Abstract
This systematic review and meta-analysis examined the efficacy of sofosbuvir-based antiviral treatment against COVID-19 (coronavirus disease 2019). PubMed, Embase, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov were searched from inception to 15 August 2021. Studies comparing the clinical efficacy and safety of sofosbuvir-based antiviral regimens (study group) with other antivirals or standard of care (control group) in patients with COVID-19 were included. Overall, 687 patients with COVID-19 were included, of which 377 patients received sofosbuvir-based treatment. Mortality was lower in the study group than in the control group [odds ratio (OR) = 0.49, 95% confidence interval (CI) 0.30–0.79; I2 = 0%]. The overall clinical recovery rate was higher in the study group than in the control group (OR = 1.82, 95% CI 1.20–2.76; I2 = 28%). The study group presented a lower requirement for mechanical ventilation (OR = 0.33, 95% CI 0.13–0.89; I2 = 0%) and intensive care unit admission (OR = 0.42, 95% CI 0.25–0.70; I2 = 0%) than the control group. Furthermore, the study group exhibited a shorter hospital length of stay [mean deviation (MD), –1.49, 95% CI –2.62 to –0.37; I2 = 56%] and recovery time (MD, –1.34, 95% CI –2.29 to –0.38; I2 = 46%) than the control group. Sofosbuvir-based treatment may help reduce mortality in patients with COVID-19 and improve associated clinical outcomes. Furthermore, sofosbuvir-based treatment was as safe as the comparator in patients with COVID-19. However, further large-scale studies are warranted to validate these findings.
Keywords: COVID-19, ICU, Mortality, SARS-CoV-2, Sofosbuvir
1. Introduction
By late August 2021, more than 210 million confirmed cases of COVID-19 (coronavirus disease 2019) had been reported, with more than 4 million documented deaths [1]. Despite the rapid development of COVID-19 vaccines, SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection remains a major challenge globally [2,3]. Therefore, identification of effective treatment regimens for patients with COVID-19 remains critical. Despite large-scale search for therapeutic regimens to manage patients with COVID-19, effective treatments against SARS-CoV-2 infection remain limited. At the time of writing, only corticosteroids and tocilizumab have been reported to reduce mortality in patients with COVID-19 by the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group [4,5]. A meta-analysis of clinical trials involving 1073 critically ill patients with COVID-19 revealed that administration of systemic corticosteroids may be associated with lower 28-day all-cause mortality compared with usual care or placebo [summary odds ratio (OR) = 0.66, 95% confidence interval (CI) 0.53–0.82] [4]. Another prospective meta-analysis of 27 clinical trials assessing patients hospitalised with COVID-19 reported that administration of interleukin-6 (IL-6) antagonists was associated with lower 28-day all-cause mortality (summary OR = 0.86, 95% CI 0.79–0.95) compared with typical care or placebo [5]. However, in addition to corticosteroids and tocilizumab, there is a persistent need for superior strategies to combat SARS-CoV-2 infection.
The clinical efficacies of several antiviral agents, including remdesivir, lopinavir/ritonavir, favipiravir, baloxavir, umifenovir and darunavir/cobicistat, as well as their combinations have been evaluated for treating patients with COVID-19 [6]; of these, only remdesivir has been approved by the US Food and Drug Administration (FDA) as a treatment for patients with COVID-19 requiring hospitalisation [7]. Although remdesivir can help improve clinical outcomes of hospitalised patients with COVID-19, no additional mortality benefit has been observed with this treatment [8]. Both hepatitis C virus (HCV) and SARS-CoV-2 are positive-sense RNA viruses, so the anti-HCV agent sofosbuvir has been repurposed as a promising treatment for COVID-19. Moreover, sofosbuvir has a broad antiviral spectrum, also exhibiting potent activity against yellow fever, Zika, dengue and chikungunya viruses. Various sofosbuvir-based regimens, including sofosbuvir/daclatasvir, sofosbuvir/ledipasvir and sofosbuvir/velpatasvir, have been assessed as potential therapeutic regimens in patients with COVID-19 [9], [10], [11], [12], [13], [14], [15], [16]. In contrast to remdesivir, an individual patient data meta-analysis revealed that sofosbuvir/daclatasvir improved clinical recovery [risk ratio (RR) = 1.34, 95% CI 1.05–1.71; P = 0.020] as well as survival (RR = 0.31, 95% CI 0.12–0.78; P = 0.013) in patients with moderate to severe COVID-19 [17]. Similar findings were reported in another meta-analysis examining four clinical studies; sofosbuvir/daclatasvir was found to be associated with lower mortality (RR = 0.31, 95% CI 0.12–0.78; P = 0.013) and reduced need for intensive care unit (ICU) admission or invasive mechanical ventilation (MV) (RR = 0.35, 95% CI 0.18–0.69; P = 0.002) [18]. However, these two meta-analyses have only focused on sofosbuvir/daclatasvir and included only three or four clinical trials [17,18]. Accordingly, no meta-analysis has assessed the effect of all sofosbuvir-based antiviral treatment, including sofosbuvir/daclatasvir, sofosbuvir/ledipasvir and sofosbuvir/velpatasvir, on the clinical outcomes in patients with COVID-19, especially in terms of mortality. Therefore, we conducted the present systematic review and meta-analysis to assess the impact of sofosbuvir-based treatment on the mortality of patients with COVID-19.
2. Methods
2.1. Study search and selection
PubMed, Embase, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov databases were searched for relevant articles from inception up to 15 August 2021. We used the following search terms, including ‘sofosbuvir’, ‘sofosbuvir/daclatasvir’, ‘sofosbuvir/ledipasvir’, ‘sofosbuvir/velpatasvir’, ‘SARS CoV 2 infection’ and ‘COVID-19’. Furthermore, we only included clinical trials that compared the clinical efficacy and safety of sofosbuvir-based treatment with other comparators for treating patients with COVID-19. Furthermore, we manually searched for additional eligible articles cited in the reference lists of the identified articles. Studies were included if they met the following inclusion criteria: (i) examined patients with COVID-19; (ii) used sofosbuvir-based treatment as the experimental drug; (iii) used alternative treatments or standard of care as comparator; and (iv) clinical outcomes, including mortality and risk of adverse events (AEs), were available. In vitro studies, case reports, case series, post-hoc analysis studies, poster or conference abstracts, and studies without available data for outcome analysis were excluded. Initially, two investigators independently screened and reviewed each study. In the case of any disagreement, a third investigator was responsible for establishing consensus. The following data were extracted from each included study: year of publication; study design; study patients; sofosbuvir-based antiviral regimen; comparative agents; clinical outcomes; and risk of AEs. This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [19].
2.2. Outcome measures
The primary outcome was all-cause mortality. Secondary outcomes included clinical recovery, hospital length of stay (LOS), ICU admission, requirement for MV and risk of AEs.
2.3. Data analysis
The Cochrane risk-of-bias tool [20] was used to assess the quality and risk of bias of the included randomised control trials (RCTs). Statistical analyses were performed using Review Manager v.5.3 (Nordic Cochrane Centre, Copenhagen, Denmark). Meanwhile, we assessed the degree of heterogeneity using Q statistic generated from χ2 test and I 2 measure. A fixed-effects model was employed when data were homogeneous, and a random-effects model was used when data were heterogeneous (I 2 > 50%). Finally, the pooled ORs and 95% CIs were calculated for outcome analysis.
3. Results
3.1. Study selection
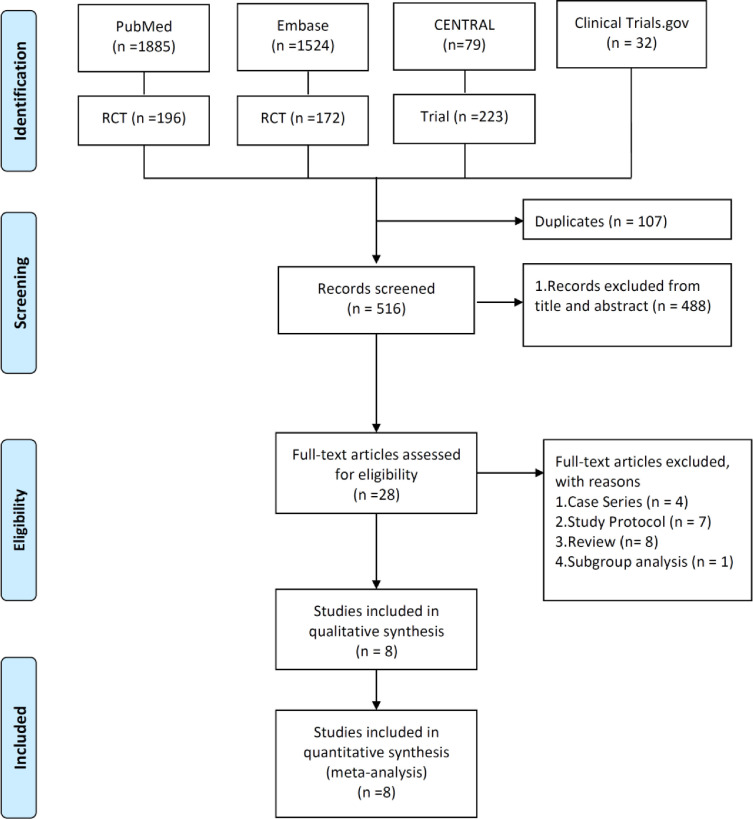
The search of online databases yielded 623 studies, of which 107 duplicate studies were excluded. In addition, 488 studies were deemed irrelevant after screening titles and abstracts as well as publications with no full-text available. Furthermore, 20 studies were excluded after the full-texts of the remaining 28 articles were screened. Finally, eight articles [9], [10], [11], [12], [13], [14], [15], [16] were included in the meta-analysis (Fig. 1 ; Appendix 1).
Fig. 1.
Flow chart of study selection. RCT, randomised controlled trial.
3.2. Study characteristics
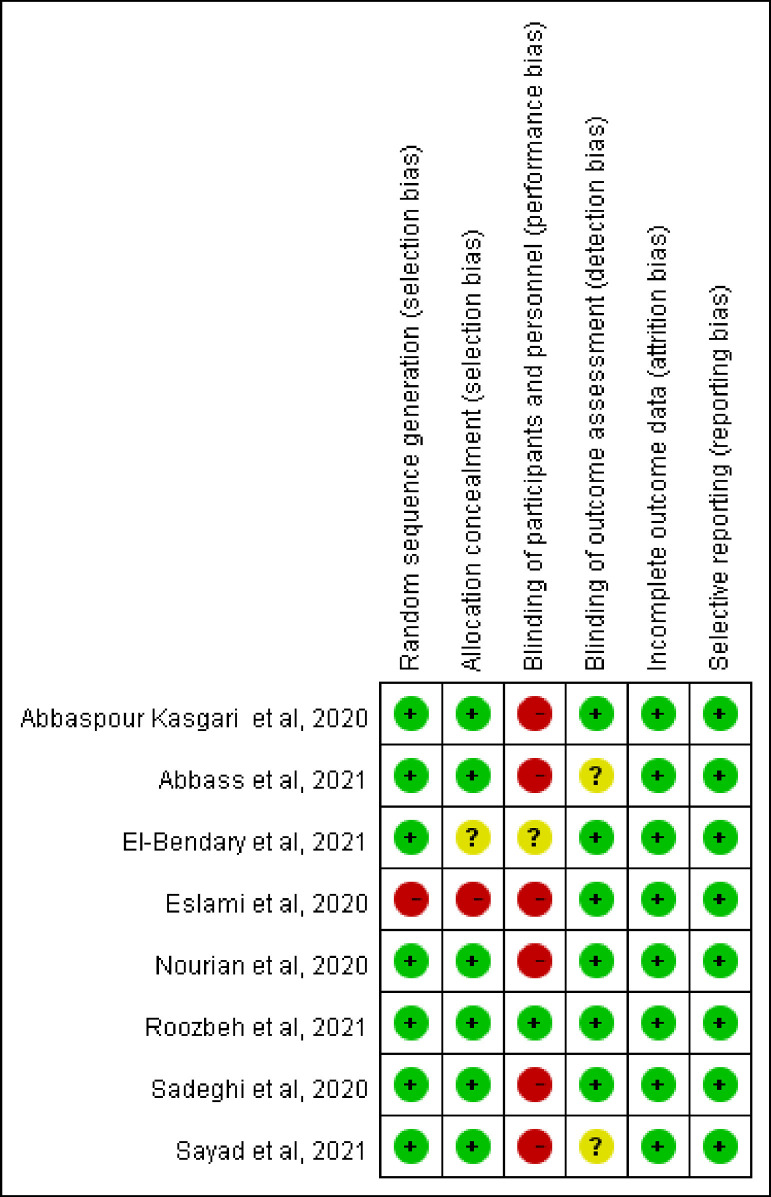
Five studies [9,[11], [12], [13], [14]] were single-centre studies, whereas three studies [10,15,16] were multicentre studies (Table 1 ). Except for two studies conducted in Egypt [10,16], all other studies were conducted in Iran [9,[11], [12], [13], [14], [15]]. Six studies [9,11,[13], [14], [15], [16]] focused on hospitalised patients, and one study [12] focused on outpatients with COVID-19. Six studies [[9], [10], [11], [12],15,16] used sofosbuvir/daclatasvir alone or in combination with ribavirin or hydroxychloroquine as experimental drugs; sofosbuvir/ledipasvir [13], sofosbuvir/velpatasvir [14] and sofosbuvir/ravidasvir [16] were used as experimental antiviral agents in the remaining studies. Overall, 687 patients with COVID-19 were included in the present study. Of these, 377 patients were allocated to receive sofosbuvir-based treatment regimens (n = 255 patients received sofosbuvir/daclatasvir; n = 42 patients received sofosbuvir/ledipasvir; n = 40 patients received sofosbuvir/velpatasvir; and n = 40 patients received sofosbuvir/ravidasvir). The risk-of-bias assessment is shown in Fig. 2 . High risk of performance bias was found in six studies [9,11,[13], [14], [15], [16]], and the study by Eslami et al. [11] also had high risk of selection bias.
Table 1.
Characteristics of the included studies
| Reference | Study design | Study site | Study population | Study drug | Control group | No. in study group | No. in control group |
|---|---|---|---|---|---|---|---|
| Abbaspour Kasgari et al., 2020 [9] | Open-label RCT | 1 centre in Iran | Hospitalised patients with moderate COVID-19 | SOF (400 mg)/DCV (60 mg) plus RBV (1200 mg) | HCQ and LPV/r with or without RBV | 24 | 24 |
| Abbass et al., 2021 [16] | Open-label RCT | 4 centres in Egypt | Moderate to severe COVID-19 | SOF (400 mg)/DCV (60 mg) or SOF (400 mg)/RDV 200 mg | Standard of care | SOF/DCV: 40; SOF/RDV: 40 | 40 |
| El-Bendary et al., 2022 [10] | Open-label RCT | 3 centres in Egypt | Adult patients with COVID-19 and pneumonia on chest CT | SOF (400 mg)/DCV (60 mg) | HCQ | 96 | 78 |
| Eslami et al, 2020 [11] | Open-label parallel trial | 1 centre in Iran | Hospitalised patients with severe COVID-19 | SOF (400 mg)/DCV (60 mg) | RBV | 35 | 27 |
| Khalili et al., 2020 [13] | Open-label RCT | 1 centre in Iran | Hospitalised patients with mild or moderate COVID-19 | SOF (400 mg)/LED (90 mg) | HCQ and ATV/r | 42 | 40 |
| Roozbeh et al., 2021 [12] | Double-blind, parallel-group, active-controlled RCT | 1 centre in Iran | Outpatients with mild COVID-19 | SOF (400 mg)/DCV (60 mg) plus HCQ | HCQ | 27 | 28 |
| Sadeghi et al., 2020 [15] | Open-label RCT | 2 centres in Iran | Hospitalised patients with moderate or severe COVID-19 | SOF (400 mg)/DCV (60 mg) | HCQ and LPV/r | 33 | 33 |
| Sayad et al., 2021 [14] | Open-label RCT | 1 centre in Iran | Hospitalised patients with moderate or severe COVID-19 | SOF (400 mg)/VEL (100 mg) | HCQ and LPV/r | 40 | 40 |
RCT, randomised controlled trial; COVID-19, coronavirus disease 2019; SOF, sofosbuvir; DCV, daclatasvir; RBV, ribavirin; HCQ, hydroxychloroquine; LPV/r, lopinavir/ritonavir; RDV, ravidasvir; CT, computed tomography; LED, ledipasvir; ATV/r, atazanavir/ritonavir; VEL, velpatasvir.
Fig. 2.
Summary of risk-of-bias assessment in the meta-analysis.
3.3. Primary outcome
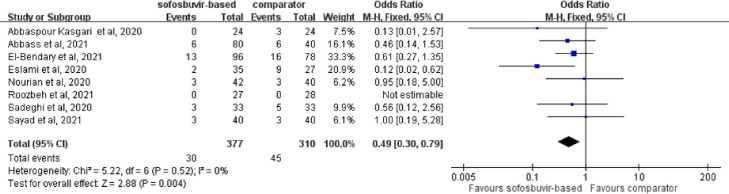
Mortality in the study group, which included patients who received sofosbuvir-based treatment, was 8.0% (30/377), which is lower than that documented in the control group (14.5%; 45/310). A significant difference in mortality rate was observed between the study and control groups using a fixed-effects model (OR = 0.49, 95% CI 0.30–0.79; I 2 = 0%) (Fig. 3 ). The lower mortality among the study group compared with the control group was unaltered using a random-effects model. In the subgroup analysis, hospitalised patients with COVID-19 receiving sofosbuvir-based treatment were associated with lower mortality than those in the control group (OR = 0.46, 95% CI 0.24–0.88; I 2 = 0%). In addition, patients receiving sofosbuvir/daclatasvir treatment were associated with a lower mortality than the comparator (OR = 0.42, 95% CI 0.23–0.75; I 2 = 0%) in the subgroup analysis of six RCTs using sofosbuvir/daclatasvir treatment as intervention [[9], [10], [11], [12],15,16].
Fig. 3.
Forest plot of the comparison of mortality between sofosbuvir-based treatment and comparators.
3.4. Secondary outcomes
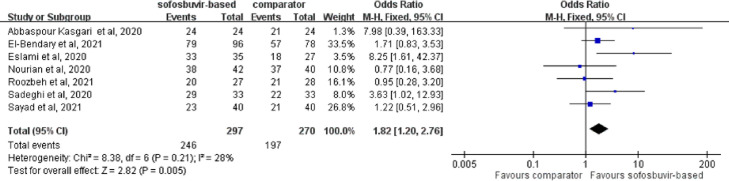
The overall clinical recovery rate was significantly higher in the study group than the control group using a fixed-effects model [82.8% (246/297) vs. 73.0% (197/270); OR = 1.82, 95% CI 1.20–2.76; I 2 = 28%] (Fig. 4 ). A similar trend was observed using a random-effects model. In addition, the study group receiving sofosbuvir-based treatment was associated with a lower rate of requiring MV (OR = 0.33, 95% CI 0.13–0.89; I 2 = 0%) and ICU admission (OR = 0.42, 95% CI 0.25–0.70; I 2 = 0%) than the control group. Furthermore, a shorter hospital LOS [mean deviation (MD), –1.49, 95% CI –2.62 to –0.37; I 2 = 56%) and a shorter recovery time (MD, –1.34, 95% CI –2.29 to –0.38; I 2 = 46%) were found in the study group than in the control group.
Fig. 4.
Forest plot of the comparison of clinical recovery between sofosbuvir-based treatment and comparators.
In the subgroup analysis of hospitalised patients with COVID-19, patients receiving sofosbuvir-based treatment were associated with a higher clinical recovery rate and lower risk of ICU admission than in the control group (clinical recovery rate: OR = 2.04, 95% CI 1.11–3.75, I 2 = 32%; ICU admission, OR = 0.30, 95% CI 0.14–0.64, I 2 = 0%). Although patients receiving sofosbuvir-based treatment had a lower risk of requiring MV than the control group, the difference did not reach statistical significance (OR = 0.36, 95% CI 0.13–1.02; I 2 = 0%). In addition, the study group exhibited a shorter hospital LOS than the control group in the subgroup analysis of hospitalised patients with COVID-19 (MD, –1.46, 95% CI –2.71 to –0.21; I 2 = 64%).
Likewise, patients receiving sofosbuvir/daclatasvir treatment were associated with a higher clinical recovery rate, lower risk of requiring MV and ICU admission, and shorter hospital LOS than the control group (clinical recovery rate, OR = 2.40, 95% CI 1.17–4.93, I 2 = 35%; requiring MV, OR = 0.27, 95% CI 0.09–0.82, I 2 = 0%; ICU admission, OR = 0.40, 95% CI 0.23–0.68, I 2 = 0%; hospital LOS, MD, –1.44, 95% CI –2.77 to –0.12, I 2 = 63%).
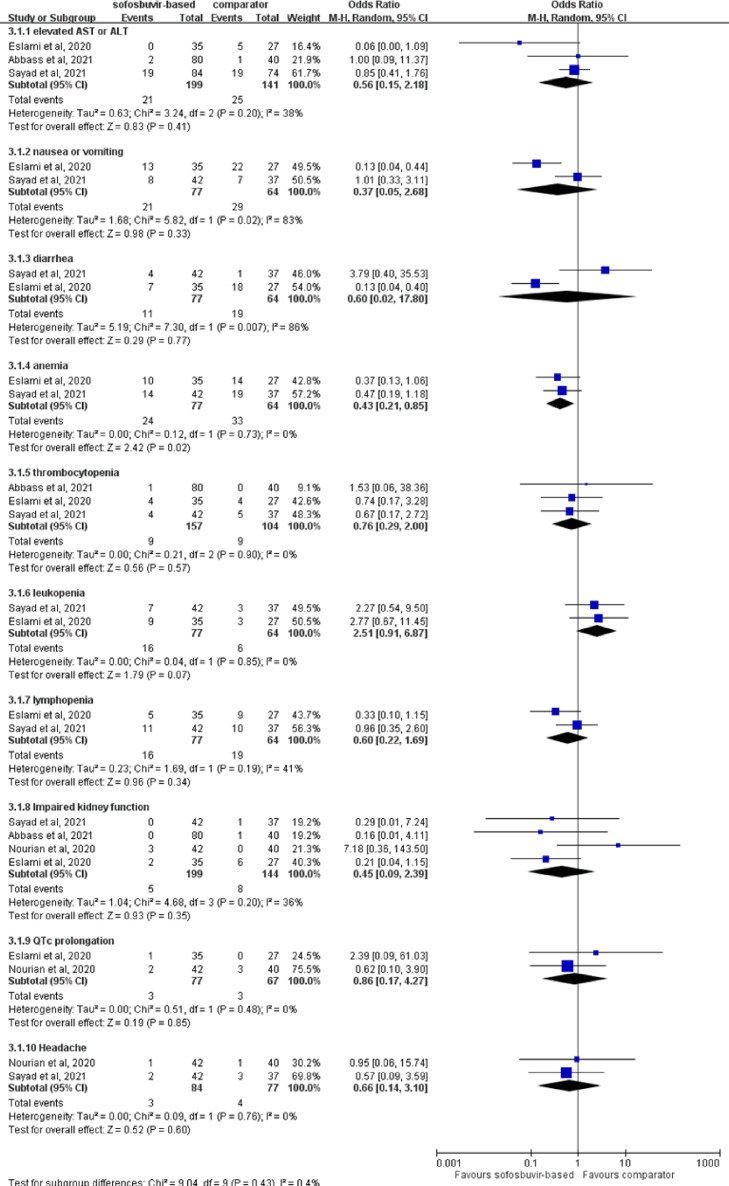
Regarding the risk of AEs, sofosbuvir-based treatment was associated with similar risk of specific AEs compared with comparators, including abnormal liver function (OR = 0.56, 95% CI 0.15–2.18; I 2 = 38%), nausea or vomiting (OR = 0.37, 95% CI 0.05–2.68; I 2 = 83%), diarrhoea (OR = 0.60, 95% CI 0.02–17.80; I 2 = 86%), anaemia (OR = 0.43, 95% CI 0.21–0.85; I 2 = 0%), thrombocytopenia (OR = 0.76, 95% CI 0.29–2.00; I 2 = 0%), leukopenia (OR = 2.51, 95% CI 0.91–6.87; I 2 = 0%), lymphopenia (OR = 0.60, 95% CI 0.22–1.69; I 2 = 41%), impaired kidney function (OR = 0.45, 95% CI 0.09–2.39; I 2 = 36%), QTc prolongation (OR = 0.86, 95% CI 0.17–4.27; I 2 = 0%) and headache (OR = 0.66, 95% CI 0.14–3.10; I 2 = 0%) (Fig. 5 ).
Fig. 5.
Forest plot of the comparison of the risk of adverse events between sofosbuvir-based treatment and comparators.
4. Discussion
In the present meta-analysis, eight articles [9], [10], [11], [12], [13], [14], [15], [16] were reviewed to assess the clinical efficacy and safety of sofosbuvir-based treatment regimens, including sofosbuvir/daclatasvir, sofosbuvir/ledipasvir, sofosbuvir/velpatasvir and sofosbuvir/ravidasvir, in the treatment of patients with COVID-19. Notably, this study revealed that sofosbuvir-based treatment could improve the clinical outcomes of patients with COVID-19, as supported by the following evidence. Most importantly, patients receiving sofosbuvir-based treatment exhibited a significantly lower mortality rate than the control group. However, this finding contrasts with a previous report investigating the clinical efficacy of another commonly used antiviral agent, namely remdesivir, which did not afford a mortality benefit in patients with COVID-19 [6,8,21]. In addition, sofosbuvir-based treatment was associated with a significantly higher clinical recovery rate, lower rate of requiring MV and ICU admission, and shorter hospital LOS and recovery time than comparators. Moreover, the clinical benefits of sofosbuvir-based treatment were consistently observed in the subgroup analysis of hospitalised patients with COVID-19. In addition, consistent with findings of previous studies [17,18], the subgroup of patients receiving sofosbuvir/daclatasvir had better clinical outcomes than those receiving comparators. Finally, we observed that sofosbuvir-based treatment regimens were not associated with a higher risk of AEs than comparators, indicating that these antiviral agents were tolerable. Overall, our findings suggest a promising role of sofosbuvir-based antiviral agents in treating patients with COVID-19. Notably, this finding could help enrich the pharmacological armamentarium against SARS-CoV-2 in this era of limited effective antiviral agents.
The clinical efficacy of sofosbuvir-based treatment against SARS-CoV-2 infection is consistent with the findings of in vitro and clinical studies [10]. Sacramento et al. have demonstrated that sofosbuvir alone and combined with daclatasvir could inhibit the replication of SARS-CoV-2 in Calu-3 cells [22]. Moreover, the authors revealed that sofosbuvir and daclatasvir could prevent neuronal apoptosis induced by SARS-CoV-2 as well as the release of cytokine storm-related inflammatory mediators such as IL-6 and tumour necrosis factor alpha (TNFα), respectively [22]. In addition, sofosbuvir was found to bind nsp12 with comparable binding energy to that of remdesivir [23]. Clinically, El-Bendary et al. have shown that sofosbuvir/daclatasvir could achieve faster eradication of SARS-CoV-2 than the standard of care [10]. However, further in vitro and in vivo studies are needed to confirm the efficacy of sofosbuvir-based regimens against SARS-CoV-2 and its variants [24].
This meta-analysis has several limitations. First, the number of studies and patients was small. Sofosbuvir/ledipasvir, sofosbuvir/velpatasvir and sofosbuvir/ravidasvir regimens were all assessed in a single study. Second, most included studies were conducted in Iran and at a single centre, therefore their findings might not be generalisable to other sites. Although Singh et al. reported a patient in India with acute leukaemia undergoing chemotherapy and receiving sofosbuvir and velpatasvir for HCV infection, which coincidentally mitigated SARS-CoV-2 infection [25], further multicentre, multinational studies are needed. Third, the study population included both outpatients and inpatients and the comparators varied. However, most of our findings were based on pooled analysis with low heterogeneity and remained consistent across different subgroup analyses. Overall, further large-scale studies are required to confirm our findings.
Based on the findings of this meta-analysis of only eight RCTs, sofosbuvir-based treatment regimens may help to reduce the mortality of patients with COVID-19 and improve associated clinical outcomes, including clinical recovery, risk of requiring MV and ICU admission, hospital LOS and recovery time. In addition, sofosbuvir-based treatment was as safe as comparators for patients with COVID-19. All of these findings suggest the potential role of sofosbuvir-based antiviral treatment against SARS-CoV-2 infection. However, further large-scale studies, especially multinational studies, are warranted to validate our findings.
Declaration of Competing Interest
None declared.
Acknowledgments
Funding
None.
Ethical approval
Not required.
Editor: Dr Jim Gray
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2022.106545.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ [accessed 28 August 2021].
- 2.Mallapaty S. COVID vaccines slash viral spread—but Delta is an unknown. Nature. 2021;596:17–18. doi: 10.1038/d41586-021-02054-z. [DOI] [PubMed] [Google Scholar]
- 3.Lai CC, Chen IT, Chao CM, Lee PI, Ko WC, Hsueh PR. COVID-19 vaccines: concerns beyond protective efficacy and safety. Expert Rev Vaccines. 2021;20:1013–1025. doi: 10.1080/14760584.2021.1949293. [DOI] [PubMed] [Google Scholar]
- 4.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CC, Chao CM, Hsueh PR. Clinical efficacy of antiviral agents against coronavirus disease 2019: a systematic review of randomized controlled trials. J Microbiol Immunol Infect. 2021;54:767–775. doi: 10.1016/j.jmii.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration (FDA). FDA approves first treatment for COVID-19. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 [accessed 10 August 2021].
- 8.Lai CC, Chen CH, Wang CY, Chen KH, Wang YH, Hsueh PR. Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2021;76:1962–1968. doi: 10.1093/jac/dkab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbaspour Kasgari H, Moradi S, Shabani AM, Babamahmoodi F, Davoudi Badabi AR, Davoudi L, et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother. 2020;75:3373–3378. doi: 10.1093/jac/dkaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Bendary M, Abd-Elsalam S, Elbaz T, El-Akel W, Cordie A, Elhadidy T, et al. Efficacy of combined sofosbuvir and daclatasvir in the treatment of COVID-19 patients with pneumonia: a multicenter Egyptian study. Expert Rev Anti Infect Ther. 2022;20:291–295. doi: 10.1080/14787210.2021.1950532. [DOI] [PubMed] [Google Scholar]
- 11.Eslami G, Mousaviasl S, Radmanesh E, Jelvay S, Bitaraf S, Simmons B, et al. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother. 2020;75:3366–3372. doi: 10.1093/jac/dkaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roozbeh F, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Merat S, Wentzel H, et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. J Antimicrob Chemother. 2021;76:753–757. doi: 10.1093/jac/dkaa501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalili H, Nourian A, Ahmadinejad Z, Emadi Kouchak H, Jafari S, Dehghan Manshadi SA, et al. Efficacy and safety of sofosbuvir/ledipasvir in treatment of patients with COVID-19; a randomized clinical trial. Acta Biomed. 2020;91 doi: 10.23750/abm.v91i4.10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayad B, Khodarahmi R, Najafi F, Miladi R, Mohseni Afshar Z, Mansouri F, et al. Efficacy and safety of sofosbuvir/velpatasvir versus the standard of care in adults hospitalized with COVID-19: a single-centre, randomized controlled trial. J Antimicrob Chemother. 2021;76:2158–2167. doi: 10.1093/jac/dkab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadeghi A, Ali Asgari A, Norouzi A, Kheiri Z, Anushirvani A, Montazeri M, et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother. 2020;75:3379–3385. doi: 10.1093/jac/dkaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbass S, Kamal E, Salama M, Salman T, Sabry A, Abdel-Razek W, et al. Efficacy and safety of sofosbuvir plus daclatasvir or ravidasvir in patients with COVID-19: a randomized controlled trial. J Med Virol. 2021;93:6750–6791. doi: 10.1002/jmv.27264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons B, Wentzel H, Mobarak S, Eslami G, Sadeghi A, Ali Asgari A, et al. Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis. J Antimicrob Chemother. 2021;76:286–291. doi: 10.1093/jac/dkaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zein AFMZ, Sulistiyana CS, Raffaello WM, Wibowo A, Pranata R. Sofosbuvir with daclatasvir and the outcomes of patients with COVID-19: a systematic review and meta-analysis with GRADE assessment. Postgrad Med J. 2021 Jun 8 doi: 10.1136/postgradmedj-2021-140287. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh S, Khera D, Chugh A, Khera PS, Chugh VK. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacramento CQ, Fintelman-Rodrigues N, Temerozo JR, Da Silva APD, Dias S, da Silva CDS, et al. In vitro antiviral activity of the anti-HCV drugs daclatasvir and sofosbuvir against SARS-CoV-2, the aetiological agent of COVID-19. J Antimicrob Chemother. 2021;76:1874–1885. doi: 10.1093/jac/dkab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elfiky AA, Azzam EB, Shafaa MW. The anti-HCV, sofosbuvir, versus the anti-EBOV remdesivir against SARS-CoV-2 RNA dependent RNA polymerase in silico. Mol Divers. 2021 Jan 3 doi: 10.1007/s11030-020-10178-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahradník J, Marciano S, Shemesh M, Zoler E, Harari D, Chiaravalli J, et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat Microbiol. 2021;6:1188–1198. doi: 10.1038/s41564-021-00954-4. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Gera A, Misra A, Mehndiratta S. SARS-CoV-2 infection in a pediatric acute leukemia patient on chemotherapy and concurrent sofosbuvir/velpatasvir for HCV. Am J Blood Res. 2021;11:286–289. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.