Abstract
Objective:
To evaluate the cardiometabolic outcomes associated with discordant visceral adipose tissue (VAT) and liver fat (LF) phenotypes in two cohorts.
Patients and Methods:
Participants in the Dallas Heart Study (DHS) underwent baseline imaging between January 2000-December 2002 and were followed for incident cardiovascular disease (CVD) and type 2 diabetes (T2DM) through 2013. Associations between VAT/LF groups (low/low, high/low, low/high, and high/high) and outcomes were assessed using multivariable-adjusted regression and were replicated in the independent UK Biobank (UKB, 2014-2020).
Results:
DHS included 2064 participants (mean age 44 (SD 9) years; 54% female; 47% black). High VAT-high LF and high VAT-low LF were associated with prevalent atherosclerosis whereas low VAT-high LF was not. Among 1731 participants without CVD/T2DM, 128 (7.4%) developed CVD and 95 (5.5%) T2DM over median 12 years. High VAT-high LF and high VAT-low LF were associated with increased risk for CVD (HR 2.0 [95% CI 1.3–3.2] and HR 2.4 [95% CI 1.4 – 4.1]) and T2DM (OR 7.8 [95% CI 3.8–15.8] and OR 3.3 [95% CI 1.4–7.8], respectively); whereas low VAT-high LF was associated with T2DM (OR 2.7 [95% CI 1.1–6.7]). In the UKB (N=22,354 April 2014-May 2020), only high VAT-low LF remained associated with CVD after multivariable adjustment for age and body mass index (HR 1.5 [95% CI 1.2 – 1.9]).
Conclusion:
Although VAT and LF are each associated with cardiometabolic risk, these observations demonstrate the importance of separating their cardiometabolic implications when there is presence or absence of one or both in an individual.
Introduction:
Approximately 42% of adults in the United States are currently estimated to have obesity.1 Abdominal obesity, in particular, has been linked to adverse cardiometabolic health outcomes.2, 3 However, there is significant heterogeneity in the manifestation of abdominal obesity, and variable manifestations of abdominal fat accumulation in visceral adipose tissue (VAT), and ectopic sites (i.e. fat deposition in non-adipose tissue containing organs) such as excess liver fat (LF), results in differing risk of developing cardiometabolic disease.4, 5 Exact mechanisms of individual variation in fat deposition are not fully understood, but multiple factors have been studied including age, race, sex, and genetics. Although both excess VAT and LF have been associated with metabolic abnormalities predictive of increased risk of cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM),6,7 it is important to separate their cardiometabolic implications when there is presence or absence of one or both in an individual patient.
Some studies suggest that while VAT is positively associated with CVD and T2DM, LF may be more variable such that individuals with elevated VAT may have a higher prevalence of T2DM when LF is elevated while, conversely, the prevalence of coronary heart disease may be higher when LF is low in individuals with high VAT.8 Although LF is an independent abdominal fat depot from VAT, the two are commonly present together, therefore, the respective contributions of excess VAT vs. LF to CVD and T2DM risk remain debated. With the rising prevalence of non-alcoholic fatty liver disease (NAFLD) in the population, it is important to understand how these two separate fat depots coassociate with cardiometabolic health.9
Therefore, we aimed to examine the associations of VAT and LF, both isolated and combined, with cardiometabolic risk variables in a cross-sectional analysis of a multiethnic North American cohort and to prospectively compare the differences between four phenotypes of high/low VAT/LF in the incidence of CVD and T2DM over longitudinal follow-up. Longitudinal analyses were then replicated in a large European cohort. We hypothesized that the high VAT-low LF phenotype would be more closely associated with atherosclerosis and CVD, whereas the high VAT-high LF phenotype would be more closely associated with metabolic disease such as T2DM and less with atherosclerosis and CVD.
Methods:
Study Population
The DHS is a multiethnic, population-based study of Dallas County residents, with over-sampling of African Americans with the goal of improving the diagnosis, prevention, and treatment of heart disease. Details of the study have been described previously.10 For the present study, we performed both a cross-sectional analysis and a longitudinal analysis. For the cross-sectional analysis, among the 3072 participants in DHS-1, we excluded participants missing VAT or LF measurements (N=803); those with excessive alcohol use (N=192), and those who died within one year of the baseline visit (N=13) to avoid confounding bias. All remaining participants (N=2064) were included for cross-sectional analyses of prevalent traditional risk factors and atherosclerotic endpoints. For biomarker and lipoprotein analysis, we further excluded individuals receiving aspirin, lipid lowering, or glucose lowering medications since these medications may influence levels of biomarkers. For longitudinal analysis of CVD and T2DM outcomes, from the 2064 individuals included in the cross-sectional analysis, we excluded an additional N=333 participants who had prevalent CVD or T2DM at study entry (Supplemental Figure 1) in order to assess incident (new-onset) outcomes among apparently healthy and non-diabetic individuals who were asymptomatic for CVD (N=1731). The study period for longitudinal analysis was from the onset of the study in January 1, 2000 through December 31, 2013. All participants provided written informed consent, and the protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
The incident CVD and T2DM analysis was replicated using data from the UK Biobank (UKB) imaging study – a population based sub study to the large UKB started in April 2014 and followed through May 2020 with detailed characterization adding imaging of the brain, heart, bones, carotid arteries and body composition of 100,000 participants.11 Written informed consent was obtained prior to study entry and the study was approved by the North West Multicenter Research Ethics Committee in the United Kingdom. Data were accessed under project ID 6569. For the present study, among the 25,000 participants first scanned, we excluded participants with missing VAT or LF and prevalent CVD or T2DM at imaging, resulting in a final sample size of 22,354.
Variable Definitions
Race/ethnicity, history of CVD, and smoking status were self-reported. Hypercholesterolemia, low high-density lipoprotein (HDL), and hypertension were defined using previously described clinical definitions.12 Diabetes was defined by a fasting glucose level ≥126 mg/dl or use of hypoglycemic medication. The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated by fasting insulin (mIU/mL) × fasting glucose (mmol/l)/22.5.13 Presence of the metabolic syndrome was defined according to the National Cholesterol Education Program’s Adult Treatment Panel III report.14 Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease Equation.15 Physical activity was derived using self-reported frequency and type of leisuretime physical activity and a standard conversion for metabolic equivalence units (METs). Excessive alcohol consumption was defined as >14 drinks/week or >4 drinks/day for men under age 65 or >7 drinks/week or >3 drinks/day for men over age 65 or women.
Body Composition Measurements
For DHS, magnetic resonance imaging (MRI) measurements of abdominal fat mass were performed as previously described using a validated method of fat mass prediction from a single MR slice at the L2-L3 intervertebral level.16 Subjects were imaged by a 1.5 Tesla MRI scanner (Intera, Philips Medical Systems, Best, The Netherlands) and abdominal adipose tissue was separated into VAT and SAT compartments by manually circumscribing contours using anatomic landmarks. Assuming an adipose tissue density of 0.9196 kg/L, fat volume was converted to mass. Hepatic triglyceride content (HTGC) was measured using 1.5T 1H magnetic resonance spectroscopy, and hepatic steatosis was defined as a HTGC >5.5% as determined for a low-risk subpopulation from this study cohort, as previously described.17 For UKB, participants were scanned in a Siemens MAGNETOM Aera 1.5T MRI scanner (Siemens Healthineers, Erlangen, Germany) using a 6-minute dual-echo Dixon Vibe protocol providing a water and fat separated volumetric data set covering neck to knees, and a multiecho Dixon acquisition for proton density fat fraction assessment in the liver. Quantification of visceral fat volume and liver fat fraction was performed using AMRA Profiler Research.18-21 Waist to hip ratio was calculated as waist circumference(cm)/hip circumference(cm). BMI was calculated as weight(kilograms)/height(meters)2. Dual-energy x-ray absorptiometry (DEXA) was used to measure total fat mass and fat-free mass of the following body compartments: head, upper and lower extremities, and trunk. Definitions of body compartments have been previously described.22
Outcomes
Biomarker measurements
Analytical methods for all biomarkers mentioned here have been previously described23, including fasting blood glucose (FBG), insulin, and plasma lipids 10, coronary artery calcium (CAC), aortic plaque burden and aortic wall thickness23, adiponectin, and high-sensitivity C-reactive protein (hs-CRP). Particle sizes and concentrations of LDL, HDL, and very low-density lipoprotein (VLDL) subclasses were measured by LipoScience, Inc. using NMR spectroscopy.
Cardiovascular Disease and Type 2 Diabetes Mellitus
CVD was defined as CV death, coronary heart disease (myocardial infarction, hospitalized unstable angina, percutaneous coronary intervention, or coronary artery bypass graft surgery), ischemic stroke, transient ischemic attack (TIA), cerebrovascular revascularization, peripheral arterial revascularization, hospitalization for systolic or diastolic heart failure, or hospitalization for atrial fibrillation. All non-fatal and fatal events were determined through December 31, 2013 using previously described methods.24 For replication in UKB, a definition as similar as possible (Supplemental Methods) was applied utilizing electronic health records data. For DHS, incident T2DM was defined by initiation of medical treatment for diabetes during the study interval, Fasting Blood Glucose (FBG) ≥126 mg/dL, non-FBG ≥200 mg/dL, or Hgb A1C ≥ 6.5%, according to updated guidelines (Hgb A1C was not measured at baseline in DHS).25 No information was available regarding time of onset of incident diabetes. For replication in UKB, a definition was applied utilizing electronic health records data to capture all cases of type 2 diabetes mellitus (ICD-10 codes E11 and E11.0 to E11.9) documented in inpatient records. No outpatient records were available for review at the time of the analysis; therefore, the definition used here is based solely on inpatient records and likely underestimates the true incidence rate of T2DM.
Statistical Analysis
Based on known racial/ethnic differences in visceral and liver fat levels, DHS participants were stratified into phenotype groups: low VAT-low LF, high VAT-low LF, low VAT-high LF, and high VAT-high LF defined by ≥ or < the median sex- and race-specific values for VAT (black and non-black) and LF (Hispanic and non-Hispanic), respectively (Supplemental Table 1).26,27 For UKB, a mainly Caucasian cohort, participants were similarly stratified into phenotype groups using median sex-specific values for VAT and LF. Dichotomous variables were compared using chi-square tests, and continuous variables were compared using Student’s t-test for normally distributed variables or the Wilcoxon rank-sum test for non-normally distributed variables.
The baseline association of VAT-LF groups with continuous biomarkers was assessed by linear regression. The β-coefficients represent the estimated change in the biomarker in each group with low VAT-low LF as referent. Logistic regression was used to assess associations of VAT-LF groups with categorical outcome variables. The odds ratio and 95% confidence interval represent the odds of the outcome for each group with low VAT-low LF as referent. To determine independent associations of VAT-LF group with cardiac and metabolic phenotypes, all models were adjusted for age, menopausal status (women only), and BMI. Cross-sectional associations with lipoproteins and inflammatory biomarkers were additionally adjusted for HOMA-IR to determine independence from insulin resistance. Cross-sectional associations with clinical and imaging variables were additionally adjusted for hypertension, diabetes, smoking, hypercholesterolemia, low HDL, glucose lowering medication, lipid-lowering medication, and aspirin to evaluate for attenuation by risk factors or medications that improve the risk factor profile. Additional secondary analyses were performed stratified by race/ethnicity based on known racial/ethnic differences in visceral and liver fat levels.
Cox proportional hazards modeling and logistic regression were used to assess longitudinal associations of VAT-LF groups with incident CVD and T2DM, respectively. The hazard ratio represents the hazard and associated 95% confidence interval of the outcome for each group with low VAT-low LF as referent, and the odds ratio and 95% confidence interval represent the odds of the outcome for each group with low VAT-low LF as referent. To determine independent associations of VAT and LF with CVD or T2DM, models were adjusted for age and BMI, and subsequently for smoking, hypercholesterolemia, hypertension, physical activity, postmenopausal status (women only), and family history of CVD (for CVD models) or family history of T2DM (for T2DM models). Additional models were performed adjusting for high sensitivity C-reactive protein (CVD outcome) and HOMA-IR (T2DM outcome) to determine independence from inflammation and insulin resistance, respectively. The assumptions of linear regression and Cox regression models were evaluated using Schoenfeld residuals. Interactions between VAT and LF were assessed for all models. For all statistical testing, a two-sided p-value <0.05 was considered statistically significant without adjustments for multiple testing. Statistical analyses were performed using SAS version 9.4 software.
Results:
The DHS study cohort included 2064 participants (mean age 44 (SD 9) years; 54% female; 47% black, 18% Hispanic). Baseline characteristics by VAT-LF group are presented in Table 1. Those with high VAT were older and more likely to have higher BMI, waist circumference, total body fat mass, cardiovascular risk factors, and metabolic syndrome, compared to the overall study population. (Table 1, p<.05 for all). Those with high VAT-low LF had a higher prevalence of existing CVD than those with high VAT-high LF (9.9% vs. 7.1%). When stratified by ethnicity, Hispanic participants were more likely to have increased LF compared to VAT, whereas white and black participants were more likely to have increased VAT compared to LF (Supplemental Figure 2). For completeness, baseline characteristics of the DHS study population stratified by normal/abnormal VAT and separately normal/abnormal LF are presented in Supplemental Tables 2 and 3, respectively.
Table 1.
Baseline Characteristics of the Study Population Stratified by Adipose Tissue Phenotype
| Overall (n = 2064) | High VAT / High LF (n=740) | High VAT / Low LF (n=293) | Low VAT / High LF (n=293) | Low VAT / Low LF (n=738) | P-Value | |
|---|---|---|---|---|---|---|
| Age, median (IQR) | 44 (37,53) | 47 (40, 54) | 48 (39, 55) | 42 (35, 51) | 41 (35, 50) | <.001 |
| Female, No. (%) | 1113 (54) | 400 (54) | 157 (54) | 157 (54) | 399 (54) | .10 |
| Race, No. (%) | ||||||
| White | 665 (32) | 254 (34) | 76 (26) | 96 (33) | 239 (32) | .11 |
| Black | 976 (47) | 329 (45) | 160 (55) | 135 (46) | 352 (48) | .04 |
| Hispanic | 378 (18) | 140 (19) | 55 (19) | 50 (17) | 133 (18) | .91 |
| Other | 45 (2) | 17 (2) | 2 (1) | 12 (4) | 14 (2) | .04 |
| Weight, median (IQR)-kg | 82 (70, 97) | 94 (84, 109) | 88 (79, 102) | 77 (68, 87) | 70 (62, 81) | <.001 |
| Height, median (IQR)-cm | 168 (160, 175) | 168 (160, 175) | 168 (161, 175) | 166 (159, 173) | 168 (160, 175) | .08 |
| Body Mass Index,median (IQR)-kg/m2 | 29 (25, 34) | 33 (30, 38) | 31 (28, 35) | 27 (25, 31) | 25 (23, 28) | <.001 |
| Waist Circumference,median (IQR)-cm | 97 (87,108) | 108 (100, 117) | 103 (96, 112) | 93 (86, 99) | 87 (79, 93) | <.001 |
| VAT, median (IQR)-kg | 2 (2, 3) | 3 (2, 3) | 3 (2, 3) | 2 (1, 2) | 1 (1,2) | <.001 |
| LF, median (IQR)-% | 4 (2, 67) | 8 (5,13) | 2 (2,3) | 5 (4,8) | 2 (1,3) | <.001 |
| DEXA | ||||||
| Fat mass,median (IQR)-kga | 25 (19, 33) | 32 (26, 41) | 28 (24, 36) | 23 (18, 29) | 19 (14, 24) | <.001 |
| Lean mass,median (IQR)-kga | 54 (46, 64) | 59 (50, 68) | 57 (48, 66) | 52 (43, 60) | 49 (42, 60) | <.001 |
| Lower body fat, median (IQR)-kga | 9 (6, 12) | 10 (8, 14) | 10 (8, 13) | 8 (6, 11) | 7 (5, 10) | <.001 |
| Truncal fat,median (IQR)-kga | 13 (9, 17) | 17 (14, 21) | 14 (12, 18) | 11 (9, 14) | 9 (6, 11) | <.001 |
| Diabetes Mellitus, No. (%)b | 231 (11) | 147 (20) | 34 (12) | 24 (8) | 26 (4) | <.001 |
| Hypercholesterolemi a, No. (%) | 279 (14) | 134 (18) | 47 (16) | 42 (14) | 56 (8) | <.001 |
| Low High-density lipoprotein cholesterol, No. (%) | 871 (42) | 407 (55) | 133 (45) | 119 (41) | 212 (29) | <.001 |
| Metabolic Syndrome, No. (%) | 734 (36) | 453 (61) | 121 (41) | 85 (29) | 75 (10) | <.001 |
| Current Smoker, No. (%)c | 515 (25) | 164 (23) | 64 (22) | 67 (23) | 220 (30) | .003 |
| Prior Cardiovascular disease, No. (%) | 136 (7) | 55 (7) | 29 (10) | 13 (4) | 39 (5) | .02 |
| Physical Activity, median (IQR)-MET min/weekd | 144 (0, 585) | 99.5 (0, 444) | 120 (0,560) | 119 (0,560) | 239 (0, 720) | <.001 |
n = 2020
n = 2063
n = 2059
n = 1918
Cardiometabolic Risk Markers, Atherosclerosis, and Traditional Risk Factors (Cross-Sectional Analysis)
When VAT and LF were continuously modeled with prevalent CVD or T2DM in the study cohort, VAT (age and BMI-adjusted HR 1.21, 95% CI 1.06-1.40), but not LF (age and BMI-adjusted HR 1.00, 95% CI 0.98-1.03) was significantly associated with CVD, and both VAT (age and BMI-adjusted HR 1.24, 95% CI 1.06-1.45) and LF (age and BMI-adjusted HR 1.33, 95% CI 1.17-1.50) were significantly associated with T2DM (Supplemental Table 4). Multivariable associations between VAT-LF groups and cardiometabolic biomarkers, atherosclerosis, and traditional CV risk factors are presented in Table 2. High VAT-low LF and low VAT-high LF were significantly and positively associated with multiple adverse metabolic biomarkers including HOMA-IR, hs-CRP, triglycerides, large VLDL cholesterol, and low HDL cholesterol. However, several dissimilar associations were observed between the two phenotypes. High VAT-low LF was associated with higher myeloperoxidase (an inflammatory marker) and aortic atherosclerosis, whereas low VAT-high LF was not associated with myeloperoxidase or aortic atherosclerosis. Many biomarkers, including several inflammatory biomarkers tested, showed no association with either high VAT-low LF or low VAT-high LF in multivariable adjusted models (Supplemental Table 5). Both high VAT-low LF and low VAT-high LF groups were associated with prevalent hypertension, metabolic syndrome, and T2DM (Table 2).
Table 2.
Multivariable-adjusted linear and logistic regression models of relation of VAT-LF phenotypes to biomarkers of cardiometabolic disease
| High VAT-High LF | High VAT-Low LF | Low VAT-High LF | |
|---|---|---|---|
| Biomarker | β-coefficient (standard error) | β-coefficient (standard error) | β-coefficient (standard error) |
|
| |||
| Glucose (mg/dL) | 0.08 (0.01)* | 0.02 (0.01) | 0.05 (0.01)* |
| HOMA-IR (units) | 0.68 (0.04)* | 0.27 (0.05)* | 0.32 (0.05)* |
| Adiponectin (ng/mL) | −0.35 (0.03)* | −0.19 (0.04)* | −0.26 (0.04)* |
| hs-CRP (mg/dL) | 0.35 (0.07)* | 0.26 (0.09)* | 0.27 (0.08)* |
| Myeloperoxidase (ng/mL) | 0.05 (0.03)* | 0.06 (0.03)* | −0.002 (0.03) |
| LDL-C (mg/dL) | 0.08 (0.02)* | 0.08 (0.03)* | 0.04 (0.03) |
| LDL Small (nm) | 0.51 (0.08)* | 0.35 (0.09)* | 0.31 (0.08)* |
| Triglycerides (mg/dL) | 0.47 (0.04)* | 0.18 (0.04)* | 0.29 (0.04)* |
| VLDL Large (nm) | 0.91 (0.05)* | 0.32 (0.06)* | 0.60 (0.06)* |
| HDL-C (mg/dL) | −0.14 (0.02)* | −0.10 (0.02)* | −0.07 (0.02)* |
| HDL Large (nm) | −0.38 (0.03)* | −0.22 (0.04)* | −0.20 (0.03)* |
| Aortic Wall Thickness (mm) | 0.09 (0.02)* | 0.06 (0.02)* | 0.03 (0.02) |
| Biomarker | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Hypertension | 2.1 (1.5, 2.9)* | 1.6 (1.1, 2.3)* | 1.5 (1.1, 2.2)* |
| Metabolic Syndrome | 6.6 (2.2, 5.9)* | 3.3 (2.3, 4.7)* | 2.9 (2.0, 4.1)* |
| Diabetes Mellitus | 3.6 (2.2, 5.9)* | 1.9 (1.1, 3.2)* | 2.2 (1.2, 3.3)* |
|
| |||
Data presented are β-coefficients (standard error) that represent the unit increase in the outcome for each phenotype group or odds ratios (95% CI) that represent the odds of the outcome for each phenotype group of High VAT-Low LF or Low VAT-High LF
Model adjusted for age + sex + race + menopausal status (women only) + body mass index (referent low VAT-low LF)
P < 0.05
LF, liver fat; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein; VAT, visceral adipose tissue; VLDL, very low density lipoprotein
Incident Cardiovascular Disease (Longitudinal Analysis)
Among the initial DHS cohort, 1731 participants did not have prevalent CVD or T2DM and were followed for a median (IQR) of 12.0 (11.5-12.7) years. Demographic characteristics of these participants are presented in Supplemental Table 6. 128 participants (7.4%) had a CVD event during follow-up (Supplemental Table 7). The high VAT-low LF group experienced the greatest frequency of CVD events (12.6%) and had the highest hazard ratio for CVD, while low VAT-high LF exhibited rates of CVD events similar to the referent low VAT-low LF (~6%) (Figure 1, Panel A). When VAT and LF were modeled continuously with CVD outcome, VAT (HR 1.24, 95% CI 1.07-1.43), but not LF (HR 0.98, 95% CI 0.84-1.14), was significantly associated with CVD, and there was no statistical interaction between VAT and LF on CVD outcome (p-interaction=.19) (Supplemental Table 8). In unadjusted models, only high VAT-high LF and high VAT-low LF were associated with incident CVD; low VAT-high LF was not associated with incident CVD even in unadjusted analysis. However, after adjustment for age and BMI, all associations with CVD were attenuated (Table 3). Findings were similar when CVD models were additionally adjusted for traditional risk factors and hs-CRP (Supplemental Table 9). There was a statistical interaction between race and VAT/LF group for incident CVD (p-interaction = .02), indicating heterogeneity of effect between race and VAT/LF groups on CVD outcomes. When stratified by race, high VAT-high LF and high VAT-low LF were significantly associated with CVD in non-black participants (HR, 95% CI: 3.2, 1.6-6.3 and HR 3.1, 1.3-7.3, respectively), but not in black participants (HR, 95% CI: 1.2, 0.8-2.0 and HR 1.3, 0.7-2.2 respectively).
Figure 1. Kaplan-Meier curves of incident cardiovascular disease events stratified by body fat phenotype in the Dallas Heart Study (panel A) and UK Biobank study (panel B).
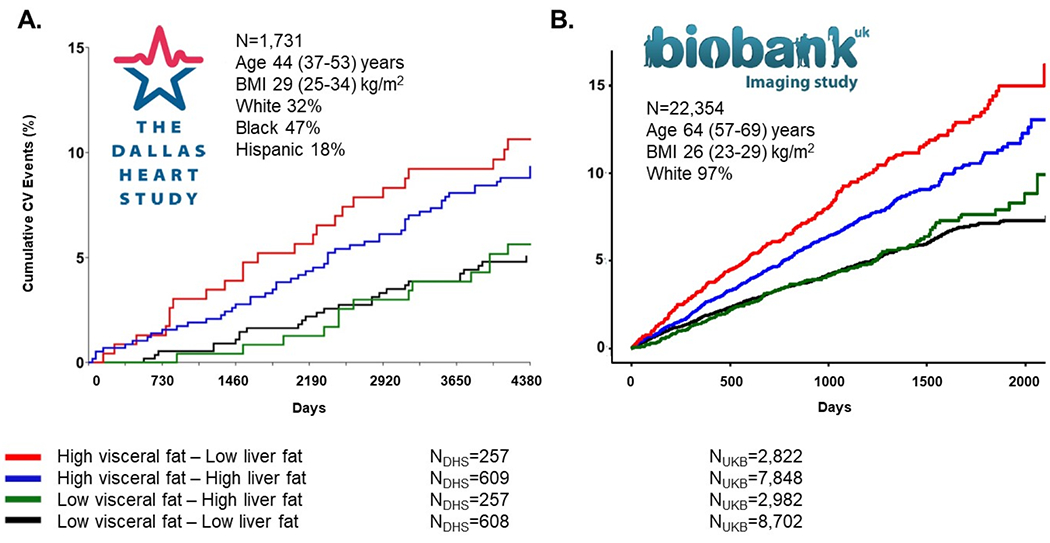
Incidence of CVD (y-axis) as a function of follow-up days (x-axis) in low VAT-low LF (black), high VAT-low LF (red), low VAT-high LF (green), and high VAT-high LF (blue) phenotypes in DHS (A) and UKB (B). High VAT/LF defined by median sex- and race-specific (black, non-black/Hispanic, non-Hispanic) values for VAT/LF in DHS and median sex-specific values for UKB. High VAT-low LF experienced the greatest rate of CVD events, while low VAT-high LF had rates of incident CVD similar to the referent group, low VAT-low LF.
Table 3.
Multivariable-Adjusted Associations of Adipose Tissue Phenotypes with Incident CVD in DHS and UKB
| Incident CVD - DHS (N=1,731) | Incident CVD - UKB (N=22,354) | |||||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | p-value | N | HR (95% CI) | p-value | |
| Model 1 | ||||||
| High VAT-High LF | 609 | 2.03 (1.28-3.21) | .002 | 7848 | 1.59 (1.35-1.87) | <.001 |
| High VAT-Low LF | 257 | 2.42 (1.41-4.13) | .001 | 2822 | 2.00 (1.65-2.43) | <.001 |
| Low VAT-High LF | 257 | 1.21 (0.63-2.31) | .55 | 2982 | 1.40 (1.12-1.75) | .003 |
| Model 2 | ||||||
| High VAT-High LF | 609 | 1.20 (0.70 – 2.06) | .50 | 7848 | 1.10 (0.90-1.34) | .34 |
| High VAT-Low LF | 257 | 1.53 (0.86 – 2.71) | .15 | 2822 | 1.50 (1.22-1.85) | <.001 |
| Low VAT-High LF | 257 | 1.04 (0.54 – 1.99) | .91 | 2982 | 1.24 (0.99-1.55) | .06 |
Hazard ratios (HR) calculated for incident CVD using Cox proportional-hazards model. Referent group is low VAT-low LF (DHS N = 608; UKB N = 8702)
Model 1 is unadjusted
Model 2 is adjusted for age and BMI
Incident Type 2 Diabetes (Longitudinal Analysis)
Incident diabetes developed in 95 (5.5%) participants in the DHS. In univariable analyses, all groups were significantly associated with incident T2DM (Table 4), with high VAT-high LF having the strongest association with incident T2DM, OR 7.8 (95% CI 3.8 – 15.8). After adjusting for age, BMI, smoking, hypercholesterolemia, hypertension, physical activity, postmenopausal status and family history of diabetes (Supplemental Table 4), all groups remained significantly associated with incident T2DM. Findings were similar when T2DM models were additionally adjusted for HOMA-IR (Supplemental Table 9).
Table 4:
Multivariable-Adjusted Associations of Adipose Tissue Phenotypes with Incident T2DM in DHS
| Incident T2DM - DHS (N=1,731) | Incident T2DM - UKB (N=22,354) | |||||
|---|---|---|---|---|---|---|
| N | OR (95% CI) | p-value | N | HR (95% CI) | p-value | |
| Model 1 | ||||||
| High VAT-High LF | 609 | 7.81 (3.85-15.85) | <.001 | 7848 | 8.22 (4.59-14.72) | <.001 |
| High VAT-Low LF | 257 | 3.26 (1.35-7.83) | .008 | 2822 | 2.75 (1.26-6.04) | .01 |
| Low VAT-High LF | 257 | 2.69 (1.08-6.71) | .03 | 2982 | 1.84 (0.73-4.60) | .20 |
| Model 2 | ||||||
| High VAT-High LF | 609 | 6.25 (2.86 – 13.67) | <.001 | 7848 | 3.68 (1.93-7.02) | <.001 |
| High VAT-Low LF | 257 | 2.72 (1.09 – 6.78) | .03 | 2822 | 1.62 (0.73-3.62) | .24 |
| Low VAT-High LF | 257 | 2.55 (1.02 – 6.40) | .05 | 2982 | 1.58 (0.63-3.98) | .33 |
Odds ratio calculated for incident T2DM using logistic regression. Hazard ratios (HR) calculated for incident CVD using Cox proportional-hazards model. Referent group is low VAT-low LF (DHS N = 608; UKB N = 8702)
Model 1 is unadjusted
Model 2 is adjusted for age and BMI
Replication in the UK Biobank cohort (Longitudinal Analysis)
The UKB included 22,354 participants without prevalent CVD or T2DM (baseline characteristics in Supplemental Table 10) who were followed for a median (IQR) of 3.1 (2.3-4.4) years. Of those, 892 (3.9%) had a CVD event and 121 (0.5%) received a diagnosis of T2DM during follow-up. Among the four phenotype groups, high VAT-low LF experienced the greatest proportion of CVD events (Figure 1, Panel B). This group was also most strongly associated with incident CVD in unadjusted analysis, HR of 2.00 (95% CI 1.65 – 2.43). This association persisted after adjustment for age and BMI, HR of 1.50 (95% CI 1.22 – 1.85) (Table 3). In adjusted analyses, neither high VAT-high LF nor low VAT-high LF were associated with incident CVD.
For the T2DM outcome, among the four phenotype groups, high VAT-high LF had the highest number of incident T2DM cases (89 versus 12, 7, and 13 in the high VAT-low LF, low VAT-high LF, and low VAT-low LF groups, respectively). This group was also most strongly associated with incident T2DM, FIR of 8.22 (95% CI 4.59 – 14.72). This association persisted after adjustment for age and BMI, HR of 3.68 (95% CI 1.93 – 7.02) (Table 4). In adjusted analyses, neither high VAT-low LF nor low VAT-high LF were associated with incident T2DM although the number of events in each group were relatively few.
Discussion:
Principal Findings:
This study examined the cardiometabolic profile and health outcomes associated with discordant phenotypes of VAT and LF in the multiethnic DHS population cohort and replicated the incident CVD and T2DM results in the large UK Biobank study. Our results indicate that VAT and LF are differentially associated with CVD and T2DM outcomes. High VAT, in the presence of high or low LF, was found to be associated with both CVD and T2DM, while high LF was not associated with CVD; moreover, a highly novel finding was that a high VAT-low LF phenotype was more robustly associated with CVD than high VAT-high LF, a finding that persisted despite multivariable adjustment when validated in the much larger UK Biobank cohort. Our findings also demonstrate consistent evidence of an association between LF and T2DM, regardless of VAT level.
In Context of Current Literature:
Previous studies have documented an association between liver fat and increased risk of CVD.28 While NAFLD has been associated with an adverse biomarker profile and increased risk of CVD, it is difficult to establish a direct relationship between NAFLD and CVD due to the close association of NAFLD with visceral obesity, metabolic syndrome, and increased levels of other ectopic fat depots. Recent studies involving detailed analyses of body fat composition showed that isolated liver fat does not pose significantly increased risk for developing CVD.9,19 Similarly, our study demonstrates no significant increased risk for CVD in subjects with elevated LF without elevated VAT. Although the association between NAFLD and T2DM has also been well documented by previous studies, the focus has been on the prevalence of NAFLD in subjects with preexisting T2DM and subsequent risk of developing cardiovascular and hepatic diseases.29-31 More recent studies have attempted to understand the bidirectional relationship between NAFLD and T2DM. Studies examining the role of NAFLD in the development of T2DM have found NAFLD to be associated with an almost two-fold increased risk of incident T2DM.31,33 Our results similarly found elevated liver fat to be associated with increased risk for incident T2DM, regardless of the concomitant visceral fat phenotype. Although the pathophysiological pathway of NAFLD and T2DM is not fully understood, possible mechanisms involve the impact of NAFLD on hepatic metabolism, including reduced hepatic extraction of insulin and increased production of hepatic glucose, linking excess liver fat to the development of T2DM.9 We found LF to be more strongly associated with triglycerides than VAT. This, along with a stronger association of large VLDL, glucose and HOMA-IR after adjustment, suggests LF is more important for circulating markers of metabolic abnormalities that are proximal to hepatic metabolism such as VLDL, insulin, and glucose levels, which is consistent with previous studies.
Possible Mechanisms for Discordant Phenotypes
Increased LF is a frequent feature of visceral obesity. However, individual variability in fat accumulation can result in discordant VAT/LF phenotypes. Exact mechanisms of individual variation in fat deposition are not fully understood, but multiple factors have been studied including age, race, sex, and genetics.9 In cases of excess LF in the setting of normal VAT, the PNPLA3 I148M and TM6SF2 E167K genetic variants have been noted to predispose individuals to excess liver fat accumulation without excess fat deposition in other depots. Interestingly, these genetic variants might be associated with protection from cardiovascular disease.34 VAT, but not LF, is associated with atherogenic apo-B containing lipoproteins and aortic wall thickness, both potentially underpinning an increased risk for CVD, an association which may be enhanced in the setting of low LF, indicating regulation of liver triglyceride partitioning may play an important role in cardiovascular health in the context of visceral obesity. Further investigations are needed to understand if high VAT-low LF is an expression of an inability to handle ectopic fat deposition via the liver associated with high CVD risk or if decrease in liver fat as consequence of liver dysfunction increases the risk for CVD.
Clinical Implications:
The differential association of VAT and LF with the development of CVD or T2DM demonstrates the value of analyzing body fat composition to understand individual risk of developing cardiometabolic disease. Racial/ethnic and other factors associated with differential fat distribution further inform patient risk. With the prevalence of obesity in the population and the recent rise in the prevalence of NAFLD, contextualizing patients’ risk for disease is limited when only anthropometry is measured. Given the risk implications, future studies of weight loss strategies, whether pharmacologic, device-based, or surgical, should incorporate assessments of fat distribution to determine the variable responses to these interventions. Furthermore, our finding that low LF in the context of high VAT confers excess risk for CVD should inform the active drug development programs for NAFLD in the current era. Based on our findings here, it is possible that lowering LF pharmacologically without concomitantly addressing high VAT could lead to paradoxically higher risk for adverse cardiovascular outcomes. This may explain why lifestyle interventions for LF are consistently linked with improved CV outcomes, since they dually lower LF and VAT together. Recognizing the importance of how one lowers LF, either concordantly with VAT or in isolation, may aid in appropriate development of effective therapies for CVD risk reduction in the future. When developing NAFLD treatments, a decrease in liver fat alone may not be sufficient to lower patients’ cardiometabolic risk. In fact, although our results are not definitive, decreasing liver fat without resolving visceral obesity may put the patient at greater risk of heart disease.
Strengths and Limitations:
Strengths of the current study include a diverse sample of adults applicable to the general population, extensive and detailed phenotyping using advanced imaging and laboratory techniques, and longitudinal follow-up in a well-validated prospective cohort. Additionally, we were able to utilize data from the UK Biobank to replicate our findings regarding cardiovascular and T2DM risk associated with discordant VAT/LF phenotypes. Several limitations of this study also merit comment. Unfortunately, the time of diabetes onset was not available in the DHS since incident T2DM was measured at follow-up appointments only. Second, since other imaging based assessments of ectopic fat were not measured in this study (e.g. epicardial, perinephric), we are unable to assess the association between other ectopic fat depots and incident CVD or T2DM. Third, the UK Biobank data for T2DM was limited to inpatient diagnosis codes only as access to the primary care UKB cohort was not available at the time of the analysis. This resulted in relatively few cases for modeling analysis when stratified into VAT/LF groups. As such, the incidence rate here likely underestimates the true incidence rate of T2DM in the UKB. Furthermore, the group hospitalized with T2DM diagnosis codes may differ from the outpatient group not hospitalized, limiting to some extent the generalizability of these findings. Despite these limitations, these data validate and support the findings in DHS and strengthen the evidence for an association of both high VAT and LF with T2DM.
Conclusions:
In summary, we found that heterogeneous manifestations of abdominal obesity impact cardiometabolic biomarker profile and health outcomes. Knowledge of patient risk for disease is limited when assessment is restricted to single, isolated fat depots. VAT is associated with an adverse atherosclerotic, dyslipidemic, dysmetabolic, and inflammatory phenotype whereas LF is more associated with metabolic abnormalities. Additionally, VAT was associated with both incident CVD and T2DM, while low VAT-high LF was significantly associated with incident T2DM, but not with increased risk of CVD events. Furthermore, high VAT-low LF most strongly associated with incident CVD, a finding replicated in the UK Biobank cohort. Further work is needed to assess the value of determining risk of cardiometabolic disease based on individual body fat composition and whether knowledge of body fat composition can serve to direct prevention and treatment strategies for individuals with obesity. In conclusion, although excess VAT and LF are individually associated with markers of cardiometabolic risk, these observations demonstrate the importance of separating their cardiometabolic implications when there is presence or absence of one or both in an individual.
Supplementary Material
Acknowledgements:
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (grant K23 DK106520 to IJN), by the National Center for Advancing Translational Sciences (grant UL1TR001105 to UT Southwestern), and by the Swedish Research Council (grant VR-2019-04751 to MB).
Declaration of Interests:
Dr. Neeland has previously received honoraria, consulting, and speaker’s bureau fees, and travel support from Boehringer-Ingelheim/Lilly Alliance, a research grant from Novo Nordisk, and has been a member of the scientific advisory board of AMRA Medical. Drs. Linge and Petersson report other from AMRA Medical AB, outside the submitted work; In addition, Drs. Linge, Borga, and Dahlqvist Leinhard have a patent pending evaluating an individual’s characteristics of at least one phenotype variable. Dr. Després reports grants from Canadian Institutes of Health Research (CIHR), outside the submitted work. Dr. Ayers reports personal fees from NIH, outside the submitted work. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute on Minority Health and Health Disparities; the National Institutes of Health; or the U.S. Department of Health and Human Services. Drs. Borga and Dahlqvist Leinhard are stockholders in AMRA Medical. All other authors have no relationship with industry/conflicts of interest to report.
Abbreviations:
- VAT
Visceral adipose tissue
- LF
Liver fat
- DHS
Dallas Heart Study
- UKB
UK Biobank
- CVD
Cardiovascular Disease
- T2DM
Type 2 Diabetes Mellitus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 3.Cornier MA, Despres JP, Davis N, et al. Assessing Adiposity: A Scientific Statement From the American Heart Association. Circulation. 2011;124:1996–2019. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular aspects of medicine. 2013;34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neeland IJ, Poirier P, Despres JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137:1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol 2011; 31(11): 2715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. The Journal of clinical endocrinology and metabolism 2010; 95(12): 5419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linge J, Whitcher B, Borga M, Dahlqvist Leinhard O. Sub-phenotyping Metabolic Disorders Using Body Composition: An Individualized, Nonparametric Approach Utilizing Large Data Sets. Obesity (Silver Spring) 2019; 27(7): 1190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neeland IJ, Ross R, Despres JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019; 7(9): 715–25. [DOI] [PubMed] [Google Scholar]
- 10.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93(12): 1473–80. [DOI] [PubMed] [Google Scholar]
- 11.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12(3): e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol 2004; 44(9): 1812–8. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28(7): 412–9. [DOI] [PubMed] [Google Scholar]
- 14.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106(25): 3143–421. [PubMed] [Google Scholar]
- 15.Levey AS, Adler S, Caggiula AW, et al. Effects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease Study. Am J Kidney Dis 1996; 27(5): 652–63. [DOI] [PubMed] [Google Scholar]
- 16.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. The American journal of clinical nutrition 1997; 65(2): 403–8. [DOI] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol 1999; 276(5 Pt 1): E977–89. [DOI] [PubMed] [Google Scholar]
- 18.Borga M, Thomas EL, Romu T, et al. Validation of a fast method for quantification of intraabdominal and subcutaneous adipose tissue for large-scale human studies. NMR in biomedicine 2015; 28(12): 1747–53. [DOI] [PubMed] [Google Scholar]
- 19.Linge J, Borga M, West J, et al. Body Composition Profiling in the UK Biobank Imaging Study. Obesity (Silver Spring) 2018; 26(11): 1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West J, Dahlqvist Leinhard O, Romu T, et al. Feasibility of MR-Based Body Composition Analysis in Large Scale Population Studies. PLoS One 2016; 11(9): e0163332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borga M, Ahlgren A, Romu T, Widholm P, Dahlqvist Leinhard O, West J. Reproducibility and repeatability of MRI-based body composition analysis. Magn Reson Med 2020; 84(6): 3146–56. [DOI] [PubMed] [Google Scholar]
- 22.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 2006; 91 (11): 4459–66. [DOI] [PubMed] [Google Scholar]
- 23.Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring, Md) 2013; 21(9): E439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neeland IJ, Turer AT, Ayers CR, et al. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol 2015; 65(19): 2150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32 Suppl 1: S62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40(6): 1387–95. [DOI] [PubMed] [Google Scholar]
- 27.Agbim U, Carr RM, Pickett-Blakely O, Dagogo-Jack S. Ethnic Disparities in Adiposity: Focus on Non-alcoholic Fatty Liver Disease, Visceral, and Generalized Obesity. Curr Obes Rep 2019; 8(3): 243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol 2016; 65(3): 589–600. [DOI] [PubMed] [Google Scholar]
- 29.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nature reviews Gastroenterology & hepatology 2017; 14(1): 32–42. [DOI] [PubMed] [Google Scholar]
- 30.Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nature reviews Endocrinology 2011; 7(8): 456–65. [DOI] [PubMed] [Google Scholar]
- 31.Radaelli MG, Martucci F, Perra S, et al. NAFLD/NASH in patients with type 2 diabetes and related treatment options. Journal of endocrinological investigation 2018; 41(5): 509–21. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018; 41(2): 372–82. [DOI] [PubMed] [Google Scholar]
- 33.Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016; 31(5): 936–44. [DOI] [PubMed] [Google Scholar]
- 34.Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019; 7(4): 313–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


