Abstract
Objective:
To understand how interpersonal trauma (IPT), stress response, and drinking to cope converge to predict stress-induced drinking, a risk factor for alcohol use disorder.
Method:
Young adults with no substance use disorder were classified into three trauma history groups: 1) IPT with PTSD (n=27); 2) IPT without PTSD (n=35); and 3) Control (no trauma-history/no PTSD; n=36). Participants completed a baseline-assessment, including a structured clinical interview, to confirm PTSD diagnosis, followed by the Trier Social Stressor Task (TSST) and an alcohol use task. Subjective units of distress and blood serum cortisol were collected at standardized timepoints throughout the tasks.
Results:
In all three groups (PTSD, IPT, control), males consumed more alcohol in the lab than females. Participants in the PTSD group had significantly higher drinking to cope motives, which were associated with greater subjective reactivity; however, neither drinking to cope motives nor subjective reactivity to the TSST predicted post-stressor alcohol consumption for those with PTSD.
Conclusions:
The interplay among trauma history, stress, and drinking among young adults is nuanced; additional lab-based studies are needed to further clarify the nuanced connection between trauma history, acute stress reactions, and alcohol use.
Keywords: stress, alcohol, drinking to cope, trauma, PTSD, cortisol
Alcohol use is common in young adults, with many engaging in problematic patterns of alcohol use that lead to a variety of unhealthy outcomes (Maggs & Schulenberg, 2002). People with histories of traumatic events, particularly interpersonal violence exposure -- including physical assault, sexual assault, and witnessed violence that result in actual or threatened serious injury or death -- are at elevated risk for developing alcohol-related problems (Hasin et al., 2007). Some research suggests a ‘dose-dependent’ effect in that more extensive or severe history of interpersonal violence exposure is associated with elevated risk for AUD symptoms (Clark & Foy, 2000; Widom et al., 2007). Further, this risk is especially prevalent in trauma-exposed individuals who develop clinically significant stress-related problems, including posttraumatic stress disorder (PTSD). For example, in a non-clinical sample of young adults, posttraumatic stress symptoms were found to account for 55% of the variance in the prediction of alcohol use (Edwards, Dunham, Ries, & Barnett, 2006). Although the connection between trauma exposure and vulnerability to developing alcohol misuse has been established, the psychological and physiological mechanisms underlying the relationship are less clear.
For trauma-exposed individuals, including people with and without PTSD, drinking may be a prominent coping strategy for managing stress and unpleasant emotions, including in the moments immediately following an acute stressor (Kaysen et al., 2014; Simpson et al., 2014). In addition to emotional distress, trauma exposure contributes significantly to allostatic load (Danese & McEwen, 2012), which is the cumulative “wear and tear” on biological stress response systems, observable through measurement of functioning and changes in physiological systems in relation to stressors (McEwen, 1998; McEwen & Seeman, 1999). Individuals who experience interpersonal trauma may be more vulnerable to the impacts of acute stressors in their daily lives (e.g., arguments with family members), which in turn can have compounding effects on their overall level of distress and risk of developing psychopathology (Marshall et al., 2010; McEwen, 2004; Rogosch, Dackis, & Cicchetti, 2011). It remains unknown how the presence or absence of trauma exposure or PTSD factor into the association between stress response and acute alcohol use.
The self-medication hypothesis offers a framework for understanding the factors involved in how distress and drinking to cope can lead to problematic alcohol use acutely and chronically. The self-medication hypothesis posits that people with histories of trauma, and in particular, symptoms of PTSD or other forms of psychopathology, experience actual or perceived relief from distress when they use alcohol, reinforcing alcohol use behavior, and leading them to rely more heavily on drinking as a coping strategy to dampen or avoid distress (Sheerin et al., 2016). Over time, this negative reinforcement pattern can result in compounding impairment and development of an alcohol use disorder (AUD). Empirical support for the self-medication hypothesis linking interpersonal trauma and alcohol has been demonstrated in a range of self-report studies (Cisler et al., 2012; Epstein, Saunders, Kilpatrick, & Resnick, 1998; Ullman et al., 2013). These findings suggest that people who have experienced interpersonal trauma may be less likely to trust others or their ability to rely on interpersonal relationships to cope, which may promote the use of maladaptive coping strategies, including drinking. While these studies provide a foundation for support of this theoretical model, studies utilizing methods that extend beyond self-report (e.g., observable drinking in the lab among people exposed and not exposed to interpersonal trauma) is needed to further clarify the roles interpersonal trauma history and PTSD play in the acute-stress and drinking relation.
Several key factors are implicated in the self-medication hypothesis that can be measured in a controlled laboratory environment, including the degree to which people endorse a general tendency to engage in problematic alcohol use in an attempt to manage or regulate distress, termed drinking to cope. Drinking to cope can be a particularly problematic reason for drinking and is consistently linked with alcohol misuse (e.g., Holahan, Moos, Holahan, Cronkite, & Randall; Merrill & Thomas, 2013). Even when amount of alcohol consumed is held constant, individuals who drink to cope (in comparison to other motives) are at greater risk of experiencing alcohol use problems (Cooper et al., 1992; Carpenter & Hasin, 1999). This relationship has been supported in samples of young adults (Kutsche et al., 2006) and adults with histories of interpersonal trauma and PTSD (e.g., Marshall-Berenz et al., 2011). Despite consistent literature on drinking to cope and drinking to reduce or dampen stress, there are mixed findings when this relationship is examined in a controlled lab setting. For instance, some studies demonstrate an inverse relationship between alcohol consumption and stress responding (e.g., Moberg & Curtin, 2009) while others have found opposite effects (Ham, Casner, Bacon, & Shaver, 2011; for review see Sayette, 2017). A recent meta-analysis found post-stressor negative affect to be significantly lower following alcohol consumption in the laboratory; however, other findings to support stress response dampening were not definitive (Bresin, 2019). Perhaps individual differences in trauma exposure and psychopathology could be impacting these mixed findings. It is possible that the impact of unique coping motives on drinking following a stressor varies based on whether people have PTSD. People with PTSD experience more frequent and severe moments of distress than people without PTSD. Therefore, people with PTSD who also are highly predisposed or motivated to use alcohol as a coping strategy may be especially vulnerable to development of problem drinking even beyond people with trauma histories but no PTSD.
The self-medication hypothesis also implicates one’s response to an acute stressor as a potential driver of stress-related alcohol use. The intensity and course of one’s acute stress responses can be measured in two phases. Stress reactivity refers to the magnitude of one’s initial reaction to a stressor. Stress regulation refers to the changes that follow the initial reaction, including during efforts to manage or cope with the reaction. Stress reactivity and regulation can be measured via multiple indices, including subjective distress via a self-report rating scale like the subjective units of distress scale (SUDs), as well as objective responses, such as change in serum cortisol. The latter serves as a reflection of the activation of the HPA axis, the primary system involved in responding to stressors (Cohen et al., 1997). The value of assessing both subjective and objective responses to a stressor is two-fold. Multiple measures can help confirm the internal validity of the stressor (that it does, in fact, evoke a stress response, experienced both psychologically and physiologically). Additionally, subjective and objective measures may reflect different aspects of the mind and body’s response to threatening or distressing stimuli. In fact, correspondence between self-reported (subjective) stress measures and physiological (objective) measures, such as cortisol response, is highly variable. The Trier Social Stress Test (TSST; Kirschbaum, 1993) is a widely used, standardized laboratory tool for eliciting an acute stress response (Dickerson & Kemeny, 2004). In a review of 49 studies where versions of the TSST were administered to adults representing a diverse range of demographic and psychiatric subgroups, significant correlations between self-reported distress and cortisol response were found in only a quarter of the studies (Campbell & Ehlert, 2012). These findings highlight the need to consider both subjective and objective indices of stress response separately when studying stress reactivity and regulation.
The intensity of stress reactivity and subsequent patterns of regulation may differ among people based on interpersonal trauma history and PTSD diagnostic status. Moreover, because reactivity and regulation represent different aspects of the stress response, it is possible that they may demonstrate different relations to alcohol use depending on trauma exposure and PTSD status--and may even interact with other factors (e.g., coping motives) to produce different patterns of post-stressor drinking. Well-controlled studies of stress reactivity in trauma-exposed participants in comparison to control participants without traumatic event history show different patterns of activation. For example, adults with trauma histories have heightened subjective distress (e.g., SUDs) to a laboratory stressor compared to adults without trauma histories (Simeon et al., 2007). Physiological responses (e.g., cortisol levels) to stressors demonstrate a more complicated pattern. Most evidence in adults with PTSD and other forms of trauma-related psychopathology show a pattern of blunted HPA reactivity (Yehuda et al., 2006) in response to standardized laboratory stressors. In contrast, other studies have found heightened HPA reactivity among trauma-exposed adults (Heim et al., 2002). To our knowledge, there has been no studies to date designed to disentangle the impact of trauma exposure versus PTSD on stress responses, while also measuring observable drinking behavior.
To date, most studies examining the relation between interpersonal trauma exposure and stress response, interpersonal trauma exposure and alcohol use, and the unique role that drinking to cope motives play in these relationships have relied on naturalistic studies and self-reported, retrospective measures. Rigorous, controlled clinical laboratory studies using well validated measures and procedures – including objective markers of stress response and alcohol use – are needed to test predictions of the self-medication hypothesis in explaining patterns of alcohol use among people with trauma exposure and PTSD. Given that young adults represent a developmental window with high vulnerability to AUD (e.g., Grant et al., 2015), lab-based studies that focus on this age group may be of particular public health value.
Current Study
The goal of the current study was to understand the mechanisms involved in the heightened risk for alcohol use problems in individuals with and without exposure to interpersonal and other forms of trauma and/or PTSD. Using the self-medication hypothesis as the theoretical model for predicting the elevated risk conveyed by interpersonal trauma exposure and PTSD in the development of AUD, the present study was conducted to empirically examine these relationships with the internal validity and experimental control afforded by a randomized clinical study. Specifically, this clinical laboratory study examined how three groups of participants differed in alcohol use behavior following a well validated stress induction paradigm (the TSST). We gave specific attention to the participants’ motives for drinking and participants’ individual patterns of stress reactivity and regulation (measured via subjective self-report and objective physiological methods). We gave specific attention to these factors due to the relevance of these constructs in the self-medication hypothesis. Three groups of participants were characterized by (1) no trauma exposure (control group), (2) interpersonal trauma exposure without PTSD (TE group), and (3) interpersonal trauma with PTSD (PTSD group), as determined by a careful assessment of traumatic event exposure and PTSD with a structured clinical interview and well-validated measures. Comparing these three groups allowed us to separate the role of psychopathology from trauma exposure in investigating drivers of post-acute stressor drinking in the laboratory.
We hypothesized that subjective stress response to the TSST would be greatest in the PTSD group and cortisol response would be blunted in the PTSD group. We further hypothesized that more elevated subjective distress or cortisol response would be associated with greater consumption of alcohol following the TSST. Finally, we hypothesized that the association between stress reactivity and drinking following the acute stressor would be strongest for the PTSD group, while also considering the unique role of drinking to cope motives.
Method
Participants
The larger sample included 281 young adults (59.2% female; 84.7% White, 6.1% African-American, 9.2% Other), between the ages of 21 and 30 years (M = 24.76, SD = 2.59), recruited through community advertisements. Participants were recruited to fill one of three study groups: non-trauma exposed (“control”), interpersonal trauma exposed without PTSD (“TE”), or with PTSD (“PTSD”).
All participants completed a comprehensive phone screen to determine: a) if the person met inclusion criteria and did not meet exclusion criteria (see below); b) whether or not the person experienced any trauma (to include/exclude from control group); and c) if the person had experienced interpersonal trauma specifically, defined as physical assault, sexual assault, and witnessed violence that resulted in actual or threatened serious injury or death. The Life Events Checklist (LEC; Gray, Litz, Hsu, & Lombardo, 2004) was the measure used to assess for traumatic event exposure during this comprehensive phone screen. Once a person was determined to be eligible for the study (designated in the control conditon if no traumatic events were reported on the LEC or in one of the trauma conditions if interpersonal trauma was endorsed on the LEC), they were scheduled for an initial in-person baseline visit. Participants were interviewed in person for the baseline visit by highly trained clinical research personnel (trained to an inter-rater reliability of >.90) using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), which is a widely-used structured clinical interview with well-established validity for diagnosing PTSD (Sheehan et al., 1998). Responses on the MINI regarding the PTSD Criterion A event (first 3 questions of the PTSD module) were compared to LEC responses from the phone screen to ensure consistency in responses. The interviewer completed the MINI diagnostic coding and the final determination of interpersonal trauma and PTSD diagnosis (to ensure accurate group membership) were staffed in the weekly study meeting with the study team investigators (trauma-specialty clinical psychologists and scientists). Participants were also administered the Clinician-Administered PTSD Scale (CAPS), a structured clinical interview that allows clinicians to make a categorical PTSD diagnosis, as well as to score symptom severity. To have been eligible for the PTSD group, participants must have reported a history of exposure to a Criteria A traumatic event that involved interpersonal violence and met criteria for PTSD, as defined by DSM-IV (exposure to a Criteria A event, endorsement of at least 1 cluster B symptom, 3 or more cluster C symptoms, at least 2 cluster D symptoms, and functional impairment) and a CAPS severity score of > 45. If individuals reported a history of more than one form of traumatic event exposure, the event personally deemed the “worst” must be interpersonal in nature.
The PTSD Checklist (PCL; Weathers, Keane, & Davidson, 2001) was also administered at the baseline visit to get a continuous measure of PTSD symptoms and as an additional check that the TE group did not have PTSD (or subthreshold PTSD). Participants were eligible for the PTSD group if they experienced an interpersonal trauma and met DSM-IV criteria for PTSD as determined by the MINI (as described above). Participants were eligible for the Trauma Exposed (TE) group if they reported a history of interpersonal trauma, did not meet criteria for PTSD during the structured clinical interview, and had a PCL score less than or equal to 25 (range: 17–85), which denotes the respondent was “asymptomatic” (Weathers et al., 2001). The mean PCL score was 38.87 (SD = 7.60) for participants in the PTSD group and the mean was 21.00 (SD = 3.03) for participants in the TE group. Participants were eligible for the Control group if they had no history of traumatic events, regardless of type, and did not meet criteria for PTSD. Among participants in the main analyses (n = 98), no participants met criteria for Obsessive-Compulsive Disorder, Social Anxiety Disorder, or Major Depressive Disorder. Among those in the PTSD group, four participants met criteria for Generalized Anxiety Disorder, one met criteria for Dysthymia, and six met criteria for Panic Disorder. Among those in the TE group, one participant met criteria for Generalized Anxiety Disorder and one participant met criteria for Panic Disorder. Among those in the control group, one participant met criteria for Panic Disorder.
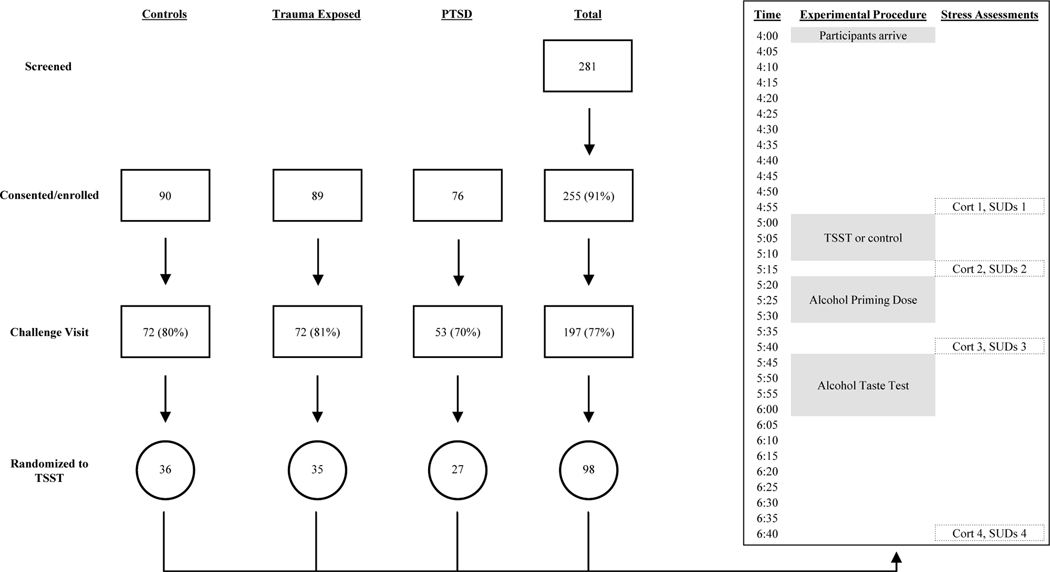
Additional eligibility criteria requirements included alcohol use. Participants must have consumed alcohol on at least 4 days in the past month but were excluded if they met criteria for alcohol dependence or any other substance use disorder (other than nicotine or caffeine dependence). Participants must have reported being a current beer drinker (i.e., consuming beer in the past month), as beer was the beverage provided in the alcohol administration laboratory task during the challenge day visit (below). Participants were excluded if they were taking any psychoactive medications, antihistamines, or medications that might alter HPA axis functioning. Individuals were also excluded if they met criteria for current major depression (which alters HPA axis functioning), lifetime psychosis, or any other medical condition that impacts HPA axis functioning (e.g., hypertension, chronic pain). Participants with severe obesity (i.e., BMI > 40) were also excluded due to potential interference of the stress hormone measures. Smokers who could not abstain from smoking for at least four hours were also excluded due to potential impairment of nicotine withdrawal on the experiment. Finally, women who were pregnant, nursing, or suspected that they may be pregnant were also excluded. Of the original 281 participants who completed an initial assessment, 255 met criteria for one of the study groups. The final sample included 72 controls, 72 trauma-exposed, and 53 PTSD participants (n=197) who returned to the laboratory after their baseline visit to complete the experimental tasks.
Procedure
Participants completed all questionnaires and interviews during their baseline visit, then returned to complete experimental tasks on a separate day, referred to here as “challenge day.” On challenge day, participants were randomly assigned to complete the Trier Social Stressor Task (TSST; Kirschbaum, Pirke, & Hellhammer, 1993; 36 control, 35 TE, and 27 PTSD) or a non-stress control condition (36 control, 37 TE, and 26 PTSD). All participants arrived to the laboratory at 4:00pm to ensure all participants underwent challenge day procedures at the same time of day to reduce the effects of diurnal timing on cortisol levels.
At 4:55pm, participants in the TSST condition received instructions for the TSST. This task includes an anticipation component, a social evaluative component (public speaking) and a performance component (mental math). The TSST is the gold standard experimental paradigm for inducing stress responses and drinking in the lab. It has been shown to induce a measurable stress response in both men and women (Kudielka et al., 2007). Participants in this condition were instructed that they would perform a behavioral personality test (i.e., discussing why they should be hired for their “dream job”) in front of three audience members who had expertise in analyzing body language. They were asked to practice this presentation for five minutes (anticipation component) and then were escorted to another room to perform their five-minute speech (evaluative component), and five-minute serial subtraction task (i.e., subtracted 13 from 1,022 as quickly and accurately as possible) (performance component). Audience members were three confederates in white laboratory coats who were told to remain flat in affect and provide no support or feedback during the participant’s presentation.
Ten minutes after the TSST, participants received a priming dose of alcohol intended to produce a blood alcohol level of .03 (based on body mass index), as is common practice when examining stress response using an alcohol administration paradigm in the laboratory (Merrill & Thomas, 2013; Thomas, Bacon, Randall, Brady, & See, 2011). Breathalyzers were used with each participant to ensure the .03 level was achieved. Fifteen minutes after priming, participants completed a mock taste test of beer, in which they were provided two glasses of beer and asked to determine whether the two beers were identical or different. Participants were told to drink as much as needed to make a decision. The experimenter provided the two glasses (glass A and glass B; 18 oz/532.3 ml each), in addition to a third glass containing eight ounces of water. The experimenter then informed participants they had 15 minutes (5:45–6:00pm) to make the determination about the beers. The experimenter then left the room and the participants were free to drink as much beer as they chose (up to 36 oz./1064.6 ml). The alcohol content of the beer provided for the taste test was 4.2% ABV.
The 98 individuals randomized to the non-stress control condition spent 15 minutes relaxing prior to receiving the priming dose of alcohol. One purpose of this control group was to validate the study design (i.e., show that the TSST elicited a stress reaction). See Figure 1 visual depiction of how participants were included in study, as well as the timeline of the challenge day visit for those in the TSST group. All procedures were approved by the University IRB.
Figure 1.
Visual depiction of how participants were included in study, and timeline of Challenge Day Visit for those in the TSST.
Measures
Stress Response (Cortisol and SUDs).
Cortisol levels were assessed using serum cortisol from blood samples collected throughout the challenge day procedures. Procedures were consistent with prior studies involving measurement of stress response in context of TSST and alcohol use paradigms (e.g., Thomas et al. 2011; Merrill & Thomas, 2013). Participants were fitted with an indwelling catheter to facilitate quick blood serum sample collection. The catheter and a blood pressure cuff were fitted at 4:15pm, and participants were allowed to acclimate to the devices and the experimental room until the stressor task began (approximately 40 minutes). Given that changes in plasma cortisol levels occur approximately 15–20 minutes after event-related changes in activation of the HPA axis (Dickerson & Kemeny, 2004), blood samples were collected 20 minutes after each time point of interest such that they generally aligned with SUDs ratings provided at the same time point of interest. For SUDs assessments, participants used a 0–10 scale (0=lowest, 10=highest) to rate how stressed they felt in that moment.
Cortisol and SUDs were collected to reflect: a) “baseline” cortisol/SUDs at the end of the acclimation period (T1), b) cortisol/SUDs at the end of the TSST (T2), and c) cortisol/SUDs 25 minutes after TSST (T3; 10 minutes after alcohol priming dose is consumed, 5 minutes before the taste test).
Assays of blood samples were conducted at the University of Texas Biological Psychiatry Analytical Lab. Blood samples (5mls per draw) were collected in BD K2 EDTA tubes and the samples were stored at –70 degrees Celsius. Samples were thawed to room temperature immediately prior to assay. Assays were conducted in accordance with instructions for Cortisol Enzyme Immunoassay Kits developed by Diagnostic Systems Laboratories, Inc. The assay has a detection limit of 0.1 mg/dL and intra-and inter-assay variation < 7%.
Covariates/Predictors Included.
Participant age, sex, and race were collected at baseline and entered as covariates in study models. A sum of 21 items assessing depressive symptoms using the Beck Depression Inventory (BDI; Beck, Steer, & Brown, 1996) also was entered as a covariate; scores on the BDI range from 0 to 63.
Participants reported their height (in inches) and weight (in pounds) during the lab visit. Using the formula (BMI=703*[weight/height2]), body mass index was calculated for each participant. Given concerns that BMI may influence cortisol levels (Fraser, Ingram, Anderson, Morrison, Davies, & Connell, 1999), the amount of alcohol individuals drank in the lab (measured in milliliters; mls), as well as the relation between drinks per drinking day and stress response, BMI was also included as a covariate in predicting all intermediate and final outcomes.
The timeline follow-back (TLFB; Sobell & Sobell, 1996) assessed alcohol use quantity (number of standard drinks) and frequency (number of drinking days) for the 30 days prior to the laboratory visit. Average drinks per drinking day (DDD) is often used in the literature (Irwin et al., 2006) to reflect an individual’s propensity to engage in heavy episodic (i.e., risky) drinking. Because of the concern that day-to-day alcohol use may affect stress response (Lovallo et al., 2000) and how much participants drank during the taste test, drinks per drinking day was used as a covariate in predicting all intermediate and final outcomes.
Fifteen items from Cooper’s (1992) Drinking Motives Questionnaire (DMQ), assessed three drinking motive subscales: coping motives, enhancement motives, and social motives. The conformity motives items from the DMQ were not included as prior literature has shown that conformity motives for drinking alcohol are not as relevant among young adults (Steinberg & Monahan, 2007). Item scales ranged from 1 (almost never/never) to 4 (almost always). Because we were most interested in testing the effects of drinking to cope on stress response and alcohol consumed in the laboratory over and above other drinking motives, a sum score for coping motives was used as an a priori predictor, with sum scores for enhancement and social motives serving as covariates.
Group Membership.
In order to determine whether associations among study variables differed by interpersonal trauma exposure or PTSD, group membership was used as the stacking variable in study models. Of the 98 participants, 28% were in the PTSD group, 36% in the TE group, and 37% in the Control group.
Data Analytic Plan
In order to better understand how stress responses might differ among these three groups, the PTSD, trauma-exposed, and controls groups were compared on all study variables using Analyses of Variance (ANOVAs) and chi-square tests for continuous and categorical outcomes, respectively. Significant group differences were probed post hoc using Tukey’s Test.
In predicting stress responses and alcohol consumed following a stressor, continuous variables were centered to reduce nonessential multicollinearity (Cohen, Cohen, West, & Aiken, 2003). Models were assessed for goodness of fit using the chi-square goodness of fit test statistic, Comparative Fit Index (CFI): ≥.95, Root Mean Square Error of Approximation (RMSEA) ≤.08, and Standardized Root Mean Square Residual (SRMR) <.08 (Hu & Bentler, 1999).
A latent difference, or change, score model (Kievit et al., 2018; Klopack & Wickrama, 2020; McArdle, 2001; McArdle, & Hamagami, 2001) was conducted in Mplus Version 7 (Muthen & Muthen, 2011) to test study questions. See Supplementary Figure for a visual depiction of the model using the latent change score method, as well as operationalization of baseline stress response, reactivity and regulation. This approach builds on an autoregressive structural equation modeling framework, such that the residual from the autoregression of a variable (assessed at t+1 on that variable at t) is a difference score when the autoregression coefficient is fixed to 1 (i.e., perfect prediction). Within these types of models, disturbances associated with each endogenous variable are set equal to 0 to facilitate model identification (Newsom, 2015). This approach was used so as to test whether alcohol use and motives predict stress response, and whether stress response predicts alcohol consumed in the laboratory following the stressor. In order to investigate whether findings within this latent change score approach differed by group, an overall model, as well as a multiple group model (stacked on group: control/TE/PTSD) was estimated. This approach allows for the examinations of study coefficients specifically for those across the three groups. That is, we estimated the latent change score model described above, while examining these study associations across our three groups of interest. Because we had a priori predictions that the associations between reactivity and regulation in the context of the TSST and alcohol consumed in the lab might vary by trauma history and/or PTSD, we ran a multiple group model within the context of a latent change score framework.
In all statistical modeling, drinks per drinking day, BMI, age, sex, race1, and depressive symptoms were entered as covariates in the prediction of baseline stress response, reactivity, regulation, and drinking in the lab. To highlight unique effects of coping motives on outcomes, as compared to social and enhancement motives were of interest, all three motive variables were included as predictors/covariates, regardless of significance. Additionally, “earlier” levels of stress response were also entered as predictors of later stress response. Specifically, in predicting SUDs/cortisol reactivity, baseline level was included as a predictor. In predicting SUDs/cortisol regulation, baseline level and reactivity were predictors. In predicting alcohol consumed in the laboratory, SUDs/cortisol baseline level, reactivity, and regulation were predictors.
Results
Descriptives and Differences between Groups on Study Variables.
See Table 1 for group differences on study variables. Of note, the PTSD group endorsed more depressive symptoms and higher coping motives compared to the control and TE groups, as well as higher enhancement motives than the control (but not TE) group. The control group also reported less drinks per drinking day compared to the TE and PTSD groups. There were no other group differences on other study variables. Supplemental Table 1 provides a breakdown of the traumatic events endorsed on the LEC in the PTSD and TE groups. The most highly endorsed interpersonal trauma experiences in both the PTSD and TE groups were physical assault and sexual assault.
Table 1.
Raw Data on Study Constructs, Split by Group.
| Overall (N=98) | Control (N=36) | TE (N=35) | PTSD (N=27) | |
|---|---|---|---|---|
|
|
||||
| % or M (SD) | % or M (SD) | % or M (SD) | % or M (SD) | |
|
| ||||
| Sex | 40.8% Males | 41.7% Males | 40% Males | 40.7% Males |
|
| ||||
| Race | 15.3% AA or Other | 11.1% AA or Other | 11.4% AA or Other | 25.9% AA or Other |
|
| ||||
| Depressive Symptoms | 5.36 (6.61) | 1.67a (2.27) | 3.57a (3.18) | 12.59b (8.00) |
|
| ||||
| Age | 24.77 (2.58) | 24.53 (2.18) | 24.80 (2.68) | 25.07 (2.84) |
|
| ||||
| Drinks/Drinking Day | 4.40 (2.75) | 3.60 (1.77) | 4.79a (3.16) | 4.91a (3.08) |
|
| ||||
| BMI | 25.30 (4.62) | 24.31 (2.74) | 25.11 (4.97) | 26.87 (5.64) |
|
| ||||
| SUDs 1 (pre-stressor) | 1.70 (1.76) | 1.56 (1.55) | 1.77 (1.79) | 1.82 (2.04) |
|
| ||||
| SUDs 2 (post +/− TSST) | 4.88 (2.54) | 4.17 (2.50) | 4.80 (2.58) | 5.59 (2.38) |
|
| ||||
| SUDs 3 (post-alcohol) | 1.69 (1.52) | 1.53 (1.32) | 1.86 (1.74) | 1.70 (1.43) |
|
| ||||
| CORT 1 (pre-stressor) | 9.15 (5.39) | 9.71 (5.28) | 9.50 (4.85) | 7.93 (4.61) |
|
| ||||
| CORT 2 (post +/− TSST) | 12.24 (5.29) | 13.66 (5.40) | 11.76 (4.60) | 10.90 (5.62) |
|
| ||||
| CORT 3 (post-alcohol) | 10.32 (4.63) | 11.45 (5.14) | 10.16 (3.59) | 9.01 (4.73) |
|
| ||||
| Coping Motives | 8.66(3.03) | 7.47a (1.75) | 7.97a (2.37) | 11.15b (3.71) |
|
| ||||
| Social Motives | 14.05(3.50) | 13.69 (3.30) | 13.66 (3.79) | 15.00 (3.29) |
|
| ||||
| Enhancement Motives | 10.11(3.16) | 9.19a (2.63) | 10.14ab (3.47) | 11.30b (3.06) |
|
| ||||
| Alcohol Consumed in the Lab | 262.63 (234.89) | 273.19 (252.44) | 247.66 (231.94) | 267.96 (212.33) |
Note. Superscripts indicate the presence of significant group differences; values not sharing a common superscript are significantly different (p <.05). Race: AA=African-American. TE = Trauma Exposed. BMI = Body Mass Index. SUDs = Subject Units of Distress. CORT = Plasma Cortisol Levels (μg/dL). Within the larger latent change score model, the first SUDs/CORT measure was the “baseline” measure, with the latent difference between the first and second (i.e., SUDs 1-SUDs 2, CORT 1-CORT 2) used to create the construct “reactivity” and the latent difference between the second and third (i.e., SUDs 2-SUDs 3, CORT 2-CORT 3) used to create the construct “regulation.”
Correlations
Supplemental Table 2 provides all zero-order Pearson (continuous variables), Tetrachoric (dichotomous variables), and Biserial (dichotomous and continuous variables) correlations among study variables. In terms of zero-order relations, group membership was not associated with any of the cortisol or SUDs baseline, reactivity, or regulation measures. However, in terms of association between drinking to cope motives and outcomes, those who reported more drinking to cope reported experiencing significantly higher baseline SUDs and less SUDs regulation.
Path Modeling
See Tables 2 and 3 for full results of all study models. Unstandardized and standardized coefficients and standard errors are presented.
Table 2.
Results of SUDs Model Predicting Reactivity, Regulation, and Drinking (N = 98)
| Baseline SUDs | SUDs Reactivity | SUDs Regulation | Alcohol Consumed in the Lab | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD | TE | Control | PTSD | TE | Control | PTSD | TE | Control | PTSD | TE | Control | |||||||||||||
| B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | |
| Race | -- | -- | -- | -- | -- | -- | −.01 (−.00) | .62 | .65 (.15) | .68 | −1.23* (−.31)* | .57 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Sex | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 1.71* (.39)* | .84 | 1.61* (.33)* | .70 | 1.87* (.37)* | 83 |
| Depressive Symptoms | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | .00 (.00) | .28 | 1.88** (.27)** | .62 | .19 (.02) | .59 | -- | -- | -- | -- | -- | -- |
| Coping Motives | .06 (.17) | .15 | .26 (.33) | .15 | .01 (.01) | .19 | 58*** (.69)*** | .15 | .25 (.22) | .21 | −.27 (−.21) | .24 | −.11 (−.17) | .10 | −.12 (−.12) | .10 | −.09 (−.08) | .10 | −.05 (−.08) | 19 | .33 (.33) | .18 | 36 31 (.25) | .31 |
| Social Motives | .02 (.03) | .11 | .05 (.10) | .10 | .15 (.31) | .09 | −.02 (−.03) | .11 | .23 (.33) | .14 | 39** (.58)** | .13 | .08 (11) | .06 | .05 (09) | .07 | −.00 (−.00) | .05 | .06 (09) | .14 | −.19 (−.30) | .12 | .10(.13) | .16 |
| Enhancement Motives | .04 (.06) | .18 | −.14 (−.27) | .13 | −.11 (−.19) | .14 | −.09 (−.09) | .18 | −.29 (−.39) | .17 | .09 (.10) | .18 | −.09 (−.11) | .10 | −.10 (−.15) | .08 | .02 (.03) | .07 | .10 (.14) | .20 | .15 (.22) | .15 | −.27 21 (−.29) | .21 |
| Baseline SUDs | -- | -- | -- | −84*** (− 55)*** | .21 | −58*** (− 41)*** | .22 | −.33 (−.23) | .20 | −.30* (−.25)* | .21 | − 32** (−.26)** | .11 | −.31** (−.24)** | .09 | .06 (.06) | .31 | .31 (.24) | .22 | − 38 (−.24) | .30 | |||
| SUDs Reactivity | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −65*** (−.84)*** | .11 | − 78*** (−.90)*** | .08 | −83*** (−.92)*** | .07 | .10 (.15) | .37 | −.07 (−.08) | .27 | −65 (−.58) | .45 |
| SUDs Regulation | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | .14(.16) | .39 | −.24(−.22) | .29 | −64(−.51) | 49 |
Note.
p<.05,
p<.01,
p<.001. As indicated in the table, unstandardized (and standardized) coefficients and standard errors are presented in the following order: PTSD, TE, and control. Sex: 0=females, 1=males. Race: 0=White, 1=Black or Other. SUDs = Subjective Units of Distress. Baseline SUDs=SUDs 1; SUDs Reactivity = latent difference between SUDs 1 and SUDs 2; SUDs Regulation = latent difference between SUDs 2 and SUDs 3. Covariate effects were removed from the model if they were not significant (p>.05) and if effects of primary predictors did not change with or without their inclusion (age, drinks per drinking day, and BMI did not have any significant effects in this model and are therefore not shown). Nonsignificant p-values less than .08 are reported for primary predictors to indicate possible trends.
Table 3.
Results of the Cortisol Model Model Predicting Reactivity, Regulation, and Drinking (N = 98)
| Baseline CORT | CORT Reactivity | CORT Regulation | Alcohol Consumed in the Lab | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD | TE | Control | PTSD | TE | Control | PTSD | TE | Control | PTSD | TE | Control | |||||||||||||
| B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | B(B) | SE | |
| Sex | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 1.75* (.40*) | .79 | 1.55* (.33*) | .69 | 2.02* (.40*) | .82 |
| Depressive Symptoms | −.46 (.28) | 1.09 | −5.05* (−.34)* | 2.41 | −3.16 (−.12) | 4.16 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Coping Motives | .36 (.28) | .35 | −.02 (.01) | .41 | 2.00** (.56)** | .68 | −.04 (−.04) | .24 | .24 (.16) | .29 | .10 (.03) | .54 | .11 (.23) | .08 | .18 (.24) | .10 | −.14 (−.10) | .31 | −.08 (−.15) | .15 | .32 (.32) | .19 | .52 (.35) | .33 |
| Social Motives | −.24 (−.17) | .26 | .12 (.09) | .25 | −.54 (−.29) | .32 | −.04 (−.04) | .18 | .15 (.16) | .19 | −.19 (−.13) | .34 | .04 (.07) | .06 | −.08 (−.16) | .07 | .13 (.18) | .24 | −.01 (−.02) | .15 | −.14 (−.23) | .12 | .00 (.00) | .15 |
| Enhancement Motives | .24 (.15) | .39 | .44 (.31) | .30 | −.30 (−.13) | .53 | .28 (.25) | .29 | −.29 (−.29) | .23 | .69 (.37) | .36 | −.15 (−.26) | .10 | −.19* (−.37*) | .08 | −.13 (−.14) | .36 | .16 (.22) | .18 | .22 (.32) | .16 | −.28 (−.29) | .24 |
| Baseline CORT | -- | -- | -- | -- | -- | -- | .08 | .14 | −.29* (−.40)* | .26 | −.46*** (−.59***) | .11 | −.14** (−.39**) | .14 | −.19*** (−.52***) | .04 | −.08 (−.22) | .11 | .01 (.03) | .10 | .11(.24) | .10 | .00 (.01) | .08 |
| CORT Reactivity | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −.28*** (−.55***) | .07 | −.38*** (−.75***) | .06 | −.16 (−.34) | .10 | .00 (.00) | .15 | .29 (.43) | .16 | .04 (.08) | .11 |
| CORT Regulation | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | .38 (.30) | .34 | .40 (.30) | .31 | .25 (.23) | .18 |
Note.
p<.05,
p<.01,
p<.001. As indicated in the table, unstandardized (and standardized) coefficients and standard errors are presented in the following order: PTSD, TE, and control. Sex: 0=females, 1=males. CORT = Plasma Cortisol Levels (µg/dL). Baseline CORT=Cort 1, CORT Reactivity = latent difference between Cort 1 and Cort 2; CORT Regulation = latent difference between Cort 2 and Cort 3. Covariate effects were removed from the model if they were not significant (p>.05) and if effects of primary predictors did not change with or without their inclusion (race, age, drinks per drinking day, and BMI did not have any significant effects in this model and are therefore not shown). Nonsignificant p-values less than .08 are reported for primary predictors to indicate possible trends.
Predicting Subjective Stress Response (SUDs) and Drinking.
Before estimating a multiple group model examining group differences in SUDs stress responses, we first determined whether model fit was better when parameters were freely estimated across groups, versus being fixed to be equal. A chi-square difference test suggested that the model allowing free parameters (χ2[4, N = 98] = 3.19) fit the data significantly better than the model fixing all parameters to be equal across groups (χ2[88, N = 98] = 112.54). Thus, we proceeded to estimate a multiple group model using the SUDs data. In order to minimize the number of estimated parameters, covariate effects were dropped if they were not significant (at p<.05) and if the effects of other predictors did not change with or without their inclusion.
Model Stacked on Group (PTSD, Trauma-Exposed, Control).
The final multiple group model with SUDs variables as predictors of alcohol consumed in the laboratory fit the data well: RMSEA = .08; CFI = .94; SRMR = .09. In all three groups, males consumed more alcohol in the lab than females.
Within the PTSD group, there were no significant predictors of baseline SUDs; however, baseline SUDs had a significant negative association with SUDs reactivity and regulation. Moreover, individuals with PTSD reporting more drinking to cope motives had significantly greater SUDs reactivity. Within the TE group, no covariates or predictors were associated with baseline SUDs. TE participants with higher baseline SUDs reported less SUDs reactivity and regulation, and those with greater SUDs reactivity showed less SUDs regulation. TE individuals with more depressive symptoms showed greater SUDs regulation. Within the control group, no covariates or predictors were associated with baseline SUDs. Control participants with higher baseline SUDs and those with greater SUDs reactivity showed less SUDs regulation. White control participants reported greater reactivity than participants identifying as Black or other. Social coping motives were positively associated with SUDs reactivity.
Predicting Cortisol Stress Response and Drinking.
Before estimating a multiple group model examining group differences in cortisol stress responses, we first determined whether model fit was better when parameters were freely estimated across groups, versus being fixed to be equal. A chi-square difference test suggested that the model allowing free parameters (χ2[4, N = 98] = 1.29) fit the data significantly better than the model fixing all parameters to be equal across groups (χ2 [88, N = 98] = 113.17). Thus, we proceeded to estimate a multiple group model using the cortisol data. In order to minimize the number of estimated parameters, covariate effects were dropped if they were not significant (at p<.05) and if the effects of other predictors did not change with or without their inclusion.
Model Stacked on Group (PTSD, Trauma-Exposed, Control).
The final multiple group model with cortisol variables as predictors of alcohol consumed in the laboratory stacked on group fit the data well: RMSEA = .02; CFI = .99; SRMR = .05. In all three groups, males consumed more alcohol in the laboratory than females.
Within the PTSD group, no predictors or covariates were associated with baseline cortisol or cortisol reactivity. Baseline cortisol and cortisol reactivity were negatively associated with cortisol regulation following the stressor. Within the TE group, depressive symptoms were negatively associated with baseline cortisol. Those with higher baseline cortisol showed less cortisol reactivity and less cortisol regulation. TE participants reporting more enhancement coping motives showed less cortisol regulation. In the control group, those reporting more coping motives had higher baseline cortisol. Control participants with higher baseline cortisol levels reported less cortisol reactivity.
Analyses among those in the Non-Stressor Condition
We estimated the SUDs and cortisol models in the non-TSST condition to determine whether the primary effects observed in the TSST models remained significant. The significant effects from the TSST model were nonsignificant in the non-TSST model. Thus, we confirmed that the observed effects were unique to the stressor condition. Moreover, we tested a latent change score model examining just the effect of the TSST condition (0 = control; 1 = TSST) on the latent change score between CORT measurements (i.e., CORT reactivity) and SUDS (i.e., SUDs reactivity). Indeed, there was a significant effect for both, such that TSST is associated with greater CORT reactivity (B = .530, p < .001) and SUDs reactivity (B = .646, p < .001), controlling for baseline response.
Discussion
The current study focused on understanding the interplay among interpersonal trauma history, coping motives, acute stress responses, and drinking among young adults, predicting that associations between these factors would vary as a function of PTSD. Although we found that young adults with PTSD with higher drinking to cope motives reported higher subjective stress reactivity following an acute stressor—and that young adult men across all three groups (PTSD. TE, and Control) drank more alcohol following an acute stressor than young adult women in the three groups, we did not observe the expected significant effect of greater quantity of alcohol consumption in the PTSD group following an acute stressor. Thus, we focus this discussion primarily on potential explanations for why our hypothesis regarding the relation between acute stress and drinking behavior in the lab was not observed in the PTSD group and “take home” implications, strengths, and limitations of the study.
Lack of Support for “Self-Medication” Among Young Adults with PTSD
Epidemiological research suggests that over 40% of individuals with PTSD also meet criteria for AUD (Pietrzak, Goldstein, Southwick, & Grant, 2011), with a particular link between interpersonal trauma and drinking problems. The self-medication hypothesis is the most common theory put forth to explain the strong connection between these conditions, where successful avoidance or “escape” from distress by way of alcohol reinforces the likelihood the person will engage in subsequent stress-induced drinking behavior. As such, we were surprised when the results of this well-controlled study of stress-induced drinking in young adults with PTSD (in comparison to those with interpersonal trauma without PTSD and those with no trauma history) did not support the acute stress-drinking connection in this group. Interestingly, there was greater endorsement of drinking to cope motives in the PTSD group in comparison to the other two groups in this study, but this “drinking to cope” behavior was not observed directly in the lab following the acute stressor. Multiple interpretations are possible. First, it is possible that acute subjective distress brought on by the anticipatory, evaluative, and performance components of the TSST does not induce immediate drinking behavior among young adults with PTSD. The distress from this type of acute stressor may not function in the same way as a trauma-specific stressor, where a young adult with PTSD who encounters a trauma cue (e.g., seeing a person that resembles their perpetrator) may be more inclined to seek alcohol to avoid the distress extending from the trauma-related memory or affect. In order words, perhaps the self-medication hypothesis only holds among people with PTSD when drinking is being negatively reinforced by avoiding trauma-specific distress. Some prior lab-based studies have utilized personalized trauma scripts (i.e., a detailed account of a participant’s worst trauma) as a method of stress induction when examining the link between acute stress and drinking proxies in trauma-exposed populations and found significant associations between stress and self-reported alcohol craving and salivation (Coffey et al., 2010).
Another possible interpretation is that there are other factors at play that we did not account for in the assessment battery. For example, some research has shown personality traits, such as anxiety sensitivity, may play a role in stress-induced drinking (e.g., Stewart, Zvolensky, & Eifert, 2001). In addition, sleep problems, which have been shown to partially mediate the relation between PTSD and substance use disorders (Vandrey, Babson, Hermann, & Bonn- Miller, 2014), may serve as an important factor to consider that was not measured in the current study. This may be particularly relevant to interpersonal trauma-related PTSD, as night-time and the dark are common trauma-related cues for victims of sexual abuse, as well as other interpersonal violence events. Alcohol use for these individuals may be reinforced when they drink at night as a way of coping (e.g., with the dark) in order to combat disturbed sleep. In sum, the lab-based stressor-drinking paradigm in the current study may not be as representative of the circumstances under which young adults with PTSD engage in (trauma-related) stress-induced drinking, and perhaps stress-induced drinking results from a dynamic interplay with other variables (such as personality traits, sleep, etc.). Despite findings not aligning with our primary prediction and beyond these interpretations offered as possibilities to explain the results in favor of the null hypothesis, study outcomes still yield important implications for addictive behavior science and future research as it relates to the self-medication hypothesis in young adult populations.
The self-medication hypothesis as a theoretical framework for understanding the relation between stress and drinking is not restricted to those with PTSD. We hypothesized that interpersonal trauma history more broadly would also affect stress and drinking responses observed in this lab-based study, but results did not support that hypothesis. Interestingly, although drinking to cope and cortisol stress reactivity were not significantly associated with alcohol consumption following an acute stressor for any of the groups, those who reported more drinking to cope reported experiencing significantly higher baseline SUDs and less SUDs regulation—suggesting a possible unique association between coping motives and stress response. Further, specific to young adults in the PTSD group, those who reported more drinking to cope had significantly greater SUDs reactivity—also not related to alcohol consumption in the current study. Future, multimethod research should attempt to clarify how drinking motives and acute stress response may interact to contribute to context-specific drinking behaviors among those with a trauma history and/or PTSD using a larger sample, and potentially focusing on trauma-specific acute stress reactions so as to enhance the ecological validity of the context in which young adults with PTSD may typically drink to cope with stress.
Strengths, Limitations, and Conclusions
A major strength of the study was use of a methodologically rigorous paradigm that had high internal validity and evidence of external validity. While many studies examine alcohol use among those with PTSD or those with trauma history, this is the first study to compare TE vs. PTSD groups in this context. This comparison helps elucidate the relative importance of interpersonal trauma exposure versus PTSD diagnosis in relation to stress responses and stress- induced drinking. Use of both objective and subjective measures of stress response is another important strength of the current study. SUDs ratings are a widely used method not just in clinical research targeting anxiety and stress disorders, but in the application of psychosocial treatment of these disorders as well. Although gathering serum cortisol is not commonplace in the course of psychotherapy, it is becoming increasingly feasible to collect other physiological biomarkers of stress response non-invasively across a wide range of settings (e.g., Adams et al., 2017). Studies such as the current one provide valuable insights into how these measures may operate differently based on patient characteristics and in relation to important clinical outcomes.
Although stringent inclusion and exclusion criteria decreased likelihood of confounds for HPA axis functioning (e.g., medications), generalizability of the findings to other populations is limited. Also, although PTSD was thoroughly assessed via a well-validated clinical interview, incident characteristics of the traumatic event histories of the TE and PTSD group, including timing, severity, or chronicity of the interpersonal violence experiences, were not assessed. Future studies may examine the degree to which timing or duration of traumatic events impact AUD risk. Additionally, the current study design did not allow for participants to be able to select which type of alcohol they would like to drink. Thus, although participants were past-month beer drinkers, beer may not be the desired alcohol that participants consume when stressed. It is also important to note that the priming dose of alcohol was administered to participants between the two time points for which regulation was calculated, thus making it difficult to discern stress regulation from alcohol-related stress dampening. Finally, our use of latent change score modeling within a multiple group analytical framework, combined with a small sample size, limited statistical power to detect small-moderate effects and to examine more nuanced group differences (e.g., by gender, age, race, and comorbid depressive symptoms) that could clarify heterogeneous patterns existing within the three groups.
While stress is a normative experience across the lifespan, emerging adulthood represents a key developmental window involving heightened vulnerability to stressors and to AUD risk (e.g., stress-induced drinking behavior). Additional work is needed to better understand the heightened risk for AUD among trauma-exposed young adults and to test whether patterns observed here generalize to people with more clinically significant drinking problems. While the current study addressed AUD risk relative to individual variability in stress drinking motives, continuation of this line of research will provide critical steps in characterizing pathways to problematic drinking and AUD in young adults and trauma-exposed populations.
Supplementary Material
Public health significance of the study: People with varying trauma history may have differing levels of vulnerability to stress-induced drinking. Although it was expected that young adults with PTSD would be at highest risk for engaging in stress-induced drinking, results suggest a more nuanced relation. For example, young adults with PTSD who report higher drinking to cope motives report higher subjective reactivity following an acute stressor; however, the relation between subjective distress, drinking motives, and drinking behavior in this population warrants more research. The nuances of trauma-specific stress response (e.g., trauma cues) should be carefully considered in future directions in this line of lab-based research with young adults with interpersonal trauma history with and without PTSD.
Footnotes
The race covariate was coded 0 = White and 1 = African American or Other. Because questions about race, and specifically White vs Black vs Other were not of substantive interest, and because we had less than 15% of the sample in two of the groups, we chose to combine the two smallest race groups to dichotomize that variable.
References
- Adams ZW, McClure EA, Gray KM, Danielson CK, Treiber FA, & Ruggiero KJ (2017). Mobile devices for the remote acquisition of physiological and behavioral biomarkers in psychiatric clinical research. J Psychiatr Res, 85, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, & Ehlert U. (2012). Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, & Hasin DS (1999). Drinking to cope with negative affect and DSM-IV alcohol use disorders: a test of three alternative explanations. J Stud Alcohol, 60(5), 694–704. [DOI] [PubMed] [Google Scholar]
- Chen FF, West SG, & Sousa KK (2006). A comparison of bifactor and second-order models of quality of life. Multivariate Behavioral Research, 1, 41, 189–225. [DOI] [PubMed] [Google Scholar]
- Cias CM, Young R, & Barreira P (2000). Loss of trust: Correlates of the comorbidity of PTSD and severe mental illness. Journal of Personal and Interpersonal Loss, 5, 103–123. [Google Scholar]
- Cisler JM, Begle AM, Amstadter AB, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG (2012). Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: Data from the NSA-R. Journal of Traumatic Stress, 25, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, & Foy DW. (2000). Trauma exposure and alcohol use in battered women. Violence Against Women, 6(1), 37–48. [Google Scholar]
- Coffey SF, Schumacher JA, Stasiewicz PR, Henslee AM, Baillie LE, & Landy N. (2010). Craving and Physiological Reactivity to Trauma and Alcohol Cues in PTSD and Alcohol Dependence. Exp Clin Psychopharmacol, 18, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. (2003). Applied multiple regression/correlation analysis for the behavioral sciences: Routledge. [Google Scholar]
- Cohen S, Kessler RC, & Gordon LU. (1997). Measuring Stress: A Guide for Health and Social Scientists. New York, NY, US: Oxford University Press. [Google Scholar]
- Cooper M, Russell M, Skinner JB, Windle M. (1992). Development and validation of a three- dimensional measure of drinking motives. Psychological Assessment, 4(2), 123–132. [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav, 106(1), 29–39. [DOI] [PubMed] [Google Scholar]
- Danielson CK, Adams ZA, & Hanson R. (2019). Risk Reduction though Family Therapy: Exposure-Based Treatment for Co-Occurring PTSD and Substance Use Problems Among Adolescents. In Back S. &. Vujanovic A. (Ed.), Posttraumatic Stress and Substance Use Disorders: A Comprehensive Clinical Handbook. New York, New York: Routledge. [Google Scholar]
- Danielson CK, Amstadter A, Dangelmaier RE, Resnick H, Saunders B, & Kilpatrick DG (2009). Trauma-related risk factors for substance abuse among male versus female young adults. Addictive Behaviors, 34, 395–399. PMCID: PMC2704020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull, 130(3), 355–391. [DOI] [PubMed] [Google Scholar]
- Edwards C, Dunham D, Ries A, & Barnett J. (2006). Symptoms of traumatic stress and substance use in a non-clinical sample of young adults. Addictive Behaviors, 31, 2094–2104. [DOI] [PubMed] [Google Scholar]
- Epstein J, Saunders BE, Kilpatrick DG, Resnick HS. (1998). PTSD as a mediator between childhood rape and alcohol use in adult women. Child Abuse & Neglect, 22(3), 223–234. [DOI] [PubMed] [Google Scholar]
- Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, & Connell JM (1999). Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension, 33(6), 1364–1368. [DOI] [PubMed] [Google Scholar]
- Gaab J, Blattler N, Menzi T, Pabst B, Stoyer S, & Ehlert U. (2003). Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology, 28(6), 767–779. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … Hasin DS (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, & Lombardo TW (2004). Psychometric properties of the life events checklist. Assessment, 11(4), 330–341. doi: 10.1177/1073191104269954 [DOI] [PubMed] [Google Scholar]
- Hasin D, Keyes KM, Hatzenbuehler ML, Aharonovich EA, Alderson D. (2007). Alcohol consumption and posttraumatic stress after exposure to terrorism: effects of proximity, loss, and psychiatric history. American Journal of Public Health, 97(12), 2268–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, & Nemeroff CB (2002). The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety, 15(3), 117–125. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Holahan CK, Cronkite RC, Randall PK (2001). Drinking to cope, emotional distress and alcohol use and abuse: A ten-year model. Journal of Studies on Alcohol, 62, 190–198. [DOI] [PubMed] [Google Scholar]
- Holzinger KJ, & Swineford F. (1937). The bi-factor method. Psychometrika, 2, 41–54. [Google Scholar]
- Hu L. & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. [Google Scholar]
- Irwin TW, Morgenstern J, Parsons JT, Wainberg M, & Labouvie E. (2006). Alcohol and sexual HIV risk behavior among problem drinking men who have sex with men: An event level analysis of timeline followback data. AIDS Behav, 10(3), 299–307. [DOI] [PubMed] [Google Scholar]
- Jones A, Button E, Rose AK, Robinson E, Christiansen P, Di Lemma L, & Field M. (2016). The ad-libitum alcohol ‘taste test’: Secondary analyses of potential confounds and construct validity. Psychopharmacology, 233, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen D, Atkins D, Simpson T, Stappenbeck CA, Blayney JA, Lee CM, & Larimer ME (2014). Proximal relationships between PTSD symptoms and drinking among female college students: results from a daily monitoring study. Psychol Addict Behav, 28(1), 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, & Lin S. (2006). Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol, 59(3), 203–209. doi: 10.1016/j.ijpsycho.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Fischer JE, Metzenthin P, Helfricht S, Preckel D, & von Kanel R. (2007). No effect of 5-day treatment with acetylsalicylic acid (aspirin) or the beta-blocker propranolol (Inderal) on free cortisol responses to acute psychosocial stress: a randomized double-blind, placebo-controlled study. Neuropsychobiology, 56(2–3), 159–166. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, & Nixon SJ (2000). Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res, 24(5), 651–658. [PubMed] [Google Scholar]
- Marshall-Berenz EC, Vujanovic AA, & Zvolensky MJ (2011). Main and interactive effects of a nonclinical panic attack history and distress tolerance in relation to PTSD symptom severity. J Anxiety Disord, 25(2), 185–191. doi: 10.1016/j.janxdis.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GN, Miles JN, & Stewart SH (2010). Anxiety sensitivity and PTSD symptom severity are reciprocally related: evidence from a longitudinal study of physical trauma survivors. J Abnorm Psychol, 119(1), 143–150. doi: 10.1037/a0018009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2004). Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Science, 1032, 1–7. doi: 10.1196/annals.1314.001 [DOI] [PubMed] [Google Scholar]
- McEwen B. & Seeman T. (1999). Protective and damaging effects of mediators of stress. Elaborating & testing the concepts of allostasis & allostatic load. Ann N Y Acad Sci, 896, 30–47. [DOI] [PubMed] [Google Scholar]
- Merrill JE, & Thomas SE (2013). Interactions between adaptive coping and drinking to cope in predicting naturalistic drinking and drinking following a lab-based psychosocial stressor. Addictive behaviors, 38(3), 1672–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun EY, von Eye A, & White H. (2009). An SEM approach for the evaluation of intervention effects using pre-post-post designs. Structural Equation Modeling, 16, 315–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B. (2009, May 28). Confidence Intervals: Unstandardized and Standardized. http://www.statmodel.com/discussion/messages/11/4331.html?1426677590. [Google Scholar]
- Muthen B, & Muthen LK. (1998-2011). MPlus Computer Software and Manual (Version Version 6.1). Los Angeles, CA: Muthen & Muthen. [Google Scholar]
- National Institutes of Health: All of Us Research Program. (2017), from https://allofus.nih.gov/
- Newsom JT (2015). Longitudinal structural equation modeling: A comprehensive introduction. Routledge. [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, & Grant BF (2011). Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from wave 2 of the National Epidemiologic Survey on alcohol and related conditions. Journal of Anxiety Disorders. 2011;25(3):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015). R: A language and environment for statistcal computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R- [Google Scholar]
- Ray LA, Mackillop J, Leggio L, Morgan M, & Hutchison KE (2009). Effects of naltrexone on cortisol levels in heavy drinkers. Pharmacol Biochem Behav, 91(4), 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch FA, Dackis MN, & Cicchetti D. (2011). Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Dev Psychopathol, 23(4), 1107–1124. doi: 10.1017/S0954579411000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JG,JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. [PubMed] [Google Scholar]
- Schulenberg JE, & Maggs JL (2002). A developmental perspective on alcohol use and heavy drinking during adolescence and the transition to young adulthood. J Stud Alcohol Suppl(14), 54–70. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, . . . Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sheerin C, Berenz EC, Knudsen GP, Reichborn-Kjennerud T, Kendler KS, Aggen SH, & Amstadter AB (2016). A population-based study of help seeking and self-medication among trauma-exposed individuals. Psychol Addict Behav, 30(7), 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Smith L, Baker BR, & Hollander E. (2007). A preliminary study of cortisol and norepinephrine reactivity to psychosocial stress in borderline personality disorder with high and low dissociation. Psychiatry Res, 149(1–3), 177–184. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Stappenbeck CA, Luterek JA, Lehavot K, & Kaysen DL (2014). Drinking motives moderate daily relationships between PTSD symptoms and alcohol use. J Abnorm Psychol, 123(1), 237–247. doi: 10.1037/a0035193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, & Sobell MB (1996). The reliability of the Alcohol TLFB when administered by telephone and by computer. Drug Alcohol Depend, 42, 49–54. [DOI] [PubMed] [Google Scholar]
- Steinberg L, & Monahan KC (2007). Age differences in resistance to peer influence. Developmental Psychology, 43, 1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Zvolensky MJ, Eifert GH (2001). Negative-reinforcement drinking motives mediate the relation between anxiety sensitivity and increased drinking behavior. Personality and Individual Differences, 3, 157–171. [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, & See RE (2011). An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology (Berl), 218(1), 19–28. doi: 10.1007/s00213-010-2163-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Merrill JE, von Hofe J, & Magid V. (2014). Coping motives for drinking affect stress reactivity but not alcohol consumption in a clinical laboratory setting. Journal of Studies on Alcohol and Drugs, 75(1), 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, & Wand GS (2009). Stress, alcohol and drug interaction: An update of human research. Addiction Biology, 14, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman SE, Relyea M, Peter-Hagene L, & Vasquez AL (2013). Trauma histories, substance use coping, PTSD, and problem substance use problems. Addict Behav, 38, 2219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Babson K, Hermann ES & Bonn-Miller MO (2014). Interactions between Disordered Sleep, Post-Traumatic Stress Disorder, and Substance Use Disorders. Int Rev Psychiatry, 26, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, & Davidson JR (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety, 13(3), 132–156. [DOI] [PubMed] [Google Scholar]
- Wemm S, Fanean A, Baker A, Blough ER, Mewaldt S, & Bardi M. (2013). Problematic drinking and physiological responses among female college students. Alcohol, 47(2), 149–157. [DOI] [PubMed] [Google Scholar]
- Widom CS, White HR, Czaja SJ, & Marmorstein NR (2007). Long-term effects of child abuse and neglect on alcohol use and excessive drinking in middle adulthood. J Stud Alcohol Drugs, 68(3), 317–326. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Yang RK, Buchsbaum MS, & Golier JA (2006). Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology, 31(4), 447–451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.