Abstract
Recently, the methodology that will serve as a basis of the standard for antifungal susceptibility testing of fermentative yeasts of the European Committee on Antibiotic Susceptibility Testing has been described. This procedure employs a spectrophotometric method for both inoculum adjustment and endpoint determination. However, the utilization of a spectrophotometer requires studies for standardization. The present work analyzes the following parameters: (i) accuracy of inoculum preparation, (ii) correlation between optical density and CFU per milliliter, (iii) influence of the wavelength on the endpoint determination, and (iv) influence of the dimethyl sulfoxide concentration on the growth kinetics. The main results can be summarized as follows: (i) inoculum preparation following the methodology recommended by the National Committee for Clinical Laboratory Standards is an exact procedure; (ii) the relationship between optical density and CFU per milliliter is linear (coefficient of determination, r2 = 0.84); (iii) MICs obtained by means of spectrophotometric readings at different wavelengths are identical (for amphotericin B, an intraclass correlation coefficient of 0.98 was obtained; for fluconazole, the intraclass correlation coefficient was 1); and (iv) a 2% concentration of dimethyl sulfoxide produces a significantly slower and lower growth curve of Candida spp. than other concentrations.
The standardization process of antifungal susceptibility testing is in progress. The Subcommittee for Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standard (NCCLS) has published an approved standard for yeasts (document M27A) (2). In Europe, another organization, the European Committee on Antibiotic Susceptibility Testing, has a Subcommittee for Antifungal Susceptibility Testing (EUCAST-AFST) that is working on this matter. Recently, the methodology that will serve as a basis of EUCAST-AFST's proposed standard was described (1). The NCCLS document M27-A recommends the spectrophotometric method for inoculum preparation and visual reading for endpoint determination (2). The visual reading is an important source of variability and inaccuracy due to the trailing phenomenon, which is caused by the partial inhibition of fungal growth that is observed with fungistatic agents. The proposed EUCAST-AFST methodology will also recommend a spectrophotometric method for inoculum preparation, and the reading of the microtitration plates will be performed by means of a spectrophotometer in order to overcome the limitations of visual reading. However, information regarding the use of these devices for antifungal susceptibility testing is limited and some variables could have an influence on both inoculum preparation and endpoint determination.
The aim of the present work was to study several parameters implicated in the use of spectrophotometers and related to antifungal susceptibility testing. Briefly, in a first set of experiments the accuracy of inoculum preparation by the spectrophotometric procedure was evaluated. Secondly, we analyzed the correlation between the optical density obtained by spectrophotometric reading and the CFU per milliliter that the microbiological cultures yielded. This experiment was designed to evaluate the limitations of spectrophotometrical procedures when especially dense or light inocula are utilized. In a third set, we assessed the influence on the endpoint determination of the wavelength employed in the experiment. In addition, we employed a spectrophotometric method to examine the influence of dimethyl sulfoxide (DMSO) on the growth kinetics of yeasts. DMSO is the solvent which is usually employed when stock solutions of antifungal agents are prepared. The details of these analyses and their implications are the subject of this work.
MATERIALS AND METHODS
Strains.
Two panels of strains were used in the experiments: (i) a reference panel formed of Candida parapsilosis ATCC 22109 (2), Candida krusei ATCC 6258 (2), Candida tropicalis MY1012 (3), Candida albicans ATCC 64550 (5), C. albicans ATCC 64548 (5), Candida glabrata ATCC 90030 (2), Candida lusitaniae CL 2819 (3), and Saccharomyces cerevisiae ATCC 9763 and (ii) a clinical panel constituted of 60 clinical strains. The majority of these isolates (n = 44) were obtained from blood cultures, and the remainder were obtained from specimens from deep sites. Isolates were identified by routine microbiological techniques and were maintained at −70°C. The clinical panel was composed of 10 isolates of each of the following species: C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, C. krusei, and C. lusitaniae. In some experiments all strains were included. In others, a selected sample from the panels was used.
Assay media.
RPMI–2% glucose is RPMI 1640 medium without sodium bicarbonate and with l-glutamine (Oxoid, Madrid, Spain). It was buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid and supplemented with 18 g of glucose per liter to reach a final concentration of 2%. Media were prepared as a double-strength solution and sterilized by filtration.
Accuracy of inoculum preparation. (i) Strains.
In this experiment the entire clinical panel, which comprised 60 isolates, was used.
(ii) Inoculum preparation.
The yeast isolates were grown on Sabouraud dextrose agar (Oxoid) for 24 h at 35°C. The inoculum preparation followed the directions of document M27-A of the NCCLS (2). Thus, the optical density (OD) of a 0.5 McFarland standard (Izasa, Madrid, Spain) at 530 nm (OD530) was measured five times on different days. The range obtained was between 0.11 and 0.14. Therefore, a suspension of each of the yeasts in sterile distilled water was adjusted in a Bausch & Lomb spectrophotometer (Pacisa S.A., Madrid, Spain) to that OD530 range. Then, this adjusted suspension was diluted 1:10, 1:100, and 1:1,000 in sterile distilled water. A 50-μl aliquot of each one of these dilutions was delivered through Autoplate 4000 (Spiral Biotech, Inc., Bethesda, Md.) onto Sabouraud agar. The next day the colony enumeration was performed by means of an automated colony counter (CIA-BEN 2.0; Spiral Biotech, Inc.).
Correlation between the OD and CFU per milliliter. (i) Strains.
In this experiment 30 strains from the clinical panel were used, 5 each of the following species: C. albicans, C. tropicalis, C. parapsilosis, C. glabrata, C. krusei, and C. lusitaniae. Testing of twelve strains was repeated twice.
(ii) Preparation of Candida suspensions.
For each of the strains 14 suspensions in sterile distilled water were prepared in a Bausch & Lomb single-beam spectrophotometer (SPEC A) (light path, 20 nm; Spectronic 20D; Pacisa S.A.). The suspensions were adjusted as closely as possible to the following OD530s: 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3, and 1.4. From each of these suspensions appropriate dilutions in sterile distilled water were prepared. Fifty microliters was delivered with the Autoplate 4000 (Spiral Biotech, Inc.) onto Sabouraud agar. The next day the colony enumeration was performed by means of an automated colony counter (CIA-BEN 2.0; Spiral Biotech, Inc.). In addition, 200 μl of each suspension was deposited into the wells of a 96-well flat-bottom microtitration plate in order to perform a new measurement of OD but using a microdilution procedure. The OD530 of each of the wells was recorded in a spectrophotometer for microtitration plate reading (SPEC B) (light path, 18 ± 2 nm; Dinex MRX II; Cultek, Madrid, Spain).
Influence of the wavelength on the MIC endpoint estimation. (i) Strains.
The same panel employed in the experiment regarding the correlation between OD and CFU/ml was used. In addition, C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were used as quality control strains.
(ii) AST.
Antifungal susceptibility testing (AST) was performed following the proposed standard of EUCAST (1). Two antifungals were used, amphotericin B (Fluka, S.A., Madrid, Spain) and fluconazole (Pfizer S.A., Madrid, Spain). Stock solutions were prepared in 100% DMSO (Sigma-Aldrich Química, Madrid, Spain) at concentrations 100 times the highest concentration to be tested and were frozen at −70°C until used. Sterile plastic microtitration plates containing flat-bottom wells were utilized. The plates contained twofold serial dilutions of the antifungal drugs and two drug-free-medium wells for sterility and growth controls, and each well was inoculated with 100 μl of the final inoculum. Each isolate was subcultured on Sabouraud dextrose agar plates at 35°C for 24 h prior to testing. The final quantity of inoculum was 0.5 × 105 to 2.5 × 105 CFU/μl. The microtitration plates were incubated at 35°C for 24 h in a humid atmosphere.
The MICs were determined at 24 h spectrophotometrically at the following wavelengths: 340, 450, 530, and 690 nm. For amphotericin B, the endpoint was defined as the minimal antifungal concentration that exerts 90% growth inhibition compared with the control well growth. For fluconazole, the endpoint was defined as the minimal antifungal concentration that exhibited 50% growth inhibition compared with the control well growth. The OD of the blank was subtracted from the OD of all wells. The MICs were compared to ascertain if they were equivalent.
Influence of DMSO concentration on the growth of yeasts. (i) Strains.
For this experiment the following strains were included: C. parapsilosis ATCC 22109, C. krusei ATCC 6258, C. tropicalis MY1012, C. albicans ATCC 64550, C. albicans ATCC 64548, C. glabrata ATCC 90030, C. lusitaniae 2819, and S. cerevisiae ATCC 9763.
(ii) GKs.
In the next set of experiments, we measured growth kinetics (GKs). The GKs of the eight strains were determined by the microdilution format in RPMI-2% glucose containing three different concentrations of DMSO: 2, 1, and 0.5%. A control growth medium without DMSO was used in all experiments. The final yeast inoculum was between 0.5 × 105 and 2.5 × 105 CFU/ml. Trays were inoculated (200 μl per well) and sealed with a gas permeable sealing membrane for microtitration plates (Breathe Easy Membrane; Sigma-Aldrich Quimica). The microplates were incubated for 48 h at 35°C inside a Labsystems IEMS Reader MFplate (Labsystems, Madrid, Spain). The reader carried out an hourly spectrophotometric reading at a wavelength of 540 nm. The hourly spectrophotometric readings were saved and analyzed with the software package Ascent Research Edition, version 2.1 (Labsystems). Curves were constructed with the help of the Sigmaplot (version 5.0) graphs package (SPSS S.L., Madrid, Spain).
Statistical analysis.
The significance of the differences in the GKs and AST between methodologies was determined by the Student t test (unpaired; unequal variance) or by the Mann-Whitney U test. Differences in proportions were determined by Fisher's exact test or by chi-square analysis. A P value of <0.01 was considered significant.
The OD in the drug-free well must be >0.2 to calculate the spectrophotometric MICs. The mean of the absorbance of eight sterility control wells was subtracted from the absorbance value obtained from each well, and then spectrophotometric MICs were calculated. The reproducibility of the results for each reading at a different wavelength was evaluated by an intraclass correlation coefficient (ICC), which compared the MICs obtained at four different wavelengths (340, 450, 530, and 690 nm). Reproducibility was calculated by means of a scales analysis, where reliability was the extent to which endpoint determinations yielded the same MICs over time. The ICC assesses reliability as an internal consistency statistic by means of interitem correlations. A two-way mixed effect model was utilized to calculate the ICC that was expressed over a maximum value of 1 and with a confidence interval of 95% (1). All statistical analyses were done with the Statistical Package for the Social Sciences (SPSS, version 10; SPSS S.L.).
RESULTS
Accuracy of inoculum preparation.
Table 1 shows the results obtained with inoculum preparations. Suspensions adjusted to OD530s ranging from 0.119 to 0.140 produced inocula containing 1 × 106 to 6.2 × 106 CFU/ml. The dilutions 1:10, 1:100, and 1:1,000 contained a number of CFU per milliliter inside the expected values (Table 1). The 99% confidence interval for the CFU per milliliter is very narrow, indicating the precision of this procedure.
TABLE 1.
Colony counts of inoculum preparation of 60 clinical isolates of Candida spp.a
| Measure | OD530 | CFU/ml in a serial dilution of:
|
||
|---|---|---|---|---|
| 1:10 | 1:100 | 1:1,000 | ||
| Mean | 0.128 | 2.2 × 105 | 2.9 × 104 | 3.5 × 103 |
| SD | 0.007 | 9.6 × 104 | 1.2 × 104 | 1.6 × 103 |
| Range | 0.119–0.140 | 1.0 × 105–6.2 × 105 | 1.1 × 104–6.5 × 104 | 6.1 × 102–7.1 × 103 |
| CI, 95%b | 0.126–0.130 | 1.9 × 105–2.4 × 105 | 2.6 × 104–3.2 × 104 | 3.1 × 103–3.9 × 103 |
| CI, 99% | 0.126–0.131 | 1.89 × 105–2.5 × 105 | 2.5 × 104–3.3 × 104 | 3.1 × 103–4.1 × 103 |
Preparation followed the spectrophotometric methodology recommended by document M27A of the NCCLS (2).
CI, confidence interval.
Correlation between the OD and CFU per milliliter.
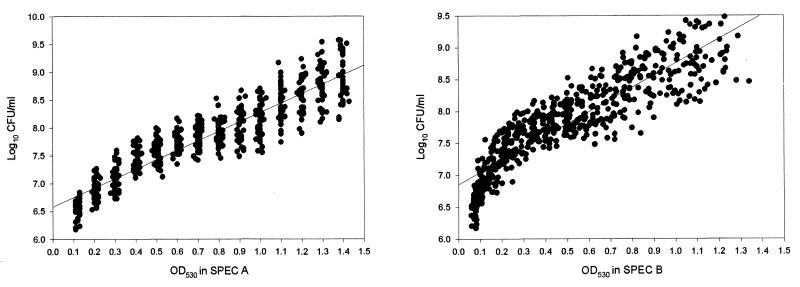
A Bausch & Lomb spectrophotometer was employed for the adjustment of the inoculum suspension (SPEC A). Then, from each suspension 200 μl was deposited in the corresponding wells of a flat-bottom microtitration plate and the OD530 was measured by a spectrophotometer designed for this procedure (SPEC B). In Table 2, the equivalence among the OD530s detected by both spectrophotometers and the corresponding CFU per milliliter for all species together is shown. The values in SPEC B were somewhat lower than those in SPEC A. The different volumes used (5 versus 0.2 ml) can explain the different OD values obtained. The correlation between OD values in SPEC A and SPEC B was excellent (coefficient of determination [r2] = 0.96). A range of OD530s of 0.11 to 1.43 produced suspensions containing 1.48 × 106 CFU/ml to 3.72 × 109 CFU/ml (Table 2). The relationship was linear with an r2 of 0.84 (Table 3 and Fig. 1). The equation of this linear regression was as follows: log10 CFU/ml = 6.57 + 1.69 OD530 (SPEC A).
TABLE 2.
Equivalence among the OD530s of SPEC A and SPEC B and colony counts
| Range of OD530 in:
|
Range of CFU/ml | |
|---|---|---|
| SPEC A | SPEC B | |
| 0.11–0.13 | 0.06–0.09 | 1.48 × 106–6.89 × 106 |
| 0.19–0.23 | 0.09–0.15 | 3.37 × 106–1.83 × 107 |
| 0.28–0.32 | 0.13–0.25 | 5.31 × 106–3.94 × 107 |
| 0.39–0.43 | 0.12–0.29 | 1.26 × 107–6.49 × 107 |
| 0.48–0.53 | 0.23–0.34 | 1.31 × 106–9.77 × 107 |
| 0.58–0.62 | 0.30–0.41 | 2.21 × 107–1.45 × 108 |
| 0.68–0.72 | 0.36–0.53 | 2.61 × 107–1.63 × 108 |
| 0.78–0.83 | 0.45–0.63 | 2.81 × 107–3.34 × 108 |
| 0.89–0.93 | 0.50–0.72 | 3.02 × 107–4.63 × 108 |
| 0.98–1.03 | 0.59–0.84 | 3.59 × 107–5.00 × 108 |
| 1.09–1.12 | 0.72–0.98 | 5.46 × 107–1.46 × 109 |
| 1.18–1.22 | 0.81–1.07 | 7.89 × 107–1.71 × 109 |
| 1.28–1.32 | 0.97–1.21 | 1.26 × 108–3.46 × 109 |
| 1.38–1.43 | 1.05–1.34 | 1.39 × 108–3.72 × 109 |
TABLE 3.
Coefficients of determination and coefficients of the linear regression equation log10 CFU/ml = log10 Y0 + aOD530 for Candida spp.a
| Species | SPEC A
|
SPEC B
|
||||
|---|---|---|---|---|---|---|
| Log10Y0 | a | r2 | Log10Y0 | a | r2 | |
| C. albicans | 6.56 | 1.41 | 0.81 | 6.79 | 1.57 | 0.76 |
| C. parapsilosis | 6.58 | 1.67 | 0.86 | 6.80 | 1.86 | 0.79 |
| C. tropicalis | 6.48 | 1.76 | 0.85 | 6.80 | 2.02 | 0.80 |
| C. glabrata | 6.71 | 1.58 | 0.85 | 6.98 | 1.78 | 0.81 |
| C. krusei | 6.54 | 1.69 | 0.89 | 6.81 | 1.89 | 0.83 |
| C. lusitaniae | 6.63 | 1.89 | 0.92 | 6.92 | 2.14 | 0.89 |
| All species | 6.57 | 1.69 | 0.84 | 6.85 | 1.9 | 0.79 |
a, coefficient of linear regression; r2, coefficient of determination.
FIG. 1.
Linear regressions obtained between log10 CFU/ml counting and OD530 measured in a single-beam spectrophotometer (SPEC A) and a device for microdilution procedures (SPEC B). Graphs were constructed with data from 30 isolates. (Left panel) The linear regression equation is log10 CFU/ml = 6.57 + 1.69 OD530 (SPEC A); the coefficient of determination is r2 = 0.84. (Right panel) The linear regression equation is log10 CFU/ml = 6.85 + 1.90 OD530 (SPEC B); the coefficient of determination is r2 = 0.79.
Similar values were obtained for SPEC B. Thus, the r2 was 0.79 and the formula of the equation was log10 CFU/ml = 6.85 + 1.90 OD530 (SPEC B) (Table 3 and Fig. 1). Table 3 also displays the r2 and the coefficients of the linear regression equation for each Candida species analyzed. For SPEC A, all r2 values were ≥0.81, and for SPEC B they were ≥0.79.
Influence of the wavelength in the MIC endpoint estimation.
Thirty isolates plus two quality control strains were employed in this experiment. Readings for quality control strains were repeated five times on different days. The influence of MICs for amphotericin B and fluconazole read at different wavelengths was analyzed. Table 4 shows the range of MICs for each species and antifungal agent. For fluconazole, the MICs were identical irrespective of the wavelength used (ICC = 1). For amphotericin B, MICs were identical for 27 strains. In two isolates a variation of one twofold dilution was obtained, and in one strain the range observed was 0.06 to 0.25 μg/ml. Despite this fact, the ICC value was 0.98 with a 95% confidence interval of 0.97 to 0.99.
TABLE 4.
Range of MICs read at different wavelengths for amphotericin B and fluconazole for 30 strains of Candida spp.
| Antifungal agent and species | Range of MICs at wavelength (nm):
|
|||
|---|---|---|---|---|
| 340 | 450 | 530 | 690 | |
| Ampho B | ||||
| C. albicans | 0.25–1.0 | 0.12–1.0 | 0.25–1.0 | 0.06–1.0 |
| C. tropicalis | 0.25–1.0 | 0.25–1.0 | 0.25–1.0 | 0.25–1.0 |
| C. parapsilosis | 0.12–1.0 | 0.12–1.0 | 0.12–1.0 | 0.12–1.0 |
| C. glabrata | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 | 0.5–1.0 |
| C. krusei | 0.06–1.0 | 0.06–1.0 | 0.06–1.0 | 0.06–1.0 |
| C. lusitaniae | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 |
| Fluconazole | ||||
| C. albicans | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 |
| C. tropicalis | 0.5–2.0 | 0.5–2.0 | 0.5–2.0 | 0.5–2.0 |
| C. parapsilosis | 0.5–2.0 | 0.5–2.0 | 0.5–2.0 | 0.5–2.0 |
| C. glabrata | 4.0–16.0 | 4.0–16.0 | 4.0–16.0 | 4.0–16.0 |
| C. krusei | 32.0–128.0 | 32.0–128.0 | 32.0–128.0 | 32.0–128.0 |
| C. lusitaniae | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 | 0.25–0.5 |
Influence of DMSO concentration on the growth of yeasts.
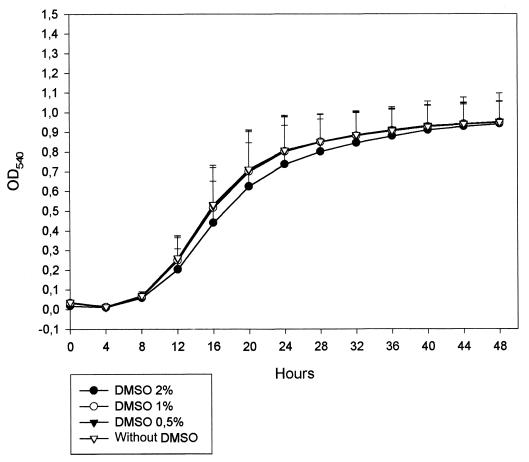
Figure 2 displays the GKs of eight ATCC strains in RPMI-2% glucose at different DMSO concentrations. The kinetics of growth are similar for all DMSO concentrations except at 2%. This concentration produced a significantly slower and lower growth curve in all species tested.
FIG. 2.
Growth curve of eight ATCC yeast isolates at different concentrations of DMSO.
DISCUSSION
The calculation of endpoint by spectrophotometric procedures presents some advantages over visual reading. The OD values stored in the computer make data analysis and endpoint reading truly quantitative, more flexible, and objective. Thus, a dose-response curve can be displayed for each isolate tested in addition to the MIC endpoint. In addition, the MIC obtained by visual determination is immovable; therefore the in vitro-in vivo correlations applied for obtaining breakpoints are managed by a fixed value. OD data stored in a computer can be calculated following different approaches, taking into consideration the clinical outcome of the patient infected by this particular strain. A clear example of this fact is shown in two recent works (4, 6). Rex et al. (4) showed that isolates with trailing growth in response to fluconazole could be identified as resistant by the NCCLS methodology. However, if the MIC was determined at 24 h and the endpoint required a less restrictive criterion (50% reduction in growth instead of 80%), MICs were better matched with in vivo response. This work was performed in a murine model of invasive candidosis, and many of the MICs were determined spectrophotometrically. The data stored in the computer allowed different calculations without the need for further testing. In addition, the dose-response curves shown in this work added a better interpretation of the animal model outcome (4). Warn et al. (6), following a similar reading approach, obtained similar conclusions and better chances to interpret the susceptibility testing results. In addition, the spectrophotometer was also used for inoculum adjustment (1, 2). However, this device can be a source of variability in results and thus a standardization process is mandatory. In the present work, information about parameters influencing AST methodology has been obtained. Inoculum adjustment following the recommendations of the NCCLS document M27-A is an exact procedure. Table 1 shows the OD530 that can be used for preparation of the inoculum suspensions. Confidence intervals for 95 and 99% indicated the precision of this methodology.
There is controversy about the equivalence among different OD530 values and CFU per milliliter. Our data show that SPEC A and SPEC B could not detect counts of CFU per milliliter of less than 106. However, above this number of CFU per milliliter there was a linear relationship between OD530 and numbers of CFU per milliliter (r2 = 0.84 for SPEC A, and r2 = 0.79 for SPEC B [Table 3 and Fig. 1]). Two linear regression equations were obtained and they could be used for calculating the number of CFU per milliliter at any OD530 between 0.11 and 1.43 for SPEC A and between 0.06 and 1.34 for SPEC B (Table 3).
Another point of discussion is the wavelength designed for reading the microtitration plates. The rational approach is to use the same wavelength as that employed for adjusting the inoculum. Unfortunately, a 530-nm filter is unusual and normally must be especially ordered. This fact raised the question of whether the use of different filters produces different MICs. The antifungal agents chosen represent two different ways of endpoint determination. Thus, a 90% inhibition coefficient was the endpoint for amphotericin B whereas a 50% inhibition coefficient was the endpoint for fluconazole. The results indicated that wavelength does not have any influence on the MIC. For amphotericin B the ICC was 0.98, and for fluconazole it was 1. In conclusion, any wavelength can be used for reading microtitration plates. Shorter wavelengths produce a higher OD for a blank well, and longer wavelengths produce the opposite. Therefore, the OD of the blank control well must be subtracted from all inoculated wells.
Finally, a 2% concentration of DMSO produced slower growth of all isolates tested. Lower concentrations did not have any significant influence. The NCCLS and EUCAST-AFST methodologies use 0.5% as the final concentration of DMSO. Although 1% could be used, it is recommended to employ the lower concentration that maintains the hydrophobic antifungal drugs dissolved.
In summary, this work complements a previous one (1). In that work, a proposition of a semiautomated methodology was described. This technique will be the basis of the standard proposition of EUCAST-AFST. In the present study, several variables have been analyzed and standardized. Useful information for a better understanding of the use of spectrophotometers as an aid for antifungal susceptibility testing is achieved.
ACKNOWLEDGMENTS
This work was supported in part by research project 99/1199 from the Instituto de Salud Carlos III. T. M. Díaz-Guerra is a fellow of the Instituto de Salud Carlos III (grant 99/4149).
REFERENCES
- 1.Cuenca-Estrella M, Diaz-Guerra T M, Mellado E, Rodriguez-Tudela J L. Influence of glucose supplementation and inoculum size on the growth kinetics and antifungal susceptibility testing of Candida spp. J Clin Microbiol. 2001;39:525–532. doi: 10.1128/JCM.39.2.525-532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 3.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Tudela J L, Berenguer J, Martinez Suarez J V, Sanchez R. Comparison of a spectrophotometric microdilution method with RPMI-2% glucose with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrob Agents Chemother. 1996;40:1998–2003. doi: 10.1128/aac.40.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warn P A, Morrissey J, Moore C B, Denning D W. In vivo activity of amphotericin B lipid complex in immunocompromised mice against fluconazole-resistant or fluconazole-susceptible Candida tropicalis. Antimicrob Agents Chemother. 2000;44:2664–2671. doi: 10.1128/aac.44.10.2664-2671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]