Abstract
Both the maternal and fetal outcomes of pregnancy vary greatly according to a pregnant woman’s community and her condition. The most devastating outcome is the death of a mother. In 2017, there were ≈295,000 maternal deaths globally with dramatic differences in maternal mortality based on geographic region, country, and women’s underlying conditions. Worldwide, the leading cause of maternal death is hemorrhage, comprising 94% of maternal deaths, with most cases occurring in low‐ or middle‐income countries. Whether a hemorrhage originates from inside the uterus (80%‐90%), from lacerations or incisions (10%‐20%), or from an underlying coagulopathy (<1%), an acute acquired coagulopathy will evolve unless the hemorrhage is controlled. In low‐ or middle‐income countries, the full range of resources to control hemorrhage is not available, but besides the usual obstetric measures, blood availability, hemostatic medication, and hematologic expertise are necessary to save mothers’ lives. Hemostasis and thrombosis experts can address the disparities in obstetric hemorrhage outcomes not only as providers but as consultants, researchers, and advocates.
Keywords: blood availability, coagulopathy, hemorrhage, maternal mortality, pregnancy
Essentials.
Worldwide, the leading cause of maternal death is obstetric hemorrhage.
Unless obstetric hemorrhage is controlled, an acute acquired coagulopathy can evolve.
In low‐ or middle‐income countries, safe blood frequently is not available.
Hemostasis and thrombosis experts can help improve the outcomes in obstetric hemorrhage.
1. INTRODUCTION
Worldwide, maternal mortality (pregnancy‐related death) is the leading reason for death among women of childbearing age. The leading cause of pregnancy‐related death is obstetric hemorrhage. Obstetric hemorrhage can occur during pregnancy or the postpartum period, but predominantly occurs during the postpartum period as a result of bleeding from the uterus (obstetric bleeding), bleeding from lacerations or incisions (surgical bleeding), or from abnormal coagulation (coagulopathy). At the time of delivery or miscarriage, the placenta should separate from the wall of the uterus and be expelled. Separation of the placenta exposes the terminal branches of the uterine arteries (the spiral arteries) and results in bleeding across the uterine surface previously occupied by the placenta. The mechanism by which bleeding from these vessels is controlled is external compression from the contraction of the interlacing muscle fibers of the uterus and from internal vasoconstriction. For bleeding to be controlled, the placenta, including any fragments, must be expelled or removed, and the walls of the uterus apposed. Approximately 80% to 90% of postpartum hemorrhage originates as obstetric bleeding, 1 10% to 20% originates as birth trauma or surgical bleeding, and <1% originates as coagulopathy. 1
2. DISPARITIES IN MATERNAL MORTALITY BY REGION AND COUNTRY
There are profound disparities in maternal mortality by region and by country. Figure 1 is a world map with maternal mortality data by country from 2017, the latest data available. 2 The maternal mortality ratio (MMR), the proportion of maternal deaths per 100 000 live births, varies from <20 per 100 000 live births in North America, Europe, Australia, and New Zealand to >1000 per 100 000 live births in the sub‐Saharan countries of Sierra Leone, Chad, and South Sudan. Figure 2 depicts the striking disparities in MMRs by region. The MMR for Western Europe in 2017 was 5, for North America was 18, for Eastern Europe and Central Asia was 19, for the Middle East and North Africa was 57, for East Asia and the Pacific was 69, for Latin America and the Caribbean was 74, for South Asia was 163, for Eastern and Southern Africa was 384, and for West and Central Africa was 674. In 2017, 295 000 women died from pregnancy‐related causes. Ninety‐four percent of these deaths occurred in low‐resource settings, and 86% occurred in sub‐Saharan Africa or Southern Asia. 2
FIGURE 1.
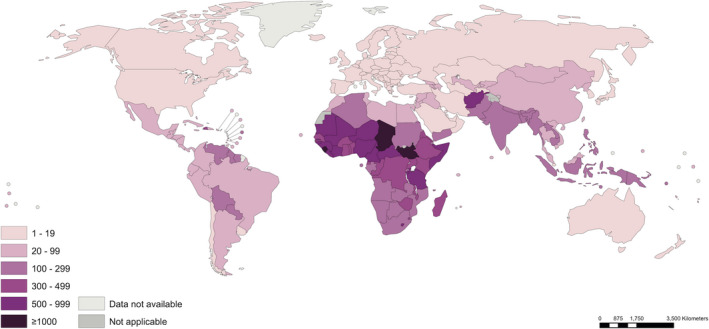
Maternal mortality ratios (maternal deaths per 100,000 live births) by country, 2017. Source: World Health Organization (WHO). https://apps.who.int/iris/bitstream/handle/10665/327596/WHO‐RHR‐19.23‐eng.pdf?ua=1. Accessed September 13, 2021. Figure used with permission of WHO
FIGURE 2.
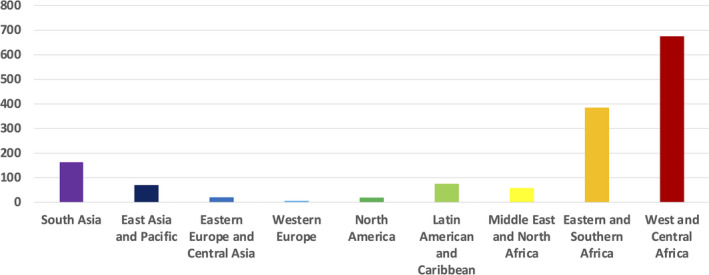
Maternal mortality ratios (maternal deaths per 100,000 live births) by region, 2017. Source: World Health Organization (WHO). https://www.who.int/reproductivehealth/publications/maternal‐mortality‐2000‐2017/en/ Data accessed February 7, 2021
3. DISPARITIES IN MATERNAL MORTALITY AMONG RACIAL AND ETHNIC GROUPS
Even within countries there are differences in maternal mortality among various racial and ethnic groups. Within the United States, a high‐income country, about 700 women die of pregnancy‐related causes each year. American Indian/Alaska Native and Black women are two to three times as likely to die from a pregnancy‐related cause than White women (Figure 3). 3 Variability in the risk of death by race/ethnicity has been attributed to several factors, including access to care, quality of care, prevalence of chronic diseases, structural racism, and implicit biases. 4 , 5 , 6
FIGURE 3.
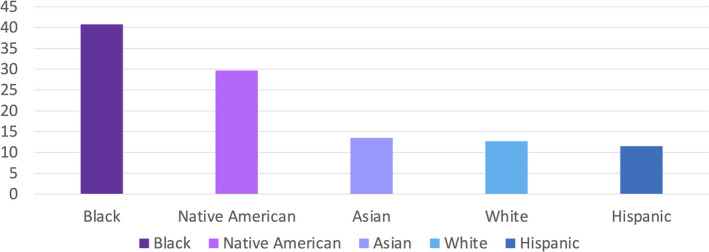
Disparities in the maternal mortality ratio (maternal deaths per 100,000 live births) in the United States by race/ethnicity, 2016. Source: US Centers for Disease Control and Prevention Racial/Ethnic Disparities in Pregnancy‐Related Deaths — Maternal Mortality Ratios. United States, 2007‐2016. https://www.cdc.gov/mmwr/volumes/68/wr/mm6835a3.htm Data accessed September 13, 2021
4. DISPARITIES IN HEMORRHAGE BY CONDITION
The leading cause of maternal mortality worldwide is hemorrhage, which accounts for 27% of all maternal deaths. 7 Even within the United States, a high‐resource setting, hemorrhage accounts for >10% of all maternal deaths. 8 Antecedents are predominantly obstetric and surgical, as there are few systemic medical conditions that increase the risk of postpartum hemorrhage (PPH). One such condition is anemia, where a hemoglobin <9.0 g/dL is associated with an adjusted odds ratio (aOR) for PPH of 2.20 (95% confidence interval [CI], 1.63‐3.15), 9 and the other is von Willebrand disease with an aOR of 3.31 (95% CI, 1.01‐10.85), 9 suggesting that anemia and underlying bleeding disorders, in general, are risk factors for PPH.
On a population basis, however, underlying bleeding disorders do not significantly contribute to the incidence of life‐threatening or massive obstetric hemorrhage. The vast majority of PPH has an obstetric or surgical origin. PPH that progresses to massive, life‐threatening hemorrhage, however, is invariably and ultimately accompanied by an acute acquired coagulopathy or “hemostatic failure,” 10 , 11 unless immediate efforts are made to replace blood loss. During a 5‐year period from 2000 to 2004 at Duke University Medical Center, among 12 476 deliveries, 30 women of the 671 who experienced a PPH also became coagulopathic. Except for one patient who had chronic hepatitis, none had any previously diagnosed abnormality of coagulation; rather, coagulopathy developed because of massive PPH from obstetric or surgical causes.
Mechanisms of the acute acquired coagulopathy that accompanies massive obstetric hemorrhage are not completely understood but include:
Hemodilution—as may occur with failure to replace clotting factors during massive transfusion 12 unless bleeding is successfully stopped and blood successfully replaced.
Severe thrombocytopenia—as occurs in the hemolysis, elevated liver enzymes, and low platelets syndrome, which increases the risk of PPH twofold. 9
Disseminated intravascular coagulation—with consumption of clotting factors and platelets as occurs in placental abruption, intrauterine fetal demise, amniotic fluid embolism, and sepsis. 13
Failure of (liver) synthetic function—as occurs with acute fatty liver of pregnancy. 14
Excessive fibrinolysis—as occurs in hemorrhagic shock. 15
Multiple and/or other mechanisms.
The treatment of massive obstetric hemorrhage requires treatment of the underlying obstetric or surgical cause. Uterine exploration, bimanual compression, uterotonics, balloon tamponade, and uterine compression sutures are used for the treatment of uterine atony, and ligatures are used for the repair of incisions, lacerations, and ruptured viscus. Uterine artery embolization and hysterectomy are used when the initial obstetric and surgical approaches have failed. 16 The initial treatment for the acute acquired coagulopathy that accompanies massive obstetric hemorrhage, however, requires blood products and hemostatic agents. Ideally, they are administered as part of a PPH‐specific massive transfusion protocol within the context of a formal obstetric hemorrhage protocol. Tranexamic acid is an antifibrinolytic agent that can be used as a relatively inexpensive and nonspecific hemostatic agent in the initial treatment of PPH and in a wide range of settings. 17
5. DISPARITIES IN BLOOD SAFETY, AVAILABILITY, AND ACCEPTANCE
Safe blood products are not uniformly available around the world. There is a marked difference in the level of access to blood between low‐ and high‐income countries (Figure 4). The whole‐blood donation rate can be viewed as an indicator for the general availability of blood in a country. The median blood donation rate in high‐income countries is 31.5 donations per 1000 people, which is usually sufficient to meet the needs of the population. In upper‐middle‐income countries, the donation rate is lower, at 15.9; in lower‐middle‐income countries, the rate is 6.8; and in low‐income countries, the rate is the lowest, at 5 donations per 1000 people. 18
FIGURE 4.
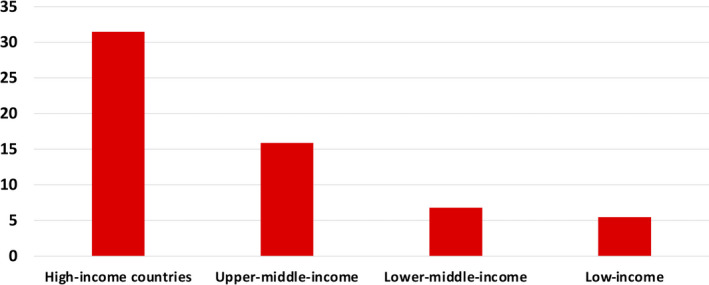
Blood donations per 1000 population by countries’ income, 2018. Sources: World Health Organization, United Nations Children’s Fund, United Nations Population Fund, World Bank Group, and the United Nations Population Division. Blood safety and availability. https://www.who.int/news‐room/fact‐sheets/detail/blood‐safety‐and‐availability. Data accessed February 7, 2021
Even in high‐income countries, not all hospital blood banks have an adequate stock of blood or blood products in case of a massive obstetric hemorrhage. A hospital blood bank’s inventory is based on a hospital’s typical usage to avoid wasting products. For a small hospital, this means a small inventory. A massive hemorrhage can result in the depletion of the inventory, resulting in (i) the need for emergency transport of blood, (ii) the need for emergency transfer of the patient, or (iii) the death of the patient. Of 181 patients with massive obstetric hemorrhage in the United Kingdom Obstetrical Surveillance System database from June 2012 to June 2013, 15 patients required >20 units of blood, 19 which is a challenge for a small or rural hospital.
The critical importance of blood to the survival of women at the time of childbirth is further demonstrated by the consequences of blood refusal, specifically by Jehovah’s Witnesses, who have an increased risk of maternal death due to blood refusal for religious reasons. In high‐income countries, women who do not accept blood would still be expected to have access to all the other resources to ensure their survival, and thus the difference in survival among those who refuse blood products can be attributed to their refusal. Three studies of maternal mortality in Jehovah’s Witnesses have revealed the profound impact of blood refusal on their risk of maternal mortality. In a study conducted at Mount Sinai Hospital in New York, the odds of maternal mortality were estimated to be 44‐fold 20 ; in a study conducted at North Middlesex Hospital in the United Kingdom, the odds were estimated to be 65‐fold 21 ; and in a study conducted in the Netherlands, the odds were estimated to be 130‐fold. 22 While these studies have very limited data, the direction and magnitude of the differences are, nonetheless, similarly positive and large. It is hard to conceive of a greater risk to maternal survival than lack of access to blood.
In a systematic review designed to estimate the impact of the lack of blood availability on maternal deaths in sub‐Saharan Africa, where blood supply is critically inadequate, Bates et al 23 found 37 studies from 15 countries that reported qualitative or quantitative information about blood transfusion services in relation to maternal mortality and/or severe maternal morbidity. In 21 of these 37 studies, the authors mentioned a direct association between maternal mortality and/or severe morbidity due to the lack of timely blood transfusions. In the 5 studies that provided quantitative information about the number of maternal deaths, a total of 184 of 713 hemorrhage deaths (26%) were due to lack of an effective emergency supply of blood. In the 6 studies that mentioned specific reasons for lack of blood, the reasons included:
Inability of the women to pay for blood in advance.
Lack of blood donors.
Unwillingness of relatives to donate blood.
Lack of supplies, blood bank, storage facilities, and transport.
The authors listed the following six action points derived from their literature review to improve timely blood transfusions and reduce maternal death:
Develop a national policy for subsidizing and sustaining blood transfusion services.
Finance emergency transfusions for poor families.
Ensure adequate stocks of blood from repeat, volunteer donors (the lowest‐risk donors).
Reduce the need for transfusion through proactive management of childbirth.
Establish a transfusion committee in each facility.
Justify the use of supplies and the use of screening tests depending on the population.
6. DISPARITIES IN HEMOSTASIS EXPERTISE
There are few data available on the distribution of hemostasis and thrombosis experts globally, but using membership in the ISTH as a surrogate, it is possible to estimate the distribution of hemostasis and thrombosis experts worldwide. Unfortunately, the numbers of practicing hematologists by country, let alone the numbers of hemostasis and thrombosis experts by country, are unknown. There are obvious limitations to using ISTH membership as a surrogate, especially since being a member of a society does not guarantee expertise. Furthermore, there are economic reasons that might account for the low number of ISTH members among physicians in low‐income countries. Nonetheless, similar economic reasons have likely precluded the training and retention of hemostasis and thrombosis experts. Subspecialists such as hematologists are known to constitute a lower proportion of the physician workforce in low‐ and middle‐income countries compared to a high‐income country such as the United States. 24 Figure 5 illustrates the distribution of ISTH members by continent. In Australia/New Zealand, there are 8.1 members per million population compared to 0.15 members per million population in Asia, 2 to 3 members per million population in Europe and North America, <1 member per million population in South American, and <0.1 member per million population in Africa. Within Africa there are further disparities. Seventy percent of the African ISTH members are located in just three countries: Egypt, Nigeria, and South Africa. Nine countries have only 1 to 7 members. Forty‐two of the 54 African countries have no members at all. 25
FIGURE 5.
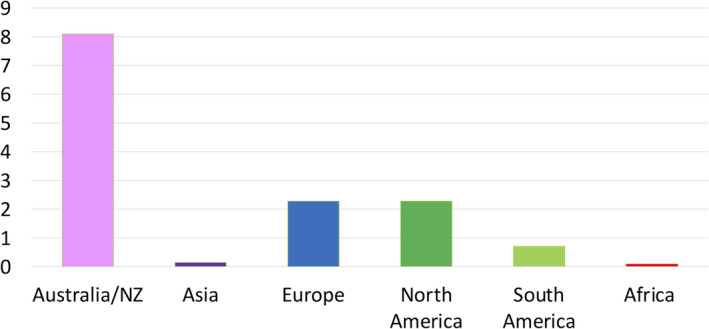
ISTH membership per million population. Source: ISTH Membership Directory. https://www.isth.org/search/ Data accessed June 16, 2021
7. ADDRESSING THE DISPARITIES IN OBSTETRIC OR POSTPARTUM HEMORRHAGE OUTCOMES
Technological advances offer hope in overcoming barriers to blood availability. For instance, aerial drone technology has been used to deliver blood. Blood is typically delivered by car or ambulance and can be delayed by poor road conditions. Only one‐third of Africans live within 2 kilometers of a road that functions year‐round. In 2016, in response to the urgent need for available blood products, the United States–based company, Zipline, launched a drone delivery operation in Rwanda and now delivers >20% of Rwanda’s blood supply outside of the capital. 26 Even in urban Montreal, aerial drone delivery of blood products was found to be faster than ground transport. 27
Clinical trials and quality improvement initiatives addressing disparities are either under way and/or have yielded or have the potential to yield improvements that can be adopted more broadly. The E‐MOTIVE (A Clinical Trial to Study the Effectiveness of a Care Bundle to Prevent Bleeding After a Woman Has Given Birth) project aims to improve the management of PPH through the implementation of a basic clinical care “bundle” of practices, which consists of (i) early detection of PPH using a calibrated drape, (ii) genital tract examination, (iii) uterine massage, (iv) oxytocic drugs, (v) intravenous fluids, and (vi) tranexamic acid. The project will be implemented and evaluated in Kenya, Nigeria, South Africa, Sri Lanka, and Tanzania. 28 The WOMAN‐2 trial (World Maternal Antifibrinolytic Trial 2) is an international randomized trial being conducted in low‐ and middle‐income countries to determine whether the hemostatic agent tranexamic acid given within 15 minutes of delivery reduces the incidence of PPH in women with moderate or severe anemia who deliver vaginally. 29 In the United States, implementation of a large‐scale quality improvement collaborative in California consisting of 17 evidence‐based recommendations for clinical care practices in the realms of (i) readiness for PPH (eg, massive transfusion protocol in place), (ii) response to PPH (eg, standard, stage‐based plans with checklists), and (iii) reporting and learning from PPH (eg, posthemorrhage reviews) reduced racial disparities in severe maternal morbidity from hemorrhage by helping ensure uniform clinical care practices for individual patients and across centers. 30
Addressing the disparities in obstetric or PPH outcomes requires a knowledgeable workforce that includes transfusion medicine and hemostasis and thrombosis experts. Hemostasis and thrombosis experts have the opportunity to address the disparities in obstetric hemorrhage outcomes. Hemostasis and thrombosis experts are leaders within their institutions, within their communities, within their countries, and internationally. Hemostasis and thrombosis experts are able to educate their colleagues and policymakers about the importance of blood safety and availability, to serve as experts in devising laboratory testing and resuscitation algorithms for PPH protocols, and to serve as relentless advocates locally and abroad. Obstetricians are prepared to implement preventive measures such as iron for the treatment of anemia during pregnancy to reduce the need for transfusion and are prepared to manage the obstetric and surgical complications that lead to a massive PPH, but if an acute acquired coagulopathy ensues, the obstetrician requires the assistance of his or her colleagues in anesthesia or critical care medicine and, ideally, receives consultation from a transfusion medicine or hemostasis and thrombosis expert when necessary. This consultation is important not only in the moment with respect to laboratory testing, blood products, and hemostatic agents, but also in the long term in planning for future events. In the era of improved communication via virtual platforms, these platforms can be used to foster education and provide consultation with a network of experts. Examples include the programs of the Foundation for Women and Girls With Blood Disorders, and the programs of the ISTH Women’s Issues in Thrombosis and Hemostasis Scientific and Standardization Committee (SSC) Subcommittee. The Women’s Issues Subcommittee, along with the Disseminated Intravascular Coagulation SSC Subcommittee, developed the Women’s Global Registry of DIC in Pregnancy (https://redcap.isth.org/surveys/?s=KFC8RN8XWC). ISTH, as an organization, already supports thrombosis and hemostasis societies in low‐ and middle‐income countries through educational courses, congresses, the ISTH Academy, webinars, and the creation of collaborations between physicians and institutions in high‐income and low‐ and middle‐income countries. This type of outreach must continue to be developed and expanded so that a wider number of patients can reap the benefits.
8. ISTH CONGRESS REPORT – RELEVANT ABSTRACTS FROM THE ISTH 2021 CONGRESS
8.1. Pertaining to disparities among racial and ethnic groups
In a retrospective cohort study,31 Davis and colleagues evaluated the association between Black race and postpartum blood transfusion using three cohorts in the United States: the National Institute of Child Health and Human Development’s (NICHD’s) Maternal Fetal Medicine Unit Network’s Cesarean Registry, the NICHD’s Coalition on Safe Labor cohorts, and a cohort of deliveries at Universal Health Services. They found that Black patients had significantly higher rates of anemia, and anemia is associated with significantly higher rates of blood transfusions postpartum, but that after adjusting for anemia, Black race was still associated with transfusion in the Cesarean Registry cohort, but not in the Coalition on Safe Labor or Universal Health Services cohorts. Given the large disparities in anemia rates by race, preconception and antepartum optimization of anemia remains an actionable lever by which postpartum transfusion disparities can be reduced.
8.2. Pertaining to disparities in hemorrhage by condition
Type O blood has been shown in the trauma literature to be associated with lower rates of von Willebrand factor (VWF), but whether type O blood increases the risk of PPH has not been confirmed, particularly for patients whose pregnancy is also complicated by thrombocytopenia. In a multicenter cohort study of 188 patients,32 consecutive patients delivering with moderate or severe thrombocytopenia or immune thrombocytopenia purpura (mean platelet count, 90 000) were matched by age, mode of delivery, and ethnicity to controls with normal platelet counts. The authors found in their primary analysis that the adjusted risk of PPH associated with type O blood was stronger in patients with thrombocytopenia (aOR, 12.7; 95% CI, 2.9‐55.3) than in patients without thrombocytopenia (aOR, 3.2; 95% CI, 1.1‐9.5). The potential synergistic association between lower levels of VWF mediated by blood type and thrombocytopenia provides an interesting mechanistic rationale for this clinical finding. Given the known difficulties in predicting PPH using conventional clinical risk factors, this work also highlights the potential for incorporation of easily identified laboratory biomarkers (such as blood type and admission platelet count) into improved PPH prediction models.
8.3. Addressing the disparities in obstetric or postpartum hemorrhage outcomes through technology
Rapid coagulation testing is helpful in the management of PPH. Many hospitals have adopted some form of viscoelastic testing (eg, thromboelastography or rotational thromboelastometry) or rapid conventional coagulation testing, but these technologies require expertise to execute the tests and maintain quality control on the analyzers and may not be an option in low‐ or middle‐income settings. Karuppiah and colleagues33 described results of an ultrasound‐based rapid coagulation analyzer that uses a disposable cartridge‐based approach to minimize skill requirements. They compared results from the new analyzer to conventional coagulation testing and found a strong correlation. Before the use of this new analyzer could be considered in low‐ or middle‐income settings, the cost of the prefilled cartridges, and the real‐world performance of this assay in PPH would need to be rigorously studied and compared to the alternatives.
Placenta accreta spectrum confers, perhaps, the highest risk of massive hemorrhage of any obstetric condition. It remains unknown, however, whether routine placement of endovascular devices such as balloon catheters or arterial sheaths for planned delivery of patients with this condition reduces morbidity or transfusion needs. In this retrospective cohort study, Bonsen and colleagues34 compared outcomes from national registries and hospitals in the Netherlands for 351 patients undergoing planned cesarean delivery for placenta accreta spectrum. They did not find statistically significant differences in estimated blood loss with application of prophylactic interventions, although the authors did highlight trends that would be clinically relevant if the findings held in larger studies (adjusted blood loss reduction of 590 mL in patients with suspected placenta accreta by imaging, or 872 mL in patients with confirmed placenta accreta by pathology). The authors plan to combine their results with that of others in a meta‐analysis, which seems like a reasonable next step toward providing evidence that could help reduce the risk of PPH in patients with this rare but profoundly high‐risk condition.
8.4. Addressing the disparities in obstetric or postpartum hemorrhage outcomes through hemostatic agents
The ideal approach to resuscitation of the patient with massive obstetric hemorrhage is unknown. In a randomized trial of 75 women having a severe hemorrhage (defined as blood loss of 40%‐52%) in the delivery or early postpartum period,35 receipt of a prothrombin complex concentrate (PCC) rather than cryoprecipitate was associated with lower transfusion requirements, lower incidence of mechanical ventilation, acute kidney injury, and gastrointestinal dysfunction. Intensive care unit stays were additionally shorter. Larger multicenter studies would be helpful to confirm these findings, but there are additional advantages to PCCs. From a safety perspective, PCCs, as opposed to cryoprecipitate, are virally inactivated. They can be reconstituted more quickly than cryoprecipitate can be thawed. Also, they have a lower infusion volume. What is particularly relevant for patients in low‐ or middle‐income settings is that PCCs do not require refrigeration, have a relatively long shelf life (3 years) and can be maintained in a pharmacy as opposed to a blood bank.
9. FUTURE DIRECTIONS
We need more experts who are interested in the unique bleeding challenges faced by women at the time of childbirth. We need more experts who are interested in PPH and willing to study the acute acquired coagulopathy that accompanies massive PPH with the same diligence that has been applied to other bleeding phenomena such as trauma‐induced coagulopathy. Besides new therapeutic options, we need a cadre of investigators around the globe. Our goal should be to make the miracles we have achieved in transfusion medicine and hemostasis and thrombosis available to every childbearing woman.
RELATIONSHIP DISCLOSURE
AHJ: research funding from Coagulant Therapeutics paid to institution; consulting fees from Octapharma and Cerus; board of directors, Foundation for Women and Girls with Blood Disorders, unpaid. JJF – National Center for Advancing Translational Sciences, UL1TR002553, funding for fellowship salary, paid to institution; Foundation for Women and Girls with Blood Disorders, funding for research activities, paid to institution. HKA: National Institutes of Health K23 HL141640, paid to institution; Society of Maternal‐Fetal Medicine Global Health Committee, International Task Force Leader, unpaid.
AUTHOR CONTRIBUTIONS
AJJ developed the concept for the paper, surveyed and interpreted the available data, wrote the original draft, and edited and approved the final version. JJF contributed critical intellectual content during the planning and revising of the paper, developed the ISTH 2021 Congress Report, and approved the final version. HKA inspired the concept for the paper, contributed critical intellectual content during the planning and revising of the paper, and approved the final version.
James AH, Federspiel JJ, Ahmadzia HK. Disparities in obstetric hemorrhage outcomes. Res Pract Thromb Haemost. 2022;6:e12656. doi: 10.1002/rth2.12656
Handling Editor: Pantep Angchaisuksiri
Contributor Information
Andra H. James, Email: andra.james@duke.edu, @andrajames031.
Homa K. Ahmadzia, @ahmadzia_k.
REFERENCES
- 1. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110(5):1368‐1373. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization , UNICEF , United Nations Population Fund , The World Bank . Trends in maternal mortality: 2000 to 2017 [Monograph]. Geneva, CH: WHO; 2019.
- 3. CDC . Infographic: racial/ethnic disparities in pregnancy‐related deaths — United States, 2007–2016 [cited 2021]. Available from: https://www.cdc.gov/reproductivehealth/maternal‐mortality/disparities‐pregnancy‐related‐deaths/infographic.html
- 4. Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453‐1463. [DOI] [PubMed] [Google Scholar]
- 5. Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61(2):387‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2(6):e323‐e333. [DOI] [PubMed] [Google Scholar]
- 8. CDC . Pregnancy mortality surveillance system. https://www.cdc.gov/reproductivehealth/maternal‐mortality/pregnancy‐mortality‐surveillance‐system.htm#causes Data accessed 7 Feb 2021
- 9. Al‐Zirqi I, Vangen S, Forsen L, Stray‐Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115(10):1265‐1272. [DOI] [PubMed] [Google Scholar]
- 10. Collins PW, Cannings‐John R, Bruynseels D, et al. Viscoelastometric‐guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double‐blind randomized controlled trial. Br J Anaesth. 2017;119(3):411‐421. [DOI] [PubMed] [Google Scholar]
- 11. Collins PW, Cannings‐John R, Bruynseels D, et al. Viscoelastometry guided fresh frozen plasma infusion for postpartum haemorrhage: OBS2, an observational study. Br J Anaesth. 2017;119(3):422‐434. [DOI] [PubMed] [Google Scholar]
- 12. Bolliger D, Gorlinger K, Tanaka KA. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology. 2010;113(5):1205‐1219. [DOI] [PubMed] [Google Scholar]
- 13. Cunningham FG, Nelson DB. Disseminated intravascular coagulation syndromes in obstetrics. Obstet Gynecol. 2015;126(5):999‐1011. [DOI] [PubMed] [Google Scholar]
- 14. Knight M, Nelson‐Piercy C, Kurinczuk JJ, Spark P, Brocklehurst P. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951‐956. [DOI] [PubMed] [Google Scholar]
- 15. Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211‐1217; discussion 7. [DOI] [PubMed] [Google Scholar]
- 16. McLintock C, James AH. Obstetric hemorrhage. J Thromb Haemost. 2011;9(8):1441‐1451. [DOI] [PubMed] [Google Scholar]
- 17. Ahmadzia HK, Phillips JM, Katler QS, James AH. Tranexamic acid for prevention and treatment of postpartum hemorrhage: an update on management and clinical outcomes. Obstet Gynecol Surv. 2018;73(10):587‐594. [DOI] [PubMed] [Google Scholar]
- 18. WHO , UNICEF , UNFPA , World Bank Group , United Nations Population Division . Blood safety and availability. https://www.who.int/news‐room/fact‐sheets/detail/blood‐safety‐and‐availability Data accessed 7 Feb 2021
- 19. Green L, Knight M, Seeney FM, et al. The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross‐sectional study. BJOG. 2016;123(13):2164‐2170. [DOI] [PubMed] [Google Scholar]
- 20. Singla AK, Lapinski RH, Berkowitz RL, Saphier CJ. Are women who are Jehovah’s Witnesses at risk of maternal death? Am J Obstet Gynecol. 2001;185(4):893‐895. [DOI] [PubMed] [Google Scholar]
- 21. Massiah N, Athimulam S, Loo C, Okolo S, Yoong W. Obstetric care of Jehovah’s Witnesses: a 14‐year observational study. Arch Gynecol Obstet. 2007;276(4):339‐343. [DOI] [PubMed] [Google Scholar]
- 22. Van Wolfswinkel ME, Zwart JJ, Schutte JM, Duvekot JJ, Pel M, Van Roosmalen J. Maternal mortality and serious maternal morbidity in Jehovah’s Witnesses in The Netherlands. BJOG. 2009;116(8):1103‐1108; discussion 8–10. [DOI] [PubMed] [Google Scholar]
- 23. Bates I, Chapotera GK, McKew S, van den Broek N. Maternal mortality in sub‐Saharan Africa: the contribution of ineffective blood transfusion services. BJOG. 2008;115(11):1331‐1339. [DOI] [PubMed] [Google Scholar]
- 24. Sriram V, Bennett S. Strengthening medical specialisation policy in low‐income and middle‐income countries. BMJ Glob Health. 2020;5(2):e002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ISTH membership Directory. https://www.isth.org/search/ Data accessed June 16, 2021.
- 26. Ling G, Draghic N. Aerial drones for blood delivery. Transfusion. 2019;59(S2):1608‐1611. [DOI] [PubMed] [Google Scholar]
- 27. Homier V, Brouard D, Nolan M, et al. Drone versus ground delivery of simulated blood products to an urban trauma center: the Montreal Medi‐Drone pilot study. J Trauma Acute Care Surg. 2021;90(3):515‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bohren MA, Lorencatto F, Coomarasamy A, et al. Formative research to design an implementation strategy for a postpartum hemorrhage initial response treatment bundle (E‐MOTIVE): study protocol. Reprod Health. 2021;18(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ker K, Roberts I, Chaudhri R, et al. Tranexamic acid for the prevention of postpartum bleeding in women with anaemia: study protocol for an international, randomised, double‐blind, placebo‐controlled trial. Trials. 2018;19(1):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Main EK, Chang SC, Dhurjati R, Cape V, Profit J, Gould JB. Reduction in racial disparities in severe maternal morbidity from hemorrhage in a large‐scale quality improvement collaborative. Am J Obstet Gynecol. 2020;223(1):123.e1‐123.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davis E, Amdur R, Ahmadzia H. Interactions of anemia with race and peripartum transfusion in three large US registries [abstract]. Res Pract Thromb Haemost. 2021;5(suppl 2):959. [Google Scholar]
- 32. Arcudi S, Ronchi A, Capecchi M, et al. The impact of the ABO blood group on postpartum haemorrhage risk among women with thrombocytopenia [abstract]. Res Pract Thromb Haemost. 2021;5(suppl 2):951‐952. [Google Scholar]
- 33. Karuppiah A, Kodali BS. Novel point of care rapid coagulation analyzer for obstetrics – quantra (SEER sonorheometry) [abstract]. Res Pract Thromb Haemost. 2021;5(suppl 2):965. [Google Scholar]
- 34. Bonsen L, Harskamp V, Feddouli S, et al. Prophylactic radiological interventions to reduce postpartum haemorrhage in patients with placenta accreta spectrum disorders [abstract]. Res Pract Thromb Haemost. 2021;5(suppl 2):968. [Google Scholar]
- 35. Sedinkin V, Klygunenko O, Volkov O. The effect of prothrombin complex concentrate and cryoprecipitate on the frequency and severity of multiple organ dysfunction syndrome in massive obstetric haemorrhage [abstract]. Res Pract Thromb Haemost. 2021;5(suppl 2):969. [Google Scholar]


