Abstract
Capsaicinoids, volatile compounds, and fatty acids were analyzed in red pepper seeds to determine any changes at different roasting temperatures. The contents of capsaicin and dihydrocapsaicin decreased as roasting temperatures increased. 3-Ethyl-2,5-dimethylpyrazine, 2,3,5,6-tetramethylpyrazine, 2-methoxy-3-(2-methylpropyl)pyrazine, 1-methylpyrrole, hexanedial, benzeneacetaldehyde, 2-acetylfuran, and butane-2,3-diol were newly detected in red pepper seeds roasted at 100 °C. Concentrations of pyrazines, pyrroles, oxygen-containing heterocyclic compounds, carbonyls, and alcohols increased rapidly in red pepper seeds as the roasting temperature increased. Such compounds could contribute roasted, grilled, and sweet odor notes to roasted red pepper seeds. Linoleic acid was the predominant fatty acid in all red pepper seeds. There were no significant differences in polyunsaturated fatty acids in red pepper seeds as roasting temperature increased. In conclusion, roasting red pepper seeds could be used in thermally processed foods because during roasting their pungency is reduced, desirable savory odors are enhanced, and the levels of polyunsaturated fatty acids remain unchanged.
Keywords: Capsicum annum L. (red pepper) seeds, Capsaicinoids, Volatile compounds, Fatty acids, Roasting, Change
Introduction
Capsicum annuum L. (red pepper) has been popularly used for flavoring and preserving processed foods worldwide due to its characteristic flavor and pungency. These properties mainly depend on the level of capsaicinoids, a family of compounds consisting of acid amides of vanillylamine and C8‐C13 branched‐chain fatty acids, primarily capsaicin and dihydrocapsaicin (Kobata et al., 1999; Srinivasan, 2016). In addition to its sensory characteristics, red pepper has several biological activities due to being rich in vitamins, minerals, capsaicinoids, and flavonoids. These include pain-release, hypolipidemic, hypocholesterolemic, antioxidant, antiinflammatory, antitumor, and anticarcinogenic effects (Anandakumar et al., 2012; Anand and Bley, 2011; Cazana et al., 1990; Ornelas-Paz et al., 2010; Manjunatha and Srinivasan, 2008; Srinivasan, 2005; Suresh et al., 2007).
Red peppers are mainly consumed in a dried form, with more than half of them is in form of the volume consumed in Korea being in the form of red pepper powder (Kim et al., 2017). Red pepper seeds have been crushed and used in the production of red pepper powder in the past, but recently only 10 to 15% of red pepper seeds are added for the production of high-quality red pepper powder with the remainder being wasted (Kim et al., 2019). However, red pepper seeds also contain sterols, saponins, phenolic compounds, and polyunsaturated fatty acids (Sung and Lee, 2016; Ananthan et al., 2018) and several studies have reported antibacterial, antioxidant, and anticorrosion activities in red pepper seed oil (Özyıldız et al., 2012; Yılmaz et al., 2015; Kurniawan and Madurani, 2015). Therefore, new applications using red pepper seeds are of substantial interest. Our previous study developed a tea product with roasted red pepper seeds and evaluated its qualities (Kim et al., 2019). Roasting is a complex process involving hundreds of chemical reactions, including Maillard or non-enzymatic browning reactions between reducing sugars and amino acids, thermal degradation of lipids, and decomposition of sugars, as well as their interactions (Schenker et al., 2002), which could produce the characteristic flavors that are differentiated from those of raw red pepper seeds. In our previous study, the sensorial pungent intensities of red pepper seeds decreased with roasting temperatures and more desirable odors were produced (Kim et al., 2019). This study profiled and analyzed capsaicinoids, volatile compounds, and fatty acids in raw and roasted red pepper seeds in an attempt to understand their changes during roasting.
Materials and methods
Chemicals
Capsaicin, dihydrocapsaicin, ethanol, nerol, n-alkanes mixtures (C8-C22), 14% boron trifluoride-methanol solution, undecanoic acid, fatty acid methyl esters (C4-C24), cis-11-vaccenic acid methyl ester, and cis-7,10,13,16,19-docosapentenoic acid methyl ester were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dichloromethane was purchased from J.T. Baker (Center Valley, PA, USA).
Sample preparation
Korean dried red pepper seeds were provided by Chaeun Co. LTD. (Yeongju, Korea) in August 2020 and stored in a deep-freezer at − 70 °C until use. Red pepper seeds were roasted in a convection oven (EDF 213 XPT, ESCO Co., Eskisehir, Turkey) at 100 and 200 °C for 2 min at each temperature. Raw and roasted seeds were placed in a blender, frozen in liquid nitrogen, and ground into a powder.
Analysis of capsaicin and dihydrocapsaicin
The ground samples (2 g) were mixed with 80 mL of 95% ethanol, heated at 85 °C for 3 h, and then cooled. Samples were made up to 100 mL with 95% ethanol and then filtered through a 0.20 μm syringe for the analysis of capsaicin and dihydrocapsaicin. The analysis was performed on u-HPLC system that consisted a LaChromUltra L-2000 U Series (Hitachi-High Technologies Co., Hitachinaka, Japan) equipped with u-HPLC pump (Model L-2200U), an autosampler with a fixed injection volume of 10 µL (Model L-2200U), EXChrome Elite software (version 3.1.8b), LaChromUltra C18 column (50 mm length × 2 mm i.d. × 2 µm film thickness), and fluorescence detector (L-2485U), which was set at an excitation wavelength of 280 nm and emission wavelength of 325 nm. The mobile phase was a mixed solution of acetonitrile and 1% acetic acid in a ratio of 60:40 (v/v), with a flow rate of 0.6 mL/min in isocratic mode. The quantification of capsaicin and dihydrocapsaicin was performed using calibration curves derived from the analysis of standard solutions. The calibration curves were established by injecting 2 µL of 0.2, 0.5, 1.0, 2.5, and 10.0 µg/mL solutions prepared by diluting capsaicin and dihydrocapsaicin stock solutions with 95% ethanol.
Analysis of volatile compounds
Ground samples (50 g) with 0.1 mL of nerol (500 ppm in dichloromethane, v/v) as an internal standard were suspended with 150 mL of dichloromethane at 300 rpm for 30 min. Volatiles in the extract were separated from non-volatiles by solvent-assisted flavor evaporation (SAFE). Volatiles in the extract were dehydrated over anhydrous sodium sulfate, concentrated on a Vigreux column (50 cm length × 3 cm i.d.) in a water bath at 45 ± 2 °C, and then placed under a slow stream of nitrogen to obtain a final volume of 0.1 mL. The volatiles in red pepper seeds were analyzed using an Agilent 7890B gas chromatography-5977B mass selective detector (Agilent Technologies Co., Palo Alto, CA, USA) equipped with an HP-5MS column (30 m length × 0.25 mm i.d. × 0.25 µm film thickness, J & W Scientific, Folsom, CA, USA). The carrier gas was helium at a constant flow rate of 1 mL/min. One microliter of the extract was injected in a splitless mode. The oven temperature was held at 40 °C for 6 min, increased to 120 °C at a rate of 3 °C/min, to 150 °C at a rate of 5 °C /min, and at a rate of 3 °C /min, and then held at 200 °C for 5 min. The injector and detector temperatures were 250 and 280 °C, respectively. The mass detector was operated in electron ionization mode with an ionization energy of 70 eV and a scan range of 30 and 500 a.m.u. Volatile compounds were identified based on comparison of their mass spectra with those of the NIST 17 (ver. 2.2) and Wiley 7.0 databases or by manual interpretation. Retention index (RI) values were compared with those reported previously (Adams, 2007; Forero et al., 2009; Yılmaz et al., 2015). The RIs of volatiles were calculated using n-alkanes (C8–C22) as external references. The quantitative analysis of volatile compounds was performed by comparing their peak areas to that of the internal standard compound (0.1 mL of 500 ppm nerol in dichloromethane, v/v) on a GC-MS total ion chromatogram.
Analysis of fatty acids
Fatty acids in red pepper seeds were methyl esterified as described by AOAC 996.06 method (AOAC, 2012) and then analyzed using an Agilent 7890B gas chromatography (Agilent Technologies Co.) equipped with aa SP™-2560 (100 m length × 0.25 mm i.d. × 0.2 µm film thickness, Supelco Inc., Bellefonte, PA, USA). The oven temperature was set to 100 °C for 4 min, increased to 240 °C at a rate of 3 °C /min, and then held at 240 °C for 20 min. The injector and flame ionization detector temperatures were 225 and 285 °C, respectively. One microliter of the extract was injected with a split ratio of 50:1. The carrier gas was nitrogen at a constant flow rate of 1 mL/min. The FAMEs were identified by comparing with mixed FAME standards and were quantified by the area percentage of each FAME.
Statistical analysis
To evaluate significant changes in the chemical compositions of red pepper seeds during their roasting, analysis of variance (ANOVA) was conducted using SPSS software (ver. 24.0; IBM, Armonk, NY, USA). The values of chemical compositions were presented as mean ± standard deviation of three replicates. Their significant differences were also indicated by performing Duncan’s multi-range test (p < 0.05).
Results and discussion
Changes in capsaicin and dihydrocapsaicin of raw and roasted red pepper seeds
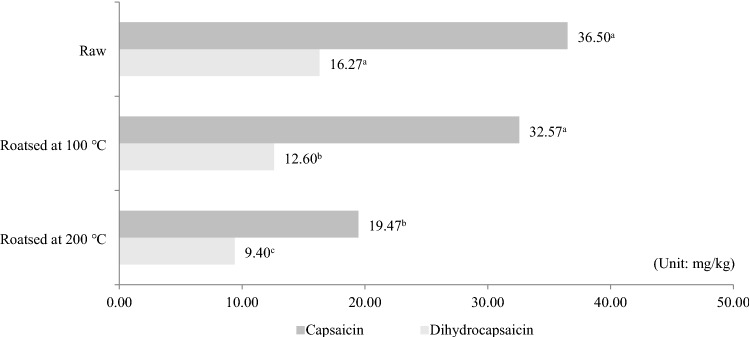
First, we investigated the effect of roasting temperatures on the capsaicin and dihydrocapsaicin concentrations of red pepper seeds. Capsaicin and dihydrocapsaicin are principal pungent compounds present in red pepper (Orellana-Escobedo et al., 2012; Surh et al., 1998; Yılmaz et al., 2015). Although consumer preferences on the pungent attribute of red pepper are considerable diverse, the concentration of these compounds is proportional to the intensity of pungency (Cliff and Green, 1996; Prescott, 1999). The capsaicin and dihydrocapsaicin concentrations in raw and roasted (100 and 200 °C) red pepper seeds in Fig. 1. There was no significant difference between the capsaicin concentrations in raw (36.50 ± 4.42 mg/kg) and roasted red peppers at 100 °C (32.57 ± 0.80 mg/kg), but the capsaicin concentration was significantly lower in red pepper seeds roasted at 200 °C (19.47 ± 1.32 mg/kg) than the other two treatments. Meanwhile the dihydrocapsaicin concentration of raw red pepper seeds (16.27 ± 1.63 mg/kg) differed significantly from the roasted red pepper seeds (100 °C: 12.60 ± 0.61 mg/kg; 200 °C: 9.40 ± 0.10 mg/kg). Wang et al. (2009) showed that in chili peppers heated to 100–260 °C, that there was no significant difference between the capsaicin and dihydrocapsaicin concentrations at temperatures below 190 °C; however, capsaicin and dihydrocapsaicin decomposed rapidly at temperatures higher than 190 °C. These results were consistent with those of the present study, in which the capsaicin and dihydrocapsaicin concentrations in red pepper seeds roasted at 200 °C were significantly changed. We previously found that pungency was an important preference attribute of red pepper seed tea (Kim et al., 2019). Based on a sensory evaluation of the pungency and preference among teas made with red pepper seeds roasted at 160, 200, or 240 °C, the pungency of the tea weakened with increasing roasting temperature; however, consumer preference was highest for the tea roasted at 200 °C, possibly due to a preference for its pungent characteristics (Kim et al., 2019).
Fig. 1.
The content of capsaicin and dihydrocapsaicin in raw and roasted red pepper seeds. There are significant differences among samples having different letters (p < 0.05)
Changes in volatile compounds of raw and roasted red pepper seeds
Volatile compounds in raw and roasted red pepper seeds were extracted by SAFE and then analyzed with GC-MS. Table 1 lists the volatile compounds identified in three red pepper seeds, their relative peak areas, and their RIs on the HP-5MS column. A total of 51 volatile compounds were identified in the red pepper seeds, including 8 pyrazines, 4 pyrroles, 9 oxygen-containing heterocyclic compounds, 6 aldehydes, 3 ketones, 13 alcohols, 4 acid and esters, 2 sulfur-containing compounds, and 2 terpene hydrocarbons. The volatile compounds in raw red pepper seeds were primarily terpenes (4.527), acids and esters (3.878) and alcohols (2.523). In a quantitative assessment, o-xylene (3.100 ± 0.605), 2-(2-butoxyethoxy)ethyl acetate (1.766 ± 0.340), and β-ocimene (1.426 ± 0.064), and dimethyl sulfoxide (1.345 ± 0.171) were the main compounds in raw red pepper seeds. o-Xylene and β-ocimene were previously found in fresh red pepper (Luning et al., 1995). In the present study, 3-ethyl-2,5-dimethylpyrazine (no. 6), 2,3,5,6-tetramethylpyrazine (no. 7), 2-methoxy-3-(2-methylpropyl)pyrazine (no. 8), 1-methylpyrrole (no. 9), hexanedial (no. 25), benzeneacetaldehyde (no. 26), 2-acetylfuran (no. 15), and butane-2,3-diol (no. 32) were not detected in raw red pepper seeds, but were formed newly in red pepper seeds roasted at 100 °C. In addition, 2-methylpyrazine (no. 1), 2,5-dimethylpyrazine (no. 2), 2-ethyl-6-methylpyrazine (no. 3), 2-ethyl-5-methylpyrazine (no. 4), 2,3,5-trimethylpyrazine (no. 5), 1H-pyrrole (no. 10), 2-methyl-1H-pyrrole (no. 11), furfural (no. 13), cyclopent-4-ene-1,3-dione (no. 14), methional (no. 23), benzaldehyde (no. 24), 1-hydroxybutan-2-one (no. 28), (Z)-hept-3-en-2-one (no. 29), 2,4-dihydroxy-2,5-dimethylfuran-3-one (no. 18), 4-methylpentan-1-ol (no. 33), o-guaiacol (no. 40), and 4-vinylguaiacol (no. 42) were more generated in red pepper seeds roasted at 200 °C. Overall, the contents of pyrazines (0.000 → 4.985 → 27.700), pyrroles (0.248 → 0.815 → 2.514), oxygen-containing heterocyclic compounds (1.980 → 2.685 → 27.198), aldehydes (0.320 → 0.706 → 10.690), ketones (0.000 → 0.000 → 0.704), and alcohols (2.523 → 8.319 → 16.483) rapidly increased in red pepper seeds as roasting temperatures increased.
Table 1.
Volatile compounds in raw and roasted red pepper seeds
| No | RI1 | Volatile compounds | Aroma description2 | Relative peak areas (mean ± SD)3 | ID5 | ||
|---|---|---|---|---|---|---|---|
| Raw | Roasted at 100 °C | Roasted at 200 °C | |||||
| Pyrazines (8) | |||||||
| 1 | 820 | 2-methylpyrazine | Nutty, roasted, chocolate, toasted | NDb4 | NDb | 1.689 ± 0.343a | MS/RI5 |
| 2 | 914 | 2,5-dimethylpyrazine | Chocolate, roasted, nutty | NDb | NDb | 10.517 ± 2.018a | MS/RI |
| 3 | 999 | 2-ethyl-6-methylpyrazine | Toasted, hazelnut-like | NDb | NDb | 0.839 ± 0.169a | MS/RI |
| 4 | 1001 | 2-ethyl-5-methylpyrazine | Nutty, roasted | NDb | NDb | 1.550 ± 0.266a | MS/RI |
| 5 | 1002 | 2,3,5-trimethylpyrazine | Roasted, potato-like | NDb | NDb | 1.905 ± 0.383a | MS/RI |
| 6 | 1077 | 3-ethyl-2,5-dimethylpyrazine | Roasted | NDc | 2.877 ± 0.257b | 4.224 ± 0.805a | MS/RI |
| 7 | 1086 | 2,3,5,6-tetramethylpyrazine | NDc | 2.078 ± 0.392b | 6.977 ± 1.148a | MS/RI | |
| 8 | 1176 | 2-methoxy-3-(2-methylpropyl)pyrazine (2-Isobutyl-3-methoxypyrazine) | Green bell-pepper note, green, earthy | NDb | 0.030 ± 0.004a | NDb | MS/RI |
| Sum of pyrazines | 0.000 | 4.985 | 27.700 | ||||
| Pyrroles (4) | |||||||
| 9 | < 800 | 1-methylpyrrole | Sweet, woody-herbaceous, coffee | NDc | 0.064 ± 0.004b | 0.306 ± 0.016a | MS/RI |
| 10 | < 800 | 1H-pyrrole | Slightly pungent, sweet, green, toasted | NDb | NDb | 0.115 ± 0.023a | MS/RI |
| 11 | 841 | 2-methyl-1H-pyrrole | NDb | NDb | 0.085 ± 0.011a | MS/RI | |
| 12 | 1067 | 1-(1H-pyrrol-2-yl)-ethanone (2-acetylpyrrole) | Bread, cracker, popcorn-like | 0.248 ± 0.032c | 0.751 ± 0.148b | 2.007 ± 0.292a | MS/RI |
| Sum of pyrroles | 0.248 | 0.815 | 2.514 | ||||
| Oxygen-containing heterocyclic compounds (9) | |||||||
| 13 | 830 | furan-2-carbaldehyde (furfural) | Sweet, bread-like, caramel-like | NDb | NDb | 0.596 ± 0.122a | MS/RI |
| 14 | 883 | cyclopent-4-ene-1,3-dione | NDb | NDb | 0.278 ± 0.028a | MS/RI | |
| 15 | 902 | 1-(furan-2-yl)ethanone (2-acetylfuran) | Floral, balsamic-cinnamic, roasted | NDb | 0.066 ± 0.003a | NDb | MS/RI |
| 16 | 912 | oxolan-2-one (γ-butyrolactone) | Sweet, slightly buttery | 0.166 ± 0.008c | 0.619 ± 0.078b | 1.704 ± 0.345a | MS/RI |
| 17 | 934 | cyclohex-2-en-1-one | 1.248 ± 0.246b | 1.416 ± 0.288b | 2.467 ± 0.076a | MS/RI | |
| 18 | 977 | 2,4-dihydroxy-2,5-dimethylfuran-3-one | NDb | NDb | 0.717 ± 0.039a | MS | |
| 19 | 989 | 2-pentylfuran | Fruity | 0.099 ± 0.015b | 0.132 ± 0.018b | 0.598 ± 0.070a | MS/RI |
| 20 | 1111 | 3-hydroxy-2-methylpyran-4-one (maltol) | Caramel-like | 0.385 ± 0.078a | 0.025 ± 0.004b | NDb | MS/RI |
| 21 | 1139 | 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one (hydroxydihydromaltol) | 0.082 ± 0.003b | 0.428 ± 0.085b | 20.838 ± 4.077a | MS/RI | |
| Sum of oxygen-containing heterocyclic compounds | 1.980 | 2.685 | 27.198 | ||||
| Aldehydes (6) | |||||||
| 22 | 886 | (Z)-2-ethenylbut-2-enal | 0.320 ± 0.058b | 0.354 ± 0.041b | 0.599 ± 0.044a | MS | |
| 23 | 905 | 3-methylsulfanylpropanal (methional) | Cooked potato-like | NDb | NDb | 0.760 ± 0.025a | MS/RI |
| 24 | 957 | benzaldehyde | Sweet, almond-like | NDb | NDb | 0.140 ± 0.016a | MS/RI |
| 25 | 1038 | hexanedial | NDb | 0.238 ± 0.037a | NDb | MS | |
| 26 | 1043 | 2-phenylacetaldehyde (benzeneacetaldehyde) | Sweet, floral | NDb | 0.115 ± 0.015b | 8.984 ± 1.007a | MS/RI |
| 27 | 1313 | (2E,4E)-deca-2,4-dienal | NDb | NDb | 0.207 ± 0.037a | MS/RI | |
| Sum of aldehydes | 0.320 | 0.706 | 10.690 | ||||
| Ketones (3) | |||||||
| 28 | < 800 | 1-hydroxybutan-2-one | Toasted | NDb | NDb | 0.057 ± 0.001a | MS |
| 29 | 938 | (Z)-hept-3-en-2-one | NDb | NDb | 0.234 ± 0.027a | MS/RI | |
| 30 | 1185 | 1-(3,4-dihydro-2H-pyridin-1-yl)ethanone | NDb | NDb | 0.413 ± 0.084a | MS | |
| Sum of ketones | 0.000 | 0.000 | 0.704 | ||||
| Alcohols (13) | |||||||
| 31 | < 800 | 3-methylbutan-1-ol (isopentyl alcohol) | 0.082 ± 0.006 b | 0.076 ± 0.002b | 0.155 ± 0.028a | MS/RI | |
| 32 | 801 | butane-2,3-diol | Creamy | NDc | 6.627 ± 0.676b | 8.388 ± 0.708a | MS/RI |
| 33 | 838 | 4-methylpentan-1-ol | NDb | NDb | 0.151 ± 0.011a | MS/RI | |
| 34 | 860 | furan-2-ylmethanol (furfuryl alcohol) | burnt | 0.067 ± 0.010b | 0.171 ± 0.015b | 1.307 ± 0.200a | MS/RI |
| 35 | 871 | hexan-1-ol | Fruity, herbal | 0.181 ± 0.006b | 0.117 ± 0.006b | 0.476 ± 0.097a | MS/RI |
| 36 | 885 | (5-methylfuran-2-yl)methanol | Caramel-like | NDb | NDb | 0.507 ± 0.044a | MS |
| 37 | 980 | oct-1-en-3-ol | Mushroom-like | 0.044 ± 0.005c | 0.073 ± 0.004b | 0.191 ± 0.004a | MS/RI |
| 38 | 1019 | 2-chlorocyclohexan-1-ol | 0.758 ± 0.154a | 0.483 ± 0.092b | NDc | MS/RI | |
| 39 | 1038 | phenylmethanol | 0.258 ± 0.004a | NDb | NDb | MS/RI | |
| 40 | 1088 | 2-methoxyphenol (o-guaiacol) | NDb | NDb | 0.296 ± 0.019a | MS/RI | |
| 41 | 1113 | 2-phenylethanol | Sweet, floral | 0.663 ± 0.107a | 0.274 ± 0.029b | NDc | MS/RI |
| 42 | 1314 | 4-ethenyl-2-methoxyphenol (4-vinylguaiacol) | Woody | NDb | NDb | 4.273 ± 0.801a | MS/RI |
| 43 | 1654 | (1S,4R)-1,6-dimethyl-4-propan-2-yl-3,4,4a,7,8,8a-hexahydro-2H-naphthalen-1-ol (α-cadinol) | 0.470 ± 0.086b | 0.497 ± 0.098b | 0.739 ± 0.110a | MS/RI | |
| Sum of alcohols | 2.523 | 8.319 | 16.483 | ||||
| Acid and Esters (4) | |||||||
| 44 | 921 | 2-ethylbutanoic acid | 1.054 ± 0.065a | NDb | NDb | MS/RI | |
| 45 | 1189 | methyl 2-hydroxybenzoate | 0.966 ± 0.191b | 0.703 ± 0.122b | 1.833 ± 0.145a | MS/RI | |
| 46 | 1370 | 2-(2-butoxyethoxy)ethyl acetate | 1.766 ± 0.340b | 2.861 ± 0.586b | 4.325 ± 0.757a | MS | |
| 47 | 1595 | [2,2,4-trimethyl-3-(2-methylpropanoyloxy)pentyl] 2-methylpropanoate | 0.092 ± 0.015b | 0.066 ± 0.005b | 0.139 ± 0.026a | MS | |
| Sum of acid and esters | 3.878 | 3.629 | 6.297 | ||||
| Sulfur-containing compounds (2) | |||||||
| 48 | 893 | methylsulfinylmethane (dimethyl sulfoxide) | Onion-like | 1.345 ± 0.171a | 0.158 ± 0.022b | 0.108 ± 0.015b | MS/RI |
| 49 | 923 | methylsulfonylmethane (dimethyl sulfone) | NDb | 0.095 ± 0.013a | NDb | MS/RI | |
| Sum of sulfur-containing compounds | 1.345 | 0.254 | 0.108 | ||||
| Terpene hydrocarbons (2) | |||||||
| 50 | 888 | 1,2-xylene (o-xylene) | Geranium, rubbery, spicy | 3.100 ± 0.605a | 0.382 ± 0.060c | 1.272 ± 0.253b | MS/RI |
| 51 | 1047 | (3E)-3,7-dimethylocta-1,3,6-triene (β-ocimene) | Warm herbaceous | 1.426 ± 0.064b | 1.611 ± 0.279b | 3.370 ± 0.512a | MS/RI |
| Sum of terpene hydrocarbons | 4.527 | 1.993 | 4.642 | ||||
1Retention indices were determined using n-alkanes C8-C22 as external standards on HP-5MS column
2Aroma description in previous reports (Lopes et al., 2021; Luning et al., 1994; Oh and Cho, 2021; Qin et al., 2011; Wang et al., 2020) was shown
3Average of contents compared to that of the internal standard mean ± standard deviation
4ND, not detected
5Identification was performed as follow: MS/RI, mass spectrum was consistent with that of Wiley mass spectrum database; MS, mass spectrum was consistent with that of Wiley mass spectrum database
6There are significant differences among samples having different letters in a row (p < 0.05)
The interaction and degradation of nonvolatile precursors in red pepper seeds, especially through the Maillard reaction and lipid degradation, could occur during thermal treatment (Schenker et al., 2002; Schieberle and Hofmann, 2002; Shibamoto, 1983). Thermal treatment produces large amounts of volatile compounds which contribute to the development of desirable aroma of roasted red pepper seeds. Pyrazines are representative nitrogen-containing heterocyclic compounds formed via the Maillard reaction and could contribute to the roasted, grilled, baked, and burnt sensory odor properties to roasted red pepper seeds (Amrani-Hemaimi et al., 1995; Schenker et al., 2002). Jung et al. (1999) previously analyzed 6 pyrazines in red pepper seed oils and found that more pyrazines were produced as the roasting time increased, similar to the findings of this study. Pyrroles are also nitrogen-containing heterocyclic compounds, which could be formed by the participation of proline and hydroxyl proline in Strecker degradation or the reaction of amino acids with furans (Rizzi, 1974; Shibamoto, 1983). In particular, 2-acetylpyrrole (no. 12), which is described as having a caramel-like odor and is one of the most abundant and frequently occurring pyrroles in thermally processed foods (Shibamoto, 1983; Schieberle and Hofmann, 2002) and was noticeably produced in red pepper seeds roasted at 200 °C in the present study. Oxygen-containing heterocyclic compounds could be generated through the predominant browning reaction of carbohydrates and could be associated with the caramel-like, sweet, fruity, and nutty odor notes of roasted red pepper seeds (Ohloff and Flament, 1979; Schieberle and Hofmann, 2002). In the present study, hydroxydihydromaltol (no. 21) was more rapidly produced in red pepper seeds roasted at 200 °C; it is often detected and described as having sweet and caramel-like odor in cooked and roasted foods (Buttery et al., 1999; Sekiwa et al., 1997; Lasekan, 2013). Carbonyls, including aldehydes and ketones, are also typical intermediates in the Maillard reaction, end products of enzymatic browning, or products of lipid oxidation (Weenen and van der Ven, 2001; Whitfield, 1992). Methional, benzaldehyde, 2-phenylacetaldehyde, and (2E,4E)-deca-2,4-dienal could be associated with boiled potato-like, sweet, bread-like, and oily odor notes in roasted red pepper seeds (Weenen and van der Ven, 2001).
Changes in fatty acids of raw and roasted red pepper seeds
The crude fat content of dried red peppers is generally 18–30% (Ku et al., 2008; Zou et al., 2015). Although the fat content varies in different parts of the red pepper (placenta: 23.4 g/100 g; seeds: 23.4 g/100 g; pericarp: 8.8 g/100 g; whole fruit: 8.9 g/100 g) (Ananthan et al., 2018), red pepper seed is particularly rich in polyunsaturated fatty acids, with linoleic acid being the primary fatty acid in both the pericarp (~ 40%) and seed (> 70%) (Kwon et al., 1998; Ananthan et al., 2018). In this study, the effect of roasting temperature on the contents of fatty acids in red pepper seeds were investigated. A total of 16 fatty acids, including 9 saturated fatty acids, 4 mono-unsaturated fatty acids, and 3 poly-unsaturated fatty acids, among 38 fatty acids were identified in raw and roasted red pepper seeds (Table 2). Quantitatively, linoleic acid was the predominant fatty acid (73.184 ± 0.528%), followed by palmitic acid (13.072 ± 0.697%) and oleic acid (9.193 ± 0.120%) with similar contents in raw seeds and seeds roasted at 100 and 200 °C. Similarly, no significant change was observed in fatty acid composition among red pepper seed oils extracted using different methods (Chouaibi et al., 2019), red pepper seed stored for different periods and with different packaging materials (Kwon et al., 1998), red pepper seeds dried following various methods (Pinar et al., 2021), and Plukenetia huayllabambana seeds roasted at different temperatures and times (Chirinos et al., 2016).
Table 2.
Fatty acids in raw and roasted red pepper seeds. (Unit: %)
| Fatty acids | Raw | Roasted at 100 °C | Roasted at 200 °C | |
|---|---|---|---|---|
| Saturated fatty acids | ||||
| Myristic acid | C14:0 | 0.141 ± 0.009a1 | 0.139 ± 0.002a | 0.136 ± 0.003a |
| Pentadecylic acid | C15:0 | 0.020 ± 0.002a | 0.021 ± 0.002a | 0.023 ± 0.002a |
| Palmitic acid | C16:0 | 13.072 ± 0.697a | 12.844 ± 0.031a | 12.734 ± 0.023a |
| Margaric acid | C17:0 | 0.085 ± 0.004a | 0.082 ± 0.002a | 0.086 ± 0.002a |
| Stearic acid | C18:0 | 1.941 ± 0.026a | 2.021 ± 0.007ab | 1.979 ± 0.020b |
| Arachidic acid | C20:0 | 0.227 ± 0.009a | 0.243 ± 0.003ab | 0.237 ± 0.003b |
| Behenic acid | C22:0 | 0.224 ± 0.010a | 0.235 ± 0.003a | 0.229 ± 0.003a |
| Tricosanoic acid | C23:0 | 0.042 ± 0.001a | 0.044 ± 0.002a | 0.045 ± 0.001a |
| Lignoceric aicd | C24:0 | 0.224 ± 0.008a | 0.237 ± 0.003ab | 0.232 ± 0.001b |
| Sum of saturated fatty acids | 15.977 | 15.865 | 15.731 | |
| Monounsaturated fatty acids | ||||
| Palmitoleic acid | C16:1 | 0.251 ± 0.003a | 0.245 ± 0.006a | 0.244 ± 0.008a |
| Oleic acid | C18:1n9c | 9.193 ± 0.120a | 9.104 ± 0.037a | 9.192 ± 0.015a |
| Vaccenic acid | C18:1n7c | 0.967 ± 0.020a | 0.960 ± 0.006a | 0.979 ± 0.008a |
| Gadoleic acid | C20:1 | 0.092 ± 0.005a | 0.092 ± 0.001a | 0.094 ± 0.002a |
| Sum of monounsaturated fatty acids | 10.502 | 10.400 | 10.509 | |
| Polyunsaturated fatty acids | ||||
| Linoleic acid | C18:2n6c | 73.184 ± 0.528a | 73.398 ± 0.033a | 73.415 ± 0.018a |
| α-Linolenic acid | C18:3n3c | 0.278 ± 0.004a | 0.278 ± 0.003a | 0.283 ± 0.002a |
| Eicosadienoic acid | C20:2 | 0.058 ± 0.002a | 0.059 ± 0.003a | 0.062 ± 0.002a |
| Sum of polyunsaturated fatty acids | 73.520 | 73.735 | 73.760 | |
1There are significant differences among samples having different letters in a row (p < 0.05)
In summary, capsaicin and dihydrocapsaicin concentrations decreased in red pepper seeds as the roasting temperature increased, showing a significant decrease at a temperature of 200 °C; however, there is no proportional relationship between pungency (i.e., capsaicinoid concentrations) and consumer preference. The concentrations of pyrazines, pyrroles, oxygen-containing heterocyclic compounds, carbonyls, and alcohols, generated via the Maillard reaction and lipid oxidation, increased in red pepper seeds as the roasting temperature increased; therefore, they may impart desirable savory odor notes to roasted red pepper seeds. However, roasting had no impact on the fatty acid concentrations of red pepper seeds, including linoleic acid. Given the lack of change in polyunsaturated fatty acid concentrations, reduced pungency, and enhanced aromas of roasted red pepper seeds, they may have useful applications in processed foods.
Acknowledgements
This research was supported by the Cooperative Research Program for Agriculture Science and Technology Department (Project No. PJ01453702) funded by the Rural Development Administration, Korea.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dahye Kim and Hyeyoung Park are Co-fisrt authors.
Contributor Information
Dahye Kim, Email: dahyekim0033@naver.com.
Hyeyoung Park, Email: qgd111@naver.com.
In Hee Cho, Email: inheecho@wku.ac.kr.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. 4. Carol Stream: Allured; 2007. [Google Scholar]
- Amrani-Hemaimi M, Cerny C, Fay LB. Mechanisms of formation of alkylpyrazines in the Maillard reaction. Journal of Agricultural and Food Chemistry. 1995;43:2818–2822. [Google Scholar]
- Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. British Journal of Anaesthesia. 2011;107:490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandakumar P, Kamaraj S, Jagan S, Ramakrishnan G, Asokkumar S, Naveenkumar C, Raghunandhakumar S, Devaki T. Capsaicin inhibits benzo(a)pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflammation Research. 2012;61:1169–1175. doi: 10.1007/s00011-012-0511-1. [DOI] [PubMed] [Google Scholar]
- Ananthan R, Subhash K, Longvah T. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chili (Capsicum chinense Jacq) Food Chemistry. 2018;238:51–57. doi: 10.1016/j.foodchem.2016.12.073. [DOI] [PubMed] [Google Scholar]
- AOAC. Official Method of Analysis of AOAC Intl. 19th ed. Method 996.06. Association of Official Analytical Chemists, Gaithersburg, MD, USA (2012)
- Buttery RG, Orts WJ, Takeoka GR, Nam Y. Volatile flavor components of rice cakes. Journal of Agricultural and Food Chemistry. 1999;47:4353–4356. doi: 10.1021/jf990140w. [DOI] [PubMed] [Google Scholar]
- Cazana FJD, Puyol MR, Caballero JP, Jimenez AJ, Duarte AM. Effect of dietary hyperlipidemia-hypercholesterolemia on rat erythrocytes. International Journal for Vitamin and Nutrition Research. 1990;60:392–397. [PubMed] [Google Scholar]
- Chirinos R, Zorrilla D, Aguilar-Galvez A, Pedreschi R, Campos D. Impact of roasting on fatty acids, tocopherols, phytosterols, and phenolic compounds present in Plukenetia huayllabambana seed. Journal of Chemistry. 2016;2016:1–10. [Google Scholar]
- Chouaibi M, Rezig L, Hamdi S, Ferrari G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Industrial Crops and Products. 2019;128:363–370. [Google Scholar]
- Cliff MA, Green BG. Sensitization and desensitization to capsaicin and menthol in the oral cavity: interactions and individual differences. Physiology and Behavior. 1996;59:487–494. doi: 10.1016/0031-9384(95)02089-6. [DOI] [PubMed] [Google Scholar]
- Forero MD, Quijano CE, Pino JA. Volatile compounds of chile pepper (Capsicum annuum L. var. glabriusculum) at two ripening stages. Flavour and Fragrance Journal. 2009;24:25–30. [Google Scholar]
- Jung MY, Bock JY, Baik SO, Lee JH, Lee TK. Effects of roasting on pyrazine contents and oxidative stability of red pepper seed oil prior to its extraction. Journal of Agricultural and Food Chemistry. 1999;47:1700–1704. doi: 10.1021/jf981028l. [DOI] [PubMed] [Google Scholar]
- Kim SW, Song SH, Shin SC, Lee MS, Chun IS. Distribution of pepper powder in mixture and its improvements. Korea Rural Economic Institute. 2017;C2017–1:1–157. [Google Scholar]
- Kim DH, Lee H, Cho IH. Characteristics of a tea made with roasted red pepper (Capsicum annum L.) seeds. Korean Journal of Food and Cookery Science. 2019;35:280–287. [Google Scholar]
- Kobata K, Sutoh K, Todo T, Yazawa S, Iwai K, Watanabe T. Nordihydrocapsiate, a new capsinoid from the fruits of a nonpungent pepper, Capsicum annuum. Journal of Natural Products. 1999;62:335–336. doi: 10.1021/np9803373. [DOI] [PubMed] [Google Scholar]
- Ku KH, Choi EJ, Park JB. Chemical component analysis of red pepper (Capsicum annuum L.) seeds with various cultivars. Journal of the Korean Society of Food Science and Nutrition. 2008;37:1084–1089. [Google Scholar]
- Kurniawan F, Madurani KA. Electrochemical and optical microscopy study of red pepper seed oil corrosion inhibition by self-assembled monolayers (SAM) on 304SS. Progress in Organic Coatings. 2015;88:256–262. [Google Scholar]
- Kwon JH, Lee GD, Byun MW, Choi KJ, Kim HK. Changes in water activity and fatty acid composition of dried red pepper during post irradiation period. Korean Journal of Food Science and Technology. 1998;30:1058–1063. [Google Scholar]
- Lasekan O. Volatile constituents of roasted tigernut oil (Cyperus esculentus L.) Journal of the Science of Food and Agriculture. 2013;93:1055–1061. doi: 10.1002/jsfa.5846. [DOI] [PubMed] [Google Scholar]
- Lopes GR, Petronilho S, Ferreira AS, Pinto M, Passos CP, Coelho E, Rodrigues C, Figueira C, Rocha SM, Coimbra MA. Insights on single-dose espresso coffee capsules’ volatile profile: from ground powder volatiles to prediction of espresso brew aroma properties. Foods. 2021;10:2508. doi: 10.3390/foods10102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luning PA, de Rijk T, Wichers HJ, Roozen JP. Gas chromatography, mass spectrometry, and sniffing port analyses of volatile compounds of fresh bell peppers (Capsicum annuum) at different ripening stages. Journal of Agricultural and Food Chemistry. 1994;42:977–983. [Google Scholar]
- Luning PA, Yuksel D, Vries R, Roozen JP. Aroma changes in fresh bell peppers (Capsicum annuum) after hot-air drying. Journal of Food Science. 1995;60:1269–1276. [Google Scholar]
- Manjunatha H, Srinivasan K. Hypolipidemic and antioxidant potency of heat processed turmeric and red pepper in experimental rats. African Journal of Food Science. 2008;2:1–6. [Google Scholar]
- Oh J, Cho IH. The aroma profile and aroma-active compounds of Brassica oleracea (kale) tea. Food Science and Biotechnology. 2021;30:1205–1211. doi: 10.1007/s10068-021-00962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohloff G and Flament I. The role of heteroatomic substances in the aroma compounds of foodstuffs. Fortschritte Der Chemie Organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. 36: 231-283 (1979)
- Orellana-Escobedo L, Ornelas-Paz JJ, Olivas GI, Guerrero-Beltran JA, Jimenez-Castro J, Sepulveda DR. Determination of absolute threshold and just noticeable difference in the sensory perception of pungency. Journal of Food Science. 2012;77:S135–S139. doi: 10.1111/j.1750-3841.2011.02589.x. [DOI] [PubMed] [Google Scholar]
- Ornelas-Paz JJ, Martínez-Burrola JM, Ruiz-Cruz S, Santana-Rodríguez V, Ibarra-Junquera V, Olivas GI, Pérez-Martínez JD. Effect of cooking on the capsaicinoids and phenolics contents of Mexican peppers. Food Chemistry. 2010;119:1619–1625. [Google Scholar]
- Özyıldız F, Karagönlü S, Basal G, Uzel A, Bayraktar O. Micro-encapsulation of ozonated red pepper seed oil with antimicrobial activity and application to nonwoven fabric. Letters in Applied Microbiology. 2013;56:168–179. doi: 10.1111/lam.12028. [DOI] [PubMed] [Google Scholar]
- Pinar H, Çetin N, Ciftci B, Karaman K, Kaplan M. Biochemical composition, drying kinetics and chromatic parameters of red pepper as affected by cultivars and drying methods. Journal of Food Composition and Analysis. 2021;102:103976. [Google Scholar]
- Prescott J. The generalizability of capsaicin sensitization and desensitization. Physiology and Behavior. 1999;66:741–749. doi: 10.1016/s0031-9384(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Qin P, Ma T, Wu L, Shan F, Ren G. Identification of tartary buckwheat tea aroma compounds with gas chromatography-mass spectrometry. Journal of Food Science. 2011;76:S401–S407. doi: 10.1111/j.1750-3841.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- Rizzi GP. Formation of N-alkyl-2-acyl-pyrroles and aliphatic aldimines in model nonenzymic browning reactions. Journal of Agricultural and Food Chemistry. 1974;22:279–282. [Google Scholar]
- Schenker S, Heinemann C, Huber M, Pompizzi R, Perren R, Escher F. Impact of roasting conditions on the formation of aroma compounds in coffee beans. Journal of Food Science. 2002;67:60–66. [Google Scholar]
- Schieberle P and Hofmann T. Flavor contribution and formation of heterocyclic oxygen-containing key aroma compounds in thermally processed foods. Heteroatomic Aroma Compounds. 207–226 (2002)
- Sekiwa Y, Kubota K, Kobayashi A. Characteristic flavor components in the brew of cooked clam (Meretrix lusoria) and the effect of storage on flavor formation. Journal of Agricultural and Food Chemistry. 1997;45:826–830. [Google Scholar]
- Shibamoto T. Heterocyclic compounds in browning and browning/nitrite model systems: occurrence, formation mechanisms, flavor characteristics and mutagenic activity. Instrumental Analysis of Foods. 1983;1:229–278. [Google Scholar]
- Srinivasan K. Spices as influencers of body metabolism: An overview of three decades of research. Food Research International. 2005;38:77–86. [Google Scholar]
- Srinivasan K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Critical Reviews in Food Science and Nutrition. 2016;56:1488–1500. doi: 10.1080/10408398.2013.772090. [DOI] [PubMed] [Google Scholar]
- Sung J, Lee J. Capsaicoside G, a furostanol saponin from pepper (Capsicum annuum L.) seeds, suppresses adipogenesis through activation of AMP-activated proein kinase in 3T3-L1 cells. Journal of Functional Foods. 2016;20:148–158. [Google Scholar]
- Suresh D, Manjunatha H, Srinivasan K. Effect of heat processing of spices on the concentrations of their bioactive principles: Turmeric (Curcuma longa), red pepper (Capsicum annuum) and black pepper (Piper nigrum) Journal of Food Composition and Analysis. 2007;20:346–351. [Google Scholar]
- Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 1998;402:259–267. doi: 10.1016/s0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xia Y, Wang J, Luo F, Huang Y. Capsaicinoids in chili pepper (Capsicum annuum L.) powder as affected by heating and storage methods. American Society of Agricultural Engineers. 2009;52:2007–2010. [Google Scholar]
- Wang X, Guo M, Song H, Meng Q. Characterization of key aroma compounds in traditional Chinese soy sauce through the molecular sensory science technique. LWT-Food Science and Technology. 2020;128:109413. [Google Scholar]
- Weenen H, van der Ven JGM. The formation of strecker aldehydes. American Chemical Society Symposium Series. 2001;794:183–195. [Google Scholar]
- Whitfield FB, Mottram DS. Volatiles from interactions of Maillard reactions and lipids. Critical Reviews in Food Science and Nutrition. 1992;31:1–58. doi: 10.1080/10408399209527560. [DOI] [PubMed] [Google Scholar]
- Yılmaz E, Arsunar ES, Aydeniz B, Güneşer O. Cold pressed capia pepperseed (Capsicum annuum L.) oils: composition, aroma, and sensory properties. European Journal of Lipid Science and Technology. 2015;117:1016–1026. [Google Scholar]
- Zou Y, Ma K, Tian M. Chemical composition and nutritive value of hot pepper seed (Capsicum annuum) grown in Northeast Region of China. Food Science and Technology. 2015;35:659–663. [Google Scholar]