Abstract
A serotyping scheme based on heat-stable surface antigens was established for 101 Campylobacter upsaliensis and 10 Campylobacter helveticus strains isolated from 261 dogs and 46 cats of different ages originating from two geographically distinct regions in Germany. The prevalence of C. upsaliensis varied between 27.8% in juvenile dogs (<12 months of age) and 55.4% in adult dogs (P < 0.05). Of the cats, 19.6% harbored C. upsaliensis, whereas 21.7% carried C. helveticus. Of the C. upsaliensis isolates from both host species, 93.1% belonged to five different serogroups, two of them being prevalent at rates of 47.5 and 27.7%, with different frequencies in both regions. Six (54.6%) of the C. helveticus isolates also belonged to serotypes found among C. upsaliensis strains, whereas five (45.4%) possessed an O antigen unique for C. helveticus. In contrast, a considerable degree of genomic diversity of the isolates was assessed by macrorestriction analyses with the endonucleases SmaI and XhoI, using pulsed-field gel electrophoresis as well as enterobacterial repetitive intergenic consensus sequence PCR (ERIC PCR). Restriction with SmaI pointed towards the existence of clonal groups associated to some extent with serotypes, while restriction with XhoI disintegrated these groups to smaller noncoherent subgroups. Analysis of ERIC PCR profiles did not exhibit any associations with serotypes. In conclusion these data demonstrate the genomic heterogeneity among C. upsaliensis strains and indicate that the combination of SmaI restriction with serotyping is a useful tool to investigate the expansion of clonal groups of C. upsaliensis.
Campylobacter upsaliensis, a catalase-negative or weakly positive thermotolerant Campylobacter species, was first isolated in 1983 from fecal samples of healthy and diarrheic dogs in Sweden (48). In cats this microorganism was identified a few years later, in 1989 (9). C. upsaliensis is also sporadically isolated from procedures done on humans or from human diseases, like abortion (16), bacteremia (39), abscess (10), gastroenteritis (11, 12, 13, 26, 52), and opportunistic infections in immunocompromised hosts (23, 43). However, its genetic characteristics and its possible pathogenic capacity for humans and small animals are far from well defined (5). According to some risk analyses, living with a dog or cat as companion is a considerable risk factor for men (14). Carrier rates of dogs and cats for C. upsaliensis between 5 and 66.2% have been reported by other investigators from different countries (3, 6, 7, 17, 30, 34, 48), with significant correlation between Campylobacter shedding and age in young diarrheic dogs reported by Burnens et al. (6). To gain further knowledge on the prevalence of this enteric pathogen in pets in Germany, its association to enteric disease, and its genomic diversity, C. upsaliensis was isolated from dogs and cats living in two distinct geographic areas in Germany over a period of approximately 1 year (November 1997 to January 1999). Fecal specimens of 261 dogs and 46 cats of different ages (healthy or suffering from enteric disease or other illness) were presented to two veterinary hospitals and were collected. The two areas of investigation were chosen by their characteristics as metropolitan (Berlin) and a rather provincial place 400 km away (situated in North Rhine-Westphalia [NRW]). With regard to previous studies on the closely related species Campylobacter helveticus occurring in cats, which was described for the first time in 1992 (49), this species was included in our investigation.
Genotypic (molecular) methods have successfully been applied to accomplish phenotypic approaches for subtyping Campylobacter species (44). Previous reports using pulsed-field gel electrophoresis (PFGE) to characterize bacteria, including Campylobacter jejuni (40, 59), C. upsaliensis (4), and Campylobacter hyointestinalis (47), on the genomic level demonstrated the high discriminatory power of this method and its usefulness for epidemiological studies. Flagellin gene polymorphism (32, 33, 38, 50) or the combination of macrorestriction analyis and serotyping according to heat-stable or heat-labile antigens has also been performed to discriminate C. jejuni and Campylobacter coli strains from each other (40, 50). In the present study C. upsaliensis isolates were characterized with respect to their species by biochemical tests confirmed by PCR. Their heat-stable antigens were determined using indirect hemagglutination (45) for the first time, to our knowledge, and the degree of their genotypic similarities was assessed by analyzing macrorestriction patterns and enterobacterial repetitive intergenic consensus sequence PCR (ERIC PCR) profiles (53).
The aim of this study was to extend knowledge of the relationship between genomic and O-antigenic diversity of the species C. upsaliensis and of the mode of expansion of this potential human enteric pathogen carried by animal hosts, especially dogs.
MATERIALS AND METHODS
Collection and cultivation of bacteria.
Rectal swabs were collected from 261 randomly selected dogs and 46 cats of different ages with and without gastroenteritic symptoms that were presented to two veterinary hospitals. The specimens were collected using Culturettes (Becton Dickinson) and transferred onto selective media within 24 h after collection. As selective media, cefoperazon-amphotericin β-teichoplanin agar (2), modified Campylobacter charcoal differential agar (21), and charcoal-based selective medium (24) were used. The plate contents were incubated for 48 to 72 h under microaerophilic conditions (5%O2, 10% CO2, and 85% N2) at 38°C.
Further cultivation was performed on Mueller-Hinton agar plates containing 5% defibrinated sheep blood (MHB). The bacteria were stored as stock cultures in thioglycolate broth containing 15% glycerol at −70°C.
Species determination.
The species of C. upsaliensis and C. helveticus isolates were determined biochemically according to the literature (20, 22, 35, 36) using the following criteria: gram negativity, spiral shape of rods, requirement of microaerophilic growth conditions, cytochrome oxidase activity, weak or no catalase activity, capacity to reduce selenite, and sensitivity to nalidixic acid and to cephalotin. The results were confirmed using species-specific PCR established for C. upsaliensis (8) and C. helveticus (27).
Indirect hemagglutination.
Sheep erythrocytes were sensitized with bacterial antigen extracted by heat (45). Briefly, bacteria harvested from MHB agar plates were suspended in 150 mM NaCl, adjusted to an optical density at 600 nm (OD600) of 1, and heated at 100°C for 1 h. After centrifugation at 6,000 × g, the antigen-containing supernatant was collected and sheep erythrocytes that had been washed with 150 mM NaCl were added to the supernatant: 5 μl of erythrocyte sediment was added to 1 ml of antigen solution, resulting in an OD540 of 3.2 to 3.4.
The erythrocyte-antigen mix was incubated at 37°C for 2 h, washed three times with 150 mM NaCl, and resuspended with the original volume of 150 mM NaCl.
The hemagglutination was performed with rabbit antisera elicited as described previously (31) against formaldehyde-inactivated bacteria of C. jejuni reference strains O:1, O:2, O:4, O:9, O:13, O:16, and O:43 (Penner); C. jejuni wild-type strains O:37 and O:40; two C. coli wild-type strains (O:20 and one that was not typeable); one Campylobacter lari strain; four wild-type C. upsaliensis strains; and one C. helveticus wild-type strain (Table 1). The C. jejuni reference strains were purchased from the Culture Collection of the University of Göteborg (CCUG), and the C. jejuni and C. coli wild-type strains were serotyped at the same place. The C. upsaliensis and C. helveticus strains were not typeable, because an O-antigen serotyping scheme did not exist for them.
TABLE 1.
Bacterial strains used for antiserum preparation
| Bacterial strain | Species | Serotype | Origin; characteristics |
|---|---|---|---|
| 10935 | C. jejuni | O:1 | CCUG; reference strain |
| 10936 | C. jejuni | O:2 | CCUG; reference strain |
| 10938 | C. jejuni | O:4 | CCUG; reference strain |
| 10945 | C. jejuni | O:13 | CCUG; reference strain |
| C. jejuni | O:16 | CCUG; reference strain | |
| C. jejuni | O:43 | CCUG; reference strain | |
| L2H | C. jejuni | O:37 | Germany; wild-type strain; species origin unknown |
| 1834 | C. jejuni | O:40 | Germany; wild-type strain; human origin |
| TU 429 | C. coli | O:20 | Pig |
| TU 440 | C. coli | O:n.t.a | Pig |
| C. lari 68 | C. lari | Chicken | |
| Ulrike 3 | C. upsaliensis | Berlin; dog | |
| H5 | C. upsaliensis | Berlin; dog | |
| H16 | C. upsaliensis | Berlin; dog | |
| H29 | C. upsaliensis | Berlin; dog | |
| K1E | C. helveticus | NRW; cat |
n.t., not typeable.
The antisera were complement inactivated (30 min, 56°C) and absorbed with washed sheep red blood cells. For hemagglutination the sera were diluted serially in microtiter plates, using 150 mM NaCl (25 μl); the same volume of sensitized erythrocyte suspension was added and incubated for 2 h at 37°C. The titers were determined macroscopically. Serum titers of 1:80 and less were neglected. In Table 1 the bacterial strains used for antiserum production are listed.
DNA preparation, DNA primers, and PCR amplification.
For PCR amplification DNA was extracted by heat. Briefly, bacteria suspended in 150 mM NaCl were adjusted to an OD600 of 1.5 and were diluted 1:4 in H2O before heating at 100°C for 5 min. The supernatant collected after centrifugation (5,700 × g) was used as template DNA solution.
The amplification reaction was performed using Ready-to-Go PCR Beads (Amersham Pharmacia Biotech, Freiburg, Germany) in a volume of 25 μl, containing finally 1 μl of DNA, 1.5 U of Taq polymerase, 10 mmol liter−1 Tris-HCl (pH 9.0), 50 mmol liter−1 KCl, 1.5 mmol liter−1 MgCl2, 200 μmol liter−1 concentrations of each dNTP, and 5 μM concentrations of each primer. Primer sequences were deduced from 23S rRNA genes for thermophilic Campylobacter species (8) and C. upsaliensis (8) and from 16S rRNA genes for C. upsaliensis and C. helveticus (27). Primers were synthesized by Amersham Pharmacia Biotech (now MWG Biotech, Ebersberg, Germany). Samples were subjected to 27 cycles of amplification in a DNA thermal cycler (Biometra, Göttingen, Germany) under conditions described by Eyers et al. (8) and Linton et al. (27) with slight modifications. Each cycle consisted of 2 min at 94°C, 1 min at 94°C, 1 min at 52 to 54°C, and 1 min at 74°C. Final elongation was performed at 74°C for 180 s. Amplified samples were analyzed by electrophoresis on 1.2% agarose gels and were visualized by ethidium bromide staining under UV light. PCR product sequence analysis was performed by Gessellschaft für Molekularbiologische Technologie, (Berlin, Germany).
The ERIC PCR was performed in a volume of 25 μl in 0.5-ml tubes with oil overlaying. The PCR mix consisted of 10 μl of bacterial genomic DNA extracted by heat, 2.5 μl of 10× PCR buffer, 3 mM MgCl2, a 25 mM concentration of each dNTP, and 50 pmol of each primer (ERIC I/ERIC II) according to Versalowic et al. (53). Samples were subjected to 95°C for 7 min followed by 30 cycles of amplification in a DNA thermal cycler (model 480; Perkin-Elmer) using the following conditions: 94°C, 1 min; 52°C, 1 min; and 72°C, 8 min. PCR products were separated by electrophoresis using precast gels [6% Poly(Nat), Elchrom Scientific AG, Weiterstadt, Germany] in a special electrophoresis apparatus with buffer recirculation (SEA 2000; Elchrom Scientific AG) at 10°C and were stained with ethidium bromide. Gels were photographed with a digital camera system (Herolab, Wiesloch, Germany). The electrophoretic patterns were analyzed using Gelcompar 4.1 Software (Applied Maths BVBA, Kortrijk, Begium; Herolab). Genetic similarities between isolates were calculated using the Ward algorithm and the Pearson correlation coefficient (for details see the Gelcompar 4.1 manual).
Macrorestriction analysis using PFGE.
Bacteria grown on MHB agar plates for 48 h were harvested in 150 mM NaCl. The suspension was adjusted photometrically to an OD600 of 1.2. Agar plugs were prepared by adding 500 μl of bacterial suspension to 700 μl of 1.2% agarose DNA-grade gels (Life Technologies, Kartsruhe, Germany), mixing thoroughly, and filling 100 μl into the plug mould. The solidified agarose plugs were incubated in ESP lysis buffer (0.5 mM EDTA, pH 9.5; 1% [wt/vol)] N-lauroyl sarcosine; and 1.8 mg of proteinase K [Roche Diagnostics, Mannheim, Germany]/ml) for 36 h at 56°C. To prepare samples for restriction endonuclease digestion, the plugs were cut into three pieces of equal size and were washed extensively in Tris-EDTA buffer (10 mM Tris and 10 mM EDTA, pH 7.5) at 4°C. Then the plug pieces were equilibrated twice with the appropriate digestion buffer at room temperature for 30 min and were incubated in 150-μl digestion buffer containing 20 U of the restriction endonuclease XhoI or SmaI overnight at temperatures recommended by the manufacturer (Roche Diagnostics). Electrophoretic separation of the DNA fragments in 1.2% (wt/vol) agarose gels was performed in a contour-clamped homogeneous electric field (CHEF DR III; Bio-Rad, Munich, Germany) apparatus under the following conditions: 6 V cm−1, pulse times ramping from 0.3 to 12 s for both enzymes, electrode angle of 120°, and a temperature of 15°C for 24 h. The running buffer contained 0.5× Tris-borate-EDTA (44.5 mM Tris; 44.5 mM boric acid; 1 mM EDTA, pH 8.0). As reference, DNA of the C. upsaliensis strain DSM 5365 digested with SmaI or XhoI was run on each gel. Finally gels were stained with ethidium bromide, viewed under UV light, and documented on Polaroid films. Photographs were scanned, and similarities between profiles, based on band positions, were calculated with Gelcompar 4.1 software (Applied Maths BVBA) using the Ward algorithm and the Dice coefficient (for details, see Gelcompar 4.1 manual) with a maximum tolerance of 1.8% and optimization of 0.5% for both enzymes. Using these parameters, the reproducibility of band profiles generated from eight duplicate strains restricted with SmaI was ≥95.5%; that of six duplicate strains restricted with XhoI was ≥95.8%. As molecular weight standard, λl concatemers (molecular size, 48.5 kb; Roche Diagnostics) were run on three lanes (both edges and the middle) of each gel.
Statistical calculations.
In spite of the fact that the mode of sample collection in a nonrandom manner does not permit calculations of true statistical significance, the differences of prevalence values were assessed by calculating 95 and 99% confidential intervals.
RESULTS
Prevalence of C. upsaliensis
From dogs in Berlin, 135 swabs were taken; 126 swabs were taken from dogs in NRW, near the cities Bielefeld and Paderborn. A total of 109 Campylobacter strains were isolated, 51 strains (37.8%) in Berlin and 58 (46.0%) in NRW. The overall prevalence of Campylobacter in juvenile dogs (<12 months of age) was 75.0%, compared to 32.7% in adult dogs from both geographic regions (P < 0.01) (Table 2). Substantial differences between dogs with and without symptoms of enteritis could not be detected.
TABLE 2.
Prevalence of Campylobacter with emphasis on C. upsaliensis in dogs and cats in Berlin and NRW, respectively
| Host species/geographic origin (illness of host) | Age (no.) | Total no. of animals (n = 307) | Results for investigated samples
|
||
|---|---|---|---|---|---|
| No. Campylobacter positive (%) (n = 131) | No. C. upsaliensis positive (%) (n = 97) | No. C. helveticus positive (%) (n = 11) | |||
| Dog/Berlin (enteritis) | Adult (35) | 50 | 14 (40.0) | 13 (37.1) | 1 |
| Juvenile (15) | 11 (73.3) | 7 (46.6) | |||
| Total | 25 (50.0) | 20 (40.0) | 1 | ||
| Dog/Berlin (without enteritis) | Adult (74) | 85 | 19 (25.7) | 18 (24.3) | |
| Juvenile (11) | 7 (63.6) | 5 (45.5) | |||
| Total | 26 (30.6) | 23 (27.1) | 0 | ||
| Dog/NRW (enteritis) | Adult (9) | 14 | 4 (44.4) | 2 (22.2) | |
| Juvenile (5) | 3 (60.0) | 2 (40.0) | |||
| Total | 7 (50.0) | 4 (28.6) | 0 | ||
| Dog/NRW (without enteritis) | Adult (87) | 112 | 30 (34.5) | 24 (27.6) | |
| Juvenile (25) | 21 (84.0) | 17 (68.0) | |||
| Total | 51 (45.5) | 41 (36.6) | 0 | ||
| Cat/Berlin (enteritis) | Adult (8) | 12 | 3 (37.5) | 1 (12.5) | 1 |
| Juvenile (4) | 2 (50.0) | ||||
| Total | 5 (41.6) | 1 (8.3) | 1 | ||
| Cat/Berlin (without enteritis) | Adult (7) | 9 | 2 (28.6) | ||
| Juvenile (2) | 2 (100.0) | 2 (100.0) | 2 | ||
| Total | 4 (44.4) | 2 (22.2) | 2 | ||
| Cat/NRW (enteritis) | Adult (1) | 1 | |||
| Juvenile (0) | |||||
| Total | 0 | 0 | 0 | ||
| Cat/NRW (without enteritis) | Adult (15) | 24 | 9 (60.0) | 4 (26.7) | 6 |
| Juvenile (9) | 4 (44.4) | 2 (22.2) | 1 | ||
| Total | 13 (54.2) | 6 (25.0) | 7 | ||
Of 109 canine Campylobacter isolates, 88 belonged to the species C. upsaliensis (80.7%). Only negligible differencies in frequency of isolation were detected between the two respective regions (84.3% [Berlin] and 77.6% [NRW]). The prevalence of C. upsaliensis among all dogs included in the investigation varied between 55.4% in juvenile and 27.8% in adult animals (P < 0.05).
Of the cats, 47.8% (22 of 46) were Campylobacter positive, 40.9% (9 of 22) of which harbored the species C. upsaliensis. However, 45.5% (10 of 22) of the Campylobacter-positive cats harbored C. helveticus, whereas this species was identified only in one dog (data not shown).
Determination of O antigens.
From 88 dogs and 9 cats, 92 canine and 9 feline C. upsaliensis strains were isolated. The strains were subjected to serotyping. Ninety-four of these 101 strains (93.1%) were typed according to their O antigens by using five antisera, four of them directed against C. upsaliensis strains and one against a C. helveticus strain as listed in Table 1. The positively reacting strains belonged to five different serotypes (preliminarily named O I to O IV and O VI), two of them being prevalent at 47.5% (O III) and 27.7% (O IV). The serotypes O I, O II, and O VI were identified at 8.9, 5.9, and 3.0%, respectively (Table 3). Serotype O II was characterized by a strong reaction with the serotype O I-recognizing antiserum, combined with a weak reaction (1:80) with the C. jejuni O:2 (Penner)-recognizing antiserum. Serotype O V was not detected among the C. upsaliensis strains tested. Of the C. upsaliensis isolates, 6.9% did not react with any antiserum. Beyond the weak reaction of serotype O II with C. jejuni O:2-recognizing antiserum, the reactivity of C. upsaliensis or C. helveticus O antigens with antisera recognizing C. jejuni O:1, O:4, O:9, O:13, O:16, O:37, and O:43, two C. coli antisera (O:20 and a non-cross-reacting strain), and one C. lari antiserum (Table 1) was not detected.
TABLE 3.
O-antigen specificities among canine and feline C. upsaliensis and C. helveticus strains in Berlin and NRW, respectively
| Host/geographic origin | No. of C. upsaliensis or C. helveticus isolatesa | No. of samples yielding each O antigen group (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | n.t.b | ||
| Dog/Berlin | 43 | 4 (9.3) | 3 (7.0) | 14 (32.6) | 12 (27.9) | 0 | 3 (7.0) | 7 (16.3) |
| Dog/NRW | 49 | 5 (10.2) | 3 (6.1) | 30 (61.2) | 11 (22.4) | 0 | 0 | 0 |
| Dog/Berlin and NRW | 92 | 9 (9.8) | 6 (6.5) | 44 (47.8) | 23 (25.0) | 0 | 3 (3.3) | 7 (7.6) |
| Cat/Berlin | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Cat/NRW | 6 | 0 | 0 | 4 | 2 | 0 | 0 | 0 |
| Cat/Berlin and NRW | 9 | 0 | 0 | 4 | 5 | 0 | 0 | 0 |
| Dog and cat/Berlin | 46 | 4 (8.7) | 3 (6.5) | 14 (30.4) | 15 (32.6) | 0 | 3 (6.5) | 7 (15.2) |
| Dog and cat/NRW | 55 | 5 (9.1) | 3 (5.5) | 34 (61.8) | 13 (23.6) | 0 | 0 | 0 |
| Dog and cat/Berlin and NRW | 101 | 9 (8.9) | 6 (5.9) | 48 (47.5) | 28 (27.7) | 0 | 3 (3.0) | 7 (6.9) |
| Dog and cat/Berlin and NRW | 11 | 0 | 3 (27.3) | 3 (27.3) | 0 | 5 (45.4) | 0 | 0 |
Only the bottom row gives C. helveticus results.
n.t., not typeable.
In contrast to C. upsaliensis, the total number of 11 C. helveticus strains could be characterized with respect to their O antigens. Six isolates belonged to the serotypes O II and O III identified among C. upsaliensis, whereas the serotypes O I, O IV, and O VI could not be identified. Five C. helveticus isolates possessed an O antigen (V) which seemed to be unique to C. helveticus.
Restriction fragment length polymorphism (RFLP).
From 101 C. upsaliensis and 11 C. helveticus strains differentiated by serotyping (see above), 31 strains yielded repeatedly unsatisfactory weak pulsed-field gel electropherograms and were omitted from further investigation. Therefore, 80 strains were subjected to RFLP analysis, 76 belonging to the species C. upsaliensis and 4 to C. helveticus. Approximately 5% of these strains possessed a very small number of recognition sites for the endonuclease SmaI or XhoI, yielding fewer than five bands. For example, the C. helveticus isolates were not digested by SmaI, whereas all of them possessed recognition sites for XhoI. These strains were not omitted from further analysis.
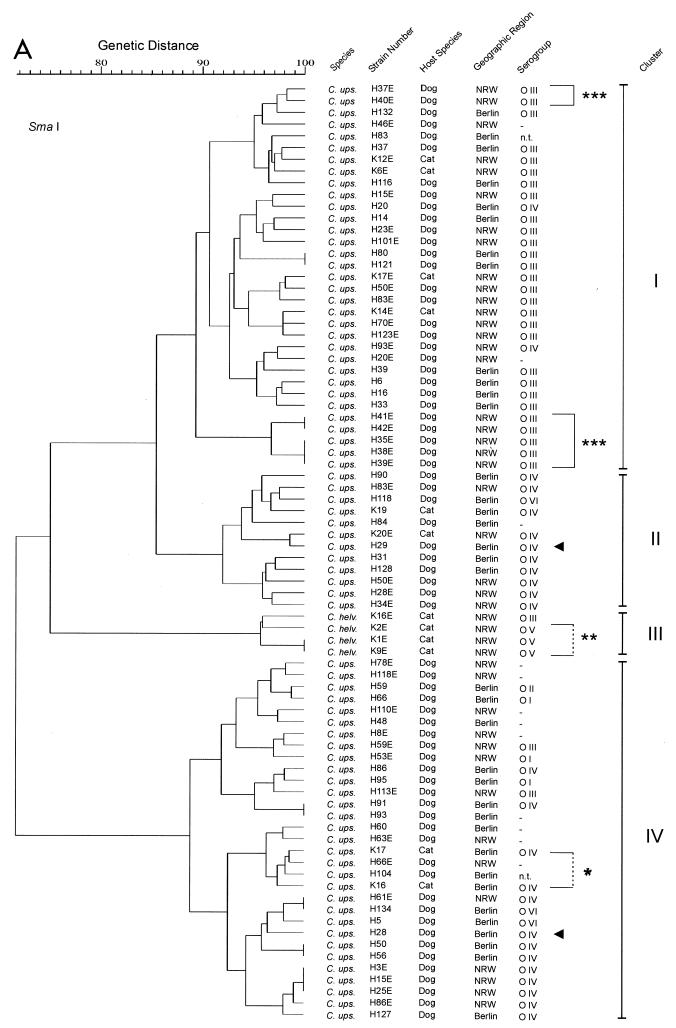
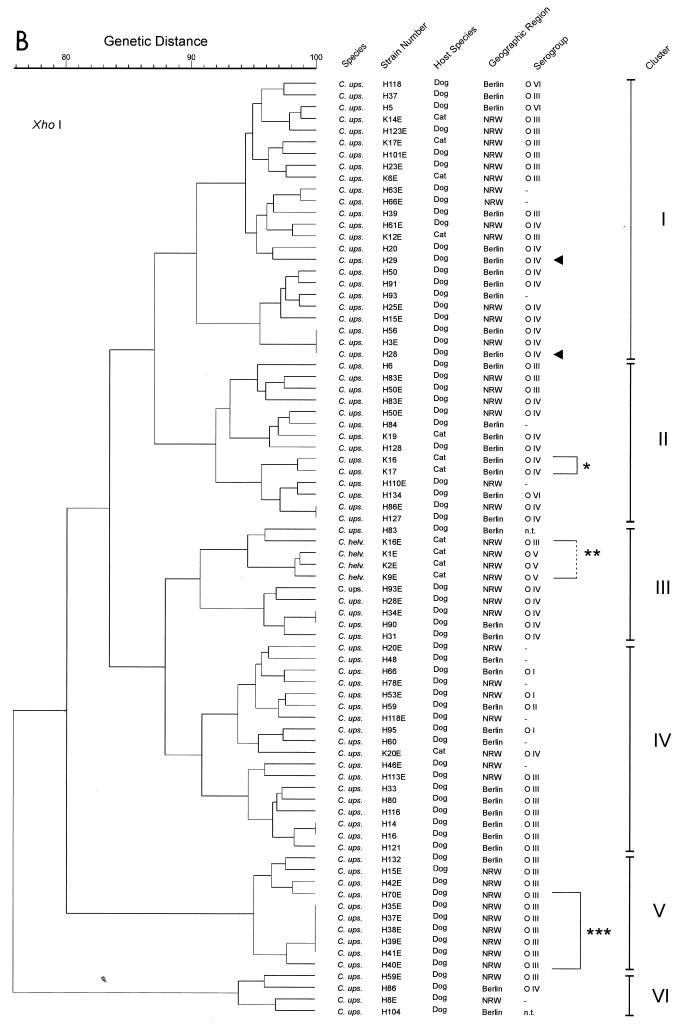
RFLP analyses with the endonucleases SmaI (recognition site, CCC↓GGG) and XhoI (recognition site, C↓TCGAG) (downward-pointing arrows indicate cutting sites) revealed a considerable degree of genomic heterogeneity among the C. upsaliensis strains, as shown in the dendrograms of genotypic similarities (Fig. 1A and B). In general, the isolates possessed a number of recognition sites for SmaI and XhoI, yielding between 10 and 20 DNA bands for either enzyme (molecular mass, up to approximately 450 kb for SmaI and 250 kb for XhoI). Almost every isolate exhibited its unique restriction pattern. Only in six cases did isolates from unrelated hosts exhibit restriction patterns identical to those of one or two other strains when digested with SmaI and in four cases when digested with XhoI (Fig. 1A and B). However, none of these pairs or groups of strains yielded identical patterns with both of the enzymes. The C. upsaliensis strains H35E, H37E, H38E, H39E, H40E, H41E, and H42E (Fig. 1, brackets and three asterisks) were collected from 8-week-old puppies belonging to one litter. Similarly, K16 and K17 (Fig. 1, bracket and asterisk) were isolated from two kittens of one litter. In contrast, H28 and H29 (Fig. 1, arrows) were isolated from unrelated dogs living together in one household. From four C. helveticus isolates (Fig. 1, bracket and two asterisks) grouped in one cluster within the dendrograms, three strains (serogroup O V) originated from cats living together in one family. Based on the similarities of SmaI-generated band profiles of ≥89%, four clusters of strains could be determined to exhibit associations to serotypes. Cluster I consisted mainly of serotype O III-positive strains. In cluster II mainly O IV-positive strains were assembled. Cluster III contained the C. helveticus isolates (O III and O V). Cluster IV was the most heterogeneous cluster, containing untypeable strains at a rate of 29%.
FIG. 1.
(A) Genotypic similarities based on macrorestriction profiles, of C. upsaliensis and C. helveticus strains isolated from dogs and cats in Berlin and NRW, that were generated by the restriction endonuclease SmaI. (B) Genotypic similarities based on macrorestriction profiles, of C. upsaliensis and C. helveticus strains isolated from dogs and cats in Berlin and NRW, that were generated by the restriction endonuclease XhoI. Where no serogorup is given (dash), the strain was tested, but not typeable (negative) with the available antisera. The single and triple asterisks indicate respective littermates, and the double asterisks indicate strains from animals living in one household but not genetically related.
Restriction with XhoI resulted in six clusters on the basis of ≥90% internal similarity. However, correlations between serotypes of strains and restriction patterns were practically not detected. When strains exhibiting ≥95% band profile similarity were grouped together, 17 clusters consisting of 2 to 10 strains were generated. Only eight of these groups contained strains expressing mainly identical O antigens. These groups were scattered rather randomly within the dendrogram.
An association of the RFLP patterns and geographic origin of the strains or host species has not been detected. The feline C. upsaliensis strains were randomly distributed among the canine strains.
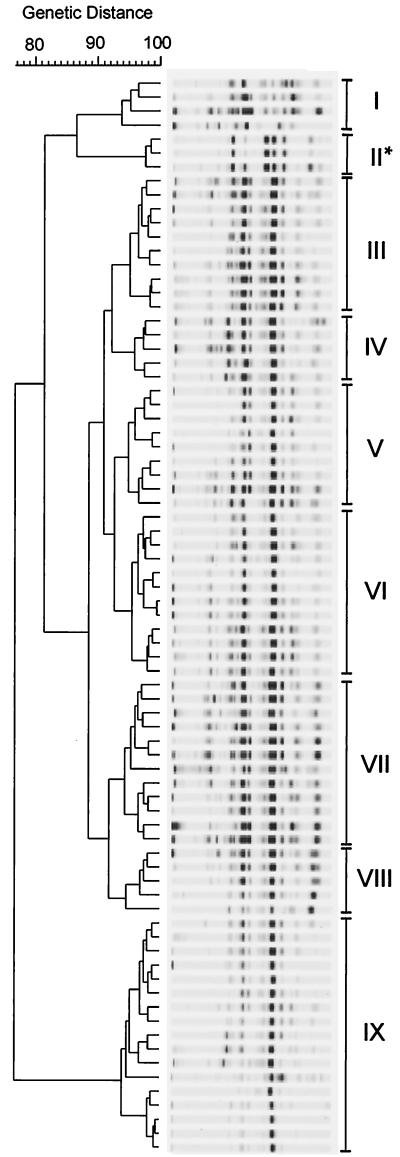
ERIC fingerprint analysis.
From the collection of 76 C. upsaliensis and 4 C. helveticus strains characterized by macrorestriction analysis, 74 C. upsaliensis and 3 C. helveticus strains were typed using ERIC PCR (Fig. 2). All bands, bright or light, were counted according to their positions and strength. Nine clusters were detected when strains exhibiting similarity rates of ≥93% were grouped together.
FIG. 2.
ERIC PCR profile analysis of canine and feline C. upsaliensis and C. helveticus strains isolated in Berlin and NRW. ∗, C. helveticus strains. I to IX represent groups of genetic relatedness based on ≥93% similarity of band positions and intensities.
The C. helveticus strains were grouped together in cluster II (Fig. 1, asterisk). Beyond that, an association between the degree of genomic similarities and origin of isolates was not detected, neither regarding the geographic area nor the host species. Furthermore, a correlation between the serotypes or the distribution of strains within the dendrogram generated on the basis of ERIC fingerprinting was not found.
Analysis of 16S rRNA sequence.
From the 76 C. upsaliensis and 4 C. helveticus strains investigated by macrorestriction analysis, 12 strains were randomly selected, three from each of the four groups of the SmaI-generated similarity tree (Fig. 1A). The numbers of the selected strains were H23E, H123E, and K12E from group I; H50E, H118, and H28E from group II; K2E, K9E, and K16E from group III (C. helveticus group); and H25E, H28, and H95 from group IV. According to Linton et al. (27), the major part of the 16S rRNA gene encoding the DNA sequence was amplified. A DNA segment of 1,169 nucleotides from each of the 12 strains was analyzed in order to determine their genetic distance. This corresponded to 80.1% of the entire gene of C. upsaliensis CCUG 14913, comprising 1,460 nucleotides beginning from nucleotide 225 (58; GenBank accession number L14628). All nine C. upsaliensis strains possessed identical nucleotide sequences compared to strain CCUG 14913, with two exceptions. Strain H95 exhibited C instead of G at nucleotide position 382 (CCUG 14913), and strain K12E showed T instead of C at nucleotide position 642 (CCUG 14913). All three C. helveticus strains also possessed identical DNA sequences. However, they differed from the C. upsaliensis DNA sequences at several nucleotide positions: G towards C at position 382, identical to the C. upsaliensis strain H95, T towards C at position 1195, and 19 changes at positions listed in Fig. 3. The C. upsaliensis strain K12E and the C. helveticus strain K16E were randomly selected for comparison.
FIG. 3.
Differences of 16S rDNA sequence between the C. upsaliensis strain K12E and the C. helveticus strain K16E.
DISCUSSION
Results of serotyping of C. upsaliensis have previously been published regarding heat-labile surface antigens (28); however, to our knowledge, typing according to heat-stable antigens has not been performed so far. Therefore, we consider this the first O-antigen typing scheme published for C. upsaliensis and C. helveticus. O-antigen typing of C. upsaliensis strains revealed that in contrast to C. jejuni and C. coli, which are divided into more than 60 O-antigenic serotypes according to Penner and Hennessy (45), this species seems to possess only a small number of different heat-stable antigens. Of the investigated strains, 93.1% were divided into only five different serotypes. Since crosswise absorption experiments have not been performed, we cannot exclude that the five heat-stable serotypes identified in the present study consist of a somewhat larger number of cross-reacting antigens. Nevertheless, the O-antigen diversity of C. upsaliensis seems to be less pronounced than that of C. jejuni. Similar to C. jejuni, where a limited number of serotypes exceed others in frequency of isolation worldwide (42, 46; W. M. Johnson, D. L. Woodward, R. Khakhria, and L. J. Price, Campylobacter, Helicobacter and related organisms. Proc. 9th Int. Workshop, p. 27, 1997), we found that the serotypes O III and O IV, identified at rates of 47.5 and 27.7%, respectively, constituted the most prevalent serotypes within the collection of isolates. After a comparison of the number of strains that exhibited the prevalent serotype O III or the rarely found serotype O VI or were untypeable, a marked difference of geographic distribution could be detected. In NRW 61.8% of all tested strains exhibited serotype O III, while none of them were O VI positive or untypeable. In contrast, of the strains collected in Berlin, only 30.4% belonged to serotype O III, 6.5% to serotype O VI, and 15.2% to the group of untypeable strains. These data point towards clonal expansion of C. upsaliensis.
Except for serotype O II, which showed a weak cross-reaction with the C. jejuni serotype O:2, the C. upsaliensis serotypes were unrelated to serotypes of other Campylobacter species available for comparison in this study. Nine C. jejuni-specific antisera mainly representing O-antigenic serotypes prevalent worldwide, two C. coli-specific antisera, and one C. lari-specific antiserum were used. The finding that C. upsaliensis strains may share some O-antigen specificity with C. jejuni or C. coli was reported before from strains in South Africa (25). These authors reported that a number of C. upsaliensis strains isolated from humans did not react with C. jejuni-specific antisera; however, they reacted strongly with antiserum against C. coli O:28. Due to the lack of availability of more than our two C. coli antisera, we were not able to confirm this reactivity in our strain collection. In contrast, 54.6% of the C. helveticus isolates shared O-antigenic structures (serotypes O II and O III) with C. upsaliensis.
In contrast to serotyping, genotyping methods (PFGE and ERIC PCR) revealed a high degree of genomic heterogeneity within the species C. upsaliensis. These data are in accordance with previous reports (4, 39, 57) and may be due to some properties of Campylobacter, which enable them to take up DNA from the environment and integrate it into the genome or undergo changes within the genome by rearrangement of autogenous DNA (15, 18, 29, 54, 55, 56). Beyond that, macrorestriction analysis with SmaI revealed a certain degree of association between serotypes and genotypic similarities. The concentration of serotype O III-positive strains in cluster I of the dendrogram especially points towards clonal expansion of at least certain subgroups within the species C. upsaliensis. In C. jejuni NCTC 11168, whose genome is totally characterized by DNA sequence analysis (41), 9 of 15 specific recognition sites of the endonuclease SmaI are located within the three copies of the 16S rRNA genes. Therefore, SmaI seems to be a very useful endonuclease for studies of the clonality of C. jejuni (19, 37, 40). The same may hold true for C. upsaliensis.
In contrast, restriction with the endonuclease XhoI caused the disintegration of these serotype-associated groups to smaller noncoherent subgroups. Therefore, due to their specific recognition sites, certain restriction endonucleases are more suitable tools to answer questions concerning clonality and evolution, while others may be more suitable for epidemiological studies due to their high discriminatory capacity.
The C. helveticus isolates were grouped together when restricted with either enzyme, despite belonging to different serotypes. As they were not digested by SmaI, they formed a separate group within the SmaI-generated dendrogram. In contrast, they possessed recognition sites for XhoI; therefore, they form only a subgroup within the XhoI-generated dendrogram.
Beyond serotypes, associations of genotypic similarities to other characteristics common for certain strain groups, like geographic or host origin (dog or cat) or enteric health status of their hosts, were not detected. These data are partly in agreement with those of Stanley et al. (51), who reported when using 16S rRNA ribotyping that C. upsaliensis isolates from healthy and diarrheic dogs were not distinguishable. However, they detected genotypic differences between human and canine strains. Unfortunately, due to the lack of human isolates, we were not able to compare canine with human isolates. These data may indicate that the pathogen is ingested by dogs and cats from the same sources, in contrast to the pattern for humans. On the other hand, it may also be a matter of adaptation of certain strains from general sources to different hosts in order to cause and maintain an infection.
ERIC sequence profile analysis demonstated the high discriminatory potential of this method, as described before (57). However, the profiles did not reveal associations to any of the above-mentioned characteristics of the strains. Again the investigated C. helveticus isolates were grouped together.
The high degree of 16S rDNA sequence similarity among the nine C. upsaliensis strains selected from the whole amplitude of the SmaI-generated tree of genetic similarity is in marked contrast to the high degree of heterogeneity of the whole genome and confirms that all the C. upsaliensis strains belong to one species. Only 1 nucleotide each was changed within the whole length of 1,169 nucleotides in two strains. With these exceptions, all 12 strains exhibited 16S rDNA sequences identical to that for the C. upsaliensis strain CCUG 14913 supplied in the database (58). The three C. helveticus strains analyzed also possessed identical sequences, compared with each other. The small number of nucleotide exchanges compared to the 16S rDNA sequence of C. upsaliensis underlines the relatedness of the two Campylobacter species. Overall, 21 of 1,169 nucleotides were changed with 19 of them between the positions 961 and 1345, compared to the 16S rDNA sequence of strain C. upsaliensis CCUG 14913.
From all these genotypic investigations, the strains which did not give satisfactory electropherograms, perhaps due to extracellular DNases or other factors, were excluded from analysis without further investigation. Therefore, the degree of genomic heterogeneity within the two species under investigation may be even higher than demonstrated in this work.
Thermophilic Campylobacter species were isolated from 41.8% of the canine fecal specimens from two distinct geographic regions in Germany, an isolation rate in accordance with other reports, where 13.8 and 50% of the samples were positive (1, 3, 6, 7, 17, 30, 34, 48). In the present study 80.7% of the isolates were identified as C. upsaliensis, and only 19.3% were identified as C. jejuni or other catalase-positive thermophilic Campylobacter or related species. Baker et al. (3) reported similar C. upsaliensis isolation rates in dogs in southern Australia. Other authors, however, isolated C. upsaliensis only at rates of 15.7 (6), 19 (17), and 7.1% (30), whereas C. jejuni was isolated at 19.2 (6), 76 (17), and 33.9% (30). Loss of viability of bacteria due to mailing conditions or the selective media used for Campylobacter isolation may contribute to the rarity of those results. In order to get reliable results, we focused on freshly collected samples, which were streaked on plates within 24 h, and did not include samples sent from elsewhere to the laboratory. Investigating two geographically separated groups of isolates, we sought to analyze differences in expansion of genomic characteristics in different bacterial populations and to prevent misinterpretations caused by generalizing certain findings which would have emerged potentially only in one group of isolates.
The prevalence of Campylobacter in juvenile and adult dogs differed significantly, in that young animals carried Campylobacter at an average isolation rate of 75.0%, whereas adults were positive only at a rate of 32.7% (P < 0.01). Similarly C. upsaliensis was isolated more than twice as often in juvenile dogs as in adult dogs (55.4 versus 27.8%; P < 0.05). Regarding the presence or absence of enteric symptoms, there was a significant difference only for the Campylobacter isolation rates detected for juvenile and adult healthy dogs, with higher frequencies in juvenile dogs. In enteritic dogs there existed only a nonsignificant tendency to higher Campylobacter rates (in juvenile animals). In general, in contrast to the findings of Burnens et al. (6), substantial differences between dogs with and without enteritic symptoms were not detected.
Taking these results together, significant differences in the incidence of Campylobacter, especially in C. upsaliensis isolation, were detected between juvenile and adult dogs without gastroenteritic symptoms, whereas a significant association with enteritis could not be found in either age group.
The number of feline isolates obtained in this study was too small to allow any further interpretation concerning associations of pathogen carriage with age or enteric health.
The potential significance of C. upsaliensis for humans is increasingly realized. The diversity of the genome renders epidemiological studies difficult to perform. Combinations of phenotypic methods, including O-antigen typing and well-considered genotypic methods, may help to follow the route of the pathogen during infection.
ACKNOWLEDGMENT
We thank M. Baumann for his help in statistical calculations.
REFERENCES
- 1.Adesiyun A A, Campbell M, Kaminjolo J S. Prevalence of bacterial enteropathogens in pet dogs in Trinidad. J Vet Med Ser B. 1997;44:19–27. doi: 10.1111/j.1439-0450.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 2.Aspinall S T, Wareing D R A, Hayward P G, Hutchinson D N. A comparison of a new campylobacter selective medium (CAT) with membrane filtration for isolation of thermophilic campylobacters including Campylobacter upsaliensis. J Clin Pathol. 1996;80:645–650. doi: 10.1111/j.1365-2672.1996.tb03269.x. [DOI] [PubMed] [Google Scholar]
- 3.Baker J, Barton M D, Lanser J. Campylobacter species in cats and dogs in South Australia. Aust Vet J. 1999;77:662–666. doi: 10.1111/j.1751-0813.1999.tb13159.x. [DOI] [PubMed] [Google Scholar]
- 4.Bourke B, Sherman P M, Woodward D, Lior H, Chan V L. Pulsed-field gel electrophoresis indicates genotypic heterogeneity among Campylobacter upsaliensis strains. FEMS Microbiol Lett. 1996;143:57–61. doi: 10.1111/j.1574-6968.1996.tb08461.x. [DOI] [PubMed] [Google Scholar]
- 5.Bourke B, Chan V L, Sherman P. Campylobacter upsaliensis: waiting in the wings. Clin Microbiol Rev. 1998;11:440–449. doi: 10.1128/cmr.11.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnens A, Angéloz-Wick B, Nicolet J. Comparison of Campylobacter carriage rates in diarrheic and healthy pet animals. J Vet Med Ser B. 1992;39:175–180. doi: 10.1111/j.1439-0450.1992.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 7.Burnens A, Nicolet J. Detection of Campylobacter upsaliensis in diarrheic dogs and cats, using a selective medium with cefoperazone. Am J Vet Res. 1992;53:48–51. [PubMed] [Google Scholar]
- 8.Eyers M, Chapelle S, Van Camp G, Goossens H, De Wachter R. Discrimination among thermophilic Campylobacter species by polymerase chain reaction amplification of 23S rRNA gene fragments. J Clin Microbiol. 1993;31:3340–3343. doi: 10.1128/jcm.31.12.3340-3343.1993. . (Erratum, 32:1620, 1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox J G, Maxwell K O, Taylor N S, Runsick C D, Edmonds P, Brenner D J. “Campylobacter upsaliensis” isolated from cats as identified by DNA relatedness and biochemical features. J Clin Microbiol. 1989;27:2376–2378. doi: 10.1128/jcm.27.10.2376-2378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudreau C, Lamothe F. Campylobacter upsaliensis isolated from a breast abscess. J Clin Microbiol. 1992;30:1354–1356. doi: 10.1128/jcm.30.5.1354-1356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goossens H, Giesendorf B A J, Vandamme P, Vlaes L, Van den Borre C, Koeken A, Quint W G V, Blomme W, Hanicq P, Koster D S, Hofstra H, Butzler J-P, Van der Plas J. Investigation of an outbreak of Campylobacter upsaliensis in day care centers in Brussels: analysis of relationships among isolates by phenotypic and genotypic typing methods. J Infect Dis. 1995;172:1298–1305. doi: 10.1093/infdis/172.5.1298. [DOI] [PubMed] [Google Scholar]
- 12.Goossens H, Pot B, Vlaes L, Van den Borre C, Van den Abbeele R, Van Naelten C, Levy J, Cogniau H, Marbehant P, Verhoef J, Kersters K, Butzler J-P, Vandamme P. Characterization and description of “Campylobacter upsaliensis” isolated from human feces. J Clin Microbiol. 1990;28:1039–1046. doi: 10.1128/jcm.28.5.1039-1046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goossens H, Vlaes L, De Boeck M, Pot B, Kersters K, Levy J, De Mol P, Butzler J-P, Vandamme P. Is “Campylobacter upsaliensis” an unrecognized cause of human diarrhoea? Lancet. 1990;335:584–586. doi: 10.1016/0140-6736(90)90359-d. [DOI] [PubMed] [Google Scholar]
- 14.Goossens H, Vlaes L, Butzler J-P, Adnet A, Hanicq P, N′Jufom S, Massart D, De Shrijver G, Blomme W. Campylobacter upsaliensis enteritis associated with canine infections. Lancet. 1991;337:1486–1487. doi: 10.1016/0140-6736(91)93182-9. [DOI] [PubMed] [Google Scholar]
- 15.Guerry P, Logan S M, Trust T. Genomic rearrangements associated with antigenic variation in Campylobacter coli. J Bacteriol. 1988;170:316–319. doi: 10.1128/jb.170.1.316-319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurgan T, Diker K S. Abortion associated with Campylobacter upsaliensis. J Clin Microbiol. 1994;32:3093–3094. doi: 10.1128/jcm.32.12.3093-3094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hald B, Madsen M. Healthy puppies and kittens as carriers of Campylobacter spp., with special reference to Campylobacter upsaliensis. J Clin Microbiol. 1997;35:3351–3352. doi: 10.1128/jcm.35.12.3351-3352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington C S, Thomson-Carter F M, Carter P E. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol. 1997;35:2386–2392. doi: 10.1128/jcm.35.9.2386-2392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington C S, Thomson-Carter F M, Carter P E. Molecular epidemiological investigation of an outbreak of Campylobacter jejuni identifies a dominant clonal line within Scottish serotype H. S. 55 populations. Epidemiol Infect. 1999;122:367–375. doi: 10.1017/s0950268899002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holländer R. Characterization of Campylobacter jejuni/coli-isolates from human faeces. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1984;258:128–134. doi: 10.1016/s0176-6724(84)80017-6. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson D N, Bolton F J. Improved blood free selective medium for the isolation of Campylobacter jejuni from fecal specimens. J Clin Pathol. 1984;37:956–957. doi: 10.1136/jcp.37.8.956-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang M N, Ederer G M. Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J Clin Microbiol. 1975;1:114–115. doi: 10.1128/jcm.1.1.114-115.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkin G A, Tee W. Campylobacter upsaliensis-associated diarrhea in human immunodeficiency virus-infected patients. J Clin Infect Dis. 1998;27:816–821. doi: 10.1086/514957. [DOI] [PubMed] [Google Scholar]
- 24.Karmali M A, Simor A E, Roscoe M, Fleming P C, Smith S S, Lane J. Evaluation of a blood-free, charcoal-based, selective medium for the isolation of Campylobacter organisms from feces. J Clin Microbiol. 1986;23:456–459. doi: 10.1128/jcm.23.3.456-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lastovica A J, Le Roux E, Penner J L. “Campylobacter upsaliensis” isolated from blood cultures of pediatric patients. J Clin Microbiol. 1989;27:657–659. doi: 10.1128/jcm.27.4.657-659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindblom G-B, Sjögren E, Hansson-Westerberg J, Kaijser B. Campylobacter upsaliensis, C. sputorum sputorum and C. concisus as common causes of diarrhea in Swedish children. Scand J Infect Dis. 1995;27:187–188. doi: 10.3109/00365549509019006. [DOI] [PubMed] [Google Scholar]
- 27.Linton D, Dewhirst F, Cleweley J P, Owen R J, Burnens A, Stanley J. Two types of 16S rRNA gene found in Campylobacter helveticus: analysis, applications and characterization of the intervening sequence found in some strains. Microbiology. 1994;140:847–855. doi: 10.1099/00221287-140-4-847. [DOI] [PubMed] [Google Scholar]
- 28.Lior H, Woodward D L. A serotyping scheme for Campylobacter upsaliensis. Microb Ecol Health Dis. 1991;4(Special issue):89. [Google Scholar]
- 29.Mills S D, Kurjanczyk L A, Shames B, Penner J L. Antigenic shifts in serotype determinants of Campylobacter coli are accompanied by changes in the chromosomal DNA restriction endonuclease digestion pattern. J Med Microbiol. 1991;35:168–173. doi: 10.1099/00222615-35-3-168. [DOI] [PubMed] [Google Scholar]
- 30.Moreno G S, Griffiths P L, Connerton I F, Park R W A. Occurrence of Campylobacters in small domestic and laboratory animals. J Appl Bacteriol. 1993;75:49–54. doi: 10.1111/j.1365-2672.1993.tb03406.x. [DOI] [PubMed] [Google Scholar]
- 31.Moser I, Hellmann E. In vitro binding of Campylobacter jejuni surface proteins to murine small intestinal cell membranes. Med Microbiol Immunol. 1989;178:217–228. doi: 10.1007/BF00202555. [DOI] [PubMed] [Google Scholar]
- 32.Nachamkin I, Bohachick K, Patton C M. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J Clin Microbiol. 1993;31:1531–1536. doi: 10.1128/jcm.31.6.1531-1536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nachamkin I, Ung H, Patton C M. Analysis of H, L, and O serotypes of Campylobacter strains by the flagellin gene typing system. J Clin Microbiol. 1996;34:277–281. doi: 10.1128/jcm.34.2.277-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson P, Sandstedt K. Campylobacter in the dog: a clinical and experimental study. Vet Rec. 1987;121:99–101. doi: 10.1136/vr.121.5.99. [DOI] [PubMed] [Google Scholar]
- 35.On S L W. Identification methods for campylobacters, helicobacters, and related organisms. Clin Microbiol Rev. 1996;9:405–422. doi: 10.1128/cmr.9.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.On S L W, Holmes B. Effect of inoculum size on the phenotypic characterization of Campylobacter species. J Clin Microbiol. 1991;29:923–926. doi: 10.1128/jcm.29.5.923-926.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.On S L W, Nielsen E M, Engbert J, Madsen M. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol Infect. 2000;120:231–237. doi: 10.1017/s0950268898008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen R J, Fitzgerald C, Sutherland K, Borman P. Flagellin gene polymorphism analysis of Campylobacter jejuni infecting man and other hosts and comparison with biotyping and somatic antigen serotyping. Epidemiol Infect. 1994;113:221–234. doi: 10.1017/s0950268800051657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen R J, Hernandez J. Genotypic variation in ‘Campylobacter upsaliensis’ from blood and faeces of patients in different countries. FEMS Microbiol Lett. 1990;72:5–10. doi: 10.1016/0378-1097(90)90335-n. [DOI] [PubMed] [Google Scholar]
- 40.Owen R J, Sutherland K, Fitzgerald C, Gibson J, Borman P, Stanley J. Molecular subtyping scheme for serotypes HS1 and HS4 of Campylobacter jejuni. J Clin Microbiol. 1995;33:872–877. doi: 10.1128/jcm.33.4.872-877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T S, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M-A, Rutherford K M, van Vliet A H M, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 42.Patton C M, Nicholson M A, Ostroff S M, Ries A A, Wachsmuth I K, Tauxe R V. Common somatic O and heat-labile serotypes among Campylobacter strains from sporadic infections in the United States. J Clin Microbiol. 1993;31:1525–1530. doi: 10.1128/jcm.31.6.1525-1530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton C M, Shaffer N, Edmonds P, Barrett T J, Lambert M A, Baker C, Perlman D M, Brenner D J. Human disease associated with “Campylobacter upsaliensis” (catalase-negative or weakly positive Campylobacter species) in the United States. J Clin Microbiol. 1989;27:66–73. doi: 10.1128/jcm.27.1.66-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patton C M, Wachsmuth I K, Evins G M, Kiehlbauch J A, Plikaytis B D, Troup N, Tompkins L, Lior H. Evaluation of 10 methods to distinguish epidemic-associated Campylobacter strains. J Clin Microbiol. 1991;29:680–688. doi: 10.1128/jcm.29.4.680-688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penner J L, Hennessy J N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penner J L, Hennessy J N, Congi R V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983;2:378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- 47.Salama S M, Tabor H, Richter M, Taylor D E. Pulsed-field gel electrophoresis for epidemiologic studies of Campylobacter hyointestinalis isolates. J Clin Microbiol. 1992;30:1982–1984. doi: 10.1128/jcm.30.8.1982-1984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandstedt K, Ursing J, Walder M. Thermotolerant Campylobacter with no or weak catalase activity isolated from dog. Curr Microbiol. 1983;8:209–213. [Google Scholar]
- 49.Stanley J, Burnens A P, Linton D, On S L W, Costas M, Owen R J. Campylobacter helveticus sp. nov., a new thermophilic species from domestic animals: characterization, and cloning of a species-specific DNA probe. J Gen Microbiol. 1992;138:2293–2303. doi: 10.1099/00221287-138-11-2293. [DOI] [PubMed] [Google Scholar]
- 50.Stanley J, Linton D, Sutherland K, Jones C, Owen R J. High-resolution genotyping of Campylobacter coli identifies clones of epidemiologic and evolutionary significance. J Infect Dis. 1995;172:1130–1134. doi: 10.1093/infdis/172.4.1130. [DOI] [PubMed] [Google Scholar]
- 51.Stanley J, Jones C, Burnens A, Owen R. Distinct genotypes of human and canine isolates of Campylobacter upsaliensis determined by 16S rRNA gene typing and plasmid profiling. J Clin Microbiol. 1994;32:1788–1794. doi: 10.1128/jcm.32.7.1788-1794.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor D E, Hiratsuka K, Mueller L. Isolation and characterization of catalase-negative and catalase-weak strains of Campylobacter species, including “Campylobacter upsaliensis,” from humans with gastroenteritis. J Clin Microbiol. 1989;27:2042–2045. doi: 10.1128/jcm.27.9.2042-2045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Versalowic J, Koeuth T, Lupinski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Taylor D E. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wassenaar T M, Fry B N, van der Zeijst B A M. Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology. 1995;141:95–101. doi: 10.1099/00221287-141-1-95. [DOI] [PubMed] [Google Scholar]
- 56.Wassenaar T M, Geilhausen B, Newell D G. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl Environ Microbiol. 1998;64:1816–1821. doi: 10.1128/aem.64.5.1816-1821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weijtens M J B M, van der Plas J, Bijker P G H, Urlings H A P, Koster D, van Logtestijn J G, Huis in't Veld J H J. The transmission of Campylobacter in piggeries; an epidemiological study. J Appl Microbiol. 1997;83:693–698. doi: 10.1046/j.1365-2672.1997.00301.x. [DOI] [PubMed] [Google Scholar]
- 58.Wesley I V, Schroeder-Tucker L, Baetz A L, Dewhirst F E, Paster B J. Arcobacter-specific and Arcobacter butzleri-specific 16S rRNA-based DNA probes. J Clin Microbiol. 1995;33:1691–1698. doi: 10.1128/jcm.33.7.1691-1698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan W, Chang N, Taylor D E. Pulsed-field electrophoresis of Campylobacter jejuni and Campylobacter coli genomic DNA and its epidemiological application. J Infect Dis. 1991;163:1068–1072. doi: 10.1093/infdis/163.5.1068. [DOI] [PubMed] [Google Scholar]