Abstract
An experimental respiratory model was used to investigate the interaction between Mycoplasma hyopneumoniae and swine influenza virus (SIV) in the induction of pneumonia in susceptible swine. Previous studies demonstrated that M. hyopneumoniae, which produces a chronic bronchopneumonia in swine, potentiates a viral pneumonia induced by the porcine reproductive and respiratory syndrome virus (PRRSV). In this study, pigs were inoculated with M. hyopneumoniae 21 days prior to inoculation with SIV. Clinical disease as characterized by the severity of cough and fever was evaluated daily. Percentages of lung tissue with visual lesions and microscopic lesions were assessed upon necropsy at 3, 7, 14, and 21 days following SIV inoculation. Clinical observations revealed that pigs infected with both SIV and M. hyopneumoniae coughed significantly more than pigs inoculated with a single agent. Macroscopic pneumonia on necropsy at days 3 and 7 was greatest in both SIV-infected groups, with minimal levels of pneumonia in the M. hyopneumoniae-only-infected pigs. At 14 days post-SIV inoculation, pneumonia was significantly more severe in pigs infected with both pathogens. However, by 21 days postinoculation, the level of pneumonia in the dual-infected pigs was similar to that of the M. hyopneumoniae-only-infected group, and the pneumonia in the pigs inoculated with only SIV was nearly resolved. Microscopically, there was no apparent increase in the severity of pneumonia in pigs infected with both agents compared to that of single-agent-challenged pigs. The results of this study found that while pigs infected with both agents exhibited more severe clinical disease, the relationship between the two pathogens lacked the profound potentiation found with dual infection with M. hyopneumoniae and PRRSV. These findings demonstrate that the relationship between mycoplasmas and viruses varies with the individual agent.
Porcine respiratory disease complex (PRDC) is an economically significant respiratory disorder characterized by slow growth, decreased feed efficiency, lethargy, anorexia, fever, cough, and dyspnea (5). Diagnostic laboratories have isolated multiple pathogens from cases of PRDC, including porcine reproductive and respiratory syndrome virus (PRRSV), Mycoplasma hyopneumoniae, swine influenza virus (SIV), Actinobacillus pleuropneumoniae, and pseudorabies virus (PRV) (5). Of these pathogens, PRRSV, M. hyopneumoniae, and SIV are most frequently detected in 10- to 22-week-old pigs with clinical signs of PRDC (5). Recent studies found that infection with M. hyopneumoniae potentiated and prolonged PRRSV-induced pneumonia based on clinical, macroscopic, and microscopic findings (12). Van Reeth et al. found that the clinical effects of PRRSV were exacerbated with concurrent infection with SIV (19). The purpose of the study reported here was to investigate the interaction between M. hyopneumoniae and SIV.
SIV infects the epithelium of the respiratory tract of pigs, inducing an acute infection with clinical signs consisting of cough, fever, lethargy, and anorexia beginning 1 to 2 days after experimental infection and lasting for 3 to 4 days (17). Macroscopic lung lesions observed in pigs with SIV are characterized by well-demarcated purplish-red lesions in the cranioventral lobes of the lungs. Microscopic lesions consist of epithelial disruption and attenuation in the bronchioles and interstitial pneumonia. Mild to moderate peribronchiolar and perivascular lymphocytic infiltration occurs at nearly all levels of the airways. Viral antigen can be detected in epithelial cells of airways and in alveoli by immunohistochemistry (IHC) (20).
M. hyopneumoniae attaches to the cilia of the respiratory epithelium of the airways. M. hyopneumoniae infection is characterized by a chronic, mild, dry, nonproductive cough beginning 10 to 14 days after experimental infection. Fever, lethargy, or anorexia is rarely observed in pigs infected only with M. hyopneumoniae. Lung lesions observed in pigs infected with M. hyopneumoniae are similar to those observed in pigs with SIV, with dark purplish areas of lung consolidation occurring primarily in the cranioventral areas of the lung. In contrast to SIV, lesions induced by M. hyopneumoniae are slow to develop, taking 2 to 3 weeks to appear. Microscopic lesions associated with M. hyopneumoniae infection consist of peribronchiolar and perivascular mononuclear infiltrates. As the disease progresses, prominent lesions consisting of extensive mononuclear cuffing and occasional lymphoid germinal center formation occur around the airways and vascular system. Mild interstitial pneumonia consisting primarily of swollen pneumocytes and alveolar edema are also occasionally observed during the acute stages of infection.
Both M. hyopneumoniae and SIV affect the epithelial cells and the mucociliary apparatus of the airways; thus, combined infections could enhance the development of secondary bacterial pneumonias. The pathogenesis of mycoplasmal pneumonia is dependent not only on the damage caused to the cilia directly by the organism but also on the effects on cells of the host's immune system (7). The study reported here found that the interaction between M. hyopneumoniae and SIV differed from that observed between M. hyopneumoniae and PRRSV. The relationship between M. hyopneumoniae and SIV appeared independent of each other, with the pneumonia induced by each pathogen following a normal course over time. Pneumonia induced by M. hyopneumoniae and SIV together lacked the overall severity of virus pneumonia potentiation observed in earlier studies with PRRSV. This report, along with previous studies, demonstrates via an in vivo model that the effect of mycoplasmas on viral infections varies with the viral agent.
MATERIALS AND METHODS
Pigs.
Seventy-two 10- to 12-day-old crossbred (landrace, large white, and duroc) pigs were obtained from a commercial herd serologically negative for PRRSV, M. hyopneumoniae and SIV. The pigs were assigned to four treatment groups and four necropsy groups with stratification by arrival weight. Pigs were fed a commercial ground feed and water ad libitum throughout the trial. The study was conducted in accordance with the guidelines of the Iowa State University Institutional Committee on Animal Care and Use.
Challenge inocula and experimental design.
The experimental design is presented in Table 1. At 5 weeks of age, a tissue homogenate containing strain 232, a derivative of M. hyopneumoniae strain 11 (105 color-changing units per ml), was administered intratracheally to pigs in the M. hyopneumoniae (MHYO)- and dual-infected (DUAL) groups at a dilution of 1:100 in 10 ml of mycoplasmal Friis medium (10). Twenty-one days later, 5 ml of SIV at 106.9 50% egg infectious doses/ml of SIV strain A/Swine/IA/40776/92 (H1N1) (generously provided by Pat Foley, National Veterinary Services Laboratory, Ames, Iowa) was administered to the SIV and DUAL groups in an aerosol dose by nebulization over a 5-min period. The hemagglutination titer of the inoculum was 1:128.
TABLE 1.
Experimental design—infection status and number of pigs necropsied on each of 4 postinfection days
| Group | Infection status (day of inoculationa) | No. of pigs necropsied at day:
|
Total no. of pigs necropsied | |||
|---|---|---|---|---|---|---|
| 3 | 7 | 14 | 21 | |||
| MHYO | M. hyopneumoniae (−21) | 5 | 5 | 5 | 5 | 20 |
| SIV | SIV (0) | 5 | 5 | 5 | 5 | 20 |
| DUAL | SIV (0) and M. hyopneumoniae (−21) | 5 | 5 | 5 | 5 | 20 |
| CONTROL | No infection | 3 | 3 | 3 | 3 | 12 |
Pigs were 8 weeks old on day 0.
Clinical evaluation.
Pigs were evaluated daily for symptoms of respiratory disease, including appetite (abdominal fill), cough, tachypnea, dyspnea, or behavioral changes for a period of 15 min, throughout the study. Rectal temperatures were measured for the first 8 days postinoculation (DPI) with SIV, after which time rectal temperatures had returned to normal. Pigs were weighed upon arrival, prior to challenge, and upon necropsy.
Serology.
Blood was collected periodically throughout the trial. Sera were tested for SIV antibodies by hemagglutination-inhibition assay by the Iowa State University Veterinary Diagnostic Laboratory (9). M. hyopneumoniae antibody levels were determined by enzyme-linked immunosorbent assay as previously described (2). Known positive and negative sera were included as controls in each plate. Readings more than two standard deviations above the mean value of the negative control were considered positive.
Pathologic examination.
Pigs were necropsied 3, 7, 14, or 21 DPI. The right rib cage was reflected and the lungs were removed and evaluated for macroscopic lesions. A portion of lung was aseptically collected for M. hyopneumoniae isolation, and additional samples were collected for fluorescent antibody (FA) assay for M. hyopneumoniae, SIV IHC, and histopathologic examination. Lesions consistent with mycoplasmal or SIV pneumonia (dark red-to-purple lobular consolidation) were sketched onto a standard lung diagram. The proportion of lung surface with lesions was determined from the diagram using a Zeiss SEM-IPS image analyzing system (10).
Tissue samples were taken from all lung lobes, fixed in 10% neutral buffered formalin, and processed and embedded in paraffin using an automated tissue processor. Lung sections were examined for peribronchiolar and perivascular lymphoid cuffing and lymphoid nodule formation. Epithelial lesions were evaluated based on disruption and attenuation of the epithelial cell lining of the bronchi and on epithelial proliferation, including polyp formation. The examiner was blinded to the treatment group and necropsy date.
M. hyopneumoniae isolation and FA detection.
Isolation of M. hyopneumoniae was performed from lung sections as described previously (10). Mycoplasma-appearing colonies were specifically identified using epi-immunofluorescence with conjugate prepared from pig antisera to M. hyopneumoniae strain 11 (4). A direct FA assay was used for detection of M. hyopneumoniae in lung tissues using a previously described method (1).
SIV antigen detection.
SIV-specific antigen was detected in lung tissues using a previously described IHC method (20). IHC was performed on sections cut from one paraffin-embedded lung tissue block which included three pieces (1 by 2 cm) of lung from the left cranial, middle, and caudal lobes.
Statistics.
Data were subjected to analysis of variance. If the P value from the analysis of variance was less than or equal to 0.05, pairwise comparisons of the different treatment groups were performed by least significant difference at the P < 0.05 rejection level.
RESULTS
Clinical disease.
Clinical disease data (coughing and rectal temperatures) are summarized in Table 2. The data from pigs necropsied 3 DPI was not included in the analysis, as there were no differences in clinical signs up to that time. Clinical disease began to be observed at approximately 3 DPI. Both SIV-inoculated groups (SIV and DUAL groups) had significantly more days with rectal temperatures of ≥104°F than either the noninfected controls (CONTROL group) or pigs infected only with M. hyopneumoniae (termed the MHYO group). Pigs inoculated with SIV and M. hyopneumoniae (DUAL) coughed for significantly more days than any of the other groups. No other significant signs associated with respiratory disease were observed. Over the course of the trial, SIV-infected pigs had significantly decreased weight gain at 7 and 14 DPI (data not shown). At the final necropsy (21 DPI), the weight gain of pigs/day in all infected groups was significantly lower than that of the CONTROL group, as shown in Table 2.
TABLE 2.
Summary (group mean ± standard deviation) of clinical observations following challenge with M. hyopneumoniae and/or SIVa
| Group | Infection status | Rectal temperatureb | Coughing scorec | Gain per dayd |
|---|---|---|---|---|
| MHYO | M. hyopneumoniae | 0.05 ± 0.09 A,B | 0.10 ± 0.23 A,B | 1.45 ± 0.11 A,B |
| SIV | SIV | 0.37 ± 0.22 B,C | 0.27 ± 0.27 B | 1.30 ± 0.16 A |
| DUAL | SIV and M. hyopneumoniae | 0.45 ± 0.22 C | 0.78 ± 0.38 C | 1.51 ± 0.10 B |
| CONTROL | No infection | 0.27 ± 0.26 B | 0.0 ± 0.0 A | 1.92 ± 0.06 C |
Within each column, values followed by different letters (A, B, C) are significantly different (P < 0.05).
Proportion of days (out of 7 total) that each pig's rectal temperature was ≥104°F.
Proportion of days (out of 7 total) that each pig was observed coughing.
Average daily weight gain (pounds) of pigs at 21 days post-SIV inoculation.
Macroscopic lesions.
Group mean percentages of lung tissue with visible pneumonia are presented in Table 3. None of the control pigs had observable pneumonia at any necropsy date. Upon necropsy at 3 DPI, pigs infected with SIV and/or M. hyopneumoniae exhibited pneumonia. Both SIV-infected groups had significantly higher percentages of pneumonia than the control group; however, no differences were observed between the SIV and the DUAL groups and the MHYO group.
TABLE 3.
Percentage of lung with visible pneumonia lesions as determined by lesion sketches and image analysis for pigs challenged with M. hyopneumoniae and/or SIV
| Group | Infection status | % of lung with visible lesions on necropsy at DPIa:
|
|||
|---|---|---|---|---|---|
| 3 | 7 | 14 | 21 | ||
| MHYO | M. hyopneumoniae | 2.1 ± 1.8 A,B | 3.6 ± 3.1 A,B | 3.3 ± 2.6 A | 7.6 ± 7.4 |
| SIV | SIV | 10.3 ± 7.4 B | 8.8 ± 5.8 B,C | 3.3 ± 2.5 A | 1.1 ± 1.4 |
| DUAL | SIV and M. hyopneumoniae | 15.0 ± 6.0 B | 9.8 ± 3.5 C | 11.4 ± 6.3 B | 4.6 ± 4.0 |
| CONTROL | No infection | 0.0 ± 0.0 A | 0.0 ± 0.0 A | 0.0 ± 0.0 A | 0.0 ± 0.0 |
Pigs were 8 weeks old on day 0. Within each column, values followed by different letters (A, B, or C) are significantly different (P < 0.05). Data are means ± standard deviations.
At 7 DPI, there was no difference in the percentage of pneumonia between the SIV and MHYO groups. However, the percentage of pigs with pneumonia in the DUAL group was significantly higher than that in the MHYO group. No difference was found between the DUAL group and the SIV group.
At 14 DPI, pigs in the DUAL group had significantly higher percentages of pneumonia than those in either the SIV or the MHYO group. The percentage of pneumonia in the SIV group was similar to that observed in the MHYO group.
At 21 DPI, no statistical differences in the percentages of pneumonia were observed between any of the groups of pigs, including the CONTROL group, due to high variability within the groups. However, the MHYO and DUAL groups tended to have higher percentages than the SIV group. The pneumonia in the group inoculated only with SIV was nearly resolved.
Microscopic lesions.
Characteristic M. hyopneumoniae lesions consisting of peribronchiolar and perivascular mononuclear infiltration were present in the majority of M. hyopneumoniae-inoculated pigs. M. hyopneumoniae-infected pigs had less inflammation at the alveolar level than either the SIV or DUAL pigs at 3 DPI (Fig. 1). Pigs in the MHYO or DUAL groups exhibited peribronchial lymphoid cell infiltrate around the large and medium airways at 14 DPI, while the SIV-infected pigs had none around the large airways and little around medium airways.
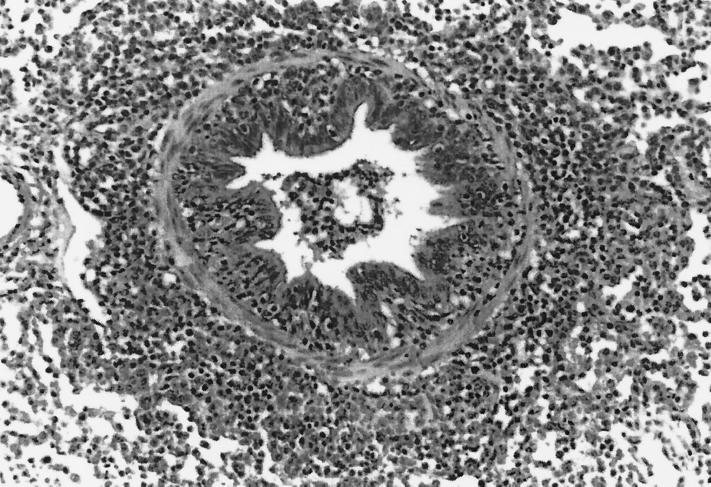
FIG. 1.
Bronchiole in the lung of a pig inoculated only with M. hyopneumoniae and euthanatized 28 DPI. The bronchiole is lined by normal tall columnar epithelial cells and is surrounded by a prominent cuff of infiltrating lymphocytes.
Lesions consistent with SIV infection were characterized by epithelial damage and mild peribronchiolar and perivascular infiltration by inflammatory cells and were present in all SIV-infected pigs. Epithelial damage was greatest in medium-sized airways at 3 DPI (Fig. 2). Damage to small to terminal airways appeared to develop slightly later, with minimal epithelial disruption at 3 DPI and more extensive lesions at 7 DPI (Fig. 3). No epithelial lesions were observed in M. hyopneumoniae-only-infected pigs. SIV-infected pigs had more epithelial lesions in the large bronchi at 3 DPI than either the CONTROL or MHYO group. However, by 7 DPI, the SIV and DUAL groups had equivalent levels of epithelial pathology, with lesions predominantly of proliferative epithelial repair in medium-sized bronchioles. Epithelial lesions had resolved in all groups by 14 DPI and were absent at 21 DPI.
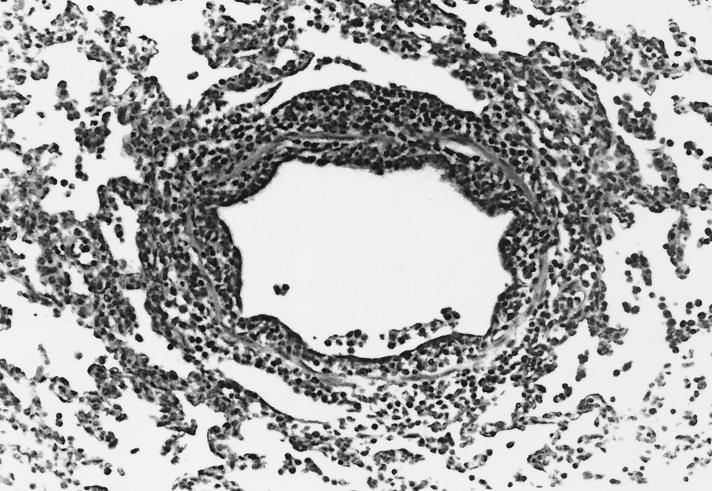
FIG. 2.
Bronchiole in the lung of a pig inoculated only with SIV and euthanatized 3 DPI. The bronchiole is lined by a thin layer of attenuated epithelium subsequent to necrosis and sloughing of epithelial cells infected with the virus. A light loose lymphocytic infiltrate surrounds the bronchiole.
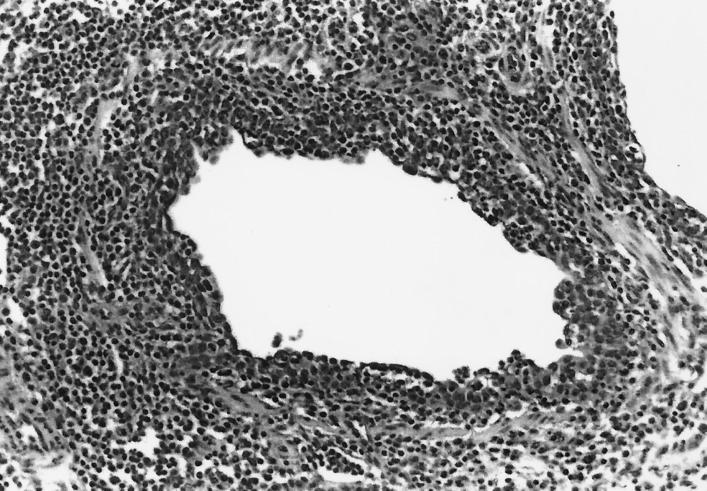
FIG. 3.
Bronchiole in the lung of a pig inoculated with both M. hyopneumoniae and SIV. The pig was euthanatized 3 DPI with SIV and 24 DPI with M. hyopneumoniae. The bronchiole exhibits the characteristic disruption and attenuation of the epithelial layer and early irregular reactive proliferation subsequent to SIV infection and intense peribronchiolar lymphocyte infiltration induced by both virus and mycoplasma.
M. hyopneumoniae isolation.
M. hyopneumoniae was isolated from all pigs in both of the M. hyopneumoniae-infected groups (MHYO and DUAL) at all necropsies. No M. hyopneumoniae was isolated from any of the pigs of the SIV and CONTROL groups. At 3 DPI, four of five and two of five pigs were positive by FA for M. hyopneumoniae antigens in MHYO and DUAL groups, respectively, and five of five and three of five at 7 DPI. M. hyopneumoniae antigen was detected by FA in all pigs in the mycoplasma-infected groups at 14 and 21 DPI.
SIV antigen detection.
The distribution of SIV antigen, as determined by IHC, was similar in both SIV and DUAL groups. Minimal viral antigen was detected in large airways at 3 DPI and none at any of the later dates. Many infected cells were present in both medium and small airways at 3 DPI but were no longer present by 7 DPI. Low numbers of irregularly scattered cells expressing viral antigen were present in the alveoli of all SIV and DUAL pigs at 3 DPI and in three of five pigs in the SIV group and two of five pigs in the DUAL group at 7 DPI. No virus was detected in the bronchioles or alveoli of any of the SIV or pigs in the DUAL group at 14 and 21 DPI.
Serology.
All pigs remained seronegative for antibodies against M. hyopneumoniae until 14 DPI (35 days after M. hyopneumoniae challenge), when two of five M. hyopneumoniae-infected pigs and three of five pigs in the DUAL group seroconverted. At 21 DPI, only one of five M. hyopneumoniae-infected pigs had seroconverted, while all of the pigs in the DUAL group were seropositive. Serum antibodies for SIV as measured by hemagglutination-inhibition assay were present in all SIV-inoculated pigs by 7 DPI, and all pigs remained positive throughout the remainder of the trial. There were no significant differences in levels of antibodies between any of the groups.
DISCUSSION
The combined effect of dual infection with SIV and M. hyopneumoniae appeared to be additive and transitory in nature, with minimal interaction between the two pathogens. This is in contrast to a previous study, where M. hyopneumoniae clearly potentiated and prolonged the pneumonia induced by PRRSV (12). At 14 DPI pigs in the DUAL group exhibited significantly more pneumonia than pigs in the SIV or MHYO group. However, the SIV-induced microscopic lesions had resolved by 14 DPI and there was no evidence of viral antigen by IHC. No differences were noted in the type or severity of microscopic lesions in the DUAL group pigs compared to those of the SIV or MHYO group pigs throughout the trial. A limitation to the study reported here was the inability to differentiate SIV-induced pneumonia from M. hyopneumoniae-induced pneumonia macroscopically. Therefore, the temporal development and resolution of SIV-induced lesions were based primarily on microscopic examination.
Clinical disease, as measured by coughing, was clearly greater in the pigs in the DUAL group. The frequency of coughing in the MHYO and SIV groups was minimal. The amount of coughing in the DUAL pigs was typical of field outbreaks of SIV.
The pathogenesis of disease induced in pigs infected with both SIV and M. hyopneumoniae appears to differ from that observed with PRRSV and M. hyopneumoniae coinfection. The relatively severe potentiation and prolongation of PRRSV-induced pneumonia by M. hyopneumoniae may be due to the inflammatory responses elicited in the course of both mycoplasmal and PRRSV pneumonia. PRRSV infects cells of the macrophage/monocytic cell lineage (15). The M. hyopneumoniae-induced infiltration of macrophages and lymphocytes into the lung parenchyma may provide a steady supply of susceptible cells for PRRSV to infect, thus potentiating and prolonging the viral pneumonia. In addition, the inflammation induced by both infections may combine to further modulate the local immune response so that clearance of PRRSV is reduced as well.
In contrast to PRRSV, SIV infects only the airway epithelium. SIV infection and the lesions induced are relatively transitory, suggesting that an effective immune response is mounted quickly (17, 18). Coinfection with M. hyopneumoniae didn't significantly alter the course of SIV. The combined pneumonias appeared more additive in nature, with minimal interaction between the two pathogens. By 21 DPI, pneumonia in the SIV group was nearly resolved and the levels of pneumonia in the DUAL and MHYO groups were similar.
As previously stated, M. hyopneumoniae decreases the function of the mucociliary apparatus (3). Disruption of airway epithelium by SIV as observed in this study would also compromise the mucociliary apparatus. Under field conditions, disruption of the mucociliary apparatus and airway epithelium would potentially lead to increased secondary infections from opportunistic organisms, leading to the increased pneumonia frequently observed with these two pathogens. In this study, no secondary infections were observed due to the high health status of the pigs and the relatively clean environment maintained in the isolation facilities, and thus the likelihood of pneumonia due to opportunistic bacteria is minimal compared to that of commercial farm operations.
It is of some interest that all DUAL-infected pigs had developed M. hyopneumoniae antibodies at 21 days post-SIV infection. Typically, experimentally infected pigs in our M. hyopneumoniae model begin seroconverting at approximately 4 weeks postinfection (13, 14). At 21 days post-SIV infection, pigs would have been infected 42 days previously with M. hyopneumoniae. It is possible that damage to the respiratory epithelium due to SIV infection could increase the rate of seroconversion to M. hyopneumoniae. However, no statistical differences in serum antibody levels was observed in this study.
Mycoplasmas in a variety of animal species are known to produce chronic and primarily silent infections. In addition, they have been identified as cofactors in a number of chronic disorders in humans, including AIDS, malignant transformation, chronic fatigue syndrome, and various arthritides (6, 8, 11, 16). The findings in this study along with previous PRRSV and M. hyopneumoniae coinfection studies suggest that the influence of M. hypopneumoniae on viral pneumonias in pigs varies with the individual viral agent. Further characterization of the differences in the interaction between mycoplasmas and viruses will ultimately improve our understanding of the mechanisms that result in the severe pneumonia observed in PRDC cases.
ACKNOWLEDGMENTS
We thank N. Upchurch, S. Siegal, T. Young, B. Erickson, T. Boettcher, R. Royer, and A. Vincent for technical assistance.
This work was supported by a grant from the Iowa Healthy Livestock Initiative.
REFERENCES
- 1.Amanfu W, Weng C N, Ross R F, Barnes H J. Diagnosis of mycoplasmal pneumoniae of swine: sequential study by direct immunofluorescence. Am J Vet Res. 1984;45:1349–1352. [PubMed] [Google Scholar]
- 2.Bereiter M, Young T F, Joo H S, Ross R F. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae in swine serum. Vet Microbiol. 1990;25:177–192. doi: 10.1016/0378-1135(90)90075-7. [DOI] [PubMed] [Google Scholar]
- 3.DeBey M C, Ross R F. Ciliostasis and loss of cilia induced by Mycoplasma hyopneumoniae in porcine tracheal organ cultures. Infect Immun. 1994;62:5312–5318. doi: 10.1128/iai.62.12.5312-5318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Giudice R A, Robillard N F, Carski T R. Immunofluorescence identification of mycoplasma on agar by use of incident illumination. J Bacteriol. 1967;93:1205–1209. doi: 10.1128/jb.93.4.1205-1209.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbur P G. Proceedings of the 15th Congress of the International Pig Veterinary Society. Nottingham, United Kingdom: Nottingham University Press; 1998. Porcine respiratory diseases; pp. 1–10. [Google Scholar]
- 6.Lo S-C. Mycoplasmas and AIDS. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 525–545. [Google Scholar]
- 7.Messier S, Ross R F, Paul P S. Humoral and cellular responses of pigs inoculated with Mycoplasmas hyopneumoniae. Am J Vet Res. 1990;51:52–58. [PubMed] [Google Scholar]
- 8.Nicolson G, Nicolson N L. Diagnosis and treatment of mycoplasmal infections in Gulf War Illness-CFIDS patients. Int J Occup Med Immunol Toxicol. 1996;5:69–78. [Google Scholar]
- 9.Palmer D F, Coleman M T, Dowdle W R, Schild G C. Immunology series No. 6. Washington, D.C.: United States Department of Health, Education and Welfare; 1975. Advanced laboratory techniques for influenza diagnosis; pp. 51–52. [Google Scholar]
- 10.Ross R F, Zimmermann-Erickson B J, Young T F. Characteristics of protective activity of Mycoplasmas hyopneumoniae vaccine. Am J Vet Res. 1984;45:1899–1905. [PubMed] [Google Scholar]
- 11.Taylor-Robinson D, Schaeverbeke T. Mycoplasmas in rheumatoid arthritis and other human arthritides. J Clin Pathol. 1996;49:781–782. doi: 10.1136/jcp.49.10.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thacker E L, Halbur P G, Ross R F, Thanawongnuwech R, Thacker B J. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999;37:620–627. doi: 10.1128/jcm.37.3.620-627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thacker E L, Thacker B J, Boettcher T B, Jayappa H. Comparison of antibody production, lymphocyte stimulation and production induced by four commercial Mycoplasma hyopneumoniae bacterins. Swine Health Prod. 1998;6:107–112. [Google Scholar]
- 14.Thacker E L, Thacker B J, Kuhn M, Hawkins P A, Waters W R. Mucosal and systemic characteristics of protective activity of a Mycoplasma hyopneumoniae bacterin. Am J Vet Res. 2000;61:1384–1389. doi: 10.2460/ajvr.2000.61.1384. [DOI] [PubMed] [Google Scholar]
- 15.Thanawongnuwech R, Young T F, Thacker B J, Thacker E L. Differential production of proinflammatory cytokines: in vitro PRRSV and Mycoplasma hyopneumoniae co-infection model. Vet Immunol Immunopathol. 2001;79:115–127. doi: 10.1016/s0165-2427(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 16.Tsai S, Wear D J, Shih J W-K, Lo S-C. Mycoplasmas and oncogenesis: persistent infection and multistage malignant transformation. Proc Natl Acad Sci USA. 1995;92:10197–10201. doi: 10.1073/pnas.92.22.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Reeth K, Nauwynck H. Proinflammatory cytokines and viral respiratory disease in pigs. Vet Res. 2000;31:187–213. doi: 10.1051/vetres:2000113. [DOI] [PubMed] [Google Scholar]
- 18.Van Reeth K, Nauwynck H, Pensaert M. Bronchoalveolar interferon-alpha, tumor necrosis factor-alpha, interleukin-1, and inflammation during acute influenza in pigs: a possible model for humans? J Infect Dis. 1998;177:1076–1079. doi: 10.1086/517398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Reeth K, Nauwynck H, Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent L L, Janke B H, Paul P S, Halbur P G. A monoclonal-antibody-based immunohistochemical method for the detection of swine influenza virus in formalin-fixed, paraffin-embedded tissues. J Vet Diagn Investig. 1997;9:191–195. doi: 10.1177/104063879700900214. [DOI] [PubMed] [Google Scholar]