Abstract
During their co-evolution with pathogens, hosts acquired defensive health strategies that allow them to maintain their health or promote recovery when challenged with infections. The cooperative defense system is a largely unexplored branch of these evolved defense strategies. Cooperative defenses limit physiological damage and promote health without having a negative impact on a pathogen’s ability to survive and replicate within the host. Here, we review recent discoveries in the new field of cooperative defenses using the model pathogens Citrobacter rodentium and Salmonella enterica. We discuss not only host-encoded but also pathogen-encoded mechanisms of cooperative defenses. Cooperative defenses remain an untapped resource in clinical medicine. With a global pandemic exacerbated by a lack of vaccine access and a worldwide rise in antibiotic resistance, the study of cooperative defenses offers an opportunity to safeguard health in the face of pathogenic infection.
Keywords: Cooperative defenses, Disease Tolerance, Anti-virulence mechanisms, Salmonella, Citrobacter rodentium
What are cooperative defenses?
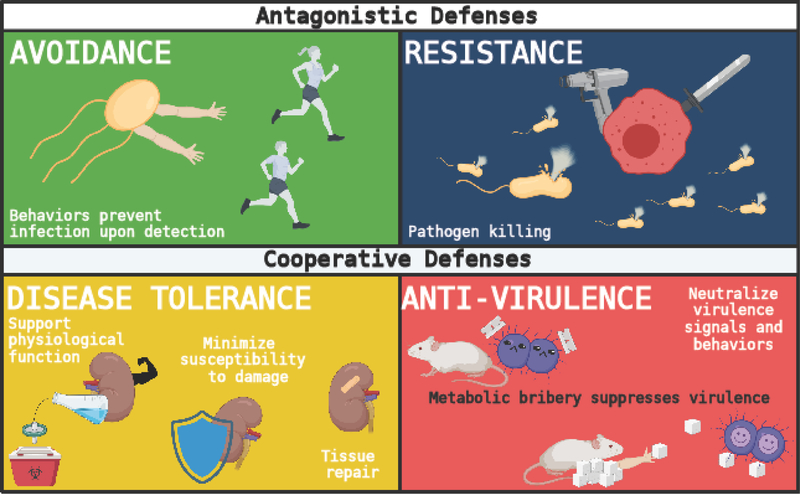
Organisms have evolved defensive health mechanisms to maintain their health (endurance) or promote recovery (resilience) when challenged with infections [1]. These mechanisms can promote endurance or resilience by antagonizing or withstanding the presence of the pathogen (Figure 1). Antagonistic defenses include avoidance and resistance mechanisms, which protect the host by interfering with a pathogen’s ability to infect or replicate inside the host. Avoidance mechanisms refer to innate or learned behaviors that prevent a host from becoming infected once the host senses a potential threat[1–3]. By contrast, resistance mechanisms eliminate pathogens that have infected the host. Pathogen clearance is largely mediated by the immune system (e.g., phagocytosis, release of antimicrobial peptides, etc.), but it is also facilitated by chemical (e.g., the secretion of hydrochloric acid by the stomach) and physical (e.g., skin and mucous membranes) barriers (Figure 1) [1,3,4].
Figure 1. Schematic of defensive health mechanisms.
Defensive health mechanisms evolved to allow hosts to maintain their health (endurance) or promote its recovery (resilience) during pathogenic infection. These mechanisms defend the health of the host by antagonizing or withstanding the infectious insult. Antagonistic defenses include avoidance strategies, which refer to innate or learned behaviors that prevent a host from becoming infected, and resistance mechanisms, which eliminate pathogens that have infected the host and are largely mediated by the immune system. Cooperative defenses encompass anti-virulence and disease tolerance mechanisms. Anti-virulence mechanisms counter pathogen or host-derived factors that can lead to disease pathogenesis. By contrast, disease tolerance mechanisms minimize host susceptibility to damage signals, support physiological function and promote tissue repair.
Historically, the vast majority of infectious disease research has focused on understanding immune mechanisms of pathogen killing. This has led to some of the most important innovations for human health, including vaccination and antimicrobial-based therapies [1,2]. Although resistance mechanisms are key to our understanding of host defense, an examination of host-pathogen interactions demonstrates that host defense against infections cannot rely solely on antagonistic strategies. Infections cause damage in the host caused by both host antagonistic mechanisms (immunopathology) and the pathogen. To survive, a host must prevent, withstand or repair this damage, demonstrating that mechanisms of pathogen killing are not sufficient to ensure survival [1,3]. The cooperative defense system enables hosts to limit physiological damage and promote health without having a negative impact on a pathogen’s ability to survive and replicate within the host, yielding an apparent cooperation between the host and the pathogen (Figure 1) [5]. Cooperative defenses encompass anti-virulence and disease tolerance mechanisms[1,4]. Anti-virulence mechanisms antagonize pathogen or host-derived factors that can lead to disease pathogenesis. For example, this can include antagonizing virulence factor expression, neutralizing host or pathogen derived toxins or dampening excessive inflammatory responses [5,6]. By contrast, disease tolerance mechanisms minimize host susceptibility to damage signals (e.g., metabolic adaptations that protect organs from infection-induced damage), support physiological function (e.g., physiological adaptations that promote functional efficiency during infection) and promote tissue repair (Figure 1) [7–11]. Here, we review recent advances in our understanding of cooperative defense mechanisms with a focus on two intestinal pathogens: Salmonella enterica and Citrobacter rodentium.
The Salmonella Typhimurium infection model
Discovered in 1885 by Theobald Smith and Daniel Elmer Salmon (after whom it was named), Salmonella continues to be one of the most important food-borne pathogens worldwide. Recent estimates indicate it is responsible for 93.8 million cases of gastroenteritis and 155,000 deaths globally each year [7,8]. The genus Salmonella consists of two species (Salmonella bongori and Salmonella enterica) and six subspecies. Salmonella enterica includes more than 2600 serovars, which can be divided into typhoidal and non-typhoidal serovars. Nontyphoidal Salmonella serovars cause acute, self-limiting gastroenteritis, often without symptoms. By contrast, infection with typhoidal serovars (e.g., S. Typhi) results in a severe and potentially fatal disseminated septicemic infection called typhoid fever. Infected individuals typically present with fever, anorexia, lethargy, and watery diarrhea, among other signs [7]. One of the most well-studied serovars is serovar Typhimurium, which causes enteric and systemic typhoid-like diseases in humans and diverse animal models. It is transmitted to new hosts via the fecal-oral route. Following oral ingestion, Salmonella adheres to and invades M cells, specialized antigen-sampling cells of the intestinal Peyer’s patches[9]. Infection is facilitated by genes harbored in Salmonella’s two main pathogenicity islands: Salmonella pathogenicity island 1 (SPI-1) and Salmonella pathogenicity island 2 (SPI-2). A type 3 secretion system (T3SS) encoded by SPI-1 is important for the gut stage and invasion step of Salmonella infection. In typhoidal salmonellosis, Salmonella disseminates via the lymphatics and bloodstream to systemic organs with the aid of a second T3SS, SPI-2 and associated effectors [10,11]. Resistance responses to Salmonella involve a complex interplay between the microbiota and the innate and adaptive immune systems. For instance, the microbiota provide colonization resistance against Salmonella via production propionate, which limits Salmonella growth by disrupting intracellular pH homeostasis [12]. Following infection, neutrophils and inflammatory monocytes are recruited to sites of infection, and this response is critical for containing bacteria at disease onset in susceptible mice[13]. Additionally, Salmonella induces a T helper 1 (THl)-biased adaptive immune response, and neutralization of the TH1 cytokine interferon-γ leads to increased bacterial burden in various organs [14].
One of the key advantages of the Salmonella model for the study of cooperative defenses is that it can be used to study not simply the acute but also the chronic, asymptomatic carrier state. Between 1–6% of patients with typhoid fever become chronic carriers of Salmonella [15,16]. These individuals continue to shed high numbers of bacteria in their stools for one year to a lifetime while showing no symptoms of disease [17]. Asymptomatic carriers are of special concern from a public health perspective as they can act as reservoirs for the spread of infectious disease. Indeed, epidemiological studies have shown that a minority of infected individuals are responsible for the majority of infections [18]. One prominent example is Mary Mallon (1869–1938). Known as “Typhoid Mary”, she was a healthy carrier of S. Typhi who infected hundreds of people, some of whom died, while working as a cook in New York[19]. As Salmonella infection induces an asymptomatic carrier state in some individuals and cooperative defenses promote asymptomatic infection [5,15,16], Salmonella is an excellent model to dissect the relationship between cooperative defenses and pathogen transmission and virulence.
Cooperative defenses during Salmonella infection
Salmonella promotes host health to increase its transmission
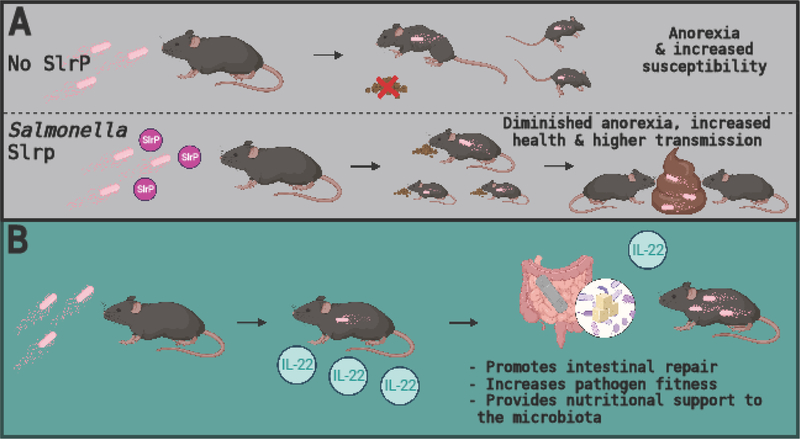
It has been proposed that pathogens have evolved mechanisms to induce cooperative defenses in their hosts to promote pathogen fitness[6]. A recent study of the role of sickness behaviors in Salmonella infection found that Salmonella evolved an effector protein, SlrP, that promotes its fitness by regulating the infection-induced anorexic response to balance virulence and transmission[6]. By inhibiting anorexia, SlrP lowers Salmonella virulence and thereby promotes survival of the host. Moreover, the authors showed that SlrP promotes host survival independent of pathogen burden. SlrP acts by inhibiting inflammasome activation and IL-1 maturation in myeloid cells of the small intestine, preventing induction of the vagus nerve-dependent anorexic program in the hypothalamus. Loss of SlrP leads to increased anorexia, enhanced dissemination to extra-intestinal organs and greater pathogen virulence, which come at the expense of pathogen transmission to new hosts (Figure 2) [6]. This study demonstrates that pathogens have evolved effectors and behavior-altering mechanisms that promote their fitness by inducing cooperative defenses in the host. Indeed, expression of SlrP by Salmonella promotes an anti-virulence defense strategy. Interestingly, these types of effectors and behavior-altering mechanisms may represent a common strategy during infection. For instance, expression of Salmonella typhoid toxin during Salmonella infection is known to increase host survival while favoring long-term colonization[20]. Additionally, behaviors traditionally ascribed to promoting virulence in pathogens, known as patterns of pathogenesis [21], can serve to promote health rather than disease [22]. For example, while gaining access to the cytosol is a common strategy to infect, replicate within and spread inside the host, protection from muscle wasting during infection by E. coli O21:H+ requires activation of an intracellular sensor [23]. Finally, it is interesting to consider whether the expression of Salmonella SlrP and similar microbial anorexia-inhibiting effectors during infection might have long-term consequences for host health. A separate study recently reported that severe infections that required hospitalization were associated with an increased risk of a subsequent diagnosis of anorexia nervosa in adolescents [24]. Adolescents who received antibiotic treatment saw an even further increase in their subsequent risk of diagnosis with anorexia nervosa. Slrp promotes cooperative defenses in the host. It may be that without effectors like Slrp to counter a hyperactive anorexic response, the health of the host is compromised not simply during infection but also long term. It is interesting to speculate that effectors like SlrP may act to prevent development of anorexia nervosa subsequent to severe infection.
Figure 2. Cooperative defenses during Salmonella infection.
(A) Salmonella effector SlrP promotes host health by reducing the infection-induced anorexic response in mice. By limiting anorexia, SlrP lowers Salmonella virulence, thereby promoting host health and survival while simultaneously increasing Salmonella transmission to new hosts. (B) Salmonella infection induces IL-22 in the host. IL-22 drives cooperative defenses by simultaneously promoting intestinal repair, pathogen fitness and providing nutritional support to the microbiota.
Why would infection induce anorexia if it is detrimental to hosts during Salmonella infection? One possible explanation, as proposed by the authors, is that while selection for sickness behaviors likely occurs at the individual host level, propelled by the benefits they confer to the individual host, the evolution of anorexia as a sickness behavior may serve to provide protection to hosts at the population level. While infection-induced anorexia is maladaptive to the individual host during Salmonella infection, the development of anorexia by individual hosts extends protection to other mice, as anorexic mice are less likely to transmit the pathogen[6]. Therefore, akin to social withdrawal, infection-induced anorexia may serve to confine the spread of a pathogen. A separate, non-mutually exclusive explanation could involve an evolutionary conflict with mechanisms that protect hosts from losing immune tolerance to dietary antigens. In 2017, a different study demonstrated that enteric infection triggers inflammatory responses to dietary antigens and development of celiac disease [25]. If infection-induced anorexia serves to minimize the loss of immune tolerance to dietary antigens, it may be that the cost of losing immune tolerance to dietary antigens is far greater to the host than the cost of maintaining anorexia during infection.
IL-22 works hard for that cooperation
While the immune system is best described in the context of mediating antagonistic defenses, it is also emerging as a critical regulator of cooperative defenses [26]. Interleukin-22 (IL-22), a member of the IL-10 family of cytokines, plays dual roles in the gastrointestinal tract (GI), where it functions to support and maintain the GI epithelial barrier as well as to facilitate barrier defense mechanisms against bacterial pathogens [27]. A recent study found a novel function for IL-22 in the promotion of cooperative defenses. Previous work had shown that although IL-22 is highly induced during acute Salmonella infection, it had no impact on cecal pathology[28]. Given the known functions of IL-22 in maintaining epithelial barrier integrity, the authors decided to investigate the role of IL-22 in a chronic model of Salmonella infection using the S. Typhimurium ΔaroA strain, which results in severe transmural inflammation and fibrosis. In contrast to the results observed with the acute infection model, the authors found that Ab-mediated IL-22 suppression following Salmonella ΔaroA infection hinders intestinal epithelial repair leading to exaggerated inflammation and delayed resolution of pathology[29]. Thus, expression of IL-22 during chronic Salmonella infection mediates a disease tolerance response. Curiously, the authors also found that Ab-mediated IL-22 suppression lowers Salmonella burden in the cecum 42 days post-infection, indicating that expression of IL-22 during infection not only acts to promote host health but also Salmonella fitness[29]. The enhanced pathogen clearance observed in mice undergoing IL-22 blockade was associated with alterations in gut microbiota composition, specifically the overgrowth of Bacteroides acidifaciens, a species previously shown to reduce Salmonella burden in the gut through vitamin B6 metabolic responses[30] (Figure 2).
Interestingly, while IL-22 specifically blocks the overgrowth of microbiota-member B. acidifaciens during chronic infection, a different study using C. rodentium showed that IL-22 is required to sustain the microbiota during the anorexic period of infection[31]. The authors demonstrated that detection of pathogen-associated molecular patters (PAMPs) resulted in rapid fucosylation of small intestine epithelial cells in mice. Following the shedding of fucosylated proteins into the gut lumen, fucose was metabolized by the microbiota. Without fucosylation, microbiota increased their virulence. By contrast, microbiota that were provided nutrients via fucosylation helped their hosts regain weight faster following immune activation, suggesting that intestinal fucosylation during infection functions as an anti-virulence defense mechanism. Based on these findings, it is tempting to speculate that IL-22 may have evolved to be an exquisite regulator of cooperative defense mechanisms in the gastrointestinal tract. Early on during infection, IL-22 acts to provide nutritional support to the microbiota, thereby suppressing virulence programs that could be induced as a result of infection-induced anorexia[31]. This could explain why a high level of IL-22 is observed during acute Salmonella infection given the lack of cecal pathology upon IL-22 blockade[28]. Later, during the chronic phase of infection, IL-22 drives intestinal epithelial repair to promote the resolution of pathology, a disease tolerance defense strategy, while simultaneously promoting Salmonella fitness, which requires suppression of a specific microbiota member[29]. Curiously, B. acidifaciens has the ability to utilize fucosylated glycans[31], indicating that while IL-22 may provide it with nutritional support early during infection, it can have a negative effect on this microbiota species at a later phase. By promoting intestinal epithelial repair in the host, supporting pathogen fitness and providing nutritional support to the microbiota, IL-22 fosters comprehensive cooperation between host, pathogen and microbiota to support health.
The Citrobacter rodentium infection model
The Gram-negative enteric bacterium Citrobacter rodentium is the causative agent of transmissible colonic hyperplasia in mice, its natural host[32,33]. Although first isolated in 1976, interest in this pathogen did not emerge until 1993 when Falkow and colleagues demonstrated that C. rodentium has the ability to attach intimately to the apical surface of the intestinal epithelium and induce the effacement of brush border microvilli, a feature known as an attaching and effacing (A/E) lesion [34,35]. These A/E lesions are virtually indistinguishable from those formed by two clinically relevant human pathogens: enteropathogenic Escherichia coli (EPEC), a leading cause of infantile diarrhea in developing countries, and enterohaemorrhagic E. coli (EHEC), which causes hemorrhagic colitis leading to hemolytic-uremic syndrome in developed countries[32,33,36]. For this reason, C. rodentium has been adopted as a model to study both the pathogenesis of and host response to infection with EPEC and EHEC.
After gaining entry via the oral route, C. rodentium utilizes the attaching and effacing mechanism to colonize the gastrointestinal tract. The pathogen attaches to the cecal patch, and from there, the infection spreads to the distal colon[37]. Visible lesions include a shrunken cecum, thickened colonic walls and the absence of normal stool in the colon. As the infection progresses, it induces a profound hyperplasia of the colonic mucosa [33]. Genetic background is a primary determinant of infection outcome. C57BL/6 and Swiss Webster mouse strains develop mild and self-limiting disease. By contrast, the mouse strains C3H and FVB develop severe disease and succumb to dehydration and electrolyte imbalances caused by diarrhea [38–41].
Cooperative defenses during Citrobacter rodentium infection
Survival means not R-spondin 2 the infection
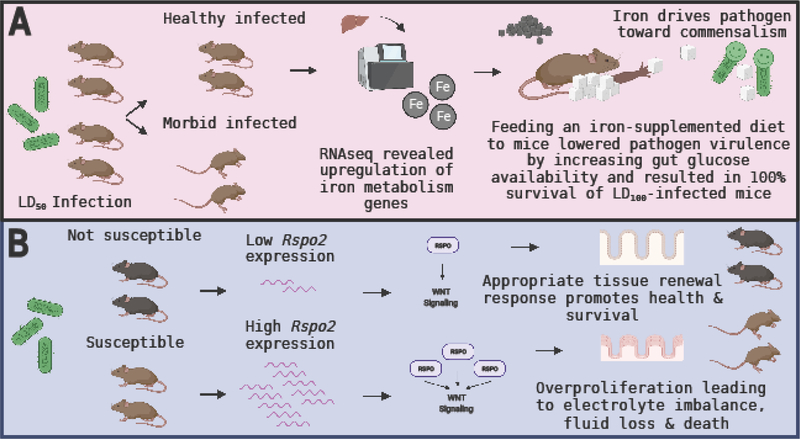
The R-spondins (RSPOs), a family of four secreted matricellular proteins, are known for their roles in potentiating or synergistically activating canonical WNT signaling[42]. Because WNT signaling plays a major role in diverse biological processes, there has been a growing interest in understanding how RSPOs regulate WNT signaling and thereby health and disease processes. Variation in the level of R-spondin 2 (Rspo2) gene expression is a major determinant of the outcome of C. rodentium infection. Susceptible mouse strains (e.g., C3H and FVB) secrete high levels of colonic RSPo2 following infection compared to strains that are not susceptible (e.g., C57BL/6) [38]. Differences in Rspo2 expression do not correlate with differences in C. rodentium burden. Instead, robust induction of Rspo2 acts to enhance canonical Wnt signaling in the colon, leading to pathological activation of Wnt signaling in this organ. Wnt signaling plays a major role in the proliferation of intestinal epithelial cell precursors, and its overactivation by RSPO2 during infection leads to excessive, uncontrolled proliferation in colonic crypt cells. This results in a poorly differentiated colonic epithelium with deficiencies in ion absorption that cause severe fluid loss and subsequently death [38,39](Figure 3). Thus, in this context, RSPo2 activity drives pathology and a decline in health (without affecting pathogen burden), which negatively impacts the ability of the host to cooperate with the pathogen.
Figure 3. Cooperative defenses during Citrobacter rodentium infection.
(A) LD50 infection is a tool to elucidate the mechanistic basis of asymptomatic infection. LD50 infection gives rise to a healthy infected and a morbid infected mouse population. This bifurcation in health trajectories can be exploited to understand the mechanisms of asymptomatic infection. In a C. rodentium LD50 mouse model, liver RNAseq revealed an upregulation of iron metabolism genes in healthy infected but not uninfected or morbid infected mice. Feeding an iron-supplemented diet to LD100-infected mice resulted in complete protection from the infection (100% survival). Mechanistic experiments revealed that iron supplementation drives insulin resistance, which increases glucose availability in the gut. High glucose lowers pathogen virulence and promote survival. Additionally, iron supplementation drove the selection of attenuated C. rodentium strains, driving the pathogen toward commensalism. (B) C. rodentium infection induces Rspo2 gene expression in the colon of mice. The level of Rspo2 expression determines infection outcome, with susceptible mouse strains expressing very high levels in contrast to non-susceptible strains. High levels of RSPo2 drive pathological overactivation of Wnt signaling, leading to uncontrolled proliferation in colonic crypt cells. This gives rise to a poorly differentiated colonic epithelium with deficiencies in ion absorption that cause severe fluid loss and subsequently death in mice.
Why would a subset of mouse strains strongly induce Rspo2 during C. rodentium infection when it is detrimental to survival? Antagonistic defenses are often referred to as a double-edged sword because, while beneficial, excessive activity can be maladaptive to the host. We propose the same may be true for cooperative defenses. The Wnt signaling pathway is important for tissue repair[43], and moderate induction of canonical Wnt signaling in the colon may be an appropriate response to infection with a pathogen that causes intestinal damage. In this scenario, the measured induction of Wnt signaling would qualify as a disease tolerance mechanism. While moderate induction of Wnt signaling may be beneficial to health and survival, excessive activation, as is the case in mouse strains that robustly induce Rspo2, is highly detrimental. Indeed, even if susceptible mice survive the infection, they are likely to face an increased risk of cancer: almost all colorectal cancers demonstrate hyperactivation of the Wnt signaling pathway [44]. In a mouse model of diethylnitrosamine (DEN)-induced injury, sustained tissue renewal processes resulted in carcinogenesis, demonstrating that there are pathological costs to sustained tolerance mechanisms [45]. For this reason, negative regulators of disease tolerance mechanisms will be critical to finetune these responses and therefore also function as part of a complex and highly regulated disease tolerance mechanism. For example, suppression of Wnt signaling by the tumor suppressor HBP1 can prevent inappropriate proliferation[46]. Thus, in this context, maladaptive overactivation of Wnt signaling can be considered the cost of an excessive disease tolerance response, similar to the way in which immunopathology is considered the cost of an excessive immune response [47].
Sweet talk your way to survival
How do we find novel ways to promote health and survival during infection? A recent study took advantage of the concept of lethal dose 50 (LD50), the dose of a pathogen that kills 50% of the host population, to address this question[5]. The LD50 of C. rodentium was administered to genetically identical mice kept in environmentally controlled conditions. Surprisingly, not only did the 50% of infected mice that survived carry the same pathogen load as those that perished, but they also remained asymptomatic. To understand the mechanistic basis of asymptomatic infection, the authors performed RNA-seq on the infected organs. Compared to uninfected and morbid infected mice, asymptomatic infected mice displayed upregulation of iron metabolism genes in their livers. Followup experiments revealed that feeding iron-supplemented diets to LD100-infected mice was sufficient to confer complete protection against C. rodentium-induced lethality. The iron diet protected mice by inducing insulin resistance, which increased glucose concentration in the intestine. In this infection model, elevated levels of intestinal glucose act as an anti-virulence mechanism. High glucose availability suppresses C. rodentium virulence, thereby promoting health and survival of infection.
While antagonistic defenses are predicted to drive the Red Queen effect between host and pathogen, leading to the oscillation of counter-adaptive strategies that promote fitness at the expense of the other, the evolutionary implications of cooperative defenses for host-pathogen co-evolution are distinct[22]. Cooperative defenses are not predicted to drive the evolution of counter-adaptive strategies in pathogen populations if the pathology being alleviated is not needed for pathogen replication or transmission. This idea has been extended to suggest that cooperative defenses may drive the evolution of benign and mutualistic host microbe relationships [47]. In support of this, the same study also showed that iron supplementation drove the selection of attenuated C. rodentium strains that acquired various mutations in their virulence factors, rendering them nonfunctional [5](Figure 3).
Aside from elucidating a novel anti-virulence mechanism, this work provided proof of concept that the LD50 model can be used to study asymptomatic infection and the cooperative defense mechanisms that enable it. Indeed, the approach can be applied to the study of infection with multiple of pathogens. Furthermore, the work highlighted how cooperative defense strategies can impose selection on pathogens in a way that drives them to commensalism in a relatively short timeframe, suggesting a new perspective on how to approach the treatment of infection. Finally, increasing intestinal glucose availability may have a dual purpose during infection. Infections often induce anorexia, which can have a negative impact on the microbiota by liming food availability[6,48]. Microbiota species are known to upregulate virulence genes in response to infection-induced anorexia[31]. By increasing glucose concentration in the intestine, iron supplementation may work not only to reduce pathogen virulence but also to reduce the induction of microbiota virulence programs. Curiously, oral rehydration therapy solutions often contain a substantial amount of sugar [49]. As infections frequently lead to dehydration (due to fever, diarrhea, vomiting, etc.), it may be that rehydration therapy not only improves patient health by supplying electrolytes and water but also by suppressing microbial virulence [50].
Prebiotics that support not only the host but also the pathogen
Galactooligosaccharides (GOS) are a type of nondigestible fiber with prebiotic activity[51]. A recent study showed that GOS supplementation protects mice from C. rodentium infection-induced colonic tissue damage[52]. Protection did not result from decreases in pathogen load, dissemination to extraintestinal tissues or inflammatory markers. In fact, improved colonic health during infection was associated with an increase in pathogen load in the colon, liver, and spleen but not the feces of GOS-treated mice. Curiously, GOS are not the only prebiotics that increase pathogen burden during infection[53]. How can GOS treatment promote health during infection despite increasing pathogen load? While the study did not provide a mechanism for the observed phenomenon, a likely explanation involves the induction of disease tolerance mechanisms by GOS. In this model, GOS-mediated disease tolerance not only offers a mechanism to explain decreased physiological susceptibility to infectious damage but also provides an explanation for the observed increase in pathogen burden, as disease tolerance mechanisms can have a positive effect on pathogen replication. The ability of disease tolerance to promote pathogen fitness while protecting the host from colonic tissue damage exemplifies true cooperation between host and pathogen. Moreover, by promoting fitness, disease tolerance can impose selection on pathogens without lowering their prevalence or starting an evolutionary arms race typical of resistance mechanisms[54]. Future studies are required to elucidate the specific mechanism(s) through which GOS supplementation may promote disease tolerance.
Concluding remarks
Preventing, withstanding and repairing infectious damage are integral parts of host defense. In this review, we summarized and discussed recent findings that inform our evolving understanding of the cooperative defense system. While the field of cooperative defenses is still in its infancy, it is already offering insights that are radically changing our understanding of host-pathogen interactions. We are learning that host-pathogen cooperation is an effective strategy to protect health during infection [5,6,29,52]. We are also discovering that the mechanisms that facilitate host-pathogen cooperation are rather complex and require exquisite regulation. Mechanisms that mediate host-pathogen cooperation, especially during enteric infection, often involve and/or affect the microbiota[29,55]. As such, any discussion of cooperative defenses should include questions on how specific cooperative defense mechanisms may affect the microbiota or how the microbiota could play a role in these mechanisms. In addition, our proposition that the cooperative defense system may be a double-edged sword, where unrestrained activity is maladaptive to the host, implies that the cooperative defense system is likely to be highly regulated, with multiple negative regulators in place. The level of regulation is likely to be on par with that of the immune system, which needs to negotiate a delicate balance between eliminating pathogens and avoiding immunopathology. This may explain why the immune system is emerging as a critical regulator of the cooperative defense system. Cooperative defenses complement antagonistic defenses in the defense of host health. Thus, it is likely that shared regulatory mechanisms exist to coordinate the efficient execution of host defense, with both defense systems exerting some level of regulatory control over the other. Finally, understanding how cooperative defenses promote host health and survival can offer different perspectives on how to approach the treatment of infection. For instance, as discussed in this review, there is now proof of concept that there are mechanisms that can drive a pathogen toward commensalism within the span of a single infection[5]. If we can develop therapies based on this idea, it will open fundamentally new avenues to treat infections. Furthermore, in the context of patients who cannot tolerate immunopathology or in the absence of effective antibiotic therapy and vaccines, asymptomatic infection mediated by cooperative defenses represents the best possible goal of medical care. Considering the enormous potential benefits, investment in these ideas is of paramount importance to the advancement of healthcare.
HIGHLIGHTS.
Cooperative defenses reveal the mechanisms of asymptomatic infection
Salmonella effector promotes host health while increasing pathogen transmission
IL-22 resolves pathology while simultaneously increasing pathogen burden
Iron-supplementation lowers pathogen virulence & drives pathogen toward commensalism
Prebiotics decrease colonic tissue damage while increasing pathogen burden
ACKNOWLEDGEMENTS
J.S.A is supported by NIH grants R01AI114929 and DP1AI144249, the NOMIS Foundation and the Keck Foundation. K.T. is supported by the NOMIS Postdoctoral Fellowship, the Pioneer Fund Postdoctoral Scholar Award and the Salk Women & Science Special Award. We also thank J. Rossi for assistance with manuscript editing. The figures were created with BioRender.
Footnotes
COI
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ayres JS: The Biology of Physiological Health. Cell 2020, 181:250–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayres JS, Schneider DS: Tolerance of infections. Annu. Rev. Immunol. 2012, 30:271–294. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Schneider DS, Soares MP: Disease tolerance as a defense strategy. Science 2012, 335:936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troha K, Ayres JS: Metabolic Adaptations to Infections at the Organismal Level. Trends Immunol. 2020, 41:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez KK, Chen GY, Schieber AMP, Redford SE, Shokhirev MN, Leblanc M, Lee YM, Ayres JS: Cooperative Metabolic Adaptations in the Host Can Favor Asymptomatic Infection and Select for Attenuated Virulence in an Enteric Pathogen. Cell 2018, 175:146–158.el5. *This work provided proof of concept that the LD50 model can be used to study asymptomatic infection and the cooperative defense mechanisms that enable it. Additionally, the work highlighted how cooperative defense strategies can impose selection on pathogens in a way that drives them to commensalism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao S, Schieber AMP, O'Connor CP, Leblanc M, Michel D, Ayres JS: Pathogen-Mediated Inhibition of Anorexia Promotes Host Survival and Transmission. Cell 2017, 168:503–516.e 12. *This study demonstrated that pathogens have evolved effectors that promote their fitness by inducing cooperative defenses in the host. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eng S-K, Pusparajah P, Mutalib N-SA, Ser H-L, Chan K-G, Lee L-H: Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Frontiers in life Science 2015, doi: 10.1080/21553769.2015.1051243. [DOI] [Google Scholar]
- 8.Palmer AD, Slauch JM: Mechanisms of Salmonella pathogenesis in animal models. Human and Ecological Risk Assessment: An International Journal 2017, doi: 10.1080/10807039.2017.1353903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monack DM, Mueller A, Falkow S: Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol 2004, 2:747–765. [DOI] [PubMed] [Google Scholar]
- 10.Azimi T, Zamirnasta M, Sani MA, Soltan Dallal MM, Nasser A: Molecular Mechanisms of SalmonellaEffector Proteins: A Comprehensive Review. Infection and Drug Resistance 2020, 13:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson KG, Holden DW: Dynamics of growth and dissemination of Salmonella in vivo. Cellular Microbiology 2010, 12:1389–1397. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, et al. : A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe 2018, 24:296–307.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CA, Silva M, Siber AM, Kelly AJ, Galyov E, McCormick BA: A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. U.S.A 2000, 97:12283–12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monack DM, Bouley DM, experimental SFTJO, 2004: Salmonella typhimurium Persists within Macrophages in the Mesenteric Lymph Nodes of Chronically Infected Nrampl+/+ Mice and Can Be Reactivated by IFNγ …. rupress.org, doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A SEARCH FOR TYPHOID CARRIERS AMONG 800 CONVALESCENTS. The Lancet 1916, 187:566–569. [Google Scholar]
- 16.Levine MM, Black RE, infectious CLTJO, 1982: Precise Estimation of the Numbers of Chronic Carriers of Salmonella typhi in Santiago, Chile, an Endemic Area. academic.oup.com,doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 17.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ: Typhoid fever. N Engl J Med 2002, 347:1770–1782. [DOI] [PubMed] [Google Scholar]
- 18.Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, et al. : Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. U.S.A 1997, 94:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marineli F, Tsoucalas G, Karamanou M, Androutsos G: Mary Mallon (1869–1938) and the history of typhoid fever. Ann Gastroenterol 2013, 26:132–134. [PMC free article] [PubMed] [Google Scholar]
- 20.Del Bel Belluz L, Guidi R, Pateras IS, Levi L, Mihaljevic B, Rouf SF, Wrande M, Candela M, Turroni S, NastasiC, et al. : The Typhoid Toxin Promotes Host Survival and the Establishment of a Persistent Asymptomatic Infection. PLoS Pathog. 2016, 12:e1005528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vance RE, Isberg RR, Portnoy DA: Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 2009, 6:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayres JS: Cooperative Microbial Tolerance Behaviors in Host-Microbiota Mutualism. Cell 2016, 165:1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schieber AMP, Lee YM, Chang MW, Leblanc M, Collins B, Downes M, Evans RM, Ayres JS: Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science 2015, 350:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breithaupt L, Köhler-Forsberg O, Larsen JT, Benros ME, Thornton LM, Bulik CM, Petersen L: Association of Exposure to Infections in Childhood With Risk of Eating Disorders in Adolescent Girls. JAMA Psychiatry 2019, 76:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, et al. : Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarville JL, Ayres JS: Disease tolerance: concept and mechanisms. Curr. Opin. Immunol. 2018, 50:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keir ME, Yi T, Lu TT, Medicine NGJOE, 2020: The role of IL-22 in intestinal health and disease. rupress.org, doi: 10.1084/jem.20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behnsen J, Jellbauer S, Wong CP, Edwards RA, George MD, Ouyang W, Raffatellu M: The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 2014, 40:262–273. *This work showed that IL-22 drives intestinal epithelial repair to promote the resolution of pathology, a disease tolerance defense strategy, while simultaneously promoting Salmonella fitness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo BC, Shin SB, Canals Hernaez D, Refaeli I, Yu HB, Goebeler V, Cait A, Mohn WW, Vallance BA, McNagny KM: IL-22 Preserves Gut Epithelial Integrity and Promotes Disease Remission during Chronic Salmonella Infection. J. Immunol. 2019, 202:956–965. [DOI] [PubMed] [Google Scholar]
- 30.Miki T, Goto R, Fujimoto M, Okada N, Hardt W-D: The Bactericidal Lectin RegIIIβ Prolongs Gut Colonization and Enteropathy in the Streptomycin Mouse Model for Salmonella Diarrhea. Cell Host Microbe 2017, 21:195–207. [DOI] [PubMed] [Google Scholar]
- 31.Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, et al. : Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 2014, 514:638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouladoux N, Harrison OJ, Belkaid Y: The Mouse Model of Infection with Citrobacter rodentium. Current Protocols in Immunology 2017, 119:19.15.1–19.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullineaux-Sanders C, Sanchez-Garrido J, Hopkins EGD, Shenoy AR, Barry R, Frankel G: Citrobacter rodentium-host-microbiota interactions: immunity, bioenergetics and metabolism. Nat Rev Microbiol 2019, 17:701–715. [DOI] [PubMed] [Google Scholar]
- 34.Schauer DB, Falkow S: Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect. Immun. 1993, 61:2486–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schauer DB, Falkow S: The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 1993, 61:4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S: Citrobacter rodentium of mice and man. Cellular Microbiology 2005, 7:1697–1706. [DOI] [PubMed] [Google Scholar]
- 37.Wiles S, Clare S, Harker J, Huett A, Young D, Dougan G, Frankel G: Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cellular Microbiology 2004, 6:963–972. [DOI] [PubMed] [Google Scholar]
- 38.Papapietro O, Teatero S, Thanabalasuriar A, Yuki KE, Diez E, Zhu L, Kang E, Dhillon S, Muise AM, Durocher Y, et al. : R-spondin 2 signalling mediates susceptibility to fatal infectious diarrhoea. Nature Communications 2013, 4:1898–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang E, Zhou G, Yousefi M, Cayrol R, Xia j, Gruenheid S: Loss of disease tolerance during Citrobacter rodentium infection is associated with impaired epithelial differentiation and hyperactivation of T cell responses. Sci Rep 2018, 8:847–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borenshtein D, Nambiar PR, Groff EB. Fox JG, Schauer DB: Development of fatal colitis in FVB mice infected with Citrobacter rodentium. Infect. Immun. 2007, 75:3271–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borenshtein D, Fry RC, Groff EB, Nambiar PR, Carey VJ, Fox JG, Schauer DB: Diarrhea as a cause of mortality in a mouse model of infectious colitis. Genome Biology 2008, 9:R122–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin Y-R, Yoon JK: The R-spondin family of proteins: emerging regulators of WNT signaling. Int J BiochemCell Biol 2012, 44:2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whyte JL, Smith AA, Helms JA: Wnt signaling and injury repair. Cold Spring Harbor Perspectives in Biology 2012, 4:a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schatoff EM, Leach BI, Dow LE: Wnt Signaling and Colorectal Cancer. Curr Colorectal Cancer Rep 2017, 13:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He G, Yu G-Y, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M: Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 2010, 17:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sampson EM, Haque ZK, Ku MC, Tevosian SG, Albanese C, Pestell RG, Paulson KE, Yee AS: Negative regulation of the Wnt-beta-catenin pathway by the transcriptional repressor HBP1. EMBO J. 2001, 20:4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayres JS: Inflammasome-microbiota interplay in host physiologies. Cell Host Microbe 2013, 14:491–497. [DOI] [PubMed] [Google Scholar]
- 48.EXTON MS: Infection-Induced Anorexia: Active Host Defence Strategy. Appetite 1997, 29:369–383. [DOI] [PubMed] [Google Scholar]
- 49.Water with Sugar and Salt. The Lancet 1978, 312:300–301. [PubMed] [Google Scholar]
- 50.Guerrant RL, Infectious BC-FC, 2003: Cholera, diarrhea, and oral rehydration therapy: triumph and indictment. academic.oup.com, doi: 10.1086/376619. [DOI] [PubMed] [Google Scholar]
- 51.Niittynen L, Kajander K, Korpela R: Galacto-oligosaccharides and bowel function. Scandinavian Journal of Food and Nutrition 2016, doi: 10.1080/17482970701414596. [DOI] [Google Scholar]
- 52.Kittana H, Quintero-Villegas MI, Bindels LB, Gomes-Neto JC, Schmaltz RJ, Segura Munoz RR, Cody LA, Moxley RA, Hostetter J, Hutkins RW, et al. : Galactooligosaccharide supplementation provides protection against Citrobacter rodentium-induced colitis without limiting pathogen burden. Microbiology (Reading) 2018, 164:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen A, Heegaard PM, Pedersen AL, Andersen JB, Sørensen RB, Frøkiær H, Lahtinen SJ, Ouwehand AC, Poulsen M, Licht TR: Some putative prebiotics increase the severity of Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol 2009, 9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitale C, Best A: The paradox of tolerance: Parasite extinction due to the evolution of host defence. Journal of Theoretical Biology 2019, 474:78–87. [DOI] [PubMed] [Google Scholar]
- 55.Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, et al. : Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 2014, 514:638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]