Abstract
Background Hypocalcemia is a rare reversible cause of dilated cardiomyopathy in pediatric population. Myocarditis is another more frequent cause of cardiomyopathy with overlapping presenting features. Cardiac magnetic resonance imaging (CMRI) is a vital modality capable of tissue characterization for the evaluation of cardiomyopathy. The present study is the first attempt to determine if any specific characteristics on CMR exist in patients with hypocalcemic dilated cardiomyopathy.
Methods A retrospective analysis of 10 cases of hypocalcemic dilated cardiomyopathy (August 2012–August 2019), among which CMRI of nine patients were analyzed. Patients were categorized in to three categories; category 1 defined as absence of edema and late gadolinium enhancement (LGE), category 2 having edema only, and category 3 with presence of both edema and LGE. A diagnosis of myocarditis was considered if both edema and LGE were present.
Results The mean age of the cohort was 5.5 ± 3.3 months. The mean ejection fraction of the cohort was 20.5 ± 6.85% that improved significantly to 35.22 ± 9.3% at the time of discharge. Five of nine patients had no edema or LGE (category 1), whereas two patients each were categorized into category 2 and 3. All cases in category 1 had normalized ventricular function on follow-up. One patient in category 2 had normal ejection fraction and one was lost to follow-up. Out of the two patients in category 3, there was one mortality and another was lost to follow-up. Of the six patients at follow-up (19 ± 11.0 months), the mean left ventricle ejection fraction improved to 56.5 ± 6.1%.
Conclusion Hypocalcemic dilated cardiomyopathy has a favorable outcome on rapid initiation of treatment. CMR can be utilized for further prognostication of these patients. Absence of edema and LGE predicts a good outcome, whereas presence of LGE and/or edema either indicates a worse prognosis or an underlying coexistent myocarditis warranting an early myocardial biopsy.
Keywords: CMR, dilated cardiomyopathy, hypocalcemia, myocardial dysfunction, myocarditis, pediatric
Introduction
Dilated cardiomyopathy is the most common form of cardiomyopathy in pediatric population, with an annual incidence of 1.13/100,000 infants and children. 1 It is defined as a form of cardiomyopathy wherein the ventricular chambers exhibit increased diastolic and systolic volume and a low (< 40%) ejection fraction. 2 3 Dilated cardiomyopathy is usually idiopathic but may arise secondary to infections, metabolic, or genetic causes. One of the rare causes of dilated cardiomyopathy in pediatric age group is hypocalcemia. 4 5 Calcium plays a pivotal role in myocardial excitation–contraction coupling, and hypocalcemia causes reduced contractility and conduction delays. 5 6 Long-standing hypocalcemia leads to myocardial dysfunction and cardiac dilatation. 6 Manifestation of hypocalcemia-induced dilated cardiomyopathy is usually acute in onset with features of congestive heart failure, 5 shock, 7 and/or neurological symptoms. Cardiac magnetic resonance imaging (CMRI) in recent times has become an important noninvasive modality that is capable of tissue characterization and is now being increasingly used for diagnosis of myocarditis. 8 Myocarditis is one of the more frequent causes of cardiomyopathy in pediatric age group and the clinical picture of both myocarditis and hypocalcemia-induced left ventricular (LV) dysfunction overlap. We attempt to determine if CMR shows any unique features among patient with hypocalcemia-induced dilated cardiomyopathy and whether it can help in prognosticating this rare subset of patients.
Materials and Methods
This is a retrospective observational study from August 2012 to August 2019. Data were retrieved from medical database. Demographic data including age, sex, weight, and height were recorded. Relevant history including the duration of illness, feeding habits, and past medical history was also recorded. Descriptive statistics were used to define demographic and baseline variables. Statistical analyses were performed using the SPSS statistical software program for Windows version 25. Continuous data was presented as mean ± standard deviation or median. Categorical data was presented as absolute numbers and percentages.
Patients were investigated as per cardiomyopathy protocol at our institute that included electrocardiogram, blood investigations, echocardiography, and CMR. Hypocalcemic dilated cardiomyopathy was diagnosed on the basis of biochemical parameters (low serum ionized and total calcium) associated with features of dilated cardiomyopathy on clinical examination, and echocardiography. CMR was performed under sedation on Achieva Philips 1.5 Tesla scanner using myocarditis protocol in pediatric patients. Pediatric patients require higher temporal resolution and spatial resolution due to their faster heart rate and small sized heart and blood vessels, respectively. As higher heart rate leads to image blurring that affects accurate ventricular volume and flow measurements, reducing the number of views to be acquired per segment and minimizing repetition time during each cardiac cycle in retrospective sequences such as balanced steady-state free precession imaging (SSFP) were done to improve the temporal resolution. Free breathing cine sequences were used in sedated patients and breadth hold cine sequences in intubated patients. Due to small size of the heart and blood vessels, slice thickness was reduced to 3 to 5 mm. The field of view was also reduced. Multiple signal averages were employed to maintain a high signal:noise ratio. To compensate for respiratory motion artifacts, averaging techniques or a respiratory navigator were applied.
Myocarditis protocol consisted of breath-hold cine images using balanced SSFP, edema-sensitive, black-blood T2-weighted short tau inversion-recovery sequences. Late gadolinium enhancement (LGE) sequences were obtained; ∼10 minutes after the contrast injection (0.10–0.12 mmol/kg gadolinium-diethylenetriamine pentaacetic acid (Magnevist; Bayer-Schering Pharma AG, Berlin, Germany). CMR acquisition was done in four chamber, vertical long axis, and short-axis view. CMR evaluation was done in terms of functional parameters, edema, and for the presence of necrosis or scar. Visual assessment was used to review images for the presence of edema and LGE. To assess the presence of edema, mean signal intensity of the myocardial region of interest (ROI) was compared with the mean signal intensity of the ROI on the skeletal muscle of the same slice, and the ratio >2 was accepted as edema positivity. LGE-positive areas were assessed by the visual examination for the presence of high signal intensity in a nonischemic distribution pattern. The CMR diagnosis of myocarditis was considered if the edema and LGE were present (2 out of 2 criteria). 9 As hyperemia imaging (pre- and postgadolinium T1 TSE sequence) is not done in infants due to poor quality imaging, not all parameters of Lake Louise criteria were taken into account. Early gadolinium enhancement (EGE) is considered useful by many experts; yet, in most centers, it is not being routinely used because of inconsistent image quality 8 and also a recent study by Chu et al indicated that removing EGE from the original Lake Louise Criteria does not significantly reduce diagnostic accuracy for myocarditis, although the positive likelihood ratio may be slightly lowered. 10
CMR findings were reviewed by two different operators from same institutions (KK and MB). Both the operators were blinded to the etiology of LV dysfunction. CMR findings were interpreted by consensus among CMR consultants. Based on edema and LGE, patients were categorized in to three categories, namely category 1 (no edema and no LGE), category 2 (only edema and no LGE), and category 3 (both edema and LGE present) ( Table 1 ).
Table 1. CMRI and follow-up.
| S. No | Age | ECHO (admission) | ECHO (follow-up) | CMRI findings | CMRI category | |||
|---|---|---|---|---|---|---|---|---|
| Edema | LGE | Effusion | Additional | |||||
| 1 | 1 mo | 20% | 50% | No | No | No | 1 | |
| 2 | 4 mo | 20% | 60% | No | No | No | 1 | |
| 3 | 4 mo | 30% | 67% | Yes (multifocal and transmural) | No | Yes | 2 | |
| 4 | 9 mo | 30% | 55% | No | No | No | 1 | |
| 5 | 7 mo | 25% | Expired | Yes (diffuse global) | Yes (septal, mid myocardial) | No | 3 | |
| 6 | 12 mo | 10% | —- | Yes (diffuse global) | Yes (transmural along anterior wall and anteroseptal region basal and mid cavity) | Yes | Ascites | 3 |
| 7 | 3 mo | 15% | — | No Images | No Images | No Images | No images | No Images |
| 8 | 4 mo | 15% | 55% | No | No | Yes | LVNC | 1 |
| 9 | 6 mo | 15% | — | Yes (multifocal transmural basal anterior and inferolateral) | No | No | 2 | |
| 10 | 2 mo | 15% | 52% | No | No | yes | 1 | |
Abbreviations: CMRI, cardiac magnetic resonance imaging; ECHO, echocardiography; LGE, late gadolinium enhancement; LVNC, left ventricle noncompaction.
Results
Baseline demographics and investigation of the cohort are depicted in Table 2 . The mean age of the cohort was 5.5 ± 3.3 months (median: 4 months) with equal sex distribution. All infants were referred from primary care centers to our institute and were admitted with features of congestive heart failure. The duration of symptoms ranged from 2 to 15 days (mean duration 8 days). There was cardiomegaly (cardiothoracic ratio > 55% on chest X-ray) in all cases. Electrocardiogram showed sinus tachycardia with normal QRS axis for age and prolonged corrected QT interval (QTc) with a mean of 511 ± 52 millisecondsec (median: 500 millisecondsec). Blood cultures and viral serology were negative in all the cases. Blood investigations revealed severe hypocalcemia (ionized calcium 0.78 ± 0.11 mmol/dL and total calcium 7.3 ± 1.1 mg/dL) and raised serum parathyroid hormone (183.2 ± 127 pg/dL) (secondary hyperparathyroidism). Serum vitamin D levels (25-hydroxy vitamin D) were low in all the cases (mean = 6.0 ng/mL). Thyroid function test was normal in all the cases except one case. On echocardiography, there was no structural heart defect in the study group except one patient who had a small fossa ovalis atrial septal defect. There was severe LV systolic dysfunction among the cohort with mean ejection fraction of 20.5 ± 6.85% (median: 20%), reduced fractional shortening (mean: 17 ± 6.9, median: 15%), and dilated LV with median LV diastolic dimension Z score +5.1 (range +3.6- +7).
Table 2. Demographic variables.
| S. No | Variables | Patient values | Normal values |
|---|---|---|---|
| 1 | Age (mo) | 5.5 ± 3.3 (median = 4) | |
| 2 | Weight (kg) | 5.97 ± 1.15 (median = 5.75) | |
| 3 | LVEF % (admission) | 20.5 ± 6.85 (median = 20) | 50–60% |
| 4 | Ionized serum calcium (mmol/dL) | 0.78 ± 0.11 (median = 0.8) | 1.1–1.3 |
| 5 | Total serum calcium (mg/dL) | 7.3 ± 1.1 (median = 7.5) | 9.6–10.6 |
| 6 | Serum magnesium (mg/dL) | 1.9 ± 0.5 (median = 1.8) | 1.7–2.2 |
| 7 | Serum phosphorus | 4.4 ± 0.76 (median = 4.3) | 3.4–4.5 |
| 8 | Serum parathormone (pg/mL) | 183.2 ± 127 (median = 137.5) | 10–65 |
| 9 | Serum alkaline phosphatase (IU/dL) | 414 ± 296 (median = 327) | 50–100 |
| 10 | QTc (msec) | 511 ± 52 (median = 500) | <440 |
| 11 | LVEF % (follow-up) | 50.1 ± 12 (median = 50) | 50–60% |
Abbreviations: LVEF, left ventricle ejection fraction; QTc, corrected QT interval.
As this was a retrospective observational study, no ethical clearance was sought.
Clinical Course
Antiheart failure therapy including digoxin, frusemide, and angiotensin-converting enzyme inhibitor was started in all patient. Intravenous calcium (10% calcium gluconate) was started as continuous intravenous infusion (dose 100–200 mg/kg/day) till serum calcium normalized, after which it was switched to oral calcium in the similar dose. High-dose therapy was given for vitamin D deficiency (60,000 IU per week for 5 weeks) that was followed by oral maintenance dose (400 IU/d). Serum ionized calcium normalized within 48 to 72 hours in all of the cases. There was decreasing trend in QTc along with improvement in total serum calcium levels and both these parameters normalized at the time of discharge. Serial echocardiography revealed improvement in ventricular function with LV ejection fraction increasing to 35.22 ± 9.3% (median: 35%) at the time of discharge. There was one in hospital mortality. This was a 7-month-old female infant admitted with congestive heart failure. She developed narrow complex tachycardia after admission that soon converted to ventricular tachycardia, which was managed with direct current cardioversion. She then went into bradycardia for which temporary pacing was started; however, she could not be resuscitated even after prolonged active cardiopulmonary resuscitation. Rest all cases were discharged home in stable condition. Three cases were lost to follow-up. The mean ventricular ejection fraction and shortening fraction (mean left ventricle ejection fraction: 56.5 ± 6.1%, median: 55%) had improved significantly at a mean follow-up duration of the study was 19 ± 11.0 months (median: 18 months) ( Table 1 ).
CMR Findings
CMR was done in all 10 patients with hypocalcemia-induced dilated cardiomyopathy; however, CMRI of one patient could not be retrieved from database and hence nine patients were analyzed. All nine cases had reduced ejection fraction, global hypokinesia, and dilated LV. Ejection fraction was 6 to 16% in 6/9 patients and 22 to 27% in 3/9 cases. As per categorization, five patients were in category 1 (i.e., no edema and no LGE), whereas there were two patients each in category 2 (i.e., edema only) and category 3 (both edema and LGE) ( Table 1 ). Additional finding of LV noncompaction was also seen in one of the patients in category 1 and two patients had pericardial effusion in category 1 and one each in category 2 and 3. In category 1 (5/9), follow-up was available in all patients and there was complete recovery in terms of ventricular function ( Table 1 ). Of the patients in category 2, edema was seen in LV wall that was patchy and multifocal in nature but there was no LGE ( Fig. 1A and 1B ). In this category, one patient showed complete recovery and one patient was lost to follow-up. In category 3 (2/9), CMR showed diffuse and global LV wall edema with LGE of LV myocardium. One patient in this category (patient 5) had mid-myocardial linear septal enhancement consistent with dilated cardiomyopathy ( Fig. 2A and 2B ). This patient succumbed 18 hours after admission and could not be revived. Another patient (patient 6) in category 3 had diffuse LV wall edema and subepicardial to mid-wall LGE along anterior wall and anteroseptal region extending from basal region to midcavity region ( Fig. 3A and 3B ). CMR findings were consistent with acute myocarditis in this patient. This patient was lost to follow-up, albeit at the time of discharge there was no significant improvement in ventricular function. Both these patients were labeled as myocarditis based on CMR findings of edema and pattern of LGE enhancement with coexistent hypocalcemia. In all patients, there was low clinical suspicion of myocarditis and viral maker evaluation did not reveal any evidence of viral myocarditis. Endomyocardial biopsy was not performed in any patient.
Fig. 1.
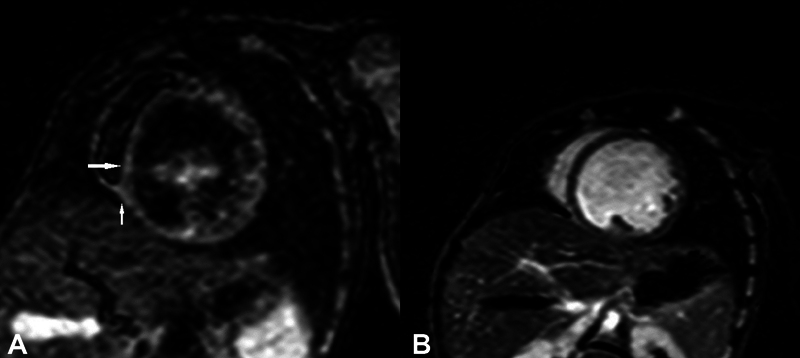
( A ) Short-axis (SA) view showing patchy myocardial edema in inferoseptal region on T2 short tau inversion-recovery images (white arrows). ( B ) SA view showing no corresponding late gadolinium enhancement on phase-sensitive inversion recovery images.
Fig. 2.
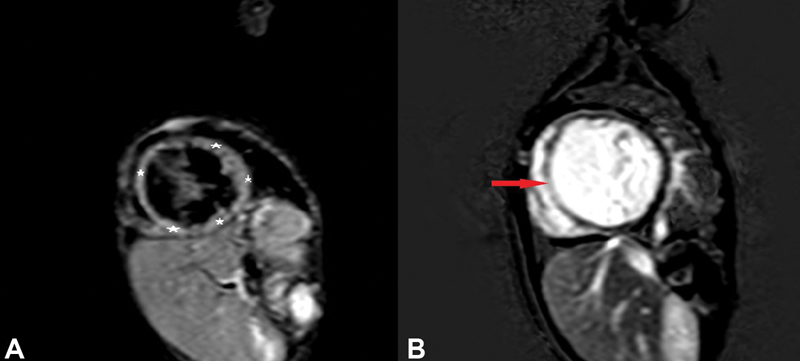
( A ) Short-axis view showing diffuse left ventricle (LV) wall edema in midcavity LV walls on T2 short tau inversion-recovery images. ( B ) Short-axis view showing transmural late gadolinium enhancement in midcavity interventricular septum (red arrow).
Fig. 3.
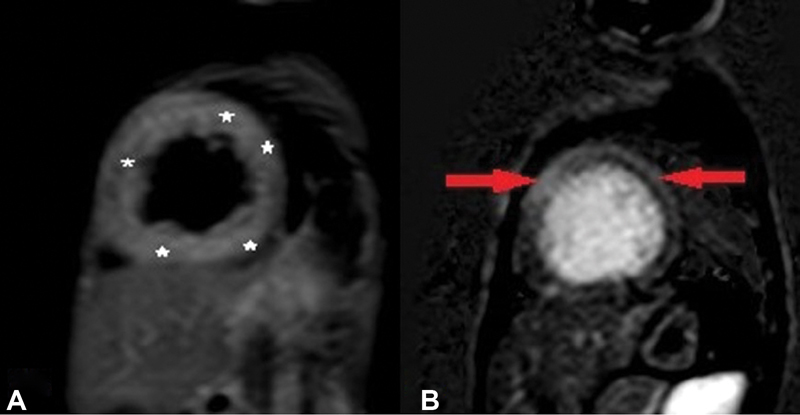
( A ) Short-axis view showing diffuse left ventricle (LV) wall edema (white asterisks) in midcavity LV walls on T2 short tau inversion-recovery images ( B ) Short-axis view showing epidural to midmyocardial late gadolinium enhancement in midcavity interventricular septum and lateral wall (red arrows).
Discussion
Among the cardiomyopathies in childhood, dilated cardiomyopathy is the most common, etiology of which can be categorized into primary form (idiopathic, familial, or genetic) and secondary form (inflammatory, toxin mediated, metabolic, or systemic). 1 Idiopathic variety, a diagnosis by exclusion, accounts for 50 to 70% of cases in pediatric age group. The discriminating feature of secondary form is that the incriminating cause (especially metabolic) itself is sometimes treatable leading to amelioration of cardiac dysfunction. Hypocalcemia is now being increasingly recognized as an important reversible cause of LV dysfunction, severe enough to present with heart failure 5 6 or shock. 7 Ionized calcium is an inotropic agent and is essential for cardiac electrical activity. Ionized calcium is required for excitation–contraction coupling that in turn allows the actin–myosin interaction via the troponin–tropomyosin complex. Low plasma calcium hence leads to decreased contractility with prolonged hypocalcemia culminating into ventricular dysfunction and cardiac dilatation. Hypocalcemia-induced dilated cardiomyopathy responds poorly to conventional cardiac failure treatment, albeit it is fairly responsive to restoration of normocalcemia. We have previously reported a series of 15 infants from the same institute with hypocalcemia cardiomyopathy, who responded dramatically to treatment with vitamin D and calcium, and cardiac function returning to normal within months except one patient, highlighting the reversible nature of hypocalcemic dilated cardiomyopathy. 5 Maiya et al reported a series of 16 cases of cardiomyopathy in children associated with vitamin D deficiency leading to hypocalcemia and reported one mortality. 11 In the present cohort, all cases with follow-up available responded to vitamin D and calcium supplementation with normalization or improvement in cardiac function. Most of the published series incriminated vitamin D as the causative agent for hypocalcemia, which was also witnessed in our cohort.
This is the first attempt to investigate the role CMR in hypocalcemia-induced dilated cardiomyopathy. CMR is now being increasingly used as a noninvasive tool for the etiological diagnosis of cardiomyopathies to avoid more invasive tissue diagnosis, especially in infants and very young children. 12 Almost all infants, young children and older children with developmental delay or apprehension undergo CMR and pediatric CMR is done under anesthesia. Goal of CMR of a sedated patient should be to avoid repeat scan due to insufficient information from MRI. 13
All the patients had reduced ejection fractions, increased end-diastolic volume, and dilated LV cavity. Follow-up showed recovery of all patients in category 1 and none of the patients showed LGE in this category, which was surprising in spite of the presence of severe nature of LV dysfunction. Since the CMR findings of edema and LGE are markers of myocardial inflammation, necrosis, and scar, absence of these findings was seen in majority of patients in this cohort. Possible explanation could lie in the series by Maiya et al in which one of the cases had undergone autopsy and revealed mild subendocardial fibrosis with inconspicuous cellular inflammatory infiltrate or myocardial necrosis, which points toward the absence of inflammation in patients with hypocalcemic dilated cardiomyopathy. 11 Absence of edema and LGE correlated with the clinical recovery of the category 1 patients and substantiates a different etiopathogenesis of hypocalcemic dilated cardiomyopathy vis-à-vis inflammatory myocarditis. CMR studies have highlighted the role of LGE in prognosis of cardiomyopathies of different etiologies. Presence of LGE correlates with a strong prediction of major adverse cardiovascular events like ventricular arrhythmias and greater risk of assist device implantation, transplantation, or death. 9 14 Absence of LGE in nonischemic cardiomyopathy predicts reduced all-cause mortality. 15 No studies are available to compare the CMR findings in hypocalcemic dilated cardiomyopathy. Few case reports of pediatric and adult hypocalcemic dilated cardiomyopathy in whom cardiac MRI were done did not reveal any edema or LGE. 16 17
Presence of both, LGE and edema, noted in two out of nine patients (category 3), out of which one patient succumbed. This patient showed diffuse LV myocardial edema with mid-wall striae septal LGE that is an indicator of poor prognosis, among all pattern of myocardial enhancement. 18 Study done by Becker et al revealed that this pattern of enhancement is due to remodeling of LV myocardium irrespective of etiology and indicates a poor prognosis. 19 Myocardial fibrosis assessed by LGE has a prognostic value in dilated cardiomyopathy and identifies patients at risk of future adverse cardiac events, including mortality irrespective of etiology, ventricular arrhythmic events, and symptomatic heart failure. 14 20 Another patient in category 3 in our cohort had CMR finding that was consistent with acute myocarditis. Lachassinne et al reported patient with hypocalcemia-induced dilated cardiomyopathy not responding to standard treatment who was finally diagnosed to have acute viral myocarditis on follow-up after 18 months. 21 This highlights the role of CMR in patients of hypocalcemic dilated cardiomyopathy with coexistent pathologies.
Two patients in category 2 had presence of edema only on CMR. Presence of edema in this category may be explained due to decreased clearance of lymph poorly contractile myocytes, heart failure itself being oxidative stress leads to myocardial inflammation, and secondary to the venous congestion. 22 However, this does not explain the absence of edema in majority of our patients. Alternatively, this subset may have edema due to mild myocardial inflammation that itself is poorly detectable with conventional inversion recovery sequences. 23 This subset of patient may be further investigated by endomyocardial biopsy to rule out the cause of underlying edema whether due to inflammation or noninflammatory transudative edema.
Early endomyocardial biopsy should be considered strongly for children showing edema and LGE in hypocalcemic dilated cardiomyopathy on CMR, for definitive diagnosis of cardiomyopathy. 24 Biopsy would further help to differentiate CMR changes that are due to coexistent myocarditis or are secondary to the hypocalcemic cardiomyopathy. This would further help to validate the role of CMR in diagnosis and prognostication of these subset of patients.
Limitations
As the study group was small in size, these results cannot be generalized; however, study on adequate number of patients needs to further validate our study. All components of Lake Louis criterion were not used due to poor image quality of T1 TSE postgadolinium enhancement sequence in pediatric patients. No endomyocardial biopsy was done to assess the presence of inflammation in patients with LGE. Newer imaging tools of CMR like T1 mapping, T2 mapping, and ECV assessment were also not done, which are now being increasingly used to myocardial tissue characterization.
Conclusion
Hypocalcemic dilated cardiomyopathy is a reversible cause of heart failure. Prompt diagnosis and treatment culminate into good response to calcium and vitamin D administration. CMR is increasingly used in pediatric patients to ascertain the cause of heart failure. CMR may play a role in the prognosis of patients with cardiomyopathy. Absence of edema and LGE predicting a good outcome and patients with presence of LGE and edema either indicating a poor prognosis or underlying co-existent myocarditis. Endomyocardial biopsy should be advised in patients showing edema and LGE to rule out underlying coexisting inflammation, for further prognosis of the patient and aid in counselling the parents.
What Is Already Known about the Subject?
Hypocalcemia-induced LV dysfunction is a rare but well-known cause of dilated cardiomyopathy in pediatric population.
What Does This Study Add?
There have been no previous studies analyzing the CMR findings in hypocalcemia-induced dilated cardiomyopathy. This study (a) analyzes the CMR findings of LV dysfunction in the subset of patients with hypocalcemia, (b) whether CMR can add to further prognosticate outcomes in these patients.
Conflicts of Interest There are no conflicts of interest.
Financial Support and Sponsorship
Nil.
Contribution of Authors
AG and SA were involved in concept, design, and manuscript preparation; KK and MB did data curation and analysis; SR did the literature search, gave inputs, and did final editing and proof reading of the draft.
References
- 1.Lipshultz S E, Sleeper L A, Towbin J A et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 2.Richardson P, McKenna W, Bristow M et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93(05):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 3.Boffa G M, Thiene G, Nava A, Dalla Volta S. Cardiomyopathy: a necessary revision of the WHO classification. Int J Cardiol. 1991;30(01):1–7. doi: 10.1016/0167-5273(91)90117-8. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz O, Olgun H, Ciftel M et al. Dilated cardiomyopathy secondary to rickets-related hypocalcaemia: eight case reports and a review of the literature. Cardiol Young. 2015;25(02):261–266. doi: 10.1017/S1047951113002023. [DOI] [PubMed] [Google Scholar]
- 5.Tomar M, Radhakrishnan S, Shrivastava S. Myocardial dysfunction due to hypocalcemia. Indian Pediatr. 2010;47(09):781–783. doi: 10.1007/s13312-010-0117-z. [DOI] [PubMed] [Google Scholar]
- 6.Fabi M, Gesuete V, Petrucci R, Ragni L. Dilated cardiomyopathy due to hypocalcaemic rickets: is it always a reversible condition? Cardiol Young. 2013;23(05):769–772. doi: 10.1017/S1047951112001850. [DOI] [PubMed] [Google Scholar]
- 7.Gupta P, Tomar M, Radhakrishnan S, Shrivastava S. Hypocalcemic cardiomyopathy presenting as cardiogenic shock. Ann Pediatr Cardiol. 2011;4(02):152–155. doi: 10.4103/0974-2069.84655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis . Friedrich M G, Sechtem U, Schulz-Menger J et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira V M, Schulz-Menger J, Holmvang G et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 10.Chu G C, Flewitt J A, Mikami Y, Vermes E, Friedrich M G. Assessment of acute myocarditis by cardiovascular MR: diagnostic performance of shortened protocols. Int J Cardiovasc Imaging. 2013;29(05):1077–1083. doi: 10.1007/s10554-013-0189-7. [DOI] [PubMed] [Google Scholar]
- 11.Maiya S, Sullivan I, Allgrove J et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life-threatening infant heart failure. Heart. 2008;94(05):581–584. doi: 10.1136/hrt.2007.119792. [DOI] [PubMed] [Google Scholar]
- 12.EACVI . Valsangiacomo Buechel E R, Grosse-Wortmann L, Fratz S et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur Heart J Cardiovasc Imaging. 2015;16(03):281–297. doi: 10.1093/ehjci/jeu129. [DOI] [PubMed] [Google Scholar]
- 13.Kellenberger C J, Yoo S J, Büchel E R. Cardiovascular MR imaging in neonates and infants with congenital heart disease. Radiographics. 2007;27(01):5–18. doi: 10.1148/rg.271065027. [DOI] [PubMed] [Google Scholar]
- 14.Becker M AJ, Cornel J H, van de Ven P M, van Rossum A C, Allaart C P, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. JACC Cardiovasc Imaging. 2018;11(09):1274–1284. doi: 10.1016/j.jcmg.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Kuruvilla S, Adenaw N, Katwal A B, Lipinski M J, Kramer C M, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7(02):250–258. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Z, Yadav S K, Liu X, Yi Q. A reversible hypocalcemic dilated cardiomyopathy caused by primary hypoparathyroidism. Asian J Med Sci. 2019;10:65–68. [Google Scholar]
- 17.Parepa I, Mazilu L, Suceveanu A, Voinea C, Tica I. Hypocalcemic cardiomyopathy - a rare heart failure etiology in adult. Acta Endocrinol (Bucur) 2019;5(01):107–112. doi: 10.4183/aeb.2019.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almehmadi F S, Nevis I, Zahrani Met al. Mid-wall striae pattern on late gadolinium enhancement imaging predicts future cardiovascular events in patients with systolic dysfunction J Am Coll Cardiol 201361(10, Suppl):E830 [Google Scholar]
- 19.Becker M A, Allaart C P, Van Der Lingen A C, Van Rossum A C, Cornel J H, Germans T. P621 Septal midwall late gadolinium enhancement indicator of left ventricular remodelling rather than specific sign of non-ischemic dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2019;20 02:jez116–jez024. [Google Scholar]
- 20.Gulati A, Jabbour A, Ismail T F et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309(09):896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 21.Lachassinne E, Gaudelus J, Lacombe F et al. [Acute heart insufficiency in an 8-month-old infant presenting with hypocalcemia and Epstein-Barr virus infection: acute myocarditis? Or primary hypokinetic dilated cardiomyopathy?] Ann Pediatr (Paris) 1992;39(03):179–183. [PubMed] [Google Scholar]
- 22.Verbrugge F H, Bertrand P B, Willems E et al. Global myocardial oedema in advanced decompensated heart failure. Eur Heart J Cardiovasc Imaging. 2017;18(07):787–794. doi: 10.1093/ehjci/jew131. [DOI] [PubMed] [Google Scholar]
- 23.Francone M, Chimenti C, Galea N et al. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging. 2014;7(03):254–263. doi: 10.1016/j.jcmg.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Cox G F, Sleeper L A, Lowe A M et al. Factors associated with establishing a causal diagnosis for children with cardiomyopathy. Pediatrics. 2006;118(04):1519–1531. doi: 10.1542/peds.2006-0163. [DOI] [PubMed] [Google Scholar]


