Abstract
For the foreseeable future, vaccines are the cornerstone in the global campaign against the Coronavirus Disease-19 (COVID-19) pandemic. As the number and fatalities due to COVID-19 decline and the lockdown anywise rescinded, we recognize an increase in the incidence of autoimmune disease post-COVID-19 vaccination. However, the causality of the most vaccine-induced side effects is debatable and, at best, limited to a temporal correlation. We herein report a case of a 51-year-old gentleman who developed Anti-Neutrophil Cytoplasmic Antibody (ANCA)-associated vasculitis (AAV) 2 week post-COVID-19 vaccination. The patient responded favorably to oral steroids and rituximab. Additionally, we conducted a case-based review of vaccine-associated AAV describing their clinical manifestations and treatment response of this emerging entity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00296-021-05069-x.
Keywords: COVID-19 vaccine, SARS-CoV-2 vaccine, ANCA-associated vasculitis, Auto-immunity, Glomerulonephritis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection induces an exaggerated immune response in susceptible individuals [1, 2]. The development of autoantibodies in a tiny minority is consequential to the release of Proteinase-3 (PR-3), Myeloperoxidase (MPO) and other antigens by the neutrophils in response to SARS-CoV-2 infection. Epitope spreading and antigen mimicry are initial triggers for antibody production [3]. In addition, host characteristics may contribute to this viral susceptibility [4]. An ideal vaccine should effectively generate a controlled and long-lasting immune response with an impeccable safety profile. In a utopian world, before the rollout, the vaccine efficacy and safety need testing in all clinical conditions, including patients with autoimmune disease those on immunosuppressive therapy, as the aforementioned cohort(s) exhibit variable immune responses to both infection [5, 6] and vaccination [7, 8]. Unfortunately, the burgeoning of COVID-19 cases worldwide and its global ramifications mandated an expeditious vaccine rollout. Henceforth, vaccine trials did not include patients with autoimmune diseases, or the development of autoimmune diseases in healthy individuals underwent inadequate scrutiny. Drawing a parallel to COVID-19 infection, researchers worldwide cast doubt over the immunogenicity of the vaccines, with an exaggerated immune response similar to an SARS-CoV-2 infection [9]. Antigen presentation, cytokine profiling, bystander activation, epitope spreading, anti-idiotypic networks, polyclonal activation of B cells are all the mechanisms that may theoretically contribute to this exaggerated immune response [9].
A growing number of reports describe the onset and recurrence of glomerular diseases like anti-Glomerular Basement Membrane (anti-GBM) disease and AAV with the widespread use of the vaccines [10]. Mainly, these occurred in individuals who were susceptible to autoimmune diseases and those in remission with these disorders. Therefore, an accepted hypothesis is that AAV develops in patients with a susceptible genetic background and a simultaneous exposure to environmental or other risk factors [11]. Previously, numerous reports have described a temporal association of AAV to influenza vaccination [12]. The aforementioned observations strengthen our credence that the SARS-CoV-2 vaccine could induce an autoimmune response analogous to the infection/influenza vaccine as causality for AAV. Furthermore, with the recent evidence suggesting booster doses of COVID-19 vaccines besides the usual dosing (in the susceptible population) [13], it is pertinent to study the association of autoimmune diseases with COVID-19 vaccination comprehensively. Therefore, we present a 51-year-old gentleman developing a new-onset AAV following the ChAdOx1 nCoV19 SARS-CoV-2 vaccine with a case-based review of the literature of similar cases.
Case report
A 51-year-old gentleman with no prior comorbidity (serum creatinine 1.2 mg/dl, before his illness) presented with a 3-day history of low-grade fever with debilitating inflammatory polyarthritis. He was a non-smoker and did not have any similar complaints in the past. He (or his contacts) had no history of COVID-19 infection. He received the first dose of ChAdOx1 nCoV-19 Vaccine (COVISHIELD- manufactured by Serum Institute of India Pvt Ltd.) 15 days before the onset of present symptoms. However, for synovitis involving multiple joints, his physical examination was unremarkable. On investigating, he had deranged kidney functions (serum creatinine- 4.8 mg/dl), proteinuria (3.4 g/day) and microscopic haematuria (erythrocyte casts and 8–10 erythrocytes/high-power field), elevated inflammatory markers (erythrocyte sedimentation rate (ESR)—46 mm/hour and C-reactive protein—7 mg/L) and PR-3 ANCA positivity. Anti-Nuclear Antibody (ANA), double-stranded DNA antibody (dsDNA), anti-GBM antibody titres were negative, and serum complement levels were within normal limits. The blood and the urine cultures were negative. Kidney biopsy suggested pauci-immune crescentic glomerulonephritis (Supplemental Figure 1). Nineteen out of the 20 glomeruli biopsied showed crescents with predominant cellular crescents. With a diagnosis of PR3-AAV, he was referred to our hospital, and we started him on oral prednisolone (60 mg/day) and rituximab (375 mg/m2 weekly for 4 weeks). We tapered oral prednisolone as per the low-dose steroid tapering schedule of the PEXIVAS trial [14]. At 20 weeks of follow-up, he had complete resolution of his constitutional symptoms and arthralgias, serum creatinine (2.3 mg/dl) and proteinuria (1.0 g/day) showing a steady decline, and micro-haematuria subsided.
Search strategy and case selection
We conducted a case-based search in PubMed, with the following search (COVID-19 vaccine OR COVID-19 OR COVID-19 vaccination OR SARS-CoV-2 vaccine OR SARS-CoV-2 OR Oxford AstraZeneca OR Moderna OR Pfizer-BioNTech OR Sputnik OR Sinopharm OR BBV152/Covaxin OR Janssen OR CoronaVac OR Novavax) AND (ANCA OR ANCA related Glomerulonephritis OR ANCA-associated glomerulonephritis OR ANCA Associated Vasculitis OR Glomerulonephritis OR MPO ANCA OR PR-3 ANCA OR Pauci-immune glomerulonephritis OR De novo vasculitis OR Anti-Neutrophil cytoplasmic antibody OR Antineutrophil cytoplasmic antibody OR Myeloperoxidase OR Anti-proteinase-3) from 1st January 2020 to 15th November 2021. We included all the case reports published in the English literature of AAV in patients aged ≥ 18 years. Cases were excluded if the AAV developed after SARS-CoV-2 infection or disease manifestations without ANCA positivity or if the ANCA report was unavailable or untested. Additionally, we included articles detected on web-based search. The title, abstracts and the full texts of the case reports were individually checked by two authors (AP and PC) and considered for evaluation if both agreed.
Statistical analysis
Descriptive statistics are used to detail the baseline characteristics of the patients. We expressed the normally distributed continuous variables as mean ± standard deviation (range), non-normally distributed variables as medians with interquartile ranges (IQR) and categorical data as proportions. All analyses were performed using Graph Pad Prism 9, San Diego, CA 92108.
Results
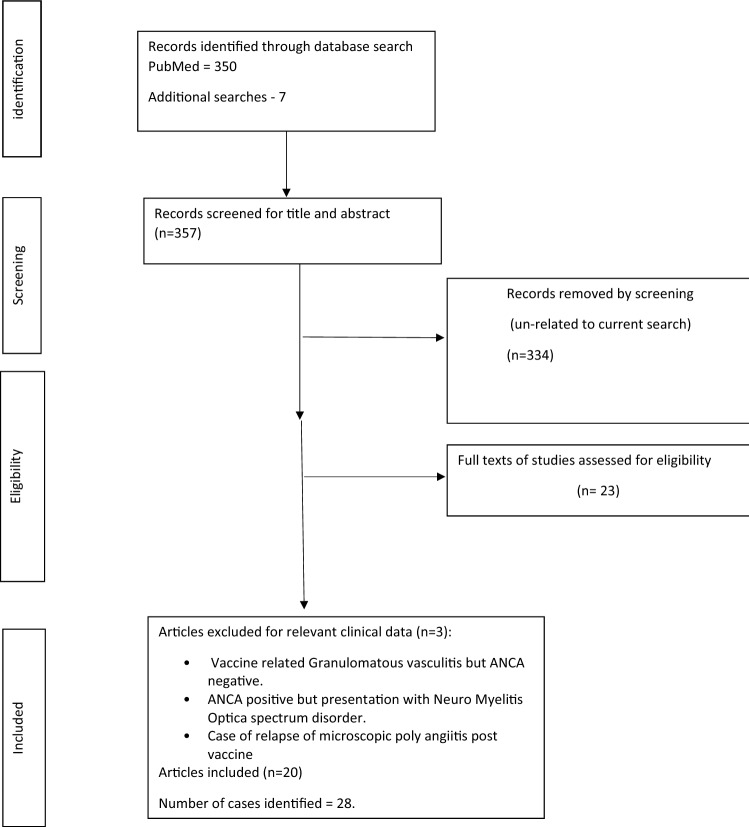
The search criteria exhibited 350 articles from PubMed. Of the 350 reports, we identified 15 cases, and an additional web-based search revealed 13 cases (Supplemental Figure 1). Also, we included our case report for review. Finally, we analyzed 29 cases for review. The median age of the patients was 71 years (IQR 54 to 78). 15 cases were males and 14 were female, respectively. The individual case details are shown in Table 1.
Table 1.
Details of AAV patients post SARS-CoV2 vaccinations
| S no | Study author | Age | Sex | Comorbidity | Relapse or new onset | Vaccine | Dose | Worsened with rechallenge | Time to onset (days) | Sero-markers | Kidney involvement | Kidney biopsy | Maximum serum creatinine (mg/dl) | Constitutional symptoms | Lung | Other clinical features | Treatment given | Follow-up duration (weeks) | Renal Outcome | General outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Anderegg et al. [31] | 81 | Male | Nil | New onset | mRNA-1273 | 1st and 2nd | Yes | NA | PR3 | AKI, microscopic haematuria, non-nephrotic range proteinuria | Crescentic GN | NA | Yes | Necrotic masses, pleural effusion | Cyc + PLEX + steroids | 3 | Improved | Improved | |
| 2 | Arjun Sekar et al.[32] | 52 | Male | HTN | New onset | mRNA-1273 | 2nd | NA | 14 | PR3 | AKI, macroscopic haematuria, non-nephrotic range proteinuria | Crescentic GN | 10.42 | Yes | NA | Rtx (1dose) f/b Cyc + steroids | 2 | Dialysis dependent | NA | |
| 3 | Shakoor et al.[33] | 78 | Female | HTN, DM, AF | New onset | mRNA-BNT162b2 | 1st and 2nd | Yes | 16—1st, 6—2nd | MPO | AKI, microscopic haematuria, non-nephrotic range proteinuria, leukocyturia | Crescentic GN | 3.54 | Yes | NA | Rtx + Steroids | 4 | Improved | Improved | |
| 4 | Dube et al. [34] | 29 | Female | congenital cystic lung disease, lung failure | New onset | mRNA-BNT162b2 | 2nd | NA | 16 | MPO | AKI, microscopic haematuria, non-nephrotic range proteinuria | Crescentic GN | 1.91 | NA | NA | Rtx + Cyc + steroids | 10 | Improved | Improved | |
| 5 | Takenaka et al. [35] | 75 | Female | DM, dyslipidaemia | New onset | mRNA-BNT162b2 | 1st | NA | 4 | MPO | NA | NA | NA | NA | NA | Right eye optic neuritis | Steroids | 4 | NA | Improved |
| 6 | Okuda et al.[36] | 37 | Female | Graves’ disease | New onset | mRNA-BNT162b2 | 1st | NA | 12 | MPO, PR-3 | Nil | NA | 0.6 | Yes | NA | Auricular chondritis, skin rash | Oral steroids | 4 | NA | Improved |
| 7 | Villa et al. [37] | 63 | Male | Nil | New onset | ChAdOx1 nCoV-19 | 1st | NA | 2 | MPO | AKI, microscopic haematuria, non-nephrotic range proteinuria | Focal pauci-immune GN | 2.91 | Yes | Alveolar haemorrhage | Cyc + steroids | 6 | Partial response | Improved | |
| 8 | Rachel David et al.[38] | 75 | Male | Renal limited MPA vasculitis | Relapse | ChAdOx1 nCoV-19 | 1st | NA | 37 | MPO | Microscopic haematuria | Crescentic GN | 6.97 | NA | Alveolar haemorrhage | Rtx + steroids | NA | Dialysis dependent | Improved | |
| 9 | Rachel David et al.[38] | 74 | Male | MPA without renal involvement | Relapse | ChAdOx1 nCoV-19 | 1st | NA | 14 | MPO | AKI | Crescentic GN | 9.97 | NA | Nil | Cyc + steroids | NA | Improved | Improved | |
| 10 | Rajib K Gupta et al.[39] | 23 | Male | Fragile-X syndrome and Interstitial Lung Disease | New onset | mRNA-1273 | 2nd | NA | 14 | MPO, Anti-GBM, ANA | AKI, microscopic haematuria, non-nephrotic range proteinuria | Crescentic GN | 14 | Yes | NA | NA | NA | NA | NA | |
| 11 | Samy Hakroush et al.[40] | 79 | Male | Hypertension, Degenerative disc disease | New onset | mRNA-BNT162b2 | 2nd | NA | 14 | MPO, ANA | Leukocyturia, microscopic Haematuria, nephrotic range proteinuria, AKI | Pauci-immune GN with myoglobin cast nephropathy | 6.57 | Yes | NA | Arthralgia | Cyc + steroids | NA | Improved | Improved |
| 12 | NattawatKlomjit et al.[41] | 82 | Female | NA | New onset | mRNA-1273 | 2nd | NA | 28 | MPO | AKI, Haematuria, Proteinuria | Crescentic GN | 3.1 | Yes | NA | Rtx + steroids | 4 | Partial response | Improved | |
| 13 | Edoardo Conticini et al.[42] | 77 | Male | MPA with renal involvement | Relapse | mRNA-BNT162b2 | 1st | NA | NA | MPO | AKI, Haematuria | NA | 1.55 | NA | GGOs with sept thickening | Steroids | NA | Improved | Improved | |
| 14 | J Prema et al.[43] | 58 | Male | Nil | New onset | BBV152 | 2nd | NA | 14 | PR3, Anti-GBM | Haemoptysis, AKI | Crescentic GN | 8.4 | NA | Alveolar haemorrhage | Cyc + PLEX + steroids | 8 | Partial response | Improved | |
| 15 | Prema et al.[43] | 45 | Male | Nil | New onset | BBV152 | 1st | NA | 12 | MPO, ANA | Haemoptysis, AKI | Crescentic GN | 9 | NA | Alveolar haemorrhage | Cyc + PLEX + steroids | 5 | Partial response | Improved | |
| 16 | Tiffany Caza et al.[44] | 76 | Male | Nil | New onset | mRNA-BNT162b2 | 2nd | NA | 11 | ANCA, ANA | AKI, Haematuria, Proteinuria | Crescentic GN | 8.6 | Nil | Nil | Rtx + steroids | 3 | Dialysis dependent | Nil | |
| 17 | Tiffany Caza et al.[44] | 81 | Female | Nil | New onset | mRNA-BNT162b2 | 2nd | NA | 2 | ANCA, ANA | AKI, haematuria, proteinuria | Crescentic GN | 3.1 | Nil | Nil | Rtx | 3 | Partial response | Nil | |
| 18 | Tiffany Caza et al.[44] | 76 | Female | Nil | New onset | mRNA-1273 | 1st | NA | 5 | ANCA, ANA | AKI, haematuria, proteinuria | Crescentic GN | 3.0 | Nil | Nil | Rtx + steroids | 5 | Partial response | Nil | |
| 19 | Tiffany Caza et al.[44] | 71 | Female | Nil | New onset | mRNA-1273 | 2nd | NA | 14 | ANCA, ANA | Haematuria, Proteinuria | Crescentic GN | 1.3 | Nil | Nil | Rtx + steroids | 1 | Nil | Nil | |
| 20 | Tiffany Caza et al.[44] | 65 | Female | Nil | New onset | mRNA-BNT162b2 | 2nd | NA | 14 | ANCA | AKI, Haematuria, proteinuria | Crescentic GN | 3.2 | Nil | Nil | Cyc + steroids | 2 | Dialysis dependent | Nil | |
| 21 | Tiffany Caza et al.[44] | 79 | Female | AAV | Relapse | mRNA-1273 | 2nd | NA | 21 | ANCA | Haematuria, Proteinuria | Crescentic GN | 1.12 | Nil | Nil | Rtx | 16 | Improved | Nil | |
| 22 | Davidovic et al.[45] | 54 | Female | Seronegative arthralgia, MPA? | New onset | mRNA-BNT162b2 | 1st and 2nd | Yes | 35 – 1st, 14—2nd | MPO | AKI, haematuria, proteinuria | Pauci-immune GN | 2.11 | Yes | Discrete opacities in radiology | Painful red eyes | Rtx + steroids | NA | Improved | Improved |
| 23 | Davidovic et al.[45] | 78 | Female | MPA | Relapse | mRNA-BNT162b2 | 2nd | NA | 2 | MPO | AKI, haematuria, proteinuria, dark urine | NA | 8.23 | Yes | Nil | Rtx + steroids | NA | Dialysis dependent | Improved | |
| 24 | Shota Obata et al.[46] | 84 | Male | CVA, colon cancer, interstitial pneumonia | New onset | mRNA-BNT162b2 | 2nd | No | 14 | MPO | Microscopic haematuria, non-nephrotic proteinuria | Pauci-immune GN | 1.22 | Yes | Worsening of interstitial pneumonia | Steroids | 8 | Improved | Improved | |
| 25 | Seif et al.[47] | 66 | Male | Hypertension, COPD, Latent TB, GCA | New Onset | mRNA-1273 | 2nd | No | 21 | MPO | Microscopic haematuria, non-nephrotic proteinuria | Crescentic GN | 2.2 | Yes | NA | Rtx + steroids | 1 | Partial response | Improved | |
| 26 | Feghali et al.[48] | 58 | male | Nil | New onset | mRNA-1273 | 2nd | No | 4 | MPO, PR-3 | AKI, Microscopic haematuria, non-nephrotic proteinuria | Crescentic GN | 4.1 | Yes | Consolidation, pleural effusion, alveolar haemorrhage | Rtx + Cyc + PLEX + steroids | 10 | Improved | Improved | |
| 27 | Chen et al.[49] | 70 | Female | UTI | New onset | mRNA-1273 | 1st | NA | 7 | MPO | AKI, Macroscopic haematuria, Nephrotic range proteinuria | Crescentic GN | 6.3 | Yes | Consolidation, GGOs | Rtx + PLEX + steroids | 3 | Partial response | Improved | |
| 28 | Rukesh Yadav et al.[50] | 54 | Female | HTN, Uterine cancer | New onset | Ad26.COV2.S | 1st | No | 12 | MPO, PR-3 | AKI, Microscopic haematuria, non-nephrotic proteinuria | Crescentic GN | 6.13 | Yes | Nil | steroids | 1 | NA | NA | |
| 29 | Index patient | 51 | Male | Nil | New onset | ChAdOx1 nCoV-19 | 1st | No | 15 | PR-3 | AKI, microscopic haematuria, nephrotic range proteinuria | Crescentic GN | 4.8 | Yes | Nil | Arthralgia | Rtx + steroids | 20 | Partial response | Improved |
AKI Acute Kidney Injury, AF Atrial Fibrillation, ANA Anti-Nuclear Antibody, ANCA Ant- Nuclear Cytoplasmic Antibody, COPD Chronic Obstructive Pulmonary Disease, CVA Cerebro-Vascular Accident, Cyc Cyclophosphamide, DM Diabetes Mellitus, GBM Glomerular Basement Membrane, GCA Giant Cell Arteritis, GGO Ground Glass Opacities, GN Glomerulonephritis, HTN Hypertension, MPA Microscopic Polyangiitis, MPO Myeloperoxidase, NA Not Available, PLEX Plasma-Exchange, PR-3 Proteinase-3, Rtx Rituximab, TB Tuberculosis, UTI Urinary Tract Infection
Vaccine
Among the eight vaccines approved to prevent SARS-CoV-2 infection by the World Health Organization, we report an association of AAV with five (2 mRNA vaccines–mRNA-1273 (Moderna) and mRNA-BNT162b2 (Pfizer-BioNTech), viral vector vaccine–ChAdOx1 nCoV-19 (Oxford AstraZeneca), Ad26.COV2.S (Johnson and Johnson) and inactivated vaccine BBV152 (Covaxin) vaccines. Most reports were secondary to mRNA vaccines (22/29), 4 with ChAdOx1 nCoV-19, 2 with BBV152 and 1 with Ad26.COV2.S vaccine. Fourteen patients had symptoms after the first dose; the remaining 15 had after the 2nd. Three patients had worsening symptoms after administering the second dose (Table 1, patient numbers 1, 3 and 22) (Fig. 1).
Fig. 1.
Search strategy algorithm
Comorbidities and ANCA status
In addition, 17 patients had prior comorbidities (05 hypertension, 02 diabetes mellitus, 03 patients interstitial lung diseases, 02 malignancies, 01 cerebrovascular accident, 01 atrial fibrillation, 01 Graves’ disease, 01 had both latent TB and Giant cell arteritis), in addition to AAV before vaccination. Twenty-four patients had a new-onset AAV. Among the ANCA subtypes, the most common association was with MPO (15 cases) ANCA alone, and four had PR-3 (4 cases) ANCA alone, 03 had dual (MPO and PR-3) positivity. One of the dual positive cases (Table 1, patient no. 6) was also on Propylthiouracil for Grave's disease and had auricular chondritis. In six cases, the ANCA subtype was not mentioned. ANA was positive besides ANCA in six cases. Prema et al. reported (Table 1, patient number 14) dual positivity for both PR-3 and GBM antibodies. Gupta et al. described a case of Fragile-X syndrome developing anti-MPO, anti-GBM antibody and ANA positivity (Table 1, patient no 10).
Symptoms
The reporting for constitutional symptoms was variable. At least 16 (55.1%) patients had constitutional symptoms at presentation. Twenty-two patients had renal involvement (93.1%) either as new-onset or recurrence of the glomerulonephritis. At least 24 (82.7%) patients had haematuria (2 among the 24 had macro-haematuria) at presentation. The median (maximum) serum creatinine was 3.82 (IQR 2.15 to 8.31) mg/dl. If we take AAV due to non- mRNA vaccines alone, the median maximum serum creatinine is 6.55 mg/dl (IQR 4.8 to 8.4). Ten patients (29.1%) had pulmonary involvement, of which 05 (17.2%) had alveolar haemorrhage (Table 1, patient numbers 7, 8, 14, 15 and 26). In addition, one patient had optic neuritis (Table 1, patient number 5), and one had auricular chondritis (Table 1, patient number 6) as the manifestation following vaccination among other organ involvement.
Relapsing disease
Five patients had relapses of AAV post-vaccination. Among them, four had prior renal involvement. All five cases had renal involvement (four had biopsy-proven crescentic glomerulonephritis). Four patients (80%) were MPO positive, and in one patient, the nature of ANCA was unavailable.
Kidney biopsies
Twenty-five patients (86.2%) underwent renal biopsies. The age (cellular, fibro cellular and fibrous), as well as the extent of crescents were variable. Twenty-one cases (84%) had crescentic glomerulonephritis (> 50% of glomeruli). In four cases (16%), the crescents were focal. Rupture of a glomerular capillary wall with periglomerular inflammation was seen in four cases (16%). Underlying tuft showed fibrinoid necrosis in 16 cases (64%). Mesangial expansion and proliferation were observed in three cases (12%). None of the biopsies reported endocapillary proliferation. Five biopsies (20%) had findings consistent with vasculitis. The information on Interstitial Fibrosis and Tubular Atrophy (IFTA) were available only in ten cases (40%). Seven (70%) and three (30%) cases had a mild and moderate degree of IFTA, respectively. Immunofluorescence findings were available for 24 cases. There were no significant deposits for immunoglobulins or complements in 18 cases (75%), fulfilling the definition criteria for pauci-immune crescentic glomerulonephritis. Linear deposits of IgG and light chains along the glomerular capillary wall were observed in three cases (Supplemental Table 1, patient numbers 10, 14 and 15). In two cases (Table 1 Patient numbers 10, 14), anti-GBM antibody titres were positive in serum, confirming coexistent Anti-GBM disease. A single patient (Supplemental Table 1, patient number 16) had 2 + intense deposits for Immunoglobulin A (IgA) in the mesangium, suggesting the diagnosis of concurrent IgA nephropathy. Similarly, another patient (Supplemental Table 1, patient number 18) had 3 + intense deposits for C3 (Complement Factor 3) in the mesangium. Furthermore, the electron microscopy showed immune complex type mesangial deposits, confirming concurrent C3 glomerulopathy. The electron microscopy findings were available in five cases. All cases had an effacement of foot processes of podocytes. The summary of renal biopsies is shown in Supplementary Table 1.
Treatment
Most patients (96.5%) received immunosuppressive therapy, including steroids, cyclophosphamide and rituximab. In addition, five patients received plasma exchange (PLEX), of whom three had a diffuse alveolar haemorrhage. At the last follow-up, at least five continued to remain dialysis-dependent.
Discussion
In the present case-based review, we highlight the clinical features and outcome of COVID-19 vaccination-induced AAV. Renal involvement is reported in over three-fourths of the cases and most patients responded favorably to immunosuppressive therapy.
Vaccine-associated autoimmunity is a well-known entity caused by cross-reactivity to antigens or adjuvants. In the current report, most vaccine-associated AAV were with mRNA vaccines. These vaccines (mRNA vaccines) may cause differential stimulation of myeloid and dendritic cells, activating the downstream pathway to produce autoinflammation [15]. mRNA vaccines have a lesser risk of infection and insertion-related mutagenesis but generate antiviral neutralising immunoglobulins and stimulate strong immune responses by activating CD8 + and CD4 + T cells [16]. Also, mRNA vaccines may cause enhanced stimulation of innate and acquired immunity compared to inactivated vaccines or natural infection [16, 17]. This new-onset autoinflammation transpires in genetically predisposed individuals; these cases with compromised immune systems have a decreased clearance of nucleic acids predisposing to Neutrophil Extracellular Traps (NETs) [18]. NETs are highly proinflammatory and provide a sustained antigenic stimulus. This NETosis is a critical step in the pathogenesis of both cytokine storms in COVID-19 infection [19] and COVID-19-triggered AAV [20]. Finally, the vaccine-induced autoimmunity associated with COVID-19-inactivated vaccines can also be related to the immune response to the SARS-CoV-2 proteins or an exaggerated response to the m RNA vaccine. Still, the exact mechanism is not fully understood. None of the patients described of COVID-19 vaccine-associated AAV had tested positive for the infection, ruling out the infective virions as the responsible triggers for the disease pathogenesis.
Glomerular diseases mentioned in association with COVID-19 vaccines include IgA nephropathy [10], podocytopathies [10], lupus nephritis [21], crescentic glomerulonephritis, anti-GBM disease [10], IgG4 disease [22], and membranous nephropathy [23]. In an observational study from Japan [24], where IgA nephropathy is more prevalent and diagnosed earlier in life than the rest of the world, gross haematuria and proteinuria on screening urine examination post-vaccination have led to the identification of recurrence and new-onset IgA nephropathy associated with COVID-19 vaccination. Thus, routine urine examination and monitoring of renal function tests after vaccination can help detect glomerular diseases in susceptible individuals. Also, most of the patients described in the current review had urine abnormalities at presentation.
The global incidence and prevalence of AAV varies from 0.4 to 24 cases per million-person-years and 300 to 421 cases per million population, respectively [25]. The involvement of the kidney in AAV varies from 54 to 97% in various studies [26, 27]. The kidney involvement in the current series is comparable to the prior reports. Kidneys followed by lungs are the commonly affected organs in COVID-19 vaccine-associated AAV. Patients who developed AAV post-vaccination responded favorably to immunosuppressive therapy. Thus, based on the current review, we recommend immunosuppressive treatment to all patients with vaccine-mediated glomerulonephritis. However, researchers need to be mindful of rituximab treated patients responding poorly to further booster doses of COVID-19 vaccination, as a susceptible population [28].
Historically, most vaccine trials fail to address the vaccine-associated autoimmunity because of the variable manifestations and long latency between the inoculation and symptomatology [29]. Also, during the earlier phase of vaccination, which was during the pandemic, worldwide lockdowns with restricted access to healthcare may account for reduced reporting of adverse events post-vaccination. Fortunately, increased patient awareness of post-vaccine symptoms, along with a decline in the infection rates, may have resulted in a surge in reporting of vaccine-related side effects.
Undoubtedly, both mass vaccination for protection against COVID-19 and heightened awareness for detecting autoimmune diseases provide the ideal platform to prevent COVID-19 infection and study the association of autoimmune diseases with vaccines. In addition, it may pave the way for studying undiagnosed pathogenic mechanisms and newer treatment options in autoimmune diseases. Kadkhoda et al. [30] reported that 57% of patients developed ANCA positivity post-COVID-19 infection. The aforementioned study is also a proof of concept for expeditious seroconversion as they tested most of the samples during hospital admission.
A temporal correlation of symptoms to vaccination forms the basis of the report; whether it equals causation considering the limited number of cases reported is debatable, short duration of follow-up, inadequate clinical and pathological data and lack of uniformity in immunosuppressive treatment are limitations to the study. To conclude, post-COVID-19 vaccination-associated AAV is rare. Grand scale vaccination against SARS-CoV-2 infection provides a suitable platform to observe and learn the association of AAV with vaccination. The number of autoimmune glomerular diseases associated with COVID-19 infection conspicuously outweighs the number of cases post-COVID-19 vaccination. Henceforth, the otherwise healthy population and individuals with autoimmune diseases in remission and on immunosuppressive therapy should be encouraged to continue with the vaccination with close monitoring of symptoms.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Figure 1: Shows fibrinoid necrosis (F) and an early crescent formation(C) (H&E × 200) (JPG 370 KB)
Funding
Nil.
Declarations
Ethical approval
No ethical clearance required as it is a case report and case-based review.
Consent for publication
Consent for publication taken from the patient for the case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arun Prabhahar, G. S. R. S. N. K. Naidu, Prabhat Chauhan, and Aravind Sekar are joint first authors.
References
- 1.Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41:509–518. doi: 10.1007/s00296-021-04792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelzo M, Cacciapuoti S, Pinchera B, et al. A transient increase in the serum ancas in patients with sars-cov-2 infection: a signal of subclinical vasculitis or an epiphenomenon with no clinical manifestations? a pilot study. Viruses. 2021;13:1–8. doi: 10.3390/v13091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta M, Weaver DF. COVID-19 as a trigger of brain autoimmunity. ACS Chem Neurosci. 2021;12:2558–2561. doi: 10.1021/acschemneuro.1c00403. [DOI] [PubMed] [Google Scholar]
- 4.Gounder AP, Boon ACM. Influenza pathogenesis: the effect of host factors on severity of disease. J Immunol. 2019;202:341–350. doi: 10.4049/jimmunol.1801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33:155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boekel L, Kummer LY, van Dam KPJ, et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3:e542–e545. doi: 10.1016/S2665-9913(21)00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27:1744–1751. doi: 10.1038/s41591-021-01469-5. [DOI] [PubMed] [Google Scholar]
- 9.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1–4. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bomback AS, KudoseVDD ST. de novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? AJKD. 2021 doi: 10.1053/j.ajkd.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott J, Hartnett J, Mockler D, Little MA. Environmental risk factors associated with ANCA associated vasculitis: a systematic mapping review. Autoimmun Rev. 2020;19:102660. doi: 10.1016/j.autrev.2020.102660. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T. Vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 2017;13:188–196. doi: 10.2174/1573397113666170517155443. [DOI] [PubMed] [Google Scholar]
- 13.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/nejmc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh M, Merkel PA, Peh C-A, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382:622–631. doi: 10.1056/nejmoa1803537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol. 2021 doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Kream RM, Stefano GB. An evidence based perspective on mRNA-SARScov-2 vaccine development. Med Sci Monit. 2020;26:1–8. doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int. 2021;100:959–965. doi: 10.1016/j.kint.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelka K, Shibata T, Miyake K, Latz E. Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol Rev. 2016;269:60–75. doi: 10.1111/imr.12375. [DOI] [PubMed] [Google Scholar]
- 19.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217:1–7. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izci Duran T, Turkmen E, Dilek M, et al. ANCA-associated vasculitis after COVID-19. Rheumatol Int. 2021;41:1523–1529. doi: 10.1007/s00296-021-04914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, et al. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021 doi: 10.1016/j.kint.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masset C, Kervella D, Kandel-Aznar C, et al. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int. 2021;100:465–466. doi: 10.1016/j.kint.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gueguen L, Loheac C, Saidani N, Khatchatourian L. Membranous nephropathy following anti–COVID-19 mRNA vaccination. Kidney Int. 2021;100:1140–1141. doi: 10.1016/j.kint.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaki K, Aoki R, Nihei Y, et al. Gross hematuria after SARS-CoV-2 vaccination: questionnaire survey in Japan. Clin Exp Nephrol. 2021 doi: 10.1007/s10157-021-02157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitching AR, Anders HJ, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Prim. 2020 doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 26.Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol. 2017;12:1680–1691. doi: 10.2215/CJN.02500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathi M, Pinto B, Dhooria A, et al. Impact of renal involvement on survival in ANCA-associated vasculitis. Int Urol Nephrol. 2016;48:1477–1482. doi: 10.1007/s11255-016-1330-z. [DOI] [PubMed] [Google Scholar]
- 28.Kronbichler A, Anders H-J, Fernandez-Juárez GM, et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol Dial Transplant. 2021;36:1160–1168. doi: 10.1093/ndt/gfab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guimarães LE, Baker B, Perricone C, Shoenfeld Y. Vaccines, adjuvants and autoimmunity. Pharmacol Res. 2015;100:190–209. doi: 10.1016/j.phrs.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadkhoda K, Laurita K. Antineutrophil cytoplasmic antibodies and their association with clinical outcomes in hospitalized COVID-19 patients. Cell Death Discov. 2021;7:19–21. doi: 10.1038/s41420-021-00671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderegg MA, Liu M, Saganas C, et al. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021;100:474–476. doi: 10.1016/j.kint.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekar A, Ruth Campbell JT, P R. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021 doi: 10.1016/j.kint.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;2:19–21. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dube GK, Benvenuto LJ, Batal I. Antineutrophil cytoplasmic autoantibody-associated glomerulonephritis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int Rep. 2021 doi: 10.1016/j.ekir.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takenaka T, Matsuzaki M, Fujiwara S, et al. myeloperoxidase anti-neutrophil cytoplasmic antibody positive optic perineuritis after mrna coronavirus disease-19 vaccine: a case report. QJM An Int J Med. 2021 doi: 10.1093/qjmed/hcab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuda S, Hirooka Y, Sugiyama M. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis after covid-19 vaccination. Vaccines. 2021 doi: 10.3390/vaccines9080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villa M, Díaz-Crespo F, Pérez de José A, et al. A case of ANCA-associated vasculitis after AZD1222 (Oxford–AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int. 2021;1222:1–2. doi: 10.1016/j.kint.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David R, Hanna P, Kenneth Lee AR. Relapsed ANCA associated vasculitis following Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination : a case series of two patients. Nephrology. 2021 doi: 10.1111/nep.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta RK, Ellis BK. Concurrent anti-GBM nephritis and ANCA-mediated glomerulonephritis after second dose of SARS-CoV-2 mRNA vaccination. Kidney Int Rep. 2021 doi: 10.1016/j.ekir.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakroush S, Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol. 2021 doi: 10.3389/fimmu.2021.762006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klomjit N, Alexander MP, Fervenza FC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021 doi: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conticini E, d’Alessandro M, Bergantini L, et al. Relapse of microscopic polyangiitis after vaccination against COVID-19: a case report. J Med Virol. 2021;93:6439–6441. doi: 10.1002/jmv.27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prema J, Muthukumaran A, Haridas N, et al. Two cases of double-positive antineutrophil cytoplasmic autoantibody and antiglomerular basement membrane disease after BBV152/Covaxin vaccination. Kidney Int Rep. 2021 doi: 10.1016/j.ekir.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caza TN, Cassol CA, Messias N, et al. Glomerular disease in temporal association to SARS-CoV-2 vaccination - a series of 29 cases. Kidney360. 2021 doi: 10.34067/kid.0005372021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidovic T, Schimpf J, Sprenger-Mähr H, et al. De novo and relapsing glomerulonephritis following SARS-CoV-2 mRNA vaccination in microscopic polyangiitis. Case Rep Nephrol. 2021;2021:1–5. doi: 10.1155/2021/8400842. [DOI] [Google Scholar]
- 46.Obata S, Hidaka S, Yamano M, Yanai M, Kunihiro Ishioka SK. MPO-ANCA-associated vasculitis after the Pfizer/BioNTech SARS-CoV-2 vaccination Shota. Clin Kidney J. 2021 doi: 10.1093/ckj/sfab181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.N Seif, Ellis, Carla L, Wadhwani S (2021) ANCA-associated glomerulonephritis and vasculitis following COVID-19 vaccination in a patient with giant cell arteritis. Kidney Week Abstr. Abstract: PO0101
- 48.Feghali EJ, Zafar M, Abid S, et al. De-Novo Antineutrophil Cytoplasmic Antibody- Associated Vasculitis Following the mRNA-1273 ( Moderna ) Vaccine for COVID-19. Cureus. 2021;3:3–7. doi: 10.7759/cureus.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C-C, Chen H-Y, Lu C-C, Lin S-H. Case report: anti-neutrophil cytoplasmic antibody-associated vasculitis with acute renal failure and pulmonary hemorrhage may occur after COVID-19 vaccination. Front Med. 2021;8:1–6. doi: 10.3389/fmed.2021.765447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav R, Shah S, Chhetri S. ANCA-associated vasculitis following Johnson and Johnson COVID-19 vaccine. Authorea. 2021 doi: 10.22541/au.163578863.32575474/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Shows fibrinoid necrosis (F) and an early crescent formation(C) (H&E × 200) (JPG 370 KB)