increase basal synaptic transmission | (Panatier, Vallee et al. 2011) |
| mGluR5 | Nucleus Accumbens | Glutamate | NMDA | Slow Inward Currents | (D'Ascenzo, Fellin et al. 2007) |
| mGluR5 and CCK2 | Dorsomedial Hypothalamus | ATP | P2X | LTP modulation of GABA synapses | (Crosby, Murphy-Royal et al. 2018) |
| Mu-opioid | Nucleus Accumbens | Glutamate | NMDA | Slow Inward Currents | (Corkrum, Rothwell et al. 2019) |
| P2Y1 | Cerebellum | Glutamate | NMDA | Activation of inhibitory interneurons | (Rudolph, Jahn et al. 2016) |
| P2Y1 | Hippocampus | Glutamate | NMDA | Frequency increase of miniature PSCs | (Santello, Bezzi et al. 2011) |
| P2Y1 | Hippocampus | Glutamate | NMDA | Frequency increase of spontaneous EPSCs | (Jourdain, Bergersen et al. 2007) |
| P2Y1 | Hippocampus | Glutamate | mGlu | Increase neuronal excitability | (Alvarez-Ferradas, Morales et al. 2015) |
| P2Y1 | Cortex | ATP | P2X | Synaptic modulation | (Lalo, Bogdanov et al. 2019) |
| P2Y | Spinal cord | Glutamate | NMDA | Slow Inward Currents | (Bardoni, Ghirri et al. 2010) |
| PAR1 | Hippocampus | Glutamate | NMDA | Slow Inward Currents | (Shigetomi, Bowser et al. 2008) |
2.2. Neurotransmitter GABA
GABA is the main inhibitory neurotransmitter in the central nervous system and act on ionotropic GABAA receptors and metabotropic GABAB receptors. There has description of expression of both types of GABA receptors in astrocytes (Charles et al., 2003; Fraser et al., 1994). In the ventral tegmental area (VTA) and the ventrobasal (VB) thalamic nucleus, the GABAB selective agonist baclofen elicited intracellular Ca2+ increases in astrocytes (Gould et al., 2014). In hippocampal slices, GABAB receptor-mediated calcium signals in astrocytes were also noted (Andersson et al., 2007; Meier et al., 2008). The mechanism of GABAB-mediated Ca2+ events was shown to involve G-proteins and Ca2+ release from internal stores (Meier et al., 2008). It is unclear, however, which G-protein is responsible for the Ca2+ response, because GABAB receptors are known to be coupled to Gi/o proteins, at least in neurons, while Ca2+ release from internal stores usually requires Gq protein activation (however see above). In hippocampal slices, activation of gabaergic interneurons evoke astrocytic calcium signals which were blocked by the GABAB receptor antagonist CGP55845A (Kang et al., 1998; Perea et al., 2016)(Table 1). Direct stimulation of astrocytes potentiated miniature inhibitory postsynaptic currents (mIPSCs) in pyramidal neurons through activation of kainate receptors in inhibitory terminals (Kang et al., 1998). Interestingly, activation of interneurons and GABAB receptor signaling in astrocytes also can potentiate glutamatergic transmission of pyramidal neurons (Perea et al., 2016). Thus, an inhibitory GABA signal has the potential to become an excitatory signal through astrocyte activation. Other aspects of astrocytic mediated synaptic modulation has also been linked to GABAB receptors in astrocytes. For example, heterosynaptic depression in CA1 hippocampus also may depend on GABAB receptor signaling in astrocytes (Andersson et al., 2007). In the striatum, GABAergic medium spiny neurons trigger astrocytic calcium elevations via GABAB receptors. Chemogenetic of the Gi pathway of striatal astrocytes resulted in acute behavioral hyperactivity and disrupted attention (Nagai et al., 2019). Results in vivo also support the role of GABAB receptors in astrocytes in brain function. Conditional knockout of GABAB receptors in astrocytes induced alterations in hippocampal oscillatory activity in vivo suggesting the direct participation of GABAB receptor signaling of astrocytes in neuronal information processing (Perea et al., 2016).
2.3. Neurotransmitter Endocannabinoids
Endocannabinoids (ECBs) are released from neurons upon depolarization induced Ca2+ influx and serves as a retrograde acting neurotransmitter (Castillo et al., 2012; Kano, 2014; Kano et al., 2009; Ohno-Shosaku et al., 2012). The first conclusive evidence supporting retrograde ECB signaling came from the observation of depolarization-induced suppression of inhibition (DSI)/excitation (DSE)(Castillo et al., 2012; Kano, 2014; Kano et al., 2009; Ohno-Shosaku et al., 2012). Later, it was discovered that the ECB system is involved not only in short-term depression, but also in long-term depression (LTD) at both excitatory and inhibitory synapses (Castillo et al., 2012; Kano, 2014; Kano et al., 2009; Ohno-Shosaku et al., 2012). Since then, the ECB system has become the most-studied retrograde signaling system in the brain. In most cases, ECB-mediated retrograde signaling starts with the production of 2-AG, in response to increased intracellular Ca2+ concentration and/or activated Gq/11-coupled receptors (Kano, 2014; Kano et al., 2009; Ohno-Shosaku et al., 2012). 2-AG is then released into and traverses the extracellular space and arrives at the presynaptic terminal where it binds to the CB1R. Activated CB1R suppresses the release of neurotransmitter in two ways: first, by inhibiting voltage-gated Ca2+ channels, which reduce presynaptic Ca2+ influx; second, by inhibiting adenylyl cyclase (AC) and the subsequent cAMP/PKA pathway, which is involved in LTD (Castillo et al., 2012; Kano et al., 2009). Albeit expressed in low levels in comparison to their neuronal counterparts, the CB1Rs in astrocytes are responsive to ECB signaling (Navarrete and Araque, 2008, 2010; Navarrete et al., 2014). ECBs released from neurons trigger the mobilization of Ca2+ from internal stores in astrocytes. Interestingly, ECB-induced astrocyte Ca2+ elevations are mediated by CB1Rs coupled to Gq/11 proteins that activate phospholipase C and produce inositol triphosphate (Busquets-Garcia et al., 2018). Several forms of synaptic plasticity have been linked with CB1R signaling in astrocytes (Table 1). In the somatosensory cortex, astrocyte CB1 receptors mediates spike-timing-dependent depression in the L4 to L2/3 synapses (Min and Nevian, 2012). In the hippocampus, astrocyte CB1 receptors are necessary for heterosynaptic short-term facilitation of synaptic transmission (Navarrete and Araque, 2010). Longer lasting forms of synaptic plasticity in some conditions can be modulated by astrocyte CB1 receptors (Robin et al., 2018). ECBs in the striatum can induce lateral heterosynaptic Potentiation in a CB1R astrocyte dependent manner (Martin et al., 2015).
2.4. Neurotransmitter Glutamate
Glutamate is the main excitatory neurotransmitter in the CNS and not surprisingly acts on astrocytes in many brain regions. Although few reports have suggested the existence of ionotropic glutamate receptors in astrocytes, it is generally accepted that glutamatergic signaling in these glial cells proceeds mainly via metabotropic glutamate receptors (mGluR). mGluRs are subclassified into three groups based on sequence homology, G-protein coupling, and ligand selectivity. Group I includes mGluRs 1 and 5, Group II includes mGluRs 2 and 3, and Group III includes mGluRs, 4, 6, 7, and 8. mGlul, mGlu3, and mGlu5 receptors are found in astrocytes in different levels depending on the developmental stage and brain region. There is ample evidence supporting the notion that mGluR5 is the most relevant glutamate receptor expressed in astrocytes in culture and in situ. Stimulation of glutamatergic neuronal afferents in hippocampal slices triggers Ca2+ waves in astrocytes inhibited by mGluR5 antagonists. In other brain regions such as the nucleus accumbens and the thalamus, mGluR5s are also critically involved in astrocytic responses to glutamatergic neurotransmission. Moreover, in vivo sensory stimulation also evoke astrocyte Ca2+ responses that are sensitive to mGluR5 antagonists. The expression of mGlu5 in astrocytes is high prenatally and decreases during development (Cai et al., 2000; Sun et al., 2013), when mGluR3 are upregulated, which has suggested that mGluR5 might play a minor role in adult stages (Sun et al.,2013). However, although the relative mGluR5/mGluR3 expression is reduced, mGluR5 receptors still play prominent functional role in astrocytes in adults, as demonstrated of mGluR5-dependent responses in a number of studies in ex vivo and in vivo preparations (Table 1). Moreover, it is clear that many receptors are expressed in astrocytes at low abundance and yet have a prominent role. For example, CBIRs in astrocytes are expressed in seemingly low levels in astrocytes in situ, but they can be detected by electron microscopy (Han et al., 2012) and they can respond to neuronal released ECBs (see above).
2.5. Neurotransmitter Norepinephrine
The neurotransmitter norepinephrine is mainly released from the locus cerueleus (LC) and has broad influence in multiple brain areas. Norepinephrine as other catecholamines are released from neurons from axonal varicosities and therefore act via “volume transmission”. Astrocytes express a1, a2 and β1 adrenergic receptors (Cahoy et al., 2008; Hertz et al., 2010) and are able to respond to norepinephrine released from neurons (Bekar et al., 2008; Ding et al., 2013). In vivo, stimulation of LC neurons trigger transient increases in cortical astrocytic Ca2+, which can be blocked with the non-specific a-adrenergic receptor blocker, phentolamine (Bekar et al., 2008). Moreover, locomotion triggers activation of astrocyte networks in multiple brain regions and this activation is blocked by a -adrenoceptor antagonists (Paukert et al., 2014).
2.6. Neurotransmitter Dopamine
While dopamine action in cultured astrocytes has been largely documented, the functional presence of dopamine receptors in situ has been elusive until recent studies that have shown astrocyte responses to dopamine in several brain areas. dopamine D2R activation of astrocytes has been shown to suppress ap-crystallin mediated neuroinflammation in vivo (Shao et al., 2013) and to decrease intracellular Ca2+ levels in hippocampal (Jennings et al., 2017) and ventral midbrain astrocytes (Xin et al., 2019), whereas D1R activation by exogenous dopamine elevates intracellular Ca2+ in hippocampal astrocytes (Jennings et al., 2017). Moreover, we have recently shown by electron microscopy the presence of D1Rs in astrocytes of the nucleus accumbens, and that their activation in vivo and in situ by synaptically-released dopamine triggers their intracellular Ca2+ elevations through GPCR signaling cascade involving IP3R2 and intracellular Ca2+ mobilization (Corkrum et al., 2020).
2.7. Neurotransmitter Acetylcholine
Acetylcholine (ACh) was the first neurotransmitter identified by Loewi by its actions on the heart (Brown, 2006). Since then, the multiple roles of ACh in synaptic communication have been identified (Picciotto et al., 2012). Cholinergic circuits have been implicated in normal and abnormal cognitive functions and disruption of cholinergic circuitry is likely to be at least partly responsible for the cognitive impairments seen in neurodegenerative disorders (Ballinger et al., 2016; Hampel et al., 2018). While the mechanism by which cholinergic signaling influences cognitive processes has been assumed to be direct cholinergic stimulation of pre- and postsynaptic neuronal receptors, a neglected area of investigation is the role of ACh in astrocytes. Muscarinic and nicotinic receptors have been identified in astrocytes (Guizzetti et al., 2008; Hernandez et al., 2014; Murphy et al., 1986; Van Der Zee et al., 1993). Cholinergic agonists or synaptically released ACh evoke Ca2+ elevations of astrocytes in hippocampal slices (Araque et al., 2002; Perea and Araque, 2005). Pharmacological approaches have revealed that muscarinic receptors mediate such effects (Araque et al., 2002; Perea and Araque, 2005). In vivo cholinergic activity evoked by sensory stimulation or electrical stimulation of the septal nucleus increases Ca2+ in hippocampal astrocytes and induces LTP of CA3-CA1 synapses (Navarrete et al., 2012). Moreover, this cholinergic-induced LTP requires activation of muscarinic receptors in astrocytes, Ca2+ elevations in astrocytes and astrocytic glutamate release (Navarrete et al., 2012). Thus in this form of synaptic plasticity in the hippocampus, astrocytes integrate afferent cholinergic activity via muscarinic receptor activation which then triggers the release of glutamate in the CA3-CA1 synapse to induce LTP (Navarrete et al., 2012). Likewise, in the mouse barrel cortex the plasticity induced by sensory stimulation concomitant with activation of cholinergic afferents from nucleus basalis of Meynert (NBM) is dependent upon muscarinic activation of astrocytes and astrocytic Ca2+ elevations (Takata et al., 2011). Moreover, pairing electrical stimulation of the NBM and visual stimulation potentiated visual responses in excitatory neurons of the primary visual cortex through activation of muscarinic AChRs in cortical astrocytes (Chen et al., 2012). Interestingly, although not the topic of this review, nicotinic receptors expressed in hippocampal astrocytes have been linked to another form of synaptic plasticity also in the hippocampus (Pabst et al., 2016). ACh released by septal projection neurons can activate astrocytes in the hilus not via muscarinic receptors but via Ca2+ -permeable nicotinic receptors. Such astrocytic activation is then followed by excitatory input to GABAergic hilar interneurons, resulting in inhibition of dentate gyrus granule cells (Pabst et al., 2016).
3. GPCRs in neurons activated by gliotransmitters
As described above, a major consequence of the GPCR activation in astrocytes by synaptically- released neurotransmitters is mobilization of the Ca2+ from internal stores. The elevated intracellular Ca2+ is known to stimulate the release of gliotransmitters, such as glutamate, D- serine, ATP, adenosine and GABA (Figure 2). Indeed, while several molecular mechanisms, probably not mutually exclusive, has been proposed to mediate gliotransmitter release (Guerra- Gomes et al., 2017; Hamilton and Attwell, 2010; Rusakov et al., 2014; Shigetomi et al., 2016; Volterra et al., 2014), a considerable amount of evidence indicates that the calcium- and SNARE protein-dependent process is a prominent mechanism underlying gliotransmitter release from astrocytes (Araque et al., 2000; Bezzi et al., 2004; Bohmbach et al., 2018; Perea and Araque, 2005; Schwarz et al., 2017); for a recent review, see (Savtchouk and Volterra, 2018).
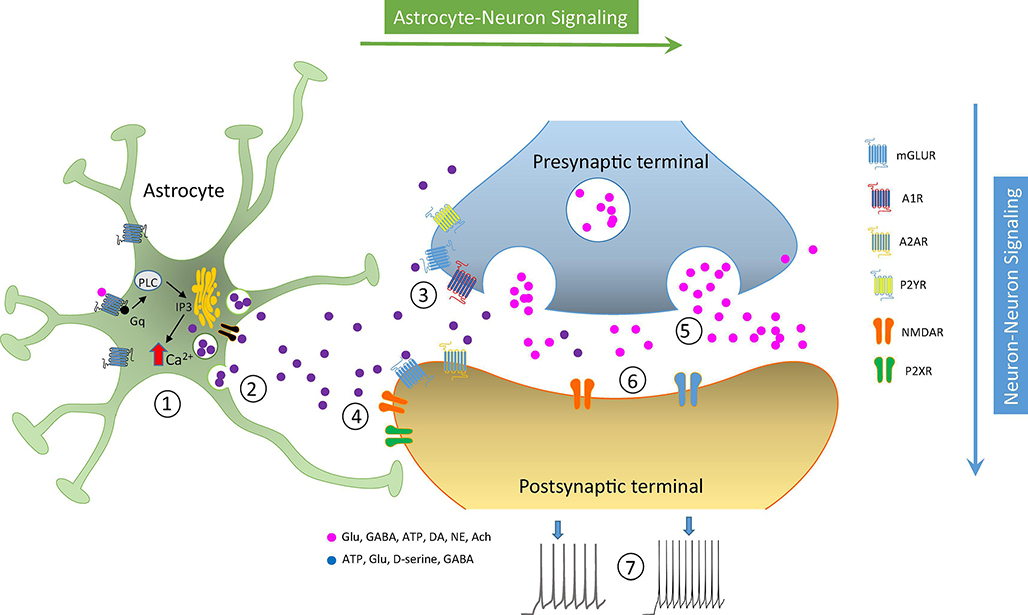
FIGURE 2: Astrocyte-Neuron Signaling at the Tripartite Synapse.
Activation of PLC cascade and resulting intracellular Ca2+ increases (1) promotes release of gliotransmitters (2) that act upon presynaptic (3) or postsynaptic (4) GPCRs or channels. Presynaptic release (5) or postsynaptic excitability are therefore regulated by astrocytic released gliotransmitters. mGluR, metabotropic glutamate receptor; P2YR, purinergic receptor type 2; A1R, adenosine receptor type 1; A2AR, adenosine receptor type 2A; NMDAR, N-methyl-D-aspartate receptor; P2XR, purinergic receptor type P2X; PLC, phospholipase C.
Astrocytes can release a plethora of neuroactive substances that can control different aspects of neuronal development and function, such as prostaglandins (Clasadonte et al., 2011), thrombospondin (Christopherson et al., 2005; Eroglu et al., 2009), or TNFa (Beattie et al., 2002; Stellwagen and Malenka, 2006). In the following paragraphs, we will focus on the regulatory effects on synaptic transmission and plasticity of gliotransmitters that are known to act through activation of GPCRs in neurons. Hence, we will not discuss other important gliotransmitters, like D-serine, which is known to be co-agonist of NMDA receptors, that have relevant effects on synaptic transmission and plasticity (Araque et al., 2014; Oliet and Mothet, 2009).
3.1. Gliotransmitter Glutamate
Glutamate was the first gliotransmitter identified as mediator of astrocyte-neuron signaling. It was originally reported to evoke neuronal calcium increases and slow inward currents (SICs) by activation of neuronal NMDA receptors (Angulo et al., 2004; Araque et al., 1998a; Fellin and Carmignoto, 2004; Parpura et al., 2004; Perea and Araque, 2005). The astrocytic glutamate was also soon reported to regulate synaptic efficacy through activation of neuronal metabotropic glutamate receptors mGluRs (Araque et al., 1998a; Araque et al., 1998b). Later studies in brain slices demonstrated that mGluR-dependent synaptic regulation by astrocytes occurs in different brain areas.
In the hippocampus, activation of type 1 mGluRs present at presynaptic terminals of the Schaffer collaterals by astrocytic glutamate induces a transient short-term enhancement of synaptic efficacy (Covelo and Araque, 2018; Gomez-Gonzalo et al., 2017; Navarrete and Araque, 2010; Perea and Araque, 2007; Perea et al., 2016). In contrast, a heterosynaptic depression in these synapses has been found to be mediated by type 2/3 mGluRs activated by astrocytic glutamate (Andersson et al., 2007). Furthermore, glutamatergic signaling from astrocytes has also been reported to be able to trigger the long-term potentiation or depression of hippocampal synaptic transmission (Adamsky et al., 2018; Gomez-Gonzalo et al., 2017; Han et al., 2012; Min and Nevian, 2012; Navarrete et al., 2012; Perea and Araque, 2007).
Similar mGluR1-dependent transient potentiation of synaptic transmission has been reported in the striatum (Martin et al., 2015). Notably, the astrocyte-induced synaptic regulation in this brain area was found to be synapse-specific, i.e., subpopulations of astrocytes that selectively respond to the activity of medium spiny neurons of the two main corticostriatal circuits —the direct and indirect pathways— specifically regulate corticostriatal synapses belonging to either pathways (Martin et al., 2015). These findings indicate that the gliotransmitter glutamate may trigger a variety of synaptic effects, depending on the specific neuronal GPCRs activated at the Tripartite Synapse.
3.2. Gliotransmitter ATP/adenosine
Purinergic signaling mediated by activation of neuronal GPCRs can also lead to synaptic regulation through several mechanisms and in different brain areas. ATP released from astrocytes is known to be metabolized by extracellular ATPases to produce adenosine, which acting on metabotropic A1 and A2A receptors regulate synaptic transmission. In addition, the direct astrocytic release of adenosine has been found to occur in experimental models of hypoxia to depress synaptic transmission in Schaffer collateral- CA1 hippocampal synapses (Martin et al., 2007). Astrocyte derived adenosine have been shown to activate neuronal presynaptic A1 or A2A receptors to distinctly regulate synaptic transmission, enhancing or depressing synaptic transmitter release, respectively. For example, activation of A1 receptors by astrocytic ATP/adenosine has been shown to depress synaptic transmission in the hippocampus (Chen et al., 2012; Covelo and Araque, 2018; Pascual et al., 2005; Perez- Rodriguez et al., 2018; Serrano et al., 2006; Zhang et al., 2003), cortex (Halassa et al., 2009), cerebellum (Brockhaus and Deitmer, 2002), retina (Newman, 2003), amygdala (Martin- Fernandez et al., 2017), and nucleus accumbens (Corkrum et al., 2019). In contrast, A2A receptor activation has been reported to enhance neurotransmission in the hippocampus (Panatier et al., 2011) and hypothalamus (Gordon et al., 2005; Gordon et al., 2009).
Remarkably, the release of ATP/adenosine from the same astrocyte can differentially affect specific neuronal circuits depending on the presynaptic receptors expressed. Indeed, we have recently shown in the centromedial nucleus of the amygdala that astrocytic ATP/adenosine depresses excitatory neurotransmission in synaptic inputs from the basolateral amygdala through activation of presynaptic A1 receptors, whereas it enhances synaptic transmission of inhibitory inputs from the central lateral amygdala through activation of presynaptic A2A receptors (Martin-Fernandez et al., 2017).
3.3. Gliotransmitter GABA
The activity of GABA transporters in astrocytes controls the extracellular levels of GABA, contributing to the magnitude of GABA tonic currents that may have several physio-pathological consequences (Cope et al., 2009; Shigetomi et al., 2011; Yu et al., 2018). In addition, astrocytes are able to release GABA (Gaidin et al., 2019; Kozlov et al., 2006; Le Meur et al., 2012; Lee et al., 2010), which acting on GPCR GABAB receptors may influence synaptic transmission. The work of Justin Lee’s group has shown novel mechanisms of GABA production and release from astrocytes (Oh and Lee, 2017; Yoon and Lee, 2014), and has reported GABA-mediated synaptic regulation in different brain areas. In the hippocampus, activation of GABAB receptors in GABAergic interneurons by astrocytic GABA leads to the disinhibition of hippocampal excitatory synapses from the perforant pathway into dentate granule neurons (Yarishkin et al., 2015), which has been suggested to have relevant implications in Alzheimer’s disease (Jo et al., 2014). In the cerebellum, astrocytic GABA regulation of parallel fiber-Purkinje cell synapses contributes to motor coordination (Woo et al., 2018).
4. Concluding remarks
In summary, the GPCR-mediated bidirectional communication between astrocytes and neurons presents a high degree of diversity (Durkee and Araque, 2019). Indeed, astrocytes respond with Ca2+ elevations to multiple neurotransmitters (e.g., glutamate, GABA, ACh, norepinephrine, etc.) that activate astrocytic GPCRs, which, in turn, stimulate the release of diverse gliotransmitters (e.g., glutamate, GABA, ATP/adenosine or D-serine). Such a high diversity of signaling grants a plethora of potential effects that may have important functional consequences. However, several questions can be formulated regarding this issue. For example, do different neurotransmitters acting on different GPCRs lead to the release of different gliotransmitters? This is interesting question remains to be investigated. On the other hand, can a single neurotransmitter acting on a specific GPCR stimulate the release of different gliotransmitters? We have recently addressed this question showing that a single hippocampal astrocyte is capable of releasing two gliotransmitters, i.e., glutamate and ATP/adenosine, in response to the stimulation of a single interneuron that signal to astrocytes through activation of astrocytic GABAB receptors (Covelo and Araque, 2018). Furthermore, we have found that the astrocytic glutamate- ad ATP/adenosine-mediated effects on synaptic transmission depended on the duration and frequency of the interneuron stimulation, indicating that astrocytes can decode neuronal inputs and integrate this information into specific gliotransmitter release (Covelo and Araque, 2018). Since the research on the bidirectional communication between astrocytes and neurons is still in its infancy it is likely that future research will provide novel and exciting new findings on the role of GPCR-mediated astrocyte neuron signaling in brain physiology and pathology.
Highlights.
Astrocytes sense synaptic activity through G protein-coupled receptor activation
GPCR activation in astrocytes increases their intracellular calcium
GPCR activation stimulates gliotransmitter release from astrocytes
Gliotransmitters regulate synaptic function by activation of neuronal GPCRs
GPCR-mediated bidirectional communication astrocyte-neuron regulates behavior
Acknowledgments
Work supported by grants from NIH-NINDS (R01NS097312), NIH-NIMH (R01MH119355) and NIH-NIDA (R01DA048822).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbracchio MP, Burnstock G, Verkhratsky A, and Zimmermann H (2009). Purinergic signalling in the nervous system: an overview. Trends Neurosci 32, 19–29. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, and Ceruti S (2006). Roles of P2 receptors in glial cells: focus on astrocytes. Purinergic Signal 2, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, et al. (2018). Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174, 59–71 e14. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, and Bergles DE (2017). Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron 93, 587–605 e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, and McCarthy KD (2008). What is the role of astrocyte calcium in neurophysiology? Neuron 59, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ (2014). Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol 30, 439–463. [DOI] [PubMed] [Google Scholar]
- Allen NJ (2019). Star Power: Astrocytes Regulate Behavior. Cell 177, 1091–1093. [DOI] [PubMed] [Google Scholar]
- Andersson M, Blomstrand F, and Hanse E (2007). Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol 585, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, and Audinat E (2004). Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci 24, 6920–6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, and Volterra A (2014). Gliotransmitters travel in time and space. Neuron 81, 728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, and Haydon PG (2000). SNARE protein-dependent glutamate release from astrocytes. J Neurosci 20, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Martin ED, Perea G, Arellano JI, and Buno W (2002). Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci 22, 2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, and Haydon PG (1998a). Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci 10, 2129–2142. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, and Haydon PG (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22, 208–215. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, and Haydon PG (1998b). Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci 18, 6822–6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs R, Miller S, Romano C, de Vries A, Chun Y, and Cotman CW (1997). Metabotropic glutamate receptor mGluR5 in astrocytes: pharmacological properties and agonist regulation. J Neurochem 69, 151–163. [DOI] [PubMed] [Google Scholar]
- Ballinger EC, Ananth M, Talmage DA, and Role LW (2016). Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 91, 1199–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, and Carmignoto G (2010). Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J Physiol 588, 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, and Malenka RC (2002). Control of synaptic strength by glial TNFalpha. Science 295, 2282–2285. [DOI] [PubMed] [Google Scholar]
- Bekar LK, He W, and Nedergaard M (2008). Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 18, 2789–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M, Behnisch T, Balschun D, Reymann KG, and Reiser G (1998). Pharmacological characterisation of metabotropic glutamatergic and purinergic receptors linked to Ca2+ signalling in hippocampal astrocytes. Neuropharmacology 37, 169–178. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, and Volterra A (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7, 613–620. [DOI] [PubMed] [Google Scholar]
- Bindocci E, Savtchouk I, Liaudet N, Becker D, Carriero G, and Volterra A (2017). Three dimensional Ca(2+) imaging advances understanding of astrocyte biology. Science 356. [DOI] [PubMed] [Google Scholar]
- Bohmbach K, Schwarz MK, Schoch S, and Henneberger C (2018). The structural and functional evidence for vesicular release from astrocytes in situ. Brain Res Bull 136, 65–75. [DOI] [PubMed] [Google Scholar]
- Boison D, Chen JF, and Fredholm BB (2010). Adenosine signaling and function in glial cells. Cell Death Differ 17, 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser DN, and Khakh BS (2004). ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci 24, 8606–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, and Deitmer JW (2002). Long-lasting modulation of synaptic input to Purkinje neurons by Bergmann glia stimulation in rat brain slices. J Physiol 545, 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA (2006). Acetylcholine. Br J Pharmacol 147 Suppl 1, S120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Bains J, and Marsicano G (2018). CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 43, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, and Hamm HE (2003). Insights into G protein structure, function, and regulation. Endocr Rev 24, 765–781. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Schools GP, and Kimelberg HK (2000). Metabotropic glutamate receptors in acutely isolated hippocampal astrocytes: developmental changes of mGluR5 mRNA and functional expression. Glia 29, 70–80. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, and Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron 76, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, et al. (2017). Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95, 531–549 e539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles KJ, Deuchars J, Davies CH, and Pangalos MN (2003). GABA B receptor subunit expression in glia. Mol Cell Neurosci 24, 214–223. [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, and Sur M (2012). Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci U S A 109, E2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, Poulain P, Hanchate NK, Corfas G, Ojeda SR, and Prevot V (2011). Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc Natl Acad Sci U S A 108, 16104–16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, and Crunelli V (2009). Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med 15, 1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, Loke K, Quintana R, Rothwell PE, Lujan R, Marsicano Gv et al. (2020). Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. In press. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrum M, Rothwell PE, Thomas MJ, Kofuji P, and Araque A (2019). Opioid-Mediated Astrocyte-Neuron Signaling in the Nucleus Accumbens. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelo A, and Araque A (2018). Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Diaz Duran R, Wang CY, Zheng H, Deneen B, and Wu JQ (2019). Brain Region-Specific Gene Signatures Revealed by Distinct Astrocyte Subpopulations Unveil Links to Glioma and Neurodegenerative Diseases. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, and Haydon PG (2007). mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A 104, 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Puerto A, Wandosell F, and Garrido JJ (2013). Neuronal and glial purinergic receptors functions in neuron development and brain disease. Front Cell Neurosci 7, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, and Volterra A (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 14, 1276–1284. [DOI] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, and Nedergaard M (2013). alpha1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doengi M, Deitmer JW, and Lohr C (2008). New evidence for purinergic signaling in the olfactory bulb: A2A and P2Y1 receptors mediate intracellular calcium release in astrocytes. FASEB J 22, 2368–2378. [DOI] [PubMed] [Google Scholar]
- Dunlap K, Holz GG, and Rane SG (1987). G proteins as regulators of ion channel function. Trends Neurosci 10, 241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee CA, and Araque A (2019). Diversity and Specificity of Astrocyte-neuron Communication. Neuroscience 396, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee CA, Covelo A, Lines J, Kofuji P, Aguilar J, and Araque A (2019). Gi/o protein coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 67, 1076–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L, Liao Z, Seye CI, and Weisman GA (2006). P2 receptors: intracellular signaling. Pflugers Arch 452, 552–562. [DOI] [PubMed] [Google Scholar]
- Erb L, and Weisman GA (2012). Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal 1, 789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, and Carmignoto G (2004). Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol 559, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, and Burnstock G (2006). Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 7, 423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DD, Mudrick-Donnon LA, and MacVicar BA (1994). Astrocytic GABA receptors. Glia 11, 83–93. [DOI] [PubMed] [Google Scholar]
- Gaidin SG, Zinchenko VP, Sergeev AI, Teplov IY, Mal’tseva VN, and Kosenkov AM (2019). Activation of alpha-2 adrenergic receptors stimulates GABA release by astrocytes. Glia. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Martin-Fernandez M, Martinez-Murillo R, Mederos S, Hernandez- Vivanco A, Jamison S, Fernandez AP, Serrano J, Calero P, Futch HS, et al. (2017). Neuron-astrocyte signaling is preserved in the aging brain. Glia 65, 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, and Bains JS (2005). Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 8, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, and Bains JS (2009). Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 64, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould T, Chen L, Emri Z, Pirttimaki T, Errington AC, Crunelli V, and Parri HR (2014). GABA(B) receptor-mediated activation of astrocytes by gamma-hydroxybutyric acid. Philos Trans R Soc Lond B Biol Sci 369, 20130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Gomes S, Sousa N, Pinto L, and Oliveira JF (2017). Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front Cell Neurosci 11, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, and Costa LG (2008). Modulation of neuritogenesis by astrocyte muscarinic receptors. J Biol Chem 283, 31884–31897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, and Haydon PG (2007). The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13, 54–63. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, and Frank MG (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, and Attwell D (2010). Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11,227–238. [DOI] [PubMed] [Google Scholar]
- Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, and Khachaturian ZS (2018). The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141, 1917–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, et al. (2012). Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Cortez I, Gu Z, Colon-Saez JO, Lamb PW, Wakamiya M, Yakel JL, and Dineley KT (2014). Research tool: Validation of floxed alpha7 nicotinic acetylcholine receptor conditional knockout mice using in vitro and in vivo approaches. J Physiol 592, 3201–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Lovatt D, Goldman SA, and Nedergaard M (2010). Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int 57, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (1992). G protein-coupled mechanisms and nervous signaling. Neuron 9, 187–195. [DOI] [PubMed] [Google Scholar]
- Holtzclaw LA, Pandhit S, Bare DJ, Mignery GA, and Russell JT (2002). Astrocytes in adult rat brain express type 2 inositol 1,4,5-trisphosphate receptors. Glia 39, 69–84. [DOI] [PubMed] [Google Scholar]
- Huang Y, and Thathiah A (2015). Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett 589, 1607–1619. [DOI] [PubMed] [Google Scholar]
- James G, and Butt AM (2001). P2X and P2Y purinoreceptors mediate ATP-evoked calcium signalling in optic nerve glia in situ. Cell Calcium 30, 251–259. [DOI] [PubMed] [Google Scholar]
- Jennings A, Tyurikova O, Bard L, Zheng K, Semyanov A, Henneberger C, and Rusakov DA (2017). Dopamine elevates and lowers astroglial Ca(2+) through distinct pathways depending on local synaptic circuitry. Glia 65, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, et al. (2014). GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med 20, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, et al. (2017). Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, and Nedergaard M (1998). Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1,683–692. [DOI] [PubMed] [Google Scholar]
- Kano M (2014). Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc Jpn Acad Ser B Phys Biol Sci 90, 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, and Watanabe M (2009). Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89, 309–380. [DOI] [PubMed] [Google Scholar]
- Khakh BS, and McCarthy KD (2015). Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol 7, a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AS, Angulo MC, Audinat E, and Charpak S (2006). Target cell-specific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci U S A 103, 10058–10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur K, Mendizabal-Zubiaga J, Grandes P, and Audinat E (2012). GABA release by hippocampal astrocytes. Front Comput Neurosci 6, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, and Lee CJ (2010). Channel-mediated tonic GaBA release from glia. Science 330, 790–796. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, and Parpura V (2008). Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56, 821–835. [DOI] [PubMed] [Google Scholar]
- Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, and Araque A (2017). Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci 20, 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, and Cena V (2007). Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia 55, 36–45. [DOI] [PubMed] [Google Scholar]
- Martin R, Bajo-Graneras R, Moratalla R, Perea G, and Araque A (2015). Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734. [DOI] [PubMed] [Google Scholar]
- Meier SD, Kafitz KW, and Rose CR (2008). Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 56, 1127–1137. [DOI] [PubMed] [Google Scholar]
- Miller SJ (2018). Astrocyte Heterogeneity in the Adult Central Nervous System. Front Cell Neurosci 12, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min R, and Nevian T (2012). Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 15, 746–753. [DOI] [PubMed] [Google Scholar]
- Molnar T, Dobolyi A, Nyitrai G, Barabas P, Heja L, Emri Z, Palkovits M, and Kardos J (2011). Calcium signals in the nucleus accumbens: activation of astrocytes by ATP and succinate. BMC Neurosci 12, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Chiang MSR, Higashimori H, Shoneye T, Iyer LK, Yelick J, Tai A, and Yang Y (2017). Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci 37, 8706–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Pearce B, and Morrow C (1986). Astrocytes have both M1 and M2 muscarinic receptor subtypes. Brain Res 364, 177–180. [DOI] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC, Fanselow MS, and Khakh BS (2019). Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 177, 1280–1292 e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, and Araque A (2008). Endocannabinoids mediate neuron-astrocyte communication. Neuron 57, 883–893. [DOI] [PubMed] [Google Scholar]
- Navarrete M, and Araque A (2010). Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Diez A, and Araque A (2014). Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 369, 20130599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Fernandez de Sevilla D, Gomez-Gonzalo M, Nunez A, Martin ED, and Araque A (2012). Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol 10, e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Maglio L, Pastor J, Garcia de Sola R, and Araque A (2013). Astrocyte calcium signal and gliotransmission in human brain tissue. Cereb Cortex 23, 1240–1246. [DOI] [PubMed] [Google Scholar]
- Newman EA (2001). Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci 21,2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA (2003). Glial cell inhibition of neurons by release of ATP. J Neurosci 23, 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, and Lee CJ (2017). Distribution and Function of the Bestrophin-1 (Best1) Channel in the Brain. Exp Neurobiol 26, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tanimura A, Hashimotodani Y, and Kano M (2012). Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist 18, 119–132. [DOI] [PubMed] [Google Scholar]
- Oliet SH, and Mothet JP (2009). Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience 158, 275–283. [DOI] [PubMed] [Google Scholar]
- Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, and Charpak S (2015). Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 18, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M, Braganza O, Dannenberg H, Hu W, Pothmann L, Rosen J, Mody I, van Loo K, Deisseroth K, Becker AJ, et al. (2016). Astrocyte Intermediaries of Septal Cholinergic Modulation in the Hippocampus. Neuron 90, 853–865. [DOI] [PubMed] [Google Scholar]
- Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, and Robitaille R (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798. [DOI] [PubMed] [Google Scholar]
- Papouin T, Dunphy J, Tolman M, Foley JC, and Haydon PG (2017). Astrocytic control of synaptic function. Philos Trans R Soc Lond B Biol Sci 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Scemes E, and Spray DC (2004). Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int 45, 259–264. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, and Haydon PG (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. [DOI] [PubMed] [Google Scholar]
- Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, and Bergles DE (2014). Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, and Araque A (2005). Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci 25, 2192–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, and Araque A (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317, 1083–1086. [DOI] [PubMed] [Google Scholar]
- Perea G, and Araque A (2010). GLIA modulates synaptic transmission. Brain Res Rev 63, 93–102. [DOI] [PubMed] [Google Scholar]
- Perea G, Gomez R, Mederos S, Covelo A, Ballesteros JJ, Schlosser L, Hernandez- Vivanco A, Martin-Fernandez M, Quintana R, Rayan A, et al. (2016). Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, and Araque A (2009). Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32, 421–431. [DOI] [PubMed] [Google Scholar]
- Perez-Rodriguez M, Arroyo-Garcia LE, Prius-Mengual J, Andrade-Talavera Y, Armengol JA, Perez-Villegas EM, Duque-Feria P, Flores G, and Rodriguez-Moreno A (2018). Adenosine Receptor-Mediated Developmental Loss of Spike Timing-Dependent Depression in the Hippocampus. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, and Webster HF (1976). The Fine Structure of the Nervous System: The Neurons and Supporting Cells (W.B. Saunders; ). [Google Scholar]
- Petravicz J, Fiacco TA, and McCarthy KD (2008). Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 28, 4967–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, and Mineur YS (2012). Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, and Yuste R (2016). Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci U S A 113, E2675–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, Bellocchio L, Soria-Gomez E, Papouin T, Varilh Mv et al. (2018). Astroglial CB1 Receptors Determine Synaptic D-Serine Availability to Enable Recognition Memory. Neuron 98, 935–944 e935. [DOI] [PubMed] [Google Scholar]
- Ross EM (1989). Signal sorting and amplification through G protein-coupled receptors. Neuron 3, 141–152. [DOI] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for Neuroscientists. Neuron 89, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Bard L, Stewart MG, and Henneberger C (2014). Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci 37, 228–242. [DOI] [PubMed] [Google Scholar]
- Santello M, Bezzi P, and Volterra A (2011). TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001. [DOI] [PubMed] [Google Scholar]
- Santello M, Cali C, and Bezzi P (2012). Gliotransmission and the tripartite synapse. Adv Exp Med Biol 970, 307–331. [DOI] [PubMed] [Google Scholar]
- Santello M, Toni N, and Volterra A (2019). Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 22, 154–166. [DOI] [PubMed] [Google Scholar]
- Savtchouk I, and Volterra A (2018). Gliotransmission: Beyond Black-and-White. J Neurosci 38, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Y, Zhao N, Kirchhoff F, and Bruns D (2017). Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways. Nat Neurosci 20, 1529–1539. [DOI] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, and Robitaille R (2006). GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci 26, 5370–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek Ev et al. (2013). Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 494, 90–94. [DOI] [PubMed] [Google Scholar]
- Sharp AH, Nucifora FC Jr., Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, and Ross CA (1999). Differential cellular expression of isoforms of inositol 4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol 406, 207–220. [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, and Khakh BS (2008). Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci 28, 6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, Ellisman MH, and Khakh BS (2013). Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141,633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Hirayama YJ, Ikenaka K, Tanaka KF, and Koizumi S (2018). Role of Purinergic Receptor P2Y1 in Spatiotemporal Ca(2+) Dynamics in Astrocytes. J Neurosci 38, 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Patel S, and Khakh BS (2016). Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol 26, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, and Khakh BS (2011). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, and Aschner M (2011). Role of astrocytes in brain function and disease. Toxicol Pathol 39, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, and Vinters HV (2010). Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, and Malenka RC (2006). Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Ferrari KD, Barrett MJP, Gluck C, Stobart MJ, Zuend M, and Weber B (2018). Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 98, 726–735 e724. [DOI] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, and Nedergaard M (2013). Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, and Hirase H (2011). Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31, 18155–18165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Zee EA, De Jong GI, Strosberg AD, and Luiten PG (1993). Muscarinic acetylcholine receptor-expression in astrocytes in the cortex of young and aged rats. Glia 8, 42–50. [DOI] [PubMed] [Google Scholar]
- Vasile F, Dossi E, and Rouach N (2017). Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct 222, 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, et al. (2003). The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci U S A 100, 4903–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Liaudet N, and Savtchouk I (2014). Astrocyte Ca(2)(+) signalling: an unexpected complexity. Nat Rev Neurosci 15, 327–335. [DOI] [PubMed] [Google Scholar]
- Weis WI, and Kobilka BK (2018). The Molecular Basis of G Protein-Coupled Receptor Activation. Annu Rev Biochem 87, 897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman GA, Woods LT, Erb L, and Seye CI (2012). P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential. CNS Neurol Disord Drug Targets 11, 722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Min JO, Kang DS, Kim YS, Jung GH, Park HJ, Kim S, An H, Kwon J, Kim J, et al. (2018). Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc Natl Acad Sci U S A 115, 5004–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Schuebel KE, Jair KW, Cimbro R, De Biase LM, Goldman D, and Bonci A (2019). Ventral midbrain astrocytes display unique physiological features and sensitivity to dopamine D2 receptor signaling. Neuropsychopharmacology 44, 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagodin S, Holtzclaw LA, and Russell JT (1995). Subcellular calcium oscillators and calcium influx support agonist-induced calcium waves in cultured astrocytes. Mol Cell Biochem 149–150, 137–144. [DOI] [PubMed] [Google Scholar]
- Yarishkin O, Lee J, Jo S, Hwang EM, and Lee CJ (2015). Disinhibitory Action of Astrocytic GABA at the Perforant Path to Dentate Gyrus Granule Neuron Synapse Reverses to Inhibitory in Alzheimer’s Disease Model. Exp Neurobiol 24, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Haroon MA, Salinas A, and Paukert M (2017). Comparison of GCaMP3 and GCaMP6f for studying astrocyte Ca2+ dynamics in the awake mouse brain. PLoS One 12, e0181113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BE, and Lee CJ (2014). GABA as a rising gliotransmitter. Front Neural Circuits 8, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G, and Khakh BS (2018). Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99, 1170–1187 e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, and Duan S (2003). ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. [DOI] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, and Parpura V (2012). Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]