Abstract
Performing antibody-based assays for secreted insulin post-sample collection usually requires a few hours to a day of assay time and can be expensive, depending on the specific assay. Secreted luciferase assays expedite results and lower the assay cost per sample substantially. Here we present a relatively underused approach to gauge insulin secretory activity from pancreatic β cells by using Gaussia luciferase genetically inserted within the C-peptide. During proteolytic processing of proinsulin the C-peptide is excised releasing the luciferase within the insulin secretory vesicle where it is co-secreted with insulin. Results can be obtained within minutes after sample collection because of the speed of luciferase assays. A limitation of the assay is that it is a relative measurement of insulin secretion and not an absolute quantitation. However, this protocol is economical, scalable, and can be performed using most standard luminescence plate readers. Analog and digital multichannel pipettes facilitate multiple steps of the assay. Many different experimental variations can be tested simultaneously. Once a focused set of conditions are decided upon, insulin concentrations should be measured directly using antibody-based assays with standard curves to confirm the luciferase assay results.
Keywords: insulin, secreted luciferase, pancreatic beta cell, diazoxide paradigm, secretion assay, gaussia
SUMMARY:
This protocol describes how to perform rapid low-cost luciferase assays in medium-throughput using an insulin-linked Gaussia luciferase as a proxy for insulin secretion from beta cells. The assay can be performed with most luminescence plate readers and multichannel pipettes.
INTRODUCTION:
The method presented here allows insulin secretion from a genetically-modified beta cell line to be assayed rapidly and affordably in 96-well plate format. The key to this protocol is a modified version of insulin with the naturally-secreted Gaussia luciferase (GLuc, ~18 kDa) inserted into the C-peptide to generate insulin-Gaussia (InsGLuc)1,2. Other larger proteins, such as GFP (~25 kDa), have been successfully inserted into the C-peptide of insulin and exhibited the expected post-translational processing from proinsulin-GFP to insulin and GFP-C-peptide3,4. For the assay in this protocol, GLuc has been codon-optimized for mammalian expression and two mutations have been introduced to enhance glow-like kinetics5,6. Multiple combinations and replicates of treatment conditions can be easily tested in 96-well plate format and the secretion results can be obtained immediately following the experiment.
A major advantage, as previously noted2, is the low cost of this luciferase-based secretion measurement (<$0.01/well) which differentiates it from the relatively higher costs and technical aspects of ELISAs (>$2/well) and homogenous time-resolved fluorescence (HTRF) or other FRET-based antibody (>$1/well) assays. In comparison to these antibody-based assays, which measure the concentration of insulin by referencing a standard curve, the InsGLuc assay measures secretory activity as a relative comparison to control wells on the plate. For that reason, every experiment requires the inclusion of proper controls. This distinction is a trade-off to allow rapid and inexpensive measurements. However, InsGLuc secretion has been demonstrated to be highly correlated with insulin secretion as measured by ELISA1,2. This technology has been scaled up for high-throughput screening1,2,7 and has led to the identification of novel modulators of insulin secretion including a voltage-gated potassium channel inhibitor7 as well as a natural product inhibitor of β cell function, chromomycin A28. The use of InsGLuc is most appropriate for researchers who plan to continually test many different treatment conditions for their impact on insulin secretion. In follow-up experiments it is necessary to repeat key findings in a parental β cell line, and optimally in murine or human islets, and measure insulin secretion using an antibody-based assay.
PROTOCOL:
1. Preparation of reagents, media and buffers
-
1.1.
MIN6 complete media: MIN6 media is prepared in 500 mL of high-glucose (4.5 g/L) DMEM (Sigma D6429) with the following additives: 15% fetal bovine serum (Sigma F4135), 100 units/mL penicillin, 100 μg/mL streptomycin, 292 μg/mL L-glutamine, and 50 μM β-mercaptoethanol. The stable line in this case is maintained in 250 μg/mL of G418.
-
1.2.
Krebs-Ringer Bicarbonate Buffer (KRBH): KRBH is used to incubate the cells with and without stimulation in order to assess insulin and/or Gaussia luciferase secretion. To prepare KRBH, make a solution containing 5 mM KCl, 120 mM NaCl, 15 mM HEPES, pH 7.4, 24 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 1 mg/mL radioimmunoassay-grade BSA. Glucose is added where specified from a 2 M stock.
-
1.3.
Coelenterazine (CTZ) stock solution: Prepare acidified methanol by adding 106 μl of concentrated HCl to 10 mL of methanol. Next, dissolve lyophilized CTZ in acidified methanol at 1 mg/mL and store at −80°C in screw cap tubes. These stocks retain sufficient activity in routine luciferase assays, even after 1 year of proper storage.
-
1.4.
GLuc assay buffer: Gaussia luciferase assay buffer was formulated based upon the literature9 as well as patent information10 to aid in half-life of the Gaussia luciferase assay in 96 well plate format. The formula is 25 mM Tris pH 8, 1 mM EDTA, 5% glycerol, 1mg/mL Na2PO4, 300 mM sodium ascorbate, 200 mM Na2SO3 in water and stocks frozen at −20°C. After thawing, buffer can be stored at 4°C.
-
1.5.
Gaussia luciferase working solution: To prepare this solution add 4.2 μL/mL of the 1 mg/mL (2.36 mM) CTZ stock solution to GLuc assay buffer. This results in a 2X working solution of 10 μM CTZ which will have a 5 μM final concentration in the assay.
-
1.6.
Viability assay buffer (relative [ATP]): Viability assay buffer can be prepared according the manufacturer’s instructions with some modifications.
-
1.6.1.
Add 100 mL of manufacturer-provided buffer (as referenced in the Table of Materials) to the substrate and mix.
-
1.6.2.
Dilute the 100 mL mixture by adding 300 mL of PBS containing 1% Triton X-100 and mix well.
-
1.6.3.
Dispense into 40 mL aliquots and store at −20°C. After thawing, the unused solution can be refrozen.
Table 1.
Buffer and stock solution recipes used to perform the presented assays.
| Krebs-Ringer Bicarbonate Buffer (KRBH) | |||
|---|---|---|---|
| Stock solution or powder | Stock | Final Concentration | 100 mL |
| KCl | 0.25 M | 5 mM | 2 ml |
| NaCl | 4 M | 120 mM | 3 ml |
| Hepes, pH 7.4 | 1 M | 15 mM | 1.5 ml |
| NaHCO3 | 0.5 M | 24 mM | 4.8 ml |
| MgCl2 | 1 M | 1 mM | 0.1 ml |
| CaCl2 | 1 M | 2 mM | 0.2 ml |
| water | H2O | 88.4 ml | |
| RIA-grade BSA | powder | 1 mg/ml | 100 mg |
| Make fresh on day of experiment. | |||
| Gaussia Assay Buffer* | |||
| Stock solution or powder | Stock | Final Concentration | 50 mL |
| Disodium phosphate | powder | 0.1% (1mg/ml) | 50mg |
| Glycerol | 40% | 5% | 6.25ml |
| Sodium Bromide | powder | 150mM | 772mg |
| EDTA pH 8 | 0.5M | 1mM | 100ul |
| Tris-HCl pH 8 | 1M | 25mM | 1.25ml |
| Ascorbic Acid* | powder | 300mM | 2.64g |
| Na2SO3** | powder | 200mM | 1.26g |
| Water | up to 50mL | ||
| Store in aliquots at −20C, thaw one at a time and keep at 4C. | |||
| *Modified recipe from Luft et al. BMC Biochemistry 2014, 15:14. | |||
| Acidified MeOH | |||
| Stock solution or powder | Stock | Final Concentration | 10 mL |
| Methanol | 100% | 10 mL | |
| HCl | 11.65 M | 1.06% | 0.106 mL |
| Coelenterazine solution | |||
| Stock solution or powder | Stock | Final Concentration | 1 mL |
| Coelenterazine | powder | 1 mg/mL (2.36 mM) | 1 mg |
| Acidified methanol | 1 mL | ||
| 4.2 ul of stock per 1 ml of Gaussia Assay Buffer results in 10 uM CTZ to be used as a 2x working solution. | |||
| For example, add 50 ul of 2x working solution to 50 ul of KRBH sample containing secreted Gaussia. | |||
2. Culture of InsGLuc MIN6 cells and seeding for secretion assays
-
2.1.
To culture MIN6 cells, trypsinize and seed the cells once per week. Change media on the cells every two to three days. Media should include appropriate selection antibiotic, such as 250 μg/mL of G418.
-
2.1.1.
To provide a sufficient number of cells weekly for experiments, maintain the cells in T75 flasks. Seeding 6 x 106 cells per T75 in 10 mL of media will typically yield 30 to 40 x 106 cells total per T75 after 7 days of culture.
-
2.2.
To prepare cells for plating into 96 well dishes, wash a confluent T75 of InsGLuc MIN6 cells twice with PBS and add 2 mL of trypsin. Incubate at 37°C for ~5 min or until the cells dissociate from the flask. Determine the cell concentration per mL.
-
2.2.1.
Dilute the cells in complete media to 1 x 106 cells/mL to result in 1 x 105 cells in 100 μL per well in a 96 well plate. The cells should be sufficiently confluent for the assay after 3-4 days. To extend the culture period, half the cell concentration can be plated. Media changes are not required prior to the day of the assay unless the cells are to be subjected to experimental treatments.
3. Glucose-stimulated Gaussia luciferase secretion assay
-
3.1.
On the day of the assay prepare enough KRBH for the experiment. Typically, 50 mL of KRBH per 96 well plate is sufficient. Prepare extra buffer if different combinations of drug treatment conditions will require KRBH for dilution.
-
3.2.
Prepare a reservoir with glucose-free KRBH. Decant the medium from 96 well plate(s) by quickly inverting the plate over a laboratory sink and then blot firmly on a stack of paper towels to remove excess medium.
-
3.3.
Using either an electronic or manual 8-channel pipette, pipette KRBH from the reservoir across the 96 well plate(s), 100 μL per well. Repeat for a total of two washes.
-
3.4.
*Optional step* acute compound treatments: If not performing drug treatments, proceed with steps 3.5 and 3.6 without modification. To test the effects of small molecules on insulin secretion, compounds can be added to the cells during the preincubation period, stimulation period, or both.
-
3.4.1.
One technique is to add compounds in batch to the KRBH in 1.5 mL tubes and use an adjustable 8-channel digital pipette to transfer the drug-KRBH from the tubes to cells in 96 well plates. If an adjustable pipette is not available, a replica 96 well plate of drug-KRBH can be made and a standard 8-channel pipette can be used to transfer buffer.
-
3.4.2.
Further modifications to the treatment paradigm can be made to treat cells for 24 h in media prior to the assay, as previously described1,8.
-
3.5.
Add 100 μL of KRBH containing the desired concentration of glucose or compounds and place the dish in the 37°C incubator for 1 h. Depending on the experimental layout, it is extremely helpful to have an electronic multichannel pipette that allows transitioning the channel distances from a column of eight 1.5-mL tubes to the 8 rows of a 96 well plate.
-
3.6.
After the 1 h preincubation, decant the buffer into the sink and blot firmly on paper towel. Add 100 μL per well of glucose-free KRBH to wash away accumulated background of Gaussia luciferase. Decant the plate again and add control and stimulatory conditions to the plate, 100 μL per well. Place the dish in the 37°C incubator for 1 h.
-
3.7.
Carefully collect 50 μL of supernatant using a multichannel pipette, changing tips between treatment conditions as necessary, and transfer the supernatant to a clean opaque white 96 well assay plate. White-walled clear-bottom plates can be used if necessary, although a significant amount of luciferase signal will be lost.
-
3.8.
After collection of 50 μL of KRBH supernatant, the sample can be assayed immediately. If necessary, samples can be sealed and stored at 4°C for a few days (GLuc activity half-life ~ 6 days) or −20°C for up to one month11,12.
4. Secreted Gaussia luciferase assay
-
4.1.
To prepare the GLuc assay working solution, pipette the required amount of CTZ stock solution (4.2 μL/mL) into GLuc assay buffer. To prevent warming the CTZ, pipette the CTZ quickly at the −80°C freezer or keep the tube on dry ice.
-
4.2.
Using an electronic multichannel pipette, quickly add 50 μL of the GLuc assay working solution per well across the 96 well dish containing the collected KRBH supernatants. If there are any droplets on the sides of any wells, briefly spin the plate in a table-top swing-bucket centrifuge.
-
4.3.
Read the luminescence in a suitable plate reader within a few minutes and read each well with a 0.1 second integration time.
REPRESENTATIVE RESULTS:
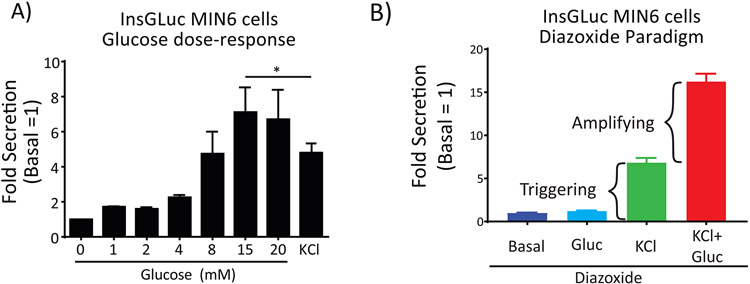
To gauge the performance of the assay under control conditions, a simple glucose dose-response curve or a stimulation using the diazoxide paradigm can be completed. In the case of the former, pre-incubating the cells for 1 h in glucose-free conditions followed by treating for 1 h with increasing glucose concentrations should result in very little secretory activity at and below 5 mM, while increased secretion is observed above 8 mM glucose (Fig. 2). Stimulation with 35 mM KCl also serves as a positive control for stimulated secretion. Inclusion of secretion-modulating drugs during the stimulation period should give the expected inhibition or potentiation of secreted GLuc activity (Fig 3). For example, diazoxide binds the KATP channel and prevents it from closing upon increased [ATP/ADP] ratio, blocking membrane depolarization and preventing secretion13. Phorbol esters like PMA activate PKC and are known amplifiers of insulin secretion14. Finally, stimulation with 1 μM epinephrine activates α2A-adrenergic receptors which in turn activate the heterotrimeric G-protein complex Gi, inhibiting membrane depolarization and insulin secretion15. It is important to recognize that while MIN6 cells are an immortal cell line, they start to lose proper glucose-induced insulin secretion responses (such as left-shifting of the response curve) after extended passaging16. For this reason, it is good practice to routinely culture all MIN6 cell lines for up to eight weeks (splitting once per week) before starting over from liquid nitrogen stocks.
Figure 2. The InsGLuc reporter is a faithful proxy of insulin secretion from MIN6 beta cells.
A) Response of MIN6 InsGLuc cells to increasing glucose concentrations and KCl (35 mM) with Gaussia luciferase secretion. Data are the mean fold luciferase activity ± SE compared to 0 mM glucose conditions for three independent experiments. *, P<0.05. Modified with permission from Kalwat MA, et al. ACS Sensors 2016 1. © 2016 American Chemical Society. B) The InsGLuc reporter in MIN6 cells exhibits the expected secretory response to the diazoxide (Dz) paradigm where 250 μM Dz treatment holds the KATP channel open, blocking membrane depolarization unless extracellular KCl (35 mM) is provided to elicit the ‘triggering’ calcium influx. Further addition of glucose (20 mM) under the Dz+KCl condition reveals the metabolic amplification of secretion that occurs without further increases in calcium influx.
Figure 3. Inclusion of secretion modulating compounds during glucose-stimulates InsGLuc secretion.
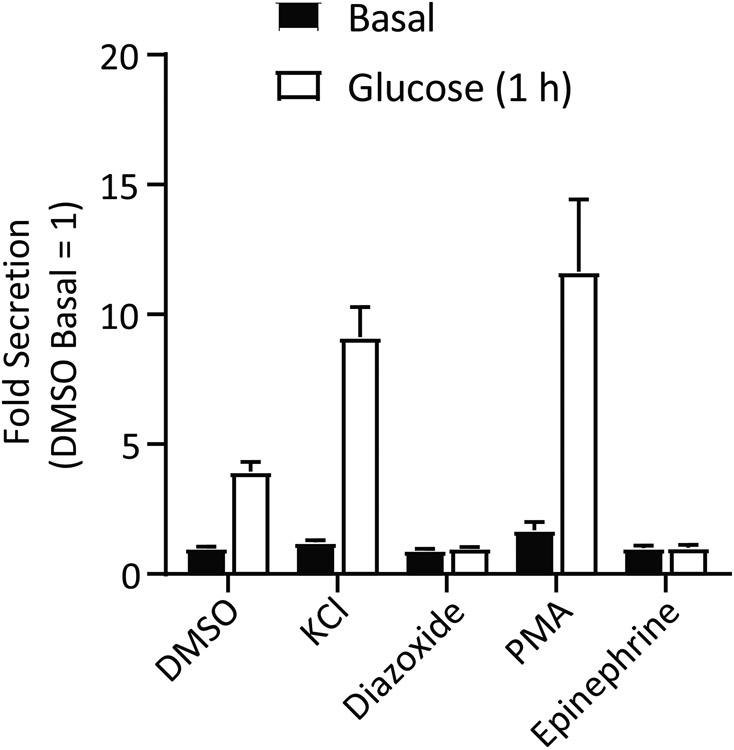
InsGLuc MIN6 cells plated in 96 well format as described were preincubated in glucose-free KRBH for 1 h. Cells were then treated with or without 20 mM glucose in the presence of DMSO (0.1%), KCl (35 mM), diazoxide (250 μM), PMA (100 nM), or epinephrine (1 μM) for 1 h. Bar graph is the mean ± SE of at least 3 independent experiments.
DISCUSSION:
Herein we present a method to rapidly assess glucose-stimulated insulin secretion responses from MIN6 β cells. For the best responses in the assay it is important to seed the MIN6 cells at the proper density and allow them to become 85-95% confluent. This improves β cell responses to glucose because of improved cell-cell contacts and synchronization and occurs both in primary islets17-21 as well as MIN6 cells16,18. To prevent losses in secretory response to glucose stimulation, it is important to maintain the cells at as low of a passage as possible and culture the cells for only 6-8 weeks prior to thawing a new vial from liquid nitrogen stocks. Modifications can be made to the plating strategy in Protocol section 2 to adapt to the available equipment as necessary. Plating InsGLuc MIN6 cells into 96-well plates for secretion assays affords a large number of wells for experimental manipulations (including replicates) as well as maintaining accuracy of plating in a normal lab setting, as plating into dishes with higher well numbers often requires special equipment usually available in high-throughput screening cores.
Current assays for insulin secretion, other than the indirect luciferase assay described here, include: ELISAs that use colorimetric readouts (direct assay), radioimmunoassays (competition assay) which use radioactive readouts, FRET-based antibody competition assay22 and HTRF23 which uses FRET between dye-linked antibodies to measure insulin directly, and DNA aptamers24. Each of these methods has its own advantages, but in general they are more expensive and/or time consuming than a luciferase assay. One key limitation of the InsGLuc assay is the fact that luminescent activity of the co-secreted luciferase is only a proxy for actual insulin secretion. Additionally, theoretically there is no expected difference in luciferase activity between Gaussia with fragments of C-peptide on its N- and C-termini or Gaussia within the proinsulin protein, as Gaussia luciferase has been successfully used as a tag to measure the secretion of other proteins25. This highlights the requirement of confirmation studies using assays that measure processed insulin specifically. Alternatives to direct and indirect measurements of insulin secretion can also be used to assess β cell function. A variety of optical reporters exist for readouts including ATP:ADP ratio, calcium influx, NAD+/NADH ratio, ERK activation, or cAMP levels26.
The future applications of InsGLuc in particular appear to be in high-throughput screening. This assay has already been used in a handful of published small screens1,2 and unpublished larger screens are either completed7 or underway. Development of other iterations of this technology may involve tagging of other secreted islet hormones with luciferases to facilitate rapid measurements, such as for glucagon or somatostatin. Modifications could be made in any case where the cell line is regenerated using alternate approaches including CRISPR/Cas9, lentivirus, or transposase-mediated insertion in any suitable beta cell line. Additional possible modifications to the original reporter may include substituting alternate secreted luciferases for Gaussia or combining multiple different secreted luciferases linked to different hormones for a multiplexed assay. Beyond cell culture, CRISPR/Cas9 technology presents the possibility of generating a mouse model where a suitable luciferase is knocked in to the C-peptide coding region of Ins2 in the genome. Such a mouse would be feasible given that transgenic mice have been created with GFP knocked in to the same C-peptide site27 and would allow measurement of endogenous β cell function with a luciferase assay in vivo or ex vivo.
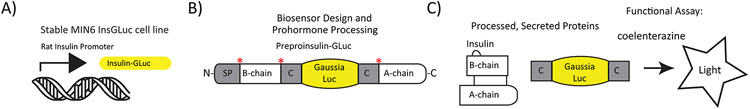
Figure 1. Description of the InsGLuc reporter.
A) First, a stable β cell line (in this case MIN6 cells) was generated expressing the Insulin-Gaussia transgene from the rat insulin promoter. B) The full protein is synthesized and packaged in insulin granules along with endogenous insulin. Prohormone convertases cleave the peptide, indicated by asterisks. C) The processed insulin and Gaussia are co-secreted and the luciferase activity is detected by the addition of CTZ in an ATP-independent, oxygen-dependent reaction.
ACKNOWLEDGMENTS:
The authors thank all current and former members of the Cobb laboratory for valuable work and discussions, and Dionne Ware for administrative assistance. Michael Kalwat is supported by a Juvenile Diabetes Research Foundation SRA-2019-702-Q-R. This work was made possible through NIH R37 DK34128 and Welch Foundation Grant I1243 to Melanie Cobb. Early parts of this work were also supported by an NIH F32 DK100113 to Michael Kalwat.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/59926.
DISCLOSURES:
The authors have nothing to disclose.
REFERENCES
- 1.Kalwat MA et al. Insulin promoter-driven Gaussia luciferase-based insulin secretion biosensor assay for discovery of beta-cell glucose-sensing pathways. ACS Sensors. 1 (10), 1208–1212, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns SM et al. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metabolism. 21 (1), 126–137, (2015). [DOI] [PubMed] [Google Scholar]
- 3.Rajan S et al. In vitro processing and secretion of mutant insulin proteins that cause permanent neonatal diabetes. American Journal of Physiology - Endocrinology and Metabolism. 298 (3), E403–410, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins S et al. Imaging Secretory Vesicles by Fluorescent Protein Insertion in Propeptide Rather Than Mature Secreted Peptide. Traffic. 3 (7), 461–471, (2002). [DOI] [PubMed] [Google Scholar]
- 5.Welsh JP, Patel KG, Manthiram K & Swartz JR Multiply mutated Gaussia luciferases provide prolonged and intense bioluminescence. Biochemical Biophysical Research Communications. 389 (4), 563–568, (2009). [DOI] [PubMed] [Google Scholar]
- 6.Tannous BA, Kim DE, Fernandez JL, Weissleder R & Breakefield XO Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Molecular Therarpy. 11 (3), 435–443, (2005). [DOI] [PubMed] [Google Scholar]
- 7.Burns SM, Wagner BK & Vetere A Compounds and methods for regulating insulin secretion. World patent WO2018175324A1 (2018).
- 8.Kalwat MA et al. Chromomycin A2 potently inhibits glucose-stimulated insulin secretion from pancreatic beta cells. Journal of General Physiology. 10.1085/jgp.201812177, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luft C et al. Application of Gaussia luciferase in bicistronic and non-conventional secretion reporter constructs. BMC Biochemistry. 15 (1), 14, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohmiya Y & Wu C Stabilizing composition and stabilizing method of coelenterazine solution for high-throughput measurement of luciferase activity. U.S. Patent 7,718,389 B2 (2010).
- 11.Tannous BA Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nature Protocols. 4 (4), 582–591, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wurdinger T et al. A secreted luciferase for ex vivo monitoring of in vivo processes. Nature Methods. 5 (2), 171–173, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henquin JC Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 49 (11), 1751–1760, (2000). [DOI] [PubMed] [Google Scholar]
- 14.Mourad NI, Nenquin M & Henquin JC Amplification of insulin secretion by acetylcholine or phorbol ester is independent of beta-cell microfilaments and distinct from metabolic amplification. Molecular & Cellular Endocrinology. 367 (1-2), 11–20, (2013). [DOI] [PubMed] [Google Scholar]
- 15.Straub SG & Sharp GW Evolving insights regarding mechanisms for the inhibition of insulin release by norepinephrine and heterotrimeric G proteins. American Journal of Physiology - Cell Physiology. 302 (12), C1687–1698, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng K et al. High passage MIN6 cells have impaired insulin secretion with impaired glucose and lipid oxidation. PLoS One. 7 (7), e40868, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Head WS et al. Connexin-36 Gap Junctions Regulate In Vivo First- and Second-Phase Insulin Secretion Dynamics and Glucose Tolerance in the Conscious Mouse. Diabetes. 61 (7), 1700–1707, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benninger RKP, Head WS, Zhang M, Satin LS & Piston DW Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. The Journal of Physiology. 589 (22), 5453–5466, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstantinova I et al. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 129 (2), 359–370, (2007). [DOI] [PubMed] [Google Scholar]
- 20.Jaques F et al. Dual effect of cell-cell contact disruption on cytosolic calcium and insulin secretion. Endocrinology. 149 (5), 2494–2505, (2008). [DOI] [PubMed] [Google Scholar]
- 21.Calabrese A et al. Connexin 36 Controls Synchronization of Ca2+ Oscillations and Insulin Secretion in MIN6 Cells. Diabetes. 52 (2), 417–424, (2003). [DOI] [PubMed] [Google Scholar]
- 22.Bielefeld-Sevigny M AlphaLISA immunoassay platform- the "no-wash" high-throughput alternative to ELISA. Assay and Drug Development Technologies. 7 (1), 90–92, (2009). [DOI] [PubMed] [Google Scholar]
- 23.Aslanoglou D, George EW & Freyberg Z Homogeneous Time-resolved Forster Resonance Energy Transfer-based Assay for Detection of Insulin Secretion. Journal of Visualized Experiments. 10.3791/57531 (135), (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafati A, Zarrabi A, Abediankenari S, Aarabi M & Gill P Sensitive colorimetric assay using insulin G-quadruplex aptamer arrays on DNA nanotubes coupled with magnetic nanoparticles. Royal Sociecy Open Science. 5 (3), 171835, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulleman JD, Brown SJ, Rosen H & Kelly JW A high-throughput cell-based Gaussia luciferase reporter assay for identifying modulators of fibulin-3 secretion. Journal of Biomolecular Screening. 18 (6), 647–658, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank JA et al. Optical tools for understanding the complexity of beta-cell signalling and insulin release. Nature Reviews Endocrinology. 14 (12), 721–737, (2018). [DOI] [PubMed] [Google Scholar]
- 27.Zhu S et al. Monitoring C-Peptide Storage and Secretion in Islet beta-Cells In Vitro and In Vivo. Diabetes. 65 (3), 699–709, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]