Abstract
Significance: Sepsis is a critical clinical syndrome with life-threatening organ dysfunction induced by a dysregulated host response to infection. Despite decades of intensive research, sepsis remains a leading cause of in-hospital mortality with few specific treatments.
Recent Advances: Toll-like receptors (TLRs) are a part of the innate immune system and play an important role in host defense against invading pathogens such as bacteria, virus, and fungi. Using a combination of genetically modified animal models and pharmacological agents, numerous preclinical studies during the past two decades have demonstrated that dysregulated TLR signaling may contribute to sepsis pathogenesis. However, many clinical trials targeting inflammation and innate immunity such as TLR4 have yielded mixed results.
Critical Issues: Here we review various TLRs and the specific molecules these TLRs sense—both the pathogen-associated and host-derived stress molecules, and their converging signaling pathways. We critically analyze preclinical investigations into the role of TLRs in animal sepsis, the complexity of targeting TLRs for sepsis intervention, and the disappointing clinical trials of the TLR4 antagonist eritoran.
Future Directions: Future sepsis treatments will depend on better understanding the complex biological mechanisms of sepsis pathogenesis, the high heterogeneity of septic humans as defined by clinical presentations and unique immunological biomarkers, and improved stratifications for targeted interventions.
Keywords: sepsis, toll-like receptors, danger-associated molecular patterns, pathogen-associated molecular patterns, innate immunity, inflammation, clinical trial
Introduction
Toll-like receptors (TLRs) represent an important component of the innate immune defense against microbial pathogens (2). They are among the first to sense pathogen invasion, activate innate immune response, control adaptive immune response (53), and maintain normal immune homeostasis. Depressed or overactivated immune responses via TLRs impair host defense against pathogens and play an important role in sepsis pathogenesis (21, 51).
Sepsis is a clinical syndrome with life-threatening organ dysfunction caused by a dysregulated host response to infection (115). In the United States, sepsis develops in more than 750,000 people annually and 210,000 of them die (9, 80). Despite the recent progress in sepsis management, such as early antibiotic coverage, aggressive fluid resuscitation, and vasopressors to maintain hemodynamics, sepsis remains the #1 cause of mortality in hospitals (32, 102, 110). There have been numerous phase 2 and 3 sepsis clinical trials of various therapies for sepsis, including anti-inflammation, anti-cytokine, and immune and coagulation modulations, most of which have failed (37, 77).
Among many possible causes, incomplete understanding of the biological mechanisms of sepsis pathogenesis may have contributed to the failed clinical trials (51, 77, 123). This article critically reviews the animal investigations on the role of TLR signaling in sepsis, the clinical trials testing the therapeutic efficacy of blocking TLR4, and the lessons we learn from these studies.
TLRs: Pattern Recognition and Signaling Pathways
Following their invasion, microbes release various pathogen-associated molecular patterns (PAMPs), such as endotoxin, lipopeptide, and nucleic acid. PAMPs are sensed by the pattern recognition receptors such as TLRs in the host cells and activate the host proinflammatory responses such as leukocyte activation, complement activation, and coagulation activation (21, 51). These host responses are extremely important to contain and eliminate microbe dissemination. However, if the responses are dysregulated and excessive, it can cause collateral damage to the host. For example, too much cytokine production (cytokine storm) can cause septic shock, coagulation activation, and subsequent consumptive coagulopathy such as disseminated intravascular coagulation.
Dead cells release damage-associated molecular pattern (DAMP) that can act on TLRs and perpetuate proinflammatory responses, causing excessive inflammation and tissue damage. Almost at the same time, the anti-inflammatory responses are initiated. These include the neuroendocrine axis of parasympathetic outflow and adrenal glands, immune cell dysfunction or death, and anti-inflammatory cytokine production. The anti-inflammatory pathways are implicated in the enhanced susceptibility to secondary infections during the later stage of sepsis.
Discovery of toll and TLR
In 1985, Nusslein-Volhard identified the Toll gene critical for the embryonic development of the fruit flies Drosophila (7, 8). A decade later, Hoffmann and colleagues found that Toll was essential to innate immunity against pathogen (69). The following year, Medzhitov and Janeway at Yale discovered that a human Toll protein was a transmembrane protein with an extracellular domain consisting of a leucine-rich repeat and cytoplasmic domain, which they coined “Toll-like receptor” (79). Subsequently, several groups discovered that mice with naturally mutated Tlr4 gene (97, 98), either a missense point mutation (Pro→His, C3H/HeJ strain) or null mutation (C57 BL/10 ScCr strain), or with specific Tlr4 gene deletion (TLR4−/−) (50), exhibited insensitivity to endotoxin, demonstrating TLR4 as the sensor for bacterial endotoxin.
TLR signaling pathways
At least 11 human and 13 mouse TLRs have been cloned and they are expressed on various types of immune and nonimmune cells, such as macrophages, monocytes, dendritic cells (DCs), lymphocytes, endothelial and epithelial cells, and cardiomyocytes. TLRs are single-spanning membrane glycoproteins with an intracellular Toll/interleukin-1 receptor (TIR) domain (58).
Based on their locations in the cell, TLRs are categorized into two groups: (i) those anchored on the plasma membranes including TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10, which mainly sense lipopeptides, peptidoglycan, lipopolysaccharide (LPS), or zymosan of bacterial and fungi origins, and (ii) those located inside the cell on the endosome membranes such as TLR3, TLR7, TLR8, TLR9, TLR11, TLR12, and TLR13, which are mainly associated with nucleic acid sensing (103), such as double-stranded (ds) RNA (TLR3) (4), single-stranded RNA (TLR7/8) (45, 73, 131), and DNA (TLR9) (49). Other known nucleic acid sensors are located in the cytoplasm and include the RNA sensors—retinoic-acid-inducible gene 1 (RIG-I) and melanoma-differentiation-associated gene 5 (MDA5), and the DNA sensors—cyclic GMP-AMP synthase and absent in melanoma 2 in the cytoplasm (103).
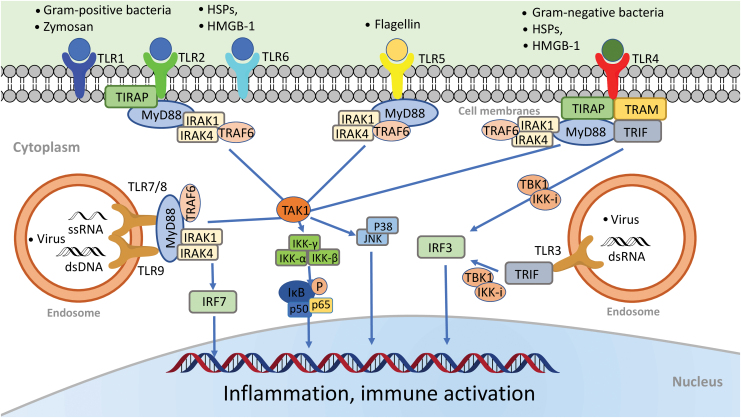
Following ligand binding, TLRs form dimers and the resulting TIR-TIR complexes trigger the downstream signaling (Fig. 1) through the specific adaptors (84), that is, MyD88 (myeloid differentiation factor 88), TIRAP (TIR domain-containing adaptor protein), Trif (TIR domain-containing adaptor inducing IFN-β–mediated transcription factor), SARM (sterile α- and heat-armadillo-motif-containing protein), and TRAM (Trif-related adaptor molecule).
FIG. 1.
TLRs: ligands and signaling pathways. All TLRs are transmembrane proteins. TLR1, TLR2, TLR4, TLR5, and TLR6 are expressed on the cell surface, whereas TLR3, TLR7/8, and TLR9 are located almost exclusively in endosomes. Different TLRs recognize different microbial components. For example, TLR2 recognizes lipopeptides or peptidoglycan, a wall component of gram-positive bacteria. It also recognizes DAMPs such as HSPs and HMGB1. TLR2 heterodimerizes either with TLR1 to recognize triacylated lipopeptide or with TLR6 to recognize diacylated lipopeptides. TLR4 senses endotoxin, a wall component of gram-negative bacteria. TLR5 senses bacterial flagellin, a protein component of flagella. TLR3 recognizes viral dsRNA, whereas TLR7 and TLR8 are the sensors for ssRNA. Finally, TLR9 senses bacterial CpG-rich hypomethylated DNA (CpG DNA) motifs. Upon ligand binding, TLRs form dimers and recruit one or more adaptor proteins, namely, MyD88, TIRAP, TRIF, or TRAM, to the cytoplasmic domains of the receptors through their TIR domain interactions. All TLRs with the exception of TLR3 signal via the MyD88-dependent pathway. TIRAP acts as a bridge to recruit MyD88 to TLR2 and TLR4 signaling, whereas TRIF is used in TLR3 signaling. In MyD88 signaling, MyD88 associates with IRAK4 and IRAK1. IRAK4 in turn phosphorylates IRAK1 and promotes their association with TRAF6, which serves as a platform to recruit and activate the kinase TAK1. Activated TAK1 activates the IKK complex, composed of IKKα, IKKβ, and IKKγ, which in turn catalyze phosphorylation and subsequent degradation of I-κB. I-κB degradation lets NF-κB (i.e., p50/p65) free to translocate from the cytoplasm to the nucleus where it activates multiple inflammatory cytokine gene expressions. The transcription factor IRF7 is activated as the downstream signaling molecule of TLR7/8 and TLR9. It is directly phosphorylated by IRAK1 and then translocated into the nucleus to induce the expression of type I IFN-α and IFN-inducible genes. In the Trif-dependent pathway, Trif activates sTBK1 and IKK-i, resulting in the IRF3 activation and translocation into the nucleus to activate the transcription of IFN-β and IFN-inducible genes. CpG, cytidine-phosphate-guanosine; DAMP, damage-associated molecular pattern; dsRNA, double-stranded RNA; HMGB1, high-mobility group box 1; HSP, heat-shock protein; IFN, interferon; IKK, I-κB kinase; IL, interleukin; IRAK, IL-1 receptor-associated kinase; IRF, interferon regulatory factor; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa B; ssRNA, single-stranded RNA; TAK1, transforming growth factor-α activated kinase 1; TIR, toll/interleukin-1 receptor; TIRAP, TIR domain-containing adaptor protein; TLR, toll-like receptor; TNF, tumor necrosis factor; TRAF6, TNF receptor-associated factor 6; TRAM, Trif-related adaptor molecule; TRIF, TIR domain-containing adaptor inducing IFN-β-mediated transcription factor. Color images are available online.
TLR signaling can be further divided into two distinct but convergent pathways: MyD88-dependent and Trif-dependent pathways. MyD88-dependent pathway is activated by all TLRs with the exception of TLR3. MyD88 pathway leads to activation of the transcription factor nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases. MyD88 recruits IL-1 receptor-associated kinase (IRAK). The IRAK1-TNF receptor-associated factor 6 (TRAF6) complex then activates transforming growth factor-α activated kinase 1 (TAK1). Activated TAK1 then phosphorylates I-κB kinase beta (IKKβ), leading to phosphorylation and degradation of I-κB, which releases the NF-κB p50/p65 subunits and results in the nuclear translocation and DNA binding of NF-κB. Trif-dependent pathway is utilized by TLR3 and TLR4. It induces type I interferon (IFN) and inflammatory cytokines through the transcription factor interferon regulatory factor 3 (IRF3).
PAMPs and DAMPs
TLRs specifically bind to a wide range of pathogens such as bacteria, fungi, and viruses through “PAMPs” recognition (Table 1) (2, 58). TLRs can also act as a stress sensor in response to noninfectious tissue injury and recognize a variety of endogenous danger molecules through “DAMPs” recognition (94).
Table 1.
Toll-Like Receptors and Ligands
| TLR | TLR localization | Adaptor molecule | PAMP | DAMP | References |
|---|---|---|---|---|---|
| TLR1 | Plasma membrane | MyD88/TIRAP | Triacyl lipopeptide soluble factors | — | (117) |
| TLR2 | Plasma membrane | MyD88/TIRAP | Lipoproteins, zymosan, atypical LPS, peptidoglycan, lipoarabinomannan | Hsp60, Hsp70, and glycoprotein 96, HMGB1, hyaluronic acid, matrix metalloproteinase 2, pancreatic adenocarcinoma upregulated factor | (5, 10, 19, 33, 38, 59, 91, 92, 112, 125, 127, 129) |
| TLR3 | Endosome | TRIF | dsRNA | mRNA | (57) |
| TLR4 | Plasma membrane and endosome | MyD88/TIRAP, TRIF/TRAM | LPS, taxol, fusion protein, envelope protein | Hsp60, Hsp70, Hsp72, Hsp22, hyaluronic acid, glycoprotein 96, heparan sulfate, fibrinogen, HMGB1 | (3, 15, 25, 55, 56, 88, 89, 92, 100, 101, 106, 111, 121, 129, 130) |
| TLR5 | Plasma membrane | MyD88 | Flagellin | HMGB1 | (43) |
| TLR6 | Plasma membrane | MyD88/TIRAP | Di-acyl lipopeptides, lipoteichoic acid, β-glucan | Amyloid β and oxidized LDL | (23, 41, 63, 126) |
| TLR7 | Endosome | MyD88 | ssRNA, imidazoquinoline, loxoribine, bropirimine | ssRNA miRNA |
(1, 44, 48, 73, 83, 131, 142, 143) |
| TLR8 | Endosome | MyD88 | ssRNA, midazoquinoline | ssRNA | (45, 83, 131) |
| TLR9 | Endosome | MyD88 | CpG-containing DNA, dsDNA, hemozoin | chromatin-IgG complex mtDNA |
(11, 20, 36, 66, 67, 76, 81, 96, 141) |
| TLR10 | Plasma membrane | MyD88 | Lipopeptides | (42) | |
| TLR11 | Endosome | MyD88 | Flagellin | (137, 138) | |
| TLR12 | Endosome | MyD88 | Profilin-like protein | (99) | |
| TLR13 | Endosome | MyD88 | 23s ribosomal RNA | (85) |
CpG, cytidine-phosphate-guanosine; DAMP, damage-associated molecular pattern; ds, double-stranded; HMGB1, high-mobility group box 1; Hsp, heat-shock protein; IFN, interferon; LDL, low-density lipoprotein; LPS, lipopolysaccharide; miRNA, microRNA; mtDNA, mitochondrial DNA; MyD88, myeloid differentiation factor 88; PAMP, pathogen-associated molecular pattern; TIRAP, TIR domain-containing adaptor protein; TLR, toll-like receptor; TRAM, Trif-related adaptor molecule; TRIF, TIR domain-containing adaptor inducing IFN-β-mediated transcription factor.
LPS, one of the best-characterized bacterial ligand, is a wall component of gram-negative bacteria. The extracellular domain of TLR4 forms a complex to act as the LPS-binding site of TLR4 (88, 89). TLR2 senses a wide range of PAMPs—including lipopeptides, peptidoglycan, and lipoteichoic acid from gram-positive bacteria. TLR2 usually forms heterodimers with TLR1 or TLR6. TLR2/6 heterodimer senses diacylated lipopeptides (125), whereas TLR1/2 distinguishes triacylated lipopeptides (127). TLR5 responds to bacterial flagella through flagellin (43). TLR11 responds to the protozoan parasite and uropathogenic bacteria through a profilin-like molecule (137, 138). TLR3 recognizes double-strand RNA, which can activate immune responses to express IFN and cytokines to exhibit antiviral and antibacterial effects. TLR7 and TLR8 recognize single-strand RNAs, as well as imidazoquinoline compounds such as guanine analogues and imiquimod (142, 143). At last, TLR9 recognizes cytidine-phosphate-guanosine (CpG) DNA motifs with unmethylated dinucleotides from bacteria and viruses (11, 96) and mitochondrial DNA (36, 76, 141).
DAMPs are produced by injured cells under both infectious (e.g., sepsis) and noninfectious (e.g., trauma, ischemic injury, and autoimmune disease) conditions (18, 29, 39, 64, 65, 71, 118, 149, 151). Some of the reported examples include heat-shock proteins, hyaluronic acid, glycoprotein 96 (Gp96), heparan sulfate, fibrinogen, HMGB1, RNA, DNA, amyloid β, and oxidized low-density lipoprotein (Table 1). These endogenous pattern molecules, once released into the extracellular space, are sensed by various TLRs and elicit the host innate immune responses.
Severe acute respiratory syndrome coronavirus 2 and innate immune receptor recognition
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible virus and has caused the current global pandemic of acute respiratory disease, named “coronavirus disease 2019” (COVID-19), that threatens the global health and safety (52). SARS-CoV-2, like other coronaviruses, contains the dsRNA that is produced during viral genome replication and transcription (60, 146) and can be recognized by several nucleic acid sensors, including RIG-I and MDA5, in the cytoplasma (70, 109) and/or by TLR3 in the endosome (78, 124). Activation of these innate immune receptors leads to the antiviral type-I IFN signaling (68).
Role of TLRs in Sepsis
Extensive preclinical work has documented the possible role of TLRs in the sepsis pathogenesis. Many of these studies have taken loss-of-function approaches, either in genetically modified mouse models or pharmacological blocking, to manipulate TLR signaling. Others have used TLR ligands to activate TLR signaling. Table 2 summarizes some of these findings.
Table 2.
Role of Toll-Like Receptors in Endotoxin Shock and Bacterial Sepsis
| TLRs | Sepsis |
|---|---|
| TLR2 | TLR2−/− mice have better survival, markedly improved cardiac function, attenuated systemic and myocardial cytokine production (147), improved global clotting function, platelet counts, and near-normal plasma tissue factor levels compared with WT mice 24 h after CLP (135). |
| Chimeric models demonstrate that it is nonhematopoietic TLR2 that contributes to neutrophil and cardiac function impairment in sepsis (150). | |
| Peptidoglycan-associated lipoprotein induces TLR2/MyD88 signaling cascade to activate cardiomyocyte dysfunction and inflammatory responses (145). | |
| TLR2 activation by bacterial lipoproteins stimulates endothelial function and coagulation pathways and contributes to endothelial activation, coagulopathy, and vascular leakage in sepsis (114). | |
| Complement factor B acts as a downstream effector of multiple TLRs and plays a critical role in bacterial sepsis (148). | |
| Circadian rhythms in immune cells mediate diurnal variations in murine sepsis severity via a TLR2-dependent mechanism (47). | |
| TLR2-mediated neutrophil depletion exacerbates bacterial sepsis (104). | |
| TLR2 and TLR4 contribute to sepsis-induced depletion of spleen dendritic cells (82, 93). | |
| TLR3 | Systemic deletion of TLR3 confers a survival benefit, improved cardiac function, and reduced inflammatory cell infiltration (28, 35). |
| TLR4 | TLR4 mediates endotoxin-induced NF-κB activation, cytokine production, and cardiac dysfunction (61, 12). Chimeric studies indicate that TLR4 in bone marrow-derived hematopoietic cells is responsible for cardiac dysfunction during endotoxin shock (13, 119, 120). |
| TLR4 signaling leads to neutrophil migration impairment and dendritic cell depletion in polymicrobial sepsis (6, 93). | |
| While TLR4 deletion clearly confers a survival benefit in endotoxin shock (97) or lethal gram-negative bacterial sepsis (105), it offers no survival benefit (26) or even deleterious effect in mild gram-negative or polymicrobial sepsis (105, 140). | |
| TLR5 | Bacterial flagellin activates innate immune response and induces marked myocardial inflammation and contractile dysfunction via TLR5 (107, 108). Flagellin-TLR5 signaling promotes endothelial repair and survival in sepsis (144). |
| TLR7 | TLR7 mediates coagulation activation and coagulopathy in murine sepsis (135). |
| Bone marrow-derived macrophages treated with TLR7 agonist (R837) exhibit marked increases in tissue factor expression (135). | |
| TLR7−/− mice have lower plasma cytokines, reduced circulatory shock, less organ injury, and significantly improved survival compared with WT mice after CLP (54). | |
| TLR9 | TLR9 contributes to cytokine production, splenic apoptosis, and kidney injury during polymicrobial sepsis (128). |
| Compared with WT mice, TLR9−/− mice exhibited lower serum inflammatory cytokine levels, higher bacterial clearance, and greater survival after CLP (95). |
CLP, cecum ligation and puncture; NF-κB, nuclear factor kappa B; WT, wild type.
TLR2
Both animal and human studies support the role of TLR2 in sepsis-induced immune and multiple organ injuries, such as cardiac, endothelial, and neutrophil dysfunction, and coagulopathy (34, 47, 93, 103, 105, 114, 135, 136, 139, 145, 147, 150). For example, peptidoglycan-associated lipoprotein, a natural TLR2 ligand and a ubiquitous gram-negative bacterial membrane protein (72), inhibits cardiomyocyte function (145) and activates endothelial function and coagulation pathways (114). In mouse models of polymicrobial sepsis, TLR2−/− mice have better survival, improved cardiac function, attenuated blood and myocardial cytokine production (147), improved clotting function (135), less immune cell depletion (82, 93), reduced mitochondrial reactive oxygen species production, and improved mitochondrial function (40) when compared with wild-type (WT) mice.
TLR3
TLR3 senses dsRNA and also endogenous RNA released from necrotic tissues and mediates acute inflammation (16). In a bacterial sepsis model, however, knockout of TLR3 confers a survival benefit and improved organ function (28, 35). Both bacterial and host RNAs are present in the circulation of septic mice and could potentially function as the agonists of TLR3. Interestingly, in a double-hit model, treatment with the TLR3 ligand poly(I:C) of animals before cecum ligation and puncture (CLP) enhances host immunity and improves the survival (24).
TLR4
Extensive reports have demonstrated the role of TLR4 in mediating cytokine storm, immune cell impairment, organ injury, and mortality in endotoxin shock or in polymicrobial sepsis (6). Animal endotoxemia induces NF-κB activation (61) that leads to robust myocardial cytokine response and myocardial dysfunction (12). This process involves signaling via TLR4, CD14, IRAK1, MyD88, and Trif. The endotoxin-mediated cardiac dysfunction may be an indirect effect secondary to immune cell activation rather than a direct effect on cardiomyocytes as in vitro treatment with LPS fails to inhibit cardiomyocyte function (120).
Chimeric models suggest that TLR4 in hematopoietic cells is responsible for cardiac dysfunction during endotoxic shock (13, 119, 120). Both MyD88 and Trif play an equally important role in endotoxin shock (31). Studies using tissue-specific MyD88 knockout models demonstrate that both cardiomyocyte- and myeloid-MyD88 play a role in mediating cardiac dysfunction and mortality during endotoxin shock (31). In animal models of bacterial sepsis, the role of TLR4 is complex and may depend on the type and severity of bacterial infection. For example, while TLR4 deletion clearly confers a survival benefit in endotoxin shock (97) or lethal gram-negative bacterial sepsis (105), it offers no survival benefit (26) or even deleterious effect in mild gram-negative or polymicrobial sepsis (105, 140).
These data seem to suggest that host may mobilize different innate immune mechanisms in endotoxemia and bacterial sepsis. Moreover, studies suggest that signaling via MyD88, but not Trif, plays a predominant role in mediating cardiac dysfunction, marked systemic inflammation, and mortality in a lethal model of bacterial sepsis, whereas MyD88 and Trif are equally important in systemic inflammation, organ dysfunction, and death during endotoxin shock (31).
Several possible mechanisms may explain the deleterious effect of TLR4 deficiency in low-grade bacterial sepsis. First, TLR4 is a part of the host immune defense against bacterial invasion. WT mice exhibit more robust neutrophil migratory and phagocytic functions compared with TLR4−/− mice. As a result of bacterial dissemination in the absence of TLR4, TLR4−/− mice have more cytokine production, bacterial load, and higher mortality. Second, TLR4 signaling may play a “preconditioning-like” role during low-grade bacterial infection. LPS pretreatment confers a cardioprotective effect against hypoxic injury (14, 17). Studies have shown that administration of low-dose endotoxin offers protection against both subsequent endotoxin challenges and polymicrobial infection (134). Finally, it should be pointed out that there is substantial difference in endotoxin sensitivity among different species. Controversies exist over whether or how well endotoxin-challenged mice mimic endotoxemia and acute inflammation in humans (22, 113, 116, 133).
TLR5
Bacterial flagellin, a TLR5 ligand, activates NF-κB-mediated inflammatory response and induces myocardial dysfunction (107, 108). In vivo, flagellin administration leads to cytokine storm, increased myocardial neutrophil infiltration, and reversible cardiac dysfunction.
TLR7/TLR8
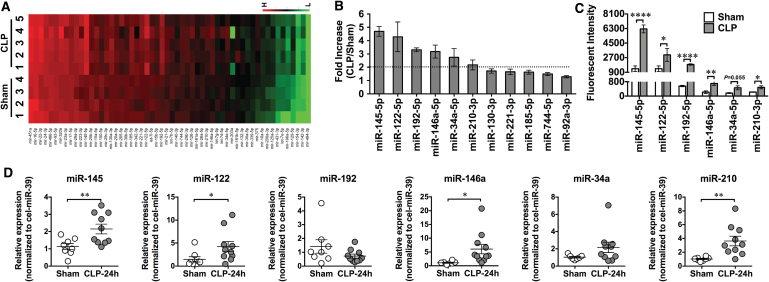
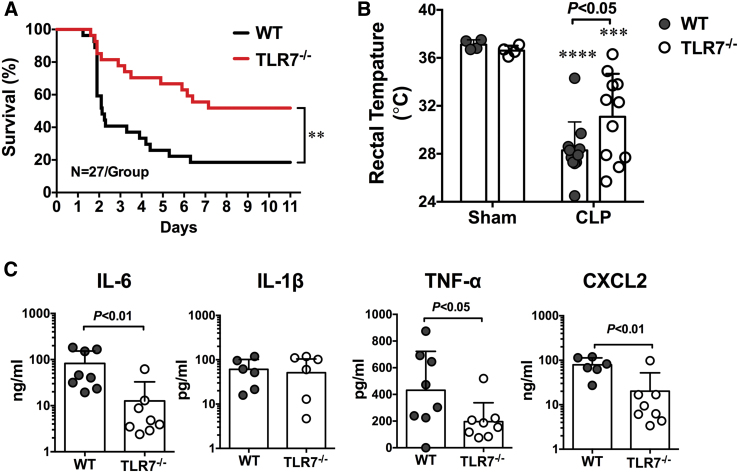
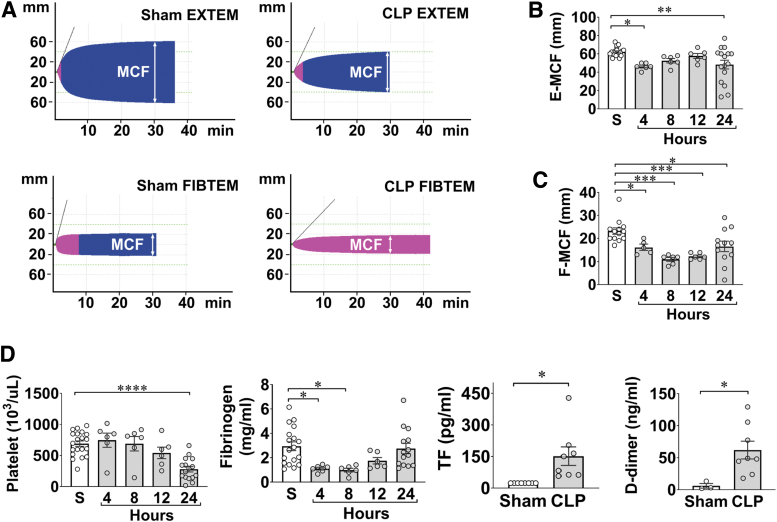
In addition to sensing viral single-stranded RNA, TLR7 may also sense endogenous extracellular RNAs released from injured cells and plays a pivotal role in murine sepsis. In a CLP model of polymicrobial sepsis, studies have found an increased plasma RNA that is closely correlated with sepsis severity (149). Plasma microRNA (miRNA) array revealed upregulation of multiple miRNAs including miR-34, miR-122, miR-145, miR-146a, miR-210 (Fig. 2) (149). Tissue RNA extracts or RNA released from injured cells or miRNA mimics induces proinflammatory cytokine production and complement activation via a TLR7-dependent mechanism (29, 30, 149). Moreover, compared with WT mice, mice deficient of TLR7 had lower plasma cytokines, reduced circulatory shock (lower core temperature), and significantly improved survival (Fig. 3) (54). Finally, similar to humans, septic mice develop sepsis-induced coagulopathy characterized by global clotting dysfunction, severe thrombocytopenia, decreased fibrinogen, and increased plasma tissue factor (TF) and D-dimers (Fig. 4) (135). TLR7−/− septic mice exhibited preserved global clotting function, platelet counts, and near-normal plasma TF concentration (135).
FIG. 2.
Plasma miRNA profiling in septic mice. (A) Heat map of miRNA array. Mice were subjected to sham surgery (n = 4) or CLP (n = 5). At 24 h, plasma was collected, and miRNAs were analyzed using a firefly miRNA array. The fluorescence intensity of 56 miRNAs was expressed from low (green) to high (red). (B) Fold change in the plasma miRNAs in CLP compared with sham mice (CLP/sham), as measured by miRNA array. (C) Mean fluorescent intensity of plasma miRNAs, as measured by miRNA array, in sham and CLP mice. Six miRNAs (miR-145, miR-122, miR-192, miR-146a, miR-34a, and miR-210) were significantly increased >2-fold in the septic mice compared with the sham control (n = 4 in sham group, n = 5 in CLP group). (D) qRT-PCR validation of miRNAs. The six target miRNAs were tested using qRT-PCR in a separate set of plasma (n = 8 in sham group, n = 10 in CLP group). *p < 0.05, **p < 0.01, ****p < 0.001. [Zou et al. (149), used with permission]. CLP, cecum ligation and puncture; miRNA, microRNA; qRT-PCR, quantitative reverse-transcriptase–polymerase chain reaction. Color images are available online.
FIG. 3.
TLR7-deficient mice have improved survival and attenuated plasma cytokine productions after polymicrobial sepsis. (A) Survival rate of WT and TLR7−/− mice during sepsis. Mice were subjected to CLP surgery and observed for survival for up to 11 days. **p < 0.01, n = 27 in WT and TLR7−/− group. (B) Rectal temperature at 24 h after CLP surgery. ***p < 0.001, ****p < 0.0001 versus sham group. Unequal variance t-test, n = 11 per group. (C) Plasma cytokines. IL-6 and TNFα are expressed as median with interquartile range and analyzed by Mann–Whitney U test. IL-1β and CXCL2 are expressed as mean ± SD and analyzed by unequal variance t-test [Jian et al. (54), used with permission]. WT, wild type. Color images are available online.
FIG. 4.
Septic mice develop global coagulopathy. Wild-type C57BL/6 mice were subjected to CLP surgery, and killed at the indicated time points for blood collection. Sham mice were killed at 24 h. (A) Representative pictures of rotational thromboelastometry traces, a hemostatic viscoelastic test. Both representative EXTEM and FIBTEM traces from sham and CLP mice are shown. EXTEM to test tissue factor-initiated clot formation; FIBTEM to test EXTEM in the presence of a platelet inhibitor, cytochalasin. MCF is marked in each tracing at 30 min. (B, C) Time course of MCF values in EXTEM assays (E-MCF) and FIBTEM assays (F-MCF) following CLP. *p < 0.05, **p < 0.01, ***p < 0.001. (D) Clotting factors in sham and septic mice. *p < 0.05, **p < 0.01, ****p < 0.0001. [Williams et al. (135), used with permission]. EXTEM, extrinsic thromboelastometry; FIBTEM, fibrinogen thromboelastometry; MCF, maximum clot firmness. Color images are available online.
TLR9
DNA and RNA isolated from Staphylococcus aureus and Escherichia coli induce rat cardiomyocyte dysfunction (87). Similarly, CpG-ODN, a TLR9 agonist, inhibits sarcomere shortening of isolated mouse cardiomyocytes. In vivo, CpG-ODN causes myocardial NF-κB activation and cytokine production. Both effects are abolished in TLR9-deficient mice (62). Compared with WT mice, TLR9−/− mice exhibited lower serum inflammatory cytokine levels, higher bacterial clearance, and greater survival after experimental peritonitis induced by CLP. Protection of TLR9−/− mice after CLP was associated with a greater number of peritoneal DCs and granulocytes than in WT controls. Adoptive transfer of TLR9−/− DCs was sufficient to protect WT mice from CLP and increased the influx of peritoneal granulocytes (95). Further studies indicate that host mitochondria-derived DNA may be responsible for TLR9 activation and contributes to sepsis-induced acute kidney injury (128).
Targeting TLRs in Sepsis: A Double-Edged Sword
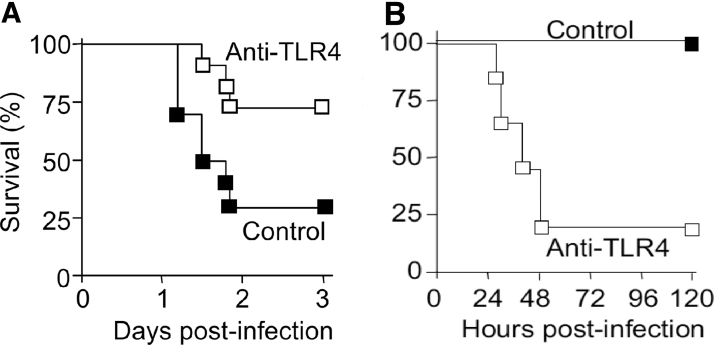
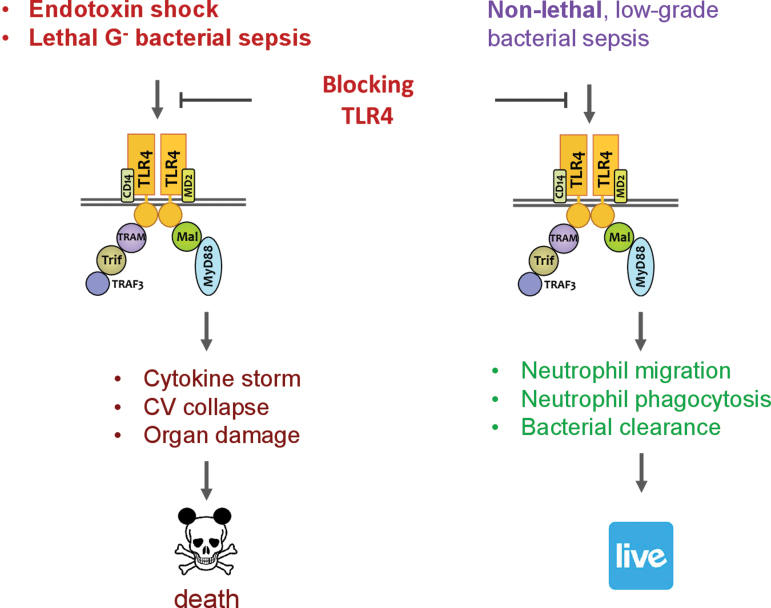
It has long been recognized that systemic inflammatory response is a major contributor to sepsis pathogenesis leading to cardiovascular collapse, multiorgan injury, and mortality. Such systemic response is highly complex and caused by both infectious and noninfectious mediators. Manipulating this process as a therapeutic strategy, while seeming attractive and logical, has proven to be a double-edge sword. One such example is targeting TLR4. Blocking or genetically deleting TLR4 effectively protects animals from endotoxin-induced circulatory shock, cardiac depression, and high mortality (27, 31, 97, 98). Moreover, in a mouse model of lethal gram-negative bacterial sepsis with more than 80% mortality, TLR4 deletion or antibody blocking proves to be beneficial—damping systemic cytokine storm (TNFα and IL-6) and markedly improving the survival of septic animals (Fig. 5) (105). However, as an essential part of innate immunity, TLR4 also plays a critical role in host defense against bacterial invasion. In nonlethal gram-negative bacterial infection or mild-form of polymicrobial sepsis, TLR4 deletion or antibody blocking proves to be deleterious and leads to increased blood bacterial loading, worse cardiac dysfunction, and higher mortality (Fig. 5) (105, 140). Thus, it is evident that sepsis severity is an important factor in determining the outcome of TLR4-targeted sepsis intervention (Fig. 6).
FIG. 5.
Effect of anti-TLR4 antibodies on the survival of mice with lethal or nonlethal gram-negative bacterial sepsis. (A) Anti-TLR4 antibodies decrease the mortality of lethal gram-negative infection. BALB/c mice were injected i.p. with anti-TLR4 or control antibodies (200 mg/kg) given before an i.p. injection of 2 × 105 cfu inoculum of Escherichia coli O18. (B) Anti-TLR4 antibodies increase the mortality of nonsevere gram-negative infections. Survival of C57BL/6 mice (n = 7 or 8) injected intranasally with 5.6 × 102 cfu of K. pneumoniae and i.p. with 40 mg/kg of anti-TLR4 or control antibodies at 24 h postinfection. p < 0.002. [Roger et al. (105), modified and used with permission].
FIG. 6.
Double-edged sword of targeting TLR4 for sepsis intervention. In lethal endotoxin shock or gram-negative bacterial sepsis, TLR4 plays a deleterious role in mediating cytokine storm, CV collapse, organ damage, and death. In contrast, in nonlethal or low-grade bacterial sepsis, TLR4 plays a beneficial defense role in neutrophil migration, neutrophil phagocytosis, and bacterial clearance. However, targeting TLR4 by genetic deletion or pharmacological inhibition would thus generate opposite outcomes. CV, cardiovascular. Color images are available online.
Targeting TLR4: The ACCESS Trials
There have been more than 100 phase 2 and phase 3 sepsis clinical trials (77). The strategies have been to manipulate the systemic inflammatory response by targeting the PAMP or endogenous inflammatory mediators such as TNFα, IL-1, eicosanoids, or platelet-activating factor, or by suppressing immune response or coagulating cascade. One of the most recent clinical trials for sepsis is eritoran. Eritoran is a synthetic lipodisaccharide, with a structure similar to LPS, that binds to MD2-TLR4 and competitively blocks LPS to TLR4 (89).
In a phase 1 trial (75), healthy volunteers were given eritoran before LPS. LPS, at a small dose of 3 ng/kg, induces very robust production of C-reactive protein, TNFα, and IL-6. Similar to animal studies, TLR4 blocking by eritoran in these healthy humans completely eliminated all clinical signs and cytokine production induced by LPS. In a subsequent phase 2 trial (122), a prospective, randomized, double-blinded, placebo-controlled multicenter study, two doses of eritoran were tested: 45 mg versus 105 mg over the course of 6 days, q12 hours, and given within 12 h of sepsis diagnosis.
The study patients had predicted risk of mortality between 20% and 80%. Among a total of 300 septic patients—100 in placebo and 200 in eritoran groups, 28-day all-cause mortality in the placebo group was 33.3%, low-dose group 32%, and high-dose group 26.6%, a substantial (20%) but not statistically significant reduction. Poststratification analysis of APACHE II score on 28-day all-cause mortality revealed that patients with less severe sepsis (score 21) probably did worse with high-dose eritoran compared with placebo. In contrast, patients with high APACHE score 4—most severe sepsis—might have been benefited from eritoran, a finding very similar to what had been seen in animal studies noted above.
It was concluded that the trend toward a lower mortality rate in patients with severe sepsis and high predicted risk of mortality should be further investigated in the phase 3 trial (ACCESS trial, NCT00334828) that involved 1961 septic patients from 197 intensive care units (ICUs) worldwide (86). Like phase 2, it was a randomized, double-blinded, placebo-controlled multicenter study. Based on the phase 2 data, the study only tests high dose at 105 mg total and given every 12 h. The study patients were highly heterogenous in terms of (i) type of infection: G−, G+, mixed, fungi, and other unknown, and (ii) infection sites: mostly in the lungs, abdomen, and genitourinary track. At the end of the 5-year trial, there was no difference in the 28-day all-cause mortality, which was about 27%–28%. The 12-month mortality was also identical between the eritoran and control groups at 40%.
Sepsis Clinical Trials: Lessons Learned
The cause of the failed ACCESS trial might be multifactorial (86): (i) patient heterogeneity: the septic patients enrolled had various severity scores and comorbidities, which could have impacted how patients responded to the treatment; (ii) only a fraction (40.7%) of the septic patients had elevated plasma endotoxin levels. Considering TLR4 as the target, this might explain why some patients failed to respond to eritoran, although a post hoc analysis did not find survival benefit in endotoxin-positive patients either; (iii) lower than anticipated mortality rate in the placebo group. In the course of the trials, the sepsis mortality rate had been gradually decreased from 40% to ∼27%, probably due to the Surviving Sepsis Campaign, which, along with other interventions, might alter the responsiveness to eritoran; (iv) too late for intervention. The time for intervention was within 12 h after initial sepsis diagnosis, which might be too late for intervention as “genie was out of bottle”; and (v) different pathogens. The study patients were infected with gram-positive, gram-negative, fungi, and mixed ones. Each of these pathogens is sensed via different TLRs and thus understandably, blocking TLR4 alone might be less efficacious.
Moving forward, the main challenges facing sepsis research are multiple. The rodent models commonly used in sepsis research are quite different from the septic patients we see in hospital. Unlike young and healthy rodents used in most laboratory research, septic patients are often old with multiple comorbidities, such as diabetes, hypercholesterolemia, hypertension, or other systemic and metabolic diseases, and are often aggressively treated with multiple medications in ICU. These underlying conditions could profoundly impact how the body responds to infection as well as to treatment. Therefore, establishing animal models that closely simulate septic patients is of paramount importance. Septic humans are highly heterogeneous in their clinical presentations of infection (sites, pathogens, severity) and responses to treatments, the underlying comorbidities, the demographics, their genetic makeup and risk factor, and their immune response to pathogen infection. Delineating these complex biological, genetic, immunological, and clinical factors in human sepsis is essential for future sepsis intervention and trial design.
Summary
Sepsis is a deadly clinical syndrome induced by a host's dysregulated immune responses to infection. Acting via pattern-recognition and converging signaling pathways with a set of adaptor molecules, kinases, and transcriptional factors, TLRs play a pivotal role in host defense against microbe pathogens by launching a proinflammatory immune response. Preclinical rodent studies have established the mechanistic role of TLR signaling in sepsis pathogenesis. Targeting these innate immune receptors and manipulating host inflammatory responses in sepsis, while attractive and logical, may yield opposite results depending on the severity of sepsis at the time of intervention. Numerous clinical trials targeting innate immunity, inflammation, and coagulation, including the eritoran ACCESS trial, have failed to demonstrate therapeutic efficacy.
Future work will be needed to better understand the complex biological mechanisms of sepsis pathogenesis, establish animal sepsis models more closely related to human conditions, identify molecular basis—biochemical and immunological risk factors and biomarkers—for clinical heterogeneity of septic patients, and carefully design human trials with clear clinical and immune stratifications and various levels of clinical outcomes.
Acknowledgments
We thank all the current and former trainees in our laboratories at the Massachusetts General Hospital/Harvard Medical School and the University of Maryland School of Medicine for their dedication and contributions.
Abbreviations Used
- APACHE II
Acute Physiology And Chronic Health Evaluation II
- CLP
cecum ligation and puncture
- COVID-19
coronavirus disease 2019
- CpG
cytidine-phosphate-guanosine
- CV
cardiovascular
- DAMPs
damage-associated molecular patterns
- DCs
dendritic cells
- ds
double-stranded
- EXTEM
extrinsic thromboelastometry
- FIBTEM
fibrinogen thromboelastometry
- Gp96
glycoprotein 96
- HMGB1
high-mobility group box 1
- HSP
heat-shock protein
- ICU
intensive care unit
- IFN
interferon
- IKK
I-κB kinase
- IL
interleukin
- IRAK
IL-1 receptor-associated kinase
- IRF
interferon regulatory factor
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- MCF
maximum clot firmness
- MD2
myeloid differentiation factor 2
- MDA5
melanoma-differentiation-associated gene 5
- miRNA
microRNA
- mtDNA
mitochondrial DNA
- MyD88
myeloid differentiation factor 88
- NF-κB
nuclear factor kappa B
- ODN
oligodeoxynucleotides
- PAMPs
pathogen-associated molecular patterns
- poly(I:C)
polyinosinic-polycytidylic acid
- qRT-PCR
quantitative reverse-transcriptase–polymerase chain reaction
- RIG-I
retinoic-acid-inducible gene 1
- SARM
sterile α- and heat-armadillo-motif-containing protein
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- ss
single-stranded
- TAK1
transforming growth factor-α-activated kinase 1
- TBK1
TRAF family member-associated NF-κB activator (TANK) binding kinase-1
- TF
tissue factor
- TIR
toll/interleukin-1 receptor
- TIRAP
TIR domain-containing adaptor protein
- TLRs
toll-like receptors
- TNFα
tumor necrosis factor α
- TRAF6
TNF receptor-associated factor 6
- TRAM
Trif-related adaptor molecule
- Trif
TIR domain-containing adaptor inducing IFN-β–mediated transcription factor
- WT
wild type
Authors' Contributions
F.C. drafted the article, L.Z. and B.W. critically edited the article, and W.C. instructed the review contents and finalized the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported, in part, by the NIH grants—R01NS110567, R01GM117233, R01GM122908, R35GM124775, and K08HL153784, by the Frontiers in Anesthesia Research Award from the International Anesthesia Research Society, and by the Faculty Research Award from the Shock Society.
References
- 1. Akira S and Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 85: 85–95, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Akira S, Uematsu S, and Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Al-Ofi E, Coffelt SB, and Anumba DO. Fibrinogen, an endogenous ligand of Toll-like receptor 4, activates monocytes in pre-eclamptic patients. J Reprod Immunol 103: 23–28, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Alexopoulou L, Holt AC, Medzhitov R, and Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Alonso H, Parra J, Malaga W, Payros D, Liu C-F, Berrone C, Robert C, Meunier E, Burlet-Schiltz O, and Rivière M. Protein O-mannosylation deficiency increases LprG-associated lipoarabinomannan release by Mycobacterium tuberculosis and enhances the TLR2-associated inflammatory response. Sci Rep 7: 1–14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alves-Filho JC, de Freitas A, Russo M, and Cunha FQ. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit Care Med 34: 461–470, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Anderson KV, Bokla L, and Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42: 791–798, 1985. [DOI] [PubMed] [Google Scholar]
- 8. Anderson KV, Jurgens G, and Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42: 779–789, 1985. [DOI] [PubMed] [Google Scholar]
- 9. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, and Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, and Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70 role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, and Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 98: 9237–9242, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baumgarten G, Knuefermann P, Nozaki N, Sivasubramanian N, Mann DL, and Vallejo JG. In vivo expression of proinflammatory mediators in the adult heart after endotoxin administration: the role of toll-like receptor-4. J Infect Dis 183: 1617–1624, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Binck BW, Tsen MF, Islas M, White DJ, Schultz RA, Willis MS, Garcia JV, Horton JW, and Thomas JA. Bone marrow-derived cells contribute to contractile dysfunction in endotoxic shock. Am J Physiol Heart Circ Physiol 288: H577–H583, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, Harken AH, and Repine JE. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci U S A 86: 2516–2520, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Byrd-Leifer CA, Block EF, Takeda K, Akira S, and Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol 31: 2448–2457, 2001. [DOI] [PubMed] [Google Scholar]
- 16. Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, and Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med 205: 2609–2621, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol 296: H1–H12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen C, Feng Y, Zou L, Wang L, Chen HH, Cai JY, Xu JM, Sosnovik DE, and Chao W. Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J Am Heart Assoc 3: e000683, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiu Y-C, Lin C-Y, Chen C-P, Huang K-C, Tong K-M, Tzeng C-Y, Lee T-S, Hsu H-C, and Tang C-H. Peptidoglycan enhances IL-6 production in human synovial fibroblasts via TLR2 receptor, focal adhesion kinase, Akt, and AP-1-dependent pathway. J Immunol 183: 2785–2792, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Coban C, Igari Y, Yagi M, Reimer T, Koyama S, Aoshi T, Ohata K, Tsukui T, Takeshita F, and Sakurai K. Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe 7: 50–61, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. The immunopathogenesis of sepsis. Nature 420: 885–891, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Copeland S, Warren HS, Lowry SF, Calvano SE, and Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 12: 60–67, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cot M, Ray A, Gilleron M, Vercellone A, Larrouy-Maumus G, Armau E, Gauthier S, Tiraby G, Puzo G, and Nigou J. Lipoteichoic acid in Streptomyces hygroscopicus: structural model and immunomodulatory activities. PLoS One 6: e26316, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis CG, Chang K, Osborne D, Walton AH, Ghosh S, Dunne WM, Hotchkiss RS, and Muenzer JT. TLR3 agonist improves survival to secondary pneumonia in a double injury model. J Surg Res 182: 270–276, 2013. [DOI] [PubMed] [Google Scholar]
- 25. Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, and Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation 105: 685–690, 2002. [DOI] [PubMed] [Google Scholar]
- 26. Echtenacher B, Freudenberg MA, Jack RS, and Mannel DN. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect Immun 69: 7271–7276, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehrentraut S, Lohner R, Schwederski M, Ehrentraut H, Boehm O, Noga S, Langhoff P, Baumgarten G, Meyer R, and Knuefermann P. In vivo Toll-like receptor 4 antagonism restores cardiac function during endotoxemia. Shock 36: 613–620, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Fattahi F, Russell MW, Malan EA, Parlett M, Abe E, Zetoune FS, and Ward PA. Harmful roles of TLR3 and TLR9 in cardiac dysfunction developing during polymicrobial sepsis. Biomed Res Int 2018: 4302726, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng Y, Chen H, Cai J, Zou L, Yan D, Xu G, Li D, and Chao W. Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J Biol Chem 290: 26688–26698, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng Y, Zou L, Yan D, Chen H, Xu G, Jian W, Cui P, and Chao W. Extracellular microRNAs induce potent innate immune responses via TLR7/MyD88-dependent mechanisms. J Immunol 199: 2106–2117, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feng Y, Zou L, Zhang M, Li Y, Chen C, and Chao W. MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology 115: 555–567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, and Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 193: 259–272, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Frasnelli ME, Tarussio D, Chobaz-Péclat V, Busso N, and So A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res Ther 7: 1–10, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao JW, Zhang AQ, Wang X, Li ZY, Yang JH, Zeng L, Gu W, and Jiang JX. Association between the TLR2 Arg753Gln polymorphism and the risk of sepsis: a meta-analysis. Crit Care 19: 416, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao M, Ha T, Zhang X, Liu L, Wang X, Kelley J, Singh K, Kao R, Gao X, Williams D, and Li C. Toll-like receptor 3 plays a central role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med 40: 2390–2399, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, and Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest 126: 859–864, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gingo MR and Morris A. HIV infection and severe sepsis: a bitter pill to swallow. Crit Care Med 43: 1779–1781, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Godefroy E, Gallois A, Idoyaga J, Merad M, Tung N, Monu N, Saenger Y, Fu Y, Ravindran R, and Pulendran B. Activation of toll-like receptor-2 by endogenous matrix metalloproteinase-2 modulates dendritic-cell-mediated inflammatory responses. Cell Rep 9: 1856–1870, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong T, Liu L, Jiang W, and Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 20: 95–112, 2020. [DOI] [PubMed] [Google Scholar]
- 40. Gong Y, Zou L, Feng Y, Li D, Cai J, Chen D, and Chao W. Importance of Toll-like receptor 2 in mitochondrial dysfunction during polymicrobial sepsis. Anesthesiology 121: 1236–1247, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goodridge HS, Wolf AJ, and Underhill DM. β-Glucan recognition by the innate immune system. Immunol Rev 230: 38–50, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guan Y, Ranoa DRE, Jiang S, Mutha SK, Li X, Baudry J, and Tapping RI. Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J Immunol 184: 5094, 2010. [DOI] [PubMed] [Google Scholar]
- 43. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, and Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103, 2001. [DOI] [PubMed] [Google Scholar]
- 44. Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, and Akira S. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol 33: 2987–2997, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, and Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303: 1526–1529, 2004. [DOI] [PubMed] [Google Scholar]
- 46. This reference has been deleted.
- 47. Heipertz EL, Harper J, Lopez CA, Fikrig E, Hughes ME, and Walker WE. Circadian rhythms influence the severity of sepsis in mice via a TLR2-dependent, leukocyte-intrinsic mechanism. J Immunol 201: 193–201, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, and Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88–dependent signaling pathway. Nat Immunol 3: 196–200, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, and Akira S. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745, 2000. [DOI] [PubMed] [Google Scholar]
- 50. Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, and Akira S. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162: 3749–3752, 1999. [PubMed] [Google Scholar]
- 51. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, and Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers 2: 16045, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hu B, Guo H, Zhou P, and Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19: 141–154, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iwasaki A and Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 5: 987–995, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Jian W, Gu L, Williams B, Feng Y, Chao W, and Zou L. Toll-like receptor 7 contributes to inflammation, organ injury, and mortality in murine sepsis. Anesthesiology 131: 105–118, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson GB, Brunn GJ, Kodaira Y, and Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168: 5233–5239, 2002. [DOI] [PubMed] [Google Scholar]
- 56. Jung ID, Jeong SK, Lee C-M, Noh KT, Heo DR, Shin YK, Yun C-H, Koh W-J, Akira S, and Whang J. Enhanced efficacy of therapeutic cancer vaccines produced by co-treatment with Mycobacterium tuberculosis heparin-binding hemagglutinin, a novel TLR4 agonist. Cancer Res 71: 2858–2870, 2011. [DOI] [PubMed] [Google Scholar]
- 57. Karikó K, Ni H, Capodici J, Lamphier M, and Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279: 12542–12550, 2004. [DOI] [PubMed] [Google Scholar]
- 58. Kawai T and Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384, 2010. [DOI] [PubMed] [Google Scholar]
- 59. Kim S, Takahashi H, Lin W-W, Descargues P, Grivennikov S, Kim Y, Luo J-L, and Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457: 102–106, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kindler E, Thiel V, and Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv Virus Res 96: 219–243, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Knuefermann P, Nemoto S, Baumgarten G, Misra A, Sivasubramanian N, Carabello BA, and Vallejo JG. Cardiac inflammation and innate immunity in septic shock: is there a role for toll-like receptors? Chest 121: 1329–1336, 2002. [DOI] [PubMed] [Google Scholar]
- 62. Knuefermann P, Schwederski M, Velten M, Krings P, Ehrentraut H, Rudiger M, Boehm O, Fink K, Dreiner U, Grohe C, Hoeft A, Baumgarten G, Koch A, Zacharowski K, and Meyer R. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: role of toll-like receptor 9. Cardiovasc Res 78: 26–35, 2008. [DOI] [PubMed] [Google Scholar]
- 63. Kruithof EKO, Satta N, Liu JW, Dunoyer-Geindre S, and Fish RJ. Gene conversion limits divergence of mammalian TLR1 and TLR6. BMC Evol Biol 7: 148, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Land W, Agostinis P, Gasser S, Garg A, and Linkermann A. Transplantation and damage-associated molecular patterns (DAMPs). Am J Transplant 16: 3338–3361, 2016. [DOI] [PubMed] [Google Scholar]
- 65. Land WG, Agostinis P, Gasser S, Garg A, and Linkermann A. DAMP—induced allograft and tumor rejection: the circle is closing. Am J Transplant 16: 3322–3337, 2016. [DOI] [PubMed] [Google Scholar]
- 66. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, and Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5: 190–198, 2004. [DOI] [PubMed] [Google Scholar]
- 67. Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-and Rothstein A. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607, 2002. [DOI] [PubMed] [Google Scholar]
- 68. Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, Wang C, Wang Y, Li L, Ren L, Guo F, Zhao Z, Zhou Z, Xiang Z, and Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun 11: 3810, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, and Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86: 973–983, 1996. [DOI] [PubMed] [Google Scholar]
- 70. Li J, Liu Y, and Zhang X. Murine coronavirus induces type I interferon in oligodendrocytes through recognition by RIG-I and MDA5. J Virol 84: 6472–6482, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, and Chao W. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem 286: 31308–31319, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang MD, Bagchi A, Warren HS, Tehan MM, Trigilio JA, Beasley-Topliffe LK, Tesini BL, Lazzaroni JC, Fenton MJ, and Hellman J. Bacterial peptidoglycan-associated lipoprotein: a naturally occurring toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J Infect Dis 191: 939–948, 2005. [DOI] [PubMed] [Google Scholar]
- 73. Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, and Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A 101: 5598–5603, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. This reference has been deleted.
- 75. Lynn M, Rossignol DP, Wheeler JL, Kao RJ, Perdomo CA, Noveck R, Vargas R, D'Angelo T, Gotzkowsky S, and McMahon FG. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis 187: 631–639, 2003. [DOI] [PubMed] [Google Scholar]
- 76. Mallavia B, Liu F, Lefrancais E, Cleary SJ, Kwaan N, Tian JJ, Magnen M, Sayah DM, Soong A, Chen J, Saggar R, Shino MY, Ross DJ, Derhovanessian A, Lynch JP, 3rd, Ardehali A, Weigt SS, Belperio JA, Hays SR, Golden JA, Leard LE, Shah RJ, Kleinhenz ME, Venado A, Kukreja J, Singer JP, and Looney MR. Mitochondrial DNA stimulates TLR9-dependent neutrophil extracellular trap formation in primary graft dysfunction. Am J Respir Cell Mol Biol 62: 364–372, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med 20: 195–203, 2014. [DOI] [PubMed] [Google Scholar]
- 78. Mazaleuskaya L, Veltrop R, Ikpeze N, Martin-Garcia J, and Navas-Martin S. Protective role of Toll-like receptor 3-induced type I interferon in murine coronavirus infection of macrophages. Viruses 4: 901–923, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Medzhitov R, Preston-Hurlburt P, and Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997. [DOI] [PubMed] [Google Scholar]
- 80. Minino AM, Heron MP, Murphy SL, and Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep 55: 1–119, 2007. [PubMed] [Google Scholar]
- 81. Nasu K and Narahara H. Pattern recognition via the Toll-like receptor system in the human female genital tract. Mediators Inflamm 2010: 976024, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Navarini AA, Lang KS, Verschoor A, Recher M, Zinkernagel AS, Nizet V, Odermatt B, Hengartner H, and Zinkernagel RM. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc Natl Acad Sci U S A 106: 7107–7112, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nuhn L, Van Hoecke L, Deswarte K, Schepens B, Li Y, Lambrecht BN, De Koker S, David SA, Saelens X, and De Geest BG. Potent anti-viral vaccine adjuvant based on pH-degradable nanogels with covalently linked small molecule imidazoquinoline TLR7/8 agonist. Biomaterials 178: 643–651, 2018. [DOI] [PubMed] [Google Scholar]
- 84. O'Neill LA and Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364, 2007. [DOI] [PubMed] [Google Scholar]
- 85. Oldenburg M, Krüger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, Bathke B, Lauterbach H, Suter M, Dreher S, Koedel U, Akira S, Kawai T, Buer J, Wagner H, Bauer S, Hochrein H, and Kirschning CJ. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance–forming modification. Science 337: 1111, 2012. [DOI] [PubMed] [Google Scholar]
- 86. Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, Jauregui L, Krell K, Pachl J, Takahashi T, Peckelsen C, Cordasco E, Chang CS, Oeyen S, Aikawa N, Maruyama T, Schein R, Kalil AC, Van Nuffelen M, Lynn M, Rossignol DP, Gogate J, Roberts MB, Wheeler JL, and Vincent JL. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309: 1154–1162, 2013. [DOI] [PubMed] [Google Scholar]
- 87. Paladugu B, Kumar A, Parrillo JE, Der S, Osman J, Mensing J, Falvo L, Xu X, and Kumar A. Bacterial DNA and RNA induce rat cardiac myocyte contraction depression in vitro. Shock 21: 364–369, 2004. [DOI] [PubMed] [Google Scholar]
- 88. Park BS and Lee J-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 45: e66-e66, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Park BS, Song DH, Kim HM, Choi B-S, Lee H, and Lee J-O. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature 458: 1191–1195, 2009. [DOI] [PubMed] [Google Scholar]
- 90. This reference has been deleted.
- 91. Park H, Lee Y, Oh Y, Jung J, Park YW, Myung K, Kim K, Koh SS, and Lim D-S. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene 30: 201–211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Park JS, Svetkauskaite D, He Q, Kim J-Y, Strassheim D, Ishizaka A, and Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377, 2004. [DOI] [PubMed] [Google Scholar]
- 93. Pene F, Courtine E, Ouaaz F, Zuber B, Sauneuf B, Sirgo G, Rousseau C, Toubiana J, Balloy V, Chignard M, Mira JP, and Chiche JD. Toll-like receptors 2 and 4 contribute to sepsis-induced depletion of spleen dendritic cells. Infect Immun 77: 5651–5658, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Piccinini AM and Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010: 672395, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Plitas G, Burt BM, Nguyen HM, Bamboat ZM, and DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med 205: 1277–1283, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pohar J, Yamamoto C, Fukui R, Cajnko M-M, Miyake K, Jerala R, and Benčina M. Selectivity of human TLR9 for double CpG motifs and implications for the recognition of genomic DNA. J Immunol 198: 2093–2104, 2017. [DOI] [PubMed] [Google Scholar]
- 97. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, and Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998. [DOI] [PubMed] [Google Scholar]
- 98. Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, and Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189: 615–625, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Raetz M, Kibardin A, Sturge CR, Pifer R, Li H, Burstein E, Ozato K, Larin S, and Yarovinsky F. Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin. J Immunol 191: 4818, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rallabhandi P, Phillips RL, Boukhvalova MS, Pletneva LM, Shirey KA, Gioannini TL, Weiss JP, Chow JC, Hawkins LD, and Vogel SN. Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. mBio 3: e00218–e00212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ramirez SR, Singh-Jasuja H, Warger T, Braedel-Ruoff S, Hilf N, Wiemann K, Rammensee H-G, and Schild H. Glycoprotein 96–activated dendritic cells induce a CD8-biased T cell response. Cell Stress Chaperones 10: 221, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, and Fiore AE. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 318: 1241–1249, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Roers A, Hiller B, and Hornung V. Recognition of endogenous nucleic acids by the innate immune system. Immunity 44: 739–754, 2016. [DOI] [PubMed] [Google Scholar]
- 104. Roger T and Calandra T. TLR2-mediated neutrophil depletion exacerbates bacterial sepsis. Proc Natl Acad Sci U S A 106: 6889–6890, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, and Calandra T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A 106: 2348–2352, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, and Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol 176: 7636–7644, 2006. [DOI] [PubMed] [Google Scholar]
- 107. Rolli J, Loukili N, Levrand S, Rosenblatt-Velin N, Rignault-Clerc S, Waeber B, Feihl F, Pacher P, and Liaudet L. Bacterial flagellin elicits widespread innate immune defense mechanisms, apoptotic signaling, and a sepsis-like systemic inflammatory response in mice. Crit Care 14: R160, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rolli J, Rosenblatt-Velin N, Li J, Loukili N, Levrand S, Pacher P, Waeber B, Feihl F, Ruchat P, and Liaudet L. Bacterial flagellin triggers cardiac innate immune responses and acute contractile dysfunction. PLoS One 5: e12687, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Roth-Cross JK, Bender SJ, and Weiss SR. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J Virol 82: 9829–9838, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, and Finfer S. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395: 200–211, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schaefer L, Babelova A, Kiss E, Hausser H-J, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, and Götte M. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115: 2223–2233, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, and Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281, 2006. [DOI] [PubMed] [Google Scholar]
- 113. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, and Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110: 3507–3512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shin HS, Xu F, Bagchi A, Herrup E, Prakash A, Valentine C, Kulkarni H, Wilhelmsen K, Warren S, and Hellman J. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J Immunol 186: 1119–1130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, and Coopersmith CM. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Takao K and Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 112: 1167–1172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, and Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169: 10–14, 2002. [DOI] [PubMed] [Google Scholar]
- 118. Tang D, Kang R, Coyne CB, Zeh HJ, and Lotze MT. PAMP s and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 249: 158–175, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tavener SA and Kubes P. Cellular and molecular mechanisms underlying LPS-associated myocyte impairment. Am J Physiol Heart Circ Physiol 290: H800–H806, 2006. [DOI] [PubMed] [Google Scholar]
- 120. Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, and Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res 95: 700–707, 2004. [DOI] [PubMed] [Google Scholar]
- 121. Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, and Simon JC. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195: 99–111, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, and Opal SM. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med 38: 72–83, 2010. [DOI] [PubMed] [Google Scholar]
- 123. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, and Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76: 16–32, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Totura AL, Whitmore A, Agnihothram S, Schafer A, Katze MG, Heise MT, and Baric RS. Toll-Like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio 6: e00638-15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, and Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem 281: 31002–31011, 2006. [DOI] [PubMed] [Google Scholar]
- 126. Tsan M-F. Toll-like receptors, inflammation and cancer. Semin Cancer Biol 16: 32–37, 2006. [DOI] [PubMed] [Google Scholar]
- 127. Tsan MF and Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol 76: 514–519, 2004. [DOI] [PubMed] [Google Scholar]
- 128. Tsuji N, Tsuji T, Ohashi N, Kato A, Fujigaki Y, and Yasuda H. Role of mitochondrial DNA in septic AKI via Toll-like receptor 9. J Am Soc Nephrol 27: 2009–2020, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Häcker H, and Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 276: 31332–31339, 2001. [DOI] [PubMed] [Google Scholar]
- 130. Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee H-G, and Wagner H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem 277: 20847–20853, 2002. [DOI] [PubMed] [Google Scholar]
- 131. Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, and Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med 202: 1575–1585, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. This reference has been deleted.
- 133. Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, and Cavaillon JM. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis 201: 223–232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, and Zingarelli B. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock 30: 267–273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Williams B, Neder J, Cui P, Suen A, Tanaka K, Zou L, and Chao W. Toll-like receptors 2 and 7 mediate coagulation activation and coagulopathy in murine sepsis. J Thromb Haemost 17: 1683–1693, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Williams DL, Ha T, Li C, Kalbfleisch JH, Schweitzer J, Vogt W, and Browder IW. Modulation of tissue Toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med 31: 1808–1818, 2003. [DOI] [PubMed] [Google Scholar]
- 137. Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, and Sher A. TLR11 Activation of dendritic cells by a protozoan profilin-like protein. Science 308: 1626, 2005. [DOI] [PubMed] [Google Scholar]
- 138. Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, and Ghosh S. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303: 1522, 2004. [DOI] [PubMed] [Google Scholar]
- 139. Zhang J, He Y, Song W, Lu Y, Li P, Zou L, and Zhong W. Lack of association between factor v leiden and sepsis: a meta-analysis. Clin Appl Thromb Hemost 21: 204–210, 2015. [DOI] [PubMed] [Google Scholar]
- 140. Zhang M, Zou L, Feng Y, Chen YJ, Zhou Q, Ichinose F, and Chao W. Toll-like receptor 4 is essential to preserving cardiac function and survival in low-grade polymicrobial sepsis. Anesthesiology 121: 1270–1280, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, and Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang Z, Ohto U, Shibata T, Krayukhina E, Taoka M, Yamauchi Y, Tanji H, Isobe T, Uchiyama S, Miyake K, and Shimizu T. Structural analysis reveals that Toll-like receptor 7 is a dual receptor for guanosine and single-stranded RNA. Immunity 45: 737–748, 2016. [DOI] [PubMed] [Google Scholar]
- 143. Zhang Z, Ohto U, Shibata T, Taoka M, Yamauchi Y, Sato R, Shukla NM, David SA, Isobe T, Miyake K, and Shimizu T. Structural analyses of Toll-like receptor 7 reveal detailed RNA sequence specificity and recognition mechanism of agonistic ligands. Cell Rep 25: 3371–3381.e5, 2018. [DOI] [PubMed] [Google Scholar]
- 144. Zhu J, Duan G, Lang L, Liu Y, Zhu J, Wang H, and Liu Y. The bacterial component flagellin induces anti-sepsis protection through TLR-5, IL-1RN and VCAN during polymicrobial sepsis in mice. Cell Physiol Biochem 36: 446–456, 2015. [DOI] [PubMed] [Google Scholar]
- 145. Zhu X, Bagchi A, Zhao H, Kirschning CJ, Hajjar RJ, Chao W, Hellman J, and Schmidt U. Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med 35: 886–892, 2007. [DOI] [PubMed] [Google Scholar]
- 146. Zielecki F, Weber M, Eickmann M, Spiegelberg L, Zaki AM, Matrosovich M, Becker S, and Weber F. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J Virol 87: 5300–5304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zou L, Feng Y, Chen YJ, Si R, Shen S, Zhou Q, Ichinose F, Scherrer-Crosbie M, and Chao W. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med 38: 1335–1342, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zou L, Feng Y, Li Y, Zhang M, Chen C, Cai J, Gong Y, Wang L, Thurman JM, Wu X, Atkinson JP, and Chao W. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J Immunol 191: 5625–5635, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zou L, Feng Y, Xu G, Jian W, and Chao W. Splenic RNA and MicroRNA mimics promote complement factor B production and alternative pathway activation via innate immune signaling. J Immunol 196: 2788–2798, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zou L, Feng Y, Zhang M, Li Y, and Chao W. Nonhematopoietic toll-like receptor 2 contributes to neutrophil and cardiac function impairment during polymicrobial sepsis. Shock 36: 370–380, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zou N, Ao L, Cleveland JC Jr., Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, and Meng X.. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol 294: H2805–H2813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]