Abstract
Cases of filler reactions after COVID‐19 vaccination have been reported. Here, we present the first case of delayed‐type reaction (DTR) to non‐hyaluronic acid Polycaprolactone dermal filler after the second dose of Sinopharm COVID‐19 vaccine which was improved with administration of topical and intralesional steroids.
Keywords: adverse effects, augmentation, case report, coronavirus, cosmetic, cosmetic filler, COVID‐19, COVID‐19 vaccines, delayed‐type reaction, dermal fillers, hyaluronic acid, hyaluronic acid fillers, non‐hyaluronic acid fillers, review, side effects, swelling, vaccination
While rare, cases of filler reactions after COVID‐19 vaccination can occur. Here, we present the first case of delayed‐type reaction (DTR) to non‐hyaluronic acid Polycaprolactone dermal filler after the second dose of Sinopharm COVID‐19 vaccine.
What do we know?
Various adverse effects associated with COVID‐19 vaccines have been reported.
Cases of filler reactions following COVID‐19 vaccination have been observed in practice.
What does this article add?
While rare, reactions to previously placed Polycaprolactone (PCL) fillers can happen following COVID‐19 vaccinations.
Individuals seeking both COVID‐19 vaccines namely Sinopharm and PCL fillers should be aware of this phenomenon.
1. INTRODUCTION
Dermal filler injections are among the most popular minimally‐invasive cosmetic procedures performed globally. 1 There exist different types of fillers such as hyaluronic acid (HA), calcium hydroxylapatite, poly‐L‐lactic acid, and collagen‐based fillers. 2 Polycaprolactone (PCL) derivatives are soft dermal fillers that are biodegradable. 3 While PCL fillers have a proven safety profile, many adverse effects associated with these fillers such as nodules, granuloma, edema, and bruising have been reported. 4 Acute type 1 hypersensitivity reactions, which are immunoglobin E (IgE)‐mediated, can occur within minutes or hours after filler injection while delayed‐type reactions (DTR), which are T‐cell mediated, can happen between hours or days after the filler placement. 5
With the onset of vaccination against the SARS‐CoV‐2 virus (COVID‐19 virus), various adverse effects associated with these vaccines were reported. As well, filler reactions following COVID‐19 vaccination have been observed in practice. 6 Herein, we present a case of DTR to previously placed PCL filler after getting the Sinopharm vaccine against COVID‐19.
2. CASE PRESENTATION
A 62‐year‐old woman without any past medical history or allergies presented to our clinic complaining of multiple nodules with stone‐like firmness in the back of her hands. She had previously received PCL filler within the back of her both hands 2 years ago. She had never experienced any complications with her hand fillers during the past 2 years. Fourteen days after getting the second dose of Sinopharm vaccine, she noticed painless swelling in the dorsum of her hands without any other symptoms. After 2 weeks, she developed multiple hard nodules in the back of her both hands and no extra‐site symptom was noticed. Notably, she did not have any complications after receiving the first dose of Sinopharm vaccine.
After taking a thorough history from our patient, it was proved that she did not suffer from any infection or trauma in that area. Furthermore, she had not started a new medication and she had not undergone any dental or medical procedures between the time of vaccination and symptoms' manifestation.
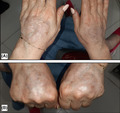
Examination revealed multiple nodules with stone‐like firmness in palpation located in the back of her hands (Figure 1). Dorsal hands soft tissue ultrasound imaging showed interstitial edema with low‐level echo fluid without any signs of collection.
FIGURE 1.
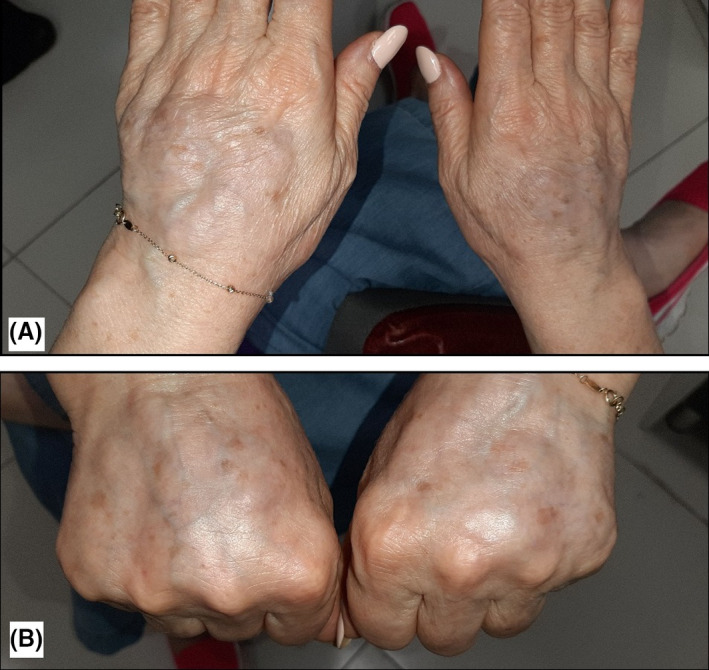
(A and B) Multiple nodules with stone‐like firmness in the back of our patient's both hands in the first visit
As a result, a DTR reaction following the second dose of COVID‐19 vaccination was made. Single dose of dexamethasone in addition to topical corticosteroid was prescribed. Gradual improvement of lesions was observed after using the topical corticosteroid. Intralesional triamcinolone injection in nodules resulted in a significant improvement. Our patient is still under treatment with topical steroids. During our 4 week follow‐up, no recurrence of the lesions was observed (Figure 2).
FIGURE 2.
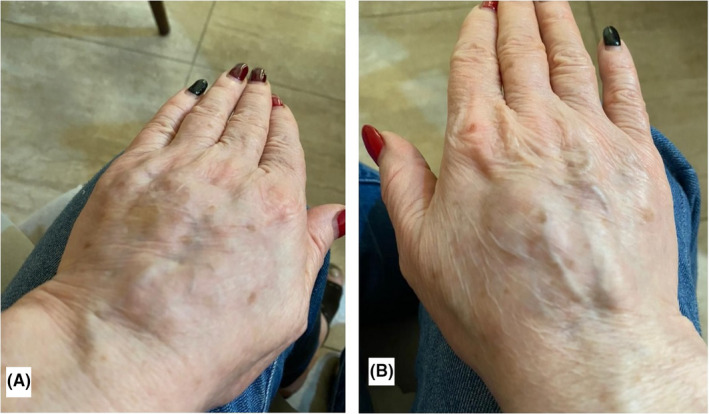
(A and B) Lesions improvement after 4 weeks of treatment
3. DISCUSSION
Recently, experts defined delayed inflammatory reactions (DIRs) as reactions occurring 2–4 weeks after injections. 6 The exact mechanisms for DTRs following the filler injection remain unclear, but various factors such as infections, filler properties, trauma, vaccinations, and injection technique are reported to be responsible. 5 PCL‐based fillers are collagen stimulators stimulating neocollagenesis providing an immediate and temporary filling effect in the target tissue. 4 In this article, we have provided the first reported case of DTR reaction to PCL filler following Sinopharm vaccination. As well, we reviewed the literature regarding the reported cases of DTR reactions after PCL placement. Furthermore, most reported cases of DTR to dermal fillers following COVID‐19 vaccinations are against HA fillers.
Polycaprolactone‐based fillers are safe and well‐tolerated choices for hand rejuvenation with minimal chance of migration. 7 In a study done by Figueiredo et al. on 5 women evaluating the efficacy and safety of a PCL‐based filler for dorsal hand rejuvenation using a visual analog scale, satisfaction was rated at 82% at 24 weeks, and patients were 88% likely to re‐treat the same procedure on average. 7
In a study done by Lin et al. reviewing 1111 treatments with PCL injections, the incidence rate of adverse effects was 4.5% such as bruising/hematoma, swelling, color change, or palpable lumps. 3 It is worth mentioning that no cases of nodule formation nor granulomas were reported in that study. There has long been a concern about collagen‐stimulating products causing nodules to develop and the injection technique is considered to be one of the most important factors in nodule formation. 3 In order to prevent nodule development, it has been recommended to use linear threading, fanning, and cross‐hatching techniques in the subcutaneous plane and to avoid injecting more than 0.2 mL. 3 Histopathology of a nodule shows an overabundance of product, as opposed to granulomas, which reflect an immune origin that depends on the immune status or specific incidents that make alterations in the immune status. 3 As well, cases of PCL‐induced granuloma have been reported in the literature.
In contrast to HA fillers which can be dissolved by injecting enzymes (hyaluronidase), PCL‐based fillers cannot be instantly removed with enzyme injections. Observation may be the best approach for treating filler‐induced nodules, because most nodules will disappear spontaneously within a year, and short‐term overcorrection might result in deformity. 8 In comparison, treatment for filler‐induced granulomas consists of observing, massaging, saline or water dilution, intralesional steroids injection, oral antibiotics administration, curettage, and lesion removal. 8 , 9 , 10 , 11 Moreover, methotrexate has been used in a case of eruptive granuloma following PCL filler placement. 10 In our case, topical and intralesional steroids were prescribed and a significant improvement of the lesions was observed.
In this article, we performed a brief review of the literature in the Pubmed/MedLine database to address complications including reported granulomas and nodules formation following PCL injection (Table 1). Unfortunately, we were not able to provide a pathologic and histologic assessment in our patient, which is considered as a limitation of our study.
TABLE 1.
Reported cases of PCL reactions presented as nodule or granuloma formations
| Reference number | First author and year of publication | Age and gender | Location | Clinical presentation and type of reaction | Interval between injection and reaction | Management | Outcome |
|---|---|---|---|---|---|---|---|
| 3 | Moon et al./2017 | 36/male | Cheek, nasolabial folds, infraorbital | Granuloma | 2 years | Doxycycline 100 mg twice daily for 1 month | Decreased lesions size |
| 9 | Skrzypek et al./2019 | 68/female | Marionette line | Granuloma | 13 months | No treatment | No follow‐up |
| 8 | Chiang et al./2021 | 57/female | Tear trough | Granuloma | 7 months | Excision | No recurrence |
| 10 | Philibert et al./2020 | 47/female | Cheeks and nasolabial folds | Eruptive granuloma | 9 months | Methotrexate, 10 mg per week for 3 months, then 20 mg per week for 9 months | Complete regression |
| 11 | Ortiz‐Álvarez et al. | 74/female | Nasolabial folds and over both zygomatic arches | Nodules which were then diagnosed with systemic sarcoidosis | 3 months | Methotrexate (20 mg subcutaneous weekly) with prednisone (0.17 mg/kg/day) | Significant improvement |
4. CONCLUSION
Cases of filler reactions after COVID‐19 vaccination have been reported. Notably, most of them were HA‐based fillers reaction to Moderna and Pfizer COVID‐19 vaccines that may well be because HA fillers are by far the most frequently used ones, and the Moderna and Pfizer COVID‐19 vaccines are also most frequently used. 13 It is worth mentioning that the authors of this manuscript have broadly worked on COVID‐19 and skin as well as cosmetic fields. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 To the best of our knowledge, this is the first reported case of nodule formation due to the PCL reaction following Sinopharm COVID‐19 vaccination and further investigations with longer follow‐ups are recommended. Our case highlights the importance of reporting similar cases in order to better understand this phenomenon and its management. Of note, individuals and physician should be aware of such reactions in practice.
CONFLICTS OF INTERESTS
None to declare.
AUTHOR CONTRIBUTION
All authors contributed to the preparation and finalization of this article. YK and AG contributed to writing the article and study design. ZA and PH contributed to literature review. AS contributed to final editing.
ETHICAL APPROVAL
This study was approved by the Medical Ethics Committee of the Rasool Akram Medical Complex.
CONSENT
Written and oral informed consent was obtained from this patient.
ACKNOWLEDGEMENTS
The authors would like to thank the Autoimmune Bullous Diseases Research Center, Tehran University of Medical Sciences, Tehran, Iran for its technical and editorial assists. The authors would like to express their gratitude to the staff of the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC) for their technical and editorial assistance.
Kalantari Y, Sadeghzadeh‐Bazargan A, Aryanian Z, Hatami P, Goodarzi A. First reported case of delayed‐type hypersensitivity reaction to non‐hyaluronic acid Polycaprolactone dermal filler following COVID‐19 vaccination: A case report and a review of the literature. Clin Case Rep. 2022;10:e05343. doi: 10.1002/ccr3.5343
Funding information
We received no funding for this project
DATA AVAILABILITY STATEMENT
All data used to support the findings of the study are included within the articles.
REFERENCES
- 1. Dadkhahfar S, Gheisari M, Kalantari Y, Zahedi K, Ehsani A, Etesami I. Motivations and characteristics of patients seeking minimally invasive cosmetic procedures in two Iranian dermatology centers: a cross‐sectional study. Int J Women’s Dermatol. 2021;7(5):737‐742. doi: 10.1016/j.ijwd.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sánchez‐Carpintero I, Candelas D, Ruiz‐Rodríguez R. Materiales de relleno: tipos, indicaciones y complicaciones [Dermal fillers: types, indications, and complications]. Actas Dermosifiliogr. 2010;101(5):381‐393. Spanish. doi: 10.1016/s1578-2190(10)70660-0 [DOI] [PubMed] [Google Scholar]
- 3. Moon SY, Eun DH, Park JH, et al. Foreign body reaction three years after injection with polycaprolactone (Ellanse®). Eur J Dermatol. 2017;27(5):549‐551. doi: 10.1684/ejd.2017.3089 [DOI] [PubMed] [Google Scholar]
- 4. Lin SL, Christen MO. Polycaprolactone‐based dermal filer complications: a retrospective study of 1111 treatments. J Cosmet Dermatol. 2020;19(8):1907‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rowland‐Warmann MJ. Hypersensitivity reaction to hyaluronic acid dermal filler following novel Coronavirus infection ‐ a case report. J Cosmet Dermatol. 2021;20(5):1557‐1562. doi: 10.1111/jocd.14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michon A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID‐19 vaccination ‐ A case report. J Cosmet Dermatol. 2021;20(9):2684‐2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Figueiredo VM. A five‐patient prospective pilot study of a polycaprolactone based dermal filler for hand rejuvenation. J Cosmet Dermatol. 2013;12(1):73‐77. doi: 10.1111/jocd.12020 [DOI] [PubMed] [Google Scholar]
- 8. Chiang CH, Peng JH, Peng HP. Filler‐induced granuloma from polycaprolactone‐based collagen stimulator injection in the tear trough area: a case report. J Cosmet Dermatol. 2021;20(5):1529‐1531. doi: 10.1111/jocd.14010 [DOI] [PubMed] [Google Scholar]
- 9. Skrzypek E, Górnicka B, Skrzypek DM, Krzysztof MR. Granuloma as a complication of polycaprolactone‐based dermal filler injection: ultrasound and histopathology studies. J Cosmet Laser Ther. 2019;21(2):65‐68. doi: 10.1080/14764172.2018.1461229 [DOI] [PubMed] [Google Scholar]
- 10. Philibert F, Gras‐Champel V, Chaby G, et al. Granulomes après injection d’Ellansé®, résolutifs sous méthotrexate [Eruptive granuloma after injection of Ellansé® successfully treated using methotrexate]. Ann Dermatol Venereol. 2020;147(8‐9):525‐529. French. doi: 10.1016/j.annder.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 11. Ortiz‐Álvarez J, Lebrón‐Martín JA, Rodríguez Fernández‐Freire L, Zulueta Dorado T, García Morillo JS. Cutaneous and ganglion sarcoidosis induced by polycaprolactone facial filler: a new expression of ASIA syndrome? Eur J Case Rep Intern Med. 2021;8(7):002652. doi: 10.12890/2021_002652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savva D, Battineni G, Amenta F, Nittari G. Hypersensitivity reaction to hyaluronic acid dermal filler after the Pfizer vaccination against SARS‐CoV‐2. Int J Infect Dis. 2021;113:233‐235. doi: 10.1016/j.ijid.2021.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michon A. Hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID‐19 vaccination ‐ A case report. J Cosmet Dermatol. 2021;20(9):2684‐2690. doi: 10.1111/jocd.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lajevardi V, Mahmoudi H, Karimi F, Kalantari Y, Etesami I. Comparing QS Nd:YAG laser alone with its combination with fractional ablative Er:YAG in tattoo removal. J Cosmet Dermatol. 2021;20(12):4078‐4080. doi: 10.1111/jocd.14076 [DOI] [PubMed] [Google Scholar]
- 15. Atefi N, Behrangi E, Mozafarpoor S, Seirafianpour F, Peighambari S, Goodarzi A. N‐acetylcysteine and coronavirus disease 2019: may it work as a beneficial preventive and adjuvant therapy? A comprehensive review study. J Res Med Sci. 2020;25:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Cytokine storm and probable role of immunoregulatory drugs in COVID‐19: a comprehensive review. Iran J Dermatol. 2020;23(Suppl. 1 (COVID‐19)):13‐18. [Google Scholar]
- 17. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Systemic retinoids in the COVID‐19 era–are they helpful, safe, or harmful? a comprehensive systematized review. Iran J Dermatol. 2020;23(Suppl. 1 (COVID‐19)):9‐12. [Google Scholar]
- 18. Sadeghzadeh‐Bazargan A, Rezai M, Nobari NN, Mozafarpoor S, Goodarzi A. Skin manifestations as potential symptoms of diffuse vascular injury in critical COVID‐19 patients. J Cutan Pathol. 2021;48(10):1266‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohamadi M, Fattahi N, Goodarzi A, et al. A comprehensive review on COVID‐19 infection and comorbidities of various organs. Acta Medica Iranica. 2021;14:4‐14. [Google Scholar]
- 20. Nobari NN, Montazer F, Seirafianpour F, Goodarzi A. Histopathologic changes and cellular events of organs systems in COVID‐19. J Cell Mol Anesth. 2021;6(1):81‐88. [Google Scholar]
- 21. Kooranifar S, Sadeghipour A, Riahi T, Goodarzi A, Tabrizi S, Davoody N. Histopathologic survey on lung necropsy specimens of 15 patients who died from COVID‐19: a large study from Iran with a high rate of anthracosis. Med J Islam Repub Iran. 2021;35(1):481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Najar Nobari N, Seirafianpour F, Dodangeh M, et al. A systematic review of the histopathologic survey on skin biopsies in patients with corona virus disease 2019 (COVID‐19) who developed virus or drug‐related mucocutaneous manifestations. Exp Dermatol. 2021;30(9):1233‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohamadi MM, Goodarzi A, Aryannejad A, et al. Geriatric challenges in the new coronavirus disease‐19 (COVID‐19) pandemic: a systematic review. Med J Islam Repub Iran. 2020;34(1):841‐848. doi: 10.47176/mjiri.34.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seirafianpour F, Mozafarpoor S, Fattahi N, et al. Treatment of COVID‐19 with pentoxifylline: could it be a potential adjuvant therapy? Dermatol Ther. 2020;33(4):e13733. doi: 10.1111/dth.13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seirafianpour F, Sodagar S, Pour Mohammad A, et al. Cutaneous manifestations and considerations in COVID‐19 pandemic: a systematic review. Dermatol Ther. 2020;33(6):e13986. doi: 10.1111/dth.13986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Najar Nobari N, Goodarzi A. Patients with specific skin disorders who are affected by COVID‐19: what do experiences say about management strategies? A systematic review. Dermatol Ther. 33(6):e13733. doi: 10.1111/dth.13986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Najar Nobari N, Seirafianpour F, Mashayekhi F, Goodarzi A. systematic review on treatment‐related mucocutaneous reactions in COVID‐19patients. Dermatol Ther. 2020;34(1). doi: 10.1111/dth.14662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalantari S, Sadeghzadeh‐Bazargan A, Ebrahimi S, et al. The effect of influenza vaccine on severity of COVID‐19 infection: an original study from Iran. Med J Islam Repub Iran. 35:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tavakolpour S, Aryanian Z, Seirafianpour F, et al. A systematic review on efficacy, safety, and treatment‐durability of low‐dose rituximab for the treatment of Pemphigus: special focus on COVID‐19 pandemic concerns. Immunopharmacology and Immunotoxicology. 2021;43(5):507‐518. doi: 10.1080/08923973.2021.1953063 [DOI] [PubMed] [Google Scholar]
- 30. Mashayekhi F, Seirafianpour F, Pour Mohammad A, Goodarzi A. Severe and life‐threatening COVID‐19‐related mucocutaneous eruptions: a systematic review. Int J Clin Pract. 2021;75(12):e14720. doi: 10.1111/ijcp.14720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lotfi Z, Haghighi A, Akbarzadehpasha A, Mozafarpoor S, Goodarzi A. Pansclerotic morphea following COVID‐19: a case report and review of literature on rheumatologic and non‐rheumatologic dermatologic immune‐mediated disorders induced by SARS‐CoV‐2. Front Med. 2021;8:728411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Behrangi E, Ghassemi M, Sadeghzadeh‐Bazargan A, Roohaninasab M, Najar Nobari N, Goodarzi A. A systematic review of clinical studies on mucocutaneous manifestations of COVID‐19: virus‐related and drug‐related. Acta Med Iran. 2021;59(12):687‐698. [Google Scholar]
- 33. Hatami P, Aryanian Z, Nicknam Asl H, Goodarzi A. Mucocutaneous adverse effects following COVID‐19 vaccination: a comprehensive review of the literature with a presentation of some cases from Iran. Iran J Dermatol. 2021;24(4). doi: 10.22034/IJD.2021.311094.1451 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of the study are included within the articles.


