Abstract
Purpose of review:
In the era of immune-oncology, a breakthrough in the field of pediatric solid tumor research has been the demonstration that immunotherapy for patients with high-risk neuroblastoma improves the event-free and overal survival. Immunotherapeutic approaches including a monoclonal antibody targeting the cell surface glycosphingolipid disialoganglioside and cytokines successfully eliminate minimal residual disease.
Recent findings:
Since this seminal discovery, clinical trials evaluating immunotherapy in combination with chemotherapy and cellular therapies have begun to demonstrate effectiveness in treatment of bulky disease. Broader knowledge has also been gained regarding immunotherapy-limiting side effects. Furthermore, biologic studies in actively treated patients have contributed to our growing understanding of the underlying immunologic processes and mechanisms of tumor response and immune evasion.
Summary:
The example of neuroblastoma is beginning to demonstrate that various immunotherapies combined with more conventional anticancer treatments can be synergistic. These advancements pose new challenges to both clinical researchers and medical provider and herald a new era in pediatric cancer therapy.
Keywords: Neuroblastoma, pediatric cancer, immunotherapy, personalized medicine
Introduction
In the era of immune-oncology, a breakthrough in the field of pediatric solid tumor research has been the demonstration that immunotherapy for patients with high-risk neuroblastoma improves the event-free survival. This review will outline the standard of care before and after the discovery of immunotherapy for these patients. Furthermore, important side effects and novel immunotherapy approaches will be reviewed.
Clinical introduction to neuroblastoma
Neuroblastoma is a developmental childhood cancer that arises from the neural crest and affects young children with an average age of 17.3 months (1, 2). Although most patients are toddlers, neuroblastoma can also occur in infants or adolescents (1). As the most common extracranial malignancy in pediatrics, it accounts for about 15% of all cancer-related deaths in children in the United States (3). About half of all patients present with features that are associated with a high risk for adverse outcomes (4). These risk categories factor in the age at diagnosis, primary tumor extent, DNA ploidy, molecular features, and histologic criteria (4, 5).
Neuroblastoma arises along the parasympathetic ganglia chain from presumptive neural crest tissue. The presenting signs and symptoms are related to the tumor location and involvement of adjacent anatomic structures. The most common areas of involvement are the adrenal glands, the abdomen, or the cervical and thoracic paraspinal areas (6). Two-third of patients have metastatic spread to the regional lymph nodes (7). Other sites of involvement are the bone, bone marrow, skin, and liver. The extent of distant metastases at diagnosis is the most important predictive factor of outcome (8, 9).
Neuroblastoma has a very heterogeneous course that ranges from spontaneous involution with no therapy to fatal outcomes despite extensive multimodal treatment. As an example, infants with stage 4S neuroblastoma present with disseminated tumors confined to the skin, liver, and bone marrow that will often regress spontaneously over time in many of these patients without therapy (5, 10, 11). In contrast, about half of all other children with stage 4 disease (dissemination to distant lymph nodes, bone, bone marrow, liver, skin and/or other organs except as defined for stage 4S) will fail to attain durable remissions and ultimately succumb to their disease despite intensive therapy that spans over 1.5 years (12–14).
Therapy regimens for patients with newly diagnosed neuroblastoma
The intensity of therapy for patients with neuroblastoma is risk-adapted. Patients with low-risk disease undergo surgery alone, which is sufficient to achieve long-term cures in more than 90% of the patients, even in cases with incomplete resection (15, 16). An exception applies to children younger than 6 months with small adrenal tumors, who can be observed (17). However, pre-emptive chemotherapy improves the outcome in infants younger than 2 months with developing hepatomegaly or baseline comorbidities (18).
Patients with intermediate-risk disease receive chemotherapy followed by surgery in some cases. In the randomized-controlled Phase III trial by the Children’s Oncology Group (COG), the duration of neoadjuvant chemotherapy was shortened when patients lacked unfavorable histology or segmental chromosomal aberrations or had a DNA index of >1. Surgery was omitted in patients who achieved a partial response with chemotherapy. The trial reported a 3-year event-free survival (EFS) that exceeded 80% and overall survival (OS) of 95% (19).
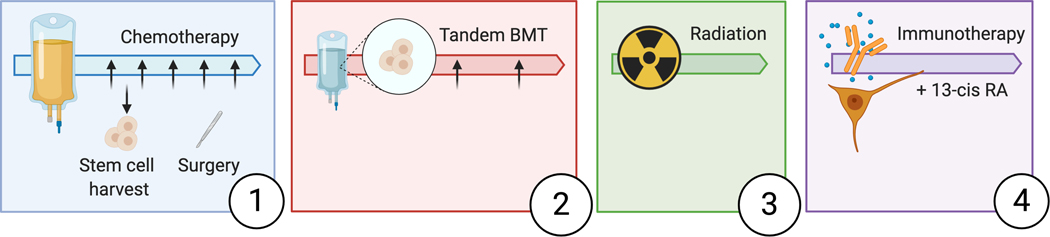
In contrast, the treatment of children with high-risk disease is complex and contains multiple consecutive phases (Figure 1). During induction, patients receive multi-agent chemotherapy (13, 14). The goal of chemotherapy is to render the tumor amenable for surgical removal and eliminate metastases. The next phase is comprised of consolidation therapy with autologous tandem bone marrow transplantations (13). Local therapy control is achieved by radiation (20). The maintenance phase of therapy integrates differentiation therapy with 13-cis-retinoic acid and immunotherapy. Patients entering this phase have attained a state of minimal residual disease (MRD), which was initially thought to be the best suited clinical scenario to test monoclonal antibody therapy and cytokines (21).
Figure 1:
Therapy regimen of patients with high-risk neuroblastoma. Patients undergo different phases of therapy starting with induction chemotherapy (1). Surgery is performed during induction. Consolidation therapy (2) consists of tandem transplant (BMT). Patients undergo radiation therapy to the primary tumor bed after consolidation therapy to prevent local tumor recurrence (3) and maintenance therapy in the end (4).
The current standard of immunotherapy for patients with neuroblastoma
The disialoganglioside GD2 is expressed at high levels on neuroblastoma cells (5–10 × 106 molecules per cell) (22). In healthy tissue, this molecule is expressed by cells of neuroectodermal origin (i.e., neurons, peripheral pain fibers, and melanocytes) (23). The restricted expression of this antigen in normal tissue made it a desirable candidate for antibody development.
Antibody-dependent cell-mediated cytotoxicity (ADCC) in cancer therapy is a process whereby tumor cells are specifically labeled with an antibody and subsequently recognized and eliminated by immune effector cells such as natural killer (NK) cells or granulocytes (24). The efficiency by which these effector cells eliminate tumor cells can be enhanced, for example, by the addition of cytokines (25). In patients with neuroblastoma, granulocyte-macrophage colony-stimulating factor (GM-CSF) was added to the immunotherapy regimen to enhance neutrophil-mediated ADCC (26, 27). Interleukin (IL)-2 was included to improve ADCC by lymphocytes (28).
IL-2 was the first immunotherapeutic agent approved for the treatment of patients with cancer (29, 30). Common regimens in pediatric immunotherapy trials include low-dose (1 × 106 IU/m2) or higher doses of IL-2 (3 to 6 × 106 IU/m2) as intravenous or subcutaneous injection (21, 31–33). Nevertheless, the benefit of IL-2 is debated owing to severe toxicities associated with higher doses, and unestablished efficacy at lower doses (34).
Historically, less than 10% of patients with high-risk neuroblastoma achieved long-term cures (1). Distant metastases and MRD in the bone marrow used to be the main culprit of late disease relapse; however, consolidation therapy with autologous bone marrow transplantation and maintenance therapy with 13-cis-retinoic acid significantly increased the progression-free survival rates to about 50% (31, 35, 36), which was further improved with the addition of immunotherapy comprised of a monoclonal antibody and cytokines (i.e., IL-2 and GM-CSF) (21). In a Phase III trial by the COG, patients who completed induction and consolidation therapy were randomized to receive immunotherapy together with 13-cis-retinoic acid or 13-cis-retinoic acid alone (21). The addition of immunotherapy to the maintenance phase significantly lengthened the 2-year EFS (66±5% vs. 46±5%) and OS (86±4% vs. 75±5%) of study participants (21) and has marked a breakthrough in recent pediatric solid tumor research. Since this seminal discovery, the anti-GD2 antibody ch14.18 (dinutuximab) without cytokines has also been combined with chemotherapy (i.e., temozolomide and irinotecan) (37). More than half of the patients with relapsed/refractory neuroblastoma randomized to the immunotherapy arm showed objective response rates (53%). In contrast, only 6% of patients treated with chemotherapy alone (i.e., temozolomide, irinotecan, and temsirolimus) achieved an objective response (37). Another Phase II trial by the St. Jude Children’s Research Hospital tested the efficacy of immunotherapy with another anti-GD2 antibody, hu14.18K322A, IL-2, and GM-CSF administered upfront with multi-agent chemotherapy to newly diagnosed patients. Thirty-two of the 42 participants (76%) had a partial response or better after two courses of treatment, which exceeded response rates in the historical control cohort from the ANBL02P1 trial, which used identical chemotherapy without hu14.18K322A/cytokines (40% [22.7–59.4]%) (38).
Recent data from a European Phase III trial has raised considerable doubt on the added benefit of subcutaneous IL-2 (31, 32, 39). In the cited study, the investigators noted increased toxicity and lack of efficacy with the addition of 3 × 106 IU/m2 of IL-2 (32). Based on this, IL-2 has been eliminated in the ongoing COG trials for newly diagnosed children with high-risk disease.
Side effects of immunotherapies in neuroblastoma
Early immunotherapy trials were conducted with the murine monoclonal anti-GD2 antibodies 3F8 (IgG3) and 14G2a (IgG2a) and the chimeric, now FDA-approved version, ch14.18 (dinutuximab) (40–42). Children receiving these antibodies experienced dose-dependent pain, fever, tachycardia, capillary leak, and occasionally, allergic reactions, hypotension, electrolyte imbalances, and gastrointestinal symptoms (40–42). The occurrence of allodynia and inflammation was attributed to the ability of the antibody to fix complement after binding to peripheral nerves, resulting in neuronal activation (43). Hu14.18K322A is a humanized anti-GD2 antibody that possesses an amino acid substitution in the constant region of the antibody designed to decrease or eliminate complement activation. Consistent with that, in a Phase I trial of hu14.18K322A, none of the participants developed capillary leak syndrome (44). Pain was still present but easier to control (45). The incidences of the other side effects were similar compared to dinutuximab. Currently, hu14.18K322A is administered as a 4-hour infusion with extensions possible if patients have persistent pain despite concomitant analgesia with intravenous narcotics (33). Per the manufacturer’s recommendation, dinutuximab is given for 10 to 20 hours (Unituxin [dinutuximab] prescribing information. Silver Spring, MD: United Therapeutics Corp; March 2017). The SIOPEN Phase II trial (APN-311–202) administered ch14.18/CHO as a long-term infusion over 10 consecutive days together with subcutaneous IL-2 at 6 × 106 IU/m2 per day as two 5-day blocks. The investigators reported lower pain scores and morphine requirements. Future studies are necessary to delineate the ideal antibody dosing and infusion schedule for patients with neuroblastoma.
Similar to previous reports, patients with neuroblastoma receiving IL-2 experience dose-dependent acute inflammatory reactions and cardio-respiratory problems caused by capillary leaking, fever, and leukocytosis (34), which is more common during co-administration with dinutuximab (21). While low-dose IL-2 is better tolerated, it potentiates the proliferation of immune-suppressive regulatory T cells (39, 46).
Other rare side effects, such as fatal hemophagocytic lymphohistiocytosis (HLH), a life-threatening hyper-inflammatory syndrome, have been associated with the experimental administration of hu14.18K322A and haploidentical adoptive NK cells during autologous bone marrow transplantation (47).
In general, the acute toxicities engendered by experimental or standard immunotherapies in patients with neuroblastoma are distinct from side effects caused by conventional chemotherapy or radiation therapy. Immunotherapy is currently administered in experienced centers with sufficient expertise and a supportive infrastructure. Optimal surveillance protocols are being developed as we learn more about the long-term sequelae of immunotherapies (e.g., autoimmune conditions) in childhood cancer survivors.
Novel immunotherapy approaches to target neuroblastoma
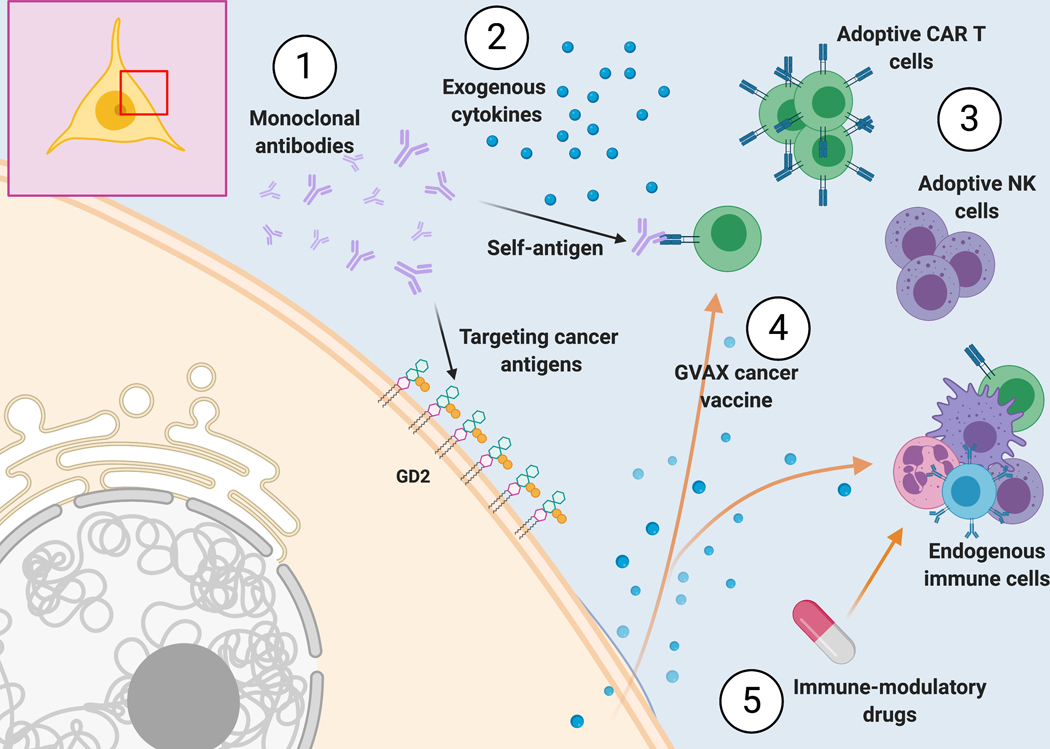
In the following section, we will review ongoing clinical trials and the rationale behind them (Figure 2).
Figure 2:
Immunotherapy approaches to target neuroblastoma. (1) Monoclonal antibodies can either target cancer antigens or serve as self-antigens (i.e., as idiotype antibody that can be bound by the variable region of other antibodies or by T or B cell receptors). (2) The administration of exogenous cytokines can directly influence the immune or cancer cells. (3) Adoptive cells are collected from a donor or the patient, can be manipulated ex vivo, and are infused back into the patient. (4) Vaccine strategies can either provide a cancer antigen to induce immunogenicity or alter the immunologic equilibrium through the release of cytokines such as GM-CSF with the GVAX vaccine. (5) Immune-modulatory drugs have a direct or indirect impact on the endogenous immune cells.
GM-CSF gene vaccine
To generate the GM-CSF gene vaccine (GVAX) against neuroblastoma, autologous neuroblastoma cells are obtained from the patient and genetically engineered to secrete human GM-CSF. The cells are subsequently irradiated to abrogate future proliferation. Upon infusion into the patient, secretion of GM-CSF is thought to induce immune activation and enhance ADCC as demonstrated in animal studies (48). In an ongoing Phase I trial, GVAX is administered with immune checkpoint blockade (ICB; i.e., nivolumab and ipilimumab) to patients with relapsed/refractory neuroblastoma (NCT04239040).
Antibody-mediated immunotherapy
Racotumomab is an anti-idiotype antibody, which can bind to the variable region of another antibody. Through this mechanism, it mimics N-glycolyl GM3 (NGcGM3) and induces an immune response similar to other cancer vaccines. NGcGM3 is a ganglioside but, unlike GD2, it is undetectable in healthy human tissues and fluids due to genetic deletion of CMP-N-acetyl hydroxylase that is required for its enzymatic synthesis during evolution (49). However, NGcGM3 is enriched in several cancers, including neuroblastoma (50). Racotumomab finished Phase I testing, which demonstrated a favorable toxicity profile. However, out of 14 participating patients, only two patients had stable disease, while 12 patients had progressive disease and died (51). Racotumomab is currently in Phase II testing (NCT02998983), where it is given to children with high-risk neuroblastoma that have achieved a complete or very good partial response after standard therapy. An additional arm contains patients with relapsed/refractory disease that receive racotumomab with metronomic chemotherapy.
Chimeric antigen receptor (CAR) T cells
The main challenges with monoclonal anti-GD2 antibody therapy is the limited persistence of the antibody (44), the lack of efficacy as monotherapy against bulky disease (52), and failure to penetrate the blood-brain barrier (53). CAR T cells have the potential to overcome all of these issues. Previous studies in adults and children with leukemia and other hematologic malignancies who received CD19 or CD22 CAR T cells demonstrated that the adoptive T cells can persist as memory CAR T cells, are effective against tumor masses (e.g., chloroma or in cases of lymphoma), and detectable in the central nervous system (54–56).
CAR T cells are made from autologous T cells that undergo genetic engineering to express a CAR. First, peripheral blood mononuclear cells are collected from the patients, and T cells are expanded and enriched in culture in the presence of cytokines. Under good manufacturing conditions, the cells are transduced with a vector, enabling them to produce a CAR that targets a tumor-specific antigen. At the end, patients receive preparative chemotherapy and are subsequently infused with the CAR T cell product. CAR T cells have achieved unprecedented responses in patients with leukemia and are now FDA-approved for several indications (57).
Multiple clinical trials with CAR T cells targeting GD2 are also underway for patients with neuroblastoma (NCT03373097, NCT02765243, NCT02761915, NCT02173093). Some of these studies performed additional modifications to the GD2 CAR T cells. For example, the cells may constitutively express IL-15 for cell activation and the inducible caspase 9 as a safety switch (NCT03721068). IL-7 is critical for T cell survival (58). Thus, some groups have engineered a constitutively activated IL-7 receptor to prolong the persistence of the adoptive CAR T cells (NCT03635632). Additional CAR T cell products target B7H3, a type I transmembrane glycoprotein molecule that is homogenously expressed on a variety of cancer cells, including neuroblastoma but very restricted in healthy tissues (NCT04483778) (59). Another target is CD171 (L1CAM; NCT02311621), which is a transmembrane adhesion molecule that is important for neural cell migration and survival and found on neuroblastoma cells (60).
Immuno-modulatory agents
In the 1960s, thalidomide was incidentally found to improve inflammatory lesions in a patient with erythema nodosum leprosum (61). Lenalidomide is a derivative of thalidomide, which lacks the neurologic side effects but has activity against various solid and hematologic malignancies (62, 63). It can modulate the immune system in many ways. For example, lenalidomide interferes with the cytokine balance by inhibiting the production of the proinflammatory cytokines TNF-α, IL-1, IL-6, IL-12 and increasing the levels of the anti-inflammatory cytokine IL-10 (64). It can also induce co-stimulation and clonal expansion of T cells through direct tyrosine phosphorylation of CD28 and activation of downstream pathways that reverse the ICB by CTLA-4 (65). Lenalidomide also enhances NK cell function and ADCC indirectly by decreasing the levels of IL-6 and tumor growth factor-β and increasing the IL-2 production of by-stander T cells (66, 67). A current clinical Phase I trial assesses the feasibility of combining lenalidomide with dinutuximab and autologous, expanded NK cells in patients with recurrent/refractory neuroblastoma (NCT02573896). Preclinical and clinical testing of other immune-modulatory small molecule combinations with immunotherapy hold promise in the future (68, 69).
Conclusion
The rapidly evolving field of immunotherapy has shaped new treatment approaches in pediatric oncology. Neuroblastoma is an example in which over a short period of time, complex regimens have evolved to treat these patients which have yielded encouraging improvements in long term survival for our patients. Despite the challenges that these new therapies pose to researchers, clinicians and our patients, immunotherapy has heralded a new era in pediatric cancer treatment with promising advances that will improve the survival and possibly lessen our patients’ long-term toxicities in the near future.
Key Bullet Points.
Immunotherapy with a monoclonal antibody targeting GD2 and cytokines eliminates minimal residual disease, thereby improving the event-free survival for patients with high-risk neuroblastoma.
Dose-limiting toxicities of anti-GD2 antibody therapy are allodynia and capillary leak syndrome, but these side effects are less common and less severe with newer generation antibodies that lack complement activation.
Current clinical trials test the feasibility and efficacy of cancer vaccines, adoptive cell transfer, and immune-modulatory treatments in patients with neuroblastoma.
New synergistic combinations of immunotherapies with more conventional anticancer treatments are promising but pose clinical challenges to researchers, clinicians, and our patients.
Acknowledgement
We thank Dr. Wayne L. Furman for his thoughtful comments.
Financial Support and Sponsorship
This work was supported by the Intramural Research Program of the NIH. The figures were generated with Biorender.com.
RN is receiving a grant (CA191207) from the Department of Defense.
Footnotes
Conflict of Interest
The authors have no conflicts of interest otherwise.
References
- 1.Goodman MT GJ, Smith MA, Olshan AF. Sympathetic nervous system tumors. Bethesda, MD: National Cancer Institute; 1999. [Google Scholar]
- 2.Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18(6):719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005;17(1):7–13. [DOI] [PubMed] [Google Scholar]
- 5.Katzenstein HM, Bowman LC, Brodeur GM, Thorner PS, Joshi VV, Smith EI, et al. Prognostic significance of age, MYCN oncogene amplification, tumor cell ploidy, and histology in 110 infants with stage D(S) neuroblastoma: the pediatric oncology group experience--a pediatric oncology group study. J Clin Oncol. 1998;16(6):2007–17. [DOI] [PubMed] [Google Scholar]
- 6.DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21(3):181–9. [DOI] [PubMed] [Google Scholar]
- 7.Brodeur GM HM, Mosse YP, Maris JM. Neuroblastoma. In: Pizzo PA PD, editor. Principles and Practice of Pediatric Oncology:. Philadelphia: Lippincott Williams & Wilkins; 2011. p. 886. [Google Scholar]
- 8.Castleberry RP, Shuster JJ, Smith EI. The Pediatric Oncology Group experience with the international staging system criteria for neuroblastoma. Member Institutions of the Pediatric Oncology Group. J Clin Oncol. 1994;12(11):2378–81. [DOI] [PubMed] [Google Scholar]
- 9.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taggart DR, London WB, Schmidt ML, DuBois SG, Monclair TF, Nakagawara A, et al. Prognostic value of the stage 4S metastatic pattern and tumor biology in patients with metastatic neuroblastoma diagnosed between birth and 18 months of age. J Clin Oncol. 2011;29(33):4358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tas ML, Nagtegaal M, Kraal K, Tytgat GAM, Abeling N, Koster J, et al. Neuroblastoma stage 4S: Tumor regression rate and risk factors of progressive disease. Pediatr Blood Cancer. 2020;67(4):e28061. [DOI] [PubMed] [Google Scholar]
- 12.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA. 2019;322(8):746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9(3):247–56. [DOI] [PubMed] [Google Scholar]
- 15.Alvarado CS, London WB, Look AT, Brodeur GM, Altmiller DH, Thorner PS, et al. Natural history and biology of stage A neuroblastoma: a Pediatric Oncology Group Study. J Pediatr Hematol Oncol. 2000;22(3):197–205. [DOI] [PubMed] [Google Scholar]
- 16.Strother DR, London WB, Schmidt ML, Brodeur GM, Shimada H, Thorner P, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children’s Oncology Group study P9641. J Clin Oncol. 2012;30(15):1842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuchtern JG, London WB, Barnewolt CE, Naranjo A, McGrady PW, Geiger JD, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children’s Oncology Group study. Ann Surg. 2012;256(4):573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twist CJ, Naranjo A, Schmidt ML, Tenney SC, Cohn SL, Meany HJ, et al. Defining Risk Factors for Chemotherapeutic Intervention in Infants With Stage 4S Neuroblastoma: A Report From Children’s Oncology Group Study ANBL0531. J Clin Oncol. 2019;37(2):115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Twist CJ, Schmidt ML, Naranjo A, London WB, Tenney SC, Marachelian A, et al. Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children’s Oncology Group Study ANBL0531. J Clin Oncol. 2019;37(34):3243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas-Kogan DA, Swift PS, Selch M, Haase GM, Seeger RC, Gerbing RB, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children’s Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56(1):28–39. [DOI] [PubMed] [Google Scholar]
- 21.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984;44(12 Pt 1):5914–20. [PubMed] [Google Scholar]
- 23.Lammie G, Cheung N, Gerald W, Rosenblum M, Cordoncardo C. Ganglioside gd(2) expression in the human nervous-system and in neuroblastomas - an immunohistochemical study. Int J Oncol. 1993;3(5):909–15. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen R, Houston J, Chan WK, Finkelstein D, Dyer MA. The role of interleukin-2, all-trans retinoic acid, and natural killer cells: surveillance mechanisms in anti-GD2 antibody therapy in neuroblastoma. Cancer Immunol Immunother. 2018;67(4):615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen R, Moustaki A, Norrie JL, Brown S, Akers WJ, Shirinifard A, et al. Interleukin-15 Enhances Anti-GD2 Antibody-Mediated Cytotoxicity in an Orthotopic PDX Model of Neuroblastoma. Clin Cancer Res. 2019;25(24):7554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, Reisfeld RA. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51(1):144–9. [PubMed] [Google Scholar]
- 27.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73(7):1936–41. [PubMed] [Google Scholar]
- 28.Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, et al. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50(17):5234–9. [PubMed] [Google Scholar]
- 29.Rosenberg SA. Immunotherapy of cancer by systemic administration of lymphoid cells plus interleukin-2. J Biol Response Mod. 1984;3(5):501–11. [PubMed] [Google Scholar]
- 30.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1617–29.30442501 **European trial questioning the benefit of subcutaneous IL-2 as part of the immunotherapy regimen for neuroblastoma. After randomization to receive either dinutuximab beta with IL-2 or dinutuximab beta alone, 38% in the first group and 12% in the latter group dropped out due to toxicities. The 3-year EFS was similar in the remainder of the patients.
- 32.Ladenstein RL, Poetschger U, Valteau-Couanet D, Gray J, Luksch R, Balwierz W, et al. Randomization of dose-reduced subcutaneous interleukin-2 (scIL2) in maintenance immunotherapy (IT) with anti-GD2 antibody dinutuximab beta (DB) long-term infusion (LTI) in front line high-risk neuroblastoma patients: Early results from the HR-NBL1/SIOPEN trial. Journal of Clinical Oncology. 2019;37(15). [Google Scholar]
- 33. Furman WL, Federico SM, McCarville MB, Shulkin BL, Davidoff AM, Krasin MJ, et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin Cancer Res. 2019;25(21):6320–8.31601569 **A single-institution Phase II trial, in which immunotherapy was combined with chemotherapy upfront in patients with newly diagnosed high-risk neuroblastoma. More than three-quarters of the patients achieved a partial response or better after two courses, prompting the COG to initiate a study to test dinutuximab and GM-CSF with upfron chemotherapy in treatment naïve patients with neuroblastoma (ANBL17P1).
- 34.Ribeiro RC, Rill D, Roberson PK, Furman WL, Pratt CB, Brenner M, et al. Continuous infusion of interleukin-2 in children with refractory malignancies. Cancer. 1993;72(2):623–8. [DOI] [PubMed] [Google Scholar]
- 35.Dini G, Lanino E, Garaventa A, Rogers D, Dallorso S, Viscoli C, et al. Myeloablative therapy and unpurged autologous bone marrow transplantation for poor-prognosis neuroblastoma: report of 34 cases. Journal of Clinical Oncology. 1991;9(6):962–9. [DOI] [PubMed] [Google Scholar]
- 36.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341(16):1165–73. [DOI] [PubMed] [Google Scholar]
- 37.Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18(7):946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29(33):4351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holger N. Lode DV-C, Gray Juliet, Luksch Roberto, Wieczorek Aleksandra, Castel Victoria, Ash Shifra, Laureys Genevieve, Papadakis Vassilios, Owens Cormac, Garaventa Alberto, Manzitti Carla, Siebert Nikolai, Sascha Troschke-Meurer Evgenia Glogova, Poetschger Ulrike, Ruth Lydia Ladenstein. Randomized use of anti-GD2 antibody dinutuximab beta (DB) long-term infusion with and without subcutaneous interleukin-2 (scIL-2) in high-risk neuroblastoma patients with relapsed and refractory disease: Results from the SIOPEN LTI-trial. J Clin Oncology. 2019;37((suppl; abstr 10014)). [Google Scholar]
- 40.Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5(9):1430–40. [DOI] [PubMed] [Google Scholar]
- 41.Handgretinger R, Baader P, Dopfer R, Klingebiel T, Reuland P, Treuner J, et al. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol Immunother. 1992;35(3):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16(6):2169–80. [DOI] [PubMed] [Google Scholar]
- 43.Sorkin LS, Otto M, Baldwin WM 3rd, Vail E, Gillies SD, Handgretinger R, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149(1):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32(14):1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anghelescu DL, Pankayatselvan V, Nguyen R, Ward D, Wu J, Wu H, et al. Bisphosphonate Use in Pediatric Oncology for Pain Management. Am J Hosp Palliat Care. 2019;36(2):138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172(11):6519–23. [DOI] [PubMed] [Google Scholar]
- 47.Epperly R, Furman W, Hines M, Santiago T, Li Y, Madden R, et al. Secondary hemophagocytic syndrome after autologous hematopoietic cell transplant and immune therapy for neuroblastoma. Pediatr Blood Cancer. 2019;66(11):e27964. [DOI] [PubMed] [Google Scholar]
- 48.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90(8):3539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95(20):11751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, Sampor C, et al. Detection of N-glycolyl GM3 ganglioside in neuroectodermal tumors by immunohistochemistry: an attractive vaccine target for aggressive pediatric cancer. Clin Dev Immunol. 2011;2011:245181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cacciavillano W, Sampor C, Venier C, Gabri MR, de Davila MT, Galluzzo ML, et al. A Phase I Study of the Anti-Idiotype Vaccine Racotumomab in Neuroblastoma and Other Pediatric Refractory Malignancies. Pediatr Blood Cancer. 2015;62(12):2120–4. [DOI] [PubMed] [Google Scholar]
- 52.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panel OKs CAR T Therapy for Leukemia. Cancer Discov. 2017;7(9):924. [DOI] [PubMed] [Google Scholar]
- 58.Morrissey PJ, Goodwin RG, Nordan RP, Anderson D, Grabstein KH, Cosman D, et al. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989;169(3):707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101(34):12640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki M, Xu H, Guo H, Nemieboka B, Wu Z, Lewis J, et al. Abstract A30: Novel T-cell engaging antibodies against L1CAM for neuroblastoma. Cancer Research. 2018;78(19 Supplement):A30-A. [Google Scholar]
- 61.Sheskin J. Thalidomide in the Treatment of Lepra Reactions. Clin Pharmacol Ther. 1965;6:303–6. [DOI] [PubMed] [Google Scholar]
- 62.Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759–69. [DOI] [PubMed] [Google Scholar]
- 63.Tempfer CB, Schultheis B, Hilal Z, Dogan A, Rezniczek GA. Thalidomide and lenalidomide for recurrent ovarian cancer: A systematic review of the literature. Oncol Lett. 2017;14(3):3327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–6. [PubMed] [Google Scholar]
- 65.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103(5):1787–90. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128(2):192–203. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, Sun J, Sheard MA, Tran HC, Wan Z, Liu WY, et al. Lenalidomide overcomes suppression of human natural killer cell anti-tumor functions by neuroblastoma microenvironment-associated IL-6 and TGFbeta1. Cancer Immunol Immunother. 2013;62(10):1637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wedekind MF, Denton NL, Chen CY, Cripe TP. Pediatric Cancer Immunotherapy: Opportunities and Challenges. Paediatr Drugs. 2018;20(5):395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Majzner RG, Heitzeneder S, Mackall CL. Harnessing the Immunotherapy Revolution for the Treatment of Childhood Cancers. Cancer Cell. 2017;31(4):476–85. [DOI] [PubMed] [Google Scholar]