Abstract
Background
Cholestatic liver injury can lead to serious symptoms and prognoses in the clinic. Currently, an effective medical treatment is not available for cholestatic liver injury. Human menstrual blood-derived stem cells (MenSCs) are considered as an emerging treatment in various diseases. This study aimed to explore the treatment effect of MenSCs in cholestatic liver injury.
Methods
The treatment effect of MenSCs on chronic cholestatic liver injury was verified in 3,5-diethoxycarbonyl-1,4-dihydroxychollidine (DDC)-induced C57/BL6 mice. Pathological, fibrosis area in the liver tissue and serum liver enzymes were tested. Proteomics and western blot were used to explore the related targets and molecular mechanisms. Adeno-associated virus (AAV) 9-infected mice were applied for verification.
Results
MenSCs markedly improved the survival rate of the DDC-treated mice (60% vs. 100%), and decreased the mouse serum aspartate aminotransferase (AST) (169.4 vs. 108.0 U/L, p < 0.001), alanine aminotransferase (ALT) (279.0 vs. 228.9 U/L, p < 0.01), alkaline phosphatase (ALP) (45.6 vs. 10.6 U/L, p < 0.0001), direct bilirubin (DBIL) (108.3 vs. 14.0 μmol/L, p < 0.0001) and total bilirubin (TBIL) (179.2 vs. 43.3 μmol/L, p < 0.0001) levels as well as intrahepatic cholestasis, bile duct dilation and fibrotic areas (16.12 vs. 6.57%, p < 0.05). The results further indicated that MenSCs repaired the DDC-induced liver tight junction (TJ) pathway and bile transporter (OATP2, BSEP and NTCP1) injury, thereby inhibiting COL1A1, α-SMA and TGF-β1 activation by upregulating liver β-catenin expression.
Conclusions
MenSC transplantation could be an effective treatment method for cholestatic liver injury in mice. MenSCs may exhibit therapeutic effects by regulating β-catenin expression.
Keywords: Chronic cholestatic liver injury, Mesenchymal stem cell, Tight junction, Bile transporter
Background
Cholestatic liver injury is a rare chronic liver disease characterized by the disruption of bile acid (BA) flow and increased BA concentration in the systemic circulation. Cholestatic liver injury mainly includes primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) [1, 2]. As the disease progresses, nonspecific symptoms, including pruritus, fatigue, clay stool or bleeding episodes, appear [3, 4]. Long-term continuous cholestasis can develop into liver fibrosis and even cirrhosis and eventually lead to death due to liver failure [5, 6]. A variety of factors, such as drugs, oxidative stress, inflammatory injury and immune disorders, are considered as the causes of cholestasis [7]. Excessive accumulation of bile components, including bile acid, cholesterol and bilirubin, in hepatic and systemic circulation is considered the major driver of liver injury. Currently, adults with different phenotypes of cholestasis have increasingly been evaluated for variants in these genes to identify specific cholestasis-related genes [8, 9].
Treatments for cholestatic liver injury include ursodeoxycholic acid (UDCA) and obeticholic acid (OCA), the only two approved drugs, as well as symptomatic treatments and liver transplantation. Long-term application of UDCA can improve the serum biochemistry and delay the progression of the histological stage. However, approximately 25%–40% of patients do not respond to UDCA [10, 11]. OCA is applied to patients who are insensitive to UDCA, although its administration is associated with several side effects [12, 13]. Additionally, some researchers have indicated that the application of OCA might be associated with an increased risk of liver failure in patients with PBC [14]. PBC and PSC represent major indications for liver transplantation (LT). However, there is a contradiction between the urgent clinical needs and donor liver shortage. In addition, numerous studies have clearly demonstrated that PBC and PSC recur after LT [15]. Therefore, new effective methods for treating cholestatic liver injury are necessary.
Mesenchymal stem cells (MSCs) are novel adult stem cells isolated from various tissues. MSCs can be used in various clinical applications, especially stem cell-based therapies [16]. MenSCs are derived from women’s menstrual blood [17]. Unlike MSCs obtained from other adult tissues such as bone marrow, amniotic fluid, and adipose tissue, MenSCs can be derived through a simple, safe, noninvasive procedure with fewer ethical problems. Compared with MSCs derived from adult bone marrow tissue, MenSCs show a higher proliferation rate [18], indicating that MenSCs may have broader clinical application prospects in the future. Investigators have suggested the effect of MSCs in promoting liver tissue repair and survival rates in acute liver failure, hepatectomy, hepatitis B virus-related acute-on-chronic liver failure, ischemic-type biliary lesions, liver fibrosis, liver transplantation and related graft-versus-host disease [19–26]. Although rash and fever (37–38 °C) that resolved without additional treatment were observed in several patients [27], no MSC transplantation-related safety issues were detected in either short- or long-term follow-up [28, 29]. All these previous studies indicate that MSC transplantation is an ideal candidate for cholestatic treatment. However, the study of the treatment efficacy of MSCs in cholestatic liver injury is limited. It is still unclear whether MSCs can be used as a treatment method and further improve the poor curative effect of drug treatment in patients with cholestasis. To solve the problem, relevant preclinical research and mechanism exploration are necessary.
This study aimed to evaluate the efficacy of MenSC transplantation in mice with DDC-induced cholestatic liver injury. The related molecular mechanism of MenSC treatment in the DDC-treated mice was further elucidated.
Materials and methods
Animals
Six- to eight-week-old male C57BL/6 mice were purchased from the Experimental Animal Center of Zhejiang Academy of Medical Sciences and housed under standard conditions.
Cell culture
MenSCs were cultured in DMEM/F12 (Gibco, Waltham, MA, USA) containing 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin (Gibco).
DDC-induced sclerosing cholangitis and MenSC transplantation
C57BL/6 mice were randomly divided into different groups and fed either a control diet (control group) or a diet containing DDC (0.1%).
To evaluate the treatment effect of MenSCs in sclerosing cholangitis, 5 × 105 cells in 500 μL of PBS were injected into mice through the tail vein in the 2nd and 4th weeks of DDC treatment. The mice received MenSC transplantation were considered as the DDC + MenSC group. DDC-fed mice injected with equal amounts of PBS were considered as the DDC group.
After 5 weeks of DDC feeding, mice were anesthetized with 1% pentobarbital sodium and sacrificed. Mouse hepatic tissues were collected immediately. Liver tissues were either fixed in paraffin or stored with frozen liquid nitrogen for further analysis. Peripheral blood was collected and centrifuged to obtain serum separation. The serum samples were stored at − 80 °C.
Surface markers and differentiation of MenSCs
The expression levels of MenSC surface markers were detected by fluorescence-activated cell sorting (FACS). The collected MenSCs (2 × 107) were washed with staining buffer (Becton Dickinson, Biosciences, San Jose, USA) and incubated for 1 h in diluted antibodies, including CD29, CD34, CD45, CD73, CD90, CD105, CD117 and human leukocyte antigen-DR (HLA-DR) (Becton Dickinson, Franklin Lakes, NJ, USA). Isotype antibodies IgG1 and IgG2a (Becton Dickinson) were applied as negative controls. An FC500 flow cytometer (Beckman Coulter, Pasadena, USA) and FlowJo software (Tree Star, Inc., Ashland, OR, USA) were applied for analysis.
A human mesenchymal stem cell osteogenic differentiation medium kit, chondrogenic differentiation medium kit, and adipogenic differentiation medium kit (Cyagen Biosciences, USA) were used to detect the differentiation potential of MenSCs.
In vivo tracking of MenSCs
XenoLight DiR (Perkin Elmer, Waltham, MA, USA) was applied to track MenSC migration in vivo. XenoLight DiR solution was diluted and incubated with MenSCs at room temperature for 20 min. Then MenSCs were washed and resuspended in PBS. The resuspended sample was then injected into the control and DDC-treated mice through the tail veins. IVIS analysis (Caliper Life Sciences, Hopkinton, MA, USA) was used after 1 d, 3 d and 7 d.
Liver function tests
Mouse hepatic function was evaluated by serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), direct bilirubin (DBIL) and total bilirubin (TBIL) levels, which were tested using commercial detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The measurement of AST, ALT, ALP, DBIL and TBIL was conducted following the instruction of the manufacturer.
Histological analysis and transmission electron microscopy
For histological analysis, liver tissues fixed in paraffin were embedded in paraffin and sectioned using a MicromHM325 rotary microtome (Thermo Fisher Scientific Life Sciences, USA) for H&E, Sirius Red and Masson staining. The fibrotic area (COL1A1/total area) was quantified using ImageJ software.
For transmission electron microscopy, liver tissues were fixed in 3% glutaraldehyde and rinsed in PBS before being placed in the secondary fixative 1% osmium tetroxide solution for 2 h. Then, the samples were rinsed 3 times in PBS and dehydrated in ethanol by density gradient. The samples were embedded in embedding agent and sectioned at 70 nm thickness. The samples were compared with uranyl acetate and lead citrate, and then examined with transmission electron microscopy (TEM).
Immunohistochemistry
Paraffin hepatic tissues were used. Samples were deparaffinized and hydrated in ethanol. Hydrogen peroxide (3%) was applied to block endogenous peroxidase. The samples were incubated with COL1A1 (1:1000; Cell Signaling Technology, 72026S) and α-smooth muscle actin (α-SMA) (1:1000; Cell Signaling Technology, 19245) antibodies at 4 °C for 10 h. Then, the samples were washed and incubated with secondary antibody (Abcam, Cambridge, United Kingdom) at 37 °C for 1 h. The samples were stained using 3,3′-diaminobenzidine solution (DAB kit, Abcam) and scanned with a NanoZoomer Digital Pathology system.
Western blot analysis
Hepatic sample lysates were extracted using RIPA buffer supplemented with cocktail protease and phosphatase inhibitor (Beyotime, Shanghai, China). The BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL) was applied to detect protein concentrations. Equal amounts of total protein were transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA) after separation on 4–20% SDS–polyacrylamide electrophoresis gels (GenScript, Nanjing, China). Transferred membranes were blocked in diluted QuickBlock buffer (Beyotime) for 30 min and then incubated at 4 °C for 10 h in the following primary antibodies: β-catenin (1:1000; Cell Signaling Technology, MA, USA, 8480S), Claudin 1 (1:1000; Proteintech Group, Inc., China, 13050-1-AP), Claudin 3 (1:500; Affinity Biosciences, Cincinnati, OH, USA, AF0129), Claudin 5 (1:1000; Affinity Biosciences, AF5216), Claudin 7 (1:1000; Proteintech, 10118-1-AP), NTCP1 (1:1000; Abcam, Cambridge, UK, ab131084), BSEP (1:100; Santa Cruz, sc-74500), OATP2 (1:100; Santa Cruz Biotech, CA, USA, sc-376424), Occludin (1:1000; Cell Signaling Technology, 91131), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:1000; Cell Signaling Technology, 5174). Then, the membranes were incubated in secondary antibodies (1:2000; Cell Signaling Technology) and detected by chemiluminescence reagents (Beyotime). ImageJ software was applied to semiquantify and analyze protein band intensities.
Protein extraction and digestion
The liver samples were separately treated with liquid nitrogen and suspended in lysis buffer [8 M urea, 50 mM Tris 8.0, 1% NP40, 1% NaDOC (sodium deoxycholate), 5 mM dithiothreitol, 2 mM EDTA, 30 mM nicotinamide, 3 µm trichostatin A, 1% cocktail protease (Sigma, P8215, for use with fungal extracts) and 1% phosphatase inhibitor cocktail (Solarbio, P1260)]. The samples were sonicated and centrifuged at 4 °C for 10 min at 20,000 g to remove tissue fragments. The protein content was detected using a 2D Quant kit (GE Healthcare Life Sciences, PA, USA). Equal amounts of protein were reduced with 5 mM dithiothreitol for 45 min at 30 °C, alkylated with 30 mM iodoacetamide for 1 h in the dark at room temperature, and then precipitated with acetone on ice. The precipitate was washed with acetone and suspended in 0.1 M triethylammonium bicarbonate (TEAB) and digested with trypsin (Promega, Madison, WI) overnight at 37 °C. Digestion was stopped with 1% trifluoroacetic acid. The peptide obtained was cleaned using a Strata X C18 SPE column (Phenomenex, Torrance, CA, USA) and vacuum-dried in a Scan-Vac maxi-beta (Labogene, Alleroed, Denmark).
TMT labeling and HPLC fractionation
The peptide samples were reconstituted in 120 μL of 0.5 M TEAB and treated using a TMTsixplex label reagent kit (Pierce, Thermo Fisher Scientific, Rockford, IL, 90068). One unit of TMT reagent (5 mg) was thawed and reconstituted in 420 μL of acetonitrile. Subsequently, four reconstituted samples were mixed separately with four TMT reagents (126, 127, 128, and 129), incubated for 2 h at room temperature and then vacuum-dried. Finally, the labeled samples were resuspended in water and mixed. The labeled peptide samples were fractionated for proteome analysis. Fractionation was performed with an XBridge Shield C18 RP column (Waters, Milford, MA, USA) in an LC20AD HPLC system (Shimadzu, Kyoto, Japan).
Construction and infection with recombinant AAV-Ctnnb1-shRNA
To deplete β-catenin expression in liver tissue, AAV9 that contained Ctnnb1 shRNA (shCtnnb1) and AAV-shControl was constructed by Genomeditech (Shanghai, China). Mice were injected via the tail vein with 150 μL of PBS containing 3 × 1011 VG of the AAV-shCtnnb1 or AAV-shControl. At 2 d after virus injection, the mice were fed a DDC or control diet for up to 5 weeks.
Statistical analysis
All data are reported as the mean ± standard deviation (SD). Student’s t test and one-way analysis of variance were used for comparisons of liver function, relative protein expression and fibrotic area among the groups. Graphs were generated using GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA). p values of less than 0.05 were considered statistically significant.
Results
Characterization and tracking of MenSCs
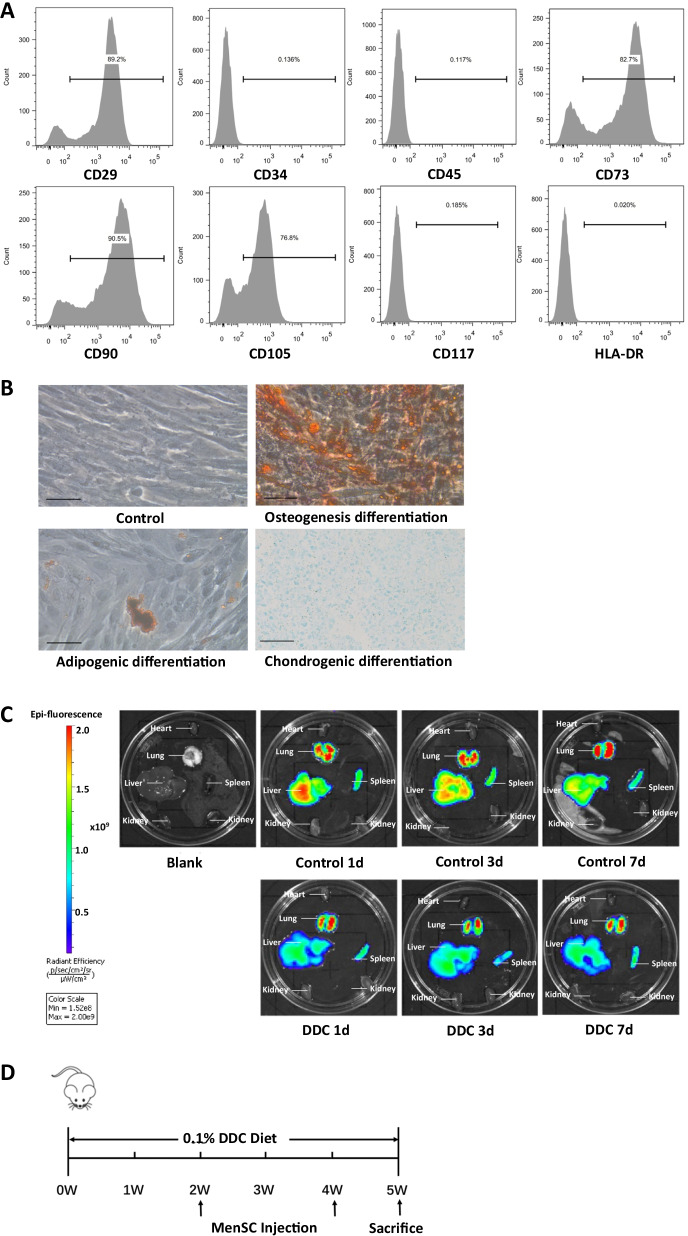
Flow cytometry was applied to identify the immunophenotype of MenSCs. The results showed positive expression of CD29, CD73, CD90 and CD105 and negative expression of CD34, CD45, CD117 and HLA-DR (Fig. 1A). MenSCs cultured in vitro were spindle-shaped and could be induced to differentiate into osteoblasts, adipocytes, and chondrocytes (Fig. 1B).
Fig. 1.
Characterization and tracking of MenSCs. A Surface markers of MenSCs were determined using flow cytometer. B Cultured MenSCs and differential potential in vitro, Alizarin red staining of osteogenesis differentiation, Oil Red O staining of adipogenic differentiation, Alcian blue staining of chondrogenic differentiation. Scale bar: 50 μm. C Analysis of DiR-labeled MenSCs after systemic administration. D Schedule of DDC feeding and MenSC transplantation
To determine the distribution and duration of MenSCs in mice, MenSCs were labeled with XenoLight DiR. Labeled MenSCs were injected into the tail veins of the control and DDC-induced mice, and their distribution was evaluated by in vivo imaging on 1d, 3d and 7d. Fluorescence was detected in lungs, liver and spleen but not in the heart or kidneys (Fig. 1C). The fluorescence could last in vivo for up to 7 days. The schedule of DDC feeding and MenSC transplantation is described above (Fig. 1D).
MenSCs reduced symptoms caused by DDC in mice
Compared with the control mice, the DDC-induced mice showed obvious weight loss, which could be significantly reduced by MenSC transplantation (Fig. 2A, C). Grossly, the livers of the DDC-treated mice were dark red, smaller and stiffer and the gallbladders were enlarged compared with those of the control mice, while MenSC treatment improved these symptoms (Fig. 2B). The survival study showed that 6 mice survived 9 weeks after modeling (6/10, 60%). MenSC transplantation improved the survival rate up to (10/10, 100%) (Fig. 2D). To evaluate liver functions, the AST, ALT, ALP, DBIL and TBIL levels in mouse serum were tested. The results showed that MenSCs could significantly reduce the levels of AST (169.4 vs. 108.0 U/L, p < 0.001), ALT (279.0 vs. 228.9 U/L, p < 0.01), ALP (45.6 vs. 10.6 U/L, p < 0.0001), DBIL (108.3 vs. 14.0 μmol/L, p < 0.0001) and TBIL (179.2 vs. 43.3 μmol/L, p < 0.0001), indicating that MenSC transplantation could restore DDC-induced mouse liver damage and liver damage-induced jaundice (Fig. 2E).
Fig. 2.
MenSCs reduced symptoms caused by DDC in mice. A Representative pictures of the mice in different groups. B The livers and gallbladder of mice in different groups. C The body weight change of mice in different groups (n = 7 for each group). D The survival rate of mice at 9 weeks (n = 10 for each group). E Serum AST, ALT, ALP, DBIL and TBIL levels in different groups (n = 7 for each group). **p < 0.01, ***p < 0.001, ****p < 0.0001
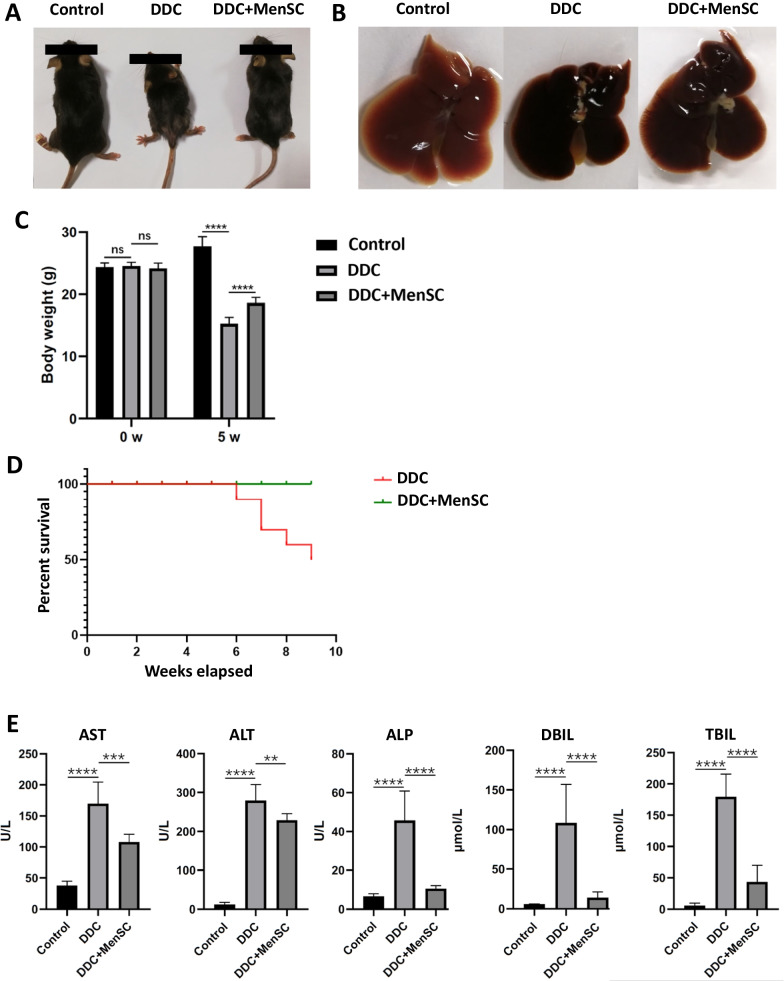
MenSC transplantation improved DDC-induced pathological changes in mouse livers
Histological assessment using H&E staining of hepatic tissues revealed periportal ductular reactions, including intrahepatic bile duct dilation and cholestasis, as well as inflammatory cell infiltration in the livers of the DDC group, and these symptoms were significantly relieved in the MenSC group (Fig. 3A). Masson and Sirius Red staining showed fibrotic changes in the hepatic sections of the DDC-treated mice (Fig. 3A). The collagen area percentage in the DDC + MenSC group (6.57%) was significantly reduced compared with the DDC group (16.12%) (p < 0.05). Additionally, the expression of α-SMA and COL1A1, markers of hepatic fibrosis, was measured by immunohistochemistry (Fig. 3B). The results indicated that α-SMA and COL1A1 expression was increased in the DDC-treated mice and significantly reduced after MenSC transplantation (p < 0.01) (Fig. 3D, E). Transmission electron microscopy of the control liver sections revealed a TJ structure, which is a thin double-stranded electron-dense structure of defined diameter (Fig. 3F). The TJ structure in the DDC livers showed less electron-dense and poorly defined margins and was partly restored in the MenSC group.
Fig. 3.
MenSC transplantation improved DDC-induced pathological changes in mouse livers. A H&E, Masson and Sirius Red staining of liver sections (n = 3 for each group). Scale bar: 250 μm. B Representative immunohistochemistry images for α-SMA and COL1A1 (n = 3 for each group). Scale bar: 250 μm. C Fibrosis area analysis measured from Masson and Sirius Red staining liver sections. D, E Semi-quantitative analysis of the expression of α-SMA and COL1A1 in liver. F Transmission electron microscopy of TJ in different groups. Scale bar: 0.5 μm for 25,000× and 200 nm for 50,000×. *p < 0.05, **p < 0.01, ***p < 0.001
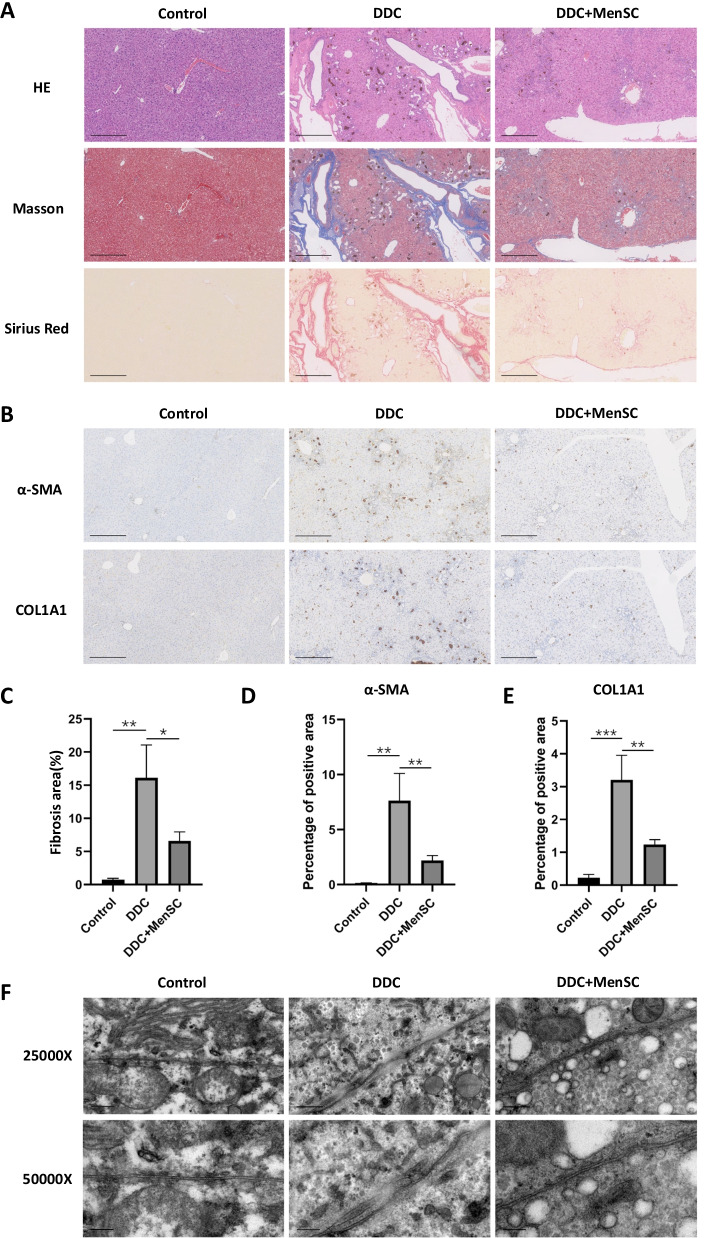
MenSC transplantation altered the liver proteomics profile of the DDC-fed mice
To further explore the molecular mechanism of the treatment efficacy of MenSC transplantation in DDC-induced cholestatic liver injury, a proteomics analysis was performed. Principal component analysis (PCA) and orthogonal partial least-squares-discriminant analysis (OPLS-DA) were used to visualize the proteomic differences in different groups. The PCA score plot revealed no outliers (Fig. 4A). Additionally, no discernible clustering was observed between the DDC and DDC + MenSC groups. The PCA score plot was characterized by the following parameters: R2X = 0.896, Q2 = 0.842. The DDC and DDC + MenSC groups were clearly distinguished in the OPLS-DA score plot (Fig. 4B, C). After analyzing the differentially expressed proteins with Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Ontology (GO) and Gene Set Enrichment Analysis (GSEA), the TJ signaling pathway attracted our attention (Fig. 4D, E).
Fig. 4.
MenSC transplantation altered the liver proteomics profile of the DDC-fed mice. A Model of a PCA plot comparing between DDC and DDC + MenSC groups (n = 6 for each group). B OPLS-DA score scatter plot comparing the DDC and DDC + MenSC groups. Each symbol represents one liver sample (n = 6 for each group). C Permutation test of the OPLS-DA model. D, E KEGG, GO and GSEA pathway enrichment analysis
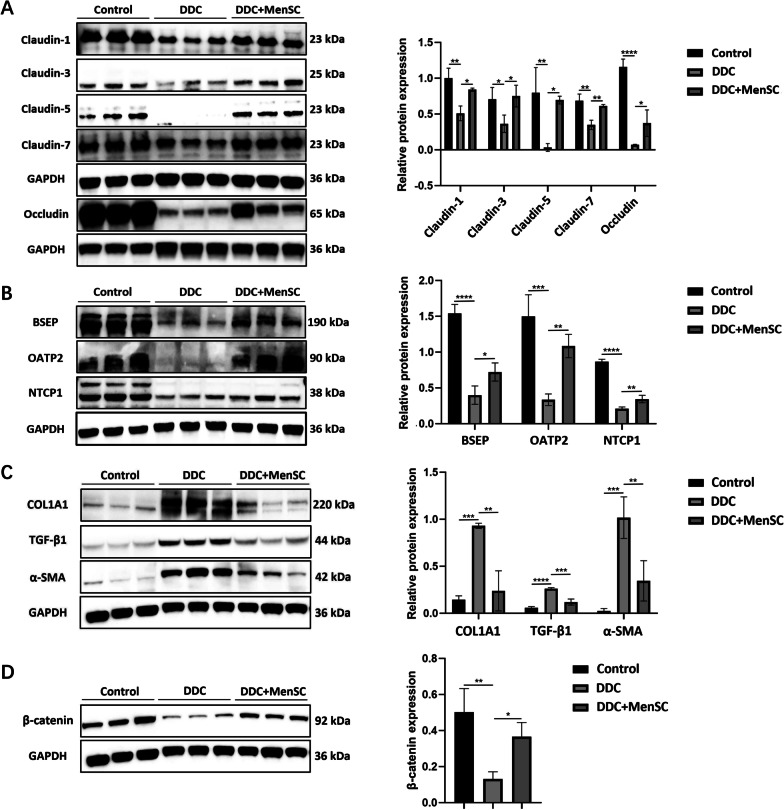
MenSCs promoted TJ- and bile transport function-related protein expression and reduced fibrosis-related protein expression
To verify our hypothesis, western blot was applied to detect the expression of related proteins. The results indicated that Claudin-1, Claudin-3, Claudin-5, Claudin-7 and Occludin levels were reduced after DDC feeding but restored by MenSC transplantation (Fig. 5A). Considering the significant efficiency of MenSC transplantation in DDC-induced hyperbilirubinemia in mice, the transport function-related proteins BSEP, OATP2 and NTCP1 were also analyzed by western blot. The results indicated that compared with those of the control mice, the BSEP, OATP2 and NTCP1 levels were decreased in the DDC group and restored in the DDC + MenSC group (Fig. 5B). Next, the expression of COL1A1, TGF-β1 and α-SMA, which are proteins related to the fibrosis pathway, was detected. According to the results, COL1A1, TGF-β1 and α-SMA levels were increased in the DDC group but decreased in the DDC + MenSC group (Fig. 5C). It was previously reported that liver-specific β-catenin knockout mice had intrahepatic cholestasis, which resembled DDC-induced symptoms. Thus, the β-catenin levels in liver tissues were measured. β-catenin was significantly inhibited in DDC group and upregulated in DDC + MenSC group (Fig. 5D).
Fig. 5.
MenSCs promoted TJ- and bile transport function-related protein expression and reduced fibrosis-related protein expression. A Western blot analysis protein levels of Claudin-1, Claudin-3, Claudin-5, Claudin-7 and Occludin in liver tissue of different groups (n = 3 for each group). B BSEP, OATP2 and NTCP1 expression in liver tissues of different groups (n = 3 for each group). C, D Liver COL1A1, α-SMA, TGF-β1 and β-catenin expression among different groups (n = 3 for each group). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Liver β-catenin deficiency inhibited the therapeutic efficacy of MenSC transplantation in DDC-induced models
To verify this hypothesis, β-catenin knockdown mice were used. Mice were infected with AAV-shControl or AAV-shCtnnb1. Green fluorescence could be detected in the liver tissues of the AAV-infected mice compared with the liver tissues of normal mice (Fig. 6A). To evaluate the β-catenin knockdown efficiency, the liver β-catenin expression levels were detected by western blot (0.11-fold, p < 0.05). Compared with the AAV-shControl mice, the AAV-shCtnnb1 mice showed lower liver β-catenin levels (Fig. 6B). Liver function tests showed that MenSC transplantation failed to downregulate serum AST, ALT, ALP, DBIL and TBIL levels (Fig. 6C). According to the H&E staining of the liver tissues, MenSCs did not significantly improve DDC-induced intrahepatic cholestasis or bile duct dilation in the AAV-shCtnnb1 mice (Fig. 6D). Additionally, MenSC failed to reduce the fibrotic area in the DDC-fed AAV-shCtnnb1 mice (Fig. 6E, F). The results indicated that liver β-catenin deficiency could strongly inhibit the treatment efficacy of MenSC transplantation in DDC-induced liver injury.
Fig. 6.
Liver β-catenin deficiency inhibited the therapeutic efficacy of MenSC transplantation in DDC-induced models. A Representative images of sections from the control and AAV-infected livers. Scale bar, 50 μm. B Expression levels of β-catenin in liver tissues of AAV-shControl and AAV-shCtnnb1 mice (n = 3 for each group). C Serum AST, ALT, ALP, DBIL and TBIL levels of AAV-shControl and AAV-shCtnnb1 mice in different groups (n = 6 for each group). D, E Representative images of H&E and Masson staining of liver tissues from AAV-shControl and AAV-shCtnnb1 mice in different groups. Scale bar, 250 μm. F Fibrosis area analysis (n = 5 for each group). *p < 0.05, **p < 0.01, ****p < 0.0001 and n.s. indicates no significance between the two indicated groups
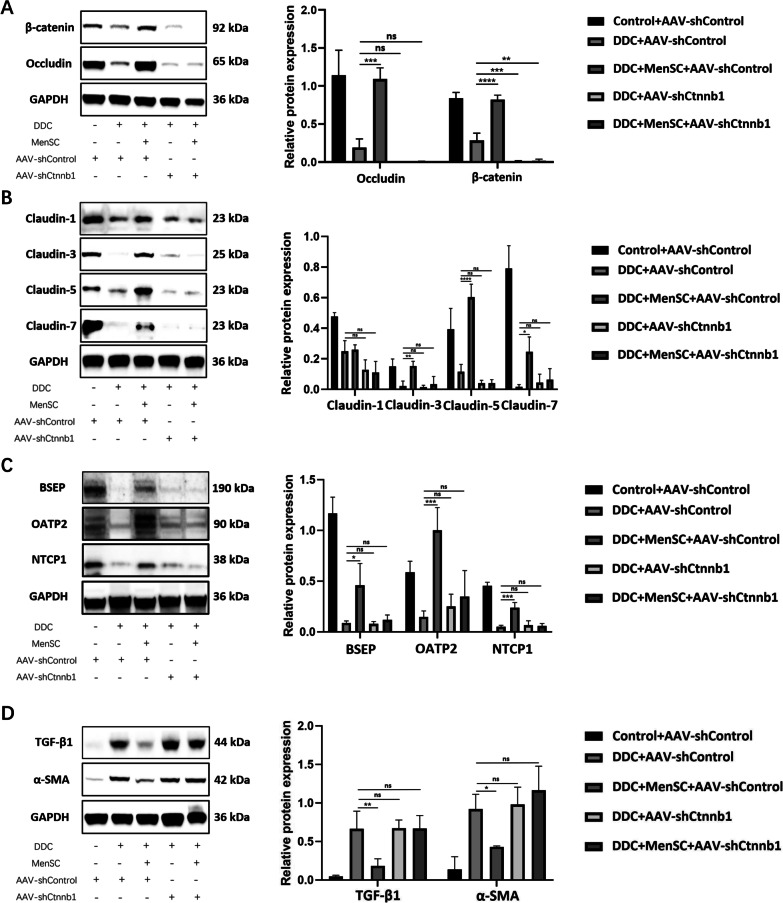
β-Catenin deficiency inhibited the regulatory effect of MenSCs on related proteins
To further explore the regulatory effect of β-catenin on related pathways and proteins, western blot was used to assess the expression of proteins. According to the results, MenSC transplantation could not restore DDC-induced damage to β-catenin, Occludin, Claudin-1, Claudin-3, Claudin-5 and Claudin-7 expression in the AAV-shCtnnb1 mice (Fig. 7A, B). Similar results were also found for the BSEP, OATP2 and NTCP1 levels (Fig. 7C). Subsequently, the TGF-β1 and α-SMA expression levels were detected. The results showed that MenSCs failed to inhibit the expression of TGF-β1 and α-SMA in the AAV-shCtnnb1 mice (Fig. 7D). These results indicated that MenSCs regulated the related proteins and pathways by upregulating β-catenin expression.
Fig. 7.
β-Catenin deficiency inhibited the regulatory effect of MenSCs on related proteins. A Western blot analysis protein levels of β-catenin and Occludin in the AAV-shControl and AAV-shCtnnb1 infected liver tissues of different groups (n = 3 for each group). B Expression of Claudin-1, Claudin-3, Claudin-5 and Claudin-7 in AAV-shControl and AAV-shCtnnb1 infected liver tissues (n = 3 for each group). C Protein levels of BSEP, OATP2 and NTCP1 (n = 3 for each group). D Expression of TGF-β and α-SMA in liver tissues (n = 3 for each group). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 and n.s. indicates no significance between the two indicated groups
Discussion
This study demonstrated that MenSCs could accumulate in the liver and attenuate the development of DDC-induced liver function injury. MenSC therapy reduced intrahepatic bile duct dilation, cholestasis and concomitant fibrosis, which were the main pathological changes in DDC mice. Injected MenSCs significantly reduced DDC-induced mouse mortality. Previous pre-clinical and clinical researches have investigated the potential treatment effect of MSCs in cholestatic liver injury. Fan et al. indicated that UC-MSCs could reduce serum levels of AST, ALT and ALP in 2-octynoic acid coupled to bovine serum albumin-induced PBC mice. Additionally, MSCs are proved to ameliorate liver inflammation by diminishing T cell function [30]. Pinheiro et al. induced cholestatic liver fibrosis by bile duct ligation in mice. According to the results, MSCs conditioned medium injection decreased levels of hepatic enzymes (AST, ALT, ALP and Albumin) and collagen deposition (p < 0.001) in liver [31]. In a clinical trial by Wang et al., PBC patients with UDCA resistant received BM-MSC transfusion. After the 12-months follow-up, the serum levels of AST, ALT, γ-GT, DBIL and IgM in PBC patients significantly decreased from baseline. While no significant changes were observed in either TBIL or ALP [32]. These conclusions are consistent with our results. Although previous studies investigated the effect of MSCs in cholestatic liver injury, less studies discussed the mechanisms in depth. Based on this situation, our following research mainly focused on exploring the related mechanisms of MenSC treatment in chronic cholestatic liver injury.
Previously, researchers hypothesized that MSC might exhibit treatment effect in liver injuries by differentiating into hepatocyte-like cells (HLCs) in vivo [33, 34]. However, in recent years, most researchers believe that the MSCs treatment effects are mainly through paracrine activities [35]. According to Chen et al., although transplanted MSCs were recruited to injured liver sites, few cells differentiated into HLCs. MSCs ameliorate hepatic injury via paracrine mediators rather than differentiation effects [18]. Thus, we mainly focused on identifying the target of MenSC treatment in DDC-induced mouse models.
In this study, we found that MenSCs could repair the TJ structure injured in the DDC-treated mice. The blood–bile barrier (BBlB) is primarily composed of TJs [36], and represents a physical barrier formed by liver epithelial cells and hepatocytes, which separates bile from blood sinusoids [37]. Loss of the BBIB is believed to be the main cause of cholestatic liver injury [38]. Although investigators have indicated that MSCs can restore TJ injury in various tissues, such as the colon, hair follicle and brain [39–41], limited researches have demonstrated the TJ repair function of MSCs in the liver. Sato et al. found that liver resident mesenchymal cells could mediate TJ assembly in mouse intrahepatic bile ducts [42]. Our study firstly demonstrated the repair effect of transplanted MSCs on liver TJs. TJ pathway (including Occludin, Claudin-1, Claudin-3, Claudin-5 and Claudin-7) was further proven to be one of the main regulators involved in DDC-induced liver damage and MenSC treatment. Previous studies have proven the regulatory effect of MSCs on related proteins, which confirmed our results. According to Zhang et al., an increase in Occludin and Claudins protein levels could improve liver histology and decrease serum TBIL levels in rats with obstructive jaundice [43]. Pan et al. found that MSC-derived exosomes ameliorated brain ischemic injury by upregulating ZO-1 and Claudin-5 levels [44]. Tak et al. indicated that superoxide dismutase 3-transduced MSCs exhibited protective effect on the epithelial TJ barrier in mouse colitis by preserving the expression of ZO-1 and Occludin [39].
Additionally, the results suggested that MenSCs could restore bile transporter (including OATP2, BSEP and NTCP1) levels inhibited by DDC feeding. Cholestasis could be partly attributed to bile transport function disorder [45, 46]. Various studies have shown that upregulation of OATP2, BSEP and NTCP levels could effectively relieve cholestasis. In the exploration of the treatment effect of UDCA in cholestatic rats, Rost et al. found that UDCA prevented impairment of liver function by restoring bile transporter levels, including Oatp1, Oatp2 and Oatp4 [47]. Xiang et al. suggested that tectorigenin could treat DDC-induced cholestasis by increasing BSEP expression through PPARγ. Blocking upregulation of BSEP expression prevented the therapeutic effect of PPAR on cholestasis [48]. According to Zhang et al., Yin-Zhi-Huang could significantly decrease the serum total bile acids and DBLT levels, and improve histological disorganization by regulating Oatp2, Ntcp, and Mrp2 expression in cholestatic rats [49]. Thus, it was speculated that MenSCs prevent impairment of the liver by restoring the TJ pathway and bile transporter expression.
Liver fibrosis is an important pathological change caused by cholestasis. An early prospective study indicated that more than 50% of patients with stage I-III PBC developed histologically confirmed cirrhosis within 4 years [50]. In the present study, MenSC transplantation reduced liver fibrosis and downregulated TGF-β1 and α-SMA expression in the DDC-treated mice. Pradhan-Sundd et al. suggested that liver fibrosis in cholestatic mice was associated with deregulation of TJs and bile transporters [38]. Although the mechanism of cholestasis leading to liver fibrosis is still unclear, we hypothesized that MenSCs may reduce liver fibrosis by restoring TJ and bile transporter functions.
Furthermore, β-catenin was demonstrated to be a key target of MenSC treatment. MenSCs promote the repair of TJs and bile transport function damage by upregulating the expression of liver β-catenin, which is inhibited by DDC, thereby inhibiting the progression of liver fibrosis. We found that hepatic β-catenin knockdown did not cause significant liver damage in normal mice but inhibited the therapeutic effect of MenSC transplantation in DDC-induced hepatic cholestasis and fibrosis. Furthermore, β-catenin knockdown inhibited MenSC regulation of TJs and bile transport function-related proteins and pathways in the DDC-treated mice.
Thompson et al. reported that the upregulation of β-catenin in mice could enhance the resolution of intrahepatic cholestasis after chronic DDC administration for 150 d [51]. According to Tao et al., mice with β-catenin-deficient hepatocytes demonstrated increased liver injury following the DDC diet [52]. Serum levels of ALT and AST were significantly increased in the KD mice after DDC feeding. These studies are consistent with our results. However, research by Saggi et al. suggested that β-catenin might play an opposite role relative to that in our study in the DDC-induced liver injury model [53]. According to Saggi et al., in mice fed a DDC diet, inhibiting β-catenin could result in decreased liver injury, as evidenced by lower percentage of liver fibrosis area and serum levels of ALP, ALT, AST and TBIL. We hypothesized that β-catenin may play various regulatory roles at DDC-induced liver injury. It means that the regulation of MSCs on β-catenin in cholestasis requires further investigation.
In addition, although it is hypothesized that MenSC transplantation could inhibit liver fibrosis induced by cholestasis, an in-depth study of the mechanism was not conducted. To date, a series of studies have indicated that β-catenin could be a protective factor against cholestasis-induced fibrosis. Interestingly, we observed that β-catenin aggravates hepatic fibrosis in carbon tetrachloride (CCl4)-induced models. Li et al. reported that inhibiting the Wnt/β-catenin signaling pathway could attenuate CCl4-induced hepatic fibrosis in rats [54]. According to Rao et al., β-catenin promotes hepatic fibrosis by activating hepatic stellate cells in CCl4-induced mouse models [55]. The different regulatory effects of β-catenin in different liver fibrosis models attracted our attention. The role of β-catenin in hepatic fibrosis induced by a variety of pathological reasons is worthy of further exploration.
Conclusion
In summary, the current work investigated the treatment effect of MenSC transplantation in DDC-induced liver injury, and the results suggest that cellular therapy is a promising strategy for cholestatic treatment. Based on the present study, we would further investigate the exact regulatory mechanism of MenSCs on β-catenin in DDC-induced liver injury. Future studies would provide ideas for improving the efficiency of MSCs in cholestasis.
Acknowledgements
Not applicable.
Abbreviations
- MenSCs
Menstrual blood-derived stem cells
- DDC
3,5-Diethoxycarbonyl-1,4-dihydroxychollidine
- PBS
Phosphate buffer saline
- AAV
Adeno-associated virus
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- DBIL
Direct bilirubin
- TBIL
Total bilirubin
- TJ
Tight junction
- PBC
Primary biliary cirrhosis
- PSC
Primary sclerosing cholangitis
- BA
Bile acid
- UDCA
Ursodeoxycholic acid
- OCA
Obeticholic acid
- LT
Liver transplantation
- MSCs
Mesenchymal stem cells
- HLA-DR
Human leukocyte antigen-DR
- α-SMA
α-Smooth muscle actin
- GAPDH
Anti-glyceraldehyde-3-phosphate dehydrogenase
- TEAB
Triethylammonium bicarbonate
- PCA
Principal component analysis
- OPLS-DA
Orthogonal partial least-squares-discriminant analysis
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- GO
Gene Ontology
- GSEA
Gene Set Enrichment Analysis
- BBIB
Blood–bile barrier
- CCl4
Carbon tetrachloride
Authors' contributions
LL, YY, and YC performed study concept and design. LZ, ST, SZ and QH performed mice feeding. ZL, FZ and QL performed mice sacrifice and tissue collection. YY and YC performed the experiments and underlying data verification. YY, FJ and YZ provided analysis, interpretation of data and statistical analysis. YY and YZ revised the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2016YFC 1101304/3).
Availability of data and materials
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD028425. All data will be made available upon reasonable request to the corresponding authors.
Declarations
Ethics approval and consent to participate
Animal experiments in this study were conducted under ethical conditions approved by the Animal Care Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, and the approval number is 2019-0002. MenSCs were provided by Professor Charlie Xiang (the First Affiliated Hospital, School of Medicine Zhejiang University). Isolation and expansion of MenSCs were approved by Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, and the approval number is 2017-623.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu H, Chen C, Ziani S, Nelson LJ, Avila MA, Nevzorova YA, et al. Fibrotic events in the progression of cholestatic liver disease. Cells. 2021;10(5):1107. doi: 10.3390/cells10051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li WK, Wang GF, Wang TM, Li YY, Li YF, Lu XY, et al. Protective effect of herbal medicine Huangqi decoction against chronic cholestatic liver injury by inhibiting bile acid-stimulated inflammation in DDC-induced mice. Phytomedicine. 2019;62:152948. doi: 10.1016/j.phymed.2019.152948. [DOI] [PubMed] [Google Scholar]
- 3.Chen HL, Wu SH, Hsu SH, Liou BY, Chen HL, Chang MH. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018;25(1):75. doi: 10.1186/s12929-018-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen PL, Ghallab A, Vartak N, Reif R, Schaap FG, Hampe J, et al. The ascending pathophysiology of cholestatic liver disease. Hepatology. 2017;65(2):722–738. doi: 10.1002/hep.28965. [DOI] [PubMed] [Google Scholar]
- 5.Chung SW, Lee JH, Kim MA, Leem G, Kim SW, Chang Y, et al. Additional fibrate treatment in UDCA-refractory PBC patients. Liver Int. 2019;39(9):1776–1785. doi: 10.1111/liv.14165. [DOI] [PubMed] [Google Scholar]
- 6.Woolbright BL. Inflammation: cause or consequence of chronic cholestatic liver injury. Food Chem Toxicol. 2020;137:111133. doi: 10.1016/j.fct.2020.111133. [DOI] [PubMed] [Google Scholar]
- 7.Ma X, Jiang Y, Zhang W, Wang J, Wang R, Wang L, et al. Natural products for the prevention and treatment of cholestasis: a review. Phytother Res. 2020;34(6):1291–1309. doi: 10.1002/ptr.6621. [DOI] [PubMed] [Google Scholar]
- 8.Nayagam JS, Williamson C, Joshi D, Thompson RJ. Review article: liver disease in adults with variants in the cholestasis-related genes ABCB11, ABCB4 and ATP8B1. Aliment Pharmacol Ther. 2020;52(11–12):1628–1639. doi: 10.1111/apt.16118. [DOI] [PubMed] [Google Scholar]
- 9.El-Araby HA, Saber MA, Radwan NM, Taie DM, Adawy NM, Sira AM. SOX9 in biliary atresia: new insight for fibrosis progression. Hepatobiliary Pancreat Dis Int. 2021;20(2):154–162. doi: 10.1016/j.hbpd.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Chascsa D, Carey EJ, Lindor KD. Old and new treatments for primary biliary cholangitis. Liver Int. 2017;37(4):490–499. doi: 10.1111/liv.13294. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C, Chazouilleres O, Belnou P, Montano-Loza AJ, Mason A, Ebadi M, et al. Long-term impact of preventive UDCA therapy after transplantation for primary biliary cholangitis. J Hepatol. 2020;73(3):559–565. doi: 10.1016/j.jhep.2020.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Manne V, Kowdley KV. Obeticholic acid in primary biliary cholangitis: where we stand. Curr Opin Gastroenterol. 2019;35(3):191–196. doi: 10.1097/MOG.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 13.Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73(1):94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorucci S, Di Giorgio C, Distrutti E. Obeticholic acid: an update of its pharmacological activities in liver disorders. Handb Exp Pharmacol. 2019;256:283–295. doi: 10.1007/164_2019_227. [DOI] [PubMed] [Google Scholar]
- 15.Carbone M, Neuberger J. Liver transplantation in PBC and PSC: indications and disease recurrence. Clin Res Hepatol Gastroenterol. 2011;35(6–7):446–454. doi: 10.1016/j.clinre.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells—sources and clinical applications. Transfus Med Hemother. 2008;35(4):272–277. doi: 10.1159/000142333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Wu Y, Wang Y, Chen L, Zheng W, Zhou S, et al. Human menstrual blood-derived stem cells mitigate bleomycin-induced pulmonary fibrosis through anti-apoptosis and anti-inflammatory effects. Stem Cell Res Ther. 2020;11(1):477. doi: 10.1186/s13287-020-01926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, et al. Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl Med. 2017;6(1):272–284. doi: 10.5966/sctm.2015-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma H, Shi X, Yuan X, Ding Y. IL-1beta siRNA adenovirus benefits liver regeneration by improving mesenchymal stem cells survival after acute liver failure. Ann Hepatol. 2016;15(2):260–270. doi: 10.5604/16652681.1193723. [DOI] [PubMed] [Google Scholar]
- 20.Despeyroux A, Duret C, Gondeau C, Perez-Gracia E, Chuttoo L, de Boussac H, et al. Mesenchymal stem cells seeded on a human amniotic membrane improve liver regeneration and mouse survival after extended hepatectomy. J Tissue Eng Regen Med. 2018;12(4):1062–1073. doi: 10.1002/term.2607. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Wang YH, Qian JQ, Wu DB, Chen EQ, Tang H. Human mesenchymal stem cells for hepatitis B virus-related acute-on-chronic liver failure: a systematic review with meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(10):1224–1229. doi: 10.1097/MEG.0000000000001156. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YC, Liu W, Fu BS, Wang GY, Li HB, Yi HM, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19(2):194–199. doi: 10.1016/j.jcyt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Hu C, Zhao L, Duan J, Li L. Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med. 2019;23(3):1657–1670. doi: 10.1111/jcmm.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detry O, Vandermeulen M, Delbouille MH, Somja J, Bletard N, Briquet A, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I–II, open-label, clinical study. J Hepatol. 2017;67(1):47–55. doi: 10.1016/j.jhep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Hu C, Li L. The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation. J Transl Med. 2019;17(1):412. doi: 10.1186/s12967-019-02167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Feng B, Xu Y, Zhu J, Feng X, Chen W, et al. Immunomodulatory effect of mesenchymal stem cells in chemical-induced liver injury: a high-dimensional analysis. Stem Cell Res Ther. 2019;10(1):262. doi: 10.1186/s13287-019-1379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66(1):209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 28.Graney BA, Lee JS. Impact of novel antifibrotic therapy on patient outcomes in idiopathic pulmonary fibrosis: patient selection and perspectives. Patient Relat Outcome Meas. 2018;9:321–328. doi: 10.2147/PROM.S144425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5(2):146–150. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- 30.Fan J, Tang X, Wang Q, Zhang Z, Wu S, Li W, et al. Mesenchymal stem cells alleviate experimental autoimmune cholangitis through immunosuppression and cytoprotective function mediated by galectin-9. Stem Cell Res Ther. 2018;9(1):237. doi: 10.1186/s13287-018-0979-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinheiro D, Dias I, Freire T, Thole AA, Stumbo AC, Cortez EAC, et al. Effects of mesenchymal stem cells conditioned medium treatment in mice with cholestatic liver fibrosis. Life Sci. 2021;281:119768. doi: 10.1016/j.lfs.2021.119768. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Han Q, Chen H, Wang K, Shan GL, Kong F, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23(20):2482–2489. doi: 10.1089/scd.2013.0500. [DOI] [PubMed] [Google Scholar]
- 33.Yu J, Cao H, Yang J, Pan Q, Ma J, Li J, et al. In vivo hepatic differentiation of mesenchymal stem cells from human umbilical cord blood after transplantation into mice with liver injury. Biochem Biophys Res Commun. 2012;422(4):539–545. doi: 10.1016/j.bbrc.2012.04.156. [DOI] [PubMed] [Google Scholar]
- 34.Shi LL, Liu FP, Wang DW. Transplantation of human umbilical cord blood mesenchymal stem cells improves survival rates in a rat model of acute hepatic necrosis. Am J Med Sci. 2011;342(3):212–217. doi: 10.1097/MAJ.0b013e3182112b90. [DOI] [PubMed] [Google Scholar]
- 35.Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roehlen N, Roca Suarez AA, El Saghire H, Saviano A, Schuster C, Lupberger J, et al. Tight junction proteins and the biology of hepatobiliary disease. Int J Mol Sci. 2020;21(3):825. doi: 10.3390/ijms21030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradhan-Sundd T, Vats R, Russell JO, Singh S, Michael AA, Molina L, et al. Dysregulated bile transporters and impaired tight junctions during chronic liver injury in mice. Gastroenterology. 2018;155(4):1218–32.e24. doi: 10.1053/j.gastro.2018.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pradhan-Sundd T, Zhou L, Vats R, Jiang A, Molina L, Singh S, et al. Dual catenin loss in murine liver causes tight junctional deregulation and progressive intrahepatic cholestasis. Hepatology. 2018;67(6):2320–2337. doi: 10.1002/hep.29585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tak LJ, Kim HY, Ham WK, Agrahari G, Seo Y, Yang JW, et al. Superoxide dismutase 3-transduced mesenchymal stem cells preserve epithelial tight junction barrier in murine colitis and attenuate inflammatory damage in epithelial organoids. Int J Mol Sci. 2021;22(12):6431. doi: 10.3390/ijms22126431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Sun P, Huang X, Chen H, Huang W, Ruan Y, et al. RNA-seq reveals tight junction-relevant erythropoietic fate induced by OCT4 in human hair follicle mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):454. doi: 10.1186/s13287-020-01976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Cao J, Duan S, Xu R, Yu H, Huo X, et al. Effect of MicroRNA-126a-3p on bone marrow mesenchymal stem cells repairing blood-brain barrier and nerve injury after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(5):104748. doi: 10.1016/j.jstrokecerebrovasdis.2020.104748. [DOI] [PubMed] [Google Scholar]
- 42.Sato A, Kakinuma S, Miyoshi M, Kamiya A, Tsunoda T, Kaneko S, et al. Vasoactive intestinal peptide derived from liver mesenchymal cells mediates tight junction assembly in mouse intrahepatic bile ducts. Hepatol Commun. 2020;4(2):235–254. doi: 10.1002/hep4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Wang XQ, Zhou YK, Ma YL, Shen TY, Chen HQ, et al. Effects of oral Lactobacillus plantarum on hepatocyte tight junction structure and function in rats with obstructive jaundice. Mol Biol Rep. 2010;37(6):2989–2999. doi: 10.1007/s11033-009-9866-y. [DOI] [PubMed] [Google Scholar]
- 44.Pan Q, Kuang X, Cai S, Wang X, Du D, Wang J, et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther. 2020;11(1):260. doi: 10.1186/s13287-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoekstra H, Tian Y, Jochum W, Stieger B, Graf R, Porte RJ, et al. Dearterialization of the liver causes intrahepatic cholestasis due to reduced bile transporter expression. Transplantation. 2008;85(8):1159–1166. doi: 10.1097/TP.0b013e31816b2465. [DOI] [PubMed] [Google Scholar]
- 46.Gijbels E, Vilas-Boas V, Annaert P, Vanhaecke T, Devisscher L, Vinken M. Robustness testing and optimization of an adverse outcome pathway on cholestatic liver injury. Arch Toxicol. 2020;94(4):1151–1172. doi: 10.1007/s00204-020-02691-9. [DOI] [PubMed] [Google Scholar]
- 47.Rost D, Herrmann T, Sauer P, Schmidts HL, Stieger B, Meier PJ, et al. Regulation of rat organic anion transporters in bile salt-induced cholestatic hepatitis: effect of ursodeoxycholate. Hepatology. 2003;38(1):187–195. doi: 10.1053/jhep.2003.50256. [DOI] [PubMed] [Google Scholar]
- 48.Xiang JQ, Yang GY, Ma CR, Wei LL, Wu H, Zhang W, et al. Tectorigenin alleviates intrahepatic cholestasis by inhibiting hepatic inflammation and bile accumulation via activation of PPAR gamma. Brit J Pharmacol. 2021;178(12):2443–2460. doi: 10.1111/bph.15429. [DOI] [PubMed] [Google Scholar]
- 49.Zhang G, Zhou Y, Rao Z, Qin H, Wei Y, Ren J, et al. Effect of Yin–Zhi–Huang on up-regulation of Oatp2, Ntcp, and Mrp2 proteins in estrogen-induced rat cholestasis. Pharm Biol. 2015;53(3):319–325. doi: 10.3109/13880209.2014.918156. [DOI] [PubMed] [Google Scholar]
- 50.Farkkila M, Rautiainen H, Karkkainen P, Karvonen AL, Nurmi H, Niemela O. Serological markers for monitoring disease progression in noncirrhotic primary biliary cirrhosis on ursodeoxycholic acid therapy. Liver Int. 2008;28(6):787–797. doi: 10.1111/j.1478-3231.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 51.Thompson MD, Awuah P, Singh S, Monga SP. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am J Pathol. 2010;177(4):1812–1822. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao GZ, Lehwald N, Jang KY, Baek J, Xu B, Omary MB, et al. Wnt/beta-catenin signaling protects mouse liver against oxidative stress-induced apoptosis through the inhibition of forkhead transcription factor FoxO3. J Biol Chem. 2013;288(24):17214–17224. doi: 10.1074/jbc.M112.445965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saggi H, Maitra D, Jiang A, Zhang R, Wang P, Cornuet P, et al. Loss of hepatocyte beta-catenin protects mice from experimental porphyria-associated liver injury. J Hepatol. 2019;70(1):108–117. doi: 10.1016/j.jhep.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, Zhu C, Li Y, Wu Q, Gao R. Mest attenuates CCl4-induced liver fibrosis in rats by inhibiting the Wnt/beta-catenin signaling pathway. Gut Liver. 2014;8(3):282–291. doi: 10.5009/gnl.2014.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao S, Xiang J, Huang J, Zhang S, Zhang M, Sun H, et al. PRC1 promotes GLI1-dependent osteopontin expression in association with the Wnt/beta-catenin signaling pathway and aggravates liver fibrosis. Cell Biosci. 2019;9:100. doi: 10.1186/s13578-019-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD028425. All data will be made available upon reasonable request to the corresponding authors.