Abstract
Fifty-six strains of Borrelia burgdorferi sensu lato, isolated from ticks and vertebrate animals in Missouri, South Carolina, Georgia, Florida, and Texas, were identified and characterized by PCR-restriction fragment length polymorphism (RFLP) analysis of rrf (5S)-rrl (23S) intergenic spacer amplicons. A total of 241 to 258 bp of intergenic spacers between tandemly duplicated rrf (5S) and rrl (23S) was amplified by PCR. MseI and DraI restriction fragment polymorphisms were used to analyze these strains. PCR-RFLP analysis results indicated that the strains represented at least three genospecies and 10 different restriction patterns. Most of the strains isolated from the tick Ixodes dentatus in Missouri and Georgia belonged to the genospecies Borrelia andersonii. Excluding the I. dentatus strains, most southern strains, isolated from the ticks Ixodes scapularis and Ixodes affinis, the cotton rat (Sigmodon hispidus), and cotton mouse (Peromyscus gossypinus) in Georgia and Florida, belonged to Borrelia burgdorferi sensu stricto. Seven strains, isolated from Ixodes minor, the wood rat (Neotoma floridana), the cotton rat, and the cotton mouse in South Carolina and Florida, belonged to Borrelia bissettii. Two strains, MI-8 from Florida and TXW-1 from Texas, exhibited MseI and DraI restriction patterns different from those of previously reported genospecies. Eight Missouri tick strains (MOK-3a group) had MseI patterns similar to that of B. andersonii reference strain 21038 but had a DraI restriction site in the spacer. Strain SCGT-8a had DraI restriction patterns identical to that of strain 25015 (B. bissettii) but differed from strain 25015 in its MseI restriction pattern. Strain AI-1 had the same DraI pattern as other southern strains in the B. bissettii genospecies but had a distinct MseI profile. The taxonomic status of these atypical strains needs to be further evaluated. To clarify the taxonomic positions of these atypical Borrelia strains, the complete sequences of rrf-rrl intergenic spacers from 20 southeastern and Missouri strains were determined. The evolutionary and phylogenetic relationships of these strains were compared with those of the described genospecies in the B. burgdorferi sensu lato species complex. The 20 strains clustered into five separate lineages on the basis of sequence analysis. MI-8 and TXW-1 appeared to belong to two different undescribed genospecies, although TXW-1 was closely related to Borrelia garinii. The MOK-3a group separated into a distinct deep branch in the B. andersonii lineage. PCR-RFLP analysis results and the results of sequence analyses of the rrf-rrl intergenic spacer confirm that greater genetic heterogeneity exists among B. burgdorferi sensu lato strains isolated from the southern United States than among strains isolated from the northern United States. The B. andersonii genospecies and its MOK-3a subgroup are associated with the I. dentatus-cottontail rabbit enzootic cycle, but I. scapularis was also found to harbor a strain of this genospecies. Strains that appear to be B. bissettii in our study were isolated from I. minor and the cotton mouse, cotton rat, and wood rat. The B. burgdorferi sensu stricto strains from the south are genetically and phenotypically similar to the B31 reference strain.
The etiological agent of Lyme disease was discovered in the tick Ixodes scapularis in New York State (7) and was subsequently described as Borrelia burgdorferi (13). We now know that B. burgdorferi is composed of a complex of genospecies known collectively as B. burgdorferi sensu lato. Nine species of the complex are known to come from Eurasia, and three are known to come from North America. The latter group consists of B. burgdorferi sensu stricto (5), Borrelia andersonii (1, 2, 15), and Borrelia bissettii (24). Of the B. burgdorferi sensu lato complex, only three species are proven to be responsible for the majority of Lyme borreliosis cases worldwide. Some clinical symptoms caused by the three species are similar, but each has also been associated predominantly with a given clinical presentation. B. burgdorferi sensu stricto, which is found in North America and Europe, is most often associated with arthritis; Borrelia garinii and Borrelia afzelii, which occur in Eurasia, are most often associated with neuroborreliosis and with late cutaneous symptoms, respectively (4, 25, 31). Currently, B. burgdorferi sensu stricto is the only species proven to cause Lyme borreliosis in humans in North America. However, the DN127 and 25015 group of strains from North America may be involved in human illness on the basis of their genetic similarities to strains from nine patients with disseminated Lyme disease in Slovenia. The DN127 and 25015 group of strains has recently been described as a new genospecies, B. bissettii (24). Interestingly, the clinical presentations of the Slovenian patients varied, from some patients having a relatively benign illness to some being severely affected (29). Moreover, some patients had variable and unpredictable serologic responses, including an apparent lack of an antibody response despite disseminated disease. Interestingly, some patients from the southern United States with Lyme disease or a Lyme disease-like disease also lack a serologic response to antigens derived from B. burgdorferi sensu stricto (9). In the present paper we report on the presence of B. bissettii, B. burgdorferi sensu stricto, and B. andersonii in the southern United States.
There is considerable genetic heterogeneity of B. burgdorferi sensu lato in the southern and western parts of the United States compared to the genetic heterogeneity of the organism in the northern parts of the United States (16, 24; T. Lin, J. H. Oliver, Jr., T. M. Kollars, Jr., and K. L. Clark, Proc. VIII Int. Conf. Lyme Borreliosis Other Emerging Tick-Borne Dis., p. 8, 1999). B. burgdorferi sensu stricto is the dominant genospecies in the northern United States and also occurs in the western and southern areas of the country (16, 22, 24). B. andersonii occurs in the eastern half of the United States and appears to exist primarily in an enzootic cycle that involves cottontail rabbits and Ixodes dentatus, a tick that bites humans, but rarely (11). B. bissettii occurs in the western and southern United States but rarely occurs in the northern region. It appears to be maintained in several enzootic transmission cycles in California and the southeastern United States and involves several tick species, including human-biting Ixodes pacificus ticks in the western half of the United States and I. scapularis ticks in the eastern half of the United States (6, 19).
Recent genetic analysis suggests that at least three genospecies of the B. burgdorferi sensu lato species complex occur in the southern United States (16; Lin et al., Proc. VIII Int. Conf. Lyme Borreliosis Other Emerging Tick-Borne Dis.). An understanding of the genetic variability present among spirochetes isolated from several species of ticks, rodents, and birds in this region is essential to obtain an understanding of the natural history of Lyme borreliosis and of the potential dangers of human infection. The purpose of our study was to investigate the genetic heterogeneity of a sample of B. burgdorferi sensu lato isolates from the southern United States by PCR-restriction fragment length polymorphism (RFLP) analysis of rrf (5S)-rrl (23S) intergenic spacer amplicons and base pair sequence analysis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains investigated in the present study were isolated from ticks (I. scapularis, I. affinis, I. dentatus, Ixodes minor, Dermacentor variabilis), rodents (cotton mouse, cotton rat, wood rat), or rabbits from Missouri, Georgia, Florida, South Carolina, and Texas (Table 1). Several strains including B-31, SH2-82 (B. burgdorferi sensu stricto), DN127 and 25015 (B. bissettii), 21038 (B. andersonii), and 20047 (B. garinii) were used as positive controls in PCR-RFLP analysis (Table 1). All isolates were low-passage (mostly third passage) isolates that had been grown in Barbour-Stoenner-Kelly (BSK) II medium at 33 to 34°C for 1 to 2 weeks (to the stationary phase of growth) and checked via dark-field microscopy for purity and concentration before they were harvested.
TABLE 1.
B. burgdorferi sensu lato strains analyzed in the present study
| Isolate | Host (sex or stage) | Source | Location | Passage no. | Genospecies |
|---|---|---|---|---|---|
| SI-1 | Peromyscus gossypinus | Bladder | Sapelo Island, McIntosh County, Ga. | 3 | B. burgdorferi sensu stricto |
| SI-3 | Sigmodon hispidus | Bladder | Sapelo Island, McIntosh County, Ga. | 3 | B. burgdorferi sensu stricto |
| SI-4 | I. scapularis (male) | Unknown | Sapelo Island, McIntosh County, Ga. | 3 | B. burgdorferi sensu stricto |
| SI-10 | I. scapularis (female) | Drag | Sapelo Island, McIntosh County, Ga. | 3 | B. andersonii |
| SI-14 | Ixodes affinis (female) | Drag | Sapelo Island, McIntosh County, Ga. | 3 | B. burgdorferi sensu stricto |
| SCI-2 | P. gossypinus | Ear clip | St. Catherines Island, Liberty County, Ga. | 3 | B. burgdorferi sensu stricto |
| SCI-4 | I. scapularis (male) | Drag | St. Catherines Island, Liberty County, Ga. | 3 | B. burgdorferi sensu stricto |
| BC-1 | I. dentatus (nymph) | Drag | Macon, Bibb County, Ga. | 3 | B. andersonii |
| SM-1 | P. gossypinus | Ear clip | St. Marys, Camden County, Ga. | 3 | B. burgdorferi sensu stricto |
| SCGT-8a | I. minor (male) | Wood rat | Georgetown County, S.C. | 3 | B. bissettii |
| SCGT-10 | Neotoma floridana | Ear clip | Georgetown County, S.C. | 3 | B. bissettii |
| SCW-30h | I. minor (nymph) | Carolina wren | Wedge Plantation, Charleston County, S.C. | 3 | B. bissettii |
| MI-2 | P. gossypinus | Bladder, ear clip | Merritt Island, Brevard County, Fla. | 3 | B. burgdorferi sensu stricto |
| MI-5 | S. hispidus | Bladder, ear clip | Merritt Island, Brevard County, Fla. | 3 | B. burgdorferi sensu stricto |
| MI-6 | S. hispidus | Ear clip | Merritt Island, Brevard County, Fla. | 3 | B. bissettii |
| MI-8 | S. hispidus | Ear clip | Merritt Island, Brevard County, Fla. | 3 | Borrelia spp. |
| MI-9 | P. gossypinus | Ear clip | Merritt Island, Brevard County, Fla. | 3 | B. bissettii |
| AI-1 | S. hispidus | Bladder, ear clip | Amelia Island, Nassau County, Fla. | 3 | B. bissettii |
| FD-1 | S. hispidus | Bladder, ear clip | Favor-Dykes, Flagler, County, Fla. | 3 | B. bissettii |
| TXW-1 | D. variabilis (male) | Coyote | Webb County, Tex. | 3 | Borrelia spp. |
| MOR-1 | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOR-2 | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOR-1b | I. dentatus (larva) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-1c | I. dentatus (larva) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-1d | I. dentatus (larva) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-1e | I. dentatus (larva) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-1f | I. dentatus (larva) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-1g | I. dentatus (larva) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-2a | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-2b | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-3a | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-3b | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-3c | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOS-4 | I. dentatus (nymph) | Rabbit | Swinton, Stoddard County, Mo. | 3 | B. andersonii |
| MOD-1 | I. dentatus (n = 2; nymph) | Rabbit | Dowd Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOD-3 | I. dentatus (n = 4; larvae) | Rabbit | Dowd Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOD-5 | I. dentatus (nymph) | Rabbit | Dowd Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-1b | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-1c | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-1d | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-1e | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-1f | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-1g | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-2-ID a | I. dentatus (larva) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-2-ID b | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-2-ID c | I. dentatus (larva) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-3a | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-3b | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-3c | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOK-3d | I. dentatus (nymph) | Rabbit | Koch Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOH-1 | I. dentatus (female) | Rabbit | Happy Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOH-2 | I. dentatus (male) | Rabbit | Happy Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOJ-1 | I. dentatus (larva) | Rabbit | Jenkins Farm, Bollinger County, Mo. | 3 | B. andersonii |
| MOG-1b | I. dentatus (nymph) | Rabbit | Gipsy, Wayne County, Mo. | 3 | B. andersonii |
| MOO-1 | I. dentatus (nymph) | Rabbit | Oran, Scott County, Mo. | 3 | B. andersonii |
| MON-1 | I. dentatus (nymph) | Rabbit | NUT Junction, Cape Giradeau County, Mo. | 3 | B. andersonii |
| B31 | I. scapularis | Shelter Island, New York, N.Y. | 3 | B. burgdorferi sensu stricto | |
| SH2-82 | I. scapularis | New York State | 7 | B. burgdorferi sensu stricto | |
| 20047 | I. ricinus | France | 3 | B. garinii | |
| DN127 | I. pacificus | California | 3 | B. bissettii | |
| 25015 | I. scapularis (larva) | White-footed mouse | Dutchess County, N.Y. | 3 | B. bissettii |
| 21038 | I. dentatus | New York State | 3 | B. andersonii |
Extraction of chromosomal DNA.
Thirty-milliliter volumes of stationary-phase cultures were washed in 0.01 M phosphate-buffered saline (pH 7.2) and 5 mM MgCl2 three times (12,000 rpm; Beckman GS-6KR, Avanti J-30I) for 10 min. Washed pellets of the spirochetes were resuspended in 250 μl of TES (50 mM Tris [pH 8.0], 50 mM EDTA, 15% [wt/vol] sucrose). Subsequently, an equal volume of 5 M NaCl as well as 0.01% (vol/vol) sodium deoxycholate was added to the cell suspension. The samples were incubated on ice for 30 min. The partially lysed cell suspension was then centrifuged at 12,000 rpm (Beckman GS-6KR, Avanti J-30I) for 10 min. After discarding the supernatant, the pellet was resuspended in 250 μl of TES; this was followed by the addition of 250 μl of 10% sodium dodecyl sulfate and 3 μl of RNase. After a 30-min incubation at 37°C, 50 μl of proteinase K (20 mg/ml) was added and the tubes were incubated at 50°C for 30 min. This solution was subsequently phenol extracted twice, phenol-chloroform-isoamyl alcohol (25:24:1) extracted once, and chloroform-isoamyl alcohol (24:1) extracted once. After the addition of 1/10 (vol/vol) volume of 3.0 M sodium acetate (pH 5.2), 2 volumes of cold ethanol were added to precipitate the nucleic acid. The DNA pellets were resuspended in 50 μl of TE buffer (10 mM Tris [pH 7.6], 1 mM EDTA); the DNA was then subjected to 0.8% agarose gel electrophoresis (the gel was stained with 0.5 μg of ethidium bromide per ml), and a clearly defined DNA band was observed under UV transillumination and the gel was photographed. The DNA concentration was determined with a UV and VIS spectrophotometer (Lambda 3B; Perkin-Elmer).
Amplification of rrf (5S)-rrl (23S) intergenic spacer amplicons.
A pair of primers, primer 1 (5′-GCG GGA GAG TAG GTT ATT-3′) and primer 2 (5′-CTA GGC ATT CAC CAT AGA CT-3′), was designed on the basis of a previously published ribosomal DNA sequence (28) and was used to amplify the variable spacer region between two conserved structures, the 3′ end of the 5S rRNA (rrf) and the 5′ end of the 23S rRNA (rrl). PCRs were performed in volumes of 50 μl containing 10 mM Tris-HCl (pH 9.0 at 25°C), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 200 μM dATP, 200 μM dGTP, 200 μM dCTP, 200 μM dTTP, 1.5 U of Taq DNA polymerase in storage Buffer A (Promega, Madison, Wis.), each primer at a concentration of 1 μM, and 10 ng of extracted DNA. Reactions were performed in a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, Mass.) for 30 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 2 min. As noted above and in Table 1, Borrelia reference strains from different genospecies (B-31, SH2-82, 20047, DN127, 25015, and 21038) were used as positive controls in PCR.
Digestion with MseI and DraI restriction endonucleases and electrophoresis.
The 241- to 258-bp rrf (5S)-rrl (23S) intergenic spacer amplicons were digested with MseI (GIBCO BRL, Life Technologies, Rockville, Md.) and DraI (Sigma Chemical Company, St. Louis, Mo.), as suggested by Postic et al. (23). Briefly, 10 μl of PCR product was digested with 4 U of restriction endonuclease MseI for 1 h at 37°C in RE act 1 buffer containing 50 mM Tris-HCl (pH 8.0) and 10 mM MgCl2. The PCR product was digested with 20 U of DraI for 1 h at 37°C in Palette buffer containing 20 mM Tris acetate (pH 7.9 at 25°C), 10 mM MgCl2, 50 mM potassium acetate, and l mM dithiothreitol. The digests were separated in a 15% acrylamide–0.75% bisacrylamide gel for 5 h at a constant voltage (100 V). The pBR322 DNA HaeIII digest marker (Sigma Chemical Company) was used as a molecular size marker. The gels were silver stained and photographed, and the dried gels were kept as permanent records. All the bands in each lane were identified by their molecular size compared to those of the bands in the molecular size standard. The exact sizes of the smaller-molecular-size bands were identified by their sequence data.
Purification of PCR products, sequence analysis, and phylogenetic analysis.
The rrf (5S)-rrl (23S) intergenic spacer amplicons were purified with the Wizard PCR preps DNA purification system (Promega). The DNA sequence of the spacer amplicon was determined by using an ABI Prism (model 377) sequencer. Sequences were aligned both manually and by using Clustal W software (12); and a phylogenetic tree was constructed by neighbor-joining (26), maximum-likelihood (8), and parsimony (30) methods by using version 4.0b4a of the Phylogenetic Analysis Using Parsimony (PAUP) package. Positive control strain B31 was used in each set of sequencing reactions. Its sequence was compared with the sequences in GenBank to eliminate the possibility of errors for each of the strains.
Retrieved sequences.
To compare the relationship between our strains and the strains in different genospecies and to conduct phylogenetic analyses, rrf-rrl intergenic spacer sequences of the following genospecies (with database accession numbers given in parentheses for the strain names given in brackets) were used. They included B. burgdorferi sensu stricto (L30127 [B31T], L30121 [212]), B. garinii L30119 [20047T], L30130 [NT29], B. afzelii (L30135 [VS461T], L30129 [J1]), B. bissettii (L30124 [CA55], L30126 [DN127T], L30122 [25015]), B. andersonii (L30120 [21133], L30118 [19952]), B. japonica (L30125 [COW611C], L30128 [HO14T]), B. valaisiana (L30134 [VS116T], L30133 [UK]), B. lusitaniae (L30131 [poti B2T], L30132 [poti B3]), and Borrelia sp. (L30123 [CA2]).
Nucleotide sequence accession numbers.
The rrf-rrl intergenic spacer nucleotide sequences of the 20 B. burgdorferi sensu lato isolates which we analyzed in the present study have been assigned the following GenBank accession numbers: AF221678 (MI-2), AF221676 (MI-5), AF227434 (MI-6), AF221680 (SI-1), AF221679 (SM-1), AF221677 (SCI-2), AF221675 (MI-9), AF221673 (SCW-30 h), AF221681 (SCGT-10), AF221683 (MOD-1), AF221686 (MOD-5), AF221688 (MOS-1b), AF221684 (SI-10), AF221685 (BC-1), AF221671 (AI-1), AF221672 (FD-1), AF221674 (MI-8), AF221682 (SCGT-8a), AF221687 (MOK-3a), AF221689 (TXW-1).
RESULTS
PCR analysis.
The intergenic spacer between the two tandem copies of the rrf-rrl ribosomal genes of B. burgdorferi sensu lato was amplified by PCR. The number of base pairs amplified varied from 241 to 258, depending on the particular strain (Table 2).
TABLE 2.
MseI and DraI restriction fragments of amplified rrf-rrl intergenic spacer amplicons
| Genospecies or genomic group | Strain | Amplicon size (bp)a | DraI RFLP pattern | DraI restriction fragment sizes (bp)a | MseI RFLP pattern | MseI restriction fragment sizes (bp)a | Nucleotide sequence accession no. |
|---|---|---|---|---|---|---|---|
| B. burgdorferi sensu stricto | B31 | 242 | A′ | 142, 43, 29, 28 | A | 105, 42, 38, 29, 28 | L30127 |
| MI-2 | 242 | A′ | 142, 43, 29, 28 | A | 105, 42, 38, 29, 28 | AF221678 | |
| MI-5 | 244 | A′ | 144, 43, 29, 28 | A | 105, 42, 40, 29, 28 | AF221676 | |
| SI-1 | 244 | A′ | 144, 43, 29, 28 | A | 105, 42, 40, 29, 28 | AF221680 | |
| SM-1 | 244 | A′ | 144, 43, 29, 28 | A | 105, 42, 40, 29, 28 | AF221679 | |
| SCI-2 | 244 | A′ | 144, 43, 29, 28 | A | 105, 42, 40, 29, 28 | AF221677 | |
| B. bissettii | DN127 | 245 | I′ | 142, 43, 33, 27 | I | 105, 42, 38, 33, 27 | L30126 |
| 25015 | 241 | K′ | 171, 43, 27 | K | 105, 42, 34, 27, 17, 12, 4 | L30122 | |
| CA55 | 214 | J′ | 142, 43, 29 | J | 105, 42, 38, 29 | L30124 | |
| MI-6 | 241 | I1′ | 142, 43, 29, 27 | I1 | 105, 42, 38, 29, 27 | AF227434 | |
| MI-9 | 241 | I1′ | 142, 43, 29, 27 | I1 | 105, 42, 38, 29, 27 | AF221675 | |
| SCW-30h | 241 | I1′ | 142, 43, 29, 27 | I1 | 105, 42, 38, 29, 27 | AF221673 | |
| SCGT-10 | 241 | I1′ | 142, 43, 29, 27 | I1 | 105, 42, 38, 29, 27 | AF221681 | |
| AI-1 | 241 | I1′ | 142, 43, 29, 27 | I2 | 105, 42, 38, 29, 20, 7 | AF221671 | |
| FD-1 | 241 | I1′ | 142, 43, 29, 27 | I1 | 105, 42, 38, 29, 27 | AF221672 | |
| SCGT-8a | 241 | K′ | 171, 43, 27 | K1 | 105, 67, 42, 27 | AF221682 | |
| B. andersonii | 21038 | 254 | L′ | No fragment | L | 118, 67, 41, 28 | L30120 |
| MOD-1 | 253 | L′ | No fragment | L3 | 116, 67, 42, 28 | AF221683 | |
| MOD-5 | 258 | L′ | No fragment | L1 | 121, 67, 42, 28 | AF221686 | |
| MOS-1b | 258 | L′ | No fragment | L1 | 121, 67, 42, 28 | AF221688 | |
| SI-10 | 252 | L′ | No fragment | L3 | 115, 67, 42, 28 | AF221684 | |
| BC-1 | 256 | L′ | No fragment | L2 | 121, 67, 40, 28 | AF221685 | |
| MOK-3a | 256 | Z′ | 185, 71 | Z | 119, 67, 42, 28 | AF221687 | |
| Borrelia spp. | MI-8 | 242 | X′ | 142, 71, 16, 13 | X | 105, 42, 38, 28, 16, 13 | AF221674 |
| TXW-1 | 244 | Y′ | 201, 43 | Y | 105, 97, 42 | AF221689 |
The exact sizes were determined from the sequences.
PCR-RFLP results.
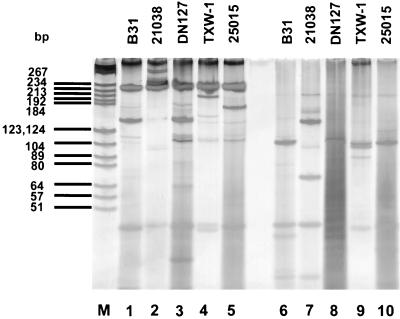
On the basis of both the MseI and the DraI restriction patterns (Fig. 1), the 56 isolates were separated into at least three genospecies that had 10 different restriction patterns (Table 3). There were four restriction patterns for B. andersonii isolates; most Missouri strains grouped into pattern L1. BC-1, the strain isolated from I. dentatus in Georgia, belonged to pattern L2. MOD-1 and, unexpectedly, SI-10, isolated from I. dentatus and I. scapularis, respectively, were of pattern L3. Pattern Z included eight strains from ticks in Missouri: MOK-3a, MOK-3b, MOK-3c, MOK-3d, MOR-2, MOS-le, MOS-1g, and MON-1. They had MseI restriction patterns similar to that of 21038, the reference strain of B. andersonii, but there was a DraI restriction site in the spacer among those strains that produced two fragments. That DraI restriction pattern made it possible to separate those strains from the others in the B. andersonii genospecies (Tables 2 and 3).
FIG. 1.
DraI restriction profiles (lanes 1, 2, 3, 4, and 5) and MseI restriction profiles (lanes 6, 7, 8, 9, and 10) of the amplified rrf-rrl spacer from strain TXW-1 and reference strains. The spacer digest was separated in a 15% acrylamide–0.75% bisacrylamide gel, stained with silver stain, and photographed. The species assignments of the strains are listed in Table 2. Lane M, molecular size markers.
TABLE 3.
MseI and DraI restriction patterns and genospecies distributions of southern strains
| Genospecies | Strains | Restriction pattern
|
|
|---|---|---|---|
| MseI | DraI | ||
| B. andersonii | MOS-1b, MOS-1c, MOS-1d, MOS-1f, MOS-2a, MOS-2b, MOS-3a, MOS-3b, MOS-3c, MOS-4, MOD-3, MOD-5, MOK-1b, MOK-1c, MOK-1d, MOK-1e, MOK-1f, MOK-1g, MOK-2-IDa, MOK-2-IDb, MOK-2-IDc, MOR-1, MOH-1, MOH-2, MOJ-1, MOG-1b, MOO-1 | L1 | L′ |
| BC-1 | L2 | L′ | |
| MOD-1, SI-10 | L3 | L′ | |
| MOK-3a, MOK-3b, MOK-3c, MOK-3d, MOR-2, MOS-1e, MOS-1g, MON-1 | Z | Z′ | |
| B. burgdorferi sensu stricto | SI-1, SI-3, SI-4, SI-14, SCI-2, SCI-4, SM-1, MI-2, MI-5 | A | A′ |
| B. bissettii | MI-6, MI-9, SCW-30h, SCGT-10, FD-1 | I1 | I1′ |
| AI-1 | I2 | I1′ | |
| SCGT-8a | K1 | K′ | |
| Borrelia spp. | MI-8 | X | X′ |
| TXW-1 | Y | Y′ | |
Despite slight differences in the MseI and DraI restriction patterns, most southeastern strains analyzed in the present study were identified as B. burgdorferi sensu stricto. Five southeastern strains (MI-6, MI-9, SCW-30h, SCGT-10, and FD-1) had identical MseI and DraI restriction patterns, and their spacers had similar nucleotide sequences; the nucleotide sequence of the spacer of strain DN127 (B. bissettii) was also similar to those of these five strains. SCGT-8a had a DraI restriction pattern identical to that of strain 25015 (B. bissettii) but a different MseI restriction pattern. AI-1 had the same DraI pattern as the other southern strains in the B. bissettii genospecies but had a distinct MseI profile. MI-8 and TXW-1 exhibited MseI and DraI restriction patterns different from each other and from those of previously reported genospecies (Tables 2 and 3; Fig. 1).
Sequence analysis of the rrf-rrl intergenic spacer amplicon.
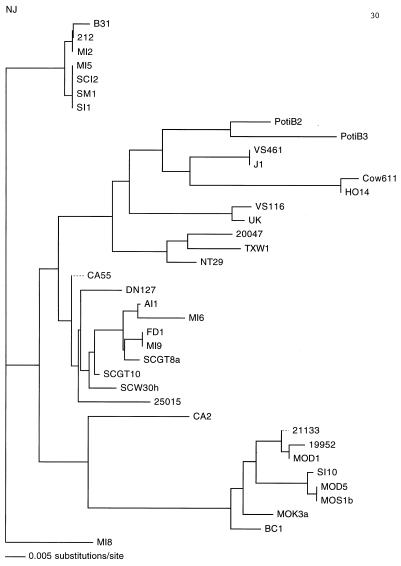
To confirm the results of PCR-RFLP analysis and to assess the DNA relatedness within and between genospecies and genogroups, the complete sequences of the rrf-rrl intergenic spacers from 20 southeastern and Missouri strains were determined. The DNA sequence analyses of rrf-rrl intergenic spacers confirmed our PCR-RFLP results. The 20 strains clustered into five separate lineages (Fig. 2). MI-8 and TXW-1 were located in separate lineages in the phylogenetic tree. TXW-1 was close to 20047, the reference strain of B. garinii; however, further studies are needed before a final conclusion concerning its taxonomic status can be made. Whether MI-8 belongs to B. burgdorferi sensu stricto or to a new genospecies will also require additional studies. MOK-3a, MOK-3b, MOK-3c, MOK-3d, MOR-2, MOS-le, MOS-1g, and MON-1 formed a subbranch in the B. andersonii lineage (Tables 2 and 3; Fig. 2 [only the results for representative strain MOK-3a are shown in Table 2 and Fig. 2]), as did strains AI-1 and SCGT-8a in the B. bissettii lineage (Tables 2 and 3; Fig. 2). Strains AI-1 and SCGT-8a had different MseI restriction patterns, and their rrf-rrl intergenic spacer regions possessed different DNA sequences. As noted earlier, their taxonomic status needs to be evaluated further. The DNA sequences of the 241- to 258-bp rrf-rrl intergenic spacer region were very stable among the strains, but about 35 bases varied. Some of the DraI and MseI restriction sites were located in various regions, thus allowing the possibility of using restriction patterns to identify and classify Borrelia species.
FIG. 2.
Phylogenetic tree determined from the DNA sequences of rrf-rrl intergenic spacer amplicons of southern strains of B. burgdorferi sensu lato. The neighbor-joining tree is constructed with PAUP software and is based on a comparison of 258-bp nucleotides of the rrf-rrl intergenic spacer sequence. The tree was compared with the trees produced by the maximum-likelihood and parsimony methods and the unweighted pair group method with arithmetic averages methods with PAUP software. The four methods produced similar results. The scale bar represents the calculated distance value.
Association between genetic variation of B. burgdorferi sensu lato strains and their animal hosts, tick vectors, and geographic sites.
Among nine strains identified as B. burgdorferi sensu stricto in Georgia and Florida, four were from cotton mice, two were from cotton rats, two were from I. scapularis ticks, and one was from an I. affinis tick (Table 4). Thirty-seven strains characterized as B. andersonii were cultured from I. dentatus ticks from Missouri and Georgia, and one strain (SI-10) from an I. scapularis tick from Georgia was also recognized as B. andersonii. Thus, there were a total of 38 B. andersonii strains, which included strains of the MOK-3a subgroup (8 strains). Seven B. bissettii strains (including AI-1 and SCGT-8a) were cultured from I. minor ticks, cotton mice, cotton rats, and wood rats from South Carolina and Florida (Table 4). Strains MI-8 and TXW-1 were isolated from a cotton rat and a D. variabilis tick from Florida and Texas, respectively (Table 4).
TABLE 4.
Relationships between Borrelia strains and their hosts, vectors, and geographic sites in the southern United States
| Genospecies (no. of strains) | Tick or vertebrate host (no. of strains) | Distribution (no. of strains) |
|---|---|---|
| B. burgdorferi sensu stricto (9) | I. affinis (1) | Georgia (7) |
| I. scapularis (2) | Florida (2) | |
| Cotton mice (4) | ||
| Cotton rats (2) | ||
| B. andersonii (38 strains including 8 strains in MOK-3a group) | I. dentatus (29) | Missouri (28) |
| I. scapularis (1) | Georgia (2) | |
| MOK-3a group (8) | I. dentatus (8) | Missouri (8) |
| B. bissettii (7 strains including AI-1 and SCGT-8a) | I. minor (1) | South Carolina (2) |
| Wood rat (1) | Florida (3) | |
| Cotton mouse (1) | ||
| Cotton rat (2) | ||
| AI-1 | Cotton rats (1) | Florida (1) |
| SCGT-8a | I. minor (1) | South Carolina (1) |
| Borrelia spp. (2) | Cotton rat (1) | Florida (1) |
| D. variabilis (1) | Texas (1) |
DISCUSSION
A large amount of genetic heterogeneity is present among B. burgdorferi sensu lato isolates from the United States (16), and there is greater variability among those from western and southern regions than among those from northern ones (16, 22, 24; Lin et al., Proc. VIII Int. Conf. Lyme Borreliosis Other Emerging Tick-Borne Dis.). Currently, three U.S. genospecies are recognized among the B. burgdorferi sensu lato complex: B. burgdorferi sensu stricto (5, 13), B. andersonii (15), and B. bissettii (24). There is evidence that additional genospecies exist in the western United States (24), and we present data herein indicating that additional ones exist in the southern United States.
It is well known that B. burgdorferi sensu stricto is pathogenic for humans, but the roles of B. andersonii and B. bissettii in human illness are unknown. It might be presumed that B. andersonii does not infect humans because its vector tick, I. dentatus, does not usually bite humans, but a recent paper reports that I. dentatus ticks attached to three humans in three different counties in North Carolina (11). Also, a partly fed I. dentatus nymph infected with an unusual B. burgdorferi strain was found to be biting a human in the northeastern United States (2). The Borrelia strain was similar to other isolates from I. dentatus ticks feeding on rabbits in that area (1), and those spirochetes were subsequently recognized as B. andersonii (15). Moreover, among 99 isolates examined, the isolate from the I. dentatus tick feeding on a human and 6 other unusual B. burgdorferi isolates from I. scapularis ticks reacted with sera from humans with early or late Lyme disease. Novel strains of borreliae occur in ticks feeding on humans, and therefore, at least some humans in that area are likely exposed to strains of borreliae other than the classic B31 type strains.
The question of the infectivities and pathogenicities of North American B. bissettii strains for humans is unanswered. However, although strain 25015 (B. bissettii) isolated from an I. scapularis tick in New York was originally thought to be infectious but not pathogenic (3), it was later confirmed to cause mild arthritis in mice (10). Strains belonging to this genospecies have been isolated from the dusky-footed wood rat (Neotoma fuscipes) and Ixodes spinipalpis and I. pacificus ticks from California (24). Also, strains were isolated from the cotton mouse, the cotton rat, the eastern wood rat, and an I. minor tick (attached to a Carolina wren) from South Carolina (Tables 1 and 4). These records of naturally occurring infections in three species of rodents, a bird species, and three species of Ixodes ticks in the southeastern United States indicate that strains of B. bissettii are not narrowly confined to particular vertebrate host or tick species and that this species is widely distributed geographically in the United States. However, strains of B. bissettii reported thus far in the United States occur in moderate climatic regions (except strain 25015 from New York State) and have been isolated from a total of four tick species, four rodent species, and one bird species. Moreover, strains genetically similar to those of B. bissettii from New York, California, South Carolina, and Florida have been isolated from several humans in Slovenia. Those patients had clinical presentations ranging from relatively benign illness to some severe afflictions. Some of the patients had variable and unpredictable serologic responses, including a lack of antibody response despite disseminated disease (29). Interestingly, some of the Lyme disease patients in the southern United States also lacked a serologic response to antigens derived from B. burgdorferi sensu stricto (9). More isolates of B. bissettii from humans and especially isolates from the United States need to be studied to allow a better understanding of its frequency and pathogenicity in humans.
B. burgdorferi sensu stricto is common in the southern United States and occurs naturally in a wide range of vertebrates and several tick species (16, 19, 22; Lin et al., Proc. VIII Int. Conf. Lyme Borreliosis Other Emerging Tick-Borne Dis.). Serologic evidence indicates that 27% of 535 cotton mice from North Carolina, South Carolina, Georgia, Florida, Alabama, and Mississippi that were tested had antibodies to B. burgdorferi (14). Moreover, cultivation of a large number of B. burgdorferi sensu stricto isolates from rodents and ticks from Georgia, Florida, and South Carolina, including a residential suburb of Charleston, S.C. (22), plus the occurrence of physician-diagnosed Lyme disease cases in Georgia and South Carolina (9), strongly suggest that B. burgdorferi sensu stricto causes some Lyme disease cases in the southern United States. Nevertheless, it also appears that alternative Borrelia species or novel uncharacterized infectious agents may account for most cases of erythema migrans lesions in the southeastern United States (9).
Of the 56 spirochetal isolates reported on here, most of those from I. dentatus ticks (from both Missouri and Georgia) appear to be B. andersonii, but genetic variation exists even among these isolates. Geographic sites, host, and tick species appear to influence the genospecies distribution of Borrelia. For example, BC-1, isolated from Georgia, showed an MseI restriction pattern slightly different from those of the Missouri strains, although they were isolated from different ticks of the same species. Moreover, some strains isolated from the same geographic site, the same tick, and the same host species exhibited different restriction patterns (e.g., MOD-1 and MOD-3 or MOD-5, MOR-1, and MOR-2). Also, different strains isolated from different ticks feeding on the same rabbit at the same geographic site had different restriction patterns. This fact suggests that coinfection of two or more B. andersonii strains probably occurs in nature. The MOK-3a group separated in a deep branch of the B. andersonii lineage in the phylogenetic tree (Fig. 2). Those strains also possessed different DraI restriction patterns within B. andersonii (Table 2). Whether they should be assigned to a subtype of B. andersonii or whether they should be recognized as a separate genospecies is uncertain. It was surprising that the SI-10 strain (from Sapelo Island, Ga.) appeared to belong to B. andersonii; however, both its restriction patterns and the nucleotide sequences of its spacer confirmed its similarity to that group of strains. It is the first B. andersonii strain isolated from I. scapularis in the South, and it is unclear whether I. scapularis serves as an enzootic vector of it on Sapelo Island.
Several southeastern strains, isolated from I. scapularis and I. affinis ticks, cotton mice, and cotton rats, were identified as B. burgdorferi sensu stricto on the basis of PCR-RFLP analysis and sequence analysis of the rrf-rrl intergenic spacer. Our results confirm that typical B. burgdorferi sensu stricto strains plus other genetically variable strains are well established in a wide range of animal hosts and presumably are naturally transmitted by at least two tick species in the southeastern United States (19). Experimentally, both species are efficient vectors in the laboratory (17, 27). Several B. burgdorferi enzootic cycles exist in widely distributed foci in the South (18–22).
Typical and atypical B. bissettii strains were identified in the present study. Nevertheless, strains MI-6, MI-9, FD-1, SCW-30h, and SCGT-10 can be placed in the B. bissettii group because of their MseI and DraI restriction patterns, which are similar to the restriction pattern of this genospecies. However, strains AI-1 and SCGT-8a also appear to be B. bissettii, but their MseI restriction patterns differ from that of B. bissettii (Table 2). Their taxonomic status needs to be evaluated further. Mathiesen et al. (16) reported that strain AI-1 clustered with DN127 (B. bissettii) by pulsed-field gel electrophoresis but did not cluster with that group on the basis of ospA sequence analysis.
Strains MI-8 and TXW-1, isolated from a cotton rat from Florida and a D. variabilis tick from Texas, respectively, possessed distinct MseI and DraI restriction patterns. Phylogenetic analysis of the rrf-rrl intergenic spacer sequence indicated that these two strains were different from each other and from other strains. Strain MI-8 segregated into a separate lineage and did not fit into any previously described genospecies. However, it was closer to the B. burgdorferi sensu stricto strains than to B. bissettii or other genospecies that we analyzed. Unexpectedly, strain TXW-1 appeared to be closer to the European B. garinii genospecies than to other genospecies. This is the first time that a strain from the United States has been reported to be close to B. garinii. Whether this strain will be found to be part of the B. garinii genospecies or an undescribed genospecies remains to be determined after additional investigations are completed. It is not known whether these two strains can infect humans. Since MI-8 was isolated from a cotton rat, it may be presumed that it serves as a natural host. It is impossible to know if the coyote is a natural host for strain TXW-1 because the D. variabilis tick had previously fed on two other hosts during the larval and nymphal stages and may have become infected at those times. The infectivities and pathogenicities of most southern strains remain to be determined.
We conclude that at least three genospecies of B. burgdorferi sensu lato and probably two additional undescribed genospecies occur among southern isolates. Most Missouri tick strains analyzed were B. andersonii. B. burgdorferi sensu stricto, B. bissettii, and B. andersonii coexist in the southern United States.
ACKNOWLEDGMENTS
This research was supported in part by grant R37 AI-24899 from the National Institutes of Health and cooperative agreement U50/CCU410282 from the Centers for Disease Control and Prevention.
We thank J. F. Anderson (Connecticut Agriculture Experiment Station), G. Baranton (Pasteur Institute, Paris, France), M. Fukunaga (Fukuyama University, Fukuyama, Japan), and L. A. Durden (Georgia Southern University) for reviewing the manuscript and G. Teltow (Texas Department of Health) for sending us the D. variabilis tick from which we isolated spirochetal strain TXW-1.
REFERENCES
- 1.Anderson J F, Magnarelli L A, LeFebure R B, Andreadis T G, McAninch J B, Perng G C, Johnson R C. Antigenically variable Borrelia burgdorferi isolated from cottontail rabbits and Ixodes dentatus in rural and urban areas. J Clin Microbiol. 1989;27:13–20. doi: 10.1128/jcm.27.1.13-20.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J F, Flavell R A, Magnarelli L A, Barthold S W, Kantor F S, Wallich R, Persing D H, Mathiesen D, Fikrig E. Novel Borrelia burgdorferi isolates from Ixodes scapularis and Ixodes dentatus ticks feeding on humans. J Clin Microbiol. 1996;34:524–529. doi: 10.1128/jcm.34.3.524-529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson J F, Barthold S W, Magnarelli L A. Infectious but nonpathogenic isolate of Borrelia burgdorferi. J Clin Microbiol. 1990;28:2693–2699. doi: 10.1128/jcm.28.12.2693-2699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assous M V, Postic D, Paul G, Nevot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 5.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 6.Brown R N, Lane R S. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science. 1992;256:1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- 7.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grumwaldt E, Davis J P. Lyme disease—a tick borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein J. PHYLIP—phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 9.Felz M W, Chandler F W, Jr, Oliver J H, Jr, Rahn D W, Schriefer M E. Solitary erythema migrans in Georgia and South Carolina. Arch Dermatol. 1999;135:1317–1326. doi: 10.1001/archderm.135.11.1317. [DOI] [PubMed] [Google Scholar]
- 10.Fikrig E, Bathold S W, Persing D H, Sun X, Kantor F S, Flavell R A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992;148:2256–2260. [PubMed] [Google Scholar]
- 11.Harrison B A, Engber B R, Apperson C S. Ticks (Acari: Ixodida) uncommonly found biting humans in North Carolina. J Vector Ecol. 1997;22:6–12. [PubMed] [Google Scholar]
- 12.Higgins D G, Sharp R. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biol Sci. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R C, Hyde F W, Schmid G P, Brenner D J. Borrelia burgdorferi sp. nov.: etiological agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 14.Magnarelli L A, Oliver J H, Jr, Hutcheson H J, Boone J L, Anderson J F. Antibodies to Borrelia burgdorferi in rodents in the eastern and southern United States. J Clin Microbiol. 1992;30:1449–1452. doi: 10.1128/jcm.30.6.1449-1452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marconi R T, Liveris D, Schwartz I. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersonii sp. nov.) isolates. J Clin Microbiol. 1995;33:2427–2434. doi: 10.1128/jcm.33.9.2427-2434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathiesen D A, Oliver J H, Jr, Kolbert C P, Tullson E D, Johnson B J B, Campbell G L, Mitchell P D, Reed K D, Telford III S R, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 17.Oliver J H, Jr, Chandler F W, Jr, Luttrell M P, James A M, Stallknecht D E, McGuire B S, Hutcheson H J, Cummins G A, Lane R S. Isolation and transmission of the Lyme disease spirochete from the southeastern United States. Proc Natl Acad Sci USA. 1993;90:7371–7375. doi: 10.1073/pnas.90.15.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver J H, Jr, Chandler F W, Jr, James A M, Sanders F H, Jr, Hutcheson H J, Huey L O, McGuire B S, Lane R S. Natural occurrence and characterization of the Lyme disease spirochete, Borrelia burgdorferi, in cotton rats (Sigmodon hispidus) from Georgia and Florida. J Parasitol. 1995;81:30–36. [PubMed] [Google Scholar]
- 19.Oliver J H., Jr Lyme borreliosis in the southern United States: a review. J Parasitol. 1996;82:926–935. [PubMed] [Google Scholar]
- 20.Oliver J H., Jr Importance of systematics to public health: ticks, microbes, and disease. Ann Mo Bot Garden. 1996;83:37–46. [Google Scholar]
- 21.Oliver J H, Jr, Kollars T M, Jr, Chandler F W, Jr, James A M, Masters E J, Lane R S, Huey L O. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1–5. doi: 10.1128/jcm.36.1.1-5.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver J H, Jr, Clark K L, Chandler F W, Jr, Lin T, James A M, Banks C W, Huey L O, Banks A R, Williams D C, Durden L A. Isolation, cultivation, and characterization of Borrelia burgdorferi from rodents and ticks in the Charleston area of South Carolina. J Clin Microbiol. 2000;38:120–124. doi: 10.1128/jcm.38.1.120-124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 24.Postic D, Marti Ras N, Lane R S, Hendson M, Baranton G. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryffel K, Péter O, Rutti B, Suard A, Dayer E. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in humans. J Clin Microbiol. 1999;37:4086–4092. doi: 10.1128/jcm.37.12.4086-4092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Sanders F H, Jr, Oliver J H., Jr Evaluation of Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis (Acari: Ixodidae) from Georgia as vectors of a Florida strain of the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1995;32:402–406. doi: 10.1093/jmedent/32.4.402. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strle F, Picken R N, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Ruzic-Sabljic E, Picken M M. Clinical findings for patients with Lyme borreliosis caused by Borrelia burgdorferi sensu lato with genotypic and phenotypic similarities to strain 25015. Clin Infect Dis. 1997;25:273–280. doi: 10.1086/514551. [DOI] [PubMed] [Google Scholar]
- 30.Swofford D L. PAUP: phylogenetic analysis using parsimony version 3.0.I. Champaign: Illinois Natural History Survey; 1992. [Google Scholar]
- 31.Van Dam A P, Kuiper H, Vos K, Widjojokusumo A, deJongh B M, Spanjaard L, Ramselaar A C P, Kramer M D, Donkert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]