Abstract
Objective:
To investigate prevalence and predictors of early depression response (EDR) in adolescents with substance use and depression receiving cognitive-behavioral therapy (CBT) for substance use; and to test the efficacy of supplemental CBT targeting depression (CBT-D) for non-EDR adolescents in an adaptive treatment approach.
Method:
Ninety-five youths at two sites (ages 14–21, mean = 17.4, SD=1.8) with alcohol or cannabis use and depressive symptoms received up to 12 sessions of CBT for substance use over 14 weeks. Assessments were at baseline, weeks four, nine, and 14. The Childrens’ Depression Rating Scale-Revised was the primary depression measure, with a reduction of 50% or more on this scale at week 4 defining EDR. The primary substance use outcomes of alcohol use, heavy alcohol use, and cannabis use frequency were assessed via interview report on the Alcohol Consumption Questionnaire and the Drug Checklist. Urinalysis provided a secondary measure of cannabis use. Non-EDR adolescents were randomized to supplemental CBT-D or enhanced depression treatment as usual (ETAU).
Results:
Thirty-five adolescents (37%, 95% CI = 27%–47%) demonstrated EDR. Fewer days of cannabis use (OR = 0.977; 95% CI = 0.961–0.992) and absence of conduct disorder (OR = 0.149; 95% CI = 0.031–0.716) predicted EDR. Frequency of drinking (F(1,82) = 11.09, η2 = 0.119, p = 0.001), heavy drinking (F(1, 82) = 19.91, η2 = 0.195, p < 0.0001) and cannabis use (F(1, 220) = 35.01, η2 = 0.137, p < 0.001) decreased over time for EDR, CBT-D and ETAU youths, with EDR adolescents evidencing earlier lower cannabis use (F(2, 220) = 4.16, η2 = 0.036, p = 0.0169). Negative (clean) urine screens increased over time (F(1, 219) = 5.10, η2 = 0.023, p = 0.0249). Comparison of CBT-D and ETAU indicated depression significantly decreased over time in both groups (F(1,48) = 64.20, η2 = 0.572, p < 0.001), with no advantage for CBT-D.
Conclusion:
Approximately one-third of adolescents with substance use and depression attain EDR during substance use treatment. Less frequent cannabis use facilitates depression response. The relatively small sample may have precluded identification of additional EDR predictors.
Clinical trial registration information:
Treatment for Teems with Alcohol Abuse and Depression; https://clinicaltrials.gov/; NCT02227589
Keywords: Depression, substance use, adolescence, adaptive treatment, cognitive-behavioral therapy
INTRODUCTION
Alcohol and cannabis use as well as depression are significant public health concerns in adolescence and emerging adulthood. Binge drinking, cannabis use frequency, and alcohol and substance use disorder prevalence increase over this developmental period.1–3 Negative consequences of alcohol or cannabis use include automobile accidents,1 risky sexual behavior,4 impaired neurocognitive5,6 and educational functioning,7 and increased risk for psychiatric and addictive disorders and suicidal behavior.6,8,9
After disruptive behavior, depression is the second most frequent comorbid disorder among adolescent substance users, affecting 20% to 30% in community samples, with higher rates in clinical samples.10,11 Depressive episodes are longer in adolescents with comorbid substance use disorders; substance-related problems are greater in depressed substance users; and the combined problems increase risk of suicidal behavior.12
To date, however, there is no standard protocol for treating young people with both alcohol or cannabis use, and depression.13 Differing treatment approaches have been evaluated, including: 1) separate interventions across mental health and substance use service systems14; 2) parallel psychosocial and pharmacological interventions15–18; 3) sequential psychosocial treatments for each problem19; and 4) psychosocial treatments targeting both problems. Overall the results have been somewhat inconsistent but tentatively suggest that services across systems are often uncoordinated,20 medication does not consistently improve depression in youths receiving psychosocial treatment for substance use,15,16,18 and psychosocial substance use treatment alone results in reduced depression for some participants.21 Further, in sequential treatment, the optimal order of treatments may depend on the severity of the depression, but retention in the second treatment is challenging regardless of order.19 Finally, psychosocial treatments targeting both problems, such as integrated cognitive behavior therapy (ICBT), have shown promise in reducing both substance use and mental health outcomes relative to available community treatment.22
We tested an approach that combines elements of the latter two approaches in an adaptive treatment model, whereby initial substance use treatment is supplemented with depression treatment only when needed. Because some youths with co-occurring substance use and depression evidence reductions in depression with substance use treatment alone, they may not need depression-specific treatment.23 Thus, an adaptive approach, an innovative type of continuity of care, may have advantages.24 Adaptive treatment has been examined with depressed adolescents25 and with substance using adolescents,26 but not with adolescents demonstrating both problems. An effective strategy for these adolescents might be to start with a substance use treatment that has been associated with improvements in depression as well as substance use for some adolescents21; and after a period of time, to provide augmenting depression-specific treatment to individuals who remain depressed. Further, if such depression treatment supplements the initial substance use treatment, using the same theoretical model, it, like ICBT, may prove superior to usual community treatment.
Cognitive-behavioral therapy (CBT) has led to early depression response (EDR) in youth with depression alone and in those with substance use and depression.15,19,27 In addition, EDR predicts later positive outcomes among individuals receiving treatment for depression.28,29 However little is known about the percentage or characteristics of depressed, substance using adolescents likely to demonstrate EDR. Although not a focus of their article Riggs and colleagues15 found that 28% of youth receiving CBT for substance use and pill placebo (versus anti-depressant medication) for depression evidenced EDR within four weeks. To our knowledge, previous studies have not attempted to characterize who these adolescent early responders are who may not need additional depression treatment. Moreover, although EDR to depression treatment predicts later outcomes in depressed adolescents and adults,30 it is not clear whether EDR during substance use treatment is sustainable and predicts continued response at the end of treatment.
In the present study, all participants received motivation enhancement therapy and cognitive behavioral therapy for substance use (MET/CBT),31,32 an intervention that led to reduced substance use in the Cannabis Youth Treatment Study.33 After four weeks, if they were early depression responders, no depression treatment was added to the ongoing MET/CBT. If not, they were randomly assigned to add either: 1) CBT for depression that supplemented MET/CBT for substance use; or 2) depression treatment-as-usual in the community. Our hypotheses were: 1) that a significant proportion of participants (25%) would be early depression responders; 2) that EDR would be associated with continued response and lower severity of depression at the end of treatment; and 3) that, among non-early depression responders, supplemental CBT targeting depression would lead to superior depression and substance use outcomes compared to treatment-as-usual.
METHOD
Participants
Participants were adolescents and emerging adults (subsequently referred to as adolescents) at two sites, (University of Connecticut, Duke University). Inclusion criteria were: 1) age 13 through 21; 2) either a DSM-IV-TR34 diagnosis of current alcohol or cannabis abuse or dependence, or use of cannabis or potentially harmful drinking (at least four drinks for male or three for female adolescents in a day) at least three times in the past 90 days; and 3) clinically significant current depression on an interview rating scale. We accepted adolescents taking anti-depressant medication if they had been on a stable dose for at least one month and still met this criterion. Exclusion criteria were: 1) suicidal or homicidal ideation with a plan, or a suicide attempt within the last month; 2) lifetime psychosis, schizophrenia, bipolar disorder, autistic disorder or intellectual disability; 3) current (past month) dependence on a substance other than alcohol, cannabis or nicotine; 4) primary (most impairing) diagnosis other than alcohol or cannabis use disorder or depression; or 5) ongoing involvement in another psychotherapy for depression or substance use.
We recruited through print and online advertisements, social media, and telephone, email, or face-to-face contacts with referral sources; from primary care, university or community mental health and substance use treatment settings, schools, and juvenile court.
Procedure
Parents of minors (under age 18) or potential participants ages 18–21 completed a telephone screen assessing entry criteria and demographic information, and explaining the study. Those who passed were offered a face-to-face informed consent and baseline assessment meeting. The Institutional Review Boards at each site approved the study.
For minors, parent participation in assessments was required as was written informed consent of parent and assent of adolescent. For those 18–21, parent participation in assessment was encouraged, and required written informed consent of both adolescent and parent.
Assessment Interviewers
Interviewers included a master’s level research clinician, a bachelor’s level research assistant, two doctoral psychologists, and two clinical psychology graduate students. Training took place during a three-day pre-enrollment meeting and a two-day mid-study meeting. Prior to conducting interviews, each assessor was required to meet inter-rater reliability criteria on two video-recorded interviews. Assessors were masked to depression treatment condition at one site. All interviews were audio-record and a subset representing each assessment point were rated by a supervisor for reliability.
Measures
Unless otherwise indicated, measures were administered at all assessments.
Demographics.
At baseline only, a structured data sheet included age, gender, race, ethnicity, highest competed grade, whether attending school, and (for parents) family income.
Substance Use.
Primary substance use outcomes were frequency of drinking, heavy drinking and cannabis use. Adolescents completed the Alcohol Consumption Questionnaire (ACQ)35 and the Drug Checklist (DC; Burke R, Kaminer Y, unpublished measure, June, 2011) in assessment interviews. The baseline assessment covered the past three months; subsequent assessments covered the interval since the previous assessment. The ACQ measures frequency of alcohol use and of heavy alcohol use, with scores ranging from zero (no use) through 11 (daily use). Inter-rater reliability (weighted Kappa) based on 10 interviews was .95 for use and 1.00 for heavy use. The DC assesses days of use of numerous substances, including cannabis, and age of first use. We converted number of days to a percentage to account for variation in intervals between assessments.
Adolescents and participating parents were interviewed separately with the Teen-Addiction Severity Index (T-ASI)36 which has good psychometric properties with adolescents in treatment.37 Baseline T-ASI assessment of past month use of substances other than alcohol, cannabis, or nicotine clarified whether those diagnosed with past year “other substance dependence” on a computerized interview (see below) remained currently dependent. At baseline, we supplemented the parent T-ASI with DSM-IV-TR34 symptom questions about adolescent mood, substance use and exclusionary disorders.
Urinalyses assessed presence of cannabinoids, cocaine, opiates, oxycontin, amphetamines, and MDMA using the 6-panel iCUP Drug Screen Test Kit, Blue Grass Drug Screen, Louisville, KY, with adulteration test strips for validity checks.
Depression.
Our primary depression outcome measure was the Children’s Depression Rating Scale-Revised (CDRS-R).38 Adolescents and parents were interviewed separately about the adolescent’s symptoms in the last week. The CDRS-R includes 14 questions and three observational items. Total scores range from 17 to 114, with 40 or higher indicating clinically significant depression,39 which was required for inclusion. Inter-rater reliability based on 25 interviews was high (ICC = .93).
Diagnoses.
Adolescents completed the computerized Voice Diagnostic Interview Schedule for Children (Voice DISC),40 which covers mood, anxiety, disruptive behavior and substance use disorders. To align diagnoses of major depressive disorder (MDD), a randomization balancing variable, with CDRS-R severity ratings, we counted a depression symptom as present if endorsed by the adolescent on the Voice DISC or by the parent on the T-ASI supplement. Other diagnoses were based solely on the DISC.
Assessment Schedule, Early Depression Response, and Randomization
Subsequent assessments were four, nine, and 14 weeks after baseline (when treatment ended), and three, six and nine months after treatment ended. We focus in this paper on EDR and short-term treatment outcomes through week 14. We defined EDR as a reduction of 50% or more on the CDRS-R from baseline to week four, a magnitude commonly used to define response.41 We chose week four based on Riggs et al15 finding of a 28% response rate at week four with a similar sample and on adult studies showing major symptom improvement by week four.42 Non-EDR adolescents were randomized to receive one of the two augmenting depression treatments, using a computerized urn randomization program,43 with balancing variables of gender, age (under 16; 16 or older), and baseline MDD. All participants continued in study substance use treatment.
Interventions
Substance Use Treatment.
Over 14 weeks, adolescents received up to 12 sessions of motivation enhancement therapy/cognitive-behavioral therapy (MET/CBT-12). Originally, MET/CBT-12 included two sessions of individual MET, followed by 10 sessions of group CBT.31,32 For this study, all sessions were individual. MET included review of substance-related problems, possible reasons to reduce or stop use, psychoeducation, goal setting and functional analysis of the substance use. CBT sessions included review of recent use and of previously assigned skill practice; new skill training; and a practice assignment. Skills included problem-solving, substance refusal, enhancing social support, managing depression, managing cravings, planning for high-risk situations, managing anger, and improving communication. We modified the original MET/CBT 12 by placing the depression management session, which introduces behavioral activation and cognitive restructuring, earlier in the session sequence, so that it would precede the study depression treatment. We also included mid-treatment progress review and an unscripted session, to permit repetition of skill training, and planning for future sessions (Kaminer Y, Barlow L, Van Linter T, unpublished manual, December, 2014).
Depression Treatment.
Non-early depression responders were randomized to CBT for depression (CBT-D) or enhanced treatment-as-usual (ETAU) in the community. CBT-D, conducted by the MET/CBT-12 therapist, consisted of up to seven additional weekly sessions, with the same structure as substance use sessions, but skills targeted depression. These included mood monitoring, behavioral activation, problem-solving, and cognitive restructuring (Curry JF, Goldston DB, Wells KC, unpublished manual, March, 2015) adapted from the Treatment for Adolescents with Depression Study (TADS) CBT manual (Curry JF, Wells KC, Brent DA et al., unpublished manual, March, 2000) These built on the MET/CBT-12 depression management module that introduced these skills.
ETAU was adapted from Esposito-Smythers.22 It included assisting the adolescent and parent to identify preferred and accessible treatment providers, and offering to send a report to the provider. Unlike Esposito-Smythers,22 we did not provide a study psychiatrist for evaluation and pharmacotherapy.
Therapists
Therapists included a social worker (site one), a psychologist and a substance use counselor (site two), each supervised by a doctoral level clinician. Initial and maintenance training occurred in the pre-enrollment and mid-study meetings noted above. Therapists were required to pass a knowledge test with at least 80% accuracy. All sessions were audio-recorded. We created a self-rating form for therapists to guide treatment fidelity across sessions, and a parallel supervisor form for reviewing session recordings. Utilization of these forms varied by site and supervisor. Session selection for review was not random, but intended to monitor fidelity across time and participants. Forms yielded overall ratings ranging from one to five, with scores of three representing acceptable fidelity. Mean scores and number of reliability ratings for the therapists were as follows: 3.50 (20 ratings), 4.36 (49 ratings), and 4.02 (74 ratings).
Data Analysis Plan
Preliminary analyses included sample descriptive statistics and comparisons between sites and between youths who attended at least one treatment session and those who dropped out before treatment. Sample means with standard deviation for continuous variables and percentage for categorical variables along with t-test, χ2 test, and Fisher’s Exact test were used. Binomial proportions and confidence intervals were used to evaluate the prevalence of EDR. Sustained depression response and lower severity at the end of treatment were evaluated using binomial proportions and paired comparisons among assessment completers.
Logistic regression models were used to determine baseline factors related to EDR following bivariate t-tests, χ2 tests, and Fisher’s Exact tests. Due to the exploratory nature of these analyses, bivariate tests were conducted initially without multiple comparison adjustments, and subsequently with such adjustments. Finally, a two-stage (generalized) mixed effect repeated measures analysis was used to compare EDR and randomized non-EDR treatment groups, over the course of treatment. In stage one, prior to examining randomized depression treatment effect, six models were considered: random intercept only, random slope only, random intercept and slope, compound symmetry covariance structure, autoregressive covariance structure, and unrestricted covariance structure. For each outcome of interest, the best random/repeated measures model based on model fit statistics such as Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) was used in stage two to evaluate treatment group fixed effects and possible interaction with linear and/or quadratic time. SAS (SAS Institute, Cary, NC) MIXED and GLIMMIX procedures were used to model continuous and dichotomous outcomes, respectively.
RESULTS
Participant demographic and clinical characteristics
As shown in Figure 1, 103 adolescents consented and met entry criteria. Of those, 95 (51 at site one; 44 at site two) began treatment. Demographic and clinical characteristics of the sample are in Table 1. The eight who dropped out before entering treatment did not differ from the study sample on these characteristics, with the exception of being more likely to have subthreshold or positive diagnoses of post-traumatic stress disorder (PTSD) (Fisher’s exact p = 0.042) or past year other substance use disorder (Fisher’s Exact p = 0.019).
FIGURE 1.
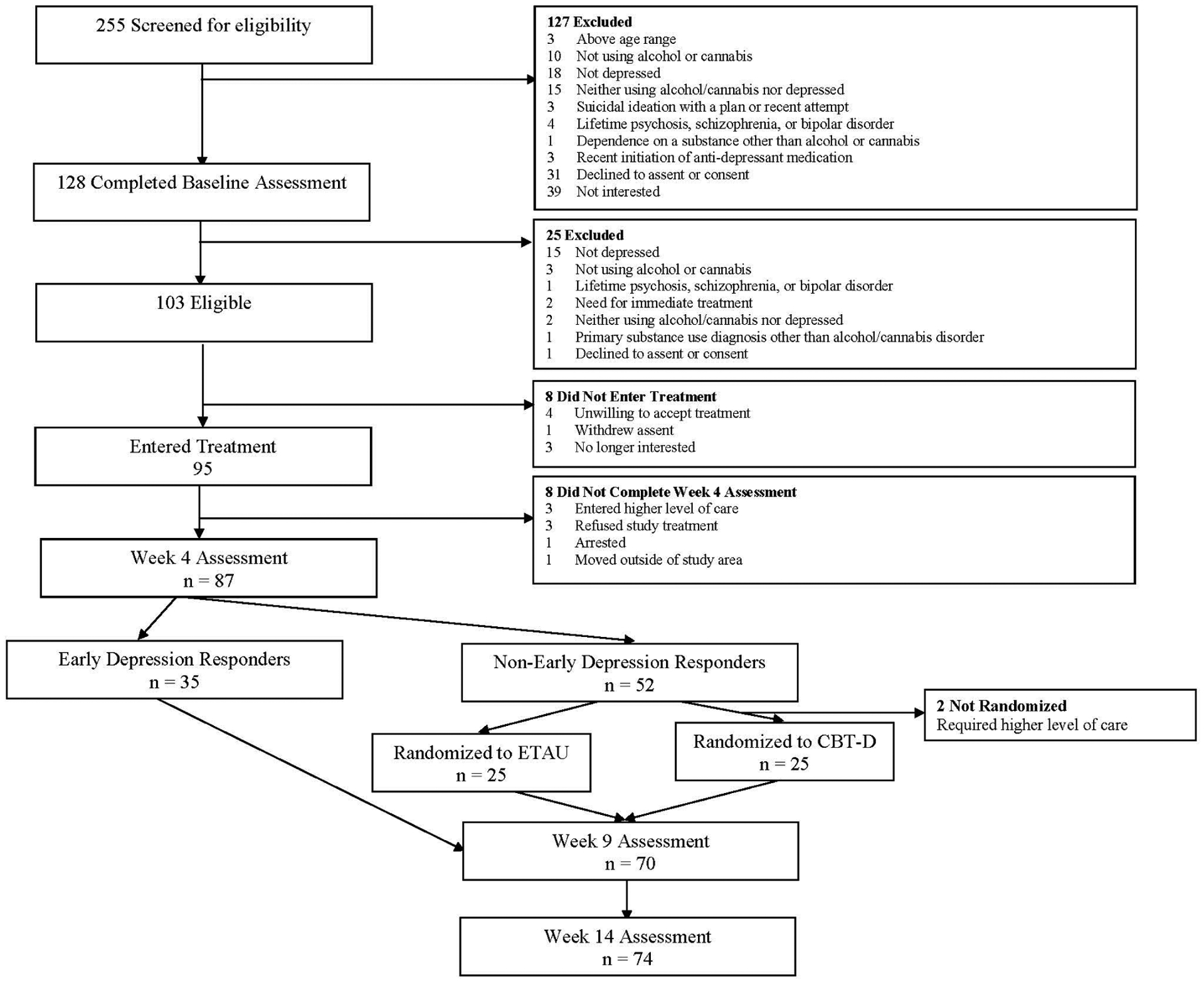
CONSORT Diagram for Adolescents in Adaptive Treatment for Substance Use and Depression
TABLE 1.
Baseline Demographic and Clinical Characteristics and Site Comparisons
| Characteristic | Mean (SD) or % (N = 95) |
Mean (SD) or % Site 1 (n = 51) |
Mean (SD) or % Site 2 (n = 44) |
Statistic | p |
|---|---|---|---|---|---|
| Gender (% male) | 67 | 61 | 75 | χ2 = 2.17 | 0.14 |
| Age (years) | 17.4 (1.8) | 17.7 (1.9) | 16.9 (1.7) | t = 2.14 | 0.04 |
| Race: | |||||
| White | 68 | 67 | 70 | χ2 = 0.16 | 0.69a |
| African American | 23 | 18 | 30 | ||
| Asian American | 3 | 6 | 0 | ||
| Native Hawaiian/Pacific Islander | 2 | 4 | 0 | ||
| Multiracial | 3 | 6 | 0 | ||
| Ethnicity: | |||||
| Hispanic | 28 | 39 | 16 | χ2 = 6.31 | 0.01 |
| Attending School | 94 | 96 | 91 | Fisher’s Exact | 0.41 |
| Highest Grade Completed | 10.6 (1.8) | 10.6 (1.9) | 10.5 (1.6) | t = 0.50 | 0.62 |
| Taking anti-depressant medication | 31 | 37 | 23 | χ2 = 2.35 | 0.12 |
| Severity of Depression (CDRS-R) | 47.1(6.8) | 46.0 (5.9) | 48.5 (7.6) | t = −1.76 | 0.08 |
| Diagnoses: | |||||
| MDD2 | 83 | 84 | 82 | χ2 = 0.11 | 0.75 |
| DD | 8 | 14 | 2 | Fisher’s exact | 0.06 |
| SP | 18 | 18 | 19 | χ2 = 0.01 | 0.90 |
| Panic | 9 | 10 | 7 | Fisher’s exact | 0.72 |
| GAD | 14 | 16 | 12 | χ2 = 0.37 | 0.54 |
| PTSD | 2 | 4 | 0 | Fisher’s exact | 0.50 |
| ADHD | 22 | 25 | 19 | χ2 = 0.64 | 0.42 |
| ODD | 16 | 22 | 9 | χ2 = 2.62 | 0.11 |
| CD | 21 | 29 | 12 | χ2 = 4.41 | 0.04 |
| AUD | 31 | 37 | 23 | χ2 = 2.35 | 0.12 |
| CUD | 83 | 88 | 77 | χ2 = 2.03 | 0.16 |
| ND | 11 | 10 | 11 | Fisher’s exact | 0.99 |
| OSUD | 12 | 12 | 11 | χ2 = 0.004 | 0.95 |
Note: Diagnosis of major depressive disorder was based on adolescent report of symptoms on the Voice Diagnostic Interview Schedule for Children and parent report of adolescent symptoms, whereas all other diagnoses were based solely on the adolescent Voice Diagnostic Interview Schedule for Children. ADHD = attention-deficit/hyperactivity disorder; AUD = alcohol use disorder; CDRS-R = Children’s Depression Rating Scale-Revised; CD = conduct disorder; CUD = cannabis use disorder; DD = dysthymic disorder; GAD = generalized anxiety disorder; MDD = major depressive disorder; ND = nicotine dependence; ODD = oppositional defiant disorder; OSUD = other substance use disorder; PD = panic disorder; PTSD = posttraumatic stress disorder; SP = social phobia.
Comparison of percentage White across sites
The sample included 67% male participants. Racial composition was 68% White, 23% African American, 8% other or multiracial. Twenty eight percent reported Hispanic ethnicity. Mean age was 17.4 years (SD = 1.8), with no participant under 14. Fifty-four participants were 14 to 17, and 41 were 18 or older. Parents participated in assessments for 76% of the sample. (There was no parent involvement in treatment.) Median income was in the $60,000 to $90,000 range.
Mean CDRS-R score was 47.1 (SD = 6.8), indicating mild to moderate depression, and 83% had MDD. Eighty-four (88%) had a substance use disorder (29 alcohol; 79 cannabis; 24 both). There were only three site differences: site one participants were on average nine months older (t = 2.14, p = 0.035), more likely to be Hispanic (χ2 = 6.31, df = 1, p = 0.012), and to have diagnoses of conduct disorder (χ2 = 4.41, df = 1, p = 0.036).
Early Depression Response (EDR)
At week four, 87 of the 95 participants completed the CDRS-R. The eight missing this assessment were considered non-EDR for purposes of these analyses. Supporting our first hypothesis, 35 participants (37%; 95% CI = 27%, 47%) demonstrated EDR with substance use treatment alone.
Predictors and Maintenance of EDR
We explored a range of baseline demographic and clinical variables as potential predictors of EDR. Demographics included site, gender, race, ethnicity, age (in years), years of education, school attendance, parent participation, and income. Clinical variables included age of first alcohol use and of first cannabis use; days of alcohol use and of cannabis use in the past three months; urinalysis negative for cannabis; use of antidepressant medication; anxiety or disruptive behavior disorders; MDD; and past year AUD, CUD, nicotine dependence, or other substance use disorder.
Because this is the first study to investigate predictors of EDR in adolescents with substance use and depression, initial analyses were exploratory, and were conducted first without adjustments, and subsequently with adjustments for multiple comparisons. Baseline variables predicting EDR in unadjusted analyses were older age of first cannabis use (t = 2.17, p = .03), negative cannabis urine drug screen (X2 = 4.524, df = 1, p = .03), fewer days of cannabis use in past three months (t = 3.53, p < .001), and absence of conduct disorder (X2 = 8.063, df = 1, p = .005). After adjusting for multiple comparisons using the False Discovery Rate (FDR), fewer days of cannabis use remained a significant predictor (FDR p = 0.016) and absence of conduct disorder was marginally significant (FDR p = 0.052). Neither older age at first cannabis use (FDR p = 0.192) nor negative cannabis urine screen (FDR p = 0.192) remained significant.
To determine whether any of the four individual predictors of EDR identified in the initial uncorrected predictor analyses (three cannabis-related variables and absence of CD) might be explained by an association with lower baseline depression severity, we analyzed their relations with baseline CDRS-R scores and diagnoses of MDD. Of the eight relations, only one was significant: negative cannabis urine screen was associated with more, not less severe depression (CDRS-R M = 50.16 (SD = 6.68) versus M = 46.39 (SD = 6.67), t = 2.20, p = 0.030).
Next, we conducted a series of multiple logistic regression analyses with EDR as the dichotomous outcome, starting with any baseline variable associated with EDR at p < .10, and then determined the most parsimonious model. Two significant predictors emerged: fewer days of cannabis use in the past three months (OR = 0.977 (CI, 0.961, 0.992)), and absence of conduct disorder (OR = 0.149 (CI, 0.031, 0.716)).
Because conduct disorder was also associated with missing the Week 4 assessment, and missed assessments were counted as non-EDR, we repeated the analysis excluding participants who had missed the Week 4 assessment. Absence of conduct disorder was retained as a predictor of EDR (X2 = 4.83, df = 1, p = .003) along with fewer days of cannabis use.
Paired comparisons between EDR and non-EDR adolescents showed that the EDR adolescents remained less depressed at week 9 (M = 28.48 (7.01) versus M = 38.36 (10.45), t = 4.69, p < .0001) and at week 14 (M = 29.36 (9.97) versus M = 34.86 (11.25), t = 2.11, p = .039), supporting our second hypothesis that EDR adolescents would maintain superior depression outcomes. However, on the dichotomous measure of responder status, the hypothesis was not supported. At week 9, 68% (17/25) (CI 47%, 85%) of EDR adolescents remained responders, and at week 14, 61% (17/28) (CI 43%, 79%) did so. By contrast, only 27% (12/24) (CI 15%, 43%) of non-EDR adolescents were responders by week 9 (X2= 10.85, df = 1, p = 0.0010), but 51% (21/43) (CI 35%, 67%) responded by week 14 (X2= 0.62, df = 1, p = 0.429). Thus, response rates improved among non-EDR and declined among EDR adolescents.
EDR, CBT-D, and ETAU Comparisons
Non-EDR participants who remained in the study (n = 50) were randomized to either CBT-D (n = 25) or ETAU (n = 25). There were no differences between these two groups in any baseline variables, with one exception. Adolescents randomized to CBT-D were significantly more depressed on the CDRS-R than those randomized to ETAU at baseline (M = 49.48 (7.66) versus M = 45.04 (6.07), t = 2.46, p = .017) and at week 4 (M = 44.12 (8.99) versus M = 38.40 (6.28), t = 2.61, p = .012).
CBT-D adolescents attended a median of four CBT-D sessions. Median number of MET/CBT-12 sessions attended was 11 for EDR, 9 for CBT-D, and 10 for ETAU adolescents. In ETAU, 10 entered psychotherapy, one began medication, two added psychotherapy plus medication, and 12 did not add a depression treatment.
The primary outcome measures (depression severity, frequency of alcohol, heavy alcohol, and cannabis use) were analyzed through week 14 for EDR, CBT-D and ETAU participants, using mixed effect repeated measures models with unrestricted covariance structures. Main and interactive effects of site were not significant. Table 2, and Figures 2 and 3 demonstrate the results.
TABLE 2.
Depression, Alcohol, and Cannabis Use from Baseline to End of Treatment
| Baseline | Week 4 | Week 9 | Week 14 | |||
|---|---|---|---|---|---|---|
| n = 85 | n = 85 | n = 70 | n = 72 | |||
| Group | Significant Effects | |||||
| Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | Mean (SD)/% | |||
| Depression | EDR | 47.26 (6.59) | 28.31 (4.48) | 28.48 (7.01) | 29.36 (9.97) | LT, QT, G, G x LT, G x QT |
| (CDRS-R) | CBTD | 49.84 (7.66) | 44.12 (8.99) | 41.10 (11.14) | 37.85 (12.98) | |
| ETAU | 45.04 (6.07) | 38.40 (6.28) | 35.87 (9.32) | 32.26 (8.99) | ||
| Alcohol | EDR | 2.71 (2.55) | 1.09 (2.05) | 1.15 (1.76) | 1.07 (1.86) | LT, QT |
| Use | CBTD | 2.75 (2.21) | 2.36 (2.43) | 1.86 (2.41) | 1.75 (2.05) | |
| (ACQ) | ETAU | 2.36 (2.31) | 1.80 (2.18) | 1.48 (2.02) | 1.30 (2.14) | |
| Heavy | EDR | 1.97 (2.62) | 0.74 (1.82) | 0.62 (1.33) | 0.46 (1.32) | LT |
| Alcohol Use | CBTD | 2.17 (2.04) | 1.44 (2.47) | 1.67 (2.27) | 1.10 (2.02) | |
| (ACQ) | ETAU | 1.88 (2.40) | 1.24 (1.79) | 1.26 (1.94) | 0.78 (1.88) | |
| % Days of | EDR | 37.08 (31.53) | 22.46 (24.94) | 18.17 (27.82) | 16.71 (25.15) | LT, QT, G, G x LT, G x QT |
| Cannabis Use | CBTD | 56.62 (35.67) | 47.36 (39.95) | 37.57 (35.03) | 27.89 (29.78) | |
| (DC) | ETAU | 58.27 (31.26) | 31.78 (29.46) | 22.81 (29.93) | 25.52 (29.27) | |
| % Cannabis | EDR | 31.43 | 51.43 | 57.69 | 53.57 | LT |
| Negative | CBTD | 24.00 | 33.33 | 33.33 | 45.00 | |
| Drug Screens | ETAU | 8.00 | 28.00 | 26.09 | 34.78 | |
Note: n = 85 adolescents who were either early depression responders, or non-early responders randomized to cognitive behavior therapy for depression or enhanced treatment as usual. All groups continued to receive motivation enhancement therapy/cognitive behavior therapy for substance abuse through week 14. ACQ = Alcohol Consumption Questionnaire; CBT-D = cognitive behavior therapy for depression; CDRS-R = Children’s Depression Rating Scale-Revised; DC = Drug Checklist; EDR = Early depression responders; ETAU = enhanced treatment as usual; G = group; LT = linear time; QT = quadratic time.
FIGURE 2. Mean Depression Severity with 95% CI During Treatment for Substance use and Depression.
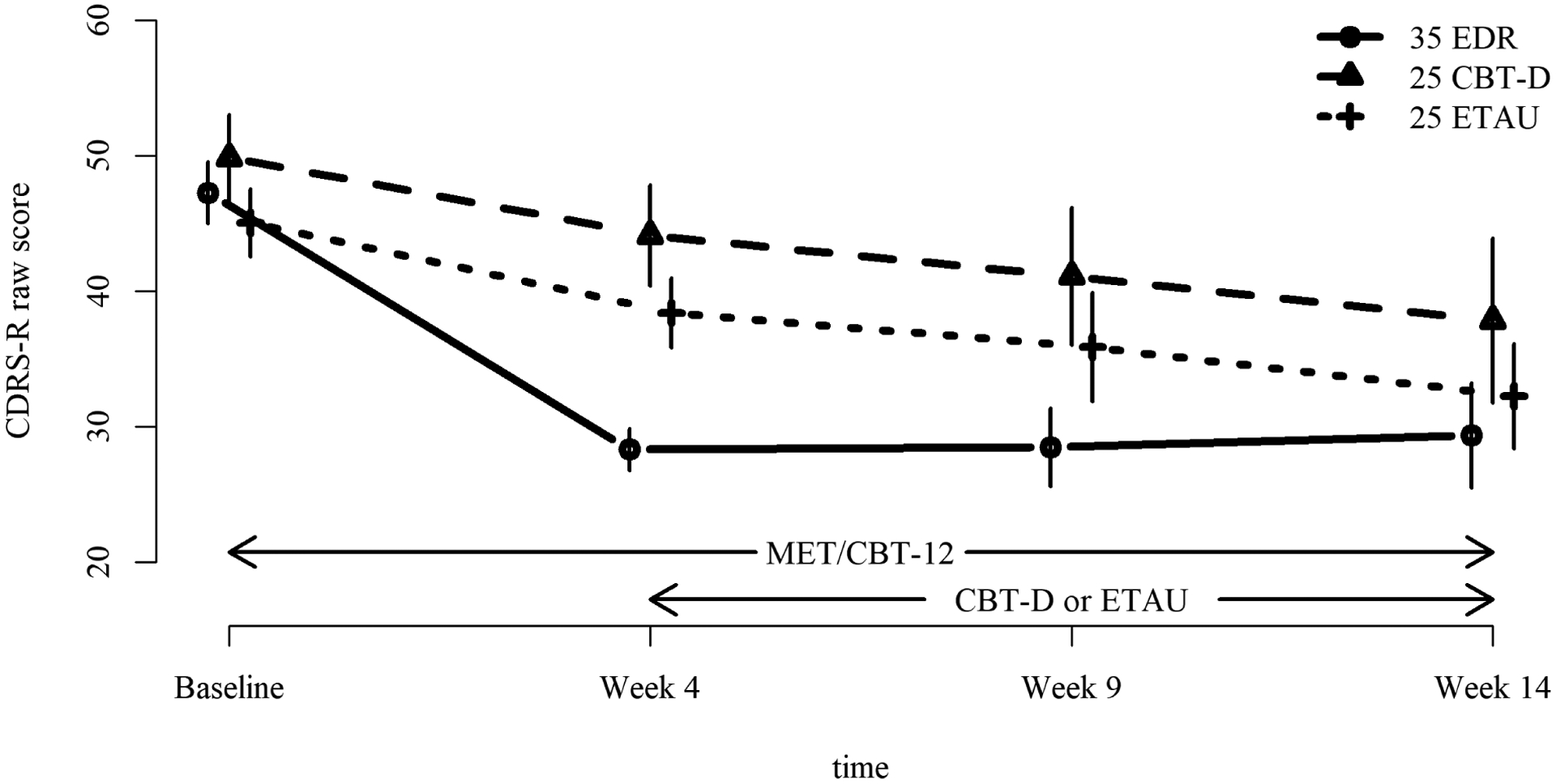
Note: This figure illustrates severity of depression in adolescents with substance use and depression over 14 weeks of treatment. All participants received up to 12 sessions of motivation enhancement therapy/cognitive behavior therapy for substance use from baseline to week 14. At week 4, those with early depression response did not add a depression treatment, whereas non-early depression responders added either cognitive behavior therapy for depression or enhanced treatment as usual. CBT-D = cognitive behavior therapy for depression; CDRS-R = Children’s Depression Rating Scale-Revised; EDR = Early Depression Responders; ETAU = Enhanced treatment as usual for depression.
FIGURE 3. Mean Percentage Days of Cannabis Use with 95% CI During Treatment for Substance Use and Depression.
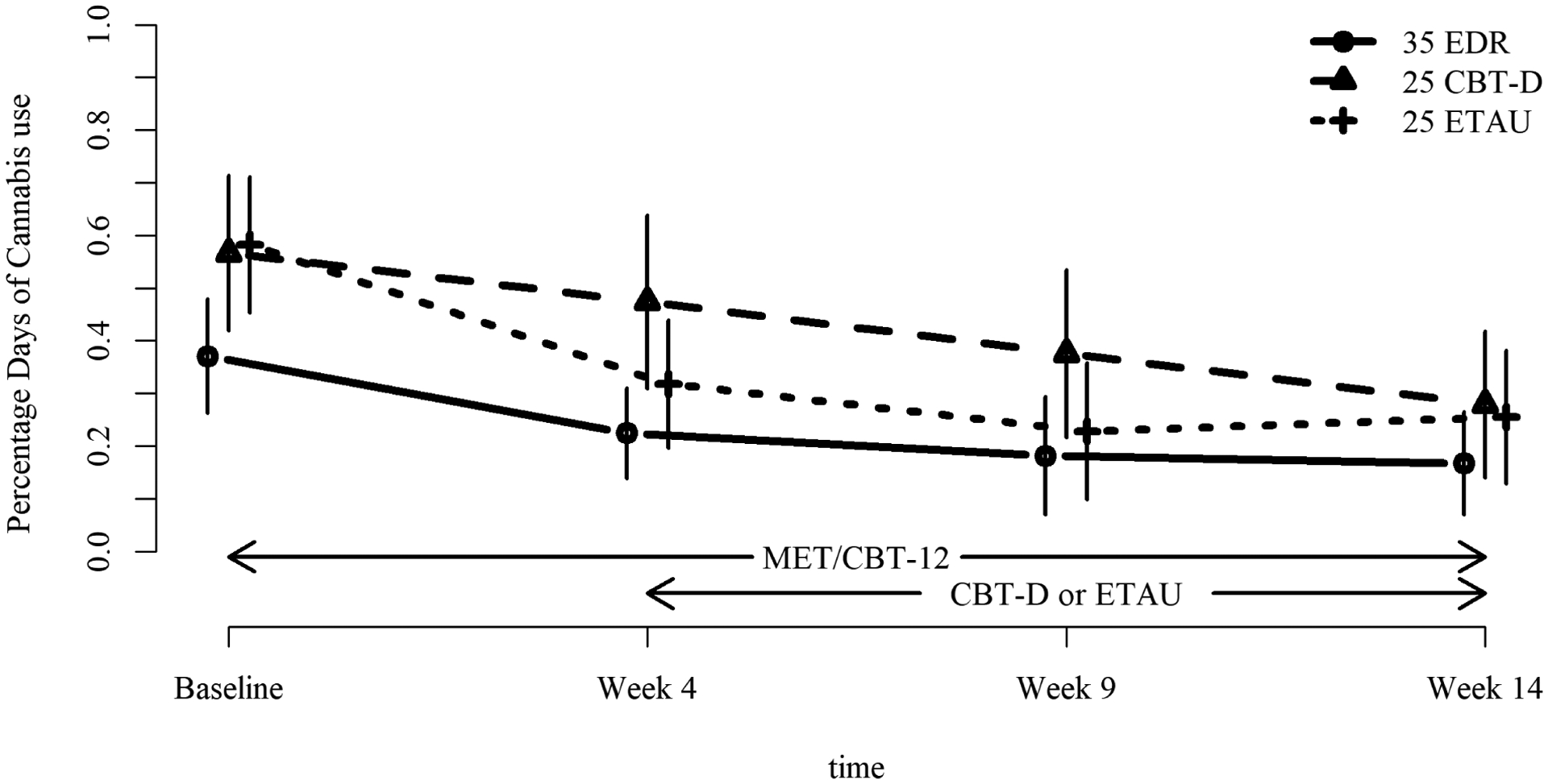
Note: This figure illustrates percentage days of cannabis use in adolescents with substance use and depression over 14 weeks of treatment. All participants received up to 12 sessions of motivation enhancement therapy/cognitive behavior therapy for substance use from baseline to week 14. At week 4, those with early depression response did not add a depression treatment, whereas non-early depression responders added either cognitive behavior therapy for depression or enhanced treatment as usual for depression. CBT-D = cognitive-behavioral therapy for depression; CDRS-R = Children’s Depression Rating Scale-Revised; EDR = Early Depression Responders; ETAU = Enhanced treatment as usual for depression.
On the CDRS-R, all effects were significant: linear time (F(1, 82) = 162.99, η2 = 0.665, p < 0.0001); quadratic time (F(1, 82) = 67.84, η2 = 0.453, p < 0.0001); group (F(2, 82) = 3.66, η2 = 0.082, p = 0.03); group-by-linear time (F(2, 82) = 34.62, η2 = 0.458, p < 0.0001); and group-by-quadratic time (F(2, 82) = 28.55, η2 = 0.410, p < 0.0001). Interpreting the highest order interaction, as shown in Figure 2, the EDR group showed more rapid decline in depression than the other groups, followed by a slight increase in depression from week 4 to week 14, whereas the other two groups showed a linear decrease.
For frequency of drinking, significant effects were found for linear and quadratic time (F(1, 82) = 11.09, η2 = 0.119, p = .001; F(2, 82) = 4.95, η2 = 0.108, p = .029), but not for group or group-by-time. Frequency of heavy drinking declined with a significant linear time effect (F(1, 82) = 19.91, η2 = 0.195, p < 0.0001)
For percentage days of cannabis use, significant effects were again found for linear time (F(1, 220) = 35.01, η2 = 0.137, p < .0001), quadratic time (F(1, 220) = 11.42, η2 = 0.049, p = 0009), group (F(2, 220) = 5.02, η2 = 0.044, p = 0.0074); group-by-linear time (F(2, 220) = 4.16, η2 = 0.036, p = 0.0169); and group-by-quadratic time (F(2, 220) = 3.80, η2 = 0.033, p = 0.0238). Percentage days of use declined in all groups but with different trajectories. As shown in Figure 3, and indicated in the predictor analyses, EDR adolescents began treatment with a lower level of use than did non-EDR adolescents. They further reduced their use, and then maintained this low level. For those later randomized to CBT-D, the initial decline was linear, whereas for those who received ETAU the decline was curvilinear, decreasing more rapidly from baseline to week 4, and then increasing slightly between weeks 9 and 14. All groups converged at week 14. Finally, negative cannabis urinalyses increased over time in a linear fashion (F(1, 219) = 5.10, η2 = 0.023, p = 0.0249) with non-significant effects for group or group-by-time.
Comparison of Depression Treatments
Mixed-effect models with random intercept and linear time slope compared only the two depression treatment groups. There were no site effects. On the CDRS-R there were significant time (F(1,48) = 64.20, η2 = 0.572, p < .0001) and group effects (F(1, 87) = 6.87, η2 = 0.073, p = 0.010), but no group-by-time effect (F(1, 87) < .01, p = 0.948). This reflects the baseline and continuing difference between groups in severity of depression, but indicates that both groups became significantly less depressed during treatment, with the same rate of improvement.
For frequency of alcohol use, there was a significant effect of time (F(1, 134) = 16.36, η2 = 0.109, p < .0001), but not of group or group-by-time. Frequency of heavy alcohol use decreased over time (F(1, 133) = 10.93, η2 = 0.076, p = 0.001), with no group or group-by-time effects.
For percentage days of cannabis use, there were significant interactions between group and linear time, and group and quadratic time (F(1, 133) = 7.05, η2 = 0.050, p = 0.009); F(1,133 = 7.03, η2 = 0.050, p = 0.009). As mentioned above, both groups reduced their cannabis use, but the CBT-D group did so in linear fashion, whereas the ETAU group showed a curvilinear pattern. Negative urine drug screens for marijuana increased significantly over time (F(1, 134) = 6.94, η2 = 0.049, p = 0.009), with no significant effects for group or group-by-time.
Remission and Abstinence
We used CDRS-R scores below 2944 and DC reports of no use since the Week 9 assessment to indicate depression remission and alcohol or cannabis abstinence, respectively, at Week 14. Depression remission characterized 46% of EDR (16/35) and 26% of non-EDR adolescents (13/50). Fifty-four percent of EDR (19/35) and 48% of non-EDR adolescents (24/50) were alcohol abstinent; 26% of EDR (9/35) and 24% of non-EDR adolescents (12/50) were cannabis abstinent.
DISCUSSION
This study examined early depression response and an adaptive treatment approach for adolescents with substance use and depression. Consistent with our first hypothesis, a substantial proportion demonstrated EDR with substance use treatment alone. Our second hypothesis that EDR would be associated with continued superior end of treatment outcomes received mixed support. Severity of depression remained significantly lower among EDR adolescents, but the proportion of responders was no longer significantly higher. Our third hypothesis that supplemental CBT for depression would yield better outcomes than depression treatment as usual was not supported.
This is the first study to focus prospectively on EDR and to evaluate its predictors among adolescents with substance use and depression. It is also the first to use adaptive treatment, whereby only non-EDR adolescents in substance use treatment received additional depression-specific intervention. Further, this is one of very few studies to evaluate a single treatment approach targeting both problems (ongoing CBT for substance use supplemented by CBT for depression) in comparison to an alternative approach.
The proportion of adolescents demonstrating EDR by week four, about one-third, was somewhat higher than that found by Riggs et al.,15 perhaps because not all of our participants had major depression or a disruptive behavior disorder, both required in that sample. Arias et al21 did not report percentage of EDR during substance use treatment, but noted that presence of depression symptoms declined from 70% of participants at baseline to 58% at month three. In comparing sequences of depression and substance use treatments for adolescents with substance use and depression, Rohde et al23 reported over half showed substantial decrease in depression by week five regardless of treatment.23 Our findings confirm that for a substantial number of these adolescents, EDR can occur during substance use treatment alone.
Severity of baseline depression did not predict EDR, but three of its four individual predictors were associated with pre-treatment cannabis use: later age of first use, less frequent recent use, and a cannabis-negative urinalysis at baseline. These findings strongly suggest that higher levels of cannabis use interfere with rapid alleviation of depression.
Why might lower cannabis use predict more rapid depression response? First, cannabis use is associated with motivational deficits,45 which may interfere with engagement in treatment goal-setting and activation that reduces depression. Second, greater cannabis use has been associated with greater stress and using to cope,46 factors that could make depression treatment more challenging. Further, the allostatic model of addiction holds that behavioral functions of substance use change as individuals move from lesser to greater involvement with substances: from positively reinforced reward-seeking to negatively reinforced compulsive behavior.47 The former behavioral functions may be associated with more treatment-responsive forms of cannabis use and depressed mood. Nevertheless, given our limited sample size, the modest odds ratio of lower cannabis use as a predictor of EDR, and our decision not to correct for multiple comparisons, replication of this finding will be critical.
Lower severity of depression persisted throughout treatment for EDR adolescents, consistent with findings for depressed individuals treated with a range of psychotherapies.48 The proportion of responders mid-treatment remained significantly higher among those with EDR, but by week 14, the gap diminished. Although part of this reduction was due to increased response rates in non-early responders, an encouraging finding, there was some regression among early responders. In a few cases, small increases in CDRS-R scores crossed the responder threshold. In others, regression may have been due to increased life stress or increased substance use.
Regarding substance use outcomes, we found a similar advantage for EDR. Days of cannabis use declined across all groups, but EDR adolescents showed the most rapid reduction and maintained a low level of use. These results are encouraging, indicating EDR is associated with reductions in cannabis use as well. It is possible that youth who are becoming less depressed are also engaging more in non-drug using activities and interacting more with non-using peers, thus supporting reduced use. Alcohol and heavy alcohol use were relatively low in this sample and declined over time, regardless of EDR or treatment group.
Contrary to expectations, adding supplemental CBT-D, theoretically and structurally consistent with ongoing substance use CBT with the same therapist, was not superior to adding community depression treatment. Neither anti-depressant medication nor characteristics of the psychotherapy in ETAU likely account for this lack of differential efficacy, as just over half of ETAU adolescents added these depression treatments. The dose of CBT-D received by our participants (Mdn = 4 sessions) may have been too small to affect more persistent depression. Both of these observations in turn reflect challenges associated with adapting treatment by adding sessions.25
By contrast, Esposito-Smythers et al. (2011)22 found integrated CBT (ICBT) superior to ETAU for adolescents who were suicidal and using substances, possibly reflecting study differences in samples or treatments. Ours was an outpatient sample, including adolescents with mild depression or substance use. Esposito-Smythers et al.22 recruited discharged inpatients with suicidality, suggesting a more impaired sample. Our adolescents had one therapist, whereas ICBT included individual and family therapists. Finally, our sample received ongoing CBT targeting substance use. Elements of that CBT, such as self-monitoring, increasing non-drug activities, problem-solving, and cognitive restructuring, may have generalized to depression for those receiving ETAU.
Limitations of this study include a relatively small sample size, which may have limited power to detect additional predictors of EDR or depression treatment differences. Our inclusion of minor adolescents and emerging adults necessitated some variability in parent involvement in study assessments, a methodological limitation. However, such involvement did not predict EDR. There were site differences in assessor masking and frequency of fidelity ratings. However, there were no site differences in outcomes. Including adolescents below full diagnostic threshold could be considered a limitation, but also reflects common clinical circumstances and thereby increases generalizability of findings. Finally, we did not test the alternative of starting with depression treatment, adding substance use treatment as needed.
Despite the limitations, the present study indicated that for a substantial number of youth with substance use and depression, treatment for substance use leads to early improvements in depression. Our findings underscore the interrelationship between cannabis use and depression over the course of treatment, highlighting the importance of attending to cannabis use when treating depressed adolescents, and the benefit to mental health providers of training in substance use treatment.
Further research should focus on optimal interventions to sustain response in EDR adolescents, and to improve depression and substance use outcomes in non-EDR adolescents. Future research could utilize a variety of methods, including ecological momentary assessment, to investigate the longitudinal interplay between changes in substance use and in depression during and after treatment, and to guide interventions accordingly.
Disclosure:
Dr. Curry has received research support from the National Center for Complementary and Integrative Health. Dr. Goldston has received research grant support from the National Institutes of Health (NIH), the Substance Abuse and Mental Health Services Administration, and the American Foundation for Suicide Prevention. Dr. Chan has received research support from the US Department of Education and NIH. Dr. Inscoe has received research support from NIH. Drs. Kaminer, Wells, Meyer and Mss. Burke and Cheek have reported no biomedical financial interests or potential conflicts of interest.
This work was supported by grants R01 AA021719 to Dr. Curry and R01 AA021735 to Dr. Kaminer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The research was performed with permission from Duke Health and UCONN Health Institutional Review Boards.
References
- 1.Chassin L, Sher KJ, Hussong A, Curran P. The developmental psychopathology of alcohol use and alcohol disorders: Research achievement and future directions. Dev Psychopathol. 2013;25(402):1567–1584. doi: 10.1017/S0954579413000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the national comorbidity survey replication-adolescent supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. Vol 170. (Substance Abuse and Mental Health Services, ed.). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.; 2019. https://www.samhsa.gov/data/ [Google Scholar]
- 4.Marshall EJ. Adolescent alcohol use: Risks and consequences. Alcohol Alcohol. 2014;49(2):160–164. doi: 10.1093/alcalc/agt180 [DOI] [PubMed] [Google Scholar]
- 5.Ellingson JM, Ross JM, Winiger E, et al. Familial factors may not explain the effect of moderate-to-heavy cannabis use on cognitive functioning in adolescents: a sibling-comparison study. Addiction. Published online 2020:1–2. doi: 10.1111/add.15207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the risks and consequences of adolescent cannabis exposure. J Am Acad Child Adolesc Psychiatry. 2017;56(3):214–225. doi: 10.1016/j.jaac.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 7.Stiby AI, Hickman M, Munafò MR, Heron J, Yip VL, Macleod J. Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction. 2015;110(4):658–668. doi: 10.1111/add.12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagot KS, Milin R, Kaminer Y. Adolescent initiation of cannabis use and early-onset psychosis. Subst Abus. 2015;36(4):524–533. doi: 10.1080/08897077.2014.995332 [DOI] [PubMed] [Google Scholar]
- 9.Gobbi G, Atkin T, Zytynski T, et al. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: A systematic review and meta-analysis. JAMA Psychiatry. 2019;76(4):426–434. doi: 10.1001/jamapsychiatry.2018.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong TD, Costello JE. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J Consult Clin Psychol. 2002;70(6):1224–1239. doi: 10.1037/0022-006X.70.6.1224 [DOI] [PubMed] [Google Scholar]
- 11.Diamond G, Panichelli-Mindel SM, Shera D, Dennis M, Tims F, Ungemack J. Psychiatric syndromes in adolescents with marijuana abuse and dependency in outpatient treatment. J Child Adolesc Subst Abus. 2006;15(4):37–54. doi: 10.1300/J029v15n04_02 [DOI] [Google Scholar]
- 12.Curry JF, Hersh J. Depressive disorders and substance use disorders. In: Kaminer Y, ed. Youth Substance Abuse and Co-Occurring Disorders. American Psychiatric Publishing; 2015:131–156. [Google Scholar]
- 13.Lichtenstein DP, Spirito A, Zimmermann RP. Assessing and treating co-occurring disorders in adolescents: Examining typical practice of community-based mental health and substance use treatment providers. Community Ment Health J. 2010;46(3):252–257. doi: 10.1007/s10597-009-9239-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman M Relationship between substance use disorders and psychiatric comorbidity. In: Kaminer Y, ed. Youth Substance Abuse and Co-Occurring Disorders. Amercian Psychiatric Publishing; 2015:21–48. [Google Scholar]
- 15.Riggs PD, Mikulich-Gilbertson SK, Davies RD, Lohman M, Klein C, Stover SK. A randomized controlled trial of fluoxetine and cognitive behavioral therapy in adolescents with major depression, behavior problems, and substance use disorders. Arch Pediatr Adolesc Med. 2007;161(11):1026–1034. doi: 10.1001/archpedi.161.11.1026 [DOI] [PubMed] [Google Scholar]
- 16.Cornelius JR, Bukstein OG, Douaihy AB, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1–2):39–45. doi: 10.1016/j.drugalcdep.2010.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelius JR, Douaihy A, Bukstein OG, et al. Evaluation of cognitive behavioral therapy/motivational enhancement therapy (CBT/MET) in a treatment trial of comorbid MDD/AUD adolescents. Addict Behav. 2011;36(8):843–848. doi: 10.1016/j.addbeh.2011.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelius JR, Bukstein OG, Wood DS, Kirisci L, Douaihy A, Clark DB. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addict Behav. 2009;34(10):905–909. doi: 10.1016/j.addbeh.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohde P, Waldron HB, Turner CW, Brody J, Jorgensen J. Sequenced versus coordinated treatment for adolescents with comorbid depressive and substance use disorders. J Consult Clin Psychol. 2014;82(2):342–348. doi: 10.1037/a0035808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito-Smythers C, Walsh A, Spirito A, Rizzo C, Goldston DB, Kaminer Y. Working with the suicidal client who also abuses substances. Cogn Behav Pract. 2012;19(2):245–255. doi: 10.1016/j.cbpra.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias AJ, Hammond CJ, Burleson JA, et al. Temporal dynamics of the relationship between change in depressive symptoms and cannabis use in adolescents receiving psychosocial treatment for cannabis use disorder. J Subst Abuse Treat. 2020;117(October 2019):108087. doi: 10.1016/j.jsat.2020.108087 [DOI] [PubMed] [Google Scholar]
- 22.Esposito-Smythers C, Spirito A, Kahler CW, Hunt J, Monti P. Treatment of co-occurring substance abuse and suicidality among adolescents: a randomized trial. J Consult Clin Psychol. 2011;79(6):728–739. doi: 10.1037/a0026074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohde P, Turner CW, Waldron HB, Brody JL, Jorgensen J. Depression change profiles in adolescents treated for comorbid depression/substance abuse and profile membership predictors. J Clin Child Adolesc Psychol. 2018;47(4):595–607. doi: 10.1080/15374416.2015.1118695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay JR. Continuing care research: What we have learned and where we are going. J Subst Abuse Treat. 2009;36(2):131–145. doi: 10.1016/j.jsat.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunlicks-Stoessel M, Mufson L, Bernstein G, et al. Critical decision points for augmenting interpersonal psychotherapy for depressed adolescents: A pilot sequential multiple assignment randomized trial. J Am Acad Child Adolesc Psychiatry. 2019;58(1):80–91. doi: 10.1016/j.jaac.2018.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminer Y, Ohannessian CMC, Burke RH. Adolescents with cannabis use disorders: Adaptive treatment for poor responders. Addict Behav. 2017;70:102–106. doi: 10.1016/j.addbeh.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 27.Renaud J, Brent DA, Baugher M, Birmaher B, Kolko DJ, Bridge J. Rapid response to psychosocial treatment for adolescent depression: A two-year follow-up. J Am Acad Child Adolesc Psychiatry. 1998;37(11):1184–1190. doi: 10.1097/00004583-199811000-00019 [DOI] [PubMed] [Google Scholar]
- 28.Lewis CC, Simons AD, Kim HK. The role of early symptom trajectories and pretreatment variables in predicting treatment response to cognitive behavioral therapy. J Consult Clin Psychol. 2012;80(4):525–534. doi: 10.1037/a0029131 [DOI] [PubMed] [Google Scholar]
- 29.Persons JB, Thomas C. Symptom severity at week 4 of Cognitive-Behavior Therapy predicts depression remission. Behav Ther. 2019;50(4):791–802. doi: 10.1016/j.beth.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Beard JIL, Delgadillo J. Early response to psychological therapy as a predictor of depression and anxiety treatment outcomes: A systematic review and meta-analysis. Depress Anxiety. 2019;36(9):866–878. doi: 10.1002/da.22931 [DOI] [PubMed] [Google Scholar]
- 31.Webb C, Scudder M, Kaminer Y, Kadden R. The Motivational Enhancement Therapy and Cognitive Behavioral Therapy Supplement: 7 Sessions of Cognitive Behavioral Therapy for Adolescent Cannabis Users, Cannabis Youth Treatment (CYT) Series, Volume 2. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2002. [Google Scholar]
- 32.Sampl S, Kadden R. Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users: 5 Sessions, Cannabis Youth Treatment (CYT) Series. Vol 1. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. http://library1.nida.ac.th/termpaper6/sd/2554/19755.pdf [Google Scholar]
- 33.Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) Study: Main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197–213. doi: 10.1016/j.jsat.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; 2000. [Google Scholar]
- 35.Cahalan D Quantifying alcohol consumption: patterns and problems. Circulation. 1981;64(3 Pt 2):7–14. [PubMed] [Google Scholar]
- 36.Kaminer Y, Bukstein O, Tarter RE. The Teen Addiction Severity Index: Rationale and reliability. Int J Addict. 1991;26(2):219–226. [DOI] [PubMed] [Google Scholar]
- 37.Kaminer Y, Wagner E, Plummer B, Seifer R. Validation of the Teen Addiction Severity Index (T-ASI): Preliminary findings. Amercian J Addict. 1993;2(3):250–254. [Google Scholar]
- 38.Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R). Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 39.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA. 2008;299(8):901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC- IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- 41.Mulder RT, Joyce PR, Frampton C. Relationships among measures of treatment outcome in depressed patients. J Affect Disord. 2003;76(1–3):127–135. doi: 10.1016/S0165-0327(02)00080-0 [DOI] [PubMed] [Google Scholar]
- 42.Ilardi SS, Craighead WE. The Role of Nonspecific Factors in Cognitive- Behavior Therapy for Depression. Clin Psychol Sci Pract. 1994;1(2):138–155. doi: 10.1111/j.1468-2850.1994.tb00016.x [DOI] [Google Scholar]
- 43.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Monogr. 1997;Supplement:70–75. [DOI] [PubMed] [Google Scholar]
- 44.Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the treatment for adolescents with depression study (TADS): Acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48(2):186–195. doi: 10.1097/CHI.0b013e31819176f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkow ND, Swanson JM, Evins AE, et al. Effects of cannabis use on human behavior, including cognition,motivation, and psychosis: A review. JAMA Psychiatry. 2016;73(3):292–297. doi: 10.1001/jamapsychiatry.2015.3278 [DOI] [PubMed] [Google Scholar]
- 46.Hyman SM, Sinha R. Stress-related factors in cannabis use and misuse: Implications for prevention and treatment. J Subst Abuse Treat. 2009;36(4):400–413. doi: 10.1016/j.jsat.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaminer Y, Winters KC. Clinical Manual of Youth Addictive Disorders. American Psychiatric Association Publishing; 2020. [Google Scholar]
- 48.Crits-Christoph P, Connolly MB, Gallop R, et al. Early improvement during manual-guided cognitive and dynamic psychotherapies predicts 16-week remission status. J Psychother Pract Res. 2001;10(3):145–154. [PMC free article] [PubMed] [Google Scholar]


