Abstract
Background:
Lung cancer screening (LCS) with low dose computed tomography (LDCT) is associated with a 20% reduction in lung cancer mortality. Psychological burden is a potential harm associated with LCS, and is a major barrier to utilization. We aimed to examine the feasibility and acceptability of a video intervention designed to reduce anxiety and promote psychological preparedness of LCS.
Patients and Methods:
This is a two group, sequential enrollment pilot study of a video intervention that integrates information on screen criteria, procedures, benefits and harms, and follow-up plan. Participants were enrolled 1-2 weeks prior to baseline LDCT, and the intervention was administered in one in-person session on the day of LDCT. Outcomes were assessed at baseline (pre-screen), immediately after LDCT, and at 1 week, 3 months, and 7 months post-screen. Outcome measures included the SF-12 (HRQOL), STAI (anxiety), psychosocial consequences of LCS (COS-LC), risk perceptions for lung cancer, and a satisfaction tool. The Student’s t-test was used for exploratory evaluations on change from baseline scores both within and between groups.
Results:
Sixteen participants (8 intervention, 8 controls) enrolled and completed the study (61.5% retention). Participants in the control group reported a significantly increased sense of dejection at 1-month and 7-months post-screen as measured by the COS-LC (p=0.01). Participants were highly satisfied with the intervention.
Conclusion:
A video intervention that promoted psychological preparedness for LCS was feasible to implement as part of an LCS program and highly accepted by participants.
Keywords: Lung cancer screening, anxiety, health-related quality of life, psychological burden, risk perceptions
Microabstract
Psychological burden is a potential harm associated with lung cancer screening, and is a major barrier to utilization. We examined the feasibility and acceptability of a video intervention designed to reduce anxiety and promote psychological preparedness of LCS. The intervention was feasible, well received, and improved certain anxiety scores compared to the control.
Introduction
Lung cancer is the most common cause of cancer death in the United States and worldwide.1 Outside of a lung cancer screening program, only about 20% of lung cancers are detected at stage I when cure is likely, and almost all of these are detected incidentally.2 Evidence from several national lung cancer screening trials, including the Early Lung Cancer Action Program (ELCAP), and the NCI-sponsored National Lung Screening Trial (NLST) supports the benefits of low-dose computed tomography (LDCT) in the early detection of lung cancer.3,4 Lung cancer screening (LCS) with LDCT may reduce lung cancer mortality in up to 65% of high-risk current and former smokers.3 These studies have led to the U.S. Preventative Services Task Force (USPSTF) recommendation endorsing LDCT in current and former smokers aged 55-80 who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.5
Psychological burden is one of the LCS-related harms highlighted by current screening guidelines.6, 7 Given the high rates of false-positive results from LDCT (39.1% had at least one false positive)8, understanding the impact and magnitude of potential screen-related psychosocial consequences is particularly important. Most LCS trials, including those conducted in the United States and Europe, assessed patient-reported outcomes (PROs) on health-related quality of life (HRQOL), anxiety, and distress. In a recent systematic review of psychological burden associated with LCS, we found that LCS may result in short-term psychological distress for individuals with positive or indeterminate scan results.9 Individuals with higher perceived risk of lung cancer experienced higher levels of distress.9, 10 Overall HRQOL did not substantially change over time or differ by LDCT results.9, 10
There has been extensive study on mammography-related anxiety, with some studies reporting psychological burden lasting several years from false positive exams, although most anxiety resolved once additional testing is performed to establish a diagnosis.11 There is little data on the magnitude of prolonged uncertainty and anxiety associated with LCS beyond a clinical trial setting.12, 13 Wiener et al evaluated the psychological impact of undergoing surveillance for indeterminate pulmonary nodules, and found that sources of distress include fear of cancer, concerns with the screening process, guilt associated with tobacco use, and uncertainty associated with screen result and potential work-up.14-17 Other factors may also impact HRQOL and anxiety in LCS. Current recommendations for screening targets high-risk populations of current and former smokers. By nature of these risk factors, the current screening population is likely to have co-morbidities, such as pulmonary and cardiovascular illnesses, that may already influence their HRQOL.18 Screen-related anxiety is especially important among smokers, a population with higher rates of mental illness compared with non-smokers.19 In the NELSON trial, current smokers reported higher levels of anxiety and lower HRQOL.20-22 Other factors, including how providers communicate cancer risk and the follow-up plan to patients, may strongly influence distress.15
Psychological burden can potentially occur at any step of the “screening cascade,” with heightened anxiety at specific time points such as receiving scan results.6,9 Given that LCS is currently recommended for smokers, a population that is already at risk for psychological issues, interventions to provide information on risk, benefits, and follow-up plans after LCS may improve patient-centered outcomes and mitigate potential screen-related psychological burden.10 While a number of shared decision making tools have been developed to facilitate patient education on LCS, there is insufficient information on whether providing LCS education reduces anxiety in patients undergoing LCS. The purpose of this study was to pilot-test a scalable video intervention to promote psychological preparedness for LCS and to determine the effect of this intervention on anxiety.
Patients and Methods
Study and Intervention Design
This was a two-group, sequential-enrollment feasibility study of a video-intervention to prepare patients for LCS. The intervention, Preparing for Lung Cancer Screening, uses a multimedia approach to provide patients with quality information related to benefits of LCS, risks for lung cancer, LCS procedures, follow-up plan for both positive and negative results, and risks of screening. The intervention content (Table 1) is delivered via a 5-minute video and 9-page handbook. It covers information on the following: 1) LCS program team and contact information; 2) reasons for screening; 3) screening eligibility; 4) how screening is performed; 5) what to expect on the day of screening; 6) what to expect after screening; 7) what to expect if result is negative; 8) what to expect if result is positive; and 9) risks of screening. At our institution, patients receive verbal counseling and a trifold pamphlet on screening as their shared decision making consultation prior to obtaining a LDCT scan.
Table 1.
Preparing for Lung Cancer Screening: Intervention Content
|
Sample and Setting
Patients who were scheduled for a LDCT and English-speaking were eligible for participation in the study. We included individuals with no history of cancer who were referred to the LCS program for screening as well as individuals with a previous cancer diagnosis who were eligible for LCS based on established criteria. Participants were recruited from the LCS program of City of Hope, a National Cancer Institute-designated comprehensive cancer center. Study procedures and protocol were approved by the Institutional Review Board. All participants provided voluntary informed consent prior to enrollment.
Outcome Measures
HRQOL was assessed using the Medical Outcomes Study Short-Form 12 (SF-12). It contains 7 items that measures the following concepts: physical functioning, role functioning, bodily pain, energy/fatigue, social functioning, mental health, emotional functioning, general health perceptions, and changes in health.23 Two validated subscales are derived from the 7 items: the physical component summary (PCS) and mental component summary (MCS) scores. The tool achieved a multiple R2 of 0.91 in the prediction of PCS-36 and 0.92 in the prediction of the MCS-36.23 The SF-12 scores were highly correlated with the MOS sample, with values of 0.904 and 0.939.23 The SF-12 was able to reproduce variance of greater than 90% for the SF-26 measures in the general US population norm.23 A higher score indicates better physical and psychological functioning. Mean scores of greater than 50 represents above average health status. The State Trait Anxiety Inventory (STAI) was used to assess distress/anxiety. The STAI include 20 items rated on a 4-point scale, with higher scores indicating greater anxiety (range 20-80). Internal consistency coefficients for the scale have ranged from .86 to .95; test-retest reliability coefficients have ranged from .65 to .75 over a 2-month interval.24 A score of > 40 indicates clinical anxiety.25 For adults aged 50 to 69 years, the median norm is 34.5 for men and 32.2 for women.26 Psychosocial impact of LCS was measured using the Consequences of Screening in Lung Cancer (COS-LC). This tool is an adapted version of the Psychological Consequences Questionnaire (PCQ), a validated measure to assess the psychological impact of mammography screening.27 The tool contains items and subscales assessing the psychosocial aspects of LCS (anxiety, sense of dejection, negative impact of LCS on behavior and sleep).12 A higher score indicates more negative psychosocial consequences of LCS.28, 29 Perceived Risk of Lung Cancer was assessed using two five-point Likert scale items on the comparative and absolute perceived risk for developing lung cancer.30 Participants who received the intervention completed a self-reported measure that assessed acceptability of the intervention. Outcomes were assessed at the following time points: a) T0 – 1 to 2 weeks before scheduled LDCT (sociodemographics, anxiety); b) T1 - immediately following LDCT (HRQOL, anxiety, psychosocial consequences of screening, perceived risk for lung cancer); c) T2 - 1-week post-screen (anxiety, psychosocial consequences of screening, satisfaction with intervention); and d) T3 and T4 - 3 and 7-months post-screen (HRQOL, anxiety, psychosocial consequences of screening).
Study Procedures
Participants were sequentially-enrolled, with participants in the control group enrolled first, followed by the intervention group. Upon informed consent, participants completed baseline surveys before screening. Participants in the control group received usual care, which included routine visits and telephone contact with the LCS program nurse practitioner and coordinator. Participants sequentially enrolled in the intervention group received the intervention on the day of their scheduled LCS screen. Participants were instructed to arrive 1-hour prior to their LDCT. The 5-minute video was viewed via a tablet. After viewing, a handbook was provided to participants, key contents were reviewed, and questions answered. Participants were instructed to contact LCS program staff with questions.
Statistical Analysis
Data were entered, audited, curated, and then analyzed using SAS®. All multi-item instruments were scored according to manuals or other formal scoring rules available. Participants’ sociodemographic and clinical characteristics were summarized and compared across groups using Fisher’s exact test for categorical data and t-tests for continuous data. Quantitative data on feasibility and acceptability of the intervention were analyzed using descriptive statistics to determine participant satisfaction with the timing, content, and delivery of the intervention. Change from baseline in outcome scores (HRQOL, psychosocial consequence of screening) were examined both within and across groups at 1-week, 3-months, and 7-months post-screen using Students t-tests. Missing data were excluded for each relevant pair-wise comparison. Due to missing data and the limited sample size, linear trends over time were insufficiently powered and therefore not assessed.
Results
A total of 26 individuals enrolled in the LCS program were eligible for the study and were invited to participate over the enrollment period. Of these, 6 declined participation (23%). Reasons for declining included being too busy (3) and being overwhelmed (3). Twenty participants provided consent and were enrolled (approximately 2 per month). Attrition rate for the 20 consented and enrolled survivors was 20%. Reasons for drop-out included being too busy (2), no longer interested (1) and unknown (1). After accounting for attrition, a total of 16 participants completed the 7-month study with evaluable data (61.5%).
Mean age was 62.5 for the control group, and 67.3 for the intervention group (Table 2). A majority of participants were non-Hispanic Whites and had at least a college education. No significant demographic differences were observed by group except for smoking history, where there were more current smokers at baseline in the intervention group. In terms of overall LCS results, 13 (81%) of participants had baseline screen results of category 1-2 based on the American College of Radiology’s Lung-CT Screening Reporting and Data System (Lung-RADS) for risk-stratification. Three participants (19%) had baseline screen results of category 4A. There were no significant differences by group for Lung-RADS category.
Table 2:
Sociodemographic and health characteristics of participants
| Control (n=8) | Intervention (n=8) | p value | |
|---|---|---|---|
| Age (mean ±SD) | 62.5 (±8.83) | 67.3 (± 8.15) | 0.27 |
| Sex (Female, N, %) | 5 (63%) | 7 (88%) | 0.20 |
| Race/ethnicity | |||
| Non-Hispanic White | 7 (87%) | 8 (100%) | 1.0 |
| Other | 1 (13%) | 0(0) | |
| Current Smoker (at baseline) | 6 (75%) | 4 (50%) | 0.61 |
| Education | |||
| HS/Vocational | 1 (13%) | 4 (50%) | 0.24 |
| Some College | 3 (38%) | 3 (38%) | |
| College | 1 (13%) | 1 (13%) | |
| Graduate School | 3 (38%) | 0(0) | |
| Employment Status | |||
| Full Time | 3 (38%) | 4 (50%) | 0.63 |
| Retired | 3 (38%) | 2 (25%) | |
| Other | 2 (25%) | 2 (25%) | |
| Household Income | |||
| <30,000 | 1 (13%) | 2 (25%) | 0.32 |
| 30,001-50,000 | 0 (0) | 3 (38%) | |
| 50,001-75,000 | 1 (13%) | 1 (13%) | |
| 75,001-100,000 | 3 (38%) | 0 | |
| >100,000 | 3 (38%) | 2 (25%) | |
| *Lung-RADS | |||
| 1 | 1 (13%) | 2 (25%) | |
| 2 | 5 (63%) | 5 (63%) | 0.14 |
| 4A | 2 (25%) | 1 (13%) |
American College of Radiology’s Lung-CT Screening Reporting and Data System
Participant Outcomes
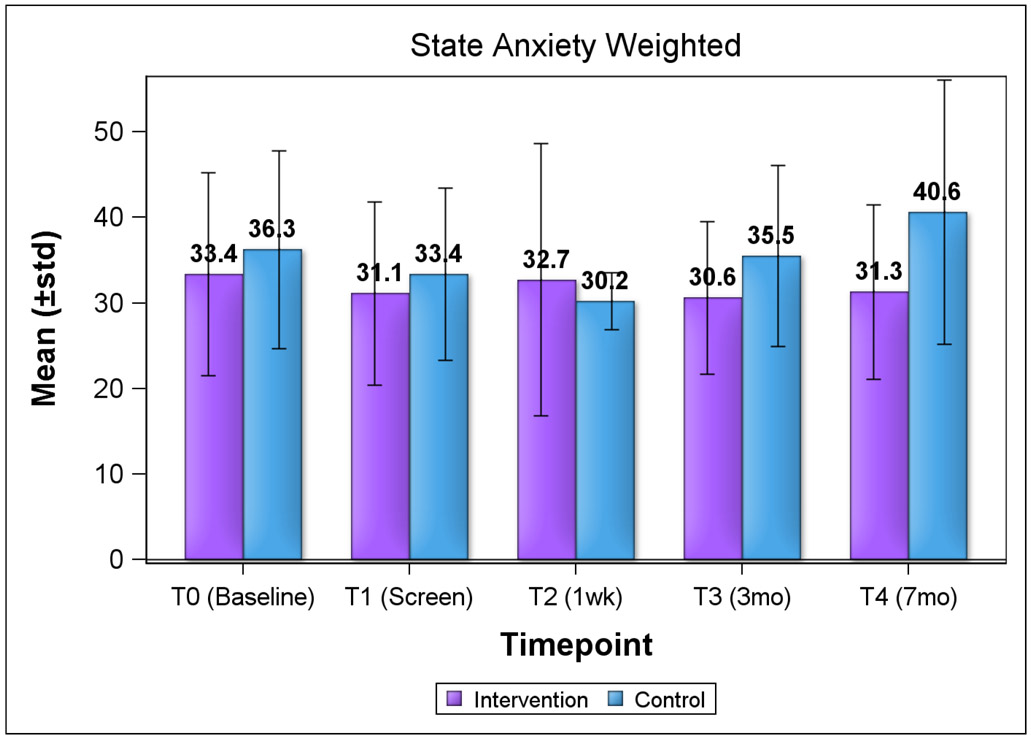
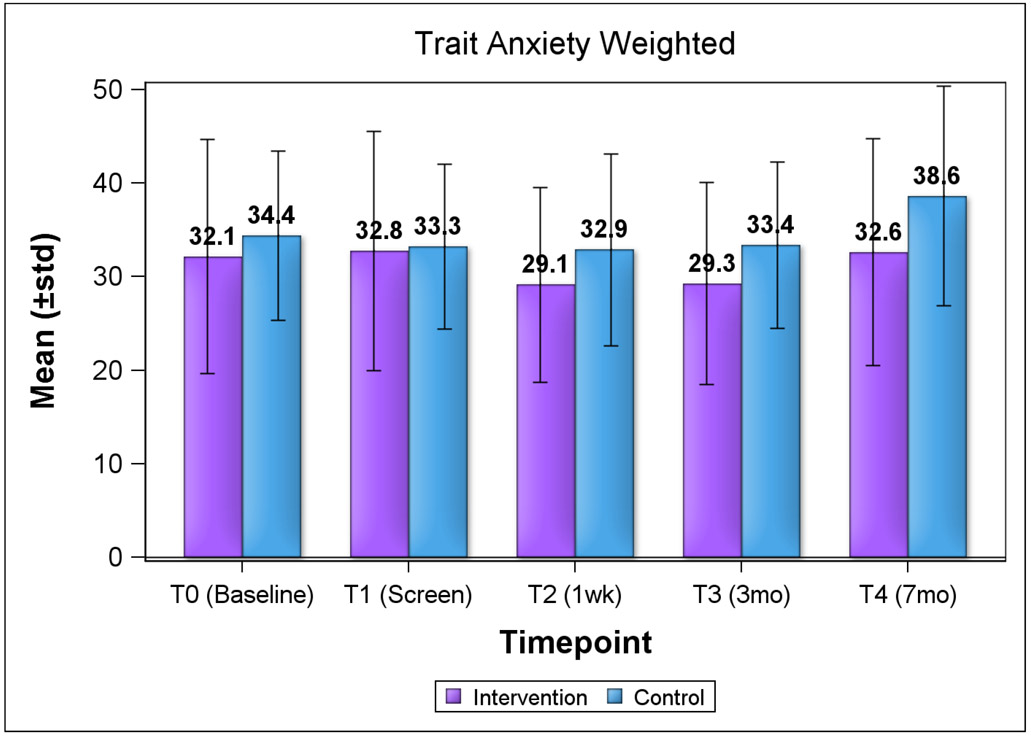
We explored HRQOL and psychosocial consequences of screening using change from baseline scores by group (Table 3). For both groups, baseline HRQOL by subscale scores (physical component and mental component summary) from the SF-12 were slightly below the mean cutoff score of 50. At both evaluation timepoints (3 months and 7 months post-screen), there were no significant changes from baseline (day of screen) both within and between groups, although most scores decreased at each subsequent timepoint, with the exception of the physical component for the control group. We observed similar trends for both lower state and trait anxiety subscale scores for the intervention group, except for state anxiety score at 1 week post-screen (Figure 1a and 1b). Anxiety scores were not clinically significant (mean of ≥40) for both groups over time. For state anxiety, mean score for the control group approached clinical significance at 7 months (40.6). Overall, the differences in HRQOL and anxiety scores by group were not statistically significant.
Table 3:
Health-related quality of life among participants (SF-12)*
| Intervention | Control | Between group p-values |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean (±SD) (N) |
Change from Baseline Mean (±SD) |
within group p- values |
Mean (±SD) (N) | Change from Baseline Mean (±SD) |
within group p-values |
|||
| *PCS | T1 (day of screen) | 48.1 (±9.8) (7) | -- | -- | 45.8 (±10.2) (8) | -- | -- | -- |
| T3 (3mo post-screen) | 46.3 (±8.1) (7) | 1.2 (±8.8) | 0.76 | 44.9 (±11.1) (8) | −1.0 (±10.0) | 0.79 | 0.69 | |
| T4 (7mo post-screen) | 37.6 (±12.9) (6) | −7.8 (±7.7) | 0.09 | 48.2 (±6.9) (7) | 0.1 (±8.0) | 0.96 | 0.12 | |
| **MCS | T1 (day of screen) | 54.4 (±8.2) (7) | -- | -- | 50.9 (±10.6) (8) | -- | -- | -- |
| T3 (3mo post-screen) | 53.2 (±10.7) (7) | 0.5 (±11.4) | 0.92 | 49.4 (±8.6) (8) | −1.5 (±11.3) | 0.71 | 0.75 | |
| T4 (7mo post-screen) | 52.4 (±6.1) (6) | 0.1 (±9.9) | 0.98 | 42.5 (±12.6) (7) | −7.2 (±16.8) | 0.30 | 0.40 | |
Physical Composite Score
Mental Composite Score
Range 0-100; higher score=better physical and psychological functioning; >50=above average health status
Only assessed at T1, T3, and T4
Figure 1. Anxiety Subscale Scores by Study Group and Timepoint (STAI).
Range 20-80; higher score=greater anxiety; ≥ 40 indicates clinical anxiety
Figure 1a. State Anxiety Scores by Group and Timepoint
Figure 1b. Trait Anxiety Scores by Group and Timepoint
For psychosocial consequences of screening (Table 4), we noted a statistically significant worse sense of dejection score at both 1-week (1.1, p=0.01) and 7-months (2.4, p=0.01) post-screen compared to the day of screen. This was not seen in the intervention group. When examined across groups, the change in the sense of dejection score was significantly higher in the control group than the intervention group at 7-months (2.4 versus 0.1, p=0.05). No significant differences within or between groups were found on either the anxiety or sleep scales.
Table 4:
Psychosocial Consequences of Screening by Study Group and Timepoint*
| Intervention | Control | Between group p-values |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean (±SD) (N) |
Change from Baseline Mean (±SD) |
within group p-values |
Mean (±SD) (N) | Change from Baseline Mean (±SD) |
within group p-values |
|||
| Anxiety (Range-0-18) | T1 (day of screen) | 4.5 (±1.8) (8) | -- | -- | 5.0 (±2.6) (8) | -- | -- | -- |
| T2 (1wk post-screen) | 4.8 (±2.4) (8) | 0.3 (±2.1) | 0.74 | 5.5 (±2.7) (8) | 0.5 (±1.1) | 0.20 | 0.76 | |
| T3 (3mo post-screen) | 4.1 (±1.6) (8) | −0.4 (±1.4) | 0.48 | 4.9 (±2.2) (8) | −0.1 (±2.0) | 0.87 | 0.78 | |
| T4 (7mo post-screen) | 4.0 (±1.4) (8) | −0.5 (±1.3) | 0.32 | 5.9 (±2.0) (8) | 0.9 (±1.6) | 0.20 | 0.11 | |
| Sense of Dejection (Range=0-18) | T1 (day of screen) | 6.5 (±2.9) (8) | -- | -- | 5.6 (±2.6) (8) | -- | -- | -- |
| T2 (1wk post-screen) | 6.4 (±2.8) (7) | 0.0 (±1.5) | 1.00 | 6.8 (±2.0) (8) | 1.1 (±1.0) | 0.01 | 0.11 | |
| T3 (3mo post-screen) | 6.0 (±1.7) (8) | −0.5 (±2.8) | 0.63 | 6.6 (±2.3) (8) | 1.0 (±1.8) | 0.15 | 0.22 | |
| T4 (7mo post-screen) | 6.6 (±2.7) (8) | 0.1 (±2.0) | 0.87 | 8.0 (±2.3) (8) | 2.4 (±2.1) | 0.01 | 0.05 | |
| Negative Impact on Behavior and Sleep (Range=0-21) | T1 (day of screen) | 7.3 (±3.0) (8) | -- | -- | 7.0 (±3.3) (8) | -- | -- | -- |
| T2 (1wk post-screen) | 6.4 (±3.2) (8) | −0.9 (±1.9) | 0.23 | 6.8 (±2.9) (8) | −0.3 (±1.7) | 0.68 | 0.49 | |
| T3 (3mo post-screen) | 5.9 (±1.9) (7) | −1.4 (±3.0) | 0.25 | 6.9 (±1.9) (8) | −0.1 (±3.2) | 0.91 | 0.43 | |
| T4 (7mo post-screen) | 6.9 (±2.5) (8) | −0.4 (±2.6) | 0.70 | 7.5 (±2.6) (8) | 0.5 (±2.4) | 0.57 | 0.50 | |
Higher score=more negative psychological consequences
Only assessed at T1, T2, T3, and T4
Immediately following LDCT, there were statistically significant differences in comparative and absolute risk perceptions for developing lung cancer by group. Sixty three percent of participants in the intervention group perceived that they have a slightly higher risk of developing lung cancer compared to others of the same age and sex, while 63% of participants perceived the risk to be “much more.” For absolute chance of developing lung cancer, half of intervention group participants perceived the risk to be “somewhat unlikely,” while 50% of the control group perceived the absolute risk to be “very likely.”
All intervention group participants completed the satisfaction tool at 1 week post-screen to assess acceptability and usability of the intervention. Overall, participants have high-scaled evaluations for the intervention (Table 6). Participants found that the intervention was helpful in understanding the purpose and procedures of LCS. The video and handbook was rated by 63% of participants as extremely helpful. Eighty-eight percent of participants felt that the timing of the intervention (on the day of LDCT) was “just right”, and all participants rated the amount of information provided through the video was “the right amount.”
Table 6.
Intervention Evaluation - 1 week post-screen (N=8)
| Item | N (%) | |
|---|---|---|
| Why it is important to screen for lung cancer | (4) Mostly understand | 1 (13) |
| (5) Completely understand | 7 (88) | |
| How the screening is done | (5) Completely understand | 8 (100) |
| What to expect on the day of the scan | (5) Completely understand | 8 (100) |
| When I will get the results of my scan | (5) Completely understand | 8 (100) |
| How my results will be given to me | (5) Completely understand | 8 (100) |
| Follow up plan based on scan results | (5) Completely understand | 8 (100) |
| Risks related to screening for lung cancer | (5) Completely understand | 8 (100) |
| Who to call if I have questions | (5) Completely understand | 8 (100) |
| Video information amount | (2) The right amount | 8 (100) |
| Overall handbook rating | (3) Moderately helpful | 1 (13) |
| (4) Mostly helpful | 2 (25) | |
| (5) Extremely helpful | 5 (63) | |
| Overall video rating | (4) Mostly helpful | 3 (38) |
| (5) Extremely helpful | 5 (63) | |
| Intervention timing | Just Right | 7 (88) |
| Too Late | 1 (13) | |
Discussion
In this pilot study, we found that a video intervention designed to support psychological preparedness for LCS was highly accepted and feasible to implement as part of an LCS program. The intervention was aimed at providing structured information on the benefits and harms (including psychological burden) of LCS. We opted to use a video format to facilitate intervention delivery, scalability and adaptability. Multiple studies have demonstrated that in a variety of settings, video interventions are superior in knowledge-transfer compared to print materials only, and may be especially suitable in populations with low health literacy.31
Our exploratory analysis of HRQOL revealed no significant difference by group except for worse physical component summary score at 7 months post-screen for the intervention group. This may be due to a higher percentage of intervention group participants who were current smokers with more limitations in physical functioning. For anxiety, we did not see a significant difference by group at all evaluation time points with the STAI. The differences, however, were clinically meaningful. For the STAI, a difference between two groups of two standard errors is generally considered to represent a large and meaningful effect.32 For example, for dejection scores at 7 months, twice the standard error is 1.9, so the difference observed of 2.1 is considered to be meaningfully different between groups. In our assessment of psychosocial consequences of screening, the anxiety subscale score from the COS-LC was statistically significant at 7 months post-screen, with less negative consequences for the intervention group. The different outcomes observed between the two measures of anxiety may be explained by survey sensitivity. The COS-LC is a condition-specific measure of LCS-specific distress, while the STAI is a more generalized anxiety measure that is not specific to cancer screening populations. Although both measures were used in LCS clinical trials in the U.S. (STAI in the NLST32) and Europe (STAI in the NELSON trial20, 21, 33, 34, COS-LC in the Danish Lung Cancer Screening Trial - DLCST28, 29), differences in long-term psychosocial consequences were observed. Inclusion of condition-specific measures is necessary to determine potential screen-related psychosocial consequences. Similar to other studies, we observed significant differences in the proportion of participants with high lung cancer risk perceptions by group. We observed these differences immediately after LDCT, suggesting that the intervention may have potential immediate effects on risk perception. Several studies in LCS populations suggest that higher risk perceptions may predict higher screen-related distress.22, 35-37
Current population-based evidence suggest that four years after the USPSTF recommendation of annual screening for lung cancer in high-risk, asymptomatic individuals, uptake of LDCT for LCS remains low and unchanged compared to 2012.38 In an analysis of data from the Health Information National Trends Survey (HINTS), patient-provider discussions about LCS remained low for both 2012 and 2014 (17% and 10%, respectively).39 Our previous research suggests that provider’s perceived barriers to LCS include concerns regarding its potential harms.40 Interventions targeting both providers and participants to promote better communication and shared decision-making may potentially attenuate the psychosocial consequences of screening and improve uptake of LCS with LDCT. Communications strategies favored by patients include 1) the provision of screen results via conversation, 2) use of plain understandable language, 3) clear explanation of nodule characteristics and what it may be, 4) provision of personalized lung cancer risk, 5) clear explanation of the follow-up plan, and 6) acknowledgement of the potential harms of screening.17, 41 Interventions should also examine potential mediators of LCS participation, including LCS health beliefs (perceived risk and benefits, barriers) and psychological variables such as stigma and distrust.42, 43
The study’s small sample size is an important limitation. This was derived to be realistic and practical for the relatively short study timeframe, and our intent was to determine whether a video intervention is practical, feasible, and acceptable to participants as part of an LCS program. Since our aims were limited to feasibility and acceptability, the analysis and comparisons by group were exploratory in nature. Non-specific factors that were not assessed may have contributed to the beneficial effects reported. A third limitation is the nature of the patient population, which was primarily Non-Hispanic White and well-educated. Findings might be different in a more diverse population. Important strengths of this study include the use of a condition-specific measure for psychological burden, which may be more sensitive to screen-related distress. Finally, the study enrolled patients receiving care in a real-world setting and outside of an LCS clinical trial context, which may be more informative and representative of the true experience of LCS participants.
In conclusion, for participants in a LCS program that participated in a video intervention to promote psychological preparedness to undergo LDCT, screen-specific anxiety was significantly lower at 7 months post-screen. This result provides preliminary evidence that screen-related distress can be potentially attenuated through structured interventions that support quality communication of the benefits and harms of LCS. As a number of a lung cancer screening shared decision making tools for LCS are developed, including video-based tools, studies are needed to test the efficacy of these interventions to mitigate the psychological consequences of screening and improve adherence to annual LCS.
Table 5.
Perceived Risk for Developing Lung Cancer Risk immediately after LDCT
| Intervention N (%) |
Control N (%) |
p-value | ||
|---|---|---|---|---|
| Risk of developing lung cancer compared to others of the same age and sex | (3) About the Same | 3 (38) | 0 (0) | 0.04 |
| (4) Slightly More | 5 (63) | 3 (38) | ||
| (5) Much More | 0 (0) | 5 (63) | ||
| Absolute chance of getting lung cancer | (1) Very unlikely | 1 (13) | 0 (0) | 0.05 |
| (2) Somewhat unlikely | 4 (50) | 0 (0) | ||
| (3) Somewhat likely | 3 (38) | 4 (50) | ||
| (4) Very likely | 0 (0) | 4 (50) | ||
Clinical Practice Points.
Lung cancer screening with LDCT may be associated with psychological distress, which may persist for months.
In this study, an educational video intervention reduced screen-specific anxiety 7 months after baseline screening.
Psychological preparedness and screen-related anxiety should be considered when implementing shared decision making tools for lung cancer screening.
Acknowledgments:
The research reported in this paper included work performed in the Survey Research Core and Biostatistics Core that are supported by the National Cancer Institute of the National Institutes of Health under award number 5K12CA001727-20 and P30CA33572. The content is solely the responsibility of the authors and do not necessarily represent the official views of the NCI or NIH.
Footnotes
Conflict of Interest
DR: Cireca, LLC consultant and grant funding; Merck, grant funding
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017 [DOI] [PubMed] [Google Scholar]
- 2.Raz DJ, Glidden DV, Odisho AY, Jablons DM. Clinical characteristics and survival of patients with surgically resected, incidentally detected lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007;2:125–130 [DOI] [PubMed] [Google Scholar]
- 3.International Early Lung Cancer Action Program I, Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage i lung cancer detected on ct screening. The New England journal of medicine. 2006;355:1763–1771 [DOI] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365:395–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2014;160:330–338 [DOI] [PubMed] [Google Scholar]
- 6.Harris RP, Sheridan SL, Lewis CL, Barclay C, Vu MB, Kistler CE, Golin CE, DeFrank JT, Brewer NT. The harms of screening: A proposed taxonomy and application to lung cancer screening. JAMA Intern Med. 2014;174:281–285 [DOI] [PubMed] [Google Scholar]
- 7.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, Sabichi AL, Smith-Bindman R, Wood DE, Qaseem A, Detterbeck FC. Benefits and harms of ct screening for lung cancer: A systematic review. JAMA : the journal of the American Medical Association. 2012;307:2418–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Lung Screening Trial Research T, Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gierada DS, Jones GC, Mahon I, Marcus PM, Sicks JD, Jain A, Baum S. Results of initial low-dose computed tomographic screening for lung cancer. The New England journal of medicine. 2013;368:1980–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu GX, Raz DJ, Brown L, Sun V. Psychological burden associated with lung cancer screening: A systematic review. Clinical lung cancer. 2016;17:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slatore CG, Sullivan DR, Pappas M, Humphrey LL. Patient-centered outcomes among lung cancer screening recipients with computed tomography: A systematic review. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2014;9:927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bond M, Pavey T, Welch K, Cooper C, Garside R, Dean S, Hyde C. Systematic review of the psychological consequences of false-positive screening mammograms. Health technology assessment. 2013;17:1–170, v-vi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodersen J, Thorsen H, Kreiner S. Consequences of screening in lung cancer: Development and dimensionality of a questionnaire. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2010;13:601–612 [DOI] [PubMed] [Google Scholar]
- 13.Tanoue LT, Tanner NT, Gould MK, Silvestri GA. Lung cancer screening. Am J Respir Crit Care Med. 2015;191:19–33 [DOI] [PubMed] [Google Scholar]
- 14.Slatore CG, Press N, Au DH, Curtis JR, Wiener RS, Ganzini L. What the heck is a "nodule"? A qualitative study of veterans with pulmonary nodules. Ann Am Thorac Soc. 2013;10:330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot?: A qualitative analysis of patients' reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143:672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. 'The thing is not knowing': Patients' perspectives on surveillance of an indeterminate pulmonary nodule. Health expectations : an international journal of public participation in health care and health policy. 2015;18:355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiener RS, Slatore CG. Real-world evidence about potential psychosocial harms of lung cancer screening. JAMA Intern Med. 2014;174:1416. [DOI] [PubMed] [Google Scholar]
- 18.Mazzone P Lung cancer screening: Examining the issues. Cleve Clin J Med. 2012;79 Electronic Suppl 1:eS1–6 [DOI] [PubMed] [Google Scholar]
- 19.Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: A model program to address this common but neglected issue. The American journal of the medical sciences. 2003;326:223–230 [DOI] [PubMed] [Google Scholar]
- 20.van den Bergh KA, Essink-Bot ML, Borsboom GJ, Scholten ET, van Klaveren RJ, de Koning HJ. Long-term effects of lung cancer computed tomography screening on health-related quality of life: The nelson trial. The European respiratory journal. 2011;38:154–161 [DOI] [PubMed] [Google Scholar]
- 21.van den Bergh KA, Essink-Bot ML, Borsboom GJ, Th Scholten E, Prokop M, de Koning HJ, van Klaveren RJ. Short-term health-related quality of life consequences in a lung cancer ct screening trial (nelson). Br J Cancer. 2010;102:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunge EM, van den Bergh KA, Essink-Bot ML, van Klaveren RJ, de Koning HJ. High affective risk perception is associated with more lung cancer-specific distress in ct screening for lung cancer. Lung Cancer. 2008;62:385–390 [DOI] [PubMed] [Google Scholar]
- 23.Ware J Jr., Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–233 [DOI] [PubMed] [Google Scholar]
- 24.Spielberger CD, Vagg PR. Psychometric properties of the stai. Journal of personality assessment. 1984;48:95–97 [DOI] [PubMed] [Google Scholar]
- 25.Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the state--trait anxiety inventory and the zung self-rating depression scale. The British journal of clinical psychology. 1983;22 (Pt 4):245–249 [DOI] [PubMed] [Google Scholar]
- 26.Spielberger C Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 27.Rijnsburger AJ, Essink-Bot ML, van As E, Cockburn J, de Koning HJ. Measuring psychological consequences of screening: Adaptation of the psychological consequences questionnaire into dutch. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2006;15:933–940 [DOI] [PubMed] [Google Scholar]
- 28.Aggestrup LM, Hestbech MS, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences of allocation to lung cancer screening: A randomised controlled trial. BMJ open. 2012;2:e000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen JF, Siersma V, Pedersen JH, Brodersen J. Psychosocial consequences in the danish randomised controlled lung cancer screening trial (dlcst). Lung Cancer. 2014;87:65–72 [DOI] [PubMed] [Google Scholar]
- 30.Montes U, Seijo LM, Campo A, Alcaide AB, Bastarrika G, Zulueta JJ. Factors determining early adherence to a lung cancer screening protocol. The European respiratory journal. 2007;30:532–537 [DOI] [PubMed] [Google Scholar]
- 31.Gysels M, Higginson IJ. Interactive technologies and videotapes for patient education in cancer care: Systematic review and meta-analysis of randomised trials. Support Care Cancer. 2007;15:7–20 [DOI] [PubMed] [Google Scholar]
- 32.Gareen IF, Duan F, Greco EM, Snyder BS, Boiselle PM, Park ER, Fryback D, Gatsonis C. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the national lung screening trial. Cancer. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Bergh KA, Essink-Bot ML, Bunge EM, Scholten ET, Prokop M, van Iersel CA, van Klaveren RJ, de Koning HJ. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (nelson trial). Cancer. 2008;113:396–404 [DOI] [PubMed] [Google Scholar]
- 34.van den Bergh KA, Essink-Bot ML, van Klaveren RJ, de Koning HJ. Informed decision making does not affect health-related quality of life in lung cancer screening (nelson trial). Eur J Cancer. 2010;46:3300–3306 [DOI] [PubMed] [Google Scholar]
- 35.Quaife SL, Janes SM. Lung cancer screening: Improving understanding of the psychological impact. Thorax. 2016;71:971–972 [DOI] [PubMed] [Google Scholar]
- 36.Park ER, Ostroff JS, Rakowski W, Gareen IF, Diefenbach MA, Feibelmann S, Rigotti NA. Risk perceptions among participants undergoing lung cancer screening: Baseline results from the national lung screening trial. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2009;37:268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinicrope PS, Rabe KG, Brockman TA, Patten CA, Petersen WO, Slusser J, Yang P, Swensen SJ, Edell ES, de Andrade M, Petersen GM. Perceptions of lung cancer risk and beliefs in screening accuracy of spiral computed tomography among high-risk lung cancer family members. Acad Radiol. 2010;17:1012–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the united states-2010 to 2015. JAMA oncology. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter-Harris L, Tan AS, Salloum RG, Young-Wolff KC. Patient-provider discussions about lung cancer screening pre- and post-guidelines: Health information national trends survey (hints). Patient Educ Couns. 2016;99:1772–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raz DJ, Wu GX, Consunji M, Nelson R, Sun C, Erhunmwunsee L, Ferrell B, Sun V, Kim JY. Perceptions and utilization of lung cancer screening among primary care physicians. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016;11:1856–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freiman MR, Clark JA, Slatore CG, Gould MK, Woloshin S, Schwartz LM, Wiener RS. Patients' knowledge, beliefs, and distress associated with detection and evaluation of incidental pulmonary nodules for cancer: Results from a multicenter survey. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016;11:700–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carter-Harris L, Ceppa DP, Hanna N, Rawl SM. Lung cancer screening: What do long-term smokers know and believe? Health expectations : an international journal of public participation in health care and health policy. 2017;20:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter-Harris L, Davis LL, Rawl SM. Lung cancer screening participation: Developing a conceptual model to guide research. Research and theory for nursing practice. 2016;30:333–352 [DOI] [PMC free article] [PubMed] [Google Scholar]