Abstract
Background:
Adipokines play a role in cardiometabolic pathways. Coronary artery calcium (CAC) progression prognosticates cardiovascular disease (CVD) risk. However, the association of adipokines with CAC progression is not well established. We examined the association of adipokines with CAC progression in a multi-ethnic cohort free of CVD at baseline.
Methods:
We included 1,904 randomly-selected adults enrolled in the Multi-Ethnic Study of Atherosclerosis who had both adipokine levels [leptin, resistin, adiponectin] and CAC by CT measured at either exam 2 (2002–2004) or exam 3 (2004–2005). CAC was previously measured at exam 1 (2000–2002) and a subset (n=566) had CAC measured at exam 5 (2010–2012). We used logistic regression to examine odds of CAC progression between exam 1 and 2/3 (defined as >0 Agatston units of change/year). We used linear mixed effect models to examine CAC progression from exam 2/3 to 5.
Results:
At exam 2/3, the mean age was 65(10) yrs; 50% women. In models adjusted for sociodemographic factors and BMI, the highest tertile of leptin, compared to lowest, was associated with an increased odds of CAC progression over the preceding 2.6yrs [OR 1.60 (95% CI: 1.10–2.33)]. In models further adjusted for visceral fat and CVD risk factors, the highest tertile of leptin was statistically significantly associated with a 4% (1–7%) greater CAC progression over an average of 7yrs. No associations were seen for resistin and adiponectin.
Conclusions:
Higher leptin levels were independently, but modestly, associated with CAC progression. Atherosclerosis progression may be one mechanism through which leptin confers increased CVD risk.
Keywords: adipokines, leptin, atherosclerosis, coronary artery calcium, cardiovascular risk
1. INTRODUCTION
Adipose tissue, particularly in the visceral space, is associated with increased risk of cardiovascular disease (CVD).1–5 It is now well recognized that adipose tissue is not just a passive reservoir of fat storage for energy; rather it is a metabolically active tissue that plays an important role in hormonal response. Adipose tissue releases endocrine proteins, known as adipokines, which play a role in metabolism and inflammation.6,7 Adipokines, including adiponectin, leptin, and resistin, have differing relationships with CVD risk. While adiponectin is thought to be protective against CVD, leptin and resistin are associated with increased CVD risk.8–11 In prior work from the Multi-Ethnic Study of Atherosclerosis (MESA), the association of leptin with incident CVD events was attenuated after accounting for body mass index (BMI) and traditional CVD risk factors,12 whereas greater resistin levels remained independently associated with CVD even after accounting for these factors.8
Coronary artery calcium (CAC), assessed by non-contrast cardiac computed tomography (CT), is a measure of total coronary atherosclerotic burden. CAC, and its progression, are strongly associated with CVD events and mortality,13–15 and provide incremental prognostic information above 10-year CVD risk scores for patients.16 Atherosclerosis progression may be one mechanism linking adipokine levels with incident CVD events. Leptin may promote calcification of vascular cells.17 Additionally, leptin receptors have been found in atherosclerotic lesions,18 further implicating its role in the process.
In a prior study from the MESA, adiponectin, leptin, and resistin levels were not found to be independently associated with CAC prevalence or severity in cross-sectional analysis.5 However, whether adipokines levels are associated with CAC progression was not evaluated in that study. The association of adipokines with CAC progression is not well established. Thus, we examined the association of adipokines with CAC progression in a multi-ethnic, community-based, cohort free of CVD at baseline to glean further insights on potential mechanisms linking adipokines with incident CVD events. Given the association of leptin and resistin with increased CVD risk, we hypothesized higher levels of these adiopkines would be associated with CAC progression over time. Similarly, given the likely protective effects of adiponectin on CVD risk, we hypothesized higher levels of adiponectin would be inversely associated with CAC progression.
2. METHODS
2.1. Study Population and Design
The MESA study design has been previously described.19 Briefly, MESA originally enrolled 6,814 men and women aged 45 to 84 years who were free of clinical CVD at baseline, between the years of 2000–2002. Participants were recruited from 6 United States communities and included 4 self-reported racial/ethnic groups (Black, Hispanic, Chinese, and non-Hispanic White individuals). After the initial exam, there has been 5 subsequent follow-up exams. The MESA study design was approved by the institutional review boards (IRB) at all field centers, and participants provided written informed consent.
For this analysis, we included a subset of randomly selected MESA participants (n=1,904) who had adipokine levels measured as part of an ancillary study at either exam 2 (2002–2004) or exam 3 (2004–2005).5,20 Participants also had a concurrent cardiac CT scan for CAC measurement at that same exam 2 or exam 3 visit. All included participants (n=1,904) had previously undergone a CAC measurement at exam 1 (2000–2002), and a subset (n=566) underwent another CT at exam 5 (2010–2012) (Figure 1). For this analysis, exam 2 or 3 (the visit with the adipokine measurement) serves as the starting point for this analysis, since adipokines were not measured at exam 1. From this visit (2 or 3), analyses of CAC progression was examined both retrospectively and prospectively.
Figure 1:
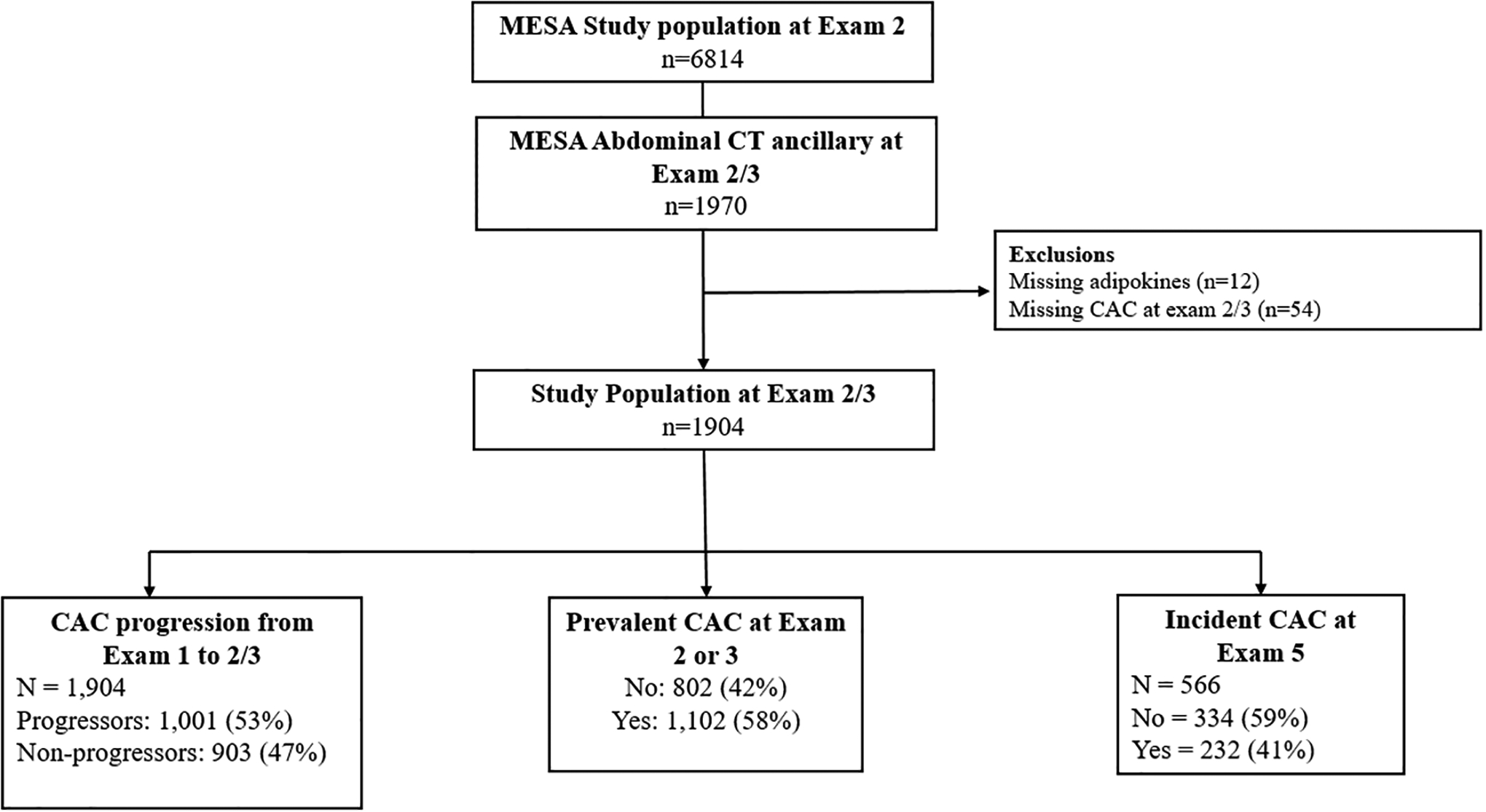
Study Flow Chart
2.2. Adipokine measurement
Adipokine levels were the exposure (independent) variables for this analysis. A random subset of participants (n=1,970) underwent abdominal CT scans at either visit 2 or visit 3 (randomly assigned) to measure abdominal aortic calcium (AAC) as part of an ancillary study.21 In a subsequent ancillary study related to body composition, adipokine levels were measured among these same participants from stored frozen serum samples obtained at the respective visits (2 or 3) of their CT scan.4,5,20,22 The adipokines (adiponectin, leptin, and resistin) were analyzed using a Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT), as previously reported.23,24 The coefficients of variation for these assays ranged from 6 to 13%.
2.3. CAC measurement
CAC progression was the primary outcome variable for this analysis. CAC was assessed by the Agatston score; prevalent or incident CAC was defined as scores > 0. As mentioned, all participants originally underwent cardiac CT scan to measure CAC at exam 1 (2000–2002). Approximately half of participants were randomly selected to get a repeat cardiac CT scan at exam 2 (2002–2004) and the other half at exam 3 (2004–2005). Finally, an additional subset who were participants of the MESA AIR ancillary study underwent repeat CT scanning at exam 5 (2010–2012). Thus, some participants in our analysis had up to 3 CT scans.
In order to leverage all the CAC data available for progression analysis, we performed three different analyses from this dataset. We examined the association of adipokines (measured at exam 2/3) with a retrospective history of CAC progression (defined below) from exam 1 to exam 2/3. Secondly, we evaluated the association of adipokines (exam 2/3) with incidence and progression of CAC (exam 2/3 to exam 5).
In supplemental analysis, we also evaluated the associated adipokines (exam 2/3) with CAC prevalence and extent from exam 2/3 as a cross-sectional assessment. The cross-sectional association of adipokines with CAC at exam 2/3 has been published,5 but that paper used slightly different regression models and covariates. Therefore we repeated the cross-sectional analysis using the same model adjustments as our current analyses for context with the prospective and retrospective results, but only included the cross-sectional analysis as supplemental material as reference
2.4. Body composition measurement
As mentioned, the adipokines were measured as part of an ancillary study focused on body composition. Abdominal CT scans from exam 2 or 3 were initially interrogated for the presence and/extent of AAC. These were subsequently assessed for the extent of visceral (VAT) and subcutaneous adipose tissue (SAT). VAT was defined as the total adipose tissue in the abdominal cavity, and SAT was defined as the total adipose tissue outside of the abdominal cavity but not within muscle tissue. As previously described, VAT and SAT were measured from the average of two CT slices obtained at L2-L3 and adjusted for height.4
2.5. Covariates
For this analysis, we included covariates that were measured at exam 2/3, the time of the adipokine measurement, unless otherwise noted. In our analysis, we considered age, sex, race, MESA field site, education (carried forward from exam 1), physical activity (MET-min-week of moderate + vigorous activity), smoking status (never, former, current), pack years of smoking, BMI, VAT, SAT, systolic blood pressure, use of medication for hypertension, total cholesterol, HDL-cholesterol, use of lipid lowering medication, diabetes status (which includes medication usage), and estimated glomerular filtration rate (eGFR).
2.6. Statistical analyses
Our study population was characterized with respect to baseline demographic and clinical factors, stratified by having a history of CAC progression between exam 1 and exam 2/3. Continuous variables were expressed as mean and standard deviations and categorical variables as percentages.
Given their non-normal distribution, adipokines were categorized into tertiles, using the lowest tertile as reference. “CAC progressors” between the first (exam 1) and second (exam 2 or 3) CT scans were defined as participants with >0 Agatston units of change per year and compared to non-progressors (i.e. participants with ≤0 Agatston units of change per year between CT scans) as has been previously defined in MESA.25 Multivariable logistic regression was used to examine the association of adipokine levels with history of CAC progression (retrospectively assessed).
Prevalent CAC was defined as CAC >0 Agatston units at exam 2/3 and measured using multivariable-adjusted Poisson regression with robust variance estimation. Multivariable-adjusted Poisson regression with robust variance estimation were also used to examine risk of incident CAC at exam 5 for participants who had respective CAC score of 0 at exam 2/3.
For the primary analysis of CAC progression, the longitudinal change in CAC scores was prospectively assessed from exams 2/3 to 5 for each of the adipokines separately. Given skew and high prevalence of zero scores, natural log of (CAC +1) was used as the outcome measure. Mixed linear effects models were used allowing for random variations in baseline CAC scores and longitudinal slope for CAC score progression across participants. The advantage of using a mixed effects model for longitudinal data is that it leverages all available CAC information from all participants, including those without follow-up measurements, to jointly model the amount of CAC at baseline and CAC progression over time. This type of analysis for CAC progression has previously been performed in MESA.26,27 Percent differences in CAC progression, compared to reference group, were calculated from exponentiated beta coefficients using formula: [Exp (β) −1]*100.
For all analyses, we used progressively adjusted regression models. Model 1 adjusted for age, sex, race/ethnicity and study site. Model 2 included model 1 variables as well as education, physical activity, smoking, pack-years of smoking and BMI. Model 2b included model 2 variables plus VAT and SAT. Model 3 included model 2 variables and systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-cholesterol, lipid-lowing medication, diabetes mellitus, and eGFR. Model 3b included model 3 variables plus VAT and SAT.
STATA 15.0 version (StataCorp LP, College Station, TX) was used for the analyses. Two-sided P values were used, with significance level set at 0.05.
3. RESULTS
3.1. Baseline characteristics
There were 1,904 participants with adipokines measured at exam 2/3 that were included in the analysis. The mean (SD) age of participants was 65 (10) years; 50% were women. There were 40% White, 13% Chinese, 21% Black, and 26% Hispanic adults (Table 1). At exam 2/3, 53% of participants had a history of interim CAC progression (>0 Agatston units of change per year) since the prior CT scan at exam 1. Individuals with a history of CAC progression were more likely to be older, male, less physically active, have greater pack year of smoking, use anti-hypertensive and lipid lowering medications, have diabetes, and have greater VAT, compared to individuals who had not had interim CAC progression. In unadjusted analysis, the mean resistin levels were higher among CAC progressors compared to non-progressors (Figure 2), but there was no significant difference in. mean leptin and adiponectin levels among CAC progressors compared to their counterparts.
Table 1.
Characteristics of study participants
| Total | CAC non-progressor | CAC progressor | P value | |
|---|---|---|---|---|
| N = 1,904 | n = 903 | n = 1,001 | ||
| Age, years | 65 (10) | 61 (9) | 68 (9) | <0.001 |
| Sex | ||||
| Women | 946 (50%) | 541 (60%) | 405 (40%) | <0.001 |
| Men | 958 (50%) | 362 (40%) | 596 (60%) | |
| Race/ethnicity | ||||
| White | 755 (40%) | 304 (34%) | 451 (45%) | |
| Chinese-American | 254 (13%) | 134 (15%) | 120 (12%) | <0.001 |
| Black | 407 (21%) | 223 (25%) | 184 (18%) | |
| Hispanic | 488 (26%) | 242 (27%) | 246 (25%) | |
| Education | ||||
| ≥ Bachelor’s degree | 690 (36%) | 329 (36%) | 361 (36%) | 0.89 |
| < Bachelor’s degree | 1,212 (64%) | 574 (64%) | 638 (64%) | |
| Physical activity, MET-min/wk | 3,536 (1,815–6,360) | 4,050 (2,145–7,110) | 3150 (1,568– 5,839) | <0.001 |
| Smoking | ||||
| Never | 878 (46%) | 483 (54%) | 395 (40%) | |
| Former | 808 (43%) | 310 (35%) | 498 (50%) | <0.001 |
| Current | 205 (11%) | 105 (12%) | 100 (10%) | |
| Pack-years of smoking if >0 | 16 (7, 34) | 15 (5, 30) | 18 (8, 38) | <0.001 |
| BMI, kg/m2 | 28 (5) | 28 (5) | 28 (5) | 0.03 |
| Systolic BP, mmHg | 124 (21) | 121 (20) | 127 (20) | <0.001 |
| Anti-hypertensive medication | 819 (44%) | 291 (33%) | 528 (54%) | <0.001 |
| Total cholesterol, mg/dL | 189 (35) | 193 (35) | 186 (36) | <0.001 |
| HDL-C, mg/dL | 52 (15) | 53 (16) | 50 (15) | <0.001 |
| Lipid-lowering medication | 468 (25%) | 152 (17%) | 316 (32%) | <0.001 |
| Diabetes | 274 (14%) | 98 (11%) | 176 (18%) | <0.001 |
| eGFR, ml/min per 1.73m2 | 73 (15) | 76 (15) | 72 (15) | <0.001 |
| SAT, cm2 | 147 (102, 205) | 150 (101, 205) | 144 (103, 206) | 0.89 |
| VAT, cm2 | 150 (91, 225) | 128 (81, 189) | 180 (108, 251) | <0.001 |
| Leptin, ng/mL | 13.4 (5.6, 28.3) | 14.3 (5.9, 30.4) | 12.5 (5.5, 27.5) | 0.10 |
| Resistin, ng/mL | 15.0 (11.9, 19.0) | 14.5 (11.3, 18.2) | 15.4 (12.2, 19.6) | 0.004 |
| Adiponectin, mcg/mL | 17.3 (11.8, 26.2) | 17.7 (11.9, 26.3) | 17.0 (11.7, 25.7) | 0.69 |
Abbreviations: BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; MET, metabolic equivalent of task; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Data were presented as mean (SD), median (IQR) or n (%).
Figure 2.
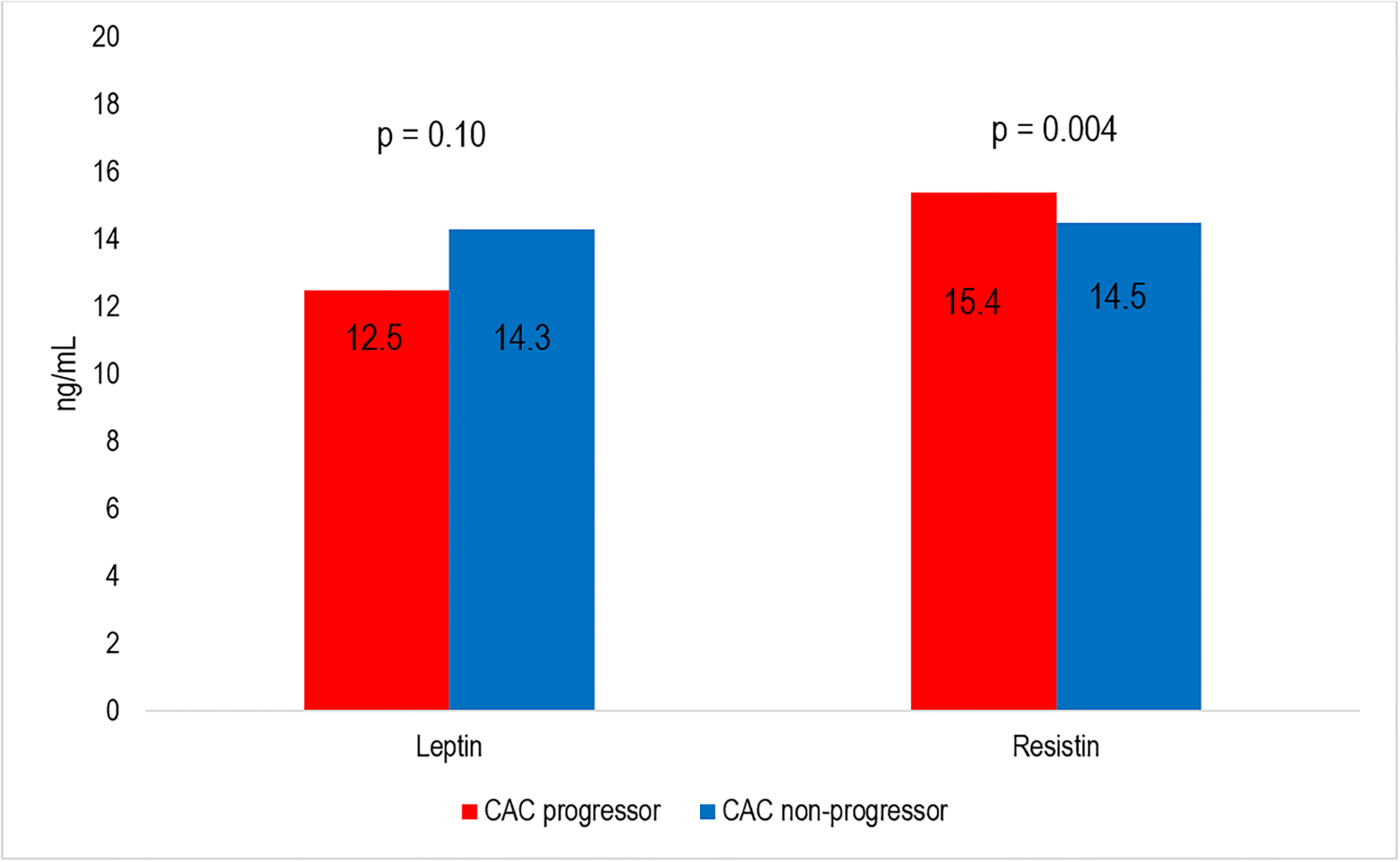
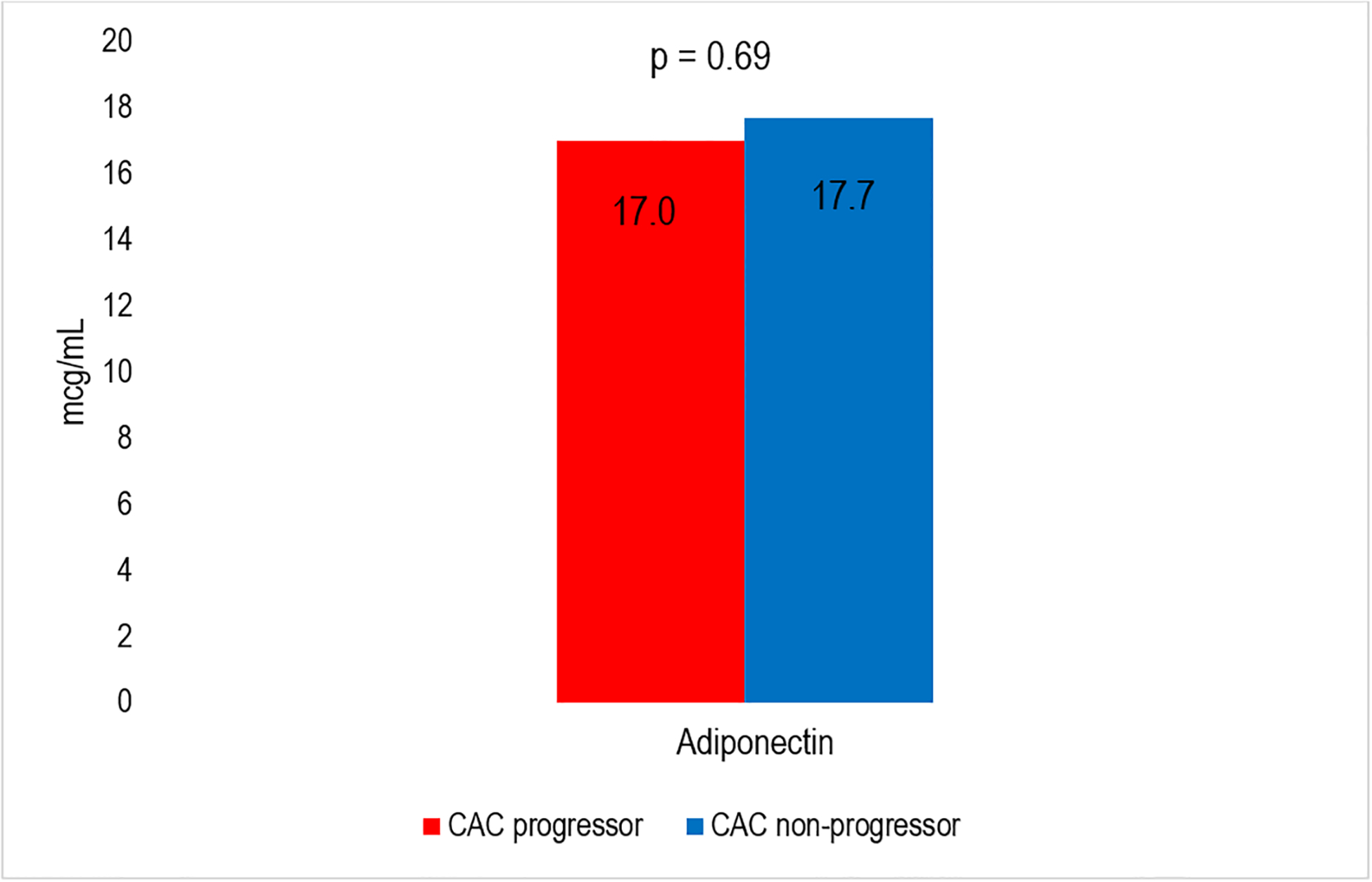
Median Adipokines levels for CAC progressors vs CAC non-progressors. Panel A shows levels for Leptin and Resitin. Panel B shows levels for Adiponectin.
3.2. Adipokines with history of CAC progression, retrospectively assessed
Table 2 shows the adjusted associations of adipokine tertiles with history of CAC progression, retrospectively assessed, after an average of 2.6 years. After adjusting for age, sex, race/ethnicity and study site (model 1), the highest tertile of leptin, compared to lowest, was positively associated with having a history of CAC progression from exam 1 to exam 2/3 (retrospectively assessed) [OR=1.99 (95% CI, 1.48, 2.69)]. This positive association persisted, but was attenuated, when adjusting for previously mentioned demographics as well as education, physical activity, smoking status, pack-years of smoking and BMI (model 2) [OR=1.60 (1.10, 2.33)]. However, the association was no longer significant with further adjustment for the visceral fat and other covariates included in models 2b, 3, and 3b.
Table 2.
Multivariable-adjusted associations of Adipokines with CAC progression (retrospectively assessed)
| Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2b | Model 3 | Model 3b | |
| Leptin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 1.37 (1.06, 1.77) | 1.24 (0.94, 1.64) | 1.14 (0.82, 1.60) | 1.16 (0.87, 1.54) | 1.10 (0.77, 1.55) |
| Tertile 3 | 1.99 (1.48, 2.69) | 1.60 (1.10, 2.33) | 1.32 (0.84, 2.09) | 1.46 (0.99, 2.15) | 1.24 (0.77, 1.99) |
| Resistin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 1.20 (0.94, 1.53) | 1.17 (0.91, 1.50) | 1.09 (0.81, 1.46) | 1.16 (0.90, 1.51) | 1.09 (0.81, 1.47) |
| Tertile 3 | 1.26 (0.99, 1.62) | 1.20 (0.93, 1.55) | 1.13 (0.84, 1.53) | 1.21 (0.92,1.58) | 1.18 (0.86, 1.62) |
| Adiponectin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 0.92 (0.72, 1.18) | 0.99 (0.77, 1.28) | 1.03 (0.76, 1.42) | 1.03 (0.79, 1.35) | 1.05 (0.76, 1.46) |
| Tertile 3 | 0.71 (0.54, 0.93) | 0.85 (0.64, 1.13) | 0.98 (0.69, 1.39) | 0.92 (0.67, 1.25) | 1.01 (0.69, 1.46) |
Adipokines and covariates were obtained at MESA visits 2 or 3 (randomly assigned) which was the baseline for this analysis.
Coronary artery calcification (CAC) progressors between first and second CT scans (median 2.4 years) were defined as participants with >0 Agatston units of change per year (and compared to non-progressor i.e. participants with ≤0 Agatston units of change per year between CT scans).
Model 1: adjusted for age, sex, race/ethnicity and study site
Model 2: model 1 + education, physical activity, smoking, pack-years of smoking and body mass index.
Model 2b: Model 2 + visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).
Model 3: model 2 + systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-cholesterol, lipid-lowing medication, diabetes mellitus, and estimated glomerular filtrate rate.
Model 3b: Model 3: VAT and SAT.
Results in bold font indicate statistical significance at p <0.05.
The highest tertile of adiponectin, compared to the lowest, had an inverse association with CAC progression from exam 1 to exam 2/3 when adjusting for the covariates included in model 1 [OR=0.71 (0.54, 0.93)]. However, this relationship was attenuated and no longer significant after further adjustment in models 2 and 3. Resistin levels were not significantly associated with CAC progression.
3.3. Cross-sectional association of adipokines with CAC prevalence and extent
There were 1,102 individuals (58%) who had prevalent CAC (score >0) at exam 2 or 3. When performing a cross-sectional analysis at exam 2/3, we found the highest tertile of leptin was associated with a greater likelihood of having prevalent CAC at exam 2/3 [PR=1.25 (1.13, 1.38)] in models adjusted for demographics (model 1) (Supplemental Table 1). Higher leptin was also associated with greater CAC extent [i.e. with CAC assessed as continuous measure as ln (CAC+1)] (Supplemental Table 2). These associations were no longer significant with further covariate adjustment in models 2 or 3. Similarly, while the highest tertile of adiponectin had a significant inverse association with prevalence of CAC at exam 2/3 when adjusting for the demographic covariates included in model 1 [PR=0.91 (0.83, 1.00)], as well as a lower percent difference in CAC extent compared to lowest tertile of adiponectin, this relationship did not remain significant when adjusting for variables in models 2 and 3. There were no significant associations between resistin levels and prevalence of CAC.
3.4. Association of adipokines with future CAC incidence and progression
There were 566 individuals who had CAC measured at exam 5; of these 232 had incident CAC (score >0). The highest tertile of leptin was associated with an increased risk of incident CAC in demographic adjusted models [RR=1.48 (1.08, 2.05)] which did not remain statistically significant after further adjustment (Table 3).
Table 3.
Multivariable-adjusted associations of Adipokines (Exam 2/3) with incident CAC (Exam 5)
| Incidence rate ratios (95% CI) | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2b | Model 3 | Model 3b | |
| Leptin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 1.24 (0.94, 1.63) | 1.12 (0.84, 1.48) | 1.15 (0.82, 1.62) | 1.12 (0.83, 1.51) | 1.19 (0.83, 1.72) |
| Tertile 3 | 1.48 (1.08, 2.05) | 1.05 (0.71, 1.56) | 1.00 (0.62, 1.60) | 1.05 (0.70, 1.58) | 1.01 (0.62, 1.66) |
| Resistin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 1.02 (0.81, 1.28) | 1.01 (0.80, 1.26) | 1.00 (0.77, 1.30) | 1.05 (0.83, 1.32) | 1.04 (0.80, 1.36) |
| Tertile 3 | 0.94 (0.73, 1.20) | 0.91 (0.71, 1.17) | 0.91 (0.69, 1.22) | 0.96 (0.74, 1.24) | 1.00 (0.74, 1.35) |
| Adiponectin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 0.87 (0.68, 1.11) | 0.93 (0.73, 1.19) | 0.95 (0.71, 1.27) | 1.00 (0.78, 1.28) | 1.03 (0.76, 1.38) |
| Tertile 3 | 0.87 (0.67, 1.13) | 0.99 (0.76, 1.29) | 1.04 (0.76, 1.44) | 1.25 (0.94, 1.66) | 1.28 (0.92, 1.79) |
Adipokines and covariates were obtained at MESA visits 2 or 3 (randomly assigned) which was the baseline for this analysis.
Incident coronary artery calcification (CAC) was defined as CAC >0 Agatston units at exam 5 and measured using multivariable-adjusted Poisson regression with robust variance estimation.
Model 1: adjusted for age, sex, race/ethnicity and study site
Model 2: model 1 + education (dichotomized), physical activity, smoking, pack-years of smoking and body mass index.
Model 2b: Model 2 + visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).
Model 3: model 2 + systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-cholesterol, lipid-lowing medication, diabetes mellitus, and estimated glomerular filtrate rate.
Model 3b: Model 3: VAT and SAT.
Results in bold font indicate statistical significance at p <0.05.
In our primary prospective analysis, we examined adipokines with CAC progression as a continuous measure [ln (CAC scores+1)], from exam 2/3 to exam 5 (Table 4). In models fully adjusted for visceral fat (VAT) and CVD risk factors, the highest tertile of leptin was associated with a 4% (1, 7) greater CAC progression over an average of 7 years, compared to the lowest tertile. No associations were seen for resistin and adiponectin in adjusted models.
Table 4.
Multivariable-adjusted associations of Adipokines with CAC progression from exam 2/3 to exam 5
| Percent difference (95% CI) | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 2b | Model 3 | Model 3b | |
| Leptin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 1 (−2, 3) | 1 (−2, 3) | 0 (−2, 3) | 1 (−1, 4) | 1 (−2, 4) |
| Tertile 3 | 3 (0, 5) | 3 (0, 5) | 3 (0, 7) | 3 (1, 6) | 4 (1, 7) |
| Resistin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 1 (−2, 3) | 0 (−2, 3) | 1 (−2, 3) | 1 (−2, 3) | 1 (−2, 4) |
| Tertile 3 | 1 (−1, 4) | 1 (−2, 4) | 1 (−2, 4) | 1 (−2, 4) | 1 (−2, 4) |
| Adiponectin | |||||
| Tertile 1 | Reference | Reference | Reference | Reference | Reference |
| Tertile 2 | 0 (−3, 2) | 0 (−3, 2) | 0 (−3, 3) | −1 (−3, 2) | 0 (−3, 3) |
| Tertile 3 | 0 (−3, 2) | 0 (−3, 2) | 1 (−2, 4) | 1.00 (0.97, 1.02) | 1 (−2, 4) |
Adipokines and covariates were obtained at MESA visits 2 or 3 (randomly assigned) which was the baseline for this analysis.
Coronary artery calcification (CAC) progression at exam 5 was measured using multivariable-adjusted linear mixed effects models using naturally log transformed (CAC +1).
Percent differences (95% CI) were calculated from exponentiated beta coefficients as follows: [Exp (β) −1]*100.
Model 1: adjusted for age, sex, race/ethnicity and study site
Model 2: model 1 + education, physical activity, smoking, pack-years of smoking and body mass index.
Model 2b: Model 2 + visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).
Model 3: model 2 + systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-cholesterol, lipid-lowing medication, diabetes mellitus, and estimated glomerular filtrate rate.
Model 3b: Model 3: VAT and SAT.
Results in bold font indicate statistical significance at p <0.05. [P = 0.036 for 1.03 (1.00, 1.05); P = 0.029 for 1.03 (1.00, 1.05); P = 0.027 for 1.03 (1.00, 1.07)].
4. DISCUSSION
4.1. Overview of study findings
In this longitudinal study from the MESA cohort, we found statistically significant positive associations between higher levels of leptin and CAC progression. In all three analyses—the retrospective analysis of the progression of CAC from exam 1 to exam 2/3, the cross-sectional analysis of the prevalence of CAC at exam 2/3, and the prospective analysis of CAC progression from exam 2/3 to exam 5—we found higher levels of leptin to be statistically significantly associated with CAC prevalence or progression in at least one of the models. However, these changes were modest and the clinical relevance of these findings is uncertain. Furthermore, some of these associations were attenuated after further adjustment for adiposity and other covariates. Notably, in our primary prospective analysis examining CAC progression from exam 2/3 to exam 5, the highest tertile of leptin remained associated with greater risk of CAC progression even after adjustment for other cardiovascular risk factors and measurements of adiposity including visceral fat measurements. On the other hand, resistin and adiponectin were not associated with CAC progression in models adjusted for adiposity.
4.2. Study findings in context with prior work
There had been limited prior studies examining the association of adipokines with vascular calcification. In a prior study from MESA, while inflammatory markers were associated with arterial calcification as assessed by the prevalence and severity of CAC and AAC, adiponectin, leptin, and resistin were not independently associated with CAC or AAC prevalence or severity in cross-sectional analysis.5 This cross-sectional analysis had been published previously, but we replicated the analysis in supplemental data with our updated models to be more comparable with our newly performed retrospective and prospective analyses. In another MESA analysis examining extra-coronary calcification (ECC), while some of the adipokines were associated with valvular and aortic calcification, leptin was not associated with measures of ECC after full covariate adjustment.20 In a cross-sectional study from a different population, while both leptin and adiponectin levels were associated with metabolic and inflammatory markers, only leptin was a significant independent predictor of CAC score among White individuals without diabetes.28 In a cross-sectional study of individuals with type 2 diabetes, plasma leptin levels were associated with CAC even after controlling for C-reactive protein and BMI.29 In sum, the aforementioned studies evaluating adipokines and CAC have shown mixed findings, were limited in temporality by cross-sectional design,5,28,29 or were limited in generalizability to almost exclusively White individuals.28 Little had been known about the association of adipokines and atherosclerotic progression over time, which is why we undertook this study. Our findings, conducted in a diverse population, do suggest that higher leptin levels may be associated with modest CAC progression. The clinical significance is unknown.
Findings from prior studies examining the association of leptin with incident clinical CVD events have also been mixed, with some studies showing increased risk while other studies found the association to be attenuated after accounting for BMI.11,12,30 On the other hand, studies have found that adiponectin is inversely associated with CVD risk.9,10,31 Our models support a positive association between leptin and CAC progression, with adiponectin illustrating a potential inverse relationship. As CAC has been shown to be strongly associated with CVD events and mortality,13–15 it is possible that leptin plays a pathophysiologic role further contributing to this process as it was found to be independently associated with CAC progression even when adjusting for smoking, diabetes, BMI, VAT, blood pressure, and cholesterol in our prospective model. As mentioned before, the pathophysiologic relationship between leptin and CVD risk is not fully understood; however, proposed mechanisms include calcification of vascular cells17 and leptin receptors having been found in atherosclerotic lesions18, suggesting direct involvement in the process. Our results further suggest that the pathophysiology of adipokines, atherosclerosis, and cardiovascular disease is complex and requires further understanding.
4.3. Strengths and Limitations
Our study included multiple strengths which may fill gaps in relation to previous data. First, we used a multi-ethnic cohort. We also used a longitudinal model meaning we evaluated the relationship between three different adipokines with CAC progression over time as opposed to cross-sectional analysis only allowing for an absolute value of CAC at one point in time. However, our study had a number of limitations. First, given the observational design, causality of associations cannot be determined. We adjusted for a number of potential confounding variables, but residual confounding may still explain in part associations seen. Second, the adipokines were only measured at one point in time (exam 2 or 3). Third, our sample size was significantly reduced for the prospective analyses. Finally, we also performed multiple analyses; thus, some of our results may have been due to chance. However, our study was meant to be exploratory and hypothesis-generating to provide mechanistic insight linking adipokines with future CVD risk.
4.4. Conclusions
In conclusion, we found in a diverse community-based cohort that higher leptin levels were independently but modestly associated with CAC progression. The clinical significance remains uncertain. Atherosclerosis progression may be one mechanism through which leptin confers increased CVD risk.
Supplementary Material
Highlights.
Adipose-related hormones called “adipokines” have been linked to incident CVD
We examined whether adipokines were associated with CAC progression
We found higher leptin levels were modestly associated with CAC progression over 7yrs
No associations were seen for resistin and adiponectin in adjusted models.
Atherosclerosis progression may be one mechanism linking leptin with incident CVD
Acknowledgements
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Disclosures:
Dr. Budoff receives grant support from General Electric. Unrelated to this work, Dr. Michos served on a Medical Advisory Board for Novartis, Novo Nordisk, Bayer, Boehringer Ingelheim, Esperion, Amarin, and Astra Zeneca. None of the other authors report any conflicts of interest.
Funding:
Drs. Michos is supported by the Amato Fund for Women’s Cardiovascular Health at Johns Hopkins. The MESA study is supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NIH/NHLBI, by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The ancillary data used in this analysis were funded by R01 HL088451. Drs. Ndumele and Michos are additionally supported for this work by an American Heart Association Strategic Focused Research Network Grant 20SFRN35120152.
REFERENCES
- 1.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol 2011;57:2461–2473. [DOI] [PubMed] [Google Scholar]
- 2.Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343–368. [DOI] [PubMed] [Google Scholar]
- 3.Fliotsos M, Zhao D, Rao VN, Ndumele CE, Guallar E, Burke GL, Vaidya D, Delaney JCA, Michos ED. Body Mass Index From Early-, Mid-, and Older-Adulthood and Risk of Heart Failure and Atherosclerotic Cardiovascular Disease: MESA. J Am Heart Assoc 2018;7:e009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes-Austin JM, Wassel CL, Jimenez J, Criqui MH, Ix JH, Rasmussen-Torvik LJ, Budoff MJ, Jenny NS, Allison MA. The relationship between adiposity-associated inflammation and coronary artery and abdominal aortic calcium differs by strata of central adiposity: The Multi-Ethnic Study of Atherosclerosis (MESA). Vasc Med 2014;19:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutheil F, Gordon BA, Naughton G, Crendal E, Courteix D, Chaplais E, Thivel D, Lac G, Benson AC. Cardiovascular risk of adipokines: a review. J Int Med Res 2018;46:2082–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma XL, Ouchi N. Role of Adipokines in Cardiovascular Disease. Circ J 2017;81:920–928. [DOI] [PubMed] [Google Scholar]
- 8.Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ, Nasir K, Criqui MH, Cushman M, McClelland RL, Allison MA. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2015;239:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;291:1730–1737. [DOI] [PubMed] [Google Scholar]
- 10.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation 2006;114:623–629. [DOI] [PubMed] [Google Scholar]
- 11.Sattar N, Wannamethee G, Sarwar N, Chernova J, Lawlor DA, Kelly A, Wallace AM, Danesh J, Whincup PH. Leptin and coronary heart disease: prospective study and systematic review. J Am Coll Cardiol 2009;53:167–175. [DOI] [PubMed] [Google Scholar]
- 12.Martin SS, Blaha MJ, Muse ED, Qasim AN, Reilly MP, Blumenthal RS, Nasir K, Criqui MH, McClelland RL, Hughes-Austin JM, Allison MA. Leptin and incident cardiovascular disease: the Multi-ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015;239:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michos ED, Blaha MJ, Blumenthal RS. Use of the Coronary Artery Calcium Score in Discussion of Initiation of Statin Therapy in Primary Prevention. Mayo Clin Proc 2017;92:1831–1841. [DOI] [PubMed] [Google Scholar]
- 14.Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, Kronmal R, Lima JAC, Liu KJ, McClelland RL, Michos E, Post WS, Shea S, Watson KE, Wong ND. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, Folsom AR, Khera A, Ayers C, Mahabadi A-A, Lehmann N, Jöckel K-H, Moebus S, Carr JJ, Erbel R, Burke GL. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res 2001;88:954–960. [DOI] [PubMed] [Google Scholar]
- 18.Kang SM, Kwon HM, Hong BK, Kim D, Kim IJ, Choi EY, Jang Y, Kim HS, Kim MS, Kwon HC. Expression of leptin receptor (Ob-R) in human atherosclerotic lesions: potential role in intimal neovascularization. Yonsei Med J 2000;41:68–75. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney T, Ogunmoroti O, Ndumele CE, Zhao D, Varma B, Allison MA, Budoff MJ, Fashanu OE, Sharma A, Bertoni AG, Michos ED. Associations of adipokine levels with the prevalence and extent of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2021;338:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbang NI, McClelland RL, Remigio-Baker RA, Allison MA, Sandfort V, Michos ED, Thomas I, Rifkin DE, Criqui MH. Associations of cardiovascular disease risk factors with abdominal aortic calcium volume and density: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2016;255:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez CP, Ogunmoroti O, Quispe R, Osibogun O, Ndumele CE, Echouffo Tcheugui J, Minhas AS, Bertoni AG, Allison MA, Michos ED. The Association Between Multiparity and Adipokine Levels: The Multi-Ethnic Study of Atherosclerosis. J Womens Health 2021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vella CA, Allison MA, Cushman M, Jenny NS, Miles MP, Larsen B, Lakoski SG, Michos ED, Blaha MJ. Physical Activity and Adiposity-related Inflammation: The MESA. Med Sci Sports Exerc 2017;49:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen-Torvik LJ, Wassel CL, Ding J, Carr J, Cushman M, Jenny N, Allison MA. Associations of body mass index and insulin resistance with leptin, adiponectin, and the leptin-to-adiponectin ratio across ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA). Ann Epidemiol 2012;22:705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fashanu OE, Bizanti A, Al-Abdouh A, Zhao D, Budoff MJ, Thomas IC, Longstreth WT Jr., Michos ED. Progression of valvular calcification and risk of incident stroke: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2020;307:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gassett AJ, Sheppard L, McClelland RL, Olives C, Kronmal R, Blaha MJ, Budoff M, Kaufman JD. Risk Factors for Long-Term Coronary Artery Calcium Progression in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2015;4:e001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanya V, Zhao D, Ouyang P, Ying W, Vaidya D, Ndumele CE, Heckbert SR, Budoff MJ, Post WS, Michos ED. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr 2019;13:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qasim A, Mehta NN, Tadesse MG, Wolfe ML, Rhodes T, Girman C, Reilly MP. Adipokines, insulin resistance, and coronary artery calcification. J Am Coll Cardiol 2008;52:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly MP, Iqbal N, Schutta M, Wolfe ML, Scally M, Localio AR, Rader DJ, Kimmel SE. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab 2004;89:3872–3878. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Guo W, Li J, Cao S, Zhang J, Pan J, Wang Z, Wen P, Shi X, Zhang S. Leptin concentration and risk of coronary heart disease and stroke: A systematic review and meta-analysis. PloS one 2017;12:e0166360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smekal A, Vaclavik J. Adipokines and cardiovascular disease: A comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:31–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


