Abstract
After decades overcoming difficult problems, antisense oligonucleotide (ASO), duplex RNA (siRNA), and messenger RNA (mRNA) nucleic acid therapeutic strategies are finally demonstrating clinical benefits. This success presents new challenges. What goals remain for basic research? Will there be an explosion of clinical applications that benefit many patients with different diseases, or will success be restricted to diseases that are ideal for the application of current technologies? The aim of this perspective is to describe a selection of the major goals for the next decade.
Keywords: antisense, siRNA, therapeutic
In 1978, Zamecnik published a report describing the use of a synthetic oligonucleotide to control gene expression [1]. These data were primitive by today's standards—at that time oligonucleotides needed to be manually prepared and simply synthesizing a 13 base oligomer was a major scientific triumph. Little was understood about how a large very un-drug like negatively charged molecule might cross the cell membrane and be active. Zamecnik's findings, however, hinted that it might be possible for synthetic nucleic acids to control the expression of any gene.
Although the potential for nucleic acid therapeutics was obvious, realizing that potential was not simple. The advent of phosphite triester synthesis methods [2,3] and efficient automated synthesis in the mid-1980s [4,5] were major steps forward, as was the founding of the field's first start-up companies in the late 1980s.
Although a foundation was being laid, progress toward therapeutics remained slow. An entire new field of science needed to be developed. How could oligonucleotides be made on a large scale? How could oligonucleotides be delivered into cells? How could chemical modifications be used to move from basic science to drug development? What constitutes a good disease target for drug development using an oligonucleotide? In which tissues would oligonucleotides be active? When RNA interference (RNAi) became an option, how would that technology fit in? When delivery of mRNA became a focus, where would it be applied in practice? Nucleic acids have also been plagued by other unpleasant surprises: off-target effects that produce confounding results, unexpected lack of translation from model species to man in terms of efficacy, metabolic stability, and safety, and sometimes unenthusiastic conditional approval. How would the long-term need for rigor be balanced against the short-term need to show results to investors?
In 1998, the first oligonucleotide drug Vitravene (fomivirsen), a 21 nucleotide 2′-deoxyphosphorothioate targeting cytomegalovirus (CMV) UL123 gene encoding the IE2 protein was approved for treatment of CMV retinitis by local intravitreal administration to the eye [6]. This was a scientific landmark but a commercial disappointment—marketing of the drug was stopped because of dramatic decrease in CMV cases due to the development of high-activity antiretroviral therapy.
In 2004, the aptamer Macugen (pegaptanib) was approved for the treatment of age-related macular degeneration and met with substantial initial success [7]. Although Macugen was rapidly superseded by competing angiogenesis inhibitors, it demonstrated that nucleic acids could have a powerful impact on disease. In 2013, the ASO Kynamro (mipomersen) was approved for hypercholesterolemia [8]. Similar to Macugen, Kynamro also lost in a competition with other therapies. Nevertheless, it was a milestone because it showed that systemically administered nucleic acids could engage with their intended target genes and control the expression of a gene involved in disease.
In 2016, the foundation of nucleic acid therapeutics shifted forever with the overwhelmingly positive clinical data and subsequent approval of Spinraza (nusinersen) [9]. Spinraza demonstrated that a nucleic acid could be administered in the central nervous system (CNS), enter the relevant cells, and control gene expression with acceptable safety. The effects on patients were profound, with children who had been facing a fatal childhood disease experiencing improvements in motor function and survival.
The approval of Spinraza was followed by the approval of several RNase H based ASOs, four splice-switching ASOs for treatment of DMD, small interfering RNAs (siRNAs) using two complementary delivery mechanisms [10–15], and then by the tremendous impact of mRNA vaccines [16,17]. The recent success of mRNA vaccines has validated the long-term investment in understanding the basic science of nucleic acids and the potential for substantial clinical impact.
In contrast to Zamecnik's experiments, which were done in cell culture, RNAi was first observed in petunia [18]. These observations were subsequently confirmed in worms by Fire and Mello [19], which led to their Nobel prize in 2006. The first mammalian RNAi cell culture experiments were done by Tuschl in 2001. Using chemically synthesized siRNAs that act through this natural RNAi pathway the first in vivo RNAi-mediated silencing in mouse liver and jejunum was demonstrated as the first in vivo proof in 2004. Just like the struggles involved in development of ASOs, after some glimpses of hope, clinical trials testing siRNAs targeting respiratory syncytial virus (RSV) genomic RNA and VEGF mRNAs were halted due to insufficient efficacy.
Finally, 20 years after the demonstration of RNAi in mammalian cells, a focused effort on liver delivery was successful, and four siRNA therapeutics have been approved in the past 36 months. To deliver therapeutic siRNAs into liver hepatocytes, a three-pronged approach was developed involving chemical modification of siRNAs, lipid nanoparticle (LNP) formulation of siRNAs for intravenous administration, and trivalent N-acetylgalactosamine (GalNAc) conjugation to siRNAs for subcutaneous administration.
The LNP strategy with a partially chemically modified siRNA resulted in the first FDA-approved RNAi therapeutic, patisiran (Onpattro®), approved in 2018. Patisiran is used to treat polyneuropathy in patients with hereditary ATTR amyloidosis.
The approval of patisiran paved the way for a whole new class of RNA-based medicines and validated LNP platform-based delivery of nucleic acids for human therapeutics including mRNA-based vaccines.
In 2019, the human therapeutic utility of the asialoglycoprotein receptor (ASGPR) and the triantennary GalNAc ligand pair for delivery of nucleic acids was fully realized. By combining the chemical modifications of oligonucleotides contributing to various flavors of nuclease resistance (the so-called Enhanced Stabilization Chemistry) along with the GalNAc ligand, human therapeutic applications of hepatocyte-targeting GalNAc-conjugated oligonucleotides was enabled for the first time. This delivery platform has revolutionized the RNA-based therapeutics field.
Three GalNAc-conjugated RNAi therapeutics have been approved so far: givosiran (Givlaari®, 2019) for treating acute hepatic porphyria, the only drug available to treat this disease, lumasiran (Oxlumo®, 2020) for the treatment of primary hyperoxaluria type 1 in both adult and pediatric populations, and Inclisiran (Lequio, 2020) for treatment of the prevalent disease hypercholesterolemia. Numerous other GalNAc-based RNAi therapeutics are advancing through human clinical trials. These agents have sustained metabolic stability, pharmacokinetics, and long duration of pharmacodynamic action after a single subcutaneous injection.
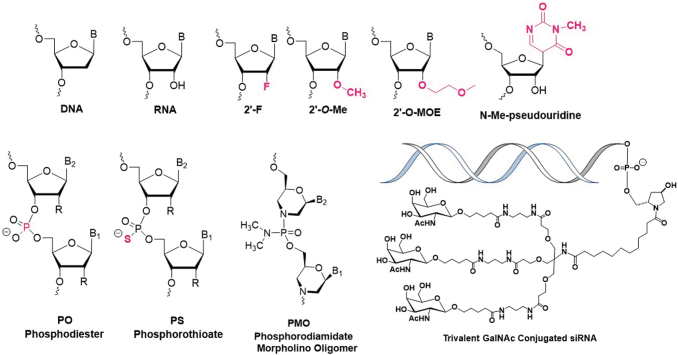
The success of ASO, siRNA, and mRNA therapeutics, powerful chemical strategies for modifying nucleic acids and improving their delivery (Fig. 1) suggest that the future has never been brighter for the development of therapeutic nucleic acids. In addition, efficient high-throughput nucleic acid sequencing and proteomic approaches for analyzing gene expression are allowing increasingly informative analyses to guide decision-making. Nevertheless, the biggest lesson from past experience is that success will never be easy, and it will always be necessary to meet scientific challenges with honest, rigorous, and innovative strategies. Here we outline potential landmarks to watch for and critical research challenges to overcome.
FIG. 1.
Chemical modifications involved in approved nucleic acids medicines.
Will other ligands replicate the success of GalNAc? What are the three biggest obstacles to successful oligonucleotide therapeutics? The cliché answer is delivery, delivery, and delivery. The single biggest discovery relevant to delivery in the past decade was the discovery that conjugation of GalNAc to duplex siRNA or an ASO could lead to large increases in potency [20,21]. GalNAc binds the high-capacity ASGPR on the surface of hepatocytes, facilitating efficient delivery to the liver hepatocytes.
Why has GalNAc remained the sole example for outstanding clinical success? The ASGPR is expressed at unusually high levels on the surface of hepatocytes. The expression of other receptors may have enough capacity to bind adequate numbers of ASOs or dsRNAs. The hepatocytes expressing ASGPR are in the liver, an organ already well suited to accumulate oligonucleotides such as phosphorothioate ASOs. The biodistribution of nucleic acids to other tissues, cell types and internalization may be too low for even a highly expressed receptor to have an effect. Moving forward, clever ligand design with appropriate pharmacokinetic properties and delivery strategies will likely need to be combined to achieve favorable outcomes that rival GalNAc conjugated oligonucleotides.
Will Lequio (Inclisiran) become the first widely used siRNA? Almost every nucleic acid drug has been developed to treat rare severe disease. Such diseases are often associated with inadequate existing therapies and high unmet need. Inclisiran is a GalNAc modified siRNA designed to reduce cholesterol levels by inhibiting the expression of PCSK9 [22]. It fits the general model for gene silencing drugs because there are patients with a genetic predisposition to high cholesterol who develop severe disease and have no other good treatment options.
Inclisiran, however, may offer more than a treatment for a rare disease. Although statins are usually associated with the treatment of high cholesterol in millions of patients, many patients are not adequately responsive and compliance with a daily regimen of oral self-administration can be problematic. Inclisiran produces a profound decrease in cholesterol levels, allowing some patients to reach goals that cannot be reached with statins. Although Inclisiran must be injected, a feature usually viewed as a negative, it has a long half-life and may need to be administered just two or three times a year. Infrequent injections may offer advantages for long-term compliance by patients and effective drug dosing. Clinical trials on thousands of patients have shown a reassuring safety profile [22].
One question is whether this new approach to maintaining healthy low levels of cholesterol levels will be acceptable to physicians and patients. Studies indicate that reduction of LDL-C to <50 mg/dL seems safe and provides greater cardiovascular benefits compared with higher levels [23]. In addition, although great strides have been made in the large-scale synthesis of oligonucleotides, it will be interesting to see whether quantities of siRNA drug sufficient for tens or hundreds of thousands of patients [24,25] can be synthesized at costs that are competitive with statin treatments and sustainable for health care systems. The potential massive impact on human health from a systemic siRNA designed to decrease cholesterol would have profound implications for all subsequent drug development efforts.
Will “N of 1” therapies demonstrate benefits to patients? If the potentially large patient population of Inclisiran is one end of the spectrum, the potential to develop drugs to treat individual patients falls at the other.
Nucleic acids are versatile and can modulate the expression of any gene. Nucleic acids are also similar to one another, making drug development more predictable. In combination, these two advantages make it possible to recognize a rare genetic disease, envision a potential nucleic acid drug, make the drug, and move through the regulatory process in months rather than years. These properties are ideal for the treatment of a severe disease that affect one or a few individuals, where time is working against patients and a drug must be developed quickly. This model for drug development was recently used to identify milasen [26]. This compound was used to treat one patient, and although it did not show definitive signs of efficacy it did demonstrate that the development of “N of 1” nucleic acid drugs is plausible.
It is now established that nucleic acids can move from identification of a genetic defect to administration to a patient within months rather than years. Physicians, patients, research scientists, and regulators have a model available for efficient cooperative development. Landmarks to watch for include an overview of how many rare genetic diseases are suitable for intervention using nucleic acids. Can drugs be developed fast enough to avoid irreversible damage? Can drugs that are developed so quickly routinely meet the high bar set for patient safety? Can the fast development of drugs be combined with the identification of compounds that are potent enough to be successful in vivo? Answering these questions over the next decade will be critical for determining the long-term value of “N of 1” therapy [27].
When will we have an adequate understanding of human miRNA mechanisms? MicroRNAs (miRNAs) are small endogenously expressed duplex RNAs that have the potential to control gene expression. Hundreds of miRNAs are expressed inside cells, and they have the potential to control almost any gene involved in disease. This potential has made them tempting targets for drug development because it is easy to envision a complementary oligonucleotide “antagomir or anti-miR” blocking the miRNA and reversing its activity.
Unfortunately, progress developing anti-miRs has been slow. Some common assumptions have proven to be unhelpful and experimental data have shown that predicting the function of miRNAs is not always straightforward [28]. Companies involved in this area have either failed, been repurposed, or maintain their efforts on a reduced scale.
We note that slow progress also characterized the development of ASOs and siRNAs. Lack of initial clinical success does not suggest that the concept is fatally flawed. However, it is likely that there is an inadequate understanding of how miRNAs function and that that lack of understanding has prevented the identification of the full range of potential disease targets. Advances in basic biology are necessary to more fully understand the subtleties of miRNA action in different cell types and disease states. The landmarks to watch for will be rigorous, well-controlled, and transparent studies that link miRNAs to disease targets and lay the foundation for well-reasoned development campaigns.
Can endosomal escape be harnessed to enhance drug potency? Anyone who has performed microscopy with a fluorophore-tagged ASO or duplex RNA is familiar with the punctate localization of tagged material to endosomes. The amount of compound released into the cytoplasm or nucleus is a small percentage (∼1%–2%) that remains compartmentalized and has not advanced since early day calculations [29,30].
Compartmentalization can be a good thing for drug development, acting as a slow-release mechanism that likely contributes to the long-lasting benefits of drugs such as Spinraza or Inclisiran. In contrast, compartmentalization leads to a requirement for higher doses to achieve any effect. A long-standing challenge in the field, therefore, is the development of chemical modifications or a dosing strategy with a nontoxic endosome releasing agent that will allow more ASO or siRNA to be immediately released into cell cytoplasm. Although such release might be unneeded or undesirable for chronic diseases, it may be necessary for acute diseases and achievement of higher short-term potencies remains a major unfulfilled need [31,32].
Can effective oral drug delivery be achieved? Systemic and local injections are appropriate for severe diseases where there is great unmet need. Similarly, as noted earlier for inclisiran, there may be cases where the long half-life of ASOs and siRNAs confers advantages with regard to patient compliance. Those points noted, safe and effective orally bioavailable drugs will always be a gold standard option for treating disease [33].
What will it take to bring about further success with RNA-based medicines in the CNS beyond Spinraza? Clearly the recent setbacks with ASOs in the CNS are notable and potency, efficacy, safety, and tolerance must be improved. Administration by intrathecal or intracerebroventricular routes are not patient-friendly, and blood–brain barrier penetration by systemic administration does not seem practical [34,35]. Uniform distribution in brain beginning in the spinal cord and spreading into cerebellum, cortex, and hippocampus needs to be achieved. This is expected to be much more difficult than delivery to cell types of liver. Finally, lack of suitable animal models and issues with translation between species is another challenge due to the enormous brain size variations between animals and humans.
The experience with Spinraza will also have more to teach us. Two other modalities have been approved for the treatment of spinal muscular atrophy [36]. One is Zolgensma, a gene therapy designed to replace the SMN2 gene. The other is Risdiplam, a small molecule with the remarkable ability to alter splicing and restore sufficient SMN2 expression. It will be important to evaluate how Spinraza competes with these other modalities or how they might be combined to improve patient outcomes.
When will RNA-based therapeutics impact cancer? This is another important area where oligonucleotide therapies offered early promise but clear-cut success has not been achieved yet [37,38]. During early days, for example, targeting the mRNAs encoding BCL-2, C-RAF, PKC-α, telomerase, VEGF, and KSP were attempted, but there is still no approved drug. Delivery to cancer tissues and cell types either by passive targeting due to enhanced permeability and retention (EPR effect) or by a ligand-conjugation targeted approach with oligonucleotides modified with improved chemistries should be revisited. Taking advantage of controlled addition of immune activation mechanisms by oligonucleotides is yet another possibility. The genetic heterogeneity within and between patients, gene mutations, and multidrug resistance also need to be addressed.
Can the success of systemic mRNA parallel the success of mRNA vaccines? The single greatest impact on human health from the field of oligonucleotide therapeutics has been the development of mRNA vaccines to treat COVID-19. It is easy to imagine that the landscape of vaccine development has been altered forever and that mRNA vaccines will become widespread.
Originally, however, much of the excitement of therapeutic mRNA was due to the potential to increase the production of a therapeutic protein as a treatment for a disease. In some circumstances, Spinraza being the highest impact example, ASOs and potentially dsRNAs can increase expression of a therapeutic protein. However, most ASOs and siRNAs act by decreasing gene expression. The potential of mRNA to lead to increased protein expression has the potential to lead to starting points for the treatments of a broader range of patients and diseases [38–40]. Landmark findings to watch for include the identification of diseases where replacement of mRNA can fill an unmet need, the development of delivery strategies for introducing mRNAs into tissues, and efficient strategies for synthesizing mRNA on the scales necessary for local or systemic administration.
Conclusion
Scientific progress seems inevitable; however, it requires a combination of inspired breakthroughs and inventive solutions to daunting practical challenges. That is the story of oligonucleotide therapeutics. There is no reason to think that the future of the field will be any different than the past. The challenges outlined in this perspective are only an incomplete list of the important problems that will be addressed in the next decade. Some challenges will see great progress, others less so. The only certainty is that success will continue to require a continued collaboration of outstanding chemistry, biology, and medicine.
Acknowledgments
M. Manoharan acknowledges the critical comments of Akshay Vaishnaw and review by Cindy Courtney. M.J. Damha acknowledges the support from the National Science and Engineering Council of Canada (Discovery Grant) and holds the Distinguished James McGill Professorship in Chemistry. M. Manoharan acknowledges the critical comments from Akshay Vaishnaw.
Author Disclosure Statement
M. Manoharan is employed by Alnylam Pharmaceuticals. M.J. Damha is a member of the Scientific Advisory Board of Aro Biotherapeutics, AUM LifeTech, and Deep Genomics.
Funding Information
D.R. Corey was supported by the National Institutes of Health (NIH; GM106151) and the Robert Welch Foundation (I-1244), and holds the Rusty Kelley Professorship in Medical Science.
References
- 1. Zamecnik PC and Stephenson ML. (1978). Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A 75:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Letsinger RL and Lunsford WB. (1976). Synthesis of thymidine oligonucleotides by phosphite triester intermediates. J Am Chem Soc 98:3655–3661. [DOI] [PubMed] [Google Scholar]
- 3. Beaucage SL and Caruthers MH. (1981). Deoxynucleoside phosphoramidites—A new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett 22:1859–1862. [Google Scholar]
- 4. Alvarado-Urbina G, Sathe GM, Liu WC, Gillen MF, Duck PD, Bender R and Ogilvie KK. (1981). Automated synthesis of gene fragments. Science (Washington, D. C., 1883-) 214:270–274. [DOI] [PubMed] [Google Scholar]
- 5. Caruthers MH. (1985). Gene synthesis machines: DNA chemistry and its uses. Science 230:281–285. [DOI] [PubMed] [Google Scholar]
- 6. Geary RS, Henry SP and Grillone LR. (2002). Fomivirsen. Clin Pharmacokinet 41:255–260. [DOI] [PubMed] [Google Scholar]
- 7. Zhou J and Rossi J. (2017). Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov 16:181–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S and Kastelein JJP. (2016). Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol 10:1011–1021. [DOI] [PubMed] [Google Scholar]
- 9. Aartsma-Rus A. (2017). FDA approval of nusinersen for spinal muscular atrophy makes 2016 the year of splice modulating oligonucleotides. Nucleic Acid Ther 27:67–69. [DOI] [PubMed] [Google Scholar]
- 10. Shen X and Corey DR. (2018). Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res 46:1584–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stein CA and Castanotto D. (2017). FDA-approved oligonucleotide therapies in 2017. Mol Ther 25:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crooke ST, Witztum JL, Bennett CF and Baker BF. (2018). RNA-targeted therapeutics. Cell Metab 27:714–739. [DOI] [PubMed] [Google Scholar]
- 13. Setten RL, Rossi JJ and Han S-P. (2019). The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov 18:421–446. [DOI] [PubMed] [Google Scholar]
- 14. Roberts TC, Langer R and Wood MJA. (2020). Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19:673–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hammond SM, Aartsma-Rus A, Alves S, Borgos SE, Buijse RAM, Collin RWJ, Covello G, Denti MA, Desviat LR, et al. (2021). Delivery of oligonucleotide-based therapeutics: challenges and opportunities. EMBO Mol Med 13:e13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, et al. (2017). Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 390:1511–1520. [DOI] [PubMed] [Google Scholar]
- 17. Tenforde MW, Patel MM, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, Gaglani M, et al. (2021). Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 Hospitalizations in the United States. medRxiv [Epub ahead of print]; DOI: 10.1101/2021.07.08.21259776. [DOI] [Google Scholar]
- 18. Napoli C, Lemieux C and Jorgensen R. (1990). Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE and Mello CC. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811. [DOI] [PubMed] [Google Scholar]
- 20. Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel'in AV, et al. (2014). Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc 136:16958–16961. [DOI] [PubMed] [Google Scholar]
- 21. Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, Schmidt K, Zhao C, Aghajan M, et al. (2014). Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res 42:8796–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, Wijngaard P, Horton JD, Taubel J, et al. (2016). A highly durable RNAi therapeutic inhibitor of PCSK9. NEJM 376:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faselis C, Imprialos K, Grassos H, Pittaras A, Kallistratos M and Manolis A. (2018). Is very low LDL-C harmful? Curr Pharm Des 24:3658–3664. [DOI] [PubMed] [Google Scholar]
- 24. (2021), The Week, Inclisiran: anti-cholesterol jab a ‘game-changer’ for heart attacks and strokes. www.theweek.co.uk/news/uk-news/953988/inclisiran-anti-cholesterol-jab-a-game-changer-for-heart-attacks-and-strokes
- 25. (2021), Medical News Today, Cholesterol-lowering jab could save over 30,000 lives. www.medicalnewstoday.com/articles/cholesterol-lowering-jab-could-save-over-30000-lives
- 26. Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, Pendergast MK, Goldkind SF, Lee EA, et al. (2019). Patient-customized oligonucleotide therapy for a rare genetic disease. NEJM 381:1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aartsma-Rus A. (2021). ‘N of 1’ therapies need a better model. Nat Med 27:939. [DOI] [PubMed] [Google Scholar]
- 28. Chu Y, Kilikevicius A, Liu J, Johnson KC, Yokota S and Corey DR. (2020). Argonaute binding within 3′-untranslated regions poorly predicts gene repression. Nucleic Acids Res 48:7439–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Behr JP. (1993). Synthetic gene-transfer vectors. Acc Chem Res 26:274–278. [DOI] [PubMed] [Google Scholar]
- 30. Brown CR, Gupta S, Qin J, Racie T, He G, Lentini S, Malone R, Yu M, Matsuda S, et al. (2020). Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res 48:11827–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Juliano RL. (2016). The delivery of therapeutic oligonucleotides. Nucleic Acids Res 44:6518–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lönn P, Kacsinta AD, Cui XS, Hamil AS, Kaulich M, Gogoi K and Dowdy. SF (2016). Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci Rep 6:32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Driscoll CM, Bernkop-Schnürch A, Friedl JD, Préat V and Jannin V. (2019). Oral delivery of non-viral nucleic acid-based therapeutics - do we have the guts for this? Eur J Pharm Sci 133:190–204. [DOI] [PubMed] [Google Scholar]
- 34. Mendonça MCP, Kont A, Aburto MR, Cryan JF and O'Driscoll CM. (2021). Advances in the Design of (Nano)Formulations for Delivery of Antisense Oligonucleotides and Small Interfering RNA: Focus on the Central Nervous System. Mol Pharmaceut 18:1491–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pawar G, Parayath NN, Sharma AA, Coito C, Khorkova O, Hsiao J, Curry WT, Amiji MM and Bleier. BS (2021). Endonasal CNS delivery system for blood-brain barrier impermeant therapeutic oligonucleotides using heterotopic mucosal engrafting. Front Pharmacol 12:660841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicolau S, Waldrop MA, Connolly AM and Mendell. JR (2021). Spinal muscular atrophy. Semin Pediatr Neurol 37:100878. [DOI] [PubMed] [Google Scholar]
- 37. Xiong H, Veedu RN and Diermeier. SD (2021). Recent advances in oligonucleotide therapeutics in oncology. Int J Mol Sci 22:3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beck JD, Reidenbach D, Salomon N, Sahin U, Ö Türeci, Vormehr M and Kranz. LM (2021). mRNA therapeutics in cancer immunotherapy. Mol Cancer 20:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chandler RJ. (2019). Messenger RNA therapy as an option for treating metabolic disorders. Proc Natl Acad Sci U S A 116:20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Truong B, Allegri G, Liu X-B, Burke KE, Zhu X, Cederbaum SD, Häberle J, Martini PGV and Lipshutz. GS (2019). Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc Natl Acad Sci U S A 116:21150. [DOI] [PMC free article] [PubMed] [Google Scholar]