Abstract
Context.—
Postmortem evaluation for neurodegenerative disease is expensive in time and materials. These challenges can be met by implementing simpler sampling protocols while preserving anatomic relations.
Objective.—
To determine the diagnostic effectiveness and cost-effectiveness of a simplified brain blocking protocol compared with the standard blocking protocol used in our Alzheimer’s Disease Research Center (ADRC).
Design.—
We prospectively compared the neuropathologic diagnoses established from our standard 19-cassette/19 brain sites ADRC protocol to a simplified 6-cassette/12 brain sites protocol in 52 consecutive cases. The simplified protocol generated 14 slides for comparison to 52 slides from our standard protocol.
Results.—
Compared with the ADRC protocol the simplified protocol produced Alzheimer Disease Neuropathologic Changes probability scores that were the same in 50 of 52 cases (r = 0.99). Staging for Lewy pathology was equivalent in 45 of 52 (r = 0.98), scoring for cerebral amyloid angiopathy was equivalent in 48 of 52 (r = 0.97), and grading for arteriolosclerosis was the same in 45 of 52 cases (r = 0.92). Progressive supranuclear palsy (n = 4), multiple system atrophy (n = 2), and corticobasal degeneration (n = 1) could be diagnosed by either protocol independently. The estimated savings per case was 72% or $1744.89 ($2436.37 [ADRC] versus $691.48 [simplified]).
Conclusions.—
The diagnosis of neurodegenerative disease at autopsy can be done accurately with a less expensive, simplified protocol. Our protocol is similar to those of previously published approaches, but it has a simpler organization scheme. This method should be valuable to institutions where autopsy cost considerations may be important.
Limited funding for autopsies generates significant monetary concerns for any center with a busy autopsy service. In the period 2017–2018 at the Massachusetts General Hospital, Boston Massachusetts, we performed 852 autopsies, 772 of which included examination of the brain. Although 205 of these cases were part of our Massachusetts Alzheimer’s Disease Research Center (MADRC), Boston Massachusetts, and received associated funding for processing of tissue, the other 567 received no outside support. Because Alzheimer disease (AD) is highly prevalent in the United States (estimated 5.7 million cases in 20181) and its burden increases significantly with age (3% of those aged 65–74 years, 17% of those aged 75–84 years, and 32% of those 85 years and older1), the ability to routinely screen for AD and other neuropathologic diseases at the time of autopsy is important from a diagnostic and public health standpoint. In a climate of increasing health costs, combined with a lack of reimbursement for autopsies, economic assessment of diagnostic methods is essential.
In 2018 we attempted to implement a condensed brain blocking protocol that results in considerable cost savings.2 We found this protocol to be cumbersome to prepare because it required sampling of 20 anatomic brain sites, with each piece assigned to a specifically designated place in 1 of 5 cassettes (4 brain sites per cassette). The small size of each piece limited examination of the anatomy. For example, the anatomic relationship between the hippocampus, parahippocampal gyrus, and occipital-temporal gyrus was not preserved, an important factor when performing Braak staging for neurofibrillary tangles. We also noted redundancies in the sampling (ie, bilateral thalamic sampling), which may be unnecessary for the diagnosis of neurodegenerative disease.
To avoid these issues while preserving the ability to reduce cost, we developed a simplified blocking protocol that required sampling 12 brain sites, with only 2 pieces of tissue placed in each cassette for a total of 6 cassettes. We found this method much easier to prepare, the blocking more intuitive to remember, and the anatomic relationships within each tissue piece better preserved. We validated the performance of this protocol by comparing it to our standard 19-cassette/19 brain sites MADRC protocol in 52 consecutive cases submitted to MADRC.
MATERIALS AND METHODS
We studied 52 consecutive submissions to our MADRC during the period of approximately 8 months with antemortem diagnoses of suspected AD (n = 18), parkinsonian-related disorder (n = 10), primary progressive aphasia (n = 6), frontotemporal dementia (n = 4), mixed dementia (n = 4), corticobasal syndrome (n = 3), cerebral amyloid angiopathy (n = 3), mild cognitive impairment (n = 3), multiple system atrophy (n = 2), normal pressure hydrocephalus (n = 1), and cases submitted as controls (n = 5; Table 3). We excluded from this study cases submitted for evaluation of amyotrophic lateral sclerosis, Huntington disease, and spinal muscular atrophy because we use a separate blocking protocol at our Alzheimer’s Disease Research Center (ADRC). Consent for autopsy, including consent for the processing of brain for research purposes, was obtained from the legal health care proxy in each case.
Table 3.
Case Details
| Case | Age y | Sex | Premortem Clinical Diagnosis | PMI, hr | Fresh Brain Weight, g |
|---|---|---|---|---|---|
| 1 | >90 | Male | AD | Unk | 937 |
| 2 | 76 | Female | AD | 12 | 820 |
| 3 | 80 | Male | AD | 12 | 1270 |
| 4 | 73 | Female | AD | 15 | 1000 |
| 5 | 66 | Female | AD | 14 | 1130 |
| 6 | 79 | Male | AD | 138 | 1360 |
| 7 | 88 | Female | AD | 24 | 1170 |
| 8 | 78 | Female | AD | 38 | 1040 |
| 9 | 74 | Male | AD | 30 | 1120 |
| 10 | >90 | Male | AD | 16 | 1270 |
| 11 | 58 | Male | AD, Presenilin-1 mutant | 30 | 1340 |
| 12 | 75 | Male | AD (PCA) | 18 | 1200 |
| 13 | 78 | Female | AD versus NPH | 28 | 1070 |
| 14 | 52 | Male | AD, Early onset | 10 | 1280 |
| 15 | 84 | Female | AD, ?lvPPA | 12 | 1180 |
| 16 | 59 | Female | AD versus FTD | 14 | 1120 |
| 17 | 89 | Female | Dementia, unclear etiology | 19 | 1170 |
| 18 | 75 | Male | Mixed dementia | 14 | 1100 |
| 19 | 85 | Male | Mixed dementia with CAA | 18 | 1330 |
| 20 | 84 | Male | AD with component of parkinsonism | 5 | 1350 |
| 21 | 84 | Female | Dementia, ?PD | 7 | 1170 |
| 22 | 67 | Female | CAA | 15 | 1240 |
| 23 | 67 | Male | CAA | 10 | 1520 |
| 24 | 62 | Female | MCI, ?CBS, ?FTLD | 6 | 1010 |
| 25 | 87 | Male | MCI | 18 | 1040 |
| 26 | 83 | Female | MCI | 12 | 970 |
| 27 | 75 | Male | CBS versus PD versus DLB versus AD versus mixed dementia | 7 | 1370 |
| 28 | 90 | Female | PD | Unk | Unk |
| 29 | 75 | Male | PD | 12 | 1440 |
| 30 | >90 | Male | PD | 36 | 1060 |
| 31 | 87 | Male | PD | 48 | 1500 |
| 32 | 75 | Female | PD | 24 | 1270 |
| 33 | 82 | Female | PDD | 12 | 1260 |
| 34 | 73 | Male | DLB | 7 | 1200 |
| 35 | 70 | Female | FTD with component of CBS | 24 | 878 |
| 36 | 70 | Male | FTD, known GRN mutation | 24 | 1040 |
| 37 | 61 | Male | FTD | 14 | 1070 |
| 38 | 73 | Male | FTD | 21 | 1000 |
| 39 | 75 | Female | svPPA | 4 | 1010 |
| 40 | 64 | Female | lvPPA | Unk | Unk |
| 41 | 70 | Male | lvPPA | 7 | 1250 |
| 42 | 79 | Male | navPPA | 12 | 1500 |
| 43 | 74 | Male | navPPA, ?PSP | 18 | 1170 |
| 44 | 70 | Female | PSP | 13 | 1040 |
| 45 | 71 | Female | PSP | 7 | 1140 |
| 46 | 62 | Male | MSA-P | 36 | 1490 |
| 47 | 69 | Female | MSA-C | 72 | 1100 |
| 48 | >90 | Female | Control;?Mixed dementia | 36 | 970 |
| 49 | >90 | Female | Control | 30 | 1080 |
| 50 | >90 | Male | Control | 36 | 1230 |
| 51 | >90 | Male | Control | Unk | 1030 |
| 52 | 89 | Male | Control | 36 | 1240 |
Abbreviations: AD, Alzheimer disease; CAA, cerebral amyloid angiopathy; CBS, corticobasal syndrome; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; FTLD, frontotemporal lobar degeneration; lvPPA, logopenic variant primary progressive aphasia; MCI, mild cognitive impairment; MSA-C, multiple system atrophy–cerebellar; MSA-P, multiple system atrophy–parkinsonian; navPPA, nonfluent/agrammatic variant primary progressive aphasia; NPH, normal pressure hydrocephalus; PCA, posterior cortical atrophy; PD, Parkinson disease; PDD, Parkinson disease dementia; PMI, postmortem interval; PSP, progressive supranuclear palsy; svPPA, semantic variant primary progressive aphasia; Unk, unknown.
Each brain was processed according to our standard MADRC protocol, with the brain divided at the midline at the time of autopsy, with one-half cut into coronal slabs for freezing at −80°C and the other half fixed in 10% buffered formalin. Following at least 1 week of formalin fixation, the fixed hemisphere was sectioned in the coronal plane at standard landmarks as previously described.3 Twelve brain sites were sampled from the formalin-fixed side and placed in 6 cassettes (hereafter called simplified protocol; Figure 1 and Table 1). Nineteen additional brain sites were sampled from the formalin-fixed side and placed in 19 cassettes for further processing, staining, and microscopic analysis, according to our standard ADRC protocol (Figure 2 and Table 2). Histologic sections corresponding to blocks in Figure 1 (1 through 6) are seen in Figure 3 (A through F).
Figure 1.
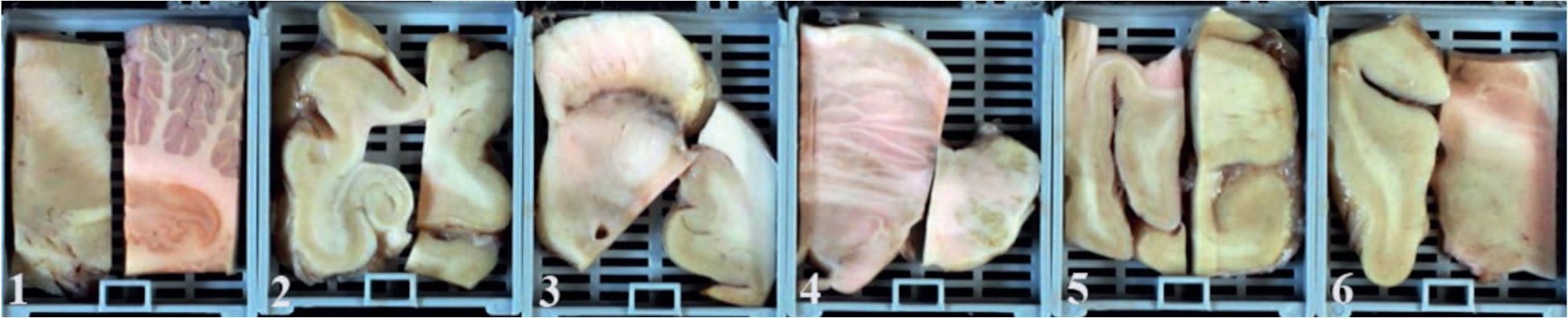
Cassettes used for the simplified protocol (numbered 1–6). 1, Cerebellum; basal ganglia; 2, hippocampus at lateral geniculate nucleus (LGN); superior frontal cortex (Brodmann Area [BA] 8, 9); 3, midbrain; cingulate gyrus (BA24); 4, medulla oblongata; pons; 5, calcarine cortex (BA17); superior frontal cortex (BA7); 6, thalamus; temporal pole (BA38). See Table 1 for staining protocol.
Table 1.
Simplified Staining Protocola
| Block | L/H&E | Tau | β-Amy | α-syn |
|---|---|---|---|---|
| 1 | Y | Y | ||
| 2 | Y | Y | Y | Y |
| 3 | Y | Y | Y | |
| 4 | Y | Y | ||
| 5 | Y | Y | ||
| 6 | Y |
Abbreviations: α-syn, α-synuclein immunohistochemistry; β-Amy, β-amyloid immunohistochemistry; L/H&E, Luxol fast blue/hematoxylin-eosin; tau, hyperphosphorylated tau immunohistochemistry.
The above numbers (1–6) correspond to the block images depicted in Figure 1.
Figure 2.
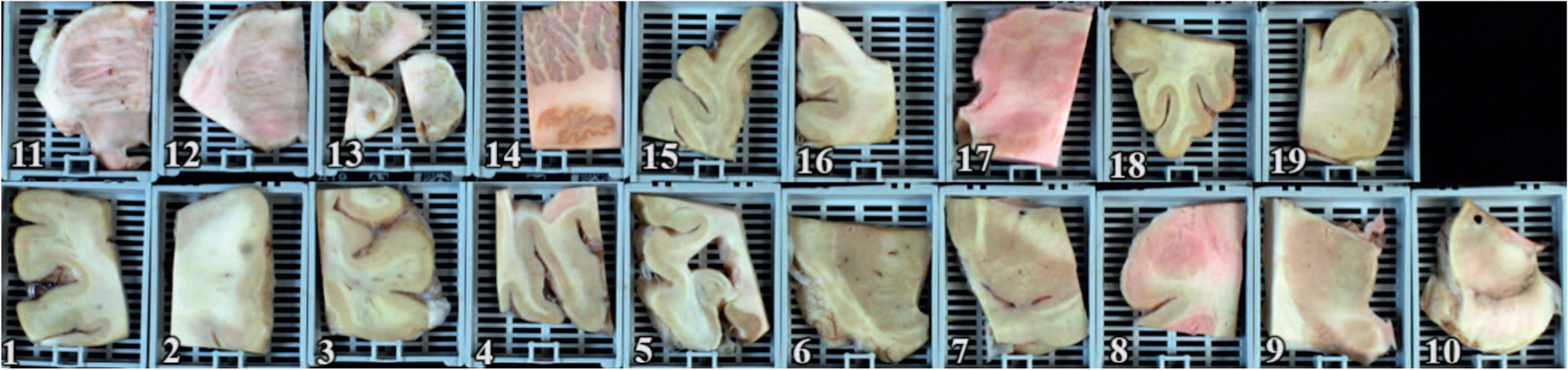
Cassettes used for the MADRC protocol (numbered 1–19). 1, Superior frontal cortex (Brodmann Area [BA] 8, 9); 2, primary motor/ sensory strip (BA 3, 4); 3, superior parietal cortex (BA7); 4, calcarine cortex (BA17); 5, hippocampus at lateral geniculate nucleus (LGN); 6, caudate, putamen, nucleus accumbens; 7, globus pallidus, putamen, substantia innominate; 8, amygdala and entorhinal cortex; 9, thalamus (level of centrum medianum); 10, midbrain; 11, upper pons (level of locus ceruleus); 12, lower pons (at inferior border of cranial nerve V); 13, medulla oblongata; 14, cerebellum (with dentate nucleus); 15, temporal pole (BA38); 16, cingulate gyrus (BA24); 17, thalamus with anterior nucleus, ventral anterior, ventral lateral, and subthalamic nucleus; 18, inferior frontal cortex (BA 10, 11, 12); 19, inferior parietal cortex (BA 39, 40). See Table 2 for staining protocol.
Table 2.
Massachusetts Alzheimer’s Disease Research Center Staining Protocola
| Block | L/H&E | Biels | Tau | β-Amy | TDP-43 | α-syn |
|---|---|---|---|---|---|---|
| 1 | Y | Y | Y | Y | Y | |
| 2 | Y | Y | ||||
| 3 | Y | Y | Y | Y | Y | |
| 4 | Y | Y | Y | Y | ||
| 5 | Y | Y | Y | Y | Y | Y |
| 6 | Y | Y | ||||
| 7 | Y | Y | ||||
| 8 | Y | Y | Y | Y | Y | Y |
| 9 | Y | Y | ||||
| 10 | Y | Y | ||||
| 11 | Y | |||||
| 12 | Y | |||||
| 13 | Y | |||||
| 14 | Y | Y | ||||
| 15 | Y | Y | ||||
| 16 | Y | Y | Y | Y | ||
| 17 | Y | |||||
| 18 | Y | |||||
| 19 | Y | Y | Y |
Abbreviations: α-syn, α-synuclein immunohistochemistry; β-Amy, β-amyloid immunohistochemistry; Biels, Bielschowsky silver stain; L/H&E, Luxol fast blue/hematoxylin-eosin; tau, hyperphosphorylated tau immunohistochemistry.
The above numbers (1–19) correspond with the block images depicted in Figure 2.
Figure 3.
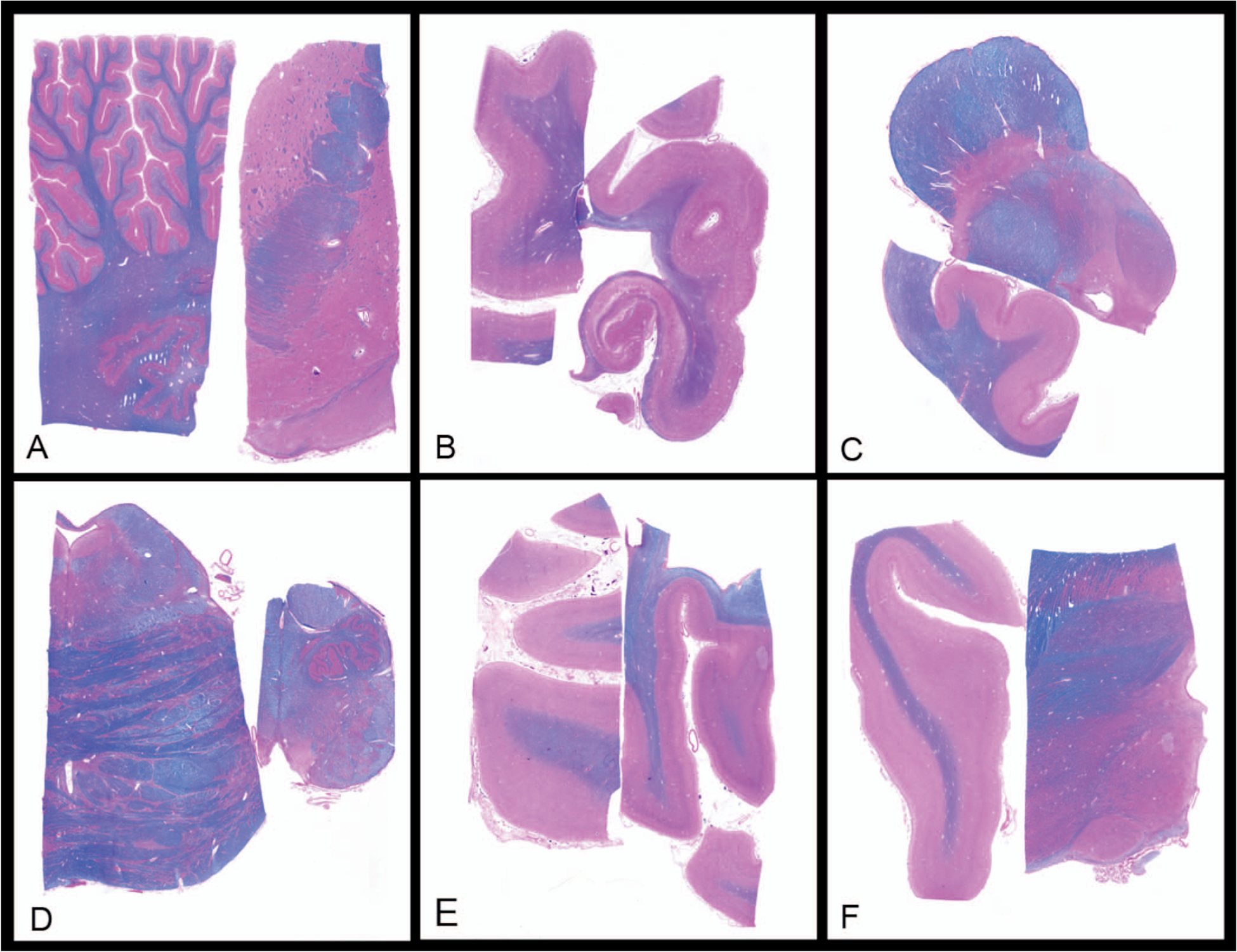
Microscopy from simplified protocol. A, Cerebellum; basal ganglia. B, Hippocampus at lateral geniculate nucleus (LGN); superior frontal cortex (Brodmann Area [BA] 8, 9). C, Midbrain; cingulate gyrus (BA24). D, Medulla oblongata; pons. E, Calcarine cortex (BA17); superior parietal cortex (BA7). F, Thalamus; temporal pole (BA38) (Luxol Fast Blue and hematoxylin-eosin, original magnification ×1).
Tissues for the simplified protocol were processed in the clinical laboratory at Massachusetts General Hospital on a Leica Peloris tissue processor (Leica Biosystems Inc, Buffalo Grove, Illinois) and were embedded in paraffin. Sections of 5 μm sections were stained with Luxol Fast Blue and hematoxylin-eosin (L/H&E) for routine assessment. Eight μm sections from selected blocks were immunostained for hyperphosphorylated tau (Polyclonal Rabbit Anti-Human Tau, Dako, Glostrup, Denmark) at a titration of 1:150 following antigen retrieval with Enzyme 1, β-amyloid (rabbit monoclonal, clone RBT-A4, BioSB, Santa Barbara, California) ready-to-use following HIER for 2.5 minutes with ER2, and α-synuclein (mouse monoclonal, clone BSB-114, BioSB) ready-to-use following HIER for 20 minutes with ER1 using a Leica Bond III automated stainer (Leica Biosystems, Wetzlar, Germany) according to the manufacturer’s instructions. The simplified protocol resulted in a total of 6 L/H&E, 2 tau, 3 β-amyloid, and 3 α-synuclein stained slides (14 slides in total; Table 1).
Tissues for the MADRC protocol were handled in our research lab. The formalin-fixed blocks were treated with 88% formic acid for 60 minutes followed by processing on a Thermo Scientific Excelsior ES tissue processor (Thermo Fisher Scientific, Waltham, Massachusetts) and embedded in paraffin. Sections 7 μm thick were stained with L/H&E for routine assessment. Staining for Bielschowsky silver stain was performed on sections from selected blocks (Table 2). Immunohistochemistry for hyperphosphorylated tau (Polyclonal Rabbit Anti-Human Tau) at a titration of 1:6000, β-amyloid (Monoclonal mouse Anti-Human Beta-Amyloid clone 6F/3D, Dako) at a titration of 1:600, α-synuclein (Mouse anti-α-Synuclein LB509, Life Technologies Corp, Frederick, Maryland) at a titration of 1:200, and p-TDP-43 (Cosmo Bio, Tokyo, Japan) at a titration of 1:3000 were performed on 7-μm–thick sections from select blocks and processed on a Leica Bond RX automated stainer (Leica Biosystems, Wetzlar, Germany) according to the manufacturer’s instructions. The MADRC protocol resulted in 19 L/H&E, 8 Bielschowsky silver, 7 tau, 6 α-synuclein, 10 β-amyloid, and 2 p-TDP-43 stained slides (52 slides in total; Table 2).
All cases were prospectively analyzed by 1 neuropathology fellow and a neuropathology attending pathologist. The MADRC and simplified protocols were evaluated concurrently but scored independently for evidence of neuropathologic disease. The extent of Alzheimer disease neuropathologic changes (ADNCs) was established using the 2012 NIAA guidelines.4 Staining for β-amyloid was used for Thal phase (scale, 0–5).5 Bielschowsky silver stain and hyperphosphorylated tau stains were used for Braak and Braak neurofibrillary tangles stage (scale, none to VI) and CERAD neuritic plaque score (scale, none to frequent) in the ADRC protocol.6–8 Only the hyperphosphorylated tau stain was used for neurofibrillary tangles staging and CERAD neuritic plaque score in the simplified protocol. The Thal, neurofibrillary tangles stage, and CERAD scores were used to generate an “ABC” score (scale, 0–3), which in turn was used to determine the probability of ADNC (not, low, intermediate, and high).
The extent of Lewy body disease was similarly determined and scored according to Braak staging (scale, 0–6) for each protocol.9–11
Comparisons for cerebrovascular disease were also performed. The extent of cerebral amyloid angiopathy was scored on a 5-point scale (scale, 0–4; 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) as described previously.12,13 Arteriolosclerosis was scored as none, mild, moderate, or severe. Microinfarcts were scored as present or not. Comparisons for macroinfarcts were not done because these require targeted sampling at the time of grossing which deviates from either protocol.
Evaluation for presence of abnormal TDP-43 staining was done in the MADRC protocol. From the cases that were positive for TDP-43 with the MADRC protocol, sections from the simplified protocol-hippocampal block were stained for TDP-43 at the research lab, for a retrospective comparison.
RESULTS
The cases included 28 men (28 of 52; 53.8%) and 24 women (24 of 52; 46.2%) with a mean age of 78 years (range, 52–102 years), mean pre–formalin fixation brain weight of 1170 g (range, 820–1520 g), and mean postmortem interval time of 22 hours (range, 4–138 hours; Table 3).
The anatomic relationships were well preserved with the simplified protocol (Figure 1). The cassette with the hippocampus and superior frontal gyrus allowed for preservation of the relationship of Ammon horn with the parahippocampal and occipital-temporal gyri as well as in some instances (depending on the degree of atrophy) a portion of the inferior temporal gyrus (Figure 1, cassette 2). The cassette with the cerebellum and basal ganglia included the cerebellar cortex, deep cerebellar white matter, and a portion of the dentate nucleus (Figure 1, cassette 1). This cassette also allowed for representation of the caudate, internal capsule, and putamen, and in some instances the globus pallidus. The cassette with the temporal cortex included thalamus and subthalamic nucleus (Figure 1, cassette 6). This area was helpful in the evaluation of progressive supranuclear palsy and corticobasal degeneration. Taking a smaller section of cingulate gyrus allowed for the entire axial half of the midbrain to be sampled in the same cassette (Figure 1, cassette 3). The cassette with the calcarine sulcus included the primary and association visual cortices (allowing for a determination of Braak V versus Braak VI) as well as the separately sampled superior parietal cortex (Figure 1, cassette 5).
The overall probability scores for ADNC were the same for the ADRC and simplified protocols in 50 of 52 cases (98% accuracy, r = 0.99), with equivalent scores of “not” (n = 9), “low” (n = 13), “intermediate” (n = 3), and “high” (n = 25; Table 4). The 2 cases that differed were both categorized as “low” with the simplified protocol and “intermediate” with the ADRC protocol. There were no differences in the “not” and “high” categories across protocols (Table 4). Examples of Thal 5, Braak VI, and CERAD frequent via immunohistochemistry from the simplified protocol are shown in Figure 4, A through C.
Table 4.
Neuropathology Results, Massachusetts Alzheimer’s Disease Research Center (MADRC; 19) Versus Simplified (6)a
| Case | Premortem Clinical Diagnosis | 19 ADNC | 6 ADNC | 19 LBD Bk | 6 LBD Bk | 19 CAA | 6 CAA | 19 AS | 6 AS | 19 Other | 6 Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AD | H | H | 0 | 0 | 2 | 2 | 2 | 2 | Mic-inf | None |
| 2 | AD | H | H | 0 | 0 | 0 | 0 | 0 | 0 | HS-TDP43 | HS-TDP43b |
| 3 | AD | H | H | 5 | 5 | 1 | 2 | 3 | 3 | Mic-inf | None |
| 4 | AD | H | H | AD-rel | AD-rel | 2 | 3 | 3 | 3 | TDP43pr | TDP43prb |
| 5 | AD | H | H | 0 | 0 | 0 | 0 | 1 | 1 | None | None |
| 6 | AD | H | H | 0 | 0 | 2 | 2 | 2 | 2 | None | None |
| 7 | AD | H | H | 0 | 0 | 3 | 3 | 3 | 3 | AHI | AHI |
| 8 | AD | H | H | AD-rel | AD-rel | 3 | 3 | 2 | 2 | None | None |
| 9 | AD | H | H | 0 | 0 | 1 | 1 | 2 | 1 | None | None |
| 10 | AD | i | L | 0 | 0 | 2 | 2 | 2 | 2 | TDP43pr | None |
| 11 | AD, Presenilin-1 mutant | H | H | 0 | 0 | 2 | 1 | 1 | 1 | None | None |
| 12 | AD (PCA) | H | H | 5 | 5 | 3 | 3 | 0 | 0 | TDP43pr | TDP43prb |
| 13 | AD versus NPH | H | H | 0 | 0 | 1 | 2 | 2 | 2 | Mic-inf | None |
| 14 | AD, early onset | H | H | 5 | 5 | 2 | 2 | 2 | 2 | None | None |
| 15 | AD, ?lvPPA | H | H | AD-rel | 0 | 3 | 3 | 2 | 2 | TDP43pr | TDP43prb |
| 16 | AD versus FTD | i | i | 6 | 6 | 0 | 0 | 0 | 0 | TDP43pr | None |
| 17 | Dementia, unclear etiology | H | H | 5 | 3 | 2 | 2 | 3 | 3 | TDP43pr | TDP43prb |
| 18 | Mixed dementia | H | H | 0 | 0 | 0 | 0 | 3 | 3 | TDP43pr | TDP43prb |
| 19 | Mixed dementia with CAA | H | H | AD-rel | 0 | 0 | 0 | 2 | 2 | AHI | AHI |
| 20 | AD with component of parkinsonism | L | L | 5 | 4 | 0 | 0 | 2 | 2 | None | None |
| 21 | Dementia, ?PD | L | L | 5 | 5 | 2 | 2 | 3 | 3 | TDP43pr | TDP43prb, Mic-inf |
| 22 | CAA | H | H | 0 | 0 | 3 | 3 | 2 | 2 | None | None |
| 23 | CAA | L | L | 0 | 0 | 4 | 4 | 3 | 3 | AHI | AHI |
| 24 | MCI, ?CBS, ?FTLD | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | None | None |
| 25 | MCI | i | i | 0 | 0 | 2 | 2 | 1 | 1 | None | None |
| 26 | MCI | H | H | AD-rel | AD-rel | 2 | 2 | 2 | 2 | TDP43pr | TDP43prb |
| 27 | CBS versus PD versus DLB versus AD versus mixed | L | L | 5 | 5 | 2 | 2 | 1 | 1 | AHI | AHI |
| 28 | PD | i | i | 6 | 5 | 1 | 1 | 2 | 2 | AHI, Mic-inf | AHI |
| 29 | PD | 0 | 0 | 5 | 4 | 0 | 0 | 2 | 2 | None | None |
| 30 | PD | 0 | 0 | 3 | 3 | 0 | 0 | 3 | 3 | PART | PART |
| 31 | PD | i | L | 4 | 4 | 0 | 0 | 3 | 3 | TDP43pr | TDP43prb |
| 32 | PD | L | L | 5 | 5 | 0 | 0 | 1 | 1 | None | None |
| 33 | PDD | L | L | 5 | 5 | 0 | 0 | 1 | 1 | AHI | None |
| 34 | DLB | H | H | 5 | 5 | 0 | 0 | 2 | 2 | None | None |
| 35 | FTD with component of CBS | H | H | 0 | 0 | 0 | 0 | 1 | 1 | None | None |
| 36 | FTD, known GRN mutation | L | L | 0 | 0 | 0 | 0 | 0 | 0 | FTLD-TDP, AHI | FTLD-TDPb, AHI |
| 37 | FTD | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | CBD | CBD |
| 38 | FTD | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | FTLD-TDP | FTLD-TDPb, AHI |
| 39 | svPPA | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | FTLD-TDP, AHI | FTLD-TDPb, AHI |
| 40 | lvPPA | H | H | 0 | 0 | 0 | 0 | 3 | 2 | None | None |
| 41 | lvPPA | H | H | AD-rel | AD-rel | 2 | 2 | 1 | 1 | None | None |
| 42 | navPPA | H | H | 0 | 0 | 0 | 0 | 1 | 1 | PSP | PSP |
| 43 | navPPA, ?PSP | L | L | 0 | 0 | 0 | 0 | 3 | 2 | PSP | PSP |
| 44 | PSP | L | L | 0 | 0 | 0 | 0 | 1 | 1 | PSP | PSP |
| 45 | PSP | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | PSP | PSP |
| 46 | MSA-P | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | MSA | MSA |
| 47 | MSA-C | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | MSA | MSA |
| 48 | Control; ?Mixed dementia | L | L | 5 | 4 | 2 | 2 | 2 | 1 | AHI | AHI |
| 49 | Control | L | L | 0 | 0 | 0 | 0 | 2 | 2 | AHI | AHI |
| 50 | Control | L | L | 0 | 0 | 0 | 0 | 3 | 2 | AHI | Mic-inf |
| 51 | Control | H | H | 5 | 5 | 3 | 3 | 3 | 2 | TDP43pr | TDP43prb |
| 52 | Control | L | L | 0 | 0 | 0 | 0 | 1 | 1 | Mic-inf | Mic-inf |
Abbreviations: ADNC, Alzheimer disease neuropathologic changes (scores: H, high; i, intermediate; L, low; 0, not); AD-rel, Alzheimer disease–related Lewy bodies; AHI, acute hypoxic/ischemic injury; AS, arteriolosclerosis (grades: 1, mild; 2, moderate; 3, severe); CAA, cerebral amyloid angiopathy (Vonsattel grades 0–4); CBD, corticobasal degeneration; CBS, corticobasal syndrome; FTD, frontotemporal dementia; FTLD, frontotemporal lobar degeneration; LBD Bk, Lewy body disease Braak staging (Braak stages 0–6); lvPPA, logopenic variant primary progressive aphasia; MCI, mild cognitive impairment; Mic-inf, microinfarct; MSA, multiple system atrophy; navPPA, nonfluent/agrammatic variant primary progressive aphasia; PART, primary age-related tauopathy; PSP, progressive supranuclear palsy; TDP43pr, TDP43-related proteinopathy; Unk, unknown.
Neuropathology results from the MADRC protocol (19) compared with the simplified protocol (6). Differences between the 2 protocols are italicized.
TDP43 immunohistochemistry done retrospectively (see Table 3 key for the clinical diagnosis acronyms).
Figure 4.
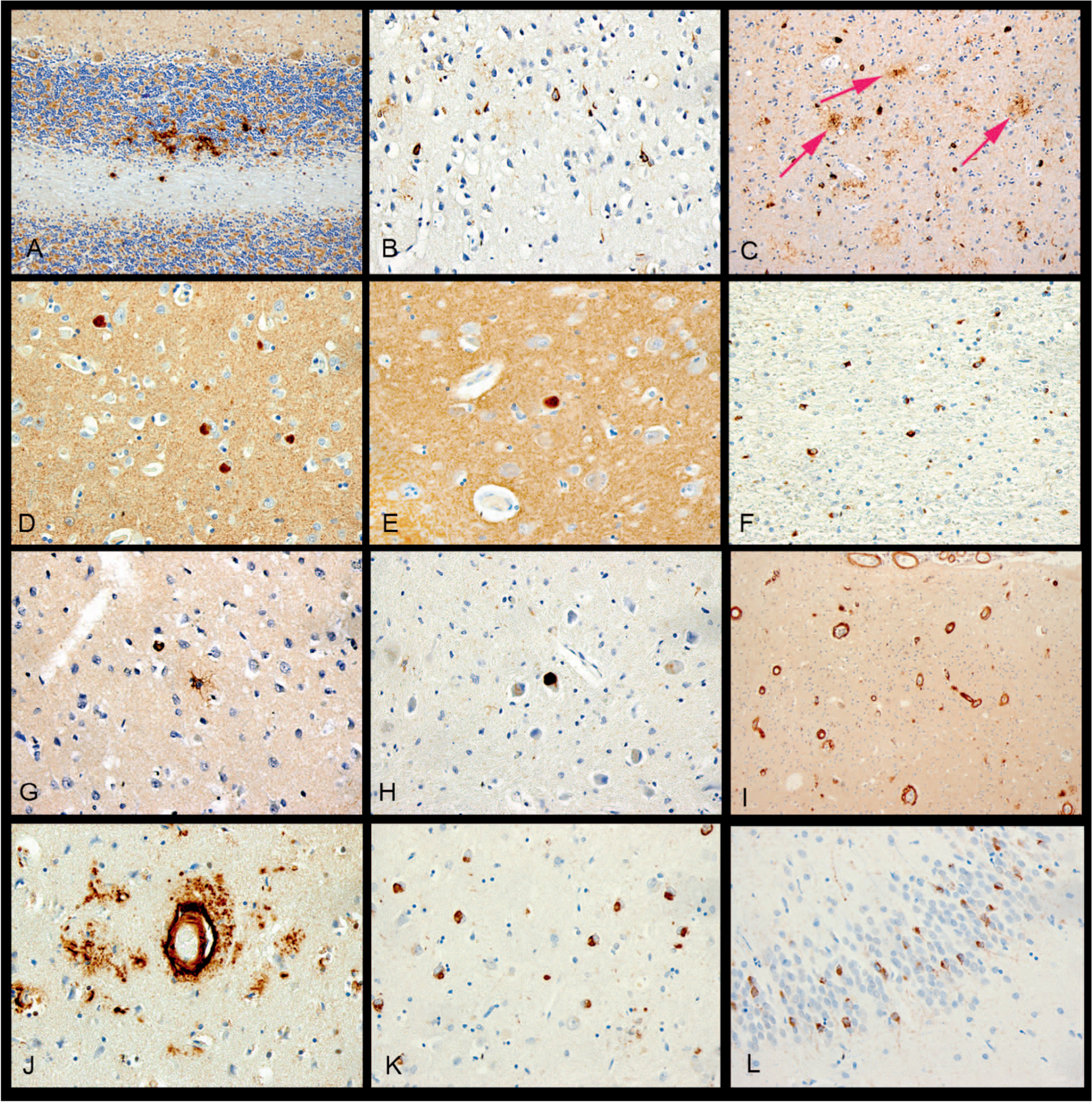
Immunohistochemistry examples from the simplified protocol. A, β-Amyloid with diffuse plaques involving cerebellum, example of Thal 5, “A3.” B, p-Tau with neurofibrillary tangles involving primary visual cortex, example of Braak VI, “B3.” C, p-Tau showing frequent neuritic plaques (arrows point to a few of these for emphasis) involving neocortex, CERAD frequent, “C3.” D, α-Synuclein with neocortical Lewy bodies in a case of Lewy body disease. E, α-Synuclein with a single Lewy body involving Cornu Ammonis subfield 1 (CA1) of the hippocampus, a location associated with Alzheimer disease. F, α-Synuclein showing cerebellar glial cytoplasmic inclusions in a case of multiple system atrophy. G, p-Tau showing a tufted astrocyte in progressive supranuclear palsy. H, p-Tau showing globose tangle in a case of corticobasal degeneration. I, β-Amyloid with cortical and leptomeningeal involvement by cerebral amyloid angiopathy (CAA). J, β-Amyloid with dyshoric CAA. K, p-TDP-43 showing neuronal cytoplasmic inlcusions (NCIs) in a case of frontotemporal lobar degeneration with TDP-43. L, p-TDP-43 demonstrating NCIs in the dentate gyrus of the hippocampus, in a location associated with Alzheimer disease (original magnifications ×200 [A, C, and F], ×400 [B, D, E, G, H, J, K, L], and ×40 [I]).
Assessment of Lewy pathology was the same for the MADRC and simplified protocols in 45 of 52 cases (87% accuracy), with equivalent scores of “none” (n = 30), Braak stages 3 (n = 1), 4 (n = 1), and 5 (n = 9), as shown in Figure 4, D; and 6 (n = 1), and “AD-associated” (n = 3), as shown in Figure 4, E. The 6 cases that differed were Braak and Braak differences of 5 versus 6 (n = 1), 4 versus 5 (n = 3), 3 versus 5 (n = 1), and “none” versus “AD associated” (n = 2). In each of the cases with differences in Braak or Lewy body scores, the simplified protocol had a lower score, but for cases assigned a Braak stage, the correlation was high (r = 0.98; Table 4).
Multiple system atrophy (n = 2; Figure 4, F), progressive supranuclear palsy (n = 4; Figure 4, G), and corticobasal degeneration (n = 1; Figure 4, H) were diagnosed with either protocol.
Scoring for cerebral amyloid angiopathy (Figure 4, I and J) was equivalent in 48 of 52 cases (92% accuracy, r = 0.97) with scores of “none” (n = 28), grade 1 (n = 2), grade 2 (n = 11), grade 3 (n = 6), and grade 4 (n = 1). Four cases differed by 1 degree only, grade 1 versus grade 2 (n = 3) and grade 2 versus grade 3 (n = 1; Table 4). Grading for arteriolosclerosis was the same in 45 of 52 cases (r = 0.92) with equivalent scores of “none” (n = 4), mild (n = 16), moderate (n = 16), and severe (n = 3). Seven cases differed by 1 degree each (Table 4), with the MADRC protocol showing a higher degree. Microinfarcts were present in 5 of 52 cases with the MADRC protocol, and in 3 of 52 cases with the simplified protocol (Table 4).
TDP-43 positivity was present in 15 of 52 cases (29%) stained in the MADRC protocol. Three cases demonstrated neuropathologic changes of FTLD-TDP-43, as shown in Figure 4, K. The other 12 had inclusions and neurites in the entorhinal cortex, amygdala, and/or hippocampus in a pattern associated with ADNC (Figure 4, L).14 Staining for TDP-43 was performed on the hippocampus/superior frontal block from the simplified protocol for these 15 cases. Of these, 13 (87%) had TDP-43 inclusions and neurites. The 2 cases that were negative in the simplified protocol either had only focal staining for TDP-43 in the amygdala and anterior entorhinal cortex (an area not represented with the simplified protocol) or had only focal TDP-43 positivity in pyramidal neurons of CA1 with the MADRC protocol.
The estimated cost for carrying out the MADRC protocol in our clinical laboratory was $2436.37, whereas that for the simplified protocol was only $691.48 (Table 5). The $1744.89 difference in cost represents a 72% reduction in expenses. Most of the savings were in the reduced costs for immunohistochemistry with the simplified protocol. The number of slides to review was decreased by 73% (14 versus 52 slides), saving significant diagnostic time compared with the MADRC protocol, which is both a diagnostic and research protocol.
Table 5.
Analysis of Estimated Cost Savingsa
| MADRC, No. | MADRC Cost, $ | Simplified, No. | Simplified, $ | |
|---|---|---|---|---|
| Gross per block ($2.75) | 19 | $52.25 | 6 | $16.50 |
| Processing per block ($3.30) | 19 | $62.70 | 6 | $19.80 |
| L/H&E stain ($33.21) | 19 | $630.99 | 6 | $199.26 |
| Bielschowsky stain ($33.21) | 8 | $265.68 | 0 | 0 |
| IHC stain ($56.99) | 25 | $1,424.75 | 8 | $455.92 |
| Total | $2,436.37 | $691.48 |
Abbreviations: L/H&E, Luxol fast blue/hematoxylin-eosin; IHC, immunohistochemistry; MADRC, Massachusetts Alzheimer’s Disease Research Center.
This analysis indicates a savings of $1744.89 or a cost reduction of 72% per case.
DISCUSSION
We have developed a cost-effective, simplified protocol, similar to the condensed technique described by Flanagan et al2 but modified with a simplified organizational scheme and larger individual pieces with better preservation of the neuroanatomy. We have validated our technique in a prospective cohort of cases submitted to the MADRC by comparing it with the combined diagnostic and research protocol as the “gold standard.” Our technique also has excellent correlation with the MADRC protocol for cerebrovascular disease, especially for cerebral amyloid angiopathy and arteriolosclerosis. This is most likely due to the larger size samples compared with previously published protocols because each of our cortical blocks included leptomeninges and subcortical white matter. Microinfarcts were detected at a slightly reduced rate compared with the MADRC protocol, likely attributable to the smaller overall volume of sampling, a limitation also present with the Flanagan method.
This protocol is optimized for the diagnosis of neurodegenerative disease. It is 98% accurate in the assessment of ADNC in our series and 100% accurate when the classification is “high” probability of AD or effectively “not” AD. The accuracy of an “intermediate” or “low” probability classification is 89%. This reduction in accuracy is of less diagnostic relevance given that an “intermediate” or “low” ADNC score will not definitively rule in or rule out the diagnosis of AD.
The simplified protocol is also very effective in detecting the presence of Lewy pathology, with an overall accuracy of 87%, with some decreased sensitivity attributed to reduced overall sampling compared with the MADRC protocol. All cases that were submitted with a clinical suspicion of Lewy pathology, as well as those that had evidence of Lewy body disease with the MADRC protocol, also had evidence of Lewy body disease with the simplified protocol. Hence, no false negatives were detected.
Although we did not prospectively test for TDP-43 with the simplified protocol, the hippocampal/superior frontal block should be stained for TDP-43 to screen for hippocampal sclerosis of aging, FTLD-TDP-43, and the TDP-43 staining associated with ADNC. In our retrospective review, 87% of the cases that showed positivity for TDP-43 in the MADRC protocol also showed positivity with the simplified protocol. Those that did not stain with the simplified protocol were cases with only focal positive staining with the MADRC protocol. In clinical practice, this stain should be performed on block 2 of the simplified protocol when the other immunohistochemical stains (β-amyloid, hyperphosphorylated tau, α-synuclein) are used.
Less common neurodegenerative disorders were also effectively diagnosed with the simplified protocol. Our cohort of cases included a number of cases with progressive supranuclear palsy and multiple system atrophy, and 1 case of corticobasal degeneration. The simplified blocking scheme allowed sufficient tissue for additional immunohistochemistry to be done when needed, with minimal additional cost. For example, for the case of multiple system atrophy we added α-synuclein to block 1 of the simplified protocol to identify glial inclusions in the cerebellar white matter, internal capsule, and putamen. Similarly, if there is concern for cerebral amyloid angiopathy, the protocol could be modified to add a β-amyloid stain to block 5 (superior parietal, occipital cortex) to increase diagnostic yield because these regions are often involved.
The potential savings when using the simplified protocol are substantial. The 72% potential reduction in cost is significant when running a busy autopsy service that is generally not directly reimbursed, or in cost-restrictive settings, such as small rural academic centers, or a medical examiner’s office. This reduction in cost is similar to a previously published alternative way of reducing the amount of tissue processed for microscopy.2 The considerable time saved by the pathologist looking at the case, having reduced the number of slides needed to make the diagnosis, is also substantial, as is the reduced technician time required in embedding and cutting sections.
Several of the authors have implemented this simplified protocol in clinical autopsy practice for older individuals where a clinical history of dementia is lacking or unclear but may potentially be of concern. The L/H&E stains are reviewed from the 6 blocks for plaques, tangles, Lewy bodies, and other pathology, and then a decision as to whether to order the immunohistochemistry is made. In many cases the immunohistochemistry is not needed and additional savings in time and money are gained.
A limitation of our study is that although we examined all cases prospectively, in most instances we were not blinded when comparing the scores across protocols. However, all slides were reviewed by a neuropathology attending with considerable experience in the field of neurodegenerative disease. Another limitation was that we did not sample the amygdala and anterior transentorhinal region in the simplified protocol. This blocking scheme would miss p-tau positive neurofibrillary tangles and/or p-TDP-43 positive inclusions isolated to those regions, as well as amygdala-only Lewy body disease. However, these scenarios may be of less clinical interest when these regions are involved in isolation. A modification of the protocol could potentially substitute the amygdala for the thalamus because the thalamus was often helpful in only a minority of cases.
A third limitation was that the stains were done in separate facilities. This, however, gave us a chance to carefully look at the differences and see where improvements in staining protocols could be made.
Although we excluded cases of Huntington disease, amyotrophic lateral sclerosis, and spinal muscular atrophy from our study, because we use different blocking protocols, a modification of the simplified protocol could be used to examine these cases. For example, in amyotrophic lateral sclerosis, a seventh block could be added to the 6-block protocol. This cassette would contain a section from the precentral gyrus and a single axial section each of cervical, thoracic, and lumbar cord. An L/H&E, glial fibrillary acid protein, ubiquitin, and TDP-43 could be done on this block, providing evidence of upper and lower motor neuron disease as well as the inclusions seen in this disease.
In summary, in any practice setting with a large volume of autopsies in older patients, the need for an economical way to rapidly assess for neurodegenerative disease is essential. This is especially important in cases where the clinical history of dementia is uncertain or lacking. This simplified protocol is sufficient to establish the diagnosis of ADNC, Lewy body pathology, cerebral amyloid angiopathy and arteriolosclerosis, as well as more rare neurodegenerative pathology. In addition, the sample sizes are volumetrically adequate to evaluate anatomic relationships necessary for the evaluation of these diseases.
Acknowledgments
This work was supported by the Department of Pathology, Brooke Army Medical Center, San Antonio, Texas. The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Air Force, the Department of the Army, the Department of Defense, or the US government.
This work was supported by grant award No. P50 AG005134. The authors have no relevant financial interest in the products or companies described in this article.
Footnotes
Presented in part as a poster presentation at the annual meeting of the American Association for Neuropathologists; June 8, 2019; Atlanta, Georgia.
References
- 1.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367–429. [Google Scholar]
- 2.Flanagan ME, Marshall DA, Shofer JB, et al. Performance of a condensed protocol that reduces effort and cost of NIA-AA guidelines for neuropathologic assessment of Alzheimer disease. J Neuropathol Exp Neurol. 2017;76(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vonsattel JP, Aizawa H, Ge P, et al. An improved approach to prepare human brains for research. J Neuropathol Exp Neurol. 1995;54(1):42–56. [DOI] [PubMed] [Google Scholar]
- 4.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thal DR, Rüb U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12): 1791–1800. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 7.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4): 479–486. [DOI] [PubMed] [Google Scholar]
- 8.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, DelTredici, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 10.Del Tredici K, Rüb U, De Vos RA, et al. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61(5):413–426. [DOI] [PubMed] [Google Scholar]
- 11.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy: sensitivity and specificity of cortical biopsy. Stroke. 1997;28(7):1418–1422. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Leurgans SE, Wang Z. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011;69(2): 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs KA, Melissa E. Murray ME, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014;127(3):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]


