Abstract
Cauda equina intradural tumors commonly reported include ependymoma, schwannoma, neurofibroma, meningioma, and drop metastasis. Hemangioblastoma of the neural axis is a rare benign vascular tumor comprising only 1.6 to 6.4% of spinal tumors, and are usually associated with Von-Hippel Lindau disease. Sporadic intradural extramedullary hemangioblastoma involving cauda equina is very rare with only countable reports, and the presence of peritumoral cyst has been reported only once. We report one such case of hemangioblastoma with a large peritumoral cyst, which was diagnosed radiologically and confirmed by histopathology following surgical excision. Pertinent radiological characteristics, diagnostic clues, treatment, and surgical outcomes are discussed.
Keywords: cauda equina tumors, cystic lesion, hemangioblastoma, intradural tumors
Introduction
Sporadic intradural extramedullary hemangioblastoma involving cauda equina is very rare with only countable reports, and the presence of peritumoral cyst has been reported only once. We report one such case of hemangioblastoma with a large peritumoral cyst, which was diagnosed radiologically and confirmed by histopathology following surgical excision. Pertinent radiological characteristics, diagnostic clues, treatment, and surgical outcomes are discussed.
Case Report
A 52-year-old female presented with gradually progressive bilateral radiculopathy (8 months duration) and claudication distance of 100 m. There were no signs of neurological dysfunction, except for diminished knee and ankle jerks. With no symptoms of low backache or any other red flag signs, the initial clinical diagnosis considered was degenerative lumbar stenosis.
Radiological Findings
Plain radiography ( Fig. 1A ) was not contributory. Magnetic resonance imaging (MRI) revealed an elongated intradural extramedullary lesion (predominantly cystic in nature) extending from the level of L1 lower end plate up to L3 vertebra. A solid intramural homogeneous nodule measuring 1.6 × 1.6 cm was present at the level of the mid-L3 vertebral body ( Fig. 1B ). The cystic component was seen on either side of the solid mural nodule and was slightly hyperintense to cerebrospinal fluid (CSF) ( Fig. 2 ). The cauda equina nerve roots were compressed and displaced to the periphery. The conus medullaris was distorted with splaying and clumping of the nerve roots. Few prominent flow voids were also noted in the region of the conus. Postcontrast images showed intense homogeneous enhancement of the mural nodule without cyst wall enhancement. There was contrast blush at the tip of the conus, most likely due to secondary tortuous vessels at the tip of the tumor ( Fig. 3 ). Screening of the whole spine and brain along with the ultrasound abdomen showed no other neoplasms.
Fig. 1.
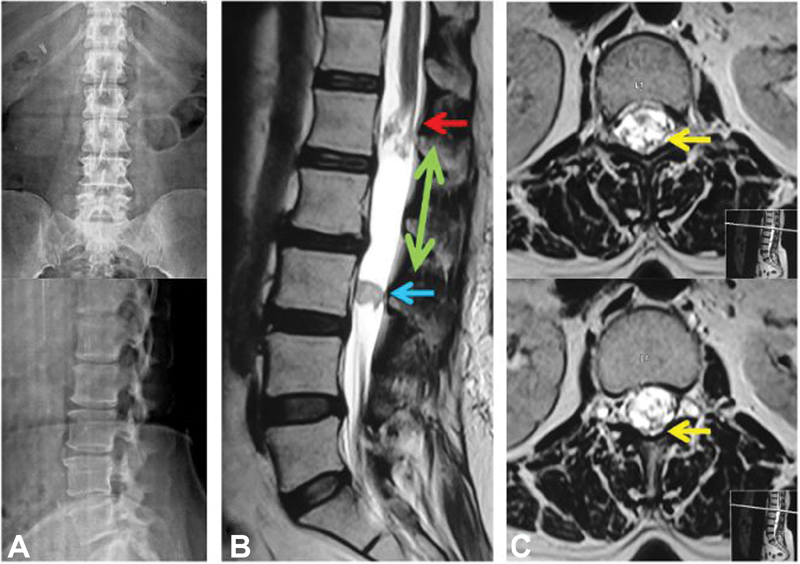
( A ) Plain radiography revealed lumbar spondylotic changes in the form of anterior disc osteophytes at L3–L4 and L4–L5 level with no evidence of instability. ( B ) Sagittal T2-weighted image shows an elongated predominantly cystic (green arrow) intradural-extramedullary lesion extending from the lower endplate of L1 to L3 vertebra. There is a solid intramural homogeneous nodule measuring 1.6 × 1.6 cm (blue arrow) at the level of the mid-L3 vertebral body. Conus is distorted with splaying and clumping of the nerve roots (red arrow) ( C ) Axial T2-weighted images showing a few prominent flow voids at the level of conus (yellow arrows).
Fig. 2.
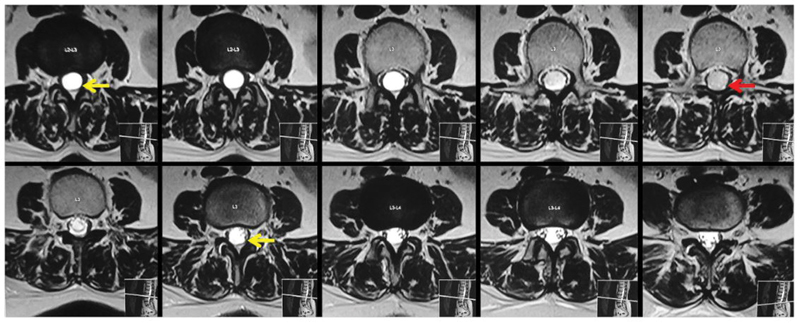
Axial T2-weighted images show the cystic component (yellow arrows) on either side of the solid mural nodule (red arrow), which is slightly hyperintense to cerebrospinal fluid. The cauda equina nerve roots are compressed and displaced to the periphery.
Fig. 3.
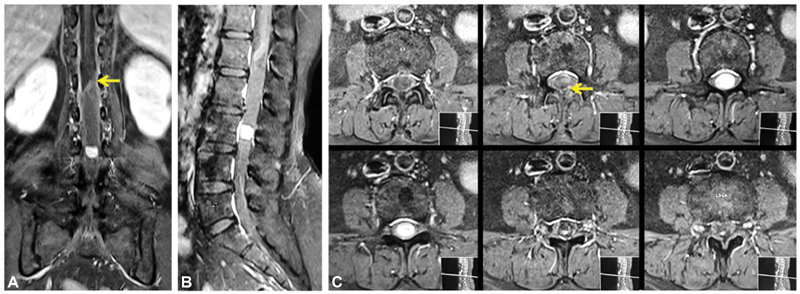
( A ) Postcontrast coronal and ( B ) sagittal image showing intense homogeneous enhancement of the mural nodule without cyst wall enhancement. ( C ) The contrast blush (yellow arrows) at the tip of the distorted conus is likely due to tortuous vessels at the tip of the tumor.
Surgical Details and Intraoperative Images
By a standard posterior approach, laminectomy was performed at two levels. Durotomy was performed, and the tumor mass, along with the cyst, was delineated. A reddish-gray mass of 1.5 × 1.5 cm size was seen ( Fig. 4 ) with a leash of vessels on the surface as well as on either side of the tumor. Following the achievement of complete hemostasis, the dural repair was performed with 6–0 Prolene. The surgical wound was closed in layers, and the postoperative period was uneventful. She remained neurologically intact with a complete recovery from her preoperative symptoms, and there has been no recurrence till the last follow-up (49 months).
Fig. 4.
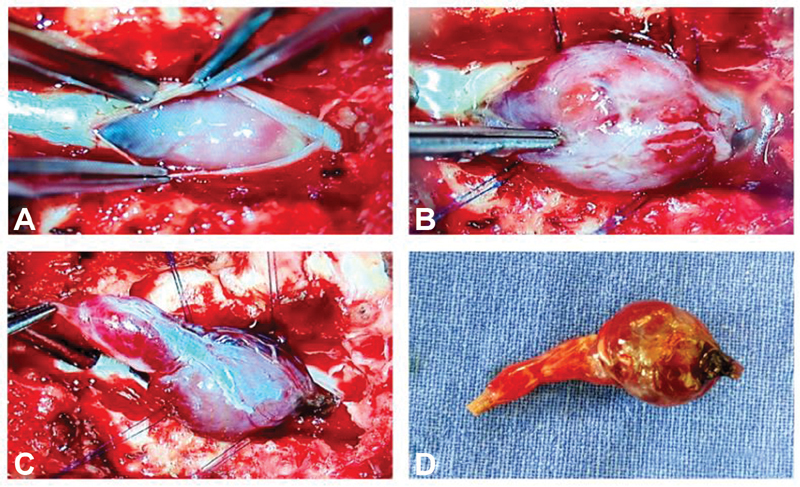
( A ) Postdurotomy surgical microscope picture before delineating the tumor. ( B ) Large tumor bulging out from the canal. ( C ) Both solid and cystic components of the tumor are very well-appreciable with a leash of vessels on the surface as well as at the poles of the tumor after delineating the tumor margins and ( D ) postexcision lesion appears shrunken in size due to decongestion.
Histology
The tumor had a striking vascular pattern with a network of blood distended capillaries interspersed with epithelioid stromal cells. Foamy cytoplasm, pleomorphic nuclei, and occasional mitoses were also noted. The stromal cells showed the tell-tale fat-filled vacuoles, characteristic of hemangioblastoma ( Fig. 5 ).
Fig. 5.
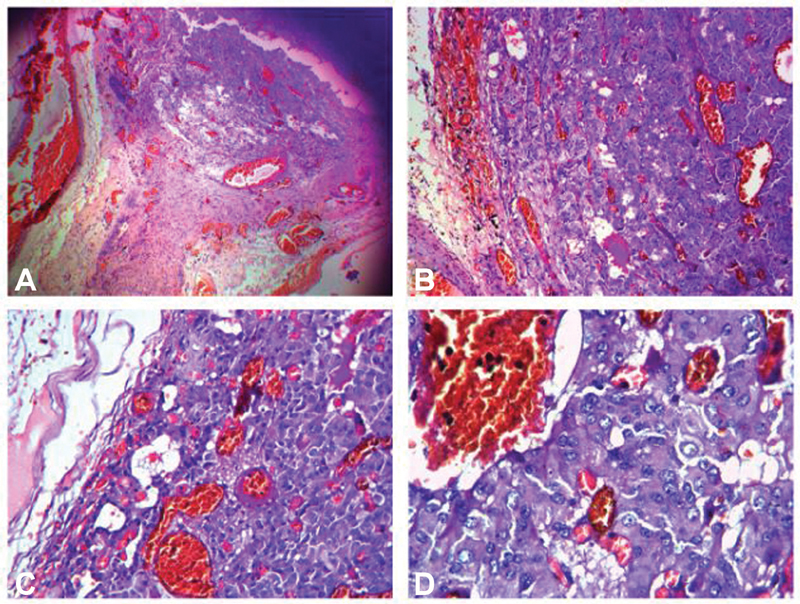
Histopathological pictures (hematoxylin and eosin × 100). ( A ) Striking vascular pattern seen with a network of blood distended capillaries interspersed with epithelioid stromal cells. ( B ) and ( C ) Stromal cells showing pleomorphic nuclei with occasional mitoses and ( D ) the tell-tale fat vacuoles.
Discussion
Epidemiology
Cauda equina tumors are rare, constituting only 10% of all spinal canal tumors, and the most common lesions are ependymoma, schwannoma, neurofibroma, meningioma, and drop metastasis. 1 Sporadic spinal hemangioblastomas are rare benign vascular tumors comprising 1.6 to 6.4% of spinal tumors. 2 3 Intradural extramedullary hemangioblastomas involving cauda equina are uncommon, with only 28 cases reported so far. 4 The presence of an associated peritumoral cyst has been reported only once, and one such case of hemangioblastoma with a large cystic component was diagnosed by MRI and entirely excised surgically. 5 We discuss pertinent literature on differentiating these rare tumors from other common tumors.
Two-thirds of spinal hemangioblastomas are sporadic, and others occur as a part of multisystem involvement in Von Hippel-Lindau disease. The majority of hemangioblastomas are intramedullary and mainly involve the thoracic and cervical cord with a frequency of 51.2 and 41.2%, respectively. 6 Intradural extramedullary lesions are rare, and there are only 28 cases reported so far, with patients ranging from 25 to 82 years. All these cases had solid enhancing or heterogeneous lesions, 16 of which originated from filum terminale. 7 In our case, the lesion was located in the cauda equina. Large tortuous vascular loops were noted in almost all except for the cases reported by Ciappetta et al and Sergides et al. 8 9
Hemangioblastomas being benign vascular neoplasms with no malignant potential are classified as World Health Organization (WHO) grade-I tumor. 10 Although hemangioblastomas are known to originate from the endothelial cells, the pathogenesis behind their sporadic development in extramedullary location is unknown. 11 In patients with VHL, germline mutations of the VHL tumor-suppressor gene located on the distal part of the short arm of chromosome 3 is known to be responsible. This regulates vascular endothelial growth factor, which explains the highly vascular nature of hemangioblastoma. 12 Patients with intradural extramedullary lesions of the lumbar canal may remain asymptomatic when small in size. Larger lesions cause compression of the conus or nerve roots resulting in symptoms ranging from low back pain, radicular symptoms, or cauda equina syndrome.
Diagnostic Features
The diagnosis of extramedullary hemangioblastoma is mainly arrived by MRI and spinal angiography. Lesions may have variable appearances in MRI depending on the size and location. Smaller lesions are solid in nature and show uniform T1 isointensity and T2 iso to hyperintensity. Larger lesions generally show heterogeneous intensity. The key imaging findings of hemangioblastomas are intense contrast enhancement and meningeal varicosities in the form of flow voids within as well as adjacent to the lesion. 13 Our case had similar characteristics with small homogeneous components that were homogeneously hyperintense on T2-weighted images. Intense enhancement was observed on contrast administration. Very few meningeal varicosities were noted at the proximal pole.
Sixty percent of cerebellar hemangioblastoma presents as cysts with mural nodule. 14 However, the peritumoral cyst in association with a hemangioblastoma at the level of cauda equina is extremely rare. 5 In our case, a sizeable peritumoral cyst extended from the level of conus to L3 vertebral level. Though the cyst did not show any enhancement, there was only minimal blush at the proximal pole, which could be secondary to meningeal varicosities. There was also a mild distortion of the conus morphology due to the cyst. The interesting findings of note were that we could confidently delineate the cystic component of the lesion because it showed a relative increase in hyperintensity as compared with adjacent CSF on T2-weighted images. This is likely because of absence of CSF flow artifact within the cyst, unlike mild inhomogeneity within the free-flowing CSF. Empty thecal sac due to peripherally displaced nerve roots is another finding that prompted us to detect the cystic component.
Close differentials for a purely cystic lesion that has CSF intensity in all the MRI sequences include cystic schwannoma, arachnoid cyst, and neuroenteric cyst. Arachnoid cyst is a purely cystic lesion without any enhancing component and is most common in the dorsal spine. Neuroenteric cyst is also a nonenhancing lesion, more common in thoracic spine having predisposition toward the ventral location. Association with vertebral anomalies like anterior spina bifida is a hallmark of these lesions. 15 Schwannomas are mostly solid or heterogeneous solid lesions; however, degeneration of the Antoni B portion of the tumor may result in cyst formation. Central ischemic necrosis also causes cystic areas within these lesions and may be responsible for a sudden increase in the size and symptoms of patients. Since degeneration/ischemic necrosis results in cystic component within the lesion, there will be rim enhancement around the cystic part on contrast administration. 16 17 18 Nonenhancing cystic component with a solid mural nodule showing intense enhancement is a hallmark feature of hemangioblastoma, as seen in our case.
Spinal angiogram plays a crucial role in the preoperative embolization of hemangioblastomas when there is evidence of extensive tumor varicosities. It also helps in differentiating spinal arteriovenous fistula and hemangioblastomas. 19 We could confidently identify hemangioblastoma in this case and did not recommend spinal angiogram since the amount of detectable tortuous vessels on MRI was minimal. Complete surgical resection of symptomatic tumors is the treatment of choice, and the tumor was removed in our case. In patients who are not surgical candidates, Zussman et al recommended intensity-modulated radiation therapy (IMRT) to reduce the size of the lesion and halt further progression. 20 Microsurgery can also be combined with IMRT for further resection as well as for precise targeting of the lesion, avoiding the cord.
There are three critical factors to be considered when diagnosing conus hemangioblastoma. First, the rare incidence in this location can lead to a misdiagnosis, such as a meningioma, schwannoma, or ependymoma. It is crucial to look for key imaging findings and derive the correct diagnosis and alert the surgeon about this highly vascular tumor for safe and effective surgical intervention. Second, if there are significant varicosities in MRI, spinal angiography may be essential to demarcate draining vein and feeding arteries. During surgery, the main draining vein should not be interrupted before all feeding arteries are occluded to avoid hemorrhage or/and neural damage, which can then result in incomplete removal of the lesion. Lastly, when hemangioblastoma is detected in such a location, it is crucial to perform whole spine MRI and screening of the brain in the same sitting. This helps to categorize the patient in to syndromic versus sporadic and also to manage accordingly. Our patient had a screening MRI of the brain and the whole spine along with an ultrasound abdomen, which was negative, indicating its sporadic nature.
We have discussed a rare case of intradural extramedullary hemangioblastoma in the lumbar canal associated with a large peritumoral cyst, emphasizing the importance of preoperative diagnosis in the optimal management of these vascular and rare tumors.
Conclusion
Intradural extramedullary sporadic hemangioblastoma is a very rare benign tumor. Key imaging features like intense enhancement of the lesion and tortuous vessels should always be looked for accurate diagnosis and prompt the surgeon about these vascular tumors. More importantly, having a clear idea of differentiating other cystic lesions provides invaluable clues to the diagnosis. Complete surgical resection was possible in our case without embolization since there were no extensive prominent flow voids associated with the lesion.
Conflicts of Interest There are no conflicts of interest.
Financial Support and Sponsorship
Ganga Orthopedic Research and Education Foundation.
References
- 1.Blaty D, Malos M, Palmrose T, McGirr S. Sporadic intradural extramedullary hemangioblastoma of the cauda equina: case report and literature review. World Neurosurg. 2018;109:436–441. doi: 10.1016/j.wneu.2017.10.104. [DOI] [PubMed] [Google Scholar]
- 2.Chu B-C, Terae S, Hida K, Furukawa M, Abe S, Miyasaka K. MR findings in spinal hemangioblastoma: correlation with symptoms and with angiographic and surgical findings. AJNR Am J Neuroradiol. 2001;22(01):206–217. [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa E, Matsumura A, Matsumuru Y, Anno I.Proximal nerve root spinal hemangioblastomas: presentation of three cases, MR appearance, and literature review Surg Neurol 20046203278–279., author reply 279 [DOI] [PubMed] [Google Scholar]
- 4.Sporadic hemangioblastoma of cauda equina: An atypical case reportIn: Surg. Neurol. Int. https://surgicalneurologyint.com/surgicalint-articles/sporadic-hemangioblastoma-of-cauda-equina-an-atypical-case-report/. Accessed November 19, 2021 [DOI] [PMC free article] [PubMed]
- 5.Sporadic hemangioblastoma of the film terminale with peritumoral cystIn: Surg. Neurol. Int. https://surgicalneurologyint.com/surgicalint-articles/sporadic-hemangioblastoma-of-the-film-terminale-with-peritumoral-cyst/. Accessed November 19, 2021 [DOI] [PMC free article] [PubMed]
- 6.Browne T R, Adams R D, Roberson G H. Hemangioblastoma of the spinal cord. Review and report of five cases. Arch Neurol. 1976;33(06):435–441. doi: 10.1001/archneur.1976.00500060041009. [DOI] [PubMed] [Google Scholar]
- 7.Gade P S, Velho V, Naik H, Bhople L. Sporadic hemangioblastoma of the filum terminale: case report and review of literature. Romanian Neurosurg. 2018;32:496–501. [Google Scholar]
- 8.Ciappetta P, Occhiogrosso G, Domenicucci M, D'Andrea G, Bastianello S, Frati A. Hemangioblastoma of the filum terminale. Case report and review of the literature. J Exp Clin Cancer Res. 2007;26(02):281–285. [PubMed] [Google Scholar]
- 9.Sergides I G, Wainwright K L, Biggs M. Incidental hemangioblastoma of the filum terminale. Acta Neurol Belg. 2009;109(01):55–56. [PubMed] [Google Scholar]
- 10.Fuller G N. The WHO classification of tumours of the central nervous system. 4th Edition. Arch Pathol Lab Med. 2008;132:906. doi: 10.5858/2008-132-906-TWCOTO. [DOI] [PubMed] [Google Scholar]
- 11.Lee J H, Kim J U, Paeng S S, Jang J S, Lee S H. Solitary hemangioblastoma at the filum terminale: a case report and review of literature. Korean J Spine. 2011;8:125–128. [Google Scholar]
- 12.Biondi A, Ricciardi G K, Faillot T, Capelle L, Van Effenterre R, Chiras J. Hemangioblastomas of the lower spinal region: report of four cases with preoperative embolization and review of the literature. AJNR Am J Neuroradiol. 2005;26(04):936–945. [PMC free article] [PubMed] [Google Scholar]
- 13.Baker K B, Moran C J, Wippold F J, II et al. MR imaging of spinal hemangioblastoma. AJR Am J Roentgenol. 2000;174(02):377–382. doi: 10.2214/ajr.174.2.1740377. [DOI] [PubMed] [Google Scholar]
- 14.Cyst With a Mural Nodule Tumor of the Brain - PubMed https://pubmed.ncbi.nlm.nih.gov/22935908/. Accessed November 19, 2021
- 15.Savardekar A, Singla N, Mohindra S, Ahuja C K, Gupta S K. Cystic spinal schwannomas: a short series of six cases. Can we predict them preoperatively? Surg Neurol Int. 2014;5 07:S349–S353. doi: 10.4103/2152-7806.139666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmar H, Patkar D, Gadani S, Shah J. Cystic lumbar nerve sheath tumours: MRI features in five patients. Australas Radiol. 2001;45(02):123–127. doi: 10.1046/j.1440-1673.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 17.Netra R, Hui M S, Gang M Z, Ming Z. Spinal cystic schwannoma: an MRI evaluation. J Coll Physicians Surg Pak. 2014;24(02):145–147. [PubMed] [Google Scholar]
- 18.Totally Cystic Intradural Schwannoma in Thoracic Region - PubMed https://pubmed.ncbi.nlm.nih.gov/28413556/. Accessed November 19, 2021 [DOI] [PMC free article] [PubMed]
- 19.Brisman J L, Borges L F, Ogilvy C S. Extramedullary hemangioblastoma of the conus medullaris. Acta Neurochir (Wien) 2000;142(09):1059–1062. doi: 10.1007/s007010070063. [DOI] [PubMed] [Google Scholar]
- 20.Zussman B, Penn D, Jeyamohan S, Werner-Wasik M, Andrews M DDW, Harrop J. Multi-modality management of an intradural-extramedullary hemangioblastoma: a case report. JHN J. 2011;6 doi: 10.29046/JHNJ.006.1.007. [DOI] [Google Scholar]


