Abstract
Purpose:
To describe the clinical outcomes of a pharmacomechanical catheter-directed venous thrombolysis (PCDT) strategy that included AngioJet rheolytic thrombectomy.
Methods:
In the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis multicenter randomized trial, physicians at 33 sites designated AngioJet as their preferred device for PCDT. In these sites, 364 patients with acute proximal lower-extremity deep vein thrombosis (DVT) were randomized to a strategy of PCDT that incorporated either AngioJet along with anticoagulation or anticoagulation alone. Relief from presenting DVT symptoms was evaluated over 30 days of follow-up. Postthrombotic syndrome (PTS), quality of life (QOL), recurrent venous thromboembolism (VTE), and safety were evaluated over 24 months of follow-up.
Results:
Within 30 days, AngioJet-PCDT led to a greater improvement in leg swelling (mean difference calf circumference 0.55 cm, P = .009), venous QOL (mean difference 6.5 Venous Insufficiency Epidemiologic and Economic Study [VEINES]-QOL points, P = .0073), and venous symptoms (mean difference 5.6 VEINES-symptoms points, P = .0134) than control, with differences most apparent in iliofemoral DVT. AngioJet-PCDT reduced PTS at 6 months (24% with AngioJet-PCDT vs 40% with control, P = .003) but did not influence PTS or QOL between 12 and 24 months. Major bleeding, pulmonary embolism, renal failure, and bradycardia were infrequent with AngioJet-PCDT (<2% each), but 24-month VTE recurrence may have been more frequent (13.9% with AngioJet-PCDT vs 6.8% with control, P = .03)
Conclusions:
In patients with acute proximal DVT, a treatment strategy that included first-line AngioJet-PCDT was reasonably safe and led to an improved symptom status and venous QOL at 1 month and reduced PTS at 6 months compared with anticoagulation alone. However, AngioJet-PCDT did not influence PTS or the QOL beyond 6 months and may have increased recurrent VTE.
Patients with acute proximal deep vein thrombosis (DVT) experience lower-extremity pain and swelling and are at high risk of developing postthrombotic syndrome (PTS) (1). Catheter-directed thrombolysis (CDT) has been used to treat DVT for many years and has been refined with the addition of percutaneous mechanical thrombectomy in efforts to enhance thrombus removal, speed treatment, and improve safety (2). Despite the wide use of various thrombectomy devices in pharmacomechanical catheter-directed venous thrombolysis (PCDT) procedures for DVT, rigorous published studies have not linked clinical outcomes to specific strategies for PCDT use.
In the Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial, the results of several PCDT methods used to treat DVT were reported in aggregate, but with limited information on the performance of specific PCDT methods (3). The AngioJet rheolytic thrombectomy system (Boston Scientific Corporation, Marlborough, Massachusetts) was used frequently in the ATTRACT trial and continues to be used in current practice (4-6). The purpose of this analysis was to delineate clinical outcomes that were associated with the use of a PCDT strategy that incorporated the AngioJet device.
MATERIALS AND METHODS
Study Design, Clinical Centers, and Patients
The ATTRACT trial was a phase III, multicenter, open-label, assessor-blinded, randomized clinical trial (registered at www.clinicaltrials.gov). Patients provided written informed consent to participate. The study was approved by the institutional review boards of all 56 participating clinical centers. The steering committee and site investigators were responsible for the conduct of the trial. The study eligibility criteria, methods, and main clinical outcomes have been previously published (3).
Prior to activation, each center was required to choose which device it preferred to use for the intrathrombus delivery of recombinant tissue plasminogen activator (rt-PA; Activase; Genentech, South San Francisco, California). Within 33 sites that chose AngioJet (“AngioJet sites,” Appendix A, available online on the article’s Supplemental Material page at www.jvir.org), patients with acute symptomatic proximal DVT extending above the popliteal vein were randomly assigned in 1:1 ratio to undergo (PCDT arm) or not undergo (control arm) PCDT therapy (Fig 1). Because PTS was known to be more frequent and severe in patients with extensive thrombus and decisions regarding the use of PCDT often consider the highest extent of thrombus, randomization was stratified by clinical center and thrombus extent (common femoral vein noncompressible = iliofemoral DVT; compressible = femoral-popliteal DVT) (7). Patients in both the treatment groups received anticoagulation and elastic compression stockings (BSN Medical, Charlotte, New Carolina). In the PCDT-arm patients, the procedures were performed by board-certified, licensed physicians whose credentials and experience with PCDT and its components (ultrasound-guided access, fibrinolytic infusions, thrombectomy device use, and venous stent placement) were reviewed and approved by an endovascular credentialing committee. Table 1 shows the baseline characteristics of randomized patients in the AngioJet sites.
Figure 1.
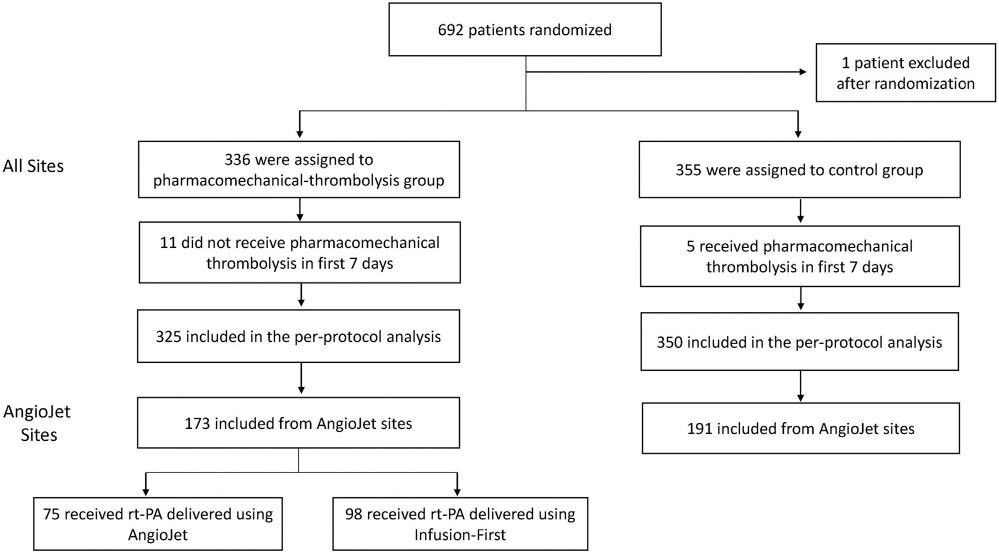
Study flow diagram. Patient flow and the use of AngioJet-PCDT in the ATTRACT trial. ATTRACT = Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis; PCDT = pharmacomechanical catheter-directed venous thrombolysis.
Table 1.
Baseline Characteristics of AngioJet Site Patients*
| Characteristic | Control arm (n = 191) |
PCDT arm (n = 173) |
|---|---|---|
| Age (y), median (range) | 54.5 (18.0, 75.0) | 51.0 (16.0, 75.0) |
| Male, n (%) | 116 (60.7) | 103 (59.5) |
| White, n (%) | 150 (78.5) | 136 (78.6) |
| Hispanic or Latino, n (%) | 8 (4.2) | 2 (1.2) |
| BMI, median (range) | 29.9 (18.5, 68.0) | 31.5 (19.1, 59.6) |
| eGFR, median (range) | 86.0 (40.0, 217.0) | 87.0 (37.0, 341.0) |
| Left index leg, n (%) | 117 (61.3) | 111 (64.2) |
| Previous DVT, n (%) | 52 (27.2) | 41 (23.7) |
| Previous DVT in the index leg, n (%) | 6 (3.1) | 2 (1.2) |
| Noncompressible CFV, n (%) | 98 (51.3) | 87 (50.3) |
| Noncompressible FV, n (%) | 172 (90.1) | 146 (84.4) |
| Noncompressible PV, n (%) | 152 (79.6) | 137 (79.2) |
BMI = body mass index; CFV = common femoral vein; DVT = deep vein thrombosis; eGFR = estimated glomerular filtration rate; FV = femoral vein; PCDT = pharmacomechanical catheter-directed venous thrombolysis; PV = popliteal vein.
Per-protocol study population.
AngioJet-PCDT Strategy
The details of PCDT in the ATTRACT trial have been presented elsewhere (3). Briefly, after venography, (a) if there was good inflow into the popliteal vein (as assessed on the procedure day), AngioJet site investigators were expected to attempt single-session therapy using AngioJet (DVX or Solent Proxi catheter) to rapidly deliver rt-PA and aspirate thrombus or (b) if popliteal vein inflow was poor or absent, investigators were expected to first infuse rt-PA through a multisidehole catheter for 6–24 hours (0.01 mg/kg/hour, not to exceed 1 mg/hour) and then use AngioJet to aspirate residual thrombus (if present). After either of the aforementioned, the physicians could use balloon maceration, catheter aspiration, angioplasty, stent placement, and/or additional rt-PA boluses or rt-PA infusion to clear residual thrombus and restore the flow. Because the use of AngioJet was intended as a central element of thrombus removal in these centers, this analysis considers the “AngioJet-PCDT strategy” to encompass all AngioJet site patients undergoing PCDT, irrespective of whether or how the device was used.
For attempted single-session PCDT (also termed as “AngioJet-mediated initial rt-PA delivery” later in this report), AngioJet was initially used to deliver a bolus dose of 1-mg rt-PA per 3–4 cm of thrombus length (estimated visually) into the thrombus, either via a power pulse with 30-minute rt-PA dwell time prior to the initiation of aspiration or the rapid lysis technique (4-6). The investigators were encouraged to use an angled guide catheter to optimize AngioJet-mediated thrombus clearance. At the physicians’ discretion, additional rt-PA delivery and aspiration could occur up to limits of 25 mg per session, 8 minutes of device activation time, and 500 mL of infusate volume. For any method, the total rt-PA dose could not exceed 35 mg for all the sessions combined.
Assessment of Clinical Outcomes
The definitions of study outcomes have been described elsewhere and are briefly presented here (3). The outcomes were assessed at 10 and 30 days and at 6, 12, 18, and 24 months after randomization. The clinicians performing the outcome assessments, adjudicators of safety and efficacy outcomes, and core laboratory evaluators of imaging studies were blinded to the treatment arm allocation.
The primary study outcome was the occurrence of PTS between 6 and 24 months, defined as a Villalta scale score of ≥5, a venous ulcer, or an unplanned endovascular procedure to treat severe venous symptoms (8). The additional outcomes included the occurrence of moderate-to-severe PTS (Villalta scale score ≥ 10 or a venous ulcer) and severe PTS (Villalta scale score ≥ 15 or a venous ulcer; modified venous clinical severity scale [VCSS] score ≥ 8), PTS severity (continuous Villalta scale and VCSS scores), general (Short Form 36) and venous disease-specific (Venous Insufficiency Epidemiologic and Economic Study-Quality of Life survey [VEINES-QOL]), quality of life (QOL), leg pain severity (Likert scale), and calf circumference (9-11). Compression ultrasound was performed at the baseline and 1-month follow-up. In addition, patients in 3 preselected AngioJet sites underwent venous duplex ultrasound at 12 months, including assessment for valvular reflux (for both superficial and deep veins, flow reversal > 0.5 seconds).
The adjudicated safety outcomes included death, bleeding, and symptomatic recurrent venous thromboembolism (VTE). Clinically overt bleeding was classified as “major” if associated with a hemoglobin drop of ≥2.0 g/dL, a transfusion of ≥2 units of red blood cells, or the involvement of a critical site (12). Less severe bleeding was classified as “minor.” Other adverse events were reported by the site investigators and did not undergo formal adjudication; however, for serious adverse events, an independent safety officer reviewed and confirmed the categorizations. The timeliness and thoroughness of event reporting were enforced via remote and in-person data monitoring.
Sample Size and Statistical Analysis
The ATTRACT trial included 692 patients (3). The study did not prespecify the sample size for AngioJet analyses or the proportion of patients expected to have AngioJet used. Comparisons were performed to evaluate the following: (a) the overall AngioJet-PCDT treatment strategy—all patients randomized to PCDT (n = 173) versus control (n = 191) in AngioJet sites (per-protocol population that excluded patients who did not receive the assigned treatment within 7 days) for proximal DVT, iliofemoral DVT, and femoral-popliteal DVT subgroups and (b) attempted single-session AngioJet-PCDT—patients in whom AngioJet was used for initial rt-PA delivery (n = 75) versus control (n = 191).
The occurrences of site investigator-reported periprocedural (defined as during or within 3 days after PCDT) adverse events and serious adverse events were also described for 183 patients in all ATTRACT sites in whom the AngioJet device was actually used (ie, including adverse events occurring in AngioJet device recipients in non-AngioJet sites). The events were defined as procedure-related if categorized as being at least possibly related to the use of rt-PA or to the study. The sites did not categorize the relatedness of the adverse events based on the AngioJet device specifically.
Differences in standard DVT treatments received and binary trial outcomes in the groups were compared using the chi-square test or Fisher exact test, as appropriate. The mean Villalta, VCSS, and QOL assessments at each visit were estimated using piecewise linear regression growth curve models adjusting for clinical center and prespecified baseline covariates (age, sex, body mass index, and race). Changes in the leg pain scores and calf circumferences of the index leg from the baseline to 10 days and from the baseline to 30 days were compared using the t test.
The analyses in this report are considered exploratory because they were not prespecified in detail and are confined to a subgroup of the main trial. For all the analyses, a 2-sided P value of .01 or lower was considered statistically significant to account for multiple testing. All the analyses were conducted using the Statistical Analysis System software, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Protocol and Treatment Adherence
The use of antithrombotic therapy and compression did not differ statistically between the 2 groups, except for the greater use of unfractionated heparin in the PCDT-arm patients, reflecting their more frequent management as in-patients (Table 2). The endovascular methods used are listed in Table 3. The mean treatment duration was 11.3 ± 10.4 hours for attempted single-session PCDT and 21.3 ± 6.3 hours for infusion-first PCDT. As noted before, the thrombus scores were 11.0 ± 5.7 before PCDT and 2.6 ± 3.9 after PCDT (P < .001) and were similar for both the PCDT methods (13).
Table 2.
Standard Care Administered to Study Patients
| Treatment | Control arm (n = 191) | PCDT arm (n = 173) | P value |
|---|---|---|---|
| Initial anticoagulant therapy (baseline), n (%) | 191 (100.0) | 173 (100.0) | 1.00 |
| Unfractionated heparin | 26 (13.6) | 62 (35.8) | <.0001 |
| Low-molecular-weight heparin | 134 (70.2) | 108 (62.4) | .10 |
| Warfarin | 151 (79.1) | 123 (71.1) | .03 |
| At 30 d, n (%) | n = 170 | n = 168 | |
| Any anticoagulant therapy | 167 (98.2) | 165 (98.2) | 1.00 |
| Antiplatelet therapy | 25 (14.7) | 17 (10.1) | .20 |
| Compression stockings used ≥3 d per wk | 147 (86.5) | 142 (84.5) | .61 |
| At 6 mo, n (%) | n = 151 | n = 152 | |
| Any anticoagulant therapy | 129 (85.4) | 116 (76.3) | .04 |
| Antiplatelet therapy | 23 (15.2) | 29 (19.1) | .37 |
| Compression stockings used ≥3 d per wk | 120 (79.5) | 112 (73.7) | .23 |
| At 24 mo, n (%) | n = 123 | n = 130 | |
| Any anticoagulant therapy | 59 (48.0) | 56 (43.1) | .43 |
| Antiplatelet therapy | 34 (27.6) | 34 (26.2) | .79 |
| Compression stockings used ≥3 d per wk | 82 (66.7) | 86 (66.2) | .93 |
PCDT = pharmacomechanical catheter-directed venous thrombolysis.
Table 3.
Endovascular Treatments in PCDT-Arm Patients
| Element of care | PCDT All patients (n = 173) |
Initial AngioJet (Good inflow) (n = 75) |
Infusion-first PCDT (Poor inflow) (n = 98) |
|---|---|---|---|
| Access site(s) | |||
| Ipsilateral tibial vein | 26 (15.0) | 9 (12.0) | 17 (17.3) |
| Internal jugular vein | 12 (6.9) | 2 (2.7) | 10 (10.2) |
| Ipsilateral popliteal vein | 141 (81.5) | 62 (82.7) | 79 (80.6) |
| Ipsilateral femoral vein | 6 (3.6) | 3 (4.0) | 3 (3.2) |
| Other vein | 16 (9.3) | 8 (10.7) | 8 (8.2) |
| Thrombolytic drug use | |||
| rt-PA duration, h (mean ± SD) | 17.0 ± 9.7 | 11.3 ± 10.4 | 21.3 ± 6.3 |
| rt-PA total dose, mg, median (IQR) | 20.7 [15.9, 27.0] | 21.4 [12.0, 28.0] | 20.7 [16.5, 25.8] |
| Additional endovascular methods | 166 (96.0) | 75 (100.0) | 91 (92.9) |
| Balloon maceration | 109 (63.0) | 48 (64.0) | 61 (62.2) |
| Aspiration thrombectomy | 31 (17.9) | 15 (20.0) | 16 (16.3) |
| Rheolytic thrombectomy | 138 (79.8) | 72 (96.0) | 66 (67.3) |
| Trellis PCDT | 1 (0.59) | 1 (1.3) | 0 (0.0) |
| Balloon angioplasty | 92 (53.2) | 43 (57.3) | 49 (50.0) |
| Stent placement | 46 (26.6) | 26 (34.7) | 20 (20.4) |
| Thrombus scores–venograms (14) | |||
| Before lysis | 11.0 (5.7) | 8.4 (4.5) | 13.0 (8,16) |
| After lysis | 2.6 (3.9) | 1.7 (2.8) | 3.4 (4.5) |
| Difference (before – after) | 8.4 (5.7) | 6.7 (4.5) | 9.8 (6.3) |
IQR = interquartile range; PCDT= pharmacomechanical catheter-directed venous thrombolysis; rt-PA = recombinant tissue plasminogen activator; SD = standard deviation.
Clinical Outcomes of AngioJet-PCDT
PTS developed in 76 (43.9%) of the 173 AngioJet-PCDT patients and 79 (41.1%) of the 191 control-arm patients (P = .62) over 24 months (Table 4). The proportion of patients with PTS differed at 6 months (24.2% with AngioJet-PCDT vs 40.0% with control, P = .003) but not subsequently. From 6 to 24 months, the point estimates for the mean Villalta scale and VCSS scores were lower in the AngioJet-PCDT patients but not statistically significant, and no differences were seen in moderate-or-severe PTS, severe PTS, venous ulcer, or QOL from 6 to 24 months or in 1-year valvular reflux (87% with AngioJet-PCDT vs 89% with control) or residual vein diameter (differences: common femoral vein −0.5 mm ± −0.8, P = .19; popliteal vein, −1.7 mm ± −1.3; P = .42) (Fig 2, Appendices B-F, available online at www.jvir.org).
Table 4.
Binary Trial Outcomes
| All proximal DVT | Control* All patients (n = 191) |
PCDT All methods (n = 173) |
P value (All methods vs control arm) |
PCDT Initial AngioJet only (n = 75) |
P value (Initial AngioJet vs control arm) |
|---|---|---|---|---|---|
| PTS between 6 and 24 mo | |||||
| Ulcer at any follow-up assessment | 9 (4.7) | 7 (4.0) | .76 | 3 (4.0) | .80 |
| Villalta scale score ≥5 without ulcer | 70 (36.6) | 69 (39.9) | .53 | 30 (40.0) | .61 |
| Late endovascular procedure only | 0 (0.0) | 0 (0.0) | 1.0† | 0 (0.0) | 1.0* |
| Total | 79 (41.4) | 76(43.9) | .62 | 33 (44.0) | .69 |
| PTS: VCSS score ≥ 4 | 64 (33.5) | 53(30.6) | .56 | 21 (28.0) | .39 |
| PTS incidence proportion according to follow-up visit | |||||
| At 6 mo | 60/150 (40.0) | 37/153 (24.2) | .003 | 18/63 (28.6) | .11 |
| At 12 mo | 42/135 (31.1) | 44/145 (30.3) | .89 | 17/56 (30.4) | .92 |
| At 18 mo | 35/117 (29.9) | 45/125 (36.0) | .31 | 13/48 (27.1) | .72 |
| At 24 mo | 41/123 (33.3) | 34/132 (25.8) | .18 | 14/49 (28.6) | .55 |
| Moderate-to-severe PTS (Villalta scale score ≥ 10) | 38 (19.9) | 29 (16.8) | .44 | 12 (16.0) | .46 |
| Moderate-to-severe PTS incidence proportion according to follow-up visit | |||||
| At 6 mo | 18/150 (12.0) | 17/153 (11.1) | .81 | 9/63 (14.3) | .65 |
| At 12 mo | 14/135 (10.4) | 17/145 (11.7) | .72 | 8/56 (14.3) | .44 |
| At 18 mo | 17/117 (14.5) | 12/125 (9.6) | .24 | 4/48 (8.3) | .28 |
| At 24 mo | 22/123 (17.9) | 13/132 (9.8) | .06 | 4/49 (8.2) | .11 |
| Severe PTS: Villalta scale score ≥ 15 | 21 (11.0) | 13 (7.5) | .25 | 7 (9.3) | .69 |
| Severe PTS: VCSS score ≥ 8 | 19 (9.9) | 11 (6.4) | .21 | 7 (9.3) | .88 |
| Major bleeding | |||||
| First 30 d | 0 (0.0) | 3 (1.7) | .11† | 0 (0.0) | 1.0* |
| Total over 24 mo | 6 (3.1) | 9 (5.2) | .32 | 4 (5.3) | .40 |
| Any bleeding | |||||
| First 30 d | 4 (2.1) | 10 (5.8) | .07 | 1 (1.3) | .99* |
| Total over 24 mo | 19 (9.9) | 23 (13.3) | .32 | 7 (9.3) | .88 |
| Symptomatic VTE | |||||
| First 30 d | 3 (1.6) | 8 (4.6) | .09 | 4 (5.3) | .09 |
| Total over 24 mo | 13 (6.8) | 24 (13.9) | .03 | 10 (13.3) | .09 |
| Death | 3 (1.6) | 2 (1.2) | .99† | 1 (1.3) | .99* |
| Venous status at 1 mo | |||||
| Noncompressible CFV, n (%) | 48/167 (28.7) | 30/167 (18.0) | .009 | 11/72 (15.2) | .004 |
| Noncompressible FV, n (%) | 110/169 (65.0) | 81/167 (48.5) | .002 | 20/72 (27.8) | <.001 |
| Noncompressible PV, n (%) | 121/169 (71.6) | 93/166 (56.0) | .001 | 27/71 (38.0) | <.001 |
| Ultrasound substudy ‡ | |||||
| Venous status at 12 mo | |||||
| Noncompressible CFV, n (%) | 4/20 (20.0) | 3/24 (12.5) | 1/11 (9.1) | ||
| Noncompressible FV, n (%) | 11/20 (55.0) | 10/25 (40.0) | 3/11 (27.2) | ||
| Noncompressible PV, n (%) | 14/20 (70.0) | 10/25 (40.0) | 3/11 (27.2) | ||
| Any reflux present, n (%) | 17/19 (89%) | 20/23 (87%) | 7/9 (78%) | ||
| Deep reflux present, n (%) | 17/19 (89%) | 20/23 (87%) | 7/9 (78%) | ||
| Iliofemoral DVT subgroup | Control (n = 100) |
PCDT (n = 95) |
P value (All patients) |
PCDT: initial AngioJet (n = 46) |
P value (Initial AngioJet) |
| PTS between 6 and 24 mo | |||||
| Ulcer at any follow-up assessment | 5 (5.0) | 5 (5.3) | - | 3 (6.5) | - |
| Villalta scale score ≥5 without ulcer | 35 (35.0) | 36 (37.9) | - | 19 (41.3) | - |
| Late endovascular procedure only | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | - |
| Total | 40 (40.0) | 41 (43.2) | .65 | 22 (47.8) | .37 |
| PTS: VCSS score ≥ 4 | 32 (32.0) | 31 (32.6) | .92 | 15 (32.6) | .94 |
| PTS incidence proportion according to follow-up visit | |||||
| At 6 mo | 29/74 (39.2) | 22/84 (26.2) | .08 | 13/40 (32.5) | .48 |
| At 12 mo | 17/68 (25.0) | 26/80 (32.5) | .32 | 13/36 (36.1) | .23 |
| At 18 mo | 18/63 (28.6) | 23/69 (33.3) | .55 | 8/31 (25.8) | .78 |
| At 24 mo | 20/66 (30.3) | 19/70 (27.1) | .68 | 9/32 (28.1) | .82 |
| Moderate-to-severe PTS (Villalta scale score ≥ 10) | 20 (20.0) | 16 (16.8) | .57 | 8 (17.4) | .71 |
| Moderate-to-severe PTS incidence proportion according to follow-up visit | |||||
| At 6 mo | 8/74 (10.8) | 12/84 (14.3) | .51 | 7/40 (17.5) | .31 |
| At 12 mo | 7/68 (10.3) | 9/80 (11.3) | .85 | 5/36 (13.9) | .59 |
| At 18 mo | 9/63 (14.3) | 8/69 (11.6) | .64 | 4/31 (12.9) | .86 |
| At 24 mo | 11/66(16.7) | 8/70 (11.4) | .38 | 2/32 (6.3) | .15 |
| Severe PTS: Villalta scale score ≥ 15 | 9 (9.0) | 9 (9.5) | .91 | 6 (13.0) | .45 |
| Severe PTS: VCSS score ≥ 8 | 9 (9.0) | 6 (6.3) | .48 | 4 (8.7) | .95 |
| Femoral-popliteal DVT subgroup | Control (n = 91) |
PCDT (n = 78) |
P value (All patients) |
PCDT: initial AngioJet (n = 29) |
P value (Initial AngioJet) |
| PTS between 6 and 24 mo † | |||||
| Ulcer at any follow-up assessment | 4 (4.4) | 2 (2.6) | - | 0 (0.0) | - |
| Villalta scale score ≥5 without ulcer | 35 (38.5) | 33 (42.3) | - | 11 (37.9) | - |
| Late endovascular procedure only | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | - |
| Total | 39 (42.9) | 35 (44.9) | .79 | 11 (37.9) | .64 |
| PTS: VCSS score ≥ 4 | 32 (35.2) | 22 (28.2) | .33 | 6 (20.7) | .14 |
| PTS incidence proportion according to follow-up visit | |||||
| At 6 mo | 31/76 (40.8) | 15/69 (21.7) | .01 | 5/23 (21.7) | .10 |
| At 12 mo | 25/67 (37.3) | 18/65 (27.7) | .24 | 4/20 (20.0) | .15 |
| At 18 mo | 17/54 (31.5) | 22/56 (39.3) | .39 | 5/17 (29.4) | .87 |
| At 24 mo | 21/57 (36.8) | 15/62 (24.2) | .13 | 5/17 (29.4) | .57 |
| Moderate-to-severe PTS (Villalta scale score ≥ 10) | 18 (19.8) | 13 (16.7) | .60 | 4 (13.8) | .47 |
| Moderate-to-severe PTS according to follow-up visit | |||||
| At 6 mo | 10/76 (13.2) | 5/69 (7.2) | .24 | 2/23 (8.7) | .57 |
| At 12 mo | 7/67 (10.4) | 8/65 (12.3) | .74 | 3/20 (15.0) | .58 |
| At 18 mo | 8/54 (14.8) | 4/56 (7.1) | .20 | 0/17 (0.0) | .09 |
| At 24 mo | 11/57 (19.3) | 5/62 (8.1) | .07 | 2/17 (11.8) | .47 |
| Severe PTS: Villalta scale score ≥ 15 | 12 (13.2) | 4 (5.1) | .07 | 1 (3.4) | .14 |
| Severe PTS: VCSS score ≥ 8 | 10 (11.0) | 5 (6.4) | .30 | 3 (10.3) | .92 |
CFV = common femoral vein; DVT = deep vein thrombosis; FV = femoral vein; PCDT = pharmacomechanical catheter-directed venous thrombolysis; PTS = postthrombotic syndrome; PV = popliteal vein; VCSS = venous clinical severity scale; VTE = venous thromboembolism.
Treatment groups are per-protocol patients.
Fisher exact test.
Comparisons adjusted for baseline compressibility status for CFV, PV, and FV at 1 month and 1 year.
Figure 2.
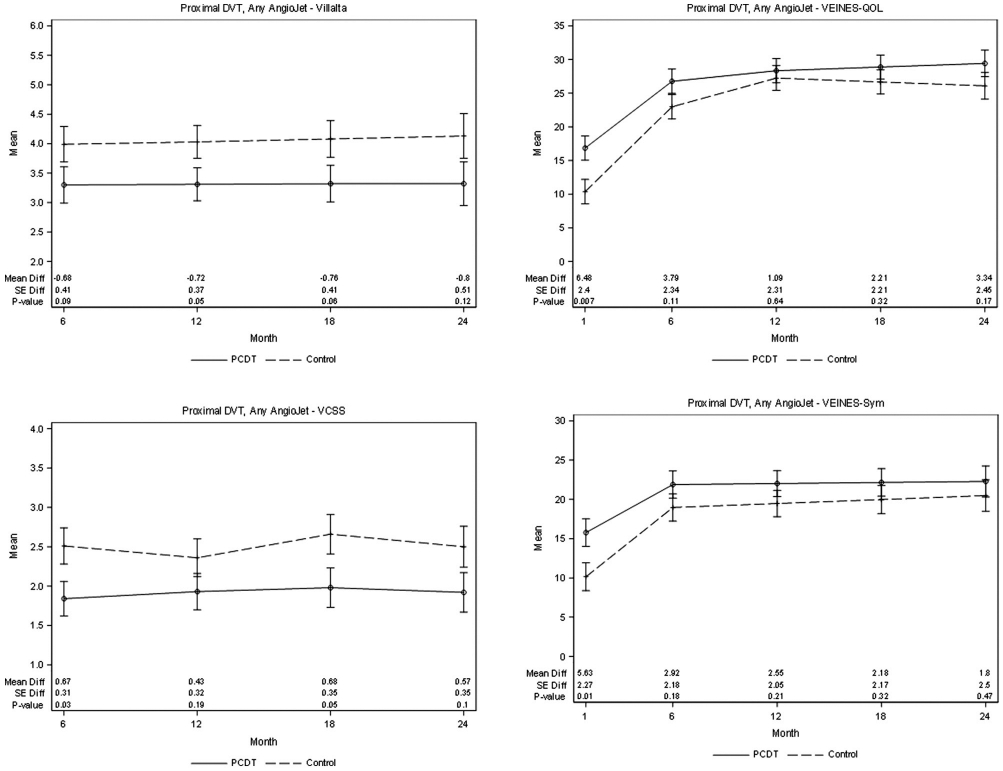
Patients with proximal DVT. Effect of any method of AngioJet-PCDT on PTS severity (mean continuous Villalta scale and VCSS scores), venous symptoms (mean VEINES-symptoms [Sym] subscale scores, adjusted for baseline), and health-related venous QOL (Mean VEINES-QOL scores, adjusted for baseline) during 24 months of follow-up. DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; PTS = postthrombotic syndrome; QOL = quality of life; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Through 30 days, patients undergoing AngioJet-PCDT experienced greater improvement in calf circumference (mean difference 0.55 cm, P = .009), venous QOL (mean difference 6.5 VEINES-QOL points, P = .0073), and overall venous symptoms (mean difference 5.6 VEINES-symptoms points, P = .0134) compared with the control-arm patients (Table 5, Fig 2). At 30 days of follow-up, AngioJet-PCDT recipients had lesser residual thrombus than control-arm patients, as shown by fewer noncompressible proximal veins (P < .01) and smaller residual vein diameters (Table 5).
Table 5.
Outcomes of AngioJet-PCDT Versus Control through 30 Days of Follow-Up
| Outcome | Control * (n = 191) |
PCDT (n = 173) |
PCDT – control difference |
|||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | Mean difference (SE) | P value | |
| All patients, any AngioJet method | ||||||
| Change in leg pain severity, to day 10† | 169 | 0.53 (0.20) | 156 | 0.13 (0.26) | −0.40 (0.33) | .22 |
| Change in leg pain severity, to day 30 | 164 | −0.09 (0.21) | 157 | −0.43 (0.28) | −0.34 (0.35) | .34 |
| Change in leg circumference (cm), to day 10 | 170 | −1.25 (0.13) | 165 | −1.61 (0.17) | −0.35 (0.21) | .09 |
| Change in leg circumference (cm), to day 30 | 166 | −1.73 (0.14) | 166 | −2.28 (0.15) | −0.55 (0.21) | .009 |
| CFV diameter (mm), at day 30 | 165 | 2.5 (6.2) | 167 | 1.4 (4.7) | −1.1 (−1.5) | .05 |
| PV diameter (mm), at day 30 | 162 | 5.4 (5.9) | 166 | 3.3 (4.0) | −2.1 (−1.9) | .0002 |
| All patients, AngioJet delivery of rt-PA | ||||||
| Change in leg pain severity, to day 10 | 169 | 0.53 (0.20) | 67 | −0.12 (0.33) | −0.65 (0.39) | .09 |
| Change in leg pain severity, to day 30 | 164 | −0.09 (0.21) | 70 | −0.51 (0.35) | −0.43 (0.41) | .30 |
| Change in leg circumference (cm), to day 10 | 170 | −1.25 (0.13) | 71 | −1.62 (0.26) | −0.37 (0.29) | .21 |
| Change in leg circumference (cm), to day 30 | 166 | −1.73 (0.14) | 72 | −2.35 (0.23) | −0.61 (0.27) | .03 |
| CFV diameter (mm), at day 30 | 165 | 2.5 (6.2) | 71 | 0.8 (2.2) | −1.7 (−4.0) | <.001 |
| PV diameter (mm), at day 30 | 162 | 5.4 (5.9) | 68 | 1.8 (2.9) | −3.6 (−0.4) | <.001 |
| Fem-Pop DVT, any AngioJet method | ||||||
| Change in leg pain severity, to day 10 | 83 | −1.39 (0.17) | 77 | −1.43 (0.27) | −0.04 (0.31) | .89 |
| Change in leg pain severity, to day 30 | 82 | −1.84 (0.19) | 77 | −2.06 (0.22) | −0.22 (0.29) | .43 |
| Change in leg circumference (cm), to day 10 | 82 | 0.79 (0.29) | 73 | 0.73 (0.41) | −0.07 (0.50) | .89 |
| Change in leg circumference (cm), to day 30 | 81 | −0.07 (0.29) | 72 | 0.13 (0.45) | 0.20 (0.53) | .71 |
| Iliofemoral DVT, any AngioJet method | ||||||
| Change in leg pain severity, to day 10 | 87 | −1.13 (0.19) | 67 | −1.76 (0.22) | −0.63 (0.29) | .03 |
| Change in leg pain severity, to day 30 | 84 | −1.63 (0.21) | 70 | −2.47 (0.22) | −0.84 (0.30) | .006 |
| Change in leg circumference (cm), to day 10 | 87 | −0.29 (0.28) | 71 | −0.40 (0.32) | −0.68 (0.43) | .11 |
| Change in leg circumference (cm), to day 30 | 83 | −0.10 (0.32) | 72 | −0.89 (0.35) | −0.80 (0.47) | .09 |
CFV = common femoral vein; DVT = deep vein thrombosis; Fem-Pop, femoral-popliteal; PCDT = pharmacomechanical catheter-directed venous thrombolysis; PV = popliteal vein; rt-PA = recombinant tissue plasminogen activator; SE = standard error.
Treatment groups are per-protocol populations.
Leg pain severity was assessed as points on a 7-point Likert scale.
Single-Session AngioJet-PCDT.
The clinical outcomes for the 75 patients who received AngioJet-mediated initial rt-PA delivery were largely similar to those for the aggregate AngioJet-PCDT patients (Table 4). Specifically, 33 (40%) of the 75 patients receiving AngioJet-mediated initial rt-PA delivery developed PTS and 12 (16%) developed moderate-or-severe PTS over 2 years, neither of which were statistically different from the control-arm patients. At 6 months, PTS was present in 18 (29%) of 63 patients in whom AngioJet-mediated rt-PA delivery was used, which was not statistically different from the control-arm patients (40%; P = .11). Between 6 and 24 months, there were no statistically significant differences in the occurrences of severe PTS or venous ulcer or in the mean Villalta scale, VCSS, VEINES-Sym, or QOL scores between the groups (Table 4, Fig 3). From the baseline to 30 days, the patients receiving AngioJet-mediated initial rt-PA delivery may have had a greater improvement in calf circumference (mean difference 0.61 cm, P = .0267), venous QOL (mean difference 7.3 VEINES-QOL scale points, P = .0215), and venous symptoms (mean difference 7.65 VEINES-Sym scale points, P = .0142) compared with the control-arm patients (Table 5).
Figure 3.
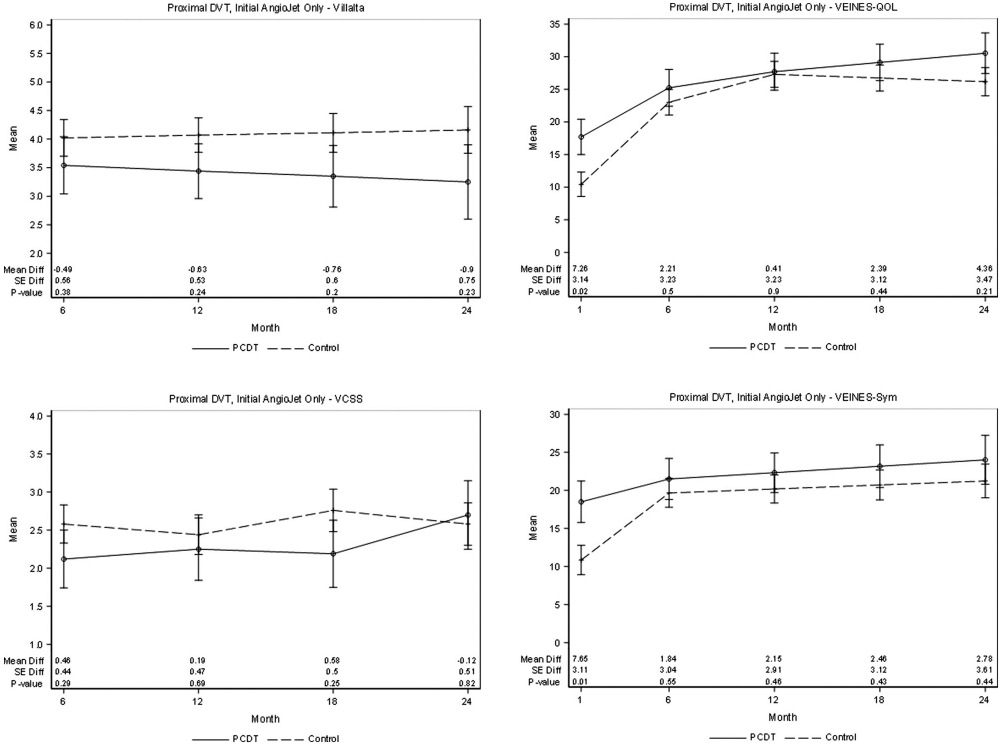
Patients with proximal DVT. Effect of attempted single-session AngioJet-PCDT on PTS severity (mean continuous Villalta scale and VCSS scores), venous symptoms (mean VEINES-symptoms [Sym] subscale scores, adjusted for baseline), and health-related venous QOL (mean VEINES-QOL scores, adjusted for baseline) during 24 months of follow-up. DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; PTS = postthrombotic syndrome; QOL = quality of life; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Adjudicated Safety Outcomes.
Within 30 days, major bleeding occurred in 3 (1.7%) of the 173 AngioJet-PCDT patients versus 0 (0.0%) of the 191 control-arm patients (P = .11), none fatal or intracranial (Table 4). Over 24 months, symptomatic recurrent VTE may have been more frequent in AngioJet-PCDT (24/173, 13.9%) versus control-arm (13/191, 6.8%) patients (P = .03). No mortality differences were seen; none of the 5 deaths (3 in control-arm and 2 in AngioJet-PCDT patients) occurred within 10 days after randomization.
Treatment Efficacy of AngioJet-PCDT in Anatomic Subgroups
Femoral-Popliteal DVT.
Except for reduced PTS at 6 months of follow-up (21.7% with AngioJet-PCDT vs 40.8% with control, P = .01), significant differences were not identified in early or late study outcomes between the AngioJet-PCDT and control-arm patients (Tables 4, 5, Fig 4).
Figure 4.
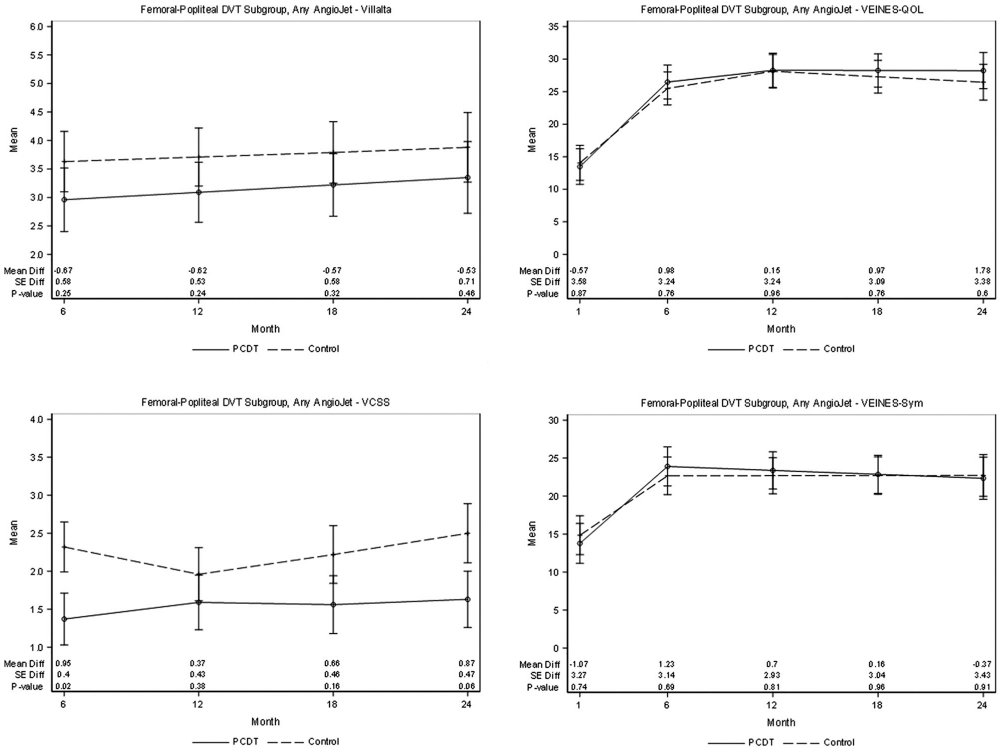
Patient subgroup with femoral-popliteal DVT. Effect of any method of AngioJet-PCDT on PTS severity (mean continuous Villalta scale and VCSS scores), venous symptoms (mean VEINES-symptoms [Sym] subscale scores, adjusted for baseline), and health-related venous QOL (mean VEINES-QOL scores, adjusted for baseline) during 24 months of follow-up. DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; PTS = postthrombotic syndrome; QOL = quality of life; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Iliofemoral DVT.
PTS developed in 41 (43.2%) of 95 AngioJet-PCDT treated iliofemoral DVT patients and 40 (40.0%) of 100 control-arm patients (P = .65) during 24 months (Table 4). The point estimates of the mean Villalta scale and VCSS scores from 6 to 24 months and of the proportions of patients with PTS at 6 months (26.2% vs 39.2%, P = .08) were nominally lower for AngioJet-PCDT than for control, but the differences were not statistically significant (Fig 5). Differences were not seen in the 2-year occurrences of moderate-or-severe PTS, severe PTS, venous ulceration, or QOL from 6 to 24 months. Improvement in early leg pain severity was greater for AngioJet-PCDT than for control, reaching statistical significance at 30 days (mean difference 0.84 Likert scale points, P = .0061) (Table 5). From the baseline to 30 days, the AngioJet-PCDT recipients had greater improvement in venous QOL (difference of 12.6 VEINES-QOL scale points, P = .0001) and symptoms (difference of 11.4 VEINES-Sym scale points, P = .0003) than the control-arm patients (Fig 5). At 30 days, the AngioJet-PCDT recipients also had less residual thrombus.
Figure 5.
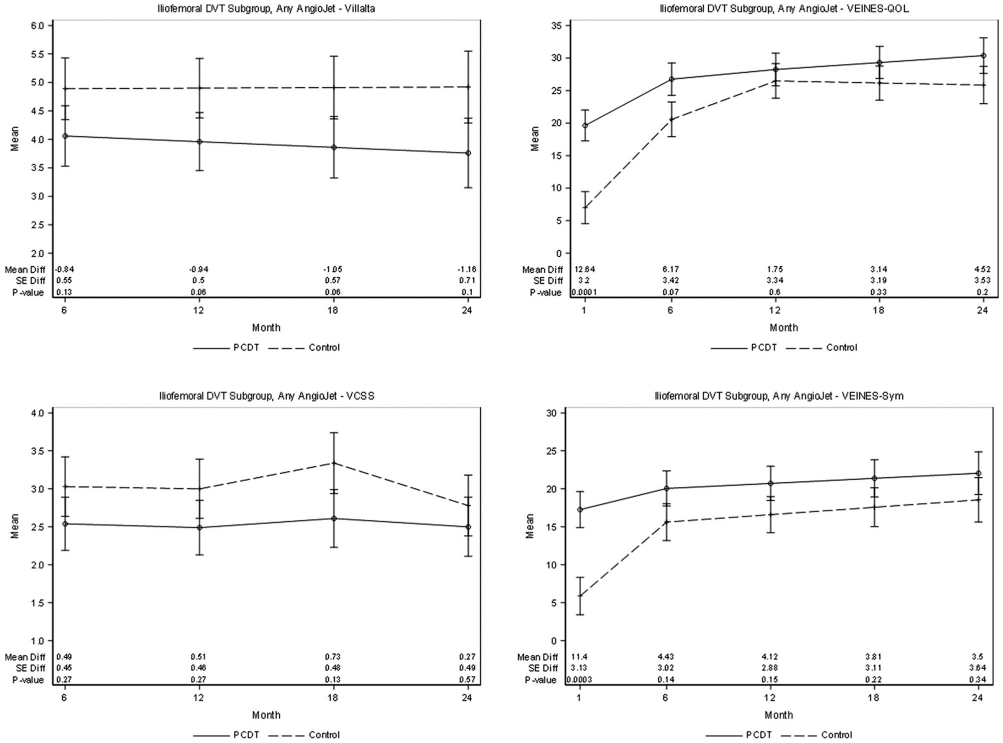
Patient subgroup with iliofemoral DVT. Effect of any method of AngioJet-PCDT on PTS severity (mean continuous Villalta scale and VCSS scores), venous symptoms (mean VEINES-symptoms [Sym] subscale scores, adjusted for baseline), and health-related venous QOL (mean VEINES-QOL scores, adjusted for baseline) during 24 months of follow-up. DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; PTS = postthrombotic syndrome; QOL = quality of life; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Investigator-Reported Adverse Events
Across all the ATTRACT sites, 25 (13.6%) of the 183 patients in whom the AngioJet device was used (any mode) experienced a total of 42 procedure-related adverse events, including 9 (4.9%) who suffered a total of 13 serious adverse events (Table 6). The procedure-related serious adverse events included 5 cases of bleeding (2 retroperitoneal [1 due to shock], 2 gastrointestinal, and 1 at the venous access site), 2 cases of symptomatic pulmonary embolism, 2 cases of acute rethrombosis, 1 case of acute renal failure, and 1 case of low hemoglobin level. In the AngioJet sites, these procedure-related serious adverse events occurred in 3 (4%) of 75 patients who underwent single-session PCDT and 6 (5.6%) of 108 patients who underwent infusion-first PCDT. One additional patient developed bradycardia, which was (maybe incorrectly) categorized as not related.
Table 6.
Procedure-Related Adverse Events in AngioJet-PCDT Recipients*
| Adverse event† | Number of events |
Number of serious adverse events |
|---|---|---|
| Bleeding at PCDT access site | 5 | 1 |
| Decreased hemoglobin or hematocrit level or red blood cell count | 5 | 1 |
| Acute rethrombosis or DVT | 3 | 2 |
| Gastrointestinal bleed or bloody vomitus | 3 | 2 |
| Nausea | 3 | 0 |
| Acute pulmonary embolism | 2 | 2 |
| Headache | 2 | 0 |
| Pain at PCDT access site | 2 | 0 |
| Retroperitoneal hematoma | 2 | 2 |
| Superficial hematoma or bruising | 2 | 0 |
| Acute renal dysfunction | 1 | 1 |
| Chest pain | 1 | 0 |
| Epistaxis | 1 | 0 |
| Guide wire tip fracture | 1 | 1 |
| Hypertension | 1 | 0 |
| Hypokalemia | 1 | 1 |
| Meralgia paresthetica | 1 | 0 |
| Pain in the right calf | 1 | 0 |
| Perforation of the popliteal vein | 1 | 0 |
| Pulmonary edema | 1 | 0 |
| Redness or irritation over the ankle | 1 | 0 |
| Swelling in the right calf | 1 | 0 |
| Vomiting | 1 | 0 |
| Total | 42 | 13 |
DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis.
All adverse events occurring during or within 3 days after the procedure that were categorized as being at least possibly related to the study drug or research study.
One additional patient had bradycardia, but this was reported as “not related” to research.
DISCUSSION
The current analysis found that in patients with acute proximal DVT, a treatment strategy that included first-line AngioJet-PCDT leads to a greater improvement in lower-extremity symptoms and venous QOL at 1 month and reduced PTS at 6 months compared with anticoagulation alone. However, beyond 6 months of follow-up, AngioJet-PCDT does not reduce PTS or improve the QOL.
Few prospective studies have evaluated the outcomes of specific device-enabled CDT strategies. A previous ATTRACT analysis found the AngioJet-PCDT strategy to provide ≥50% thrombus removal in 96% of treated patients, as did the multicenter Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths (PEARL) registry (4,13). In the BERN Ultrasound-assisted Thrombolysis for Ilio-Femoral deep vein thrombosis versUs standard catheter directed thromboLysis (BERNUTIFUL) trial, 48 patients with acute iliofemoral DVT underwent CDT using a standard rt-PA dosing via an ultrasound catheter (Ekowave; Boston Scientific Corporation, Marlborough, Massachusetts) (14). In this trial, no differences were found in thrombus removal, safety, PTS, or QOL at 1 year between patients randomized to have and not have the ultrasound energy delivered. The Ultrasound-Accelerated Catheter-Directed Thrombolysis Versus Anticoagulation for the Prevention of Post-Thrombotic Syndrome (CAVA) trial randomized 184 patients with acute iliofemoral DVT to undergo and not undergo ultrasound-assisted CDT, and no benefit in PTS or QOL were found at 1 year (15). Other randomized trials have evaluated traditional infusion CDT or have not reported device-specific outcomes (16,17).
Hence, the current randomized trial analysis links discrete clinical benefits to the use of a specific device-enabled CDT strategy for the management of acute DVT. Within the first 30 days, the AngioJet-PCDT strategy provided enhanced recovery from DVT, as shown by the significant improvements in leg swelling, venous symptoms, and venous QOL (3). Like the overall study, these benefits were sizable in patients with iliofemoral DVT (12.6 VEINES-QOL and 11.4 VEINES-Sym points, P < .001) but not discernable in patients with femoral-popliteal DVT (18,19).
The use of the AngioJet-PCDT strategy was associated with a range of adverse events, as expected for a catheter-based procedure. Major bleeding was infrequent (1.7%) and likely attributable to rt-PA and anticoagulation, in line with the overall ATTRACT (1.7%) and PEARL (3.6%) studies (3,4). As in the PEARL registry, serious adverse events plausibly related to AngioJet (pulmonary embolism, renal failure, low hemoglobin level, and bradycardia) occurred at very low (0%–2%) rates. In the ATTRACT trial, patients with pre-existing renal dysfunction were excluded, and there were protocol limits on device activation time, infusate volume, and hydration before the procedure. To avoid gaps in VTE treatment, the investigators were instructed not to stop anticoagulation before PCDT unless it was supratherapeutic. To prevent bradycardia, the protocol encouraged the limitation of the duration of AngioJet activation to 30-second runs with 10-second rest periods.
The AngioJet-PCDT strategy provided a clinical benefit for 6 months but not beyond. The absolute PTS risk reduction at 6 months was 16% with the AngioJet-PCDT strategy (P = .003 vs control), in line with the overall trial (13%), and was slightly more prominent for patients with femoral-popliteal DVT (19%, P = .01 vs control) than for patients with iliofemoral DVT (13%, P = .08 vs control) (3). However, there was no 6-month QOL benefit, and from 12 to 24 months, the PCDT benefits upon PTS severity, moderate-or-severe PTS, and QOL that were seen in patients with iliofemoral DVT in the overall ATTRACT trial were absent, most likely because of the smaller sample size and smaller observed differences between the AngioJet-PCDT and control-arm patients.
The aforementioned pattern (early efficacy that did not translate into late benefits) was apparent in both patients treated with AngioJet-mediated rt-PA delivery and recipients of infusion-first PCDT. AngioJet-mediated rt-PA delivery yielded shorter treatment times (difference 10 hours), with 45% of the patients treated in less than 4 hours (like the 36% treated in <6 hours in the PEARL registry), suggesting that this may be an efficient strategy to use when the thrombus extent is favorable (ie, good inflow) (3,4). However, majority of the patients still needed overnight infusion. Major bleeding with PCDT was less frequent in the ATTRACT trial than in other large CDT studies, perhaps because of the reduced rt-PA dose and treatment time (3). If future refinements can enable single-session treatment to be achieved more often, safety may be further enhanced.
The reasons why the AngioJet-PCDT strategy did not improve long-term outcomes and the reason for PCDT’s limited long-term effectiveness in the ATTRACT trial overall remain unclear. Although the thrombus was successfully cleared initially, recurrent VTE may have been more frequent in the AngioJet-PCDT arm than in the control arm over 24 months (P = .03). At 1 year, residual vein diameters in the AngioJet-PCDT arm were not much different from the control arm, unlike the overall ATTRACT trial, in which significant differences were seen in residual thrombus burden (20). It is unclear as to what extent these findings simply reflect chance versus the biological effects of AngioJet, the overall PCDT treatment, or antithrombotic therapy, but it seems possible that rethrombosis (overt and/or subclinical) may have undermined the long-term effects of AngioJet-PCDT and overall PCDT in the ATTRACT trial. Although a venogram analysis has shown that AngioJet-PCDT is highly effective in removing thrombus and the protocol actively encouraged AngioJet use with an angled guiding catheter, the incomplete removal of peripheral thrombi could be a potential contributor to late rethrombosis (13). Stent placement was not assigned by randomization so comparisons are subject to selection bias, but 24-month recurrent VTE did not differ between patients who received stents (15.0%) and patients who did not (11.8%) (P = .43). Device-mediated endothelial injury has traditionally been considered a short-lived phenomenon; however, the pathophysiology of PTS involves a complex transition by which early venous inflammation leads to late fibrosis, reduced vein wall elasticity, and impaired valve function. The biological effects of catheter-based interventions upon such processes have not yet been carefully examined.
The study applied a similar antithrombotic therapy to the patients in both the treatment arms, with adherence to the published guidelines and flexibility to provide physician-directed care. During initial therapy, unfractionated heparin was used more frequently in the PCDT-arm patients, but its antithrombotic efficacy is known to be similar to that of low-molecular-weight heparin. If some clinicians use the ultrasound extent of residual vein thrombus as a factor for making clinical decisions, a shorter duration of anticoagulation could be a real-world effect of PCDT use. In the current analysis, fewer patients in the AngioJet-PCDT arm may have been anticoagulated at 6 months than in the control arm; however, this apparent difference was small (9%, reflecting a switch to antiplatelet therapy in half of the cases), not statistically significant, and absent at other time points. Most patients received warfarin in the long term—although some studies have suggested that rivaroxaban is more effective for PTS prevention (inconclusive), its limited use in this study was balanced between the treatment arms. Among other avenues, it is possible that after further study, improved antithrombotic regimens enhance the results of PCDT and AngioJet-PCDT.
This analysis has limitations. First, because multiple endovascular methods were used and some infusion-first PCDT patients did not require AngioJet to be used, the findings are applicable to the AngioJet-PCDT treatment strategy used, not solely to the AngioJet device. The randomization of patients to the AngioJet-PCDT arm versus that to anticoagulation alone in the same sites allowed these groups to be compared, but patients were not randomized to different PCDT methods because any resulting comparisons would have had low statistical power and it was believed that allowing physicians to use the device of their choice would have enabled the study to better represent the outcomes of PCDT as used in clinical practice. Therefore, different PCDT methods were not directly compared; the collective outcomes reported for AngioJet-PCDT may not reflect the outcomes of each method of using the device. Second, to an extent, clinical practice has since evolved to incorporate updated AngioJet catheters (eg, Zelante), new thrombectomy devices, dedicated venous stents, and increased use of intravascular ultrasound. However, notwithstanding provider enthusiasm for new approaches, none of these interventions has yet been shown to be superior to the methods used to treat acute DVT in the ATTRACT trial. Old and new AngioJet models work based on the same mechanistic platform and should, thus, have common biological effects. Third, there was limited imaging follow-up, and blood samples were not analyzed for genomic and other predictive biomarkers, reducing insight into the reasons why the AngioJet-PCDT strategy did not improve long-term outcomes. Fourth, as noted previously, there was substantial loss to follow-up over 24 months, with an imbalance between the treatment arms that could have affected treatment effect estimates (3). However, the results of a sensitivity analysis of the primary outcome (PTS) that used multiple imputation (data not shown), as previously described, were consistent with the results presented (3). Finally, this analysis involved substantial multiple statistical testing in a medium-sized study; so, caution should be applied to avoid overinterpreting subgroup findings that could stem from chance and low statistical power.
In conclusion, in patients with acute proximal DVT, a treatment strategy that included first-line AngioJet-PCDT was effective in increasing early symptom improvement compared with standard therapy alone but did not reduce PTS beyond 6 months. This analysis may provide a useful benchmark against which to evaluate future PCDT strategies for DVT.
RESEARCH HIGHLIGHTS.
A subset analysis of the ATTRACT randomized prospective trial was performed to compare patients who underwent pharmacomechanical catheter-directed venous thrombolysis (PCDT) using the AngioJet device with patients treated with anticoagulation alone.
Three hundred sixty-four patients from 33 sites were included.
At 1 month, patients treated with AngioJet-PCDT showed a greater improvement in swelling, symptoms, and the quality of life (QOL), with the greatest benefit for iliofemoral thrombosis. At 6 months, the incidence of postthrombotic syndrome (PTS) was lower.
Advantages did not persist, and PTS and QOL were equivalent at 12 and 24 months.
The recurrence of thrombosis was higher at 24 months in patients who underwent treatment with AngioJet-PCDT.
STUDY DETAILS.
Study type:
Multicenter, prospective, randomized controlled trial
Study phase:
III
ACKNOWLEDGMENTS
The ATTRACT trial was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) for the clinical coordinating center (U01-HL088476 to Washington University in St. Louis) and data coordinating center (U01-HL088118 to McMaster University, Hamilton, ON); the Washington University Center for Translational Therapies in Thrombosis, which was supported by a grant from the NHLBI (U54-HL112303); the Washington University Institute of Clinical and Translational Sciences, which was supported by a grant from the National Center for Advancing Translational Sciences (UL1-TR00044810); Boston Scientific; Covidien (now Medtronic); Genentech; the Society of Interventional Radiology Foundation; the Canada Research Chairs Program (tier 1 support to Dr. Susan Kahn); the CanVECTOR Network, funded by Canadian Institutes of Health Research (CDT-142654; to Dr. Kahn); the Heart and Stroke Foundation of Canada (Investigator Award to Dr. Clive Kearon); and a Jack Hirsh Professorship in Thrombosis (to Dr. Kearon). BSN Medical donated the compression stockings. The authors thank Dr. Andrei Kindzelski (NHLBI Project Officer), Dr. Clive Kearon (Chair, Data Coordinating Center), and the entire network of investigators and study staff at the ATTRACT trial coordinating centers, core laboratories, and clinical centers (see Appendix).
S.V. received grants from the National Heart Lung and Blood Institute, Boston Scientific, Covidien (now Medtronic), Genentech, BSN Medical, for the ATTRACT trial and grants from Medi USA (in-kind support, no financial support) and Cook Medical (to institution) for unrelated research. A.S. received support from the American Heart Association, outside the submitted work. None of the other authors have identified a conflict of interest.
ABBREVIATIONS
- ATTRACT
Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis
- CDT
catheter-directed thrombolysis
- DVT
deep vein thrombosis
- PCDT
pharmacomechanical catheter-directed venous thrombolysis
- PEARL
Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths
- PTS
postthrombotic syndrome
- QOL
quality of life
- rt-PA
recombinant tissue plasminogen activator
- VCSS
venous clinical severity scale
- VEINES
Venous Insufficiency Epidemiologic and Economic Study
- VEINES-QOL
Venous Insufficiency Epidemiologic and Economic Study-Quality of Life
- VTE
venous thromboembolism
Appendix
APPENDIX A. THE ATTRACT TRIAL–STUDY LEADERSHIP, INVESTIGATORS, AND LEAD SONOGRAPHERS
Steering Committee
Samuel Z. Goldhaber, MD (Chair) Harvard Medical School
David J. Cohen, MD, MSc St. Luke’s Mid America Heart Institute
Anthony J. Comerota, MD University of Michigan
Heather L. Gornik, MD, MHS, RVT Cleveland Clinic Heart & Vascular Institute
Michael R. Jaff, DO Harvard Medical School
Jim Julian, MMath McMaster University
Susan R. Kahn, MD, MSc McGill University, Jewish General Hospital
Clive Kearon, MB, PhD McMaster University
Stephen Kee, MD (SIR Foundation) UCLA Medical Center
Andrei L. Kindzelski, MD, PhD National Heart, Lung, and Blood Institute
Lawrence Lewis, MD Washington University in St. Louis
Elizabeth Magnuson, ScD St. Luke’s Mid America Heart Institute
Mahmood K. Razavi, MD St. Joseph’s Vascular Institute
Timothy P. Murphy, MD Brown University
Suresh Vedantham, MD (Principal Investigator [PI]) Washington University in St. Louis
CLINICAL COORDINATING CENTER
Mallinckrodt Institute of Radiology, Washington University in St. Louis, United States
DATA COORDINATING CENTER
Ontario Clinical Oncology Group, McMaster University, Hamilton, Canada
HEALTH ECONOMIC CORE LABORATORY
Mid America Heart Institute, St. Luke’s Hospital, Kansas City, United States
VASCULAR ULTRASOUND CORE LABORATORY
VasCore, Massachusetts General Hospital, Boston, United States
ANGIOJET SITES IN THE ATTRACT TRIAL
Albert Einstein Medical Center: Paul Brady—site PI, Marvin Schatz, Mindy Horrow, Peyman Markazi, Leli Forouzan, Terence A.S. Matalon, and David Hertzog
Allegheny General Hospital: Swapna Goday—site PI, Margaret Kennedy—previous site PI, Robert Kaplan, Thomas Campbell, Jamie Hartman, Elmer Nahum, and Arvind Venkat
Central DuPage Hospital: Joseph Schneider—site PI, Stanley Kim, Farrah Hashemi, Joseph Boyle, Nilesh Patel, and Michael Verta
Christiana Care Hospital: Daniel Leung—site PI, Marc Garcia—previous site PI, Phillip Blatt, Jamil Khatri, Dave Epstein, Randall Ryan, Tom Sweeny, Michael Stillabower, George Kimbiris, Tuhina Raman, Paul Sierzenski, Lelia Getto, Michael Dignazio, Paul Sierzenski, and Mark Horvath
Cleveland Clinic Foundation: Heather Gornik—site PI, John Bartholomew, Mehdi Shishehbor, Frank Peacock, Douglas Joseph, Soo Hyum Kim, Natalia Fendrikova-Mahlay, Daniel Clair, Sean Lyden, Baljendra Kapoor, Gordon McLennon, Gregory Pierce, James Newman, James Spain, Amanjiit Gill, Aaron Hamilton, Anthony Rizzo, and Woosup Park
Danbury Hospital: Alan Dietzek—site PI, Ira Galin, Dahlia Plummer, Richard Hsu, Patrick Broderick, Andrew Keller, and Sameer Sayeed
Eastern Connecticut Hematology and Oncology Associates: Dennis Slater—site PI, Herb Lustberg, Jan Akus, Robert Sidman, Mandeep Dhami, Phillip Kohanski, Anca Bulgaru, Renuka Dulala, James Burch, Dinesh Kapur, and Jie Yang
Forsyth Medical Center: Stephen Motew—site PI, Robin Royd-Kranis, Raymond Workman, Scott Kribbs, Gerald Hogsette, Phillip Moore, Bradley Thomason, William Means, Richard Bonsall, John Stewart, and Daniel Golwya
Georgetown University: Thomas Chang—site PI, Karun Sharma—previous site PI, Sandra Allison, Fil Banovac, Emil Cohen, Brendan Furlong, Craig Kessler, Mike McCullough, and Jim Spies
Maine Medical Center: Paul Kim—site PI, Marc Jacquet, Thomas Dykes, Joseph Gerding, Christopher Baker, Mark Debiasto, Derek Mittleider, George Higgins III, Steven Amberson, Roger Pezzuti, and Thomas Gallagher PA-C
Massachusetts General Hospital: Robert Schainfeld—site PI, Stephan Wicky—previous site PI, Sanjeeva Kalva, Gregory Walker, Gloria Salazar, Benjamin Pomerantz, Virenda Patel, Christopher Kabrhel, Shams Iqbal, Suvranu Gangull, Rahmi Oklu, and Scott Brannan
Mayo Clinic: Sanjay Misra—site PI, Haraldur Bjarnason – previous site PI, Aneel Ashrani, Michael Caccavale, Chad Fleming, Jeremy Friese, John Heit, Manju Kalra, Thanila Macedo, Robert McBane, Michael McKusick, Andrew Stockland, David Woodrum, and Waldemar Wysokinski
Medical College of Wisconsin/Froedtert Hospital and Clinics: Parag Patel—site PI, William Rilling, Sean Tutton, Robert Hieb, Eric Hohenwalter, M. Riccardo Colella, James Gosset, Sarah White, Brian Lewis, Kellie Brown, Peter Rossi, and Gary Seabrook
Medical University of South Carolina: Marcelo Guimaraes—site PI, J. Bayne Selby, William McGary, Christopher Hannegan, Jacob Robison, Thomas Brothers, Bruce Elliott, Nitin Garg, M. Bret Anderson, Renan Uflacker, Claudio Schonholz, Laurence Raney, and Charles Greenberg
Oregon Health & Science University: John Kaufman—site PI, Frederick Keller, Kenneth Kolbeck, Gregory Landry, Erica Mitchell, Robert Barton, Thomas DeLoughery, Norman Kalbfleisch, Renee Minjarez, Paul Lakin, Timothy Liem, Gregory Moneta, Khashayar Farsad, Ross Fleischman, and Loren French
Phoenix Heart & Cardiovascular: Rajul Patel—site PI, Rahul Malhotra—previous site PI, Stanley Kim, Farah Hashemi, Joseph Boyle, Nilesh Patel, Marvin Padnick, Melissa Gurley, Fred Cucher, Ronald Sterrenberg, G. Reshmaal Deepthi, and Gomes Cumaranatunge
Southern Illinois University: Kim Hodgson—site PI, Robert McLafferty—previous site PI, Douglas Hood, Colleen Moore, and David Griffen
St. Elizabeth Healthcare Edgewood (KY): Darren Hurst—site PI, David Lubbers, Daniel Kim, Brent Warren, Jeremy Engel, and Damodhar Suresh
St. Luke’s Hospital and Health Network: Jamie Thomas—site PI, Justin Psaila, Michael Ringold, Jay Fisher, Any Lipcomb, and Timothy Oskin
St. Vincent Medical Group: Kannan Natarajan—site PI, Stewart Bick, Jeffrey Cooke, Ann Hedderman, Anne Greist, Lorrie Miller, Brandon Martinez, Vincent Flanders, and Mark Underhill
Stanford University Medical Center: Lawrence Hofmann—site PI, Daniel Sze, William Kuo, John Louie, Gloria Hwang, David Hovsepian, Nishita Kothary, Caroline Berube, Donald Schreiber, and Brooke Jeffrey
Staten Island University Hospital: Jonathan Schor—site PI, Jonathan Deitch, Kuldeep Singh, Barry Hahn, Brahim Ardolic, and Shilip Gupta
The Reading Hospital: David Sacks—site PI, Robert Guay, Mark Scott, Karekin Cunningham, Adam Sigal, Terrence Cescon, Nick Leasure, and Thiruvenkatasamy Dhurairaj
University of Iowa: Melhem Sharafuddin—site PI, Steven Lentz, Andrew Nugent, William Sharp, Timothy Kresowik, Rachel Nicholson, Shiliang Sun, Fadi Youness, and Luigi Pascarella
University of Illinois, Chicago: Charles Ray—site PI, Martha-Gracia Knuttinen—previous site PI, James Bui, Ron Gaba, Valerie Dobiesz, Ejaz Shamim, Sangeetha Nimmagadda, David Peace, Aarti Zain, and Alison Palumto
University of Michigan Hospitals and Health Centers: David Williams—site PI, Joseph Gemmete, Venkataramu Krishnamurthy, Wojciech Cwikiel, Kyung Cho, James Schields, Ranjith Vellody, Paula Novelli, Narasimham Dasika, Thomas Wakefield, John Rectenwald, Peter Henke, Jeffrey Desmond, James Froehlich, and Minhajuddin Khaja
University of North Carolina: Stephan Moll—site PI, Matthew Mauro, Joseph Stavas, Charles Burke, Robert Dixon, Hyeon Yu, Blair Keagy, Kyuny Kim, Raj Kasthuri, and Nigel Key
University of Pittsburgh: Rabih Chaer—site PI, Michael Makaroun, Robert Rhee, Jae-Sung Cho, Donald Baril, Luke Marone, Margaret Hseih, Kristian Feterik, Roy Smith, Geetha Jeyabalan, and Jennifer Rogers
University of Virginia Health System: John Angle—site PI, Alan Matsumoto, Nancy Harthun, Ulku Turba, Wael Saad, Brian Uthlaut, Srikant Nannapaneni, David Ling, Saher Sabri, John Kern, B. Gail Macik, George Hoke, Auh Wahn Park, James Stone, Benjamin Sneed, Scott Syverud, Kelly Davidson, Aditya Sharma, Ziv Haskal, and Luke Wilkins
Wake Forest Baptist Health: Randolph Geary—site PI, Matthew Edwards, Christopher Godshall, and Pavel Levy
Weill Cornell Medical College: Ronald Winokur—site PI, Akhilesh Sista—previous site PI, David Madoff, Kyungmouk Lee, Bradley Pua, Maria DeSancho, Raffaele Milizia, and Jing Gao
Western Penn Allegheny Health System: Swapna Goday—site PI, Margaret Kennedy—previous site PI, Robert Kaplan, Thomas Campbell, Gordon McLean, Jamie Hartman, Elmer Nahum, and Sanualah Khalid
Washington University in St. Louis*: Suresh Vedantham—site PI, Larry Lewis, Nael Saad, Mark Thoelke, Robert Pallow, Seth Klein, and Gregorio Sicard
ATTRACT ULTRASOUND SUBSTUDY: PARTICIPATING SITES AND LEAD SONOGRAPHERS
Cleveland Clinic Foundation: Alia G. Grattan and Kathleen MacDonald
Massachusetts General Hospital: Kathryn Lane Contis, Kathleen Hannon, Andrea Mattoon, and Caroline Yarnevich
Washington University in St. Louis*: John Gibson and Deborah Wehrle
APPENDIX B.
GENERAL QUALITY OF LIFE (36-ITEM SHORT FORM SURVEY PHYSICAL COMPONENT SCORE) IN ANGIOJET SITE PATIENTS
| Change in physical component score | Control* (n = 191) |
PCDT (n = 173) |
PCDT: control difference |
|||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | Mean difference (SE) | P value | |
| All proximal DVT | ||||||
| At 1 mo | 168 | 6.10 (0.90) | 167 | 7.04 (0.90) | 0.94 (1.28) | .46 |
| At 6 mo | 150 | 11.22 (0.88) | 152 | 11.16 (0.88) | −0.06 (1.25) | .96 |
| At 12 mo | 133 | 11.71 (0.87) | 144 | 11.50 (0.87) | −0.21 (1.23) | .87 |
| At 18 mo | 117 | 12.19 (0.93) | 124 | 11.83 (0.94) | −0.36 (1.32) | .79 |
| At 24 mo | 121 | 12.67 (1.06) | 129 | 12.16 (1.06) | −0.51 (1.50) | .73 |
| All proximal DVT (initial AngioJet only) | ||||||
| At 1 mo | 168 | 6.09 (0.88) | 73 | 7.71 (1.35) | 1.62 (1.61) | .32 |
| At 6 mo | 150 | 11.20 (0.87) | 63 | 10.32 (1.34) | −0.88 (1.60) | .58 |
| At 12 mo | 133 | 11.66 (0.87) | 55 | 11.10 (1.34) | −0.56 (1.60) | .73 |
| At 18 mo | 117 | 12.12 (0.94) | 47 | 11.89 (1.46) | −0.23 (1.73) | .89 |
| At 24 mo | 121 | 12.58 (1.07) | 47 | 12.67 (1.67) | 0.09 (1.98) | .96 |
| Iliofemoral DVT subgroup | ||||||
| At 1 mo | 86 | 5.60 (1.29) | 91 | 8.43 (1.24) | 2.84 (1.79) | .11 |
| At 6 mo | 74 | 10.75 (1.29) | 84 | 10.97 (1.23) | 0.21 (1.78) | .90 |
| At 12 mo | 66 | 11.65 (1.28) | 80 | 11.21 (1.23) | −0.44 (1.78) | .80 |
| At 18 mo | 63 | 12.55 (1.37) | 68 | 11.45 (1.32) | −1.10 (1.91) | .56 |
| At 24 mo | 66 | 13.45 (1.54) | 68 | 11.70 (1.49) | −1.76 (2.15) | .41 |
| Femoral-popliteal DVT subgroup | ||||||
| At 1 mo | 82 | 6.66 (1.25) | 78 | 5.45 (1.31) | −1.21 (1.81) | .51 |
| At 6 mo | 76 | 11.71 (1.20) | 68 | 11.40 (1.27) | −0.30 (1.75) | .86 |
| At 12 mo | 67 | 11.72 (1.17) | 64 | 11.79 (1.24) | 0.07 (1.70) | .97 |
| At 18 mo | 54 | 11.72 (1.26) | 56 | 12.17 (1.32) | 0.45 (1.82) | .81 |
| At 24 mo | 55 | 11.73 (1.44) | 61 | 12.55 (1.49) | 0.82 (2.07) | .69 |
DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; SE = standard error.
Treatment groups are per-protocol patients.
APPENDIX C.
CONTINUOUS TRIAL OUTCOMES FOR PATIENTS WITH PROXIMAL DVT: ANGIOJET-PCDT (ANY METHOD) VERSUS CONTROL
| Outcome | Control* (n = 191) |
PCDT (n = 173) |
PCDT: control difference |
|||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | Mean difference (SE) | P value | |
| Villalta scale score | ||||||
| At 6 mo | 150 | 3.99 (0.30) | 153 | 3.30 (0.31) | −0.68 (0.41) | .09 |
| At 12 mo | 135 | 4.03 (0.28) | 145 | 3.31 (0.28) | −0.72 (0.37) | .05 |
| At 18 mo | 117 | 4.08 (0.31) | 125 | 3.32 (0.31) | −0.76 (0.41) | .06 |
| At 24 mo | 123 | 4.13 (0.38) | 132 | 3.32 (0.37) | −0.80 (0.51) | .12 |
| VCSS score | ||||||
| At 6 mo | 146 | 2.51 (0.23) | 151 | 1.84 (0.22) | 0.67 (0.31) | .03 |
| At 12 mo | 132 | 2.36 (0.24) | 139 | 1.93 (0.23) | 0.43 (0.32) | .19 |
| At 18 mo | 115 | 2.66 (0.25) | 121 | 1.98 (0.25) | 0.68 (0.35) | .05 |
| At 24 mo | 112 | 2.50 (0.26) | 121 | 1.92 (0.25) | 0.57 (0.35) | .10 |
| VEINES-QOL | ||||||
| At 1 mo | 168 | 10.37 (1.82) | 167 | 16.85 (1.81) | 6.48 (2.40) | .007 |
| At 6 mo | 150 | 22.98 (1.82) | 152 | 26.76 (1.80) | 3.79 (2.34) | .11 |
| At 12 mo | 133 | 27.24 (1.84) | 144 | 28.33 (1.80) | 1.09 (2.31) | .64 |
| At 18 mo | 117 | 26.66 (1.80) | 125 | 28.88 (1.77) | 2.21 (2.21) | .32 |
| At 24 mo | 121 | 26.09 (1.98) | 129 | 29.42 (1.95) | 3.34 (2.45) | .17 |
| VEINES symptoms | ||||||
| At 1 mo | 168 | 10.15 (1.78) | 167 | 15.78 (1.77) | 5.63 (2.27) | .01 |
| At 6 mo | 150 | 18.97 (1.74) | 152 | 21.89 (1.72) | 2.92 (2.18) | .18 |
| At 12 mo | 133 | 19.48 (1.68) | 144 | 22.02 (1.66) | 2.55 (2.05) | .21 |
| At 18 mo | 117 | 19.98 (1.78) | 125 | 22.16 (1.75) | 2.18 (2.17) | .32 |
| At 24 mo | 121 | 20.49 (2.01) | 129 | 22.29 (1.97) | 1.80 (2.50) | .47 |
DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; QOL = quality of life; SE = standard error; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Treatment groups are per-protocol patients.
APPENDIX D.
CONTINUOUS TRIAL OUTCOMES FOR PATIENTS WITH PROXIMAL DVT: PCDT (INITIAL ANGIOJET ONLY) VERSUS CONTROL
| Outcome | Control* (n = 191) |
PCDT (Initial AngioJet only) (n = 75) |
PCDT: control difference |
|||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | Mean difference (SE) | P value | |
| Villalta scale score | ||||||
| At 6 mo | 150 | 4.02 (0.32) | 72 | 3.54 (0.50) | −0.49 (0.56) | .38 |
| At 12 mo | 135 | 4.07 (0.30) | 63 | 3.44 (0.48) | −0.63 (0.53) | .24 |
| At 18 mo | 117 | 4.11 (0.34) | 56 | 3.35 (0.54) | −0.76 (0.60) | .20 |
| At 24 mo | 123 | 4.16 (0.41) | 48 | 3.25 (0.65) | −0.9 (0.75) | .23 |
| VCSS score | ||||||
| At 6 mo | 146 | 2.58 (0.25) | 62 | 2.12 (0.38) | 0.46 (0.44) | .29 |
| At 12 mo | 132 | 2.44 (0.26) | 52 | 2.25 (0.41) | 0.19 (0.47) | .69 |
| At 18 mo | 115 | 2.76 (0.28) | 45 | 2.19 (0.44) | 0.58 (0.50) | .25 |
| At 24 mo | 112 | 2.58 (0.28) | 43 | 2.70 (0.45) | −0.12 (0.51) | .82 |
| VEINES-QOL | ||||||
| At 1 mo | 168 | 10.44 (1.87) | 73 | 17.70 (2.71) | 7.26 (3.14) | .02 |
| At 6 mo | 150 | 23.02 (1.96) | 63 | 25.23 (2.82) | 2.21 (3.23) | .50 |
| At 12 mo | 133 | 27.29 (1.99) | 55 | 27.70 (2.85) | 0.41 (3.23) | .90 |
| At 18 mo | 117 | 26.73 (1.98) | 48 | 29.12 (2.79) | 2.39 (3.12) | .44 |
| At 24 mo | 121 | 26.17 (2.17) | 47 | 30.53 (3.11) | 4.36 (3.47) | .21 |
| VEINES symptoms | ||||||
| At 1 mo | 168 | 10.85 (1.92) | 73 | 18.50 (2.73) | 7.65 (3.11) | .01 |
| At 6 mo | 150 | 19.66 (1.89) | 63 | 21.50 (2.69) | 1.84 (3.04) | .55 |
| At 12 mo | 133 | 20.19 (1.85) | 55 | 22.34 (2.60) | 2.15 (2.91) | .46 |
| At 18 mo | 117 | 20.72 (1.96) | 48 | 23.18 (2.79) | 2.46 (3.12) | .43 |
| At 24 mo | 121 | 21.24 (2.21) | 47 | 24.02 (3.20) | 2.78 (3.61) | .44 |
DVT = deep vein thrombosis; PCDT= pharmacomechanical catheter-directed venous thrombolysis; QOL = quality of life; SE = standard error; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Treatment groups are per-protocol patients.
APPENDIX E.
FEMORAL-POPLITEAL DVT SUBGROUP: CONTINUOUS TRIAL OUTCOMES, ANY ANGIOJET-PCDT VERSUS CONTROL
| Outcome | Control* (n = 91) |
PCDT (n = 78) |
PCDT: control difference |
|||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | Mean difference (SE) | P value | |
| Villalta scale score | ||||||
| At 6 mo | 76 | 3.63 (0.53) | 69 | 2.96 (0.56) | −0.67 (0.58) | .25 |
| At 12 mo | 67 | 3.71 (0.51) | 65 | 3.09 (0.53) | −0.62 (0.53) | .24 |
| At 18 mo | 54 | 3.79 (0.54) | 56 | 3.22 (0.55) | −0.57 (0.58) | .32 |
| At 24 mo | 57 | 3.88 (0.61) | 62 | 3.35 (0.63) | −0.53 (0.71) | .46 |
| VCSS score | ||||||
| At 6 mo | 75 | 2.32 (0.33) | 68 | 1.37 (0.34) | 0.95 (0.40) | .02 |
| At 12 mo | 66 | 1.96 (0.35) | 62 | 1.59 (0.36) | 0.37 (0.43) | .38 |
| At 18 mo | 52 | 2.22 (0.38) | 55 | 1.56 (0.38) | 0.66 (0.46) | .16 |
| At 24 mo | 49 | 2.50 (0.39) | 58 | 1.63 (0.37) | 0.87 (0.47) | .06 |
| VEINES-QOL | ||||||
| At 1 mo | 82 | 14.06 (2.68) | 78 | 13.49 (2.75) | −0.57 (3.58) | .87 |
| At 6 mo | 76 | 25.49 (2.53) | 68 | 26.47 (2.62) | 0.98 (3.24) | .76 |
| At 12 mo | 67 | 28.12 (2.57) | 64 | 28.27 (2.64) | 0.15 (3.24) | .96 |
| At 18 mo | 54 | 27.28 (2.52) | 56 | 28.25 (2.56) | 0.97 (3.09) | .76 |
| At 24 mo | 55 | 26.44 (2.73) | 61 | 28.22 (2.77) | 1.78 (3.38) | .60 |
| VEINES symptoms | ||||||
| At 1 mo | 82 | 14.86 (2.56) | 78 | 13.79 (2.62) | −1.07 (3.27) | .74 |
| At 6 mo | 76 | 22.68 (2.47) | 68 | 23.91 (2.56) | 1.23 (3.14) | .69 |
| At 12 mo | 67 | 22.69 (2.38) | 64 | 23.39 (2.44) | 0.70 (2.93) | .81 |
| At 18 mo | 54 | 22.71 (2.48) | 56 | 22.87 (2.51) | 0.16 (3.04) | .96 |
| At 24 mo | 55 | 22.72 (2.74) | 61 | 22.35 (2.77) | −0.37 (3.43) | .91 |
DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; QOL = quality of life; SE = standard error; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Treatment groups are per-protocol patients.
APPENDIX F.
ILIOFEMORAL DVT SUBGROUP: CONTINUOUS TRIAL OUTCOMES, ANGIOJET-PCDT (ANY METHOD) VERSUS CONTROL
| Outcome | Control* (n = 100) |
PCDT (n = 95) |
PCDT: control difference |
|||
|---|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | Mean difference (SE) | P value | |
| Villalta scale score | ||||||
| At 6 mo | 74 | 4.89 (0.54) | 72 | 4.06 (0.53) | −0.84 (0.55) | .13 |
| At 12 mo | 68 | 4.90 (0.52) | 63 | 3.96 (0.51) | −0.94 (0.50) | .06 |
| At 18 mo | 63 | 4.91 (0.55) | 56 | 3.86 (0.54) | −1.05 (0.57) | .06 |
| At 24 mo | 66 | 4.92 (0.63) | 48 | 3.76 (0.61) | −1.16 (0.71) | .10 |
| VCSS score | ||||||
| At 6 mo | 86 | 3.03 (0.39) | 62 | 2.54 (0.35) | 0.49 (0.45) | .27 |
| At 12 mo | 74 | 3.00 (0.39) | 52 | 2.49 (0.36) | 0.51 (0.46) | .27 |
| At 18 mo | 66 | 3.34 (0.40) | 45 | 2.61 (0.38) | 0.73 (0.48) | .13 |
| At 24 mo | 63 | 2.78 (0.40) | 43 | 2.50 (0.39) | 0.27 (0.49) | .57 |
| VEINES-QOL | ||||||
| At 1 mo | 86 | 7.00 (2.46) | 73 | 19.64 (2.36) | 12.64 (3.20) | .0001 |
| At 6 mo | 74 | 20.58 (2.67) | 63 | 26.75 (2.5) | 6.17 (3.42) | .07 |
| At 12 mo | 66 | 26.48 (2.66) | 55 | 28.23 (2.49) | 1.75 (3.34) | .60 |
| At 18 mo | 63 | 26.16 (2.61) | 48 | 29.3 (2.46) | 3.14 (3.19) | .33 |
| At 24 mo | 66 | 25.85 (2.87) | 47 | 30.37 (2.74) | 4.52 (3.53) | .20 |
| VEINES symptoms | ||||||
| At 1 mo | 86 | 5.87 (2.47) | 73 | 17.27 (2.37) | 11.40 (3.13) | .0003 |
| At 6 mo | 74 | 15.63 (2.44) | 63 | 20.06 (2.30) | 4.43 (3.02) | .14 |
| At 12 mo | 66 | 16.61 (2.38) | 55 | 20.72 (2.25) | 4.12 (2.88) | .15 |
| At 18 mo | 63 | 17.58 (2.56) | 48 | 21.39 (2.44) | 3.81 (3.11) | .22 |
| At 24 mo | 66 | 18.56 (2.93) | 47 | 22.06 (2.81) | 3.50 (3.64) | .34 |
DVT = deep vein thrombosis; PCDT = pharmacomechanical catheter-directed venous thrombolysis; QOL = quality of life; SE = standard error; VCSS = venous clinical severity scale; VEINES = Venous Insufficiency Epidemiologic and Economic Study; VEINES-QOL = Venous Insufficiency Epidemiologic and Economic Study-Quality of Life.
Treatment groups are per-protocol patients.
Footnotes
Appendices A-F can be found by accessing the online version of this article on www.jvir.org and selecting the Supplemental Material tab.
Washington University was an AngioJet Site for 50% of each year (pre-specified periods).
Contributor Information
Suresh Vedantham, Mallinckrodt Institute of Radiology, Washington University, St. Louis.
Amber Salter, Division of Biostatistics, Washington University, St. Louis.
Samantha Lancia, Division of Biostatistics, Washington University, St. Louis.
Lawrence Lewis, Department of Emergency Medicine, Washington University, St. Louis.
Siddhant Thukral, School of Medicine, University of Missouri, Kansas City, Missouri.
Susan R. Kahn, Department of Medicine, McGill University, Division of Internal Medicine & Center for Clinical Epidemiology, Jewish General Hospital, Montreal, Quebec, Canada..
REFERENCES
- 1.Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008; 149:698–707. [DOI] [PubMed] [Google Scholar]
- 2.Vedantham S, Vesely TM, Sicard GA, et al. Pharmacomechanical thrombolysis and early stent placement for iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2004; 15:565–574. [DOI] [PubMed] [Google Scholar]
- 3.Vedantham S, Goldhaber SZ, Julian J, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med 2017; 377:2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia MJ, Lookstein R, Malhotra R, et al. Endovascular management of deep vein thrombosis with rheolytic thrombectomy: final report of the prospective multicenter PEARL (Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths) registry. J Vasc Interv Radiol 2015; 26:777–785. [DOI] [PubMed] [Google Scholar]
- 5.Cynamon J, Stein EG, Dym RJ, Jagust MB, Binkert CA, Baum RA. A new method for aggressive management of deep vein thrombosis: retrospective study of the power pulse technique. J Vasc Interv Radiol 2006; 17:1043–1049. [DOI] [PubMed] [Google Scholar]
- 6.Garcia M, Dignazio M, Kimbris G, Beeravolu A, Peters A, Veasey J. ATTACK-DVT: Angiojet and TPA thrombolysis: a closer look at combined therapy for the treatment of DVT. J Vasc Interv Radiol 2007; 18:S21. [Google Scholar]
- 7.Vedantham S, Grassi CJ, Ferral H, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol 2006; 17:417–34. [DOI] [PubMed] [Google Scholar]
- 8.Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost 2009; 7:884–888. [DOI] [PubMed] [Google Scholar]
- 9.Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg 2010; 52:1387–1396. [DOI] [PubMed] [Google Scholar]
- 10.Lamping DL, Schroter S, Kurz X, Kahn SR, Abenhaim L. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg 2003; 37:410–19. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein SL, Bijur PE, Gallagher EJ. Relationship between intensity and relief in patients with acute severe pain. Am J Emerg Med 2006; 24: 162–166. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694. [DOI] [PubMed] [Google Scholar]
- 13.Razavi MK, Salter A, Goldhaber SZ, et al. Correlation between post-procedure residual thrombus and clinical outcome in deep vein thrombosis patients receiving pharmacomechanical thrombolysis in a multicenter randomized trial. J Vasc Interv Radiol 2020; 31:1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelberger RP, Stuck A, Spirk D, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis: 1-year follow-up data of a randomized-controlled trial. J Thromb Haemost 2017; 15:1351–1360. [DOI] [PubMed] [Google Scholar]
- 15.Notten P, ten Cate-Hoek AJ, Arnoldussen CW, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol 2020; 7:e40–e49. [DOI] [PubMed] [Google Scholar]
- 16.Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38. [DOI] [PubMed] [Google Scholar]
- 17.Sharifi M, Bay C, Mehdipour M, Sharifi J. Thrombus obliteration by rapid percutaneous endovenous intervention in deep venous occlusion (TORPEDO) trial: midterm results. J Endovasc Ther 2012; 19: 273–280. [DOI] [PubMed] [Google Scholar]
- 18.Comerota AJ, Kearon C, Gu CS, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis: analysis from a stratified multicenter randomized trial. Circulation 2019; 139:1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kearon C, Gu CS, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis in acute femoral-popliteal deep vein thrombosis: analysis from a stratified randomized trial. Thromb Haemost 2019; 119:633–634. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg I, Vedantham S, Salter A, et al. Relationships between the use of pharmacomechanical catheter-directed thrombolysis, sonographic findings, and clinical outcomes in patients with acute proximal DVT: results from the ATTRACT multicenter randomized trial. Vasc Med 2019; 24:442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


