Abstract
Purpose:
To report trends in the prevalence of early graft failure after endothelial keratoplasty in the United States (U.S.).
Methods:
Descemet Membrane Endothelial Keratoplasty (DMEK) and Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) graft volumes were collected from records maintained by 6 major eye banks in the U.S. from January 1, 2013 to December 31, 2018. The prevalence and presumed cause of early graft failures (defined as a graft with persistent edema or re-grafted within 8 weeks after keratoplasty) each year were sourced from surgeon-reported adverse events. Failed graft cases from three of the eye banks were compared to non-failures at the donor and recipient level to perform subset analysis of factors associated with early graft failure.
Results:
51,887 endothelial keratoplasty tissues were distributed during the study period; 72% were DSAEK grafts. The total number of early graft failures reported for DMEK was 168/14,284 (1.18%) and 322/37,603 for DSAEK (0.86%). Early DMEK failures decreased from 2013 (7.69%) to 2018 (0.68%). In generalized linear mixed model analyses adjusting for donor tissue characteristics, recipient age, and diagnosis, an association of borderline significance was found between higher donor age and early failure (odds ratio (OR), [95% confidence interval]): 1.03 [1.00, 1.05] (unit change of 1 year) and DSAEK (OR 1.02 [1.00, 1.04]; unit of change 1 year) cases.
Conclusions:
The proportion of early graft failures in DMEK decreased over time and was comparable to failure rates in DSAEK at the end of the study period. Surgeon learning curve might have played a role.
Keywords: Primary graft failure, early re-graft, endothelial keratoplasty, Descemet membrane endothelial keratoplasty, DMEK, Descemet stripping endothelial keratoplasty, DSAEK, eye bank
Introduction
The total number of annual keratoplasty procedures performed in the United States (U.S.) has increased by about 20% over the last decade, from 42,606 procedures in 2009 to 51,336 in 2019.1 This change coincided with increasing numbers of endothelial keratoplasty (EK) procedures, which has been the most commonly performed type of keratoplasty procedure in the U.S. since 2012. At present, Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) is performed more frequently than Descemet Membrane Endothelial Keratoplasty (DMEK). However, the annual numbers of DMEK have increased over the past 5 years as more surgeons adopt the procedure.1 In addition, the adoption of DMEK has also been facilitated by the availability of pre-stripped and pre-loaded tissue from eye banks. Given the rising number of EK procedures performed annually in the U.S. and evolving tissue preparation processes at eye banks, we wished to explore the extent to which early graft failures might be contributing to the rising case numbers.
In 1990, the Eye Bank Association of America (EBAA) implemented a system requiring participating eye banks to track reported adverse events resulting from ocular tissue transplantation. Surgeons voluntarily report adverse events using forms provided by EBAA-accredited eye banks as one of six types of events: graft failure (including early graft failure and early re-graft), ocular infections, systemic infection in a recipient, corneal dystrophy, ocular malignancy, and refractive surgery in the donor tissue. A form is distributed at the time of surgery, and additional forms are sent between 3 and 6 months postoperatively in compliance with EBAA standards.2 For adverse events, the source eye bank leads an investigation, partnering with the reporting surgeon and the distributing or processing eye bank to determine the reason for graft failure, such as donor tissue abnormalities, issues with surgical manipulation, or recipient factors. Those cases that are determined to be tissue-related are determined to be primary graft failure (PGF) and reported to the EBAA as such. Similarly, the eye banks also report cases of early re-grafts to the EBAA. However, eye banks maintain records for all reported early graft failures and early re-grafts to monitor outcomes. It was the aim of the present investigation to examine trends in early failures inclusive of early re-grafts and PGF.
In the current literature, the PGF rate for PK has been reported as less than 1% for uncomplicated keratoplasty indications like endothelial failure3 and slightly higher at 1.98% inclusive of all indications.4 Comparatively, average early graft failure rates for DSAEK have been reported as slightly higher at 5% (range, 0%–29%) compared to 1.9% (range, 0%–12.5%) for DMEK, although the estimates range in sample size and patient population.5,6 In the Cornea Preservation Time Study (CPTS), 45 instances of early graft failure occurred among 1330 DSAEK procedures, for a total prevalence of 3.38%.7 In a separate study of adverse reaction reports, the rates of PGF were higher for EK at 11/10,000 (0.11%) compared to 6.9/10,000 (0.069%) for PK.8 The higher early graft failure rates in EK compared to PK likely reflects complex graft preparation, increased handling of tissue during preparation, and several additional steps of tissue processing such as precutting/pre-stripping, marking, and pre-loading EK tissue per surgeon preference. In addition, the learning curve and technical difficulty of EK procedures, which have been described in previous studies,9–12 are greater than PK.13 Thus, intraoperative surgical trauma from the technical challenge in learning EK may play a greater role in early graft failures for EK procedures compared with PK.4
While reasons for poor outcomes in EK include poor visual outcomes relating to donor tissue/host interface abnormalities14 and immunologic graft rejection,15 there remains a paucity of data on the frequency and causes of early graft failure in a large cohort of transplanted tissues. The data we examine in this study are representative of a broad group of surgeons, patients, and donor tissues nationally. Thus, the purpose of this study is to report the epidemiology of early graft failure inclusive of early re-grafts and PGF using data from six large eye banks geographically distributed throughout the U.S., representing approximately 30 percent of all EK grafts transplanted in the U.S. during the study time frame. In addition, we explore possible donor tissue and recipient-level factors correlated with early graft failure in EK.
Methods
Description of Dataset
The study protocol was submitted and deemed exempt by the Johns Hopkins University School of Medicine Institutional Review Board. All study activities adhered to the principles of the Declaration of Helsinki. Six eye banks were contacted for data collection and agreed to participate in the study, including Advancing Sight (Birmingham, AL), CorneaGen (Seattle, WA), Eversight (Ann Arbor, MI), Lions VisionGift (Portland, OR), Saving Sight (Kansas City, MO), and VisionFirst (Carmel, IN).
Partnering eye banks shared data generated from surgeon reports of either early graft failure or early re-grafts for Descemet Stripping Endothelial Keratoplasty (DSEK), DSAEK, DMEK, and Descemet Membrane Automated Endothelial Keratoplasty (DMAEK) procedures. For the purposes of this study, early graft failure was defined as “corneal edema present from the time of keratoplasty that did not clear after eight weeks including tissue-related causes (aka PGF), surgical or recipient factors, and also cases that were re-grafted in less than eight weeks.”2
De-identified data on donors, donor tissue, and recipients for tissue distributed in the U.S. from each eye bank were collected for surgeries between January 1, 2013, and December 31, 2018. Graft failure data from eye banks were sourced from adverse reaction forms, post-operative outcomes forms, and any personal email or phone communication from the surgeon to the eye bank outside of these forms. These reports included the following recipient information: surgical indication, age, and gender. The following donor tissue information was available: donor age, cause of death, mated case status, endothelial cell count, death to cooling time, death to preservation time, and death to surgery time. Data pertaining to the surgical procedure included the type of transplant (PK, DSEK/DSAEK, and DMEK/DMAEK), the determined cause of early graft failure, and the type of transplant performed after the initial graft failure if known. For the purposes of this study, the small number of DSEK grafts was grouped with DSAEK grafts for analysis; similarly, the small number of DMAEK grafts were grouped together with DMEK grafts.
Data were obtained from each eye bank regarding the total number of grafts supplied to surgeons in the U.S. for each year for DSAEK and DMEK in order to calculate the proportion of corneal transplantations resulting in early graft failure. Additionally, three of the six eye banks were also able to provide donor tissue and recipient information described above for all DMEK and DSAEK procedures without reports of early graft failure for the same time period to serve as a comparison group for a subset analysis.
The overall cumulative incidence and the yearly incidence of early graft failure over the 5-year study period for DSAEK and DMEK was calculated. The causes of early graft failure were divided into the following categories: surgical manipulation, donor tissue issues, recipient factors (including surgical indication and patient compliance), and unknown/other based on the eye banks’ investigation of adverse reactions reported.
Statistical Analysis
For demographic variables and clinical outcomes, the student’s t-test or Wilcoxon rank sum test were used to compare continuous risk factors, while Fisher’s exact tests or chi-squared testing was used to compare binary or categorical risk factors. Incidence rate and cause of failure were plotted on a yearly basis for early graft failures. For the purposes of our statistical analysis, significance is defined as p < 0.05. Statistical analyses and plots were completed using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). For the three eye banks for which non-failure data was obtained, a generalized linear mixed effects model16 with eye banks as random intercept was used to fit early graft failure versus non-failure grafts on factors that achieved statistical significance (p<0.2 on univariate analysis). Initial ECD and ECD post-cut were standardized by the mean and standard deviation for inclusion in the model.
Results
A total of 51,887 tissues—37,603 (72%) for DSAEK and 14,284 (28%) for DMEK—were distributed by the participating eye banks from January 1, 2013, to December 31, 2018. The yearly number of tissues distributed for DSAEK and DMEK is shown in Supplemental Figure 1. The number of DMEK tissues distributed increased from 403 in 2013 to 5,228 in 2018. The total number of early graft failures reported was 168 (1.17% of all grafts) for DMEK, 322 (0.86%) for DSAEK, and 152 (0.50%) for PK (Supplemental Figure 2). In 2013, 7.69% of DMEK cases experienced early graft failures, compared to 0.61% of DSAEK cases. By 2018, these rates changed to 0.68% for DMEK and 1.06% for DSAEK (Figure 1). The most frequently reported cause of early graft failures in DMEK was surgical manipulation (n=66, 39%), followed by donor tissue (n=48, 29%), recipient factors (n=32, 19%), and other/unknown (n=21, 13%; Figure 2a). In 2013, 51.6% of reported DMEK early graft failures were deemed to be due to surgical manipulation. By 2018, this rate had decreased to 19.4%. In comparison, the most frequently reported cause of early graft failures for DSAEK was recipient factors (n=120, 37%), followed by donor tissue factors (n=112, 35%), surgical manipulation (n=45, 14%), and other/unknown (n=45, 14%; Figure 2b). In 2018, both DMEK and DSAEK failures were most frequently attributed to donor tissue factors (Figure 2b).
Figure 1.
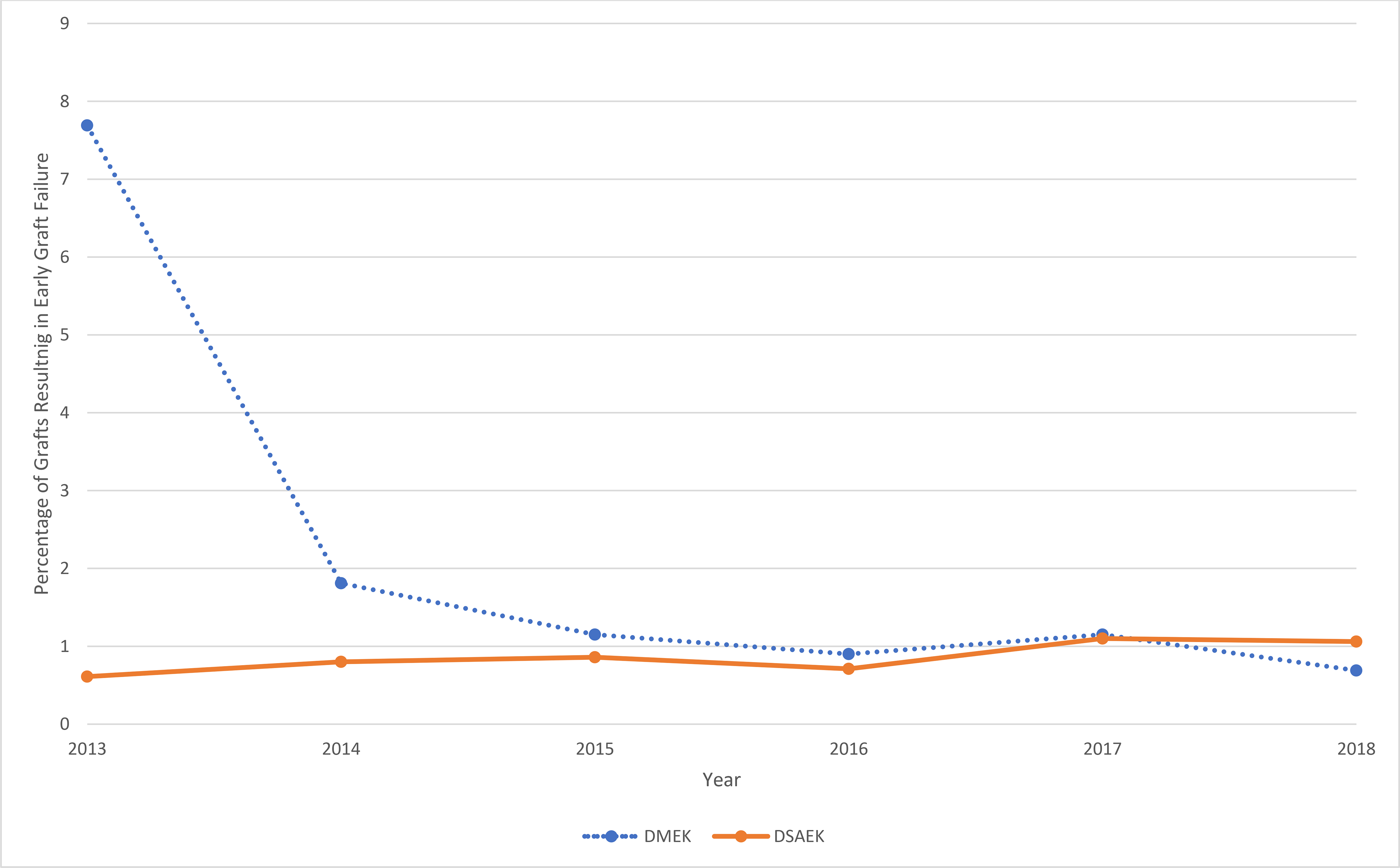
Proportion of endothelial grafts resulting in early graft failure by year from six eye banks in the United States, 2013 to 2018. The dashed blue line represents DMEK (inclusive of both DMEK and DMAEK), while the solid orange line represents DSAEK (inclusive of DSEK and DSAEK).
DMEK = Descemet Membrane Endothelial Keratoplasty
DMAEK = Descemet Membrane Automated Endothelial Keratoplasty
DSEK = Descemet Stripping Endothelial Keratoplasty
DSAEK = Descemet Stripping Automated Endothelial Keratoplasty
Figure 2a.
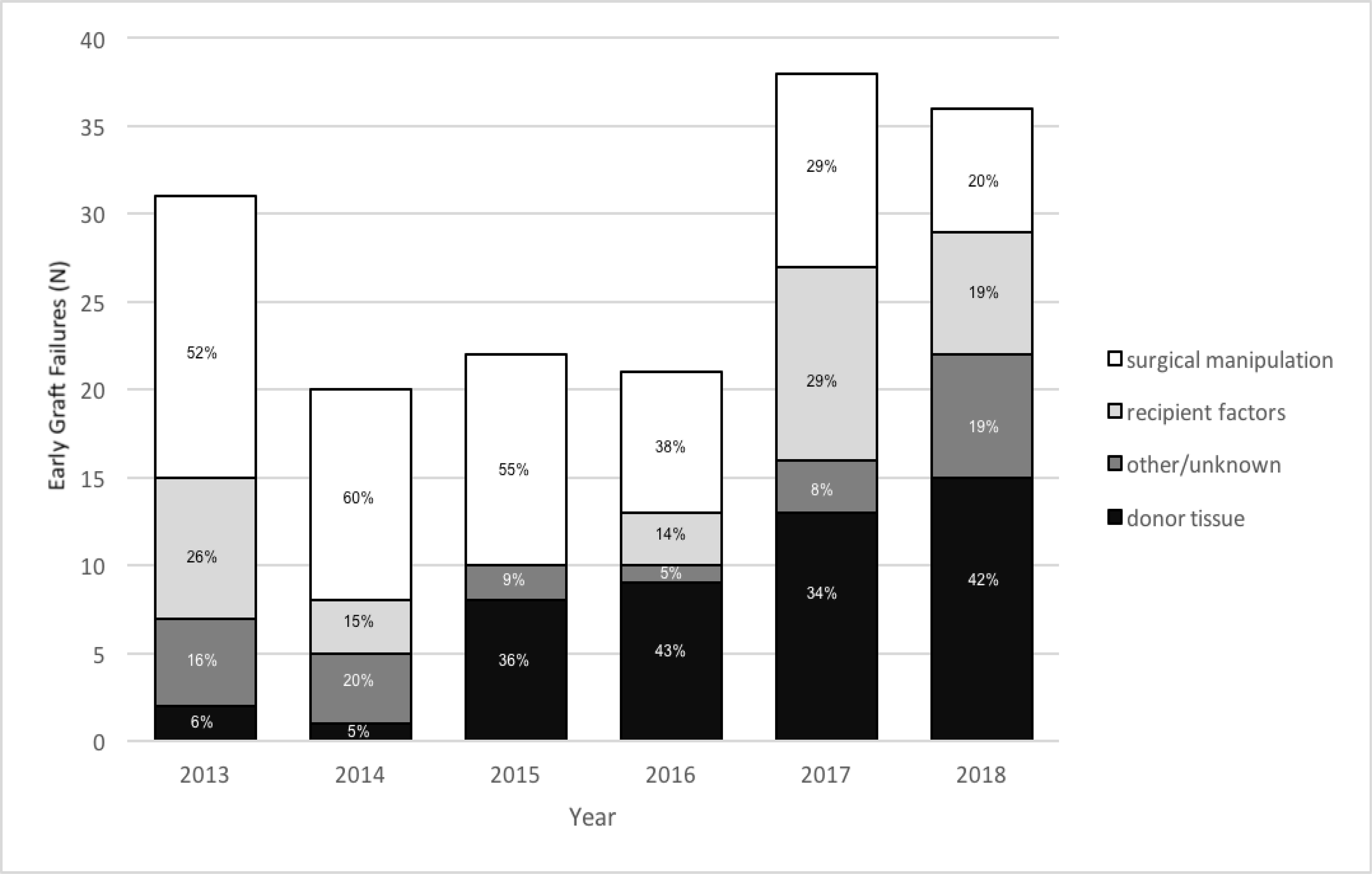
Cause of early graft failure (inclusive of early re-grafts) for DMEK/DMAEK from 2013 to 2018.
DMEK = Descemet Membrane Endothelial Keratoplasty
DMAEK = Descemet Membrane Automated Endothelial Keratoplasty
Figure 2b.
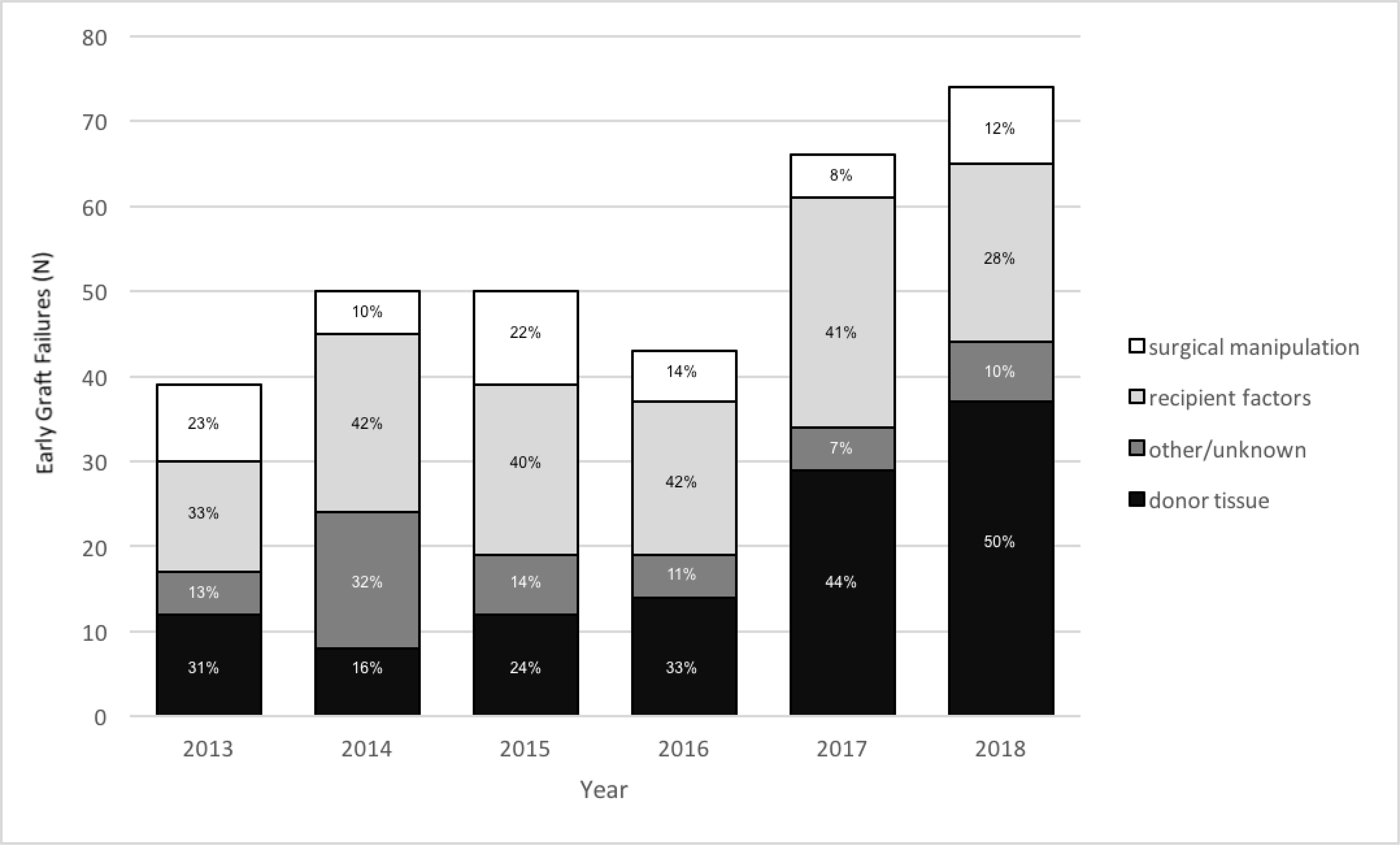
Cause of early graft failure (inclusive of early re-grafts) for DSEK/DSAEK from 2013 to 2018.
DSEK = Descemet Stripping Endothelial Keratoplasty
DSAEK = Descemet Stripping Automated Endothelial Keratoplasty
Donor and Recipient Factors Associated with DMEK and DSAEK Early Failure or Early Re-Graft
The univariate analysis comparing donor tissue and recipient factors between DMEK cases with and without early graft failure and re-graft is shown in Table 1. Among the three eye banks that provided data for both failed and non-failed grafts, there were 29,002 transplanted tissues, representing 55.9% of the overall study cohort. Of this subset, 63.8% (n=18,493) were DSAEK cases. Cases of DMEK with failure had a slightly higher donor age (65.2 ± 7.5 vs. 63.7 ± 7.9, p=0.05), although this finding was of borderline significance. In addition, the proportions of indications for surgery differed (p=0.01; Table 1). There were no significant differences, however, in any other donor tissue or recipient factors studied (Table 1). DSAEK cases with early graft failure or re-graft had a higher donor age (60.2 ± 12.4 vs. 55.9 ± 13.5, p=0.001) and a lower ECD post-cut (2,743 ± 246 vs. 2805 ± 288, p = 0.04). Initial ECD, death to cooling, and death to preservation, recipient age, and recipient sex were not significantly different between the failure and non-failure groups for DSAEK. However, the proportions of donor cause of death was different between the two groups (p=0.004; Table 1).
Table 1.
Univariate analysis of donor and recipient characteristics for DMEK and DSAEK cases with and without early graft failure.
| DMEK cases with early failure | DMEK cases without early failure | P-value | DSAEK cases with early failure | DSAEK cases without early failure | P-value | |
|---|---|---|---|---|---|---|
| N | 109 | 10,481 | 104 | 18,389 | ||
| Donor Factors | ||||||
| Donor Age, years [mean (SD); range] | 65.2 (7.5); 44–81 | 63.7 (7.9); 6–80 | 0.05 | 60.2 (12.4); 14–78 | 55.9 (13.5); 2–80 | 0.001 |
| Cause of Death (%) | 0.80 | 0.004 | ||||
| Cancer | 30 (27.8) | 2431 (23.2) | 34 (33.0) | 3325 (18.1) | ||
| Cerebrovascular Accident | 11 (10.2) | 1058 (10.1) | 8 (7.8) | 1743 (9.5) | ||
| Heart disease | 37 (34.3) | 4185 (39.9) | 31 (30.1) | 7516 (40.9) | ||
| Respiratory disease | 15 (13.9) | 1338 (12.8) | 13 (12.6) | 1954 (10.6) | ||
| Trauma | 6 (5.6) | 472 (4.5) | 8 (7.8) | 1948 (10.6) | ||
| Other | 9 (8.3) | 997 (9.5) | 9 (8.7) | 1903 (10.3) | ||
| Initial ECD cells/mm2 [mean (SD)] | 2765 (299) | 2807 (279) | 0.12 | 2769 (312) | 2812 (304) | 0.15 |
| Post-cut ECD cells/mm2 [mean (SD)] | 2758 (265) | 2767 (265) | 0.78 | 2743 (247) | 2805 (288) | 0.04 |
| Death To cooling time, hours [median (IQR)] | 2.52 (1.59, 3.83) | 2.72 (1.90, 3.83) | 0.22 | 2.63 [1.71, 3.33] | 2.70 [1.95, 3.80] | 0.15 |
| Death To preservation time, hours [median (IQR)] | 9.60 (7.72, 13.00) | 10.3 (7.62, 14.17) | 0.33 | 10.8 [7.42, 14.6] | 11.1 [7.97, 15.2] | 0.21 |
| Recipient Factors | ||||||
| Recipient age, years [mean (SD)] | 70.3 (10.3) | 68.9 (10.22) | 0.14 | 72.13 (12.22) | 71.88 (11.78) | 0.83 |
| Recipient sex, male (%) | 36 (34.6) | 2375 (39.2) | 0.39 | 42 (43.3) | 5843 (42.5) | 0.95 |
| Recipient diagnosis (%) | 0.01 | 0.11 | ||||
| Post cataract surgery edema | 11 (10.1) | 655 (6.2) | 20 (19.2) | 3437 (18.7) | ||
| Ectasias/thinning | 0 (0.0) | 35 (0.3) | 0 (0.0) | 85 (0.5) | ||
| Endothelial dystrophies | 80 (73.4) | 7502 (71.6) | 39 (37.5) | 8621 (46.9) | ||
| Repeat corneal transplant | 10 (9.2) | 537 (5.1) | 17 (16.3) | 1709 (9.3) | ||
| Other/unknown | 8 (7.3) | 1752 (16.7) | 28 (26.9) | 4537 (24.7) | ||
Footnote: The data presented in this table is from the three eye banks from which both failure and non-failure data were available.
ECD = endothelial cell density
SD = standard deviation
IQR = interquartile range
DMEK = Descemet Membrane Endothelial Keratoplasty
DSAEK = Descemet Stripping Automated Endothelial Keratoplasty
For DMEK, the generalized linear mixed effects (GLME) regression model found that donor age increase of 1 year (odds ratio (OR) [95% confidence interval]:1.03 [1.00,1.05]; p=0.06), standardized initial ECD (OR 0.95 [0.78,1.18]; p=0.68), recipient age increase of 1 year (OR 1.02 [0.98, 1.04]; p=0.10) and recipient diagnoses compared to a diagnosis of cancer (p=0.16, 0.86, and 0.13) were not significantly associated with early graft failure and re-graft for DMEK (Table 2).
Table 2.
Results of Generalized Linear Mixed Effects (GLME) regression models for early graft failures in DMEK and DSAEK.*
| Factor | DMEK | DSAEK | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Donor Factors | ||||||
| Age (1 year unit change) | 1.03 | (1.00–1.05) | 0.06 | 1.02 | (1.00–1.05) | 0.03 |
| COD: cerebrovascular accident | 0.41 | (0.17–1.02) | 0.05 | |||
| COD: heart disease | 0.50 | (0.29–0.84) | <0.001 | |||
| COD: respiratory disease | 0.96 | (0.48–1.95) | 0.92 | |||
| COD: trauma | 0.59 | (0.24–1.43) | 0.24 | |||
| COD: other | 0.515 | (0.22–1.21) | 0.13 | |||
| Initial ECD** | 0.96 | (0.78–1.18) | 0.68 | 1.21 | (0.87–1.68) | 0.27 |
| Post-cut ECD** | 0.849 | (0.61–1.18) | 0.34 | |||
| Death time to cooling (hours) | 0.96 | (0.86–1.08) | 0.54 | |||
| Recipient Factors | ||||||
| Age (1-year unit change) | 1.02 | (1.00,1.04) | 0.10 | |||
| Diagnosis: Ectasia/thinning § | ||||||
| Diagnosis: Endothelial dystrophies | 0.63 | (0.33,1.20) | 0.156 | 0.539 | (0.30,0.96) | 0.04 |
| Diagnosis: Repeat corneal transplant | 1.08 | (0.44,2.68) | 0.861 | 1.44 | (0.70,2.95) | 0.318 |
| Diagnosis: Other/unknown | 0.47 | (0.18,1.24) | 0.128 | 1.17 | (0.63,2.17) | 0.614 |
Only factors achieving statistical significance of p<0.2 from the univariate analysis in Table 1 were included in the model (with factors not included in the models in gray). The reference group for Donor COD is a cause of death from cancer; the reference group for Recipient Diagnosis is a diagnosis of post-cataract surgery edema.
ECD values were standardized for this model, where the unit increase is 1 standard deviation change of ECD.
Excluded due to unstable and non-converging regression estimates obtained due to there being zero cases of recipient diagnosis of Ectasia or Thinning in the subset analysis group.
DMEK = Descemet Membrane Endothelial Keratoplasty
DSAEK = Descemet Stripping Automated Endothelial Keratoplasty
ECD = endothelial cell density
COD = Cause of Death
OR = odds ratio
CI = confidence interval
SE = standard error
For DSAEK, the GLME model found that donor age was significantly associated with the failure rate (OR 1.02 [1,1.05] per 1 year increase in donor age; p=0.03) and that recipient diagnosis of endothelial dystrophies had lower odds of early failure compared to post-cataract surgery edema (OR 0.54 [0.30,0.96]; p= 0.04). The initial ECD (OR 1.21 [0.85,1.68]; p=0.27), post-cut ECD (OR 0.85 [0.61,1.18]; p=0.34), or death time to cooling (OR 0.96 [0.86,1.08)]; p=0.54) were not associated with early failure (Table 2).
Discussion
This large sample of data reported by eye banks provides a real-world view of the early success rate of DMEK and DSAEK over a broad group of surgeons and patients during the years coinciding with early adoption for DMEK in the U.S. This study, which includes approximately 30% of the total EK procedures performed in the U.S., found an overall rate of early graft failure of 1.17% for DMEK and 0.86% for DSAEK compared to 0.5% for PK. Although the DMEK failure rate was higher at 7.69% in 2013 (the year DMEK numbers began increasing in the U.S.), the later data from 2018 demonstrate a much lower rate of 0.68%, which is comparable to DSAEK.
The decreased prevalence of early graft failure from 2013 to 2018 for DMEK observed in this study may reflect progression on the surgical learning curve that has been previously reported for this specific procedure.17,18 The fact that surgical trauma as a cause for graft failure for DMEK decreased from 84% to 28% over the course of the study period is also consistent with this hypothesis. Given the availability of corneal tissue and the low cost of corneal transplantation relative to many other surgical procedures, the slightly increased early graft failure rate for DMEK or DSAEK demonstrated in our study (0.68% and 1.06%, respectively, in 2018) compared to PK (0.20% in 2018) may represent an acceptable cost and risk/benefit given the increased wound integrity, decreased risk of endophthalmitis, and rapidity of visual rehabilitation associated with EK.
Our study also identified a trend toward an increasing proportion of cases being attributed to donor tissue for both DSAEK and DMEK in the latter years. The overall improvement in outcomes suggests that this reflects a decrease in operative complications with improved techniques and surgeon experience rather than an increase in compromised tissue. In recent years, eye banks have implemented innovations such as preloaded tissues, endothelium-in versus endothelium-out graft loading, and storage of grafts in media to prevent post-operative fungal infections.19 Thus, the impact of some of these tissue processing and preservation changes need ongoing monitoring, as is already done with the EBAA Ocular Adverse Reaction Reporting System (OARRS). However, we believe that the more comprehensive approach of trying to capture all failure events (regardless of tissue attribution) might be helpful to monitor trends in outcomes as more surgeons continue to adopt DMEK in the U.S. and abroad.
In the CPTS study, 45 (3.4%) of 1,330 grafts performed between 2012 and 2014 for Fuchs endothelial dystrophy or bullous keratopathy resulted in early graft failure. That study found that donor diabetes and the presence of operative complications were associated with early graft failure when adjusting for recipient diagnosis, sex, donor age, pre-cut ECD and prior glaucoma surgeries.7 The present study design is limited in its ability to assess many of the factors comprehensively analyzed in the CPTS; however, it provides rates of early failures over a broader group of surgeons and patients and surgical indications. We were able to analyze donor tissue and limited recipient factors in our multivariable analysis, which compared DMEK and DSAEK failures versus non-failures from each eye bank. In contrast to the CPTS, higher donor age was associated with early DSAEK failures in our multivariable models. As mentioned above, the present study could not adjust for all of the factors that were accounted for in the CPTS, and thus the implications of this finding remain uncertain.
There are several important limitations to address when considering results from this study. Our study found an overall low rate of early graft failures, but since submission of adverse events is a voluntary process for surgeons, it is possible that our study is affected by underreporting.8 In addition to variability in surgeon reporting, there can be variation in how eye banks solicit and collect adverse reaction reports. We hoped to overcome this limitation by collecting and analyzing data from multiple eye banks. Our collaborating eye banks estimated that they received post-operative outcomes or adverse reaction forms on approximately 53% to 89% of tissues placed during our study period. If we were to accordingly reduce the denominator used for our rates of early failures by 30%, the early DMEK failure rate would be approximately 1.6% and 1.2% for DSAEK, which is still lower than prior literature.5–7,20 It is also possible that surgeons were less inclined to report early graft failure in cases of known intraoperative complications, surgical manipulation, or patient-level factors when compared to cases with perceived issues with donor tissues in cases of early graft failure. Thus, our finding that surgical manipulation is less frequently a cause of early failure in more recent years may be biased. Like the CPTS, a previous study of repeat keratoplasty in Medicare beneficiaries demonstrated a higher rate of early re-grafts of 3.6%.20 However, the Medicare study included DMEK and DSEK procedures in a cohort of patients over the age of 65. Thus, it is possible that the differences in re-graft rates seen between this Medicare study and our present investigation can be attributable to differences in the study population in addition to underreporting.
Another limitation of our study is that the subset analysis was limited to three of the six collaborating eye banks that had this retrospective data available for the study analysis and thus, findings that were observed may not be generalizable to all eye banks. We also did not have information pertaining to tissue processing at the eye bank prior to surgery such as pre-loading, marking, or preservation media; thus, we could not account for issues relating to processing in our analysis. Finally, the analysis is limited to factors available in this retrospective review of adverse reaction reports data and does not include comprehensive operative or post-operative information or even donor characteristics such as diabetes status. Nonetheless, the methodology presented in this paper enables monitoring of quality improvement efforts as they continue to unfold at eye banks and as surgeons evolve and refine their EK techniques in the U.S. and abroad. Eye banks in the U.S. play an important role in tissue preparation for certain other countries. For example, in Canada, not all eye banks have the capability to undertake advanced tissue processing, meaning that much of the pre-processed tissue is imported from the U.S.21 As more surgeons adopt DMEK and eye banks potentially assume a greater role in tissue processing as a result, the data presented in our study may serve as useful benchmarks.
Supplementary Material
Supplemental Figure 1. Number of endothelial keratoplasty tissues distributed by year.
Supplemental Figure 2. Proportion of penetrating keratoplasty grafts resulting in early graft failures by year from six eye banks in the United States, 2013 to 2018.
Acknowledgements
The authors would like to thank Wilbeth Flojo of CorneaGen, Seattle, WA, Michael O’Keefe of Eversight in Ann Arbor, MI, and Alan Blake at Advancing Sight in Birmingham, AL, for their efforts toward data collection.
Funding/Support:
This work was supported by the National Institutes of Health (P30EY001765, Wilmer Biostats Core).
Conflicts of Interest and Disclosure of Funding
• MJF has no financial disclosures to report.
• JAC has no financial disclosures to report.
• XL has no financial disclosures to report.
• ME is employed by Lions VisionGift.
• CS is CEO of Lions VisionGift.
• CM is employed by Eversight.
• MST is employed by Eversight.
• PJ is Director of Quality Improvement and Regulatory Affairs for Saving Sight.
• KBJ is employed by VisionFirst.
• TF is President/CEO of VisionFirst.
• LKB is employed by VisionFirst.
• MMM is President and CEO of CorneaGen.
• DBG has no financial disclosures to report.
• EKA has no financial disclosures to report.
• DS is a consultant for Alcon and receives grant support from the National Eye Institute and American Academy of Ophthalmology/Hoskins Center unrelated to this work.
Footnotes
Presentations:
Preliminary data from this manuscript was accepted for a poster presentation at the Association for Research in Vision and Ophthalmology (ARVO) meeting in May 2020 as well as a paper presentation for the World Cornea Congress meeting in May 2020. Both meetings were cancelled due to coronavirus disease-19 (COVID-19) related restrictions.
References
- 1.Eye Bank Association of America. 2020 Eye banking statistical report. 2021. Available at: https://restoresight.org/what-we-do/publications/statistical-report/. Accessed May 17, 2021.
- 2.Eye Bank Association of America. Guidance Document for Investigating and Reporting Adverse Reactions to the EBAA. 2017. Available at: http://restoresight.org/wp-content/uploads/2017/07/OARRS-Guidance_07_2017.pdf. Accessed May 17, 2021.
- 3.Gal RL, Dontchev M, Beck RW, et al. The effect of donor age on corneal transplantation outcome results of the cornea donor study. Ophthalmology. 2008;115:620–626.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelmus KR, Stulting RD, Sugar J, et al. Primary corneal graft failure. A national reporting system. Medical Advisory Board of the Eye Bank Association of America. Arch Ophthalmol. 1995;113:1497–1502. [DOI] [PubMed] [Google Scholar]
- 5.Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology. 2018;125:295–310. [DOI] [PubMed] [Google Scholar]
- 6.Lee WB, Jacobs DS, Musch DC, et al. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–1830. [DOI] [PubMed] [Google Scholar]
- 7.Terry MA, Aldave AJ, Szczotka-Flynn LB, et al. Donor, recipient, and operative factors associated with graft success in the Cornea Preservation Time Study. Ophthalmology. 2018;125:1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelstein SL, DeMatteo J, Stoeger CG, et al. Report of the Eye Bank Association of America Medical Review Subcommittee on Adverse Reactions reported from 2007 to 2014. Cornea. 2016;35:917–926. [DOI] [PubMed] [Google Scholar]
- 9.Keane MC, Mills RA, Coster DJ, et al. Is there evidence for a surgeon learning curve for endothelial keratoplasty in Australia? Clin Exp Ophthalmol. 2017;45:575–583. [DOI] [PubMed] [Google Scholar]
- 10.Parekh M, Ruzza A, Romano V, et al. Descemet membrane endothelial keratoplasty learning curve for graft preparation in an eye bank using 645 donor corneas. Cornea. 2018;37:767–771. [DOI] [PubMed] [Google Scholar]
- 11.Sella R, Einan-Lifshitz A, Sorkin N, et al. Learning curve of two common Descemet membrane endothelial keratoplasty graft preparation techniques. Can J Ophthalmol. 2019;54:467–472. [DOI] [PubMed] [Google Scholar]
- 12.Varadaraj V, Woreta FA, Stoeger CG, et al. Surgeon preference for endothelial keratoplasty techniques. Cornea. 2020;39:2–7. [DOI] [PubMed] [Google Scholar]
- 13.Dickman MM, Peeters JM, van den Biggelaar FJ, et al. Changing practice patterns and long-term outcomes of endothelial versus penetrating keratoplasty: A prospective Dutch registry study. Am J Ophthalmol. 2016;170:133–142. [DOI] [PubMed] [Google Scholar]
- 14.Letko E, Price DA, Lindoso EM, et al. Secondary graft failure and repeat endothelial keratoplasty after Descemet’s stripping automated endothelial keratoplasty. Ophthalmology. 2011;118:310–314. [DOI] [PubMed] [Google Scholar]
- 15.Wu EI, Ritterband DC, Yu G, et al. Graft rejection following descemet stripping automated endothelial keratoplasty: features, risk factors, and outcomes. Am J Ophthalmol. 2012;153:949–957.e1. [DOI] [PubMed] [Google Scholar]
- 16.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J of Statistical Software [Online]. October 2015; 67:1–48. Available from: Foundation for Open Access Statistics, Los Angeles, CA. Accessed May 17, 2021. [Google Scholar]
- 17.Dapena I, Ham L, Droutsas K, et al. Learning curve in Descemet’s membrane endothelial keratoplasty: First series of 135 consecutive cases. Ophthalmology. 2011;118:2147–2154 [DOI] [PubMed] [Google Scholar]
- 18.Dunker SL, Veldman MHJ, Winkens B, et al. Real-world outcomes of DMEK: A prospective Dutch registry study. Am J Ophthalmol. 2021;222:218–225. [DOI] [PubMed] [Google Scholar]
- 19.Chiang TT, Shtein RM, McCoy K, et al. Cost-benefit and cost-utility analysis of amphotericin B supplementation of corneal storage media with endothelial keratoplasty-prepared tissue. Cornea. 2020;39:422–430. [DOI] [PubMed] [Google Scholar]
- 20.Zafar S, Wang P, Woreta FA, et al. Risk factors for repeat keratoplasty after endothelial keratoplasty in the Medicare population. Am J Ophthalmol. 2021;221:287–298. [DOI] [PubMed] [Google Scholar]
- 21.Bae SS, Rocha G, Humphreys C, et al. A National Consensus Forum on improving cornea donation and transplantation access in Canada. BMC Proc. 2021;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Number of endothelial keratoplasty tissues distributed by year.
Supplemental Figure 2. Proportion of penetrating keratoplasty grafts resulting in early graft failures by year from six eye banks in the United States, 2013 to 2018.


