Abstract
The association between artificial sweetener (AS) consumption and the risk of organ-specific cancers has been debated for decades. We hypothesized that AS consumption is associated with reduced risk of gastrointestinal (GI) cancers. We aimed to test this hypothesis by conducting a systematic review with meta-analysis of the association between AS and GI cancers. We searched four databases for comparative studies of AS consumption (exposed) versus no consumption (nonexposed) and the odds or risk of GI luminal or non-luminal cancer (primary outcome). Estimates were pooled using a random-effects model. Studies were evaluated for quality, bias, and heterogeneity. We analyzed 8 (4 prospective, 4 case-control) studies comprising data on 1,043,496 individuals, among whom 3271 pancreatic, 395 gastric, 304 esophageal, 3008 colorectal, and 598 oropharyngeal cancers occurred. While there was no significant association between AS consumption and odds of GI cancer overall, AS consumption was associated with 19% reduced likelihood of luminal GI cancer (OR 0.81, 95% CI:0.68–0.97). There was no association between AS consumption and non-luminal GI cancer. Meta-regression demonstrated no difference in effect estimates based on study type. Based on this first meta-analysis of AS and GI cancer, we demonstrated that AS consumption is associated with a significantly lower likelihood of luminal, but not non-luminal, GI cancer.
Keywords: meta-analysis, systematic review, epidemiology, environment and public health, digestive system neoplasm, nutrition therapy
1. Introduction
The use of artificial sweeteners (AS) as low-calorie chemical substitutes for sugar-sweetened foods and beverages has become increasingly widespread over the past several decades.[1–4] According to the most recent reports, which reflect data from nearly a decade ago, at least one-third of adults and children in the United States (US) reported regular AS consumption, with similar trends demonstrated in Europe and Australia, and even higher rates in South American countries.[1–4] Notably, rates of AS consumption have continued to rise. This is in large part attributed to the increasing availability and introduction of newer AS, as well as the rising obesity epidemic and the increased use of “diet” and “low-calorie” products, especially beverages.[5] Prior to 2014, there were five Federal Drug Administration (FDA)-approved AS: saccharin, which was the earliest AS discovered (1879); aspartame (FDA-approved 1981); acesulfame (FDA-approved 1988); neotame (FDA-approved 2002); and sucralose (FDA-approved 1998), which is the most commonly used AS.[3,6] As of 2014, the FDA approved a sixth AS, advantame. Since their introduction, there have been mixed data regarding the possible health effects associated with AS, with most studies demonstrating null associations or generally favoring a slight positive influence on metabolic parameters including insulin sensitivity.[7–10] Although the supporting body of literature is heterogeneous, AS use is more common among populations specifically trying to reduce their sugar intake, such as individuals with diabetes and obesity participating in diet modifications for intentional weight loss, which complicates the picture. The underlying mechanisms for AS and altered metabolic parameters might relate to both biological (e.g. altered metabolism and insulin sensitivity, microbiome changes) and nonbiological (e.g. altered compensatory nutritional intake, including paradoxically increased consumption of more sugar-sweetened and processed foods) factors.[11–13]
The controversy surrounding the health risks and benefits of AS extends also to their carcinogenic potential (or lack thereof). Whether AS are associated with cancer risk has been a topic of debate for nearly half a century and one which was spurred initially by experimental studies demonstrating an increased risk of bladder cancer in rodents exposed to high doses of saccharin. This observation was supported by early epidemiological studies in humans, but has not borne out in subsequent, larger epidemiological studies.[14–20] Since these first investigations in the 1970s-1980s, there have been several more studies analyzing the association between AS and solid and liquid malignancies, especially urinary tract, brain and hematopoietic cancers.[21, 22] While saccharin was the first AS and therefore has a larger body of literature compared to other AS types, more recent studies, including experimental studies, have also analyzed the association between newer AS, such as aspartame, but again with mixed results.[23,24] Human studies remain limited, particularly studies analyzing the association between AS and gastrointestinal (GI) neoplasia specifically. Indeed, dietary factors have long been studied for their role in the development and progression of neoplasia, particularly of the GI tract since this is the first point of direct contact and digestion. Notably, while sugar-sweetened beverages have been associated with increased risk of pancreatic cancer,[21,25] increased risk of colon cancer recurrence,[26] and possibly increased risk of gastric cancer,[27] the association between AS and GI cancers is less defined. One Italian case-control study reported no association between aspartame or saccharin consumption and pancreatic cancer,[21] while other studies have reported a positive association. There are mixed data for luminal GI cancer as well, especially colorectal cancer.[28] Whether there is a differential association based on luminal tract (e.g. colorectal, esophageal, gastric) versus non-luminal tract (e.g. pancreatic, hepatocellular or other hepatobiliary) GI malignancies or based on AS type and sources (e.g. AS-containing beverages) is therefore unclear.
A better understanding of downstream biological and non-biological consequences of AS is fundamental, particularly the association with GI cancer risk, given the increased use of AS in our society and the need to identify modifiable cancer risk determinants in order to improve health outcomes. Based on the observation that consumption of sugar-sweetened products is associated with increased risk of several GI cancers and recurrence, we hypothesized that AS consumption is associated with reduced risk of GI cancers, perhaps due to a direct biological effect (e.g. microbiome changes) or indirect effect of less consumption of sugar-sweetened products. We aimed to test the primary hypothesis by 1) systematically reviewing the literature for comparative studies estimating the association between AS consumption and GI cancers overall, as well as based on anatomic location within the GI tract, and 2) performing a meta-analysis of these studies as appropriate.
2. Methods
This study was conducted in compliance with the guidelines as detailed in the Cochrane Handbook[29] and the Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[30]
2.1. Data Sources and Searches
In consultation with a certified biomedical librarian, we searched four publicly available databases—Pubmed, Embase, Medline, and Web of Science—from their inception dates through July 7th, 2019. We searched key words including “neoplasm”, “cancer”, “malignancy”, “tumor”, “carcinoma”, “artificial sweetener”, “diet soda”, “sugar substitute”, “diet beverage”, and related terms. Multiple search strings were included to capture studies of any cancer type associated with AS, as well as narrower search strings focused specifically on GI cancers associated with AS. The full search strings are provided in the Supplemental Material. Animal studies were excluded. To remain broad in our initial catchment, we did not restrict our initial search to studies published only in the English language; however, there were no articles published solely in a non-English language identified in our search. References of included studies, review articles or other relevant articles were also manually searched for any additional studies.
2.2. Inclusion/Exclusion Criteria
The following inclusion criteria were applied to all studies: (1) confirmed diagnosis of gastrointestinal cancer (primary outcome); (2) comparative study design with distinct comparison between individuals exposed versus not exposed to AS (primary exposure), as well as sufficient details to determine exposed versus nonexposed categories; (3) sufficient detail to calculate or determine effect estimates (e.g. odds ratio); (4) AS as defined by any consumption of food or drinks containing artificial sweetener or packets with mention of specific sweetener type such as saccharin or aspartame or any consumption of AS as long as this was distinctly quantified; (5) full text article available in English. Case series and non-comparative study designs were excluded. Studies that did not meet above criteria were excluded from analysis.
2.3. Data Abstraction
Initial screening for eligibility and data abstraction processes were independently conducted by three reviewers (AT, GH, and SJ) using the Covidence web-based platform. Any discrepancies were resolved by consensus with final arbitration by SCS. Standardized data collection forms were generated and included the following information: primary author(s) last name(s); date of publication; country of origin; study design; study population, eligibility criteria, study interval; type(s) of cancer; study definition for exposure vs non-exposure to AS, including threshold cutoff for exposed vs nonexposed determination; counts and demographics (age, sex) of exposed vs nonexposed; exposure (AS) type and method of measurement; number of cancer cases, type of cancer, histology (e.g. adenocarcinoma), method of confirmation for cancer diagnosis (e.g. histology) and data source (e.g. cancer registry).
2.4. Primary Exposure and Outcome Categorization
The primary exposure for this analysis was AS consumption. Studies differed in the type of AS (e.g. aspartame, saccharin), the formulation/quantification (e.g. diet drinks vs packets or grams of AS consumed), as well as threshold cutoffs for qualifying someone as a consumer (“exposed”) vs non-consumer (“nonexposed”) of AS; these study-specific details were documented as described above (Table 1). To maintain consistency, we categorized AS exposure as “any” vs. “none”. Studies varied in AS type as well as definition of exposure. Of the studies that examined diet beverages, the least specific was Bao et al. where the nonexposed group was simply defined as “never-drinkers” of diet soda. Similarly, Bosetti et al. nonspecifically designated groups as “users and nonusers” of saccharin and aspartame. As best able, we quantified the amount of AS consumed.
Table 1.
Details of studies meeting inclusion criteria
| Author, Year, Region Study Type | Cancer Type | Type of Sweetener | Study Period (End of follow-up) | Study Population (Mean Age: Exposed, Unexposed) | Male/Female (Exposed, Unexposed) Total N | Selection of Controls | Exposure Duration and Amount1 | Cancer Data Source/Method of Confirmation |
|---|---|---|---|---|---|---|---|---|
|
Schernhammer et al, 2005, USA Prospective Cohort |
Pancreatic | Diet Soft Drinks | 1976–1998; 1986 – 1990 (2000) |
Women from Nurses’ Health Study (NHS) cohort age 30–55; Men from Health Professionals Follow up Study (HFPS) cohort age 40–75 at entry/questionnaire (47.2, 47.5) | 27270M/44049F, 22094M/44745F Total N: 138158 |
N/A | Separated into groups based on drink frequency (almost never = reference; <1/month vs. greater) | Questionnaire, confirmed with medical records; received follow up questionnaire biennially |
|
Bao et al, 2008, USA Prospective Cohort |
Pancreatic | Diet soft drinks | 1995–2003 (2003) |
American Association of Retired Persons (AARP) members (61.8, 62.5) | 119957 M/99855 F, 164119 M/103991 F Total N: 487922 |
N/A | Quintiles of various sweetener amounts/quantified as “never” vs. quintiles of soft drinks per day; for analysis, categorized as “never” vs. “ever" | Cancer registry |
|
Navarrete-Munoz et al, 2016, Europe3 Prospective Cohort |
Pancreatic | Diet soft drinks | 1992–2000 (varied; 2004–2009)2 |
10 European Countries (50.3, 53.3) | 40185M/100945F, 60901M/166340F Total N: 368371 |
N/A | Intake in the year leading up to diagnosis /unexposed cutoff of <0.1gram/day4 | Cancer Registry |
|
Hodge et al, 2018, Australia Prospective Cohort |
Gastric, colorectal | Diet soft drinks | 1990–1994; 2003–2007 (2013) |
Melbourne Collaborative Cohort Study (53.4, 55.1) | 3460M/5472F, 10641M/16020F Total N: 35593 |
N/A | Intake in the year leading up to diagnosis /unexposed cutoff <1 "time" per month | Victorian Cancer Registry, Australian Institute of Health and Welfare, National Death Index, Australian Cancer Database and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database |
|
Norell et al, 1986, Sweden Case-Control |
Pancreatic | Artificial sweetener, no other details provided | 1982–1984 (N/A) |
3 surgical centers in Sweden (unavailable) | Unavailable Total N: 234 |
Age-matched, selected from parish registries5 | Not specified for threshold (yes/no) | Pathology, radiology |
|
Bosetti et al, 2009, Italy Case-Control |
Gastric, pancreatic | Saccharin, Aspartame | 1991–2007 (N/A) |
Hospitalized patients in Italy (63, 63) | Unavailable Total N, Gastric: 772 Total N, Pancreatic: 977 |
Age, sex, and study center-matched controls admitted for acute, non-neoplastic disorders | Unavailable (simplifies to nonusers vs users) | Histologically confirmed, hospital cases from four Italian regions |
|
Chan et al, 2009, USA Case-Control |
Pancreatic | Diet Soft Drinks/Low calorie beverages | 1995–1999 (N/A) |
San Francisco residents (unavailable) | Unavailable Total N: 22276 |
Age and sex matched | Intake studied for the year leading up to patienťs diagnosis/ various cutoffs with lowest <1 serving per month | Northern California Cancer Center Registry |
|
Gallus et al, 2007, Italy Case-Control |
Oropharyngeal, esophagus, colon, rectum | Saccharin, and “other” (mostly aspartame) | 1991–2004 (N/A) |
Hospitalized patients in Italy, interviewed during hospital stay (unavailable) | Unavailable Total N (Oropharyngeal): 2089 Total N (Esophagus): 1046 Total N (Colorectal): 61077 |
Matched controls admitted for acute, non-neoplastic disorders in some, but otherwise not specified | Intake studied for the two years leading up to diagnosis/unexposed defined as 0 "sachet or tablet" per day | Histologically confirmed, hospital cases from four Italian regions |
All studies used Food Frequency Questionnaires (FFQ), which varied based on the specific study (see also text)
Follow-up was considered completed up to the end of 2009 for Germany, France, and Greece; mid-2008 for Cambridge; 2008 for Turin, Norway, and Sweden; 2007 for Denmark, Netherlands, Murcia, Navarra, and Oxford; 2006 for Florence, Varese, Ragusa, Naples, Granada, and San Sebastian; and 2004 for Asturias.
Raw numbers differed when comparing supplemental material to limited numbers in published study. Since raw numbers were needed, N’s for calculations were all gathered from supplemental material.
12oz beverage = 336g
Study included both “population” and “hospital” controls (note: “Hospital” controls were patients admitted to the hospital with inguinal hernias). Population controls were used for this meta-analysis given more similar to controls in other studies.
Control N’s differ by +/−1, but no explanation is provided in the study. The largest number reported for the same population of controls was used to calculate total N. But for individual OR calculation, and meta-analyses, individual control N’s were respected for each group as seen in Table 2.
Colon and rectal cancers cases were separated for calculations OR’s in original paper, compared to the same group of controls (N = 4154). In total N calculations, controls (N=4154) were only counted once.
Abbreviations: USA, United States of America; N/A, not applicable; M, male; F, female
The primary outcome for this study was GI cancers, both overall and stratified by luminal vs non-luminal. Luminal GI tract cancers included any cancer occurring in the luminal GI tract, such as oropharyngeal, esophageal, gastric, small intestinal, and colorectal, while non-luminal GI cancers included pancreatic and hepatobiliary including hepatocellular carcinoma. If a study included several cancer types, we abstracted data separately for each cancer type. Of note, all cancer cases were independent and only the first primary cancer was considered for all studies.
2.5. Quality and Risk of Bias and Assessment
We used the Newcastle-Ottawa Scale (NOS) for quality and risk of bias assessment for non-randomized studies, including respective templates for case-control and cohort studies as appropriate.[31] No randomized controlled trials were identified. Studies were each independently assessed and scored by AT and SJ, with discrepancies adjudicated by a third arbiter (SCS). Studies were categorized as “high-quality” if the NOS score was at least 7 out of a possible maximum score of 9. [31]
2.6. Qualitative Synthesis and Quantitative Statistical Analysis
Reviewers recorded and organized the details of each study, including cancer type, study design, country/region, AS used and exposure amount, study population information and data sources. The odds of GI cancer in AS exposed vs nonexposed were calculated and reported as odds ratios (OR) for each included study based on raw numbers provided by contributing studies. A random-effects model was used to pool risk estimates from included studies, which was reported as OR and 95% confidence interval (95% CI). In addition to the overall meta-analysis including all studies, separate analyses were also performed for luminal cancers, non-luminal cancers, and for studies that included only beverages. Subanalysis based on AS type (e.g. saccharin, aspartame) was also planned a priori, but this was not possible since the included studies did not provide these granular details. A meta-regression analysis was planned a priori to evaluate for influence of study type (prospective cohort and case-control studies) on the observed effect estimates.
Publication bias for the primary outcome was assessed with Egger’s test and the use of a funnel plot. Chi-squared and I2 tests were utilized to determine heterogeneity among studies.[32] Heterogeneity between studies is indicated by Chi-squared test with p-value <0.15, while the I2 test provides a graded scale for heterogeneity interpretation: I2 cut-offs of <30%, 30–59%, 60–75%, and >75% indicate low, moderate, substantial, and considerable heterogeneity, respectively.[32]
RevMan 5.1 (Review Manager Version 5.1, Copenhagen, Denmark) and Comprehensive MetaAnalysis (version 2.0; Biostat, Englewood, NJ) software were used for these analyses.
3. Results
The flow diagram for study eligibility and ultimate inclusion is illustrated in Figure 1. The search identified 6106 studies, from which 3272 duplicate studies were removed, yielding a total of 2834 unique articles for eligibility review. A total of 2775 articles were removed for irrelevance based on title/abstract screening alone. Review of the references of the full-texts meeting inclusion criteria as well as relevant reviews yielded an additional 3 studies which also met full eligibility criteria.[33–35] The full-texts of each of the remaining 62 studies were reviewed for eligibility. From these 62 total studies, 54 studies were excluded for the following reasons: lack of GI cancer data (n=41); AS exposure (n=7); insufficient detail, such as not reporting the data inputs necessary to calculate numbers of exposed versus nonexposed (n=5); and ineligible study design (n=1). Attempts at contacting authors for additional details were unsuccessful. Thus, data from the 8 studies meeting full inclusion criteria were extracted for qualitative synthesis and quantitative meta-analysis.
Figure 1.
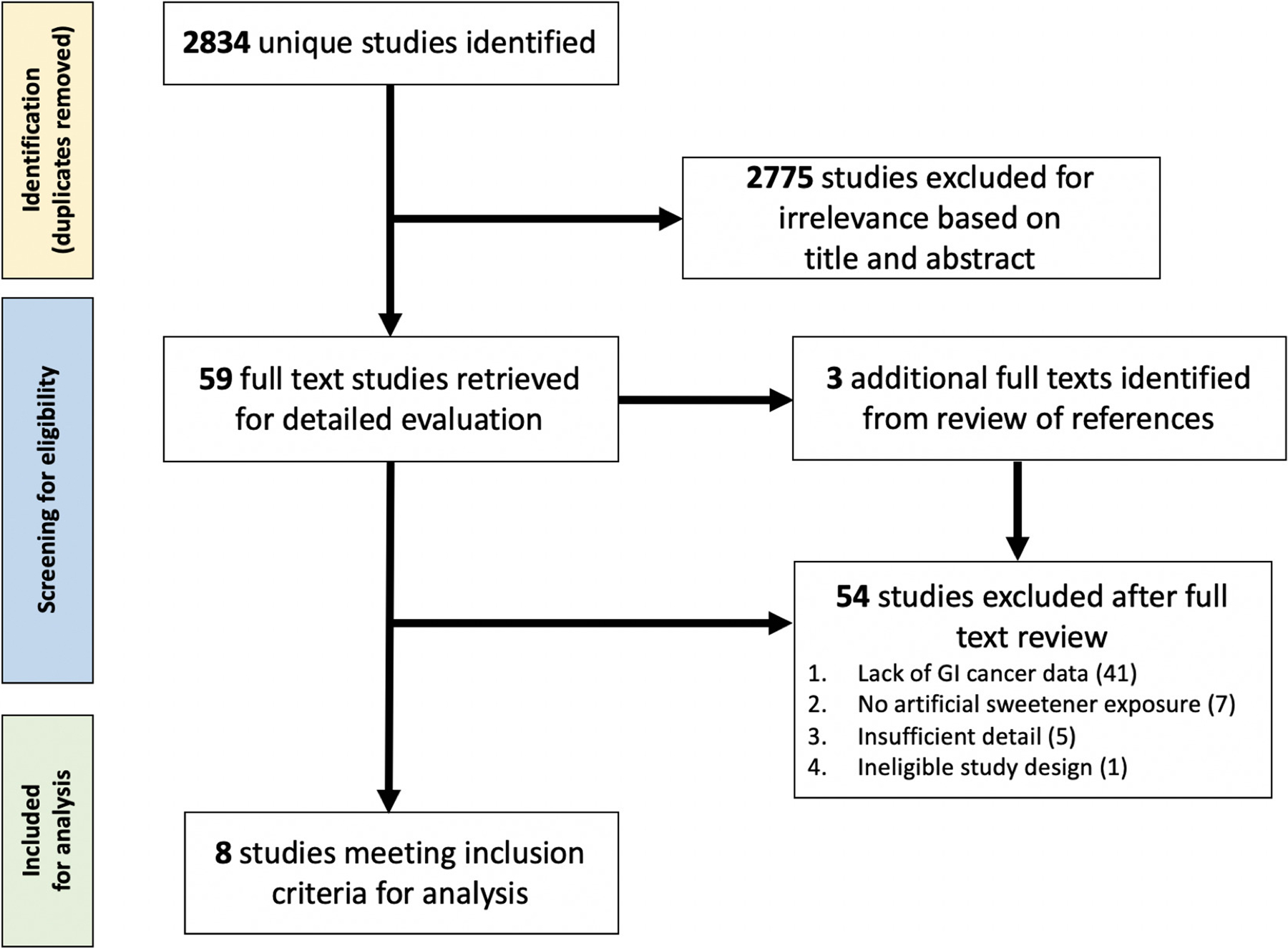
Flowchart of study inclusion
3.1. Qualitative synthesis of included studies
Details of the included studies are summarized in Table 1. Of the 8 included studies, 4 were prospective cohort studies[36–39] and 4 were case-control studies[21,40–42]. No randomized-controlled or interventional studies were identified. Of the included studies, 4 were from European countries[21, 37, 40, 41], 3 from the US[36, 39, 42], and 1 from Australia[38]. All studies included only adult patients. Three studies included more than one type of GI cancer.[21, 38, 41] All studies used cancer incidence as the primary outcome.
In total, 6 studies analyzed pancreatic cancer, 2 analyzed gastric cancer (one cardia[38], other study[21] anatomic location was not distinctly specified, but is suspected to be cardia and noncardia), and 1 study[41] analyzed esophageal, colon, rectal, and oropharyngeal cancer each as independent outcomes. Four of the studies did not specifically state the GI cancer histology[21, 36, 38, 41], while the other 4 studies, all of which were pancreatic cancer studies, specifically stated exocrine pancreatic adenocarcinoma histology.[37, 39, 40, 42] Other than pancreatic cancer, no other analyses of non-luminal GI cancers met criteria for inclusion. Of note, while the study by Hodge et al. captured liver cancer as an outcome, the investigators only analyzed those cancers with at least 100 cases; thus, effect estimates and other details were not provided for liver cancer in this study since there were only 52 cases. Our attempts to contact the study authors were unsuccessful.
The longest study time interval extended from 1976–2010, with most studies spanning 1990–2007. The populations for the cohort studies were derived from already established population-based cohorts including the American Association for Retired Persons (AARP, males and females aged 50–71 at entry)[39], the Melbourne Collaborative Cohort Study (MCCS, males and females age 40–79),[38] Health Professionals Follow-up Study (HPFS, males only aged 40–75 at entry), and Nurses’ Health Study (NHS, females only aged 30–55 at entry),[36] and the European Prospective Investigation into Cancer and nutrition (EPIC, males and females age 47–59 at entry, although some centers recruited only females) cohort.[37] The two Italian case-control studies included hospitalized patients,[21, 41] while the other 2 case-control studies included outpatients[42] and patients seen in surgical centers[40]. Three studies specified that controls were age and sex matched,[21, 40, 42] 2 studies[21, 41] used inpatient controls admitted for “acute, non-neoplastic disorders”, and 1 study[40] selected controls from parish registries which included both inpatient and outpatient controls separately. One study did not provide details on the selection of controls.[42]
All studies used food frequency questionnaires (FFQ) to ascertain AS exposure. Several of the studies used previously validated FFQs with many modeled from the Block questionnaire and some also commercially available.[37, 39, 42, 43] Three studies, two from Italy and one from Australia, used country-specific FFQs validated for their respective country’s population.[44, 45, 46] The measure of exposure also varied significantly among studies. All 4 prospective cohort studies included diet AS-sweetened soft drinks as the AS exposure of interest. Of the 4 case-control studies, Chan et al. was the only study that examined diet AS-sweetened soft drinks. The remaining 3 case-control studies included exposure to saccharin, aspartame and unspecified AS. No studies provided the level of granularity to determine outcome according to AS type. Both Navarrete-Munoz et al. and Hodge et al. analyzed diet soda as the AS exposure, which they defined differently; the former defined AS exposure as ≥0.1 grams/day, while Hodge et al. had a lower limit of exposure of “1 time per month” for diet soft drink consumption. Chan et al. and Schernhammer et al. used a cut off of “1 serving per month” of diet carbonated beverages (with variation based on subcategories), while Bao et al. simply defined nonexposed group as “never-drinkers” of diet soda. Gallus et al. defined exposure as anything more than 0 “sachets [small packets] or tablets” of saccharin or “other artificial sweeteners”. Both Bosetti et al. and Norell et al. were binary in their categorization, designating groups simply as “users and nonusers” of saccharin and aspartame, or “yes” or “no” regarding AS use, respectively.
All individual studies provided risk estimates adjusted for demographics and relevant confounders, although the exact covariates varied. Only one study did not mention the exact covariates included in the model.[40] Otherwise, all studies at a minimum adjusted for age, sex, body mass index (BMI), caloric intake, smoking, education, and history of diabetes, while most also adjusted for alcohol and physical activity as well. We were unable to perform a separate meta-analysis of the adjusted odds ratios for these studies since the categorization of comparative subgroups varied across all studies, for example study-specific quantiles of sweetener intake; thus, pooling these studies would not be appropriate.
3.2. Quantitative Analysis
In total, 1,043,496 people overall were included for analysis, including 1,030,044 from prospective cohort and 13,452 from case-control studies. Across all studies, 3271 pancreatic, 395 gastric, 304 esophageal, 3008 colorectal, and 598 oropharyngeal cancers cases occurred.
Based on meta-analysis of all 8 studies of GI luminal and non-luminal (pancreatic) cancers, there was no significant association between AS exposure and odds of GI cancer overall (OR 0.96, 95% CI: 0.88–1.05) (Supplemental Figure 1, Supplemental Table 1 and 2). There was also no association between AS exposure and non-luminal GI cancer, i.e. pancreatic cancer (OR 1.04, 95% CI: 0.95–1.14) (Figure 2). However, compared to non-exposure, AS exposure was associated with 19% significantly lower odds of luminal GI cancer (OR 0.81, 95% CI: 0.68–0.97) (Figure 3a). The directionality and significance of the association was maintained when the individual analysis for oropharyngeal cancers (study-specific OR for oropharyngeal cancer: 0.39, 95% CI: 0.23–0.64) was specifically excluded from the meta-analysis (OR 0.90, 95% CI: 0.81–0.99) (Figure 3b).
Figure 2.
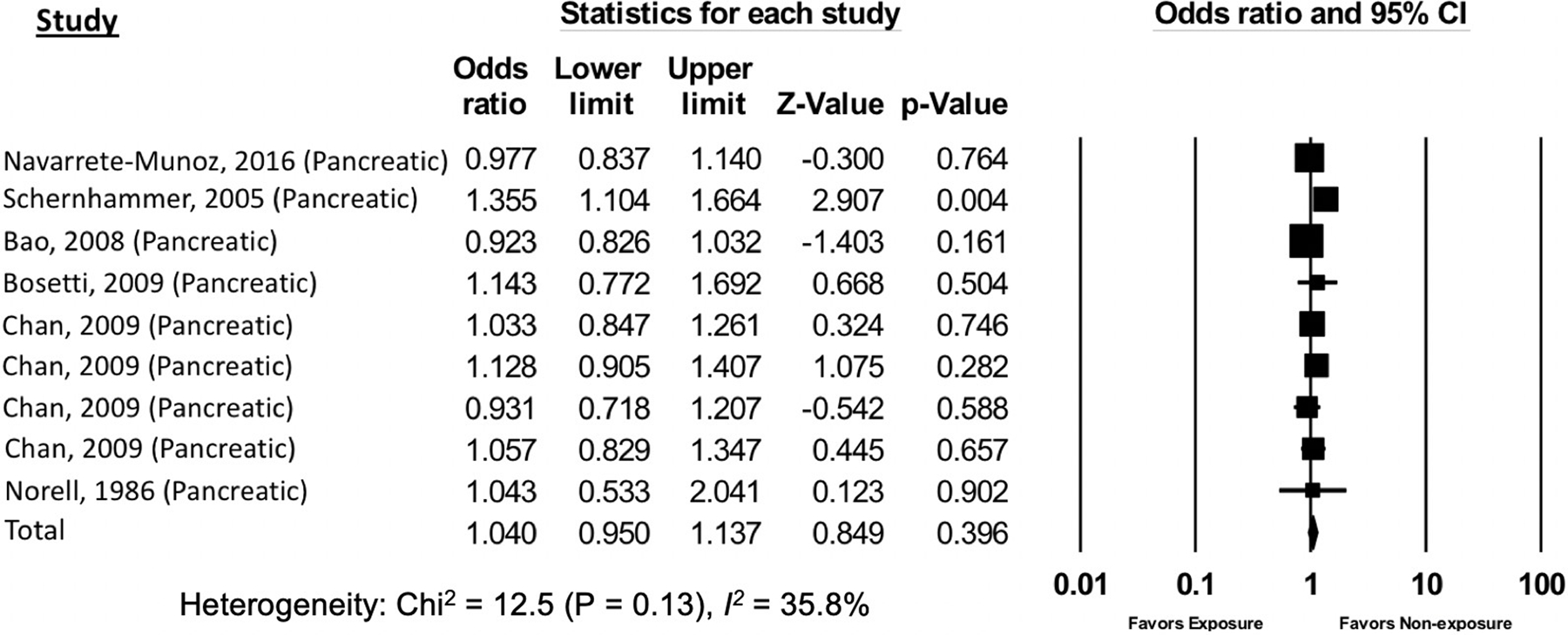
The association of artificial sweetener consumption with all non-luminal gastrointestinal cancers
Figure 3.
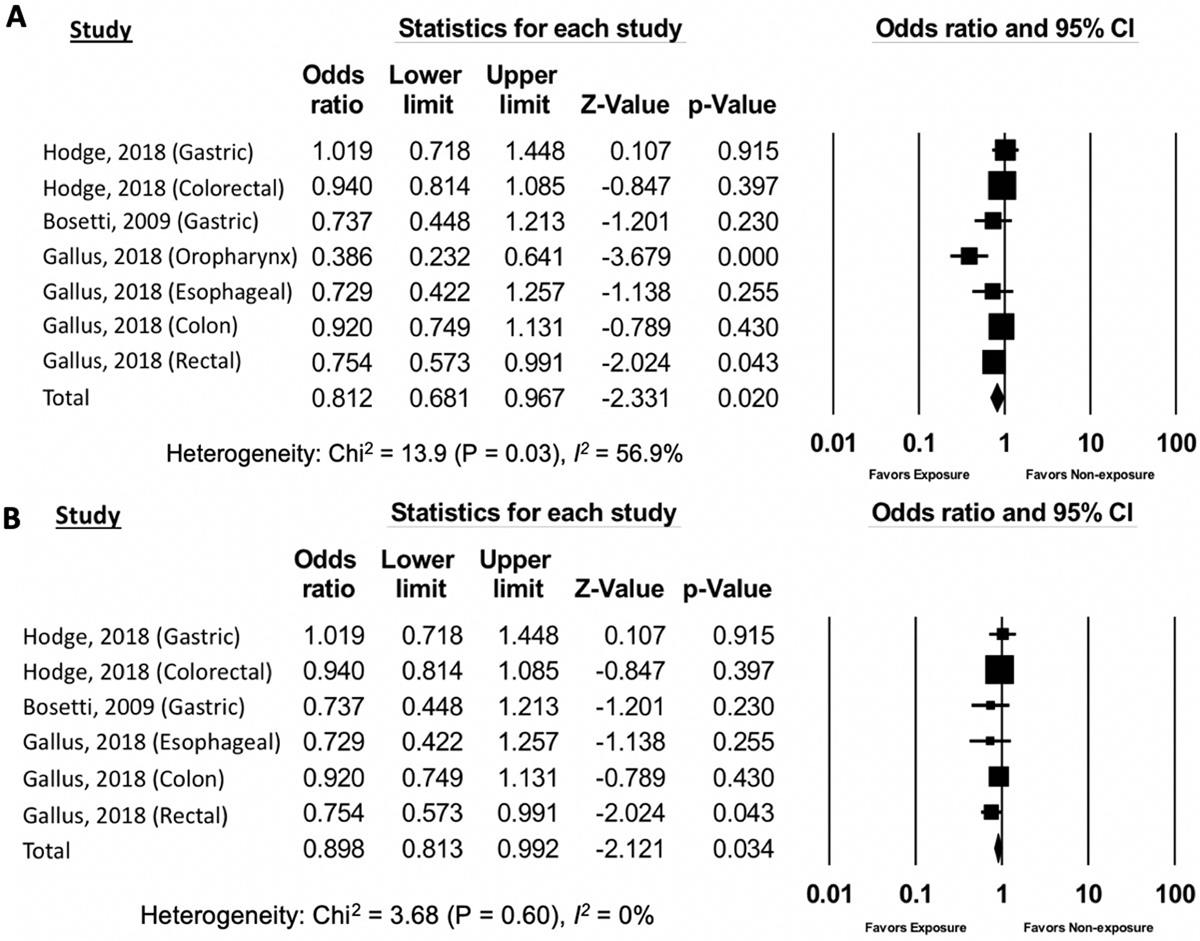
The association of artificial sweetener consumption with luminal gastrointestinal cancers (3a: oropharyngeal cancers included, 3b: oropharyngeal cancers excluded
Based on a subgroup analysis of the studies that included consumption of diet beverages containing AS, there was no association between diet beverages and odds of GI cancer (OR 1.02, 95% CI: 0.91–1.13) (Supplemental Figure 2, Supplemental Table 3). Of note, of the 5 separate studies included in the sub-analysis, 4 analyzed pancreatic cancer while Hodge et al. analyzed gastric cancer and colorectal cancer separately. Removing the study by Hodge et al. did not alter the outcome of the analysis (data not shown).
Meta-regression analysis of prospective versus case-control study design demonstrated no significant difference in effect size between the two study types (p=0.47, Supplemental Figure 3). Overall, the meta-analysis results were potentially most influenced by limitations of exposure ascertainment, including variation of AS measurement and cutoffs, and by the inability to separate AS by type.
3.3. Publication bias and heterogeneity
The funnel plot generated from the included studies was symmetrical, suggesting no significant publication bias. This was confirmed by Egger’s test (p=0.42, Supplemental Figure 4). There was no statistically significant heterogeneity (Chi-squared test p =0.60; I2=0%) among the studies including in the two primary meta-analyses—that is the association between AS exposure and odds of GI luminal (Chi-squared test p =0.60; I2=0%, Figure 3b) or non-luminal (Chi-squared test p =0.13; I2=35.8%; Figure 2) cancer. However, including the one study of AS exposure and odds of oropharyngeal cancer [41] in the GI luminal cancer meta-analysis increased the heterogeneity estimates significantly, such that there was moderate heterogeneity (Chi-squared test p =0.03; I2=56.9%; Figure 3a). The secondary analyses of the association between AS exposure and all luminal or non-luminal GI cancer and AS-sweetened diet beverages and all GI cancer demonstrated substantial heterogeneity that was statistically significant (Supplemental Figure 1 and 2).
3.4. Quality Assessment
Based on the NOS risk of bias assessment tool for case-control and cohort studies, all studies were considered “good quality” and achieved a score ≥7.[31] The scores for each subcategory of selection, comparability and exposure categories are provided in Supplemental Table 2. All case-control studies received a score of 7 and all prospective cohort studies received a score of 8.
4. Discussion
We conducted a comprehensive systematic review and identified 4305 cases of luminal and 3271 cases of non-luminal GI cancer among 1,043,496 people, the majority derived from prospective cohort studies. The corresponding meta-analysis demonstrated that while AS intake was associated with a modest, but statistically significant lower likelihood of luminal GI tract cancer, there was no association with pancreatic cancer, which was the only non-luminal GI cancer meeting inclusion criteria for the analysis. There was no difference in effect estimates based on prospective versus case-control study design. To our best knowledge, this is the first meta-analysis analyzing the association between AS, including a separate analysis of diet beverages, and GI cancers. Overall, these data, which represent over 1 million people globally, are congruent with the majority of contemporary epidemiological data demonstrating no association between AS and increased risk of cancer, although the modest reduction in luminal GI tract cancer warrants further investigation, ideally with well-designed interventional studies.
There has been a plethora of human studies over the past few decades analyzing the impact of each of the FDA-approved AS on various non-malignant and primarily metabolic health parameters, such as weight gain and glucose metabolism. While heterogeneity across study designs and populations complicates direct comparison, no AS has consistently been associated with adverse health effects and, based on FDA scrutiny, all are designated as safe for consumption.[47] However, the carcinogenic potential of AS remains a point of controversy and is not rigorously studied for gastrointestinal cancers. Some of the byproducts of AS, for example formaldehyde as a metabolic byproduct of aspartame, or additional components of diet beverages, such as 4-methylimidazole, are established carcinogens and provide biological plausibility through mechanisms identified in experimental studies, such as DNA damage, chromosomal aberrations and mitotic errors.[48, 49] This is especially the case for GI cancers given their intimate role in digestion and direct contact with byproducts of digestion. However, the quantity and duration of exposure for which these byproducts are clinically significant with respect to cancer risk in humans is not precisely defined and complicates our understanding; while it is unlikely, based on current evidence, that standard daily consumption of AS is associated with clinically significant risk, excessive consumption particularly in someone with additional predisposing risk factors, might be relevant. To this end, all human studies including those in this meta-analysis, have included participants with non-excessive consumption with the threshold for “AS exposure” relatively low (e.g. more than one sachet [small packet] or tablet per day).
Other considerations perhaps explaining the association we observed between AS and lower odds of luminal GI tract cancer are the indirect effects of AS consumption, such as reduced intake of sugar-sweetened foods, especially beverages. There is some evidence to suggest that AS might positively affect metabolic parameters including insulin sensitivity, so long as compensatory caloric intake does not occur and shroud these potential benefits.[5, 50–52] Accordingly, it is plausible that the corresponding decrease in sugar-sweetened food/beverages might explain, in part, the observed association of AS with lower odds of GI luminal tract cancer. Sugar-sweetened beverages in particularly have been associated with obesity, weight gain, glucose intolerance and overt diabetes mellitus type II, each of which are independently and positively associated with colorectal cancer risk.[53] Indeed, colorectal cancer, which accounted for the largest weight in the meta-analysis of GI luminal tract cancers, is one of the cancers that is most consistently associated with sugar-containing food/beverages, with the mechanism of carcinogenesis hypothesized to relate to heightened synthesis of insulin-like growth factor, as well as possibly secondary bile acid production and gut microbiome changes.[47, 54–59] AS might also be associated with microbiome changes, although these are not as well-defined.[11, 12] The findings from a large prospective cohort study analyzing the association between nutritional intakes and lifestyle habits and the risk of subsequent obesity-related cancers, including colorectal and gastric cardia cancer, supports the hypothesis that compensatory decrease in sugar-sweetened foods/beverages might confound the association between AS and lower odds of GI tract cancer. Based on this prospective analysis of over 35,500 participants without diabetes in Australia followed for over three decades including linkage to the Australian Cancer Database and the Victorian Cancer Registry, sugar-sweetened beverages were associated with a 28% increased risk of colorectal cancer after adjusting for several relevant confounders, including age, sex, waist circumference, alcohol, smoking, physical activity, and Mediterranean diet score; however, while there was a trend for a protective association between AS and colorectal cancer, this was not statistically significant after adjusting for sugar-sweetened beverages in addition to the other factors listed.[38]
The association between sweetened beverages and risk of pancreatic cancer has been mixed, with several studies showing an increased risk,[25] but others showing a null association.[37] There also has been no consistent association between AS or diet beverages and pancreatic cancer after adjusting for relevant confounders, including sugar-sweetened beverages and obesity, which is confirmed by our meta-analysis; although, one case-control study did demonstrate a suggestive trend for AS and reduced odds of pancreatic cancer (adjusted OR 0.62, 95% CI: 0.37–1.04), which was significant for individuals at least 60 years old and also among those with caloric intakes ≥2100 calories/day (study-specific caloric intake threshold per Bosetti et al.).[21] Notably, long-term consumption of sugar-sweetened but not artificially sweetened beverages has been associated with increased risk of cancer-related mortality.[60] While we excluded analyses of cancer-related mortality, no study in our search analyzed GI cancer-related mortality specifically. Our finding that AS are associated with significantly lower odds of luminal GI tract cancer therefore holds importance irrespective of whether it is via biological mechanisms such as microbiome changes or via indirect mechanisms through reduction in sugar-sweetened beverage intake and improved metabolic parameters. Our findings, particularly in the context of non-GI cancer literature, supports not only the safety of AS with respect to GI cancer risk, but also supports the current public health recommendations to limit sugar-sweetened food/beverages.
In addition to our comprehensive search strategy, our study has several other strengths. This is the first meta-analysis analyzing the association between AS and luminal and non-luminal GI tract cancers. All studies stated which confounders were adjusted for in the respective study’s analysis, including metabolic and lifestyle parameters such as physical activity. Outcome assessment was appropriate, since all studies included only histologically confirmed cancer cases. For studies which included multiple cancers, we were able to appropriately separate cases from controls and ensure that cases were independent of each other, without duplication of entries (e.g. more than one type of cancer). There was no statistically significant heterogeneity across the included studies. Notwithstanding, our study does have limitations, most of which are inherent limitations of meta-analyses. One primary limitation is with respect to exposure measurement and categorization. The included studies used FFQs, which have the intrinsic limitation that these are based on subjective self-report rather than direct confirmed measurements of intake; that said, each of the FFQs were validated as appropriate metrics of actual intake. The FFQs also varied across the studies, but again were validated for the population in which these instruments were used. We therefore do not think that this significantly influenced our findings, as also evidenced by the lack of significant heterogeneity across included studies. The exposure threshold and duration also varied, and we are therefore unable to comment on whether there is a dose-dependent association between AS and luminal GI tract cancers. There was also an insufficient number of studies and granularity of details to perform separate meta-analyses based on AS type (e.g. saccharin, aspartame), although we acknowledge the relevance. While we conducted a separate analysis for diet AS-containing beverages, we are not able to fully adjust for unmeasured additives or other possible unmeasured confounders. There is also the potential for recall bias in the case-control studies, although we conducted a meta-regression for study design and did not identify and difference in effect between prospective cohort vs. case-control studies. In addition to clarifying dose threshold for effect and the influence of AS type, future studies, and distinctly experimental studies, should focus on identifying putative mechanisms underlying our observations.
In conclusion, we demonstrated that AS consumption is associated with a reduced likelihood of luminal GI tract cancers, but not pancreatic cancer. Given the very high prevalence of current AS use in US, which is projected to only increase in the face of the obesity epidemic and rising rates of diabetes, it is critical that we better define both the biological and non-biological mechanisms underlying this observation. Rigorous scrutiny of these findings would be best achieved with an interventional study design, although the logistical limitations, including prolonged follow up time for the outcome occurrence (i.e. luminal GI tract cancer) are acknowledged. That said, given the potential public health implications of providing evidenced-based guidance regarding the cancer reducing benefit, or lack thereof, of one of the mostly commonly consumed food/beverage additives would have great value from a public health promotion standpoint with respect to reducing cancer burden.
Supplementary Material
5.1. Sources of Support
SCS is funded by the Agency for Healthcare Research (AHRQ) and Quality and Patient-Centered Outcomes Research Institute (PCORI) [grant number: K12 HS026395], a 2019 American Gastroenterological Association Research Scholar Award, and Veterans Affairs Career Development Award [grant number: ICX002027A01]. The content is solely the responsibility of the listed authors and does not necessarily represent the official views of the funding agencies listed.
Abbreviations
- AARP
American Association for Retired Persons
- AS
artificial sweeteners
- BMI
body mass index
- CI
confidence interval
- EPIC
European Prospective Investigation into Cancer and nutrition
- FDA
Federal Drug Administration
- FFQ
food frequency questionnaires
- GI
gastrointestinal
- HPFS
Health Professionals Follow-up Study
- NHS
Nurses’ Health Study
- NOS
Newcastle-Ottawa Scale
- OR
odds ratios
- PRISMA
Preferred Items for Systematic Reviews and Meta-Analyses
- US
United States
Footnotes
Author Declarations
The authors have no potential conflicts (financial, professional, nor personal) that are relevant to this manuscript. This work was accepted for abstract presentation at the Annual American College of Gastroenterology national conference in October 2020.
6. References
- 1.Durán Agüero S, Record Cornwall J, Encina Vega C, Salazar de Ariza J, Cordón Arrivillaga K, Cereceda Bujaico Mdel P, et al. Consumption of carbonated beverages with nonnutritive sweeteners in Latin American university students. Nutr Hosp 2014;31:959–65. doi: 10.3305/nh.2015.31.2.8026. [DOI] [PubMed] [Google Scholar]
- 2.Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes 2013;8:294–306. doi: 10.1111/j.2047-6310.2013.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav 2016;164:446–50. doi: 10.1016/j.physbeh.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr 2012;96:640–6. doi: 10.3945/ajcn.112.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Després JP, Hu FB, et al. Low-Calorie Sweetened Beverages and Cardiometabolic Health: A Science Advisory From the American Heart Association. Circulation 2018;138:e126–e40. doi: 10.1161/CIR.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 6.United States Food and Drug Administration. Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States. (Accessed 23 April 2020 at https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states)
- 7.Roberts JR. The paradox of artificial sweeteners in managing obesity. Curr Gastroenterol Rep 2015;17:423. doi: 10.1007/s11894-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 8.Pepino MY. Metabolic effects of non-nutritive sweeteners. Physiology & behavior 2015;152:450–5. doi: 10.1016/j.physbeh.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crichton G, Alkerwi A, Elias M. Diet Soft Drink Consumption is Associated with the Metabolic Syndrome: A Two Sample Comparison. Nutrients 2015;7:3569–86. doi: 10.3390/nu7053569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira MA. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: a review of the evidence. Nutr Rev 2013;71:433–40. doi: 10.1111/nure.12038. [DOI] [PubMed] [Google Scholar]
- 11.Feijó FM, Ballard CR, Foletto KC, Batista BAM, Neves AM, Ribeiro MFM, et al. Saccharin and aspartame, compared with sucrose, induce greater weight gain in adult Wistar rats, at similar total caloric intake levels. Appetite 2013;60:203–7. doi: 10.1016/j.appet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181–6. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 13.Pearlman M, Obert J, Casey L. The Association Between Artificial Sweeteners and Obesity. Curr Gastroenterol Rep 2017;19:64. doi: 10.1007/s11894-017-0602-9. [DOI] [PubMed] [Google Scholar]
- 14.Weihrauch MR, Diehl V. Artificial sweeteners--do they bear a carcinogenic risk? Ann Oncol 2004;15:1460–5. doi: 10.1093/annonc/mdh256. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong B, Doll R. Bladder cancer mortality in diabetics in relation to saccharin consumption and smoking habits. Br J Prev Soc Med 1975;29:73–81. doi: 10.1136/jech.29.2.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howe GR, Burch JD, Miller AB, Morrison B, Gordon P, Weldon L, et al. Artificial sweeteners and human bladder cancer. Lancet 1977;2:578–81. doi: 10.1016/s0140-6736(77)91428-3. [DOI] [PubMed] [Google Scholar]
- 17.Hoover RN, Strasser PH. Artificial sweeteners and human bladder cancer. Preliminary results. Lancet 1980;1:837–40. doi: 10.1016/s0140-6736(80)91350-1. [DOI] [PubMed] [Google Scholar]
- 18.Cartwright RA, Adib R, Glashan R, Gray BK. The epidemiology of bladder cancer in West Yorkshire. A preliminary report on non-occupational aetiologies. Carcinogenesis 1981;2:343–7. doi: 10.1093/carcin/2.4.343. [DOI] [PubMed] [Google Scholar]
- 19.Wynder EL, Stellman SD. Artificial sweetener use and bladder cancer: a case-control study. Science 1980;207:1214–6. doi: 10.1126/science.7355283. [DOI] [PubMed] [Google Scholar]
- 20.Dybing E, Sanner T. Species differences in chemical carcinogenesis of the thyroid gland, kidney and urinary bladder. IARC scientific publications 1999:15–32. (no doi available) [PubMed] [Google Scholar]
- 21.Bosetti C, Gallus S, Talamini R, Montella M, Franceschi S, Negri E, et al. Artificial sweeteners and the risk of gastric, pancreatic, and endometrial cancers in Italy. Cancer Epidemiol Biomarkers Prev 2009;18:2235–8. doi: 10.1158/1055-9965.EPI-09-0365. [DOI] [PubMed] [Google Scholar]
- 22.Lim U, Subar AF, Mouw T, Hartge P, Morton LM, Stolzenberg-Solomon R, et al. Consumption of aspartame-containing beverages and incidence of hematopoietic and brain malignancies. Cancer Epidemiol Biomarkers Prev 2006;15:1654–9. doi: 10.1158/1055-9965.EPI-06-0203. [DOI] [PubMed] [Google Scholar]
- 23.Soffritti M, Belpoggi F, Degli Esposti D, Lambertini L, Tibaldi E, Rigano A. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ Health Perspect 2006;114:379–85. doi: 10.1289/ehp.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagiwara A, Fukushima S, Kitaori M, Shibata M, Ito N. Effects of three sweeteners on rat urinary bladder carcinogenesis initiated by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Gan 1984;75:763–8. (no doi available) [PubMed] [Google Scholar]
- 25.Genkinger JM, Li R, Spiegelman D, Anderson KE, Albanes D, Bergkvist L, et al. Coffee, tea, and sugar-sweetened carbonated soft drink intake and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Cancer Epidemiol Biomarkers Prev 2012;21:305–18. doi: 10.1158/1055-9965.EPI-11-0945-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guercio BJ, Zhang S, Niedzwiecki D, Li Y, Babic A, Morales-Oyarvide V, et al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). PLoS One 2018;13:e0199244. doi: 10.1371/journal.pone.0199244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Vecchia C, Bosetti C, Negri E, Franceschi S. Refined sugar intake and the risk of gastric cancer. Int J Cancer 1998;78:130–1. doi: . [DOI] [PubMed] [Google Scholar]
- 28.Mahfouz EM, Sadek RR, Abdel-Latief WM, Mosallem FA, Hassan EE. The role of dietary and lifestyle factors in the development of colorectal cancer: case control study in Minia, Egypt. Cent Eur J Public Health 2014;22:215–22. doi: 10.21101/cejph.a3919. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook. [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ottawa Hospital Research Institute. 2019. (Accessed December 22, 2019, at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.)
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2013;97:517–23. doi: 10.3945/ajcn.112.050997. [DOI] [PubMed] [Google Scholar]
- 34.Ewertz M, Gill C. Dietary factors and breast-cancer risk in Denmark. Int J Cancer 1990;46:779–84. doi: 10.1002/ijc.2910460505. [DOI] [PubMed] [Google Scholar]
- 35.Bunin GR, Kushi LH, Gallagher PR, Rorke-Adams LB, McBride ML, Cnaan A. Maternal diet during pregnancy and its association with medulloblastoma in children: a children’s oncology group study (United States). Cancer Causes Control 2005;16:877–91. doi: 10.1007/s10552-005-3144-7. [DOI] [PubMed] [Google Scholar]
- 36.Schernhammer ES, Hu FB, Giovannucci E, Michaud DS, Colditz GA, Stampfer MJ, et al. Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol Biomarkers Prev 2005;14:2098–105. doi: 10.1158/1055-9965.EPI-05-0059. [DOI] [PubMed] [Google Scholar]
- 37.Navarrete-Muñoz EM, Wark PA, Romaguera D, Bhoo-Pathy N, Michaud D, Molina-Montes E, et al. Sweet-beverage consumption and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2016;104:760–8. doi: 10.3945/ajcn.116.130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodge AM, Bassett JK, Milne RL, English DR, Giles GG. Consumption of sugar-sweetened and artificially sweetened soft drinks and risk of obesity-related cancers. Public Health Nutr 2018;21:1618–26. doi: 10.1017/S1368980017002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao Y, Stolzenberg-Solomon R, Jiao L, Silverman DT, Subar AF, Park Y, et al. Added sugar and sugar-sweetened foods and beverages and the risk of pancreatic cancer in the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr 2008;88:431–40. doi: 10.1093/ajcn/88.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norell SE, Ahlbom A, Erwald R, Jacobson G, Lindberg-Navier I, Olin R, et al. Diet and pancreatic cancer: a case-control study. Am J Epidemiol 1986;124:894–902. doi: 10.1093/oxfordjournals.aje.a114479. [DOI] [PubMed] [Google Scholar]
- 41.Gallus S, Scotti L, Negri E, Talamini R, Franceschi S, Montella M, et al. Artificial sweeteners and cancer risk in a network of case-control studies. Ann Oncol 2007;18:40–4. doi: 10.1093/annonc/mdl346. [DOI] [PubMed] [Google Scholar]
- 42.Chan JM, Wang F, Holly EA. Sweets, sweetened beverages, and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control 2009;20:835–46. doi: 10.1007/s10552-009-9323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 44.Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel MT, La Vecchia C, et al. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Annals of epidemiology 1996;6:110–8. doi: 10.1016/1047-2797(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 45.Ireland P, Jolley D, Giles G, O’Dea K, Powles J, Rutishauser I, et al. Development of the Melbourne FFQ: a food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pacific journal of clinical nutrition 1994;3:19–31. (no doi available) [PubMed] [Google Scholar]
- 46.Bassett JK, English DR, Fahey MT, Forbes AB, Gurrin LC, Simpson JA, et al. Validity and calibration of the FFQ used in the Melbourne Collaborative Cohort Study. Public Health Nutr 2016;19:2357–68. doi: 10.1017/S1368980016000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. Journal of the National Cancer Institute 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 48.Yilmaz S, Ucar A. A review of the genotoxic and carcinogenic effects of aspartame: does it safe or not? Cytotechnology 2014;66:875–81. doi: 10.1007/s10616-013-9681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rycerz K, Jaworska-Adamu JE. Effects of aspartame metabolites on astrocytes and neurons. Folia neuropathologica 2013;51:10–7. doi: 10.5114/fn.2013.34191. [DOI] [PubMed] [Google Scholar]
- 50.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, et al. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes care 2012;35:1798–808. doi: 10.2337/dc12-9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 53.Popkin BM. Understanding global nutrition dynamics as a step towards controlling cancer incidence. Nature reviews Cancer 2007;7:61–7. doi: 10.1038/nrc2029. [DOI] [PubMed] [Google Scholar]
- 54.Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. The Proceedings of the Nutrition Society 2012;71:181–9. doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Uchida K, Ohnaka K, Morita M, Toyomura K, Kono S, et al. Sugars, sucrose and colorectal cancer risk: the Fukuoka colorectal cancer study. Scandinavian journal of gastroenterology 2014;49:581–8. doi: 10.3109/00365521.2013.822091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. The Proceedings of the Nutrition Society 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 58.Kruis W, Forstmaier G, Scheurlen C, Stellaard F. Effect of diets low and high in refined sugars on gut transit, bile acid metabolism, and bacterial fermentation. Gut 1991;32:367–71. doi: 10.1136/gut.32.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiseman M The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. The Proceedings of the Nutrition Society 2008;67:253–6. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 60.Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, et al. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019;139:2113–25. doi: 10.1161/CIRCULATIONAHA.118.037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


