Abstract
Background.
A case of human infection with Plasmodium knowlesi has been recently discovered in Thailand. To investigate the prevalence of this malaria species, a molecular-based survey was performed.
Methods.
Blood samples from 1,874 patients were determined for Plasmodium species by microscopy and nested PCR. Characterization of P. knowlesi was done by sequencing the merozoite surface protein-1 (msp-1) gene.
Results.
Of all species identified, P.falciparum, P.vivax, P.malariae, P.ovale and P.knowlesi contributed 43.52, 68.08, 1.37, 1.03 and 0.57%, respectively. Mixed species infections were more common in northwestern and southwestern regions bordering Myanmar (23-24%) than in eastern and southern areas (3-5%). In southern and southwestern regions, mixed species infections had significantly higher prevalence in dry than in rainy seasons (p<0.003). P.knowlesi was found in 10 patients, mostly from southern and southwestern areas. Nine of these were co-infected with either P.falciparum or P.vivax. Most of the P.knowlesi Thai isolates were more related with malaria from macaques than isolates from Sarawak patients. The msp-1 sequences of isolates from the same endemic area differed and possessed novel sequences, indicating genetic polymorphism in P.knowlesi infecting humans.
Conclusions.
This survey highlighted the widespread distribution of P.knowlesi in Thailand, albeit at low prevalence and mostly occurring as cryptic infections.
Keywords: epidemiology, malaria diagnosis, Plasmodium knowlesi, mixed species malaria infection, Thailand, merozoite surface protein-1, small subunit ribosomal RNA
Introduction
The effectiveness of malaria control undoubtedly relies on integrative interventions involving both malaria parasites and mosquito vectors. The foundation for such a control strategy requires accurate epidemiological data. Microscopy-based detection of malaria is the mainstay for routine diagnosis and has been widely implemented for early case detection and management in Thailand [1]. However, precision and reliability of the microscopy method seem to depend largely on the quality of the stained blood films, parasite density and the expertise of microscopists. Comparative studies have consistently demonstrated the superiority of polymerase chain reaction (PCR)-based method to microscopy-based diagnosis of malaria in both sensitivity and specificity. Importantly, mixed species infections in Thailand and other endemic areas of Asia have been detected in <2% by microscopy, whereas compelling evidence indicates that a large number of mixed species infections were undetected [2, 3]. Underestimation of mixed species infections has a significant impact on malaria control because transmission of cryptic parasites could be persistent and treatment of infected patients can be inadequate. In addition, interpretation of the efficacy of malaria control measures can be misleading.
Plasmodium knowlesi is known to circulate among Southeast Asian monkeys, especially crab-eating (Macaca fascicularis) and pig-tailed macaques (M. nemestrina), which are populated at various natural habitats in these regions [4]. Our recent survey has shown that macaques living in mangrove forests in southern Thailand harbor a number of nonhuman primate malaria species [5]. Recent discoveries of natural P. knowlesi infections in humans in southern Thailand, Malay Peninsula, Malaysian Borneo and the Philippines have highlighted that a ‘fifth’ human malaria species could be of public health importance in these regions [6–10]. Because morphological resemblance in trophozoites, schizonts and gametocytes between P. knowlesi and P. malariae, and in ring stage between P. knowlesi and P. falciparum, P. knowlesi malaria is often misdiagnosed by conventional microscopy, leading to underestimation of its prevalence. Since P. knowlesi infection has a 24 h erythrocytic cycle and disease progression may be fast, timely diagnosis and treatment may be essential. The report of at least four fatal cases of malaria in Malaysia probably caused by P. knowlesi underpins its contribution to the burden of human malaria [8].
The majority of malaria cases in Thailand is distributed in border areas with neighboring countries. The objectives of this study are to determine the distribution of human malaria species and P. knowlesi infections in malaria patients living in four disease endemic areas of Thailand with high annual parasite incidence (API) bordering Myanmar, Cambodia and Malaysia. We evaluated the status of malaria infection by both conventional microscopy and PCR. Our results have shown that malaria species distribution and the prevalence of mixed species infections differed both geographically and seasonally. Furthermore, we have detected additional P. knowlesi infections, the majority of which was co-infected with other species.
Materials and Methods
Study populations
From October 2006 to September 2007, we collected blood samples from 1,874 patients with febrile symptoms who attended malaria clinics at northwestern (Tak Province, n=675), eastern (Chantaburi Province, n=261), southwestern (Prachuab Khirikhan Province, n=276), and southern (Yala, n=286, and Narathiwat Provinces, n=376) areas of Thailand (Figure 1). For malaria diagnosis, Giemsa-stained thick and thin blood films were made and additional 200 μl of finger-prick blood were collected from each patient. This study has been approved by the Institutional Review Board of Faculty of Medicine, Chulalongkorn University.
Figure 1.
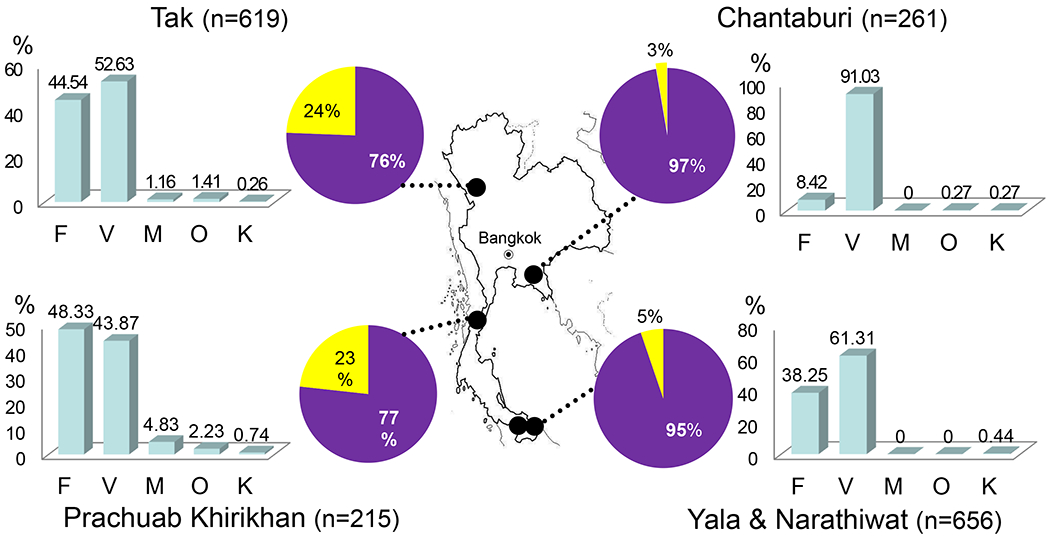
Map of Thailand showing the locations of the sample collection sites in northwestern (Tak Province), southwestern (Prachuab Khirikhan Province), southern (Yala and Narathiwat Provinces) and eastern areas (Chantaburi Province). The Pie diagrams represent percentage of single (filled) and mixed species (unfilled) malaria infections. Distribution of malaria species (F=P. falciparum, V=P. vivax, M=P. malariae, O=P. ovale and K=P. knowlesi) for each endemic area as determined by the species-specific nested polymerase chain reaction is shown in bar graphs.
Malaria diagnosis by microscopy and PCR
Malaria diagnosis by microscopy was performed by experienced microscopists who were blinded to the results of PCR detection. For each slide, the thick blood film was examined for at least 200 leucocytes, and the thin blood film for at least 200 microscopic fields with the 100 X objective. For molecular diagnosis, DNA was extracted from the 200 μl of blood by using a QIAGEN DNA minikit (Hilden, Germany). Malaria species was identified by nested PCR using Plasmodium genus-specific outer primers derived from the small subunit ribosomal RNA (SSU rRNA) gene (M18SF0: 5’-CCATTAATCAAGAACGAAAGTTAAGG-3’ and M18SR0: 5’-CAAGGAAGTTTAAGGCAACAACA-3’) for primary PCR and one pair of the species-specific inner primers (P. falciparum, PF18SF: 5’-CATCTTTCGAGGTGACTTTTAG-3’ and PF18SR: 5’-GTTTTTTACTCTATTTCTCTCTTC-3’; P. vivax, PV18SF: 5’-GAATTTTCTCTTCGGAGTTTATTC-3’ and PV18SR: 5’-TAACAGTTTCCCTTTCCCTTTTCTAC-3’, P. malariae, PM18SF: 5’-GAGACATTCATATATATGAGTG-3’ and PM18SR: 5’-GTTTTTTTTAATAAAAACGTTCTTTTCCC-3’; P. ovale, PO18SF: 5’-GAAAAFFCCTTTTGGAAATTTCTTAG-3’ and PO18SR: 5’-GATACATTATAGTGTCCTTTTCCC-3’ and P. knowlesi, PK18SF: 5’-GAGTTTTTCTTTTCTCTCCGGAG-3’ and PK18SR: 5’-ACGTTAAATGTGATTCCTTTCCC-3’) for nested PCR. Thirty-five and 25 cycles (94°C for 40 s, 60°C for 30 s and 72°C for 1 min) were performed for the primary and nested PCR, respectively. The PCR products were separated in 1% and 2 % agarose gels for primary and nested PCR, respectively, stained with ethidium bromide and visualized under a UV light.
Genetic diversity of P. knowlesi
Genetic diversity of P. knowlesi isolates was evaluated by using the SSU rRNA gene and the merozoite surface protein-1 gene of P. knowlesi (Pkmsp-1). DNA sequences of the P. knowlesi SSU rRNA gene were determined from PCR products that were subcloned into Promega’s pGEM-T Easy Vector Systems (Madison, WI), using 10 recombinant plasmid clones for each isolate. Sequencing analysis was performed from both directions for each template. Pkmsp-1 was amplified by using primers from regions equivalent to conserved blocks 1 and 5 of the P. vivax merozoite surface protein-1 gene (Pvmsp-1) (PKMSP1F: 5’-ATTGGAAAAATGGAAGCCTTCA-3’ and PKMSP1R: 5’-CTTCCAATTTTATTTTGCTC-3’) [11]. The Pkmsp-1 fragment was sequenced directly from purified PCR product [12]. Whenever singletons occurred, the sequence was re-determined using PCR products from two independent amplifications from the same DNA samples.
Data analysis
Sequences were aligned by the CLUSTAL X program with minor manual adjustment made by visual inspection [13]. Phylogenetic construction was performed by the neighbor-joining method using the maximum composite likelihood with 1,000 bootstrap iterations as implemented in the MEGA version 4.0 software [14].
Results
Demography and malaria patients
Of the 1874 febrile patients examined in this study, 1751 patients harbored malaria parasites in their blood circulation detected by both microscopic and PCR examinations. The sex ratio of the malaria patients was 2.25:1 (male:female). The age range was from 1 to 81 years (mean=25.54, median=23 and mode=19 years). The majority of the patient population was Thai (56%), while the remainders were cross-border migratory foreigners (36% from Myanmar and 8% from Cambodia).
Comparison of the diagnostic methods
Microscopic examination detected malaria parasites in 1695 patients. The remaining 179 microscopy-negative patients were from Tak and Prachuab Khirikhan Provinces. Microscopic diagnosis revealed that P. vivax was the most prevalent species (59.5%), followed by P. falciparum (39.9%) and P. malariae (0.2%). Neither P. ovale nor P. knowlesi infection was detected. Microscopy also detected 40 cases of mixed infections by P. falciparum and P. vivax.
We re-evaluated the 1874 blood samples using the nested PCR method (Table 1). Consistent with previous findings, the PCR method offered higher sensitivity in parasite detection. While the 1695 samples positive by microscopy were also positive by PCR, 56 of the 179 malaria-negative samples by microscopy were positive by PCR. Of these 56 samples (31.3%), the most prevalent cryptic infection was due to P. vivax (n=32), followed by P. falciparum (n=17), co-infection of P. falciparum and P. vivax (n=5), co-infection of P. malariae and P. ovale (n=1) and triple-infection of P. falciparum, P. vivax and P. ovale (n=2). The two methods yielded remarkable discrepancy in parasite species identification. Of the 656 P. falciparum positives by microscopy, 494 samples (75.3%) yielded concordant results by PCR, whereas 38 samples were found to be single infections by P. vivax (n=32), P. malariae (n=2), P. ovale (n=2) and co-infections of P. vivax and P. malariae (n=2). All the remaining 124 isolates contained P. falciparum and cryptic infections with P. vivax (n=108), P. malariae (n=5), P. ovale (n=2), P. knowlesi (n=5), P. vivax and P. malariae (n=2), and P. vivax and P. ovale (n=2). Likewise, of the 996 cases diagnosed with P. vivax infections by microscopy, 871 cases were confirmed to contain P. vivax single infections by PCR, whereas 27 samples were misdiagnosed by microscopy and contained single infections with P. falciparum (n=23), P. malariae (n=1), and P. ovale (n=1), and a mixed infection with P. falciparum and P. malariae (n=1), and a triple infection with P. falciparum, P. malariae and P. ovale (n=1). Although the remaining 98 isolates were also confirmed by the PCR method to contain P. vivax, these samples were co-infected with P. falciparum (n=80), P. malariae (n=6), P. knowlesi (n=4), P. ovale (n=3), P. falciparum and P. malariae (n=2), P. falciparum and P. ovale (n=2), and P. falciparum, P. malariae and P. ovale (n=1). Interestingly, one of three cases diagnosed to be P. malariae infection was identified to be P. knowlesi by PCR. Further discrepancy between the two methods existed in the detection of mixed-species infections. Of the 40 cases of mixed infections with P. falciparum and P. vivax, only eight were confirmed by PCR. The rest of these samples were single infection of either P. falciparum (n=11) or P. vivax (n=21).
Table 1.
Diagnosis of Plasmodium species by microscopy and PCR methods of 1751 malaria patients in Thailand.
| Microscopy-based detection |
|||||||
|---|---|---|---|---|---|---|---|
| PCR-based detection | P.faciparum | P.vivax | P.malariae | P. ovale | P.falciparum and P.vivax | Negative | Total cases by PCR |
| P.falciparum | 505 | 21 | - | - | 1 | 10 | 537 |
| P.vivax | 42 | 889 | - | - | 2 | 31 | 964 |
| P.malariae | 1 | 1 | 2 | - | - | - | 4 |
| P.ovale | 2 | 1 | - | - | - | - | 3 |
| P.knowlesi | - | - | 1 | - | - | - | 1 |
| P.falciparum and P.vivax | 110 | 81 | - | - | 5 | 4 | 200 |
| P.falciparum and P.malariae | 5 | 1 | - | - | - | - | 6 |
| P.falciparum and P.ovale | 2 | - | - | - | - | - | 2 |
| P.falciparum and P.knowlesi | 5 | - | - | - | - | - | 5 |
| P.vivax and P.malariae | 2 | 6 | - | - | - | - | 8 |
| P.vivax and P.ovale | 1 | 3 | - | - | - | - | 4 |
| P.vivax and P knowlesi | - | 4 | - | - | - | - | 4 |
| P.malariae and P.ovale | - | - | - | - | - | 1 | 1 |
| P.falciparum, P.vivax and P.malariae | 2 | 2 | - | - | - | - | 4 |
| P.falciparum, P. vivax and P.ovale | 2 | 3 | - | - | - | 2 | 7 |
| P.falciparum, P.vivax, P.malariae and P.ovale | - | 1 | - | - | - | - | 1 |
|
| |||||||
| Total cases by microscopy | 679 | 1013 | 3 | 0 | 8 | 48 | 1751 |
Taken together, of the 1874 febrile patients, perfect agreement of results from microscopy and PCR detection was obtained in 79.9% of samples while underdiagnosis was observed in 14.8%, overdiagnosis in 1.7% and misdiagnosis in 3.5%.
Regional and seasonal variations in malaria epidemiology
The epidemiology of malaria in Thailand showed regional difference in terms of species distribution and prevalence of mixed-species infections (Figure 1). Among single species infection, P. vivax outnumbered P. falciparum in 3 of 4 regions examined and the most remarkable difference was observed in eastern province. In terms of mixed species infections, the two provinces bordering Myanmar had much higher prevalence (23-24%). In comparison, the eastern region had only 3% mixed species infections. In southern region, where malaria prevalence increased considerably during the past decade, mixed species infections reached 5%. In agreement with the predominant status of P. vivax and P. falciparum in Thailand, co-infections of P. vivax and P. falciparum predominated over other combinations of mixed species infections in all regions.
The occurrence of mixed species infections in samples collected from Tak Province was significantly higher in dry season (November to April) than in rainy season (May to October) (χ2 = 18.61, p=0.000, odd ratio=2.28, 95%CI=1.56-3.34). The difference remains significant when either single infections of P. falciparum (χ2 = 26.36, p=0.000, odd ratio=3.37, 95%CI=2.10-5.41) or P. vivax (χ2 = 8.55, p=0.003, odd ratio=1.85, 95%CI=1.22-2.80) were considered separately. On the contrary, no significant seasonal difference in the rate of mixed species infections was observed among samples collected in other regions of Thailand (χ2 = 2.23, p=0.135, odd ratio=1.42, 95% CI=0.90-2.07).
P. knowlesi infections
Although microscopy did not detect P. knowlesi in any samples examined, ten samples (0.6% of all positives) contained P. knowlesi parasite by the PCR method (Table 2). The case BMC1-51 from Narathiwat Province displayed ring stages, ‘band’ shaped trophozoites, and ovale-shape macrogametocytes in otherwise normal erythrocytes, and thus was diagnosed as P. malariae by microscopy. However, PCR using the SSU rRNA gene target clearly identified this sample as single infection with P. knowlesi. Additional nine samples morphologically identified to be either P. falciparum or P. vivax also were PCR positive for P. knowlesi, indicating of mixed species infections. While it was not possible to determine the parasite densities of each species in these mixed infections, the overall parasite density was low (geometric mean=3273 parasites/μl, ranges=320 to12640 parasites/μl). Case BMC-51 that contained single P. knowlesi infection had a parasite density of 13,120 parasites/μl.
Table 2.
Demography and parasitological profiles of Plasmodium knowlesi-positive samples.
| Case | Age (yr) | Sex | Ethnics | Location (Province) | Stages of parasites | Microscopy-based diagnosis | Parasite density (per μl) | PCR-based diagnosis | Monkey in vicinity | Period of infection (season) |
|---|---|---|---|---|---|---|---|---|---|---|
| BSN-9 | 48 | M | Thai | Prachuab Khirikhan | Ring (some with double chromatin), Schizont, Gametocyte | P. vivax | 8,000 | P. vivax, P. knowlesi | Yes | Rainy |
| BSN-93 | 45 | M | Thai | Prachuab Khirikhan | Ring, Growing trophozoite, Gametocyte | P. vivax | 13,120 | P. vivax, P. knowlesi | Yes | Rainy |
| BMC1-51 | 26 | M | Thai | Narathiwat | Ring, Growing trophozoite (band-shaped), Gametocyte | P. malariae | 12,640 | P. knowlesi | Yes | Dry |
| MC1-26 | 39 | M | Thai | Narathiwat | Ring (some with double chromatin) | P. falciparum | 5,120 | P. falciparum, P. knowlesi | Yes | Rainy |
| YL-68 | 35 | F | Thai | Yala | Ring (some with double chromatin) | P. falciparum | 5,760 | P. falciparum, P. knowlesi | Yes | Rainy |
| YL-71 | 17 | F | Thai | Yala | Ring, Growing trophozoite | P. falciparum | 1,920 | P. falciparum, P. knowlesi | Yes | Dry |
| YL-217 | 19 | M | Thai | Yala | Ring, Growing trophozoite, Schizont | P. vivax | 2,240 | P. vivax, P. knowlesi | Yes | Rainy |
| CTRA-9 | 46 | M | Thai | Chantaburi | Ring, Growing trophozoite | P. falciparum | 6,560 | P. falciparum, P. knowlesi | No | Rainy |
| TSY-1141 | 27 | M | Myanmar | Tak | Growing trophozoite | P. vivax | 320 | P. vivax, P. knowlesi | No | Rainy |
| TSY-1154 | 12 | M | Myanmar | Tak | Ring (some with double chromatin and some with multiple infection) | P. falciparum | 1,600 | P. falciparum, P. knowlesi | No | Rainy |
Most (8/10) P. knowlesi-positive samples occurred in the rainy season and 8/10 were male patients (Table 2). Southern and southwestern regions of Thailand had the highest number (7/10) of P. knowlesi malaria cases and all of these lived in villages where macaque monkeys were reared as pets and for coconut picking. All P. knowlesi-infected cases had uncomplicated malaria symptoms for 1 to 7 days (mean=3.9) prior to attending malaria clinics and the mean axillary temperature was 38.2°C (range=37.7 to 38.6°C). All P. knowlesi-positive patients responded well to antimalarial treatment for either P. vivax or P. falciparum.
Genetic diversity of P. knowlesi
To reaffirm the presence of P. knowlesi in malaria populations of Thailand, we determined the type A sequence of SSU rRNA gene spanning ~530 bp of variable domain 7 and its flanking regions and compared with those of human or macaque isolates reported elsewhere [6, 7, 15, 16]. Result revealed three sequence types among the Thai isolates, one of which was unique (isolate CTRA-9 from Chantaburi Province) and the others were identical with either human isolates from Sarawak (isolates YL-217 from Yala Province and TSY-1154 from Tak Province) or those derived from macaques (the remaining isolates). Phylogenetic tree has placed isolate CTRA-9 within the same clade as most other Thai isolates that shared sequences with those from macaques (Figure 2). A separate cluster containing isolates YL-217, TSY-1154 and most isolates from Sarawak was noted [7]. However, these 2 clades did not receive high bootstrap support.
Figure 2.
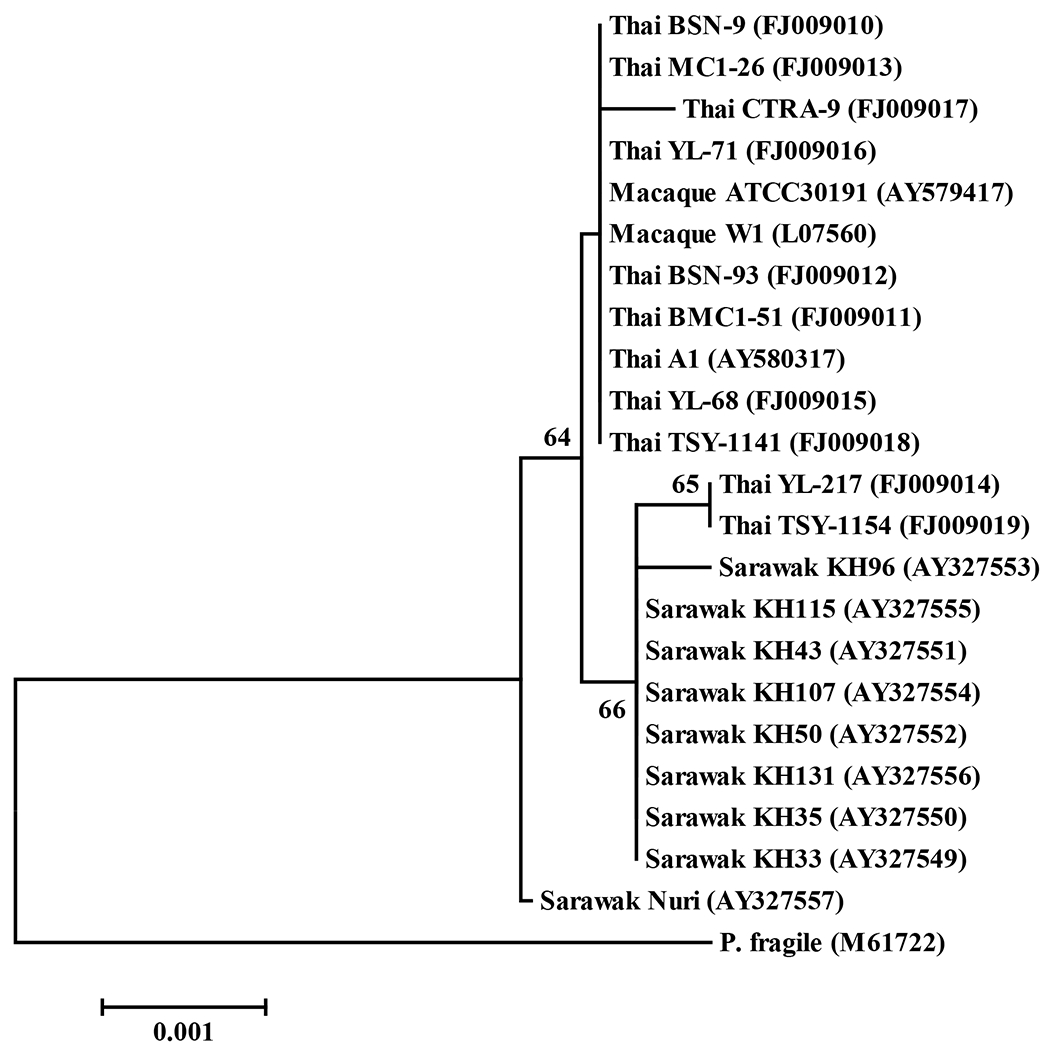
Neighbor-joining tree inferred from the type A sequences of the small subunit ribosomal RNA gene of Plasmodium knowlesi from Thai and Sarawak human isolates comparing with those from macaque origins (ATCC30191 and W1) using maximum composite likelihood model with the orthologous sequence of P. fragile as an outgroup. Percentage of 1000 bootstrap replicates supports over 50% is shown along the branches. Scale underneath the tree indicates the number of base substitutions per site. Isolate names are listed along with their respective GenBank accession numbers.
To further determine whether the two P. knowlesi isolates BMC1-51 and MC1-26 that were collected from the same endemic area in Narathiwat Province and had identical SSU rRNA sequence were identical parasites, we sequenced their Pkmsp-1 gene. Sequence comparison reveals that these two isolates differed and possessed distinct novel sequences in regions equivalent to block 2 of Pvmsp-1 with unique substitutions in blocks 3 and 5 (Figure 3) [11]. It is noteworthy that the Pkmsp-1 sequence of isolate A1 originally from the first human case of knowlesi malaria in Thailand was more related to sequence of Malayan strain from macaque and thus remarkably different from the two isolates in this analysis [6, 12, 17].
Figure 3.
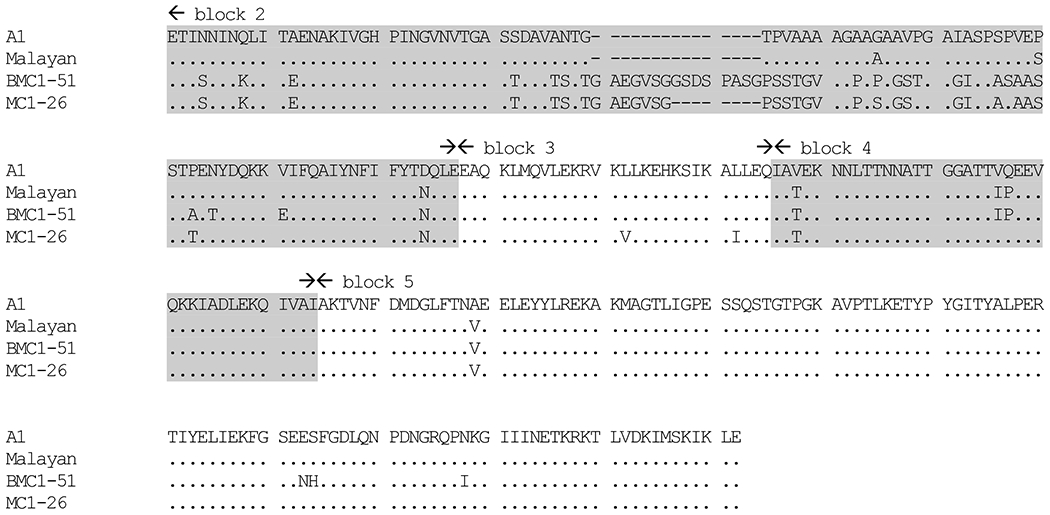
Amino acid sequence comparisons of merozoite surface protein-1 of Plasmodium knowlesi from human origins (A1, BMC-51 and MC1-26) and a macaque origin (Malayan strain). Identical and deleted residues are indicated by dots and dashes, respectively. Boundaries of blocks follow those of the msp-1 gene of P. vivax [11]. Shaded and unshaded regions denote variable and conserved blocks, respectively.
Discussions
Laboratory diagnosis of malaria in routine practice in disease endemic countries relies on microscopic detection of parasites in blood smears. In malaria clinics of Thailand, the microscopy-based method has been used as the sole procedure for malaria diagnosis during the past decades, during which annual malaria incidence has been reported to continuously decline [18]. Although we did not conduct a systematic assessment of malaria infections in microscopy-negative febrile patients in this study, we detected by PCR as high as 31% of these samples collected during high transmission period containing submicroscopic malaria infections. Furthermore, the PCR method also detected misdiagnosed malaria cases as well as the presence of P. ovale and P. knowlesi infections in patients. These data suggest that the current report of malaria epidemiology in Thailand based on microscopy per se had certain degrees of inaccuracy, and underdiagnosis and misdiagnosis should bear significant consequences, especially on the poor and vulnerable patients in terms of inappropriate medical management, adverse psychosocial impact and economic loss [19].
During the past four decades, malaria incidence rate in Thailand showed an overall tendency of reduction along with the decline in the ratio of P. falciparum to P. vivax from 4.1 in 1965 to 0.6 in 2006 [20]. The remarkable decline in P. falciparum cases may be resulted from close disease monitoring, extensive surveillance of drug resistance, and corresponding timely changes of nation-wide antimalarial drug policy. However, the slow decline in P. vivax cases was less understood, and could be due to the intrinsic biological properties of the parasite, development of resistance to current therapeutics, and changes in vectors community structures [21–26]. Overall, this study revealed considerable heterogeneity of the spatial distribution of parasite species and their seasonal dynamics. Whereas P. falciparum to P. vivax ratio was higher in the western provinces (Prachuab Khirikhan, 1.10 and Tak, 0.85), it was much lower in the southern provinces (0.62) and extremely low in the eastern province (0.09). In terms of seasonal variation, this ratio was higher in rainy season than in dry season.
The PCR method detected significantly more cases of mixed infections. Mixed species infections tend to be overlooked by microscopic diagnosis especially when one parasite species is present at a low parasitemia. For example, in Thailand, about 33% of cryptic P. vivax infections became clinically patent following treatment of P. falciparum whereas about 13% of P. vivax isolates had cryptic P. falciparum infections [27, 28]. Our study has shown that the distribution and prevalence of mixed species infections varied across geographic regions of Thailand, which is probably correlated with the annual parasite incidence (API) rate per 1000 individuals. Tak Province had the highest API among the study sites (15.5), whereas the API of southern and eastern regions during the period of study was 4.5 and 2.8, respectively [20]. It is noteworthy that mixed species infections are more prevalent in western Thailand bordering Myanmar. These findings have clinical significance because malaria in areas bordering Myanmar requires scrutiny for adequate drug treatment.
Annual malaria transmission in Thailand follows a bimodal pattern, peaking in May-July and October-November. Intriguingly, the number of mixed species infections in dry season was significantly more than that in the rainy season, especially in Tak and Prachuab Khirikhan Provinces. Malaria from the most prevalent period in the rainy season to the least prevalent in dry season experiences significant reductions (4.0 and 7.6 folds in 2006 and 2007, respectively). A substantial reduction in number of malaria cases in dry season is expected to cause a decrease in the parasite population size. Consequently, it is expected that genetic diversity of parasite population after the dry season is reduced, akin to the bottleneck-like effect. However, analysis of the genes encoding polymorphic merozoite surface protein-2 and sporozoite-threonine-asparagine-rich protein of P. falciparum collected in 2006 and 2007 in the same endemic areas did not show evidence of significant reduction in haplotype diversity of these genes as well as the merozoite surface protein-1 locus of P. vivax [29, Putaporntip et al, unpublished]. Although host immune pressure could contribute to sequence diversification by positive selection, such influence does not generate a rapid change and, thus, could not be observed within a few generations of parasites based on an in vitro study [30]. In addition to the observation of mixed species infections, mixed strain infections are also prevalent in some of the endemic areas of Thailand (such as Tak province) [31]. This could have contributed to the maintenance of the effective number of variants in the gene pool of each malaria species. Moreover, other factors such as intrinsic properties of the parasites or seasonal changes in the population structures and feeding behaviors of mosquito vectors may also help maintain the genetic diversity through different seasons.
Despite that the transmissibility of P. knowlesi from macaques to humans has been established under experimental conditions over seven decades ago, the first and second naturally acquired human infections are reported in 1965 and 1971. To date, P. knowlesi-infected patients have been found in a wide geographic range. Surveillance of knowlesi malaria in humans in Malaysia has shown an uneven distribution of infected cases with higher prevalence in Sarawak than in the mainland Malaysia [8]. In contrast, our large-scale survey in Thailand revealed a very limited number of malaria cases caused by P. knowlesi. It is noteworthy that single infections of P. knowlesi are rare in Thailand while most of the positive cases were co-infected with other malaria species. In Malaysian Borneo, P. knowlesi infections have often been misdiagnosed due to its morphological similarity to P. malariae infections. However, this was not the case in Thailand since most of the microscopy-based diagnosis as P. malariae were correct as confirmed by PCR. Yet, the low prevalence of P. knowlesi in Thailand, morphological similarity with P. malariae, and the cryptic nature of this species as masked by other common malaria parasites make accurate diagnosis of P. knowlesi infections difficult.
Analysis of type A sequence of the SSU rRNA gene spanning variable block 7 revealed that most of the Thai isolates were more closely related to monkey strains while a few Thai isolates were placed within the same cluster as those from Sarawak patients, suggesting that genetic heterogeneity within P. knowlesi exhibits geographic variations. Sequence analysis of blocks 2 to 5 of Pkmsp-1 gene from the first human isolate of P. knowlesi in Thailand comparing with that originated from macaque natural host showed 97.5% amino acid sequence identity [12, 17]. Additional analysis in this study incorporating 2 Thai isolates from the same endemic area that shared the same sequence of block 7 of the SSU rRNA locus revealed novel sequences of block 2 of the Pkmsp-1 gene, indicating that the extent of polymorphism in this locus is more extensive than previously known. The existence of genetic polymorphism in Pkmsp-1 of human isolates could suggest that transmission of P. knowlesi to humans was not from a single origin. The widespread distribution of P. knowlesi in Thailand, albeit at low prevalence, reflects a wide distribution of its mosquito vectors along with the presence of macaque reservoirs [4, 5, 32].
It is noteworthy that all P. knowlesi positive samples in this survey had low parasite density. To date there is no evidence for sequestration of mature parasite stages of P. knowlesi in visceral circulation, the number of parasites in peripheral blood would reflect actual parasite burden in infected hosts. Recent report has suggested that P. knowlesi infection with high parasite burden in some Sarawak patients could probably turn into fatal complications [8]. In this regard, proper diagnosis and surveillance of P. knowlesi in humans are undoubtedly of public health importance.
Acknowledgements
We are grateful to all patients who donated their blood samples for this survey and to Malee Charoenkorn, Chutinan Areekul, Rattiporn Kosuvin, Kriangkrai Karnchaisri, Urassaya Pattanawongse, Jittrakul Suwancharoen and staff at malaria clinics for assistance in field works.
Financial supports:
The National Research Council of Thailand (grant for fiscal years 2006-2007) to SJ and CP.
The Fogarty International Center, NIH (grant D43TW006571) to CP and LC.
Footnotes
References
- 1.Malikul S The current situation of the anti-malaria programme in Thailand. Southeast Asian J Trop Med Public Health 1988;19:355–9. [PubMed] [Google Scholar]
- 2.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol 2004;20:233–40. [DOI] [PubMed] [Google Scholar]
- 3.Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol 2004;20:333–9. [DOI] [PubMed] [Google Scholar]
- 4.Fooden J Systematic review of Southeast Asian long-tail macaques, Macaca fascicularis (Raffles, [1821]). Fieldiana Zoology 1995;81:1–206. [Google Scholar]
- 5.Seethamchai S, Putaporntip C, Malaivijitnond S, Cui L, Jongwutiwes S. Malaria and Hepatocystis species in wild macaques, southern Thailand. Am J Trop Med Hyg 2008;78:646–53. [PubMed] [Google Scholar]
- 6.Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis 2004;10:2211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh B, Kim Sung L, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004;363:1017–24. [DOI] [PubMed] [Google Scholar]
- 8.Cox-Singh J, Davis TM, Lee KS, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis 2008;46:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng OT, Ooi EE, Lee CC, et al. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis 2008;14:814–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luchavez J, Espino F, Curameng P, et al. Human Infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis 2008;14:811–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putaporntip C, Jongwutiwes S, Sakihama N, et al. Mosaic organization and heterogeneity in frequency of allelic recombination of the Plasmodium vivax merozoite surface protein-1 locus. Proc Natl Acad Sci USA 2002;99:16348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putaporntip C, Jongwutiwes S, Iwasaki T, Kanbara H, Hughes AL. Ancient common ancestry of the merozoite surface protein 1 of Plasmodium vivax as inferred from its homologue in Plasmodium knowlesi. Mol Biochem Parasitol 2006;146:105–8. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997;25:4876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 2007;24:1596–9. [DOI] [PubMed] [Google Scholar]
- 15.Waters AP, Higgins DG, McCutchan TF. Evolutionary relatedness of some primate models of Plasmodium. Mol Biol Evol 1993;10:914–23. [DOI] [PubMed] [Google Scholar]
- 16.Leclerc MC, Hugot JP, Durand P, Renaud F. Evolutionary relationships between 15 Plasmodium species from new and old world primates (including humans): an 18S rDNA cladistic analysis. Parasitology 2004;129:677–84. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe K, Escalante A, Sakihama N, et al. Recent independent evolution of msp1 polymorphism in Plasmodium vivax and related simian malaria parasites. Mol Biochem Parasitol 2007;156:74–9. [DOI] [PubMed] [Google Scholar]
- 18.Zhou G, Sirichaisinthop J, Sattabongkot J, et al. Spatio-temporal distribution of Plasmodium falciparum and P. vivax malaria in Thailand. Am J Trop Med Hyg 2005;72:256–62. [PubMed] [Google Scholar]
- 19.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet 2004;364:1896–8. [DOI] [PubMed] [Google Scholar]
- 20.Annual Statistics, Division of Vector-Borne Diseases, Ministry of Public Health, Thailand. Available at: http://www.thaivbd.org. Accessed 30 June 2008. [Google Scholar]
- 21.Charoenlarp P, Harinasuta T. Relapses of vivax malaria after a conventional course of primaquine and chloroquine: Report of 2 cases. Southeast Asian J Trop Med Public Health 1973;4:135–7. [PubMed] [Google Scholar]
- 22.Looareesuwan S, Buchachart K, Wilairatana P, et al. Primaquine-tolerant vivax malaria in Thailand. Ann Trop Med Parasitol 1997;91:939–43. [DOI] [PubMed] [Google Scholar]
- 23.Krotoski WA. Frequency of relapse and primaquine resistance in Southeast Asian vivax malaria. N Engl J Med 1980;303:587. [DOI] [PubMed] [Google Scholar]
- 24.Somboon P, Lines J, Aramrattana A, Chitprarop U, Prajakwong S, Khamboonruang C. Entomological evaluation of community-wide use of lambdacyhalothrin-impregnated bed nets against malaria in a border area of north-west Thailand. Trans R Soc Trop Med Hyg 1995;89:248–54. [DOI] [PubMed] [Google Scholar]
- 25.Somboon P, Suwonkerd W, Lines JD. Susceptibility of Thai zoophilic anophelines and suspected malaria vectors to local strains of human malaria parasites. Southeast Asian J Trop Med Public Health 1994;25:766–70. [PubMed] [Google Scholar]
- 26.Apiwathnasor C, Prommongkol S, Samung Y, Limrat D, Rojruthai B. Potential for Anopheles campestris (Diptera: Culicidae) to transmit malaria parasites in Pa Rai subdistrict (Aranyaprathet, Sa Kaeo Province), Thailand. J Med Entomol 2002;39:583–6. [DOI] [PubMed] [Google Scholar]
- 27.Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet 1987;2:1052–5. [DOI] [PubMed] [Google Scholar]
- 28.Siripoon N, Snounou G, Yamogkul P, Na-Bangchang K, Thaithong S. Cryptic Plasmodium falciparum parasites in clinical P. vivax blood samples from Thailand. Trans R Soc Trop Med Hyg 2002;96:70–1. [DOI] [PubMed] [Google Scholar]
- 29.Jongwutiwes S, Putaporntip C, Karnchaisri K, Seethamchai S, Hongsrimuang T, Kanbara H. Positive selection on the Plasmodium falciparum sporozoite threonine-asparagine-rich protein: analysis of isolates mainly from low endemic areas. Gene 2008;410:139–46. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann EH, Malafronte RS, Moraes-Avila SL, et al. Origins of sequence diversity in the malaria vaccine candidate merozoite surface protein-2 (MSP-2) in Amazonian isolates of Plasmodium falciparum. Gene 2006;376:224–30. [DOI] [PubMed] [Google Scholar]
- 31.Cui L, Mascorro CN, Fan Q, et al. Genetic diversity and multiple infections of Plasmodium vivax malaria in Western Thailand. Am J Trop Med Hyg 2003;68:613–9. [DOI] [PubMed] [Google Scholar]
- 32.Malaivijitnond S, Hamada Y, Varavudhi P, Takenaka O. The current distribution and status of macaques in Thailand. Nat Hist J Chula Univ 2005;5:35–45. [Google Scholar]


