Abstract
Objective:
Chronic alcohol use increases risk of alcohol withdrawal symptoms (AW) as well as craving and abstinence-related distress symptoms, in those entering alcohol use disorder (AUD) treatment. Here, we examined whether AW and alcohol craving in AUD patients entering outpatient treatment prospectively predicts future heavy drinking days/week (HDD) and additional alcohol use outcomes during 8-weeks of outpatient treatment, and their relationship to abstinence symptoms of depression, anxiety and sleep difficulties.
Methods:
Participants were 80 treatment-seeking adults with current DSM-5 AUD (39% female; 43% White; 20–60 years) who completed assessments of AW and alcohol craving and also alcohol abstinence symptoms of depression, anxiety, and sleep quality at treatment intake. Participants were prospectively followed using daily diaries for alcohol use intake during 8-week of standardized weekly relapse prevention counseling to support recovery.
Results:
After accounting for demographic and pretreatment alcohol use, greater alcohol craving at treatment entry predicted higher HDD (p<.013) as well as greater drinking days (DD: p<.004), average drinks per drinking day/week (AvgD: p<.001) and relapse to heavy drinking (p<.05), while higher levels of pretreatment AW symptoms interacted with treatment week to predict greater HDD (p<.018). Abstinence symptoms of depression, anxiety, and sleep difficulties were associated with AW and craving but did not predict any drinking-related outcomes.
Conclusions:
These results provide evidence that increased AW and craving may serve as prognostic indicators of greater risk of heavy drinking in outpatient treatment. Findings suggest the need to evaluate AW and craving at outpatient treatment entry and develop targeted treatments to specifically address the effects of AW and craving on drinking outcomes in outpatient AUD treatment.
Keywords: alcohol withdrawal, craving, alcohol use disorder, treatment, abstinence, relapse
1. INTRODUCTION
Alcohol misuse and Alcohol Use Disorder (AUD) are significantly associated with adverse consequences and global disease burden (Griswold et al., 2018). Although there are efficacious treatments for AUD, treatment failure and high relapse rates remain a significant issue in AUD treatment (Sinha, 2011). The current FDA approved medications in the treatment of AUD have modest efficacy and heterogeneity in clinical symptomology of treatment-entering AUD patients contributes to the variability in the clinical course of AUD (Maisto, Kirouac and Witkiewitz, 2014). Recent initiatives aimed at addressing this heterogeneity to improve treatment of AUD (Litten et al., 2015; Witkiewitz et al., 2019) suggests a critical need to identify AUD features that differentiate those at increased risk for treatment failure and develop treatments targeted for those who are at risk of relapse to improve treatment outcomes and increase treatment success rates. For example, new research suggests that medication efficacy of gabapentin and prazosin for AUD treatment is moderated by alcohol withdrawal symptoms (AW) at treatment entry (Anton et al., 2020; Sinha et al., 2021). Previous work also suggests that alcohol craving at treatment entry levels may moderate naltrexone effects in AUD treatment (Monterosso et al., 2001). Notably, AW and alcohol craving are common clinical features of AUD among patients entering treatment. However, systematic evaluation of these symptoms for their impact on predicting AUD treatment response has been limited thus far.
Recent research has demonstrated that early alcohol abstinence is associated with disruptions in the stress and reward brain neurocircuitry resulting from chronic alcohol use (Koob, 2003). In humans, chronic alcohol effects on stress biology has been documented by disruptions in prefrontal-striatal brain function and in peripheral autonomic and hypothalamic-pituitary-adrenal axis functioning (Duka et al., 2011; Milivojevic and Sinha, 2018; Blaine et al., 2020) that are accompanied by increased risk of associated clinical symptoms such as AW, craving, greater distress, including depression, anxiety and sleep difficulties (Fox et al., 2007; Sinha et al., 2011; Milivojevic and Sinha, 2018). This profile of stress biological dysfunction as well as associated distress symptoms characterize chronic alcohol-related stress pathophysiology in AUD, which is also associated with greater subjective stress and alcohol cue reactivity (Fox et al., 2007; Sinha et al., 2011), and increased risk of relapse that jeopardizes alcohol recovery (Sinha, 2011). Despite existing evidence of relapse and treatment failure risk in those showing such AUD-related stress pathophysiology (Sinha, 2001; Koob and Schulkin, 2018; Milivojevic and Sinha, 2018), research to specifically assess whether stress-related clinical features of AUD significantly impact alcohol use outcomes in outpatient treatment has lagged behind.
Thus, a prospective observational study was conducted to examine whether specific AUD clinical features of AW and craving predicted subsequent risky drinking in AUD patients entering early outpatient treatment. On the basis of previous work (Sinha, 2011; Litten et al., 2015; Milivojevic and Sinha, 2018; Sinha et al., 2020), we selected AW and craving as co-primary predictors to assess their specific prospective prediction of the primary outcome of heavy drinking days/week (HDD) during treatment, after accounting for baseline levels of alcohol intake prior to study entry, abstinence days after study entry and prior to first treatment session, age, gender, race and education. We chose HDD as the primary outcome due to increasing emphasis on HDD and risky drinking more generally as an clinically informative outcome in clinical trials that aligns more closely with patient goals (Falk et al., 2010, 2019; Witkiewitz et al., 2020). In addition, specific alcohol-related covariates of recent alcohol consumption for 90 days prior to study entry, and abstinence days between intake and first treatment session were selected on the basis of previous work indicating their association with drinking outcomes during treatment (Sinha et al., 2011; Blaine et al., 2020), but more importantly, to assess specifically the effect of AW and craving over and above the quantitative effects of chronic alcohol use and abstinence/recovery days. Based on previous work (Sinha, 2001; Koob and Schulkin, 2018; Milivojevic and Sinha, 2018), we predicted that high levels of AW and craving would prospectively predict greater number of HDD during treatment. Additionally, we conducted secondary analyses to determine if AW and craving predicted other alcohol use outcomes (average drinks per drinking day for each week [AvgD], number of drinking days [DD] for each week, time to dropout, time to lapse, and time to relapse) to determine consistency of effects of the primary predictors across drinking outcomes. Finally, because of the close association between AW and craving and other alcohol abstinence features of depression, anxiety and sleep difficulties, we conducted post-hoc exploratory analyses to assess the association between these commonly reported abstinence-related distress symptoms and AW, craving and also alcohol use at intake and prospectively with alcohol use outcomes.
2. Method
2. 1. Participants
Participants were 80 AUD treatment-seeking community adults with current moderate to severe AUD (38.8% female; 42.5% White; aged 20–60 years; Mage = 36.6, SD = 11.24) who were recruited from the Greater New Haven area. Participants were recruited using flyers, brochures, newspapers, website announcements, social media, and referrals from local addiction treatment facilities (see Supplementary Materials for eligibility and exclusion criteria). The study protocol was approved by the Yale University School of Medicine’s Human Investigation Committee and registered on ClinicalTrials.gov (NCT02616094) to study the clinical and neurobiological predictors of AUD treatment outcome.
2. 2. Procedure
Interested individuals were interviewed and screened via telephone by trained research assistants to determine their eligibility. Participants first completed additional in-person intake screenings (including urine toxicology screens for recent alcohol and drug use), provided informed consent, and completed measures of AW, craving, depression, anxiety, and sleep difficulties at intake. Two eligible participants completed intake procedures and opted to receive medical detoxification at Yale’s Clinical Neuroscience Research Unit (CNRU) prior to initiating outpatient treatment. All participants were then prospectively followed while they participated in weekly standard manualized evidence-based behavioral treatment as described below for eight weeks with a trained master’s level addiction counselor or clinical psychologist to reduce craving, alcohol intake and relapse risk while initiating and maintaining alcohol recovery. Alcohol intake was measured with daily diaries via mobile phone and corroborated with weekly timeline follow-back assessments using the Substance Use Calendar method (Miller and Del Boca, 1994).
2. 3. Measures and Materials
2. 3. 1. Initial visits and baseline assessments at intake.
At intake, participants provided demographic information, including age, gender, race, annual household income, and years of education. Participants also completed the Clinical Institute of Withdrawal Assessment for Alcohol-revised (CIWA-Ar; (Sullivan et al., 1989)), Alcohol Urge Questionnaire (AUQ; (Bohn, Krahn and Staehler, 1995)), Hamilton Anxiety Scale (HAS; (Maier et al., 1988)), Beck Depression Inventory (BDI; (Beck et al., 1961)), and the Pittsburgh Sleep Quality Index (PSQI; (Buysse et al., 1988)). Internal consistency estimates of reliability were satisfactory for all these measures (see Table 1). Baseline alcohol intake for 90 days prior to study entry was assessed using the 90-day Substance Use Calendar (SUC; (Miller and Del Boca, 1994)). In addition, alcohol use was assessed daily after study entry up to the first treatment session to account for days of abstinence prior to the 8-week treatment. Finally, the Structured Clinical Interview for DSM-5 (SCID-5; (First et al., 2015)) was administered to assess DSM-5 criteria for AUD and other psychiatric conditions (see Supplemental Methods).
Table 1.
Demographics and Clinical Characteristics at Intake, Overall and Broken Down by Females and Males
| Range | n | Overall (N = 80) |
Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | n | Mean | SD | n | |||
| Demographics | ||||||||||
| Gender (% Female) | 0–1 | 31 | 38.75 | – | – | – | – | – | – | – |
| Age (in years) | 20–60 | 80 | 36.55 | 11.24 | 36.00 | 10.10 | 31 | 36.90 | 12.00 | 49 |
| Race (% White) | 0–1 | 34 | 32.5 | – | 32.26 | – | 10 | 48.98 | – | 24 |
| Income | 1–8 | 80 | 3.20 | 1.72 | 3.00 | 1.46 | 31 | 3.33 | 1.86 | 49 |
| Education (in years) | 9–20 | 80 | 13.82 | 2.31 | 14.26 | 2.38 | 31 | 13.63 | 2.25 | 49 |
| SES (in percentile) | 0–100 | 80 | 49.59 | 31.06 | 50.81 | 31.3 | 31 | 48.81 | 31.2 | 49 |
| Psychiatric Disorders a | ||||||||||
| Lifetime major depressive disorder (%) | 0–1 | 30 | 37.5 | – | 48.39 | – | 15 | 30.61 | – | 15 |
| Lifetime anxiety disorder (%) | 0–1 | 13 | 16.25 | – | 19.35 | – | 6 | 14.29 | – | 7 |
| Lifetime PTSD (%) | 0–1 | 19 | 23.75 | – | 35.48 | – | 11 | 16.33 | – | 8 |
| Alcohol Use | ||||||||||
| Years of alcohol use | 0.3–40 | 79 | 12.66 | 10.59 | 9.78 | 9.71 | 31 | 14.51 | 10.80 | 49 |
| Past 90-day average drinks per drinking day | 1.33–39.30 | 76 | 6.37 | 4.79 | 5.00 | 2.85 | 31 | 7.17 | 5.50 | 49 |
| Past 90-day % drinking days | 0–100 | 79 | 60.31 | 29.03 | 56.14 | 26.4 | 31 | 62.87 | 30.5 | 49 |
| Past 90-day % heavy drinking days | 0–100 | 79 | 39.26 | 30.89 | 33.38 | 25.8 | 31 | 42.86 | 33.4 | 49 |
| Age at onset of alcohol use | 6–28 | 80 | 15.20 | 3.12 | 16.00 | 3.41 | 31 | 14.7 | 2.84 | 49 |
| Other Drug Use | ||||||||||
| Past 90-day tobacco use (in cigarettes) | 0.25–25 | 38 | 7.60 | 6.65 | 7.65 | 7.06 | 13 | 7.57 | 6.59 | 25 |
| Past 90-day marijuana use (in joints) | 0–20 | 27 | 1.65 | 3.77 | 0.73 | 0.79 | 11 | 2.11 | 4.84 | 16 |
| Past 90-day cocaine use (in grams) | 0–50 | 12 | 10.94 | 16.79 | 24.72 | 23.4 | 10 | 4.05 | 6.69 | 8 |
| Alcohol Abstinence-Related Symptoms | ||||||||||
| Alcohol Withdrawal (CIWA; α = .71) | 0–15 | 80 | 4.55 | 3.84 | 4.06 | 3.84 | 31 | 4.86 | 3.84 | 49 |
| Craving (AUQ; α = .92) | 1–7 | 80 | 2.81 | 1.70 | 2.92 | 1.63 | 31 | 2.74 | 1.76 | 49 |
| Depression (BDI; α = .93) | 0–50 | 80 | 10.97 | 10.92 | 12.39 | 9.97 | 31 | 10.08 | 11.50 | 49 |
| Anxiety (HAM-A; α = .85) | 0–29 | 79 | 10.16 | 7.72 | 10.06 | 8.47 | 31 | 10.23 | 7.29 | 49 |
| Sleep Difficulties (PSQI; α = .74) | 0–17 | 80 | 8.04 | 4.09 | 8.06 | 4.12 | 31 | 8.02 | 4.12 | 49 |
Note. Income = annual household income, where 1 = “$0” and 8 = “More than $200,000”
Lifetime DSM-5 diagnosis as determined by the SCID for DSM-5; SD = standard deviation. Means and associated SDs that differ significantly between men and women at the level of p < .05 are shown in bold. labe
2. 3. 2. Weekly behavioral counseling and assessments during 8-week treatment.
Participants participated in once-weekly treatment sessions for 8 weeks with empirically validated standardized 12-Step and relapse prevention approach as outlined in the NIAAA Project MATCH manuals (Kadden, R. et al., 1994; Nowinski, Baker and Carroll, 1994). In addition, twice-weekly sessions were conducted to assess their alcohol use with timeline follow-back assessments using the 7-day SUC (Miller and Del Boca, 1994) (see Supplemental Methods). Participants also received contingency management (CM) reinforcing treatment attendance, in the form of ‘fishbowl’ draws in values of $1, $5, $10, and $25, in acordance with fishbowl CM techniques (Petry et al., 2000). All patients were referred to suitable continued treatment and aftercare at the end of the 8-week study treatment period.
2. 3. 3. Daily Alcohol Use Surveys.
Participants also reported their daily alcohol intake during treatment using brief surveys administered in a smartphone application (MetricWire, Inc.). Surveys on the total number of drinks consumed (beer, wine, and liquor) were obtained from daily morning and evening prompts triggered on their smartphones every day at 8:00 a.m. and 8:00 p.m. We calculated an index of the total number of drinks per day Overall, compliance was acceptable (69%), consistent with other daily diary studies monitoring everyday alcohol use (Piasecki, 2019).
2. 3. 4. Clinical predictors and drinking outcomes.
The co-primary predictors of interest were AW and alcohol craving and the primary outcome was heavy drinking days/week (HDD). Heavy drinking days were operationalized as days in which patients had 4+ drinks (women) and 5+ drinks (men). Participants reported their daily alcohol use during treatment using diary reports via mobile phone and weekly timeline follow-back assessments using the 7-day SUC. Note that if drinking data from daily surveys via mobile phone were missing, timeline follow-back data obtained using the 7-day SUC was utilized to obtain alcohol intake data weekly. To assess consistency in effects of AW and craving predictors on alcohol intake, secondary measures of drinking outcomes, namely drinking days/week (DD), the average drinks per drinking day for each week (AvgD), time to dropout (i.e., time to withdraw from the study), time to lapse (i.e., time to first drink), and time to relapse (i.e., time to first heavy drinking day) were included.
3. Data Analytic Approach
To determine the extent to which varying levels of AW and craving prior to treatment initiation were prospectively associated with HDD during treatment, linear mixed-effects (LME) regression models were estimated in R (R Core Team, 2020) using the ‘lme4’ (Bates et al., 2015) and ‘lmerTest’ (Kuznetsova, Brockhoff and Christensen, 2017) packages. A single separate model was utilized to examine the effects of each of the co-primary predictors of AW and craving using continuous scores for each, and their interactions with treatment week (week 1–8) on HDD1. Thus, a separate LME regression model included age, gender, race, education, baseline total drinks, days of abstinence, AW and treatment week, as well as AW X treatment week effect on primary HDD outcome during treatment. Similarly, a separate LME regression model including age, gender, race, education, baseline total drinks, days of abstinence, Craving and treatment week, as well as Craving X treatment week assessed the effects on primary HDD outcome (2 LME models). We corrected for multiple comparisons using a Bonferroni correction across the two co-primary predictor models for HDD, considering significant effects to be below p < 0.025 (0.05/2). Each of the two LME models specified a random intercept varying by participant and were estimated using restricted maximum likelihood (REML) estimation. Between-person continuous covariates were grand-mean-centered. We used t-tests and 95% confidence intervals (CIs), along with Satterthwaite-approximated denominator degrees of freedom to determine the significance of fixed-effect parameters at the level of p < .05. Both full model and primary predictors variance contributions was provided with marginal and conditional R2 (Rights et al., 2019) using ‘partR2’ package (Stoffel et al., 2020). To aid in the interpretation, the estimated marginal means of the primary HDD outcome were calculated based on the ±1 standard deviation levels of the craving and AW predictors.
Similar to the model described above for the primary HDD drinking outcome, we conducted analyses for the secondary drinking outcomes during treatment using a separate LME regression model for AW and a separate model for craving with the covariates specified above, to assess their effect on number of drinking days per week (DD) and separately for average drinks per drinking day per week (AvgD) (4 LME regression models). For the secondary drinking outcomes of time to drop out, time to lapse and time to relapse (3 outcomes), we utilized separate right-censored Cox Proportional-Hazards (CPH) regression models in R using the ‘survival’ (Therneau, 2021) and ‘survminer’ (Kassambara et al., 2020) packages for each of the pretreatment AW and craving predictors to assess their prospective effect on time to dropout, time to lapse, and time to relapse during treatment. Thus, separate models were estimated to examine the effects of baseline AW and craving on each time-to-event outcome (total of 6 models). All CPH models were estimated using partial likelihood (PL) estimation. The day of lapse or relapse for patients who withdrew from the study without having reported a lapse or relapse was considered censored at the day that they withdrew (i.e., lapse coded as a 0 but the study day was censored at point of dropout). Efron’s approximation method was used to handle ties. Wald χ2 and 95% CIs were used to determine the significance of hazard ratios (HRs) at the level of two-tailed p < .05.
Additional post-hoc exploratory LMEs, same as the separate LME regression models described above, were conducted to assess the separate effects of anxiety, depression, sleep problems and alcohol intake at pre-treatment and also to assess whether depression, anxiety and sleep problems predicted alcohol use outcomes during treatment.
3. 1. Covariate Adjustment:
Given the evidence that alcohol use outcomes during treatment could be influenced by the number of abstinence days (Blaine et al., 2020) and levels of recent alcohol use (Breslin et al., 1997) upon study entry, all models controlled for the effects of the number of abstinence days after study entry and prior to the first of the 8 treatment sessions and past 90-day alcohol use upon study entry, in addition to age, sex, race, and education.
4. Results
4.1. Description of the Analytic Sample
Table 1 summarizes demographic information and clinical characteristics of the sample overall and separately for men and women. Overall, the sample consisted of 80 treatment-entering patients with current DSM-5 moderate to severe AUD who were middle-aged (Mage = 36.6, SD = 11.24), predominantly male (62.2%), and non-White (57.5%). Participants regularly consumed alcohol for 12.66 (SD = 10.59) years, on average, with a mean age at onset of drinking at 15.20 (SD = 3.12) years. During the 90 days prior to study entry, participants reported, on average, 6.37 (SD = 4.79) drinks per drinking day, 60.31 (SD = 29.03) percent drinking days, and 39.26 (SD = 30.89) percent of heavy drinking days. On average, participants reported 14.04 (SD = 62.26) abstinence days between intake and prior to the 8-week outpatient treatment initiation. Most participants reported having experienced some degree of AW symptoms (90%), craving (80%), depression (83.4%), anxiety (94.9%), and sleep difficulties (98.8%) at treatment entry. No sex differences were found between men and women in their levels of pretreatment alcohol abstinence-related symptoms (all ps > .05). Participants completed, on average, 7.42 sessions (SD = 1.60) throughout the entire treatment period, with most participants (83.8%) remaining in the study until the end of treatment.
4.2. Pretreatment Alcohol Craving ad AW Predicting Percent Heavy Drinking Days
Pretreatment Alcohol Craving:
Alcohol craving levels at treatment entry significantly predicted higher HDD overall through the 8-weeks of treatment (F1,71.65= 6.53, p = .013; Conditional R2: Full Model=0.66, Alcohol Craving=0.63; Marginal R2: Full Model=0.08; Alcohol Craving= 0.05) (Figure 1a). Thus, in the real-world context, individuals with a low craving score (−1 SD = 1.79) had approximately 0.78 (95% CI: 0.−1.76) heavy drinking days on average per week, whereas an individual with a high craving score (+1 SD = 5.24) would have 1.28 (95% CI: 0.66–1.89) heavy drinking days per week. Notably, other than the co-primary predictor of alcohol craving, none of the covariates of age, sex, race, education, baseline drinking and pretreatment abstinence days yielded a significant effect on HDD during treatment. These data indicate that alcohol craving accounted for the majority of variance in HDD each week over the 8-week period.
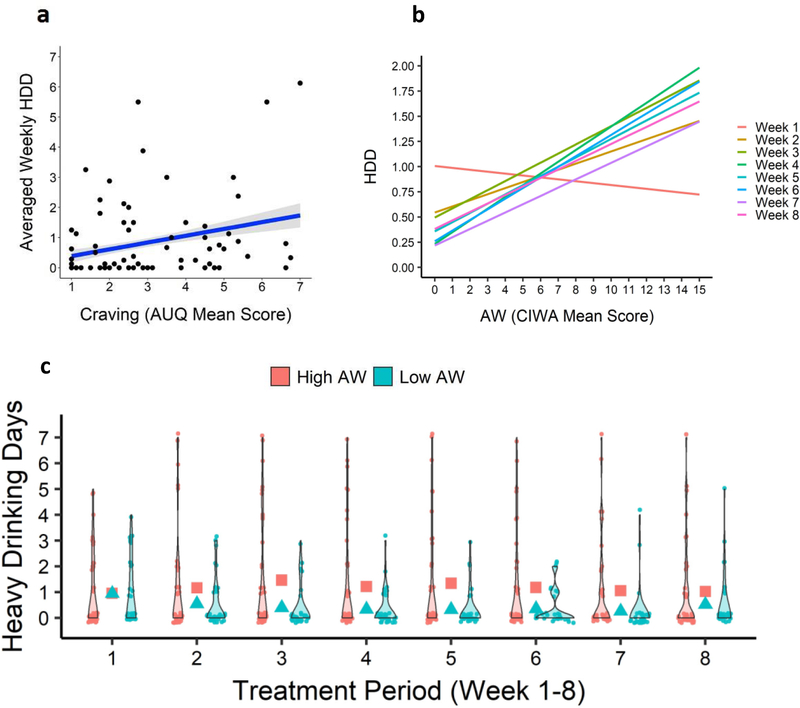
Figure 1.
Pretreatment alcohol craving and AW predicted subsequent heavy drinking days during treatment. 1a: Higher the AUQ Craving score, greater the HDD (p <.013) during treatment. Scores on the AUQ were averaged so that possible scores would fall between 1–7; 1b: Regression lines for AW continuous score X each treatment week is shown representing CIWA mean score in the sample by number of HDD each week, with greater the baseline AW being associated with higher HDD weekly after week 1, while lower AW predicted progressive reductions in HDD weekly (p < .018). 1c: Violin plots showing distribution of number of HDD for each treatment week for the Low (Blue data points and mean in Blue squares) and High (Red data points and Red squares) AW scores, using the median split cut-off of 3.5 to define low and high groups. Total scores for AW (measured by the CIWA) ranges between 0–67, although the range for the current sample was 0–15 as shown in Fig 1b.
Pretreatment Alcohol Withdrawal Symptoms (AW):
Continuous scores of AW at treatment entry interacted with treatment week to predict significantly higher HDD during treatment (F7,474.82= 2.44, p = .018; Conditional R2: Full Model=0.67, AW X Treatment Week=0.65; Marginal R2: Full Model=0.07; AX x Treatment Week= 0.05). During the first study week, an individual who experienced low AW (−1 SD = 1.56) at baseline experienced 0.98 (95% CI: 0.76 – 1.19) heavy drinking days per week; similarly, a high AW score (+1 SD = 9.62) was associated an average of 0.82 (95% CI: 0.54 – 1.10) heavy drinking days. However, assessing the effect of AW at Week 6, low AW was associated with 0.42 (95% CI: −0.01 – 0.86) but high AW was associated with an average of 1.82 (95% CI: 0.70 – 1.85) heavy drinking days. Again, the covariates did not contribute significantly to the variance in HDD outcomes over 8 weeks in this model. As shown in Figure 1b, individuals with higher AW at treatment entry maintained had a higher frequency of HDD each week, whereas individuals low in AW had fewer HDD episodes as they progressed from week 1 through week 8 in the study. Figure 1c also provides actual raw number of heavy drinking days for each treatment week for the high and low AW individuals.
4.3. Secondary Analyses of Pretreatment Alcohol Craving and AW on Secondary Alcohol Use Outcomes.
Pretreatment Alcohol Craving:
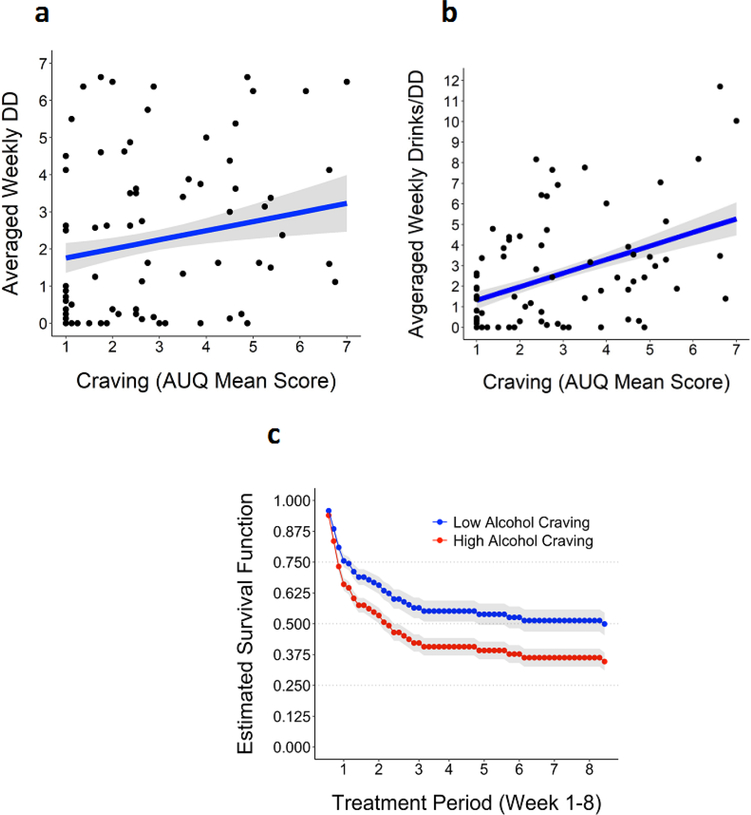
Pretreatment craving significantly predicted higher DD during treatment (F1,70.65= 9.29, p < .004; Conditional R2: Full Model=0.77, Alcohol Craving=0.71; Marginal R2: Full Model=0.13; Alcohol Craving= 0.07) and AvgD (F1,70.23= 12.07, p < .001, Conditional R2: Full Model=0.53 Alcohol Craving=0.49; Marginal R2: Full Model=0.11; Alcohol Craving= 0.08) (see Figure 2a–2b). Furthermore, higher pretreatment alcohol craving levels significantly predicted a higher risk of subsequent time to relapse to heavy drinking during treatment in the CPH models (HR: 1.20; 95% CI [1.00–1.44], p = .049), where each one-unit increase in craving at intake was associated with a 20% increase in the risk of subsequent relapse to heavy drinking during treatment (see Figure 2c). Pretreatment craving did not significantly predict risk of subsequent time to alcohol lapse or dropout during treatment (all ps > .05).
Figure 2.
Pretreatment alcohol craving predicting subsequent secondary alcohol use outcomes during treatment. Scores on the AUQ were averaged so that possible scores would fall between 1–7, which is the Likert scale range for each AUQ item. 2a-2b: Higher AUQ craving scores predicted significant greater DD (p <.004) and AvgD (p<.001) during treatment; 2c: Baseline AUQ alcohol craving (continuous scores) predicted risk of relapse to heavy drinking during treatment (p = .049). The survival function (or model-estimated predicted probability) is depicted across the entire treatment period (week 1–8). AUQ scores were median split at 2.5 to define high and low alcohol craving groups.
Pretreatment Alcohol Withdrawal Symptoms (AW):
Pretreatment AW continuous scores did not significant predict DD, AvgD, nor risk of subsequent lapse, relapse, or dropout during treatment (all ps > .05).
4.4. Post-Hoc Exploratory Analyses
Association of Pretreatment AW and Craving with Abstinence Symptoms and Alcohol Intake:
Pretreatment levels of AW and alcohol craving were moderately positively correlated (r = .32, p < .004). Pretreatment AW and alcohol craving were moderately to strongly associated with depression, anxiety, and sleep difficulties at treatment entry (mean value |r| = .42; range .32 to .65; all p’s < .05; see Table S1 and Figure S1). Craving was moderately correlated with pretreatment HDD (r =.30, p <.007). Higher AW tended to be related to increased baseline HDD but this association was not significant (r = .21, p =.063).
Pretreatment Depression, Anxiety and Sleep Difficulties on Alcohol Use Outcomes:
Although moderately to strongly correlated with AW and craving, pretreatment symptoms of depression, anxiety, and sleep difficulties did not significantly predict any drinking-related outcome during treatment after accounting for demographic and pretreatment alcohol use covariates (all ps > .05).
5. Discussion
This prospective observational study examined whether chronic alcohol intake-related AUD clinical features of AW and alcohol craving at pretreatment prospectively predicted alcohol treatment outcome, specifically heavy drinking days as the primary outcome and also secondary drinking outcomes of number of drinking days/week and average drinks/per drinking day each week in AUD patients entering outpatient treatment. Higher levels of pretreatment craving predicted a higher number of heavy drinking days across all weeks in the 8-week treatment period. Furthermore, higher pretreatment levels of AW interacted with treatment weeks to predict greater number of heavy drinking days each week for weeks 2–8. Remarkably, none of the other demographic (age, sex, education, race) or baseline drinking and pre-treatment days of abstinence variables exerted any significant influence on weekly drinking outcomes during the 8-week treatment period, either in the craving model or the AW model. While it is possible that other temperament, personality or genetic factors not assessed in the current study may also influence treatment outcomes, it was notable that alcohol craving and AW each accounted for over the majority of the variance in the primary number of heavy drinking days and secondary drinking outcomes of number of drinking days and average drinks per drinking day during the 8-week treatment in each of their respective models. Notably these significant effects of pretreatment craving and AW occurred even in the context of the expected improvements in drinking outcomes with weekly empirically validated efficacious behavioral counseling being provided. Furthermore, higher craving also predicted swifter relapse to heavy drinking. Thus, the findings suggest that while the empirically validated 8-weeks of behavioral counseling treatment was effective in reducing alcohol misuse among patients with little or no craving or AW, it did not improve risky drinking in patients with higher levels of pretreatment AW or those with higher alcohol craving. The findings for HDD were significant even after adjusting for multiple comparisons and suggest that pretreatment AW and alcohol craving as assessed via Clinical Institute of Withdrawal Assessment for Alcohol (CIWA-Ar) and Alcohol Urge Questionnaire (AUQ), may serve as clinical prognostic indicators of alcohol use outcomes and AUD treatment response.
Current findings indicating that pre-treatment alcohol craving predicted heavy alcohol use during outpatient treatment are consistent with previous research assessing alcohol use and heavy drinking (Mchugh et al., 2017; Schlauch et al., 2019), and relapse (Higley et al., 2011; Sinha et al., 2011). Findings also indicated that baseline levels of AW interacted with treatment week to predict alcohol use and heavy drinking (O’Connor et al., 1991; Sinha et al., 2021). The significant interaction reflected sustained reductions in alcohol intake across treatment weeks in patients with little or no AW symptoms, but no such reductions were observed in those with higher levels of AW at treatment entry, and these effects were independent of demographic and baseline drinking variables. This is significant as it suggests that key AUD-related clinical features of craving and AW represent significant disease processes that impact AUD clinical course and drinking outcome during outpatient treatment.
Previous research has shown alcohol craving and AW are associated with disruptions in brain and peripheral stress physiology that also co-occur with additional abstinence related distress symptoms such as anxiety, depression and sleep problems, which together may represent stress pathophysiology of AUD beyond that attributable to chronic alcohol intake levels and drinking patterns (Koob, 2003; Fox et al., 2007; Duka et al., 2011; Blaine et al., 2018, 2020; Milivojevic and Sinha, 2018). Furthermore, preclinical and clinical research indicates that these alcohol-related changes in stress neurobiology involve alterations in noradrenergic, corticotrophin releasing factor (CRF) dopaminergic and Gamma-Aminobutyric Acid (GABA) pathways among others, that in turn, affect increased alcohol craving and relapse risk (Koob and Volkow, 2016; Koob and Schulkin, 2019). Recent pharmacotherapy development has focused on targeting individual differences in AW as well as stress-induced craving related changes that promote relapse risk and treatment failure. For example, evidence shows the utility of Gabapentin treatment for AUD in those with high AW (Anton et al., 2020) and we also reported that Prazosin, a noradrenergic alpha-1 antagonist showed benefit in AUD treatment outcomes only in those with significant AW and not in those with no or very few AW (Sinha et al., 2021). The prazosin findings are also consistent with human laboratory and real world ecological momentary assessment studies showing that Prazosin improves disruption in peripheral stress physiology and also reduced stress- and alcohol -cue- induced craving (Fox et al., 2012; Milivojevic et al., 2020), and that day-to-day stress and related craving significantly impacts increases in next day drinking in early treatment for AUD (Wemm et al., 2020). These findings underscore the need to assess AUD clinical features such as AW and craving at treatment entry as these indicators add not only significant heterogeneity in clinical presentation (Litten et al., 2015), but also support targeted treatment development for those with higher levels of craving and AW can be of benefit in improving AUD drinking outcomes.
Consistent with our previous work (Sinha et al., 2021) additional post-hoc exploratory analyses also revealed that higher baseline levels of AW and craving were associated with symptoms of depression, anxiety, and sleep difficulties at treatment entry, and depression and sleep difficulties were also associated with baseline levels of alcohol intake. Despite these positive associations with craving, AW, and pretreatment drinking, post-hoc exploratory analyses showed that baseline symptoms of depression, anxiety, and sleep difficulties did not predict subsequent alcohol use outcomes during 8-weeks of behavioral AUD treatment. These results suggest that these symptoms are related, but conceptually and functionally distinct constructs. Nevertheless, attesting to their clinical significance in AUD treatment, these clinical symptoms are associated with higher alcohol craving in AUD patients, thereby increasing the risk of drinking episodes during treatment (Witkiewitz, Bowen and Donovan, 2011; Wemm et al., 2020). Given that individuals high in AW and craving at treatment entry are more likely to experience symptoms of depression, anxiety, and sleep difficulties during treatment, these additional clinical symptoms may further reduce their ability to cope with stressful events and resist alcohol cravings, thereby increasing their risk of relapse. Thus, these findings support the need to evaluate and monitor clinical symptoms associated with AUD-related stress pathophysiology during outpatient treatment and indeed throughout treatment to promote AUD treatment success. The findings also suggest that assessments of AW and craving at treatment entry may serve as relevant modifiable clinical prognostic factors influencing AUD drinking outcome, while broader, transdiagnostic AUD-related clinical phenotypes of negative mood or emotionality or measures of depression, anxiety, and sleep difficulties may be associated with AW and craving but may not directly impact drinking outcomes in treatment.
The current study has several strengths that address common shortcomings of AUD treatment studies. First, the current study used daily diary self-reports to capture daily drinking in close temporal proximity to the actual drinking experiences, which augments the validity and accuracy of weekly timeline follow-back drinking data obtained during treatment by reducing recall bias (Piasecki, 2019). Moreover, the hypotheses were tested using linear mixed-effects (LME) regression models which factors in week-by-week variance in drinking outcomes to more powerfully model prospective treatment effects. Furthermore, this approach also has many advantages over more traditional regression-based techniques for analyzing data from treatment-based daily diary studies (Shiffman, Stone and Hufford, 2008), including its ability to handle missing observations without excluding the entire patient’s data while still providing unbiased parameter estimates. An additional strength is that we chose to focus the riskiest level of drinking, namely heavy drinking days, as our primary outcome of interest to emphasize the clinical utility of these measures. Finally, even though baseline 90 days of recent past alcohol use and number of abstinence days prior to treatment initiation have been shown to influence AUD treatment outcomes (Breslin et al., 1997; Blaine et al., 2020), current findings build on previous work indicating the clinical relevance of alcohol withdrawal (Malcolm et al., 2000; Schuckit, 2014) and craving (Fazzino et al., 2013) in AUD treatment course by showing that the effects of AW and craving on subsequent alcohol use outcomes during treatment were present even after accounting for any potential influence of these alcohol use measures prior to the 8-week treatment period.
The current study has some limitations that need to be discussed and addressed in future research. First, we collected data from 80 treatment-entering AUD patients, consisting of mostly men, which may have prevented us from having adequate statistical power for detecting differences between men and women. Because previous research has shown some evidence of sex differences in the chronic course of AUD (Peltier et al., 2019; Guinle, 2020), future studies should replicate our findings with larger samples of women to adequately test for gender differences. In addition, the current study relied exclusively on patients’ reports on their alcohol intake during treatment. Although there is evidence for the validity of drinking self-report data in patients receiving treatment for AUD (Mundle et al., 1999; Babor et al., 2000), researchers should nevertheless replicate the current findings using more objective measures obtained from wearable technology with biosensors and other biochemical measures of recent alcohol intake as additional outcome measures in future studies. Also, future research may benefit from advanced statistical techniques such as multivariate approaches, including structural equation modeling, to account for the potentially covarying effects or multiple related drinking variables, AW, craving and other abstinence symptoms. Finally, future research to replicate current findings and also determine specific cut-off scores for CIWA-Ar and AUQ that are sensitive to treatment response could be of benefit to clinicians in outpatient treatment settings to help them identify patients at risk for relapse and poor alcohol use outcomes during treatment, to further improve AUD treatment efficacy.
In conclusion, the current study provides evidence that increased levels pre-treatment craving and AW place patients with AUD at increased risk for treatment failure, thus jeopardizing their recovery efforts. Our findings extend previous research showing that higher levels of craving at treatment entry predict heavy alcohol use and a higher risk of subsequent relapse to heavy drinking during outpatient treatment, and also provide the first evidence that AW at treatment entry interacted with treatment week to predict treatment response. Individuals with more AW symptoms at treatment entry maintained high levels of alcohol use, whereas those individuals low in withdrawal showed improvements with evidence-supported AUD behavioral therapy with significant reductions in their alcohol use during treatment. These findings are consistent with the notion that AW and craving may serve as useful and clinically meaningful prognostic indicators of treatment response, and suggest the need for both assessment of AW and craving at treatment entry, and the development of treatments that specifically target AW and craving to facilitate early alcohol recovery and improve drinking outcomes in AUD.
Supplementary Material
Highlights.
Alcohol withdrawal symptoms (AW) and craving are common symptoms of alcohol use disorder (AUD) in patients entering outpatient AUD treatment, but they have not been systematically evaluated as predictors of drinking outcomes in outpatient AUD treatment.
Participants completed assessments of AW and craving and also for depression, anxiety and sleep difficulties at treatment entry and were prospectively followed using daily diaries to assess primary outcome of heavy drinking days as well as additional secondary outcomes of any drinking days and average drinks per day for an 8-week outpatient treatment period.
After controlling or baseline drinking, abstinence days and demographic variables, alcohol craving and AW each prospectively predicted heavy drinking days and additional secondary drinking outcomes, while depression, anxiety and sleep difficulties were not associated with drinking outcomes.
Alcohol craving and AW may serve as prognostic indicators of AUD treatment outcome and need to be specifically addressed in AUD treatment.
Acknowledgements
We thank Rachel Hart, Ryan Douglas and Chloe Larkin for their assistance with recruitment and data collection.
Author Disclosures
This work was funded by grants R01-AA013892 and R01-AA026514 (PI: Rajita Sinha) from the National Institutes of Health (NIH) and National Institute on Alcohol Abuse and Alcoholism (NIAAA). The analyses and results described in this manuscript were presented in poster format at the 44th Annual Research Society on Alcoholism Scientific Meeting/ISBRA Congress.
Footnotes
Conflict of Interest Statement
All authors declare no financial relationships with commercial interests.
Daily drinking data was aggregated to obtain average weekly heavy drinking and secondary drinking measures to match with weekly treatment provided. Time (treatment week) was treated as a discrete, within-subject variable (week 1–8) in the analyses. We adopted this modeling approach based on two main methodological and practical considerations. First, given that treatment was provided on a weekly basis, aggregating HDD at the weekly level allows best to characterize treatment responses throughout the entire treatment period. Note that recent work (Hallgren, Atkins and Witkiewitz, 2016) suggests that analyses of clinical trials based on aggregated drinking data produced nearly identical Type I error rates, statistical power, and bias in estimating treatment effects, compared to analyses using completely disaggregated daily drinking data. Second, obtaining aggregate drinking data over the week over weekly intervals and specifying treatment week as a discrete, within-subject variable allows the interpretation of the main effects of interest as averaged effects across the entire treatment period.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton RF et al. (2020) ‘Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients with Alcohol Withdrawal Symptoms: A Randomized Clinical Trial’, JAMA Internal Medicine, 180(5), pp. 728–736. doi: 10.1001/jamainternmed.2020.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF et al. (2000) ‘Talk is cheap: Measuring drinking outcomes in clinical trials’, Journal of Studies on Alcohol, 61(1), pp. 55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- Bates D et al. (2015) ‘Fitting linear mixed-effects models using lme4’, Journal of Statistical Software, 67(1). doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Beck AT et al. (1961) ‘An inventory for measuring depression’, Archives of general psychiatry, pp. 561–571. [DOI] [PubMed] [Google Scholar]
- Blaine SK et al. (2018) ‘Alcohol, Stress, and Glucocorticoids: From Risk to Dependence and Relapse in Alcohol Use Disorders’, Neuropharmacology, Aug 1;122:136–147. doi: 10.1016/j.neuropharm.2017.01.037. Epub 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK et al. (2020) ‘Association of Prefrontal-Striatal Functional Pathology with Alcohol Abstinence Days at Treatment Initiation and Heavy Drinking after Treatment Initiation’, American Journal of Psychiatry, 177(11), pp. 1048–1059. doi: 10.1176/appi.ajp.2020.19070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD and Staehler BA (1995) ‘Development and Initial Validation of a Measure of Drinking Urges in Abstinent Alcoholics’, Alcoholism: Clinical and Experimental Research, 19(3), pp. 600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Breslin FC et al. (1997) ‘Toward a stepped care approach to treating problem drinkers: The predictive utility of within-treatment variables and therapist prognostic ratings’, Addiction, 92(11), pp. 1479–1489. doi: 10.1111/j.1360-0443.1997.tb02869.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1988) ‘The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research’, Psychiatry Research, 28, pp. 193–213. [DOI] [PubMed] [Google Scholar]
- Duka T et al. (2011) ‘Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics’, Biological Psychiatry. 70(6), pp. 545–552. doi: 10.1016/j.biopsych.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D et al. (2010) ‘Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcohol clinical trials’, Alcoholism: Clinical and Experimental Research, 34(12), pp. 2022–2034. doi: 10.1111/j.1530-0277.2010.01290. [DOI] [PubMed] [Google Scholar]
- Falk DE et al. (2019) ‘Evaluation of Drinking Risk Levels as Outcomes in Alcohol Pharmacotherapy Trials: A Secondary Analysis of 3 Randomized Clinical Trials’, JAMA Psychiatry, 76(4), pp. 374–381. doi: 10.1001/jamapsychiatry.2018.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino TL et al. (2013) ‘A Daily Process Examination of the Bidirectional Relationship Between Craving and Alcohol Consumption as Measured Via Interactive Voice Response’, Alcoholism: Clinical and Experimental Research, 37(12), pp. 2161–2167. doi: 10.1111/acer.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M et al. (2015) Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Fox HC et al. (2007) ‘Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals’, Alcoholism: Clinical and Experimental Research, 31(3), pp. 395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC et al. (2012) ‘Prazosin Effects on Stress- and Cue-Induced Craving and Stress Response in Alcohol-Dependent Individuals: Preliminary Findings’, Alcoholism: Clinical and Experimental Research, 36(2), pp. 351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MG et al. (2018) ‘Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016’, The Lancet, 392(10152), pp. 1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinle MIB, Sinha R (2020) ‘The Role of Stress, Trauma, and Negative Affect in the Development of Alcohol Misuse and Alcohol Use Disorders in Women’, Alcohol Research: Current Reviews, Aug 20, 40(2):05. doi: 10.35946/arcr.v40.2.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, Atkins DC and Witkiewitz K (2016) ‘Aggregating and analyzing daily drinking data in clinical trials: A comparison of type I errors, power, and bias’, Journal of Studies on Alcohol and Drugs, 77(6), pp. 986–991. doi: 10.15288/jsad.2016.77.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE et al. (2011) ‘Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals’, Psychopharmacology, 218(1), pp. 121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadden R et al. (1994) Cognitive-behavioral coping skills therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Project MATCH Monograph Series, Vol. 3. DHHS Publication No. 94–3724. Rockville, MD: NIAAA. [Google Scholar]
- Kassambara et al. (2020) ‘survminer: Drawing Survival Curves using ‘ggplot2’. [Google Scholar]
- Koob GF (2003) ‘Alcoholism: Allostasis and Beyond’, Alcoholism: Clinical & Experimental Research, 27(2), pp. 232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF and Schulkin J (2018) ‘Addiction and stress: An allostatic view’, Neuroscience & Biobehavioral Reviews, 106(August), pp. 245–262. doi: 10.1016/j.neubiorev.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Koob GF and Schulkin J (2019) ‘Addiction and stress: An allostatic view’, Neuroscience and Biobehavioral Reviews, 106(August 2018), pp. 245–262. doi: 10.1016/j.neubiorev.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Koob GF and Volkow ND (2016) ‘Neurobiology of addiction: A neurocircuitry analysis’, The Lancet Psychiatry. Elsevier Ltd, 3(8), pp. 760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB and Christensen RHB (2017) ‘lmerTest Package: Tests in Linear Mixed Effects Models ‘, Journal of Statistical Software, 82(13). doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Litten RZ et al. (2015) ‘Heterogeneity of alcohol use disorder: Understanding mechanisms to advance personalized treatment’, Alcoholism: Clinical and Experimental Research, 39(4), pp. 579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- Maier W et al. (1988) ‘The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders’, Journal of Affective Disorders, 14(1), pp. 61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Kirouac M and Witkiewitz K (2014) ‘Alcohol use disorder clinical course research: informing clinicians’ treatment planning now and in the future’, Journal of studies on alcohol and drugs, 75(5), pp. 799–807. doi: 10.15288/jsad.2014.75.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R et al. (2000) ‘Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification’, Alcohol, 22(3), pp. 159–164. doi: 10.1016/S0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- Mchugh RK et al. (2017) ‘Association between a brief alcohol craving measure and drinking in the following week’, 111(6), pp. 1004–1010. doi: 10.1111/add.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V et al. (2020) ‘Effects of Prazosin on Provoked Alcohol Craving and Autonomic and Neuroendocrine Response to Stress in Alcohol Use Disorder’, Alcoholism: Clinical and Experimental Research, 44(7), pp. 1488–1496. doi: 10.1111/acer.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic V and Sinha R (2018) ‘Central and Peripheral Biomarkers of Stress Response for Addiction Risk and Relapse Vulnerability’, Trends in Molecular Medicine. Elsevier Ltd, 24(2), pp. 173–186. doi: 10.1016/j.molmed.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR and Del Boca FK (1994) ‘Measurement of drinking behavior using the Form 90 family of instruments’, Journal of Studies on Alcohol, 55(SUPPL. 12), pp. 112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Monterosso JR et al. (2001) ‘Predicting treatment response to naltrexone: The influence of craving and family history’, American Journal on Addictions, 10(3), pp. 258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Mundle G et al. (1999) ‘Treatment outcome in alcoholism - A comparison of self-report and the biological markers carbohydrate-deficient transferrin and γ-glutamyl transferase’, European Addiction Research, 5(2), pp. 91–96. doi: 10.1159/000018972. [DOI] [PubMed] [Google Scholar]
- Nowinski J, Baker S and Carroll KM (1994) Twelve step facilitation therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Project MATCH Monograph Series, Vol. 1. DHHS Publication No. 94–3722. Rockville, MD: NIAAA. [Google Scholar]
- O’Connor PG et al. (1991) ‘Social and clinical features as predictors of outcome in outpatient alcohol withdrawal’, Journal of General Internal Medicine, 6(4), pp. 312–316. doi: 10.1007/BF02597427. [DOI] [PubMed] [Google Scholar]
- Peltier MR et al. (2019) ‘Sex differences in stress-related alcohol use’, Neurobiology of Stress. Elsevier, 10(February), p. 100149. doi: 10.1016/j.ynstr.2019.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM et al. (2000) ‘Give them prizes, and they will come: Contingency management for treatment of alcohol dependence’, Journal of Consulting and Clinical Psychology, 68(2), pp. 250–257. doi: 10.1037/0022-006X.68.2.250. [DOI] [PubMed] [Google Scholar]
- Piasecki TM (2019) ‘Assessment of Alcohol Use in the Natural Environment’, Alcoholism: Clinical and Experimental Research, 43(4), pp. 564–577. doi: 10.1111/acer.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rights JD et al. (2019). ‘Quantifying explained variance in multilevel models: An integrative framework for defining R-squared measures’, Psychological Methods, 24(3), pp.309–338. [DOI] [PubMed] [Google Scholar]
- R Core Team (2020) ‘R: A Language and Environment for Statistical Computing’. Vienna, Austria. Available at: https://www.r-project.org/. [Google Scholar]
- Schlauch RC et al. (2019) ‘The role of craving in the treatment of alcohol use disorders: The importance of competing desires and pretreatment changes in drinking’, Drug and Alcohol Dependence. Elsevier, 199(April), pp. 144–150. doi: 10.1016/j.drugalcdep.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA (2014) ‘Recognition and Management of Withdrawal Delirium (Delirium Tremens)’, New England Journal of Medicine, 371(22), pp. 2109–2113. doi: 10.1056/nejmra1407298. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA and Hufford MR (2008) ‘Ecological momentary assessment’, Annual Review of Clinical Psychology, 4, pp. 1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Sinha R (2001) ‘How does stress increase risk of drug abuse and relapse?’, Psychopharmacology, 158(4), pp. 343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R et al. (2011) ‘Effects of adrenal sensitivity, stress- and cue-induced craving,and anxiety on subsequent alcohol relapse and treatment outcomes’, Archives of General Psychiatry, 68(9), pp. 942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2011) ‘New findings on biological factors predicting addiction relapse vulnerability’, Current Psychiatry Reports, 13(5), pp. 398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R et al. (2021) ‘Moderation of Prazosin’s Efficacy by Alcohol Withdrawal Symptoms’, American Journal of Psychiatry, May 1;178(5):447–458. doi: 10.1176/appi.ajp.2020.20050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel MA et al. (2020). ‘partR2: Partitioning R2 in generalized linear mixed models’, bioRxiv. doi: 10.1101/2020.07.26.221168, https://www.biorxiv.org/content/10.1101/2020.07.26.221168v1. [DOI] [PMC free article] [PubMed]
- Sullivan JT et al. (1989) ‘Assessment of Alcohol Withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar)’, British Journal of Addiction, 84(11), pp. 1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Therneau T (2021) ‘A Package for Survival Analysis in R. R package version 3.2–11’. Available at: https://cran.r-project.org/package=survival. [Google Scholar]
- Wemm SE et al. (2020) ‘A day-by-day prospective analysis of stress, craving and risk of next day alcohol intake during alcohol use disorder treatment’, Drug and Alcohol Dependence, Nov 1;204:107569. doi: 10.1016/j.drugalcdep.2019.107569. Epub 2019 Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K et al. (2019) ‘Advancing Precision Medicine for Alcohol Use Disorder: Replication and Extension of Reward Drinking as a Predictor of Naltrexone Response’, Alcoholism: Clinical and Experimental Research, 43(11), pp. 2395–2405. doi: 10.1111/acer.14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K et al. (2020) ‘World Health Organization risk drinking level reductions are associated with improved functioning and are sustained among patients with mild, moderate and severe alcohol dependence in clinical trials in the United States and United Kingdom’, Addiction, 115(9), pp. 1668–1680. doi: 10.1111/add.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S and Donovan DM (2011) ‘Moderating effects of a craving intervention on the relation between negative mood and heavy drinking following treatment for alcohol dependence’, Journal of Consulting and Clinical Psychology, 79(1), pp. 54–63. doi: 10.1037/a0022282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.