Abstract
Introduction and importance
En-plaque-meningioma (EPM) is characterized by its flat growth along the bony contour. It accounts for 2–9% of all meningiomas. Very few grade II or III EPM cases were reported. Surgical resection of sphenoid wing EPM is especially challenging as the tumour tends to invade the cavernous sinus, and/or the orbit, and their neurovascular structures. Consequently, tumours in such locations have a higher rate of recurrence. We report the clinical course and management of a patient suffering a second recurrence of grade II EPM. The clinical course of grade II EPM, and the management of multiple recurrences of EPM are scarcely reported in the literature.
Case presentation
A 53-year-old male with a history of three previous surgeries for EPM presented with decreased vision in the right eye. Brain magnetic resonance imaging (MRI) showed progression of a sphenoid wing meningioma invading the left optic nerve, indicating a second recurrence of the tumour.
Clinical discussion
We reviewed the literature discussing the clinical course of grade II EPM, and cases suffering multiple recurrences. Only a few cases were found with varying clinical course and management. In our case, surgical intervention was necessary to save the patient's vision. A modified orbitozygomatic craniotomy was performed. A small residual tumour invading the cavernous sinus was left for treatment with stereotactic radiosurgery.
Conclusion
Sphenoid wing EPM is challenging pathology to manage, especially grade II tumours which are rarely encountered. Multimodality treatment with surgery and radiotherapy offers EPM patients the best chance of treatment.
Keywords: En plaque, Meningioma, Sphenoid wing, Grade II, Recurrent meningioma, Atypical meningioma
Highlights
-
•
En-plaque-meningioma (EPM) accounts for 2–9% of meningiomas.
-
•
Clinical course of grade II EPM is scarcely discussed in the literature.
-
•
Management of multiple EPM recurrences needs a multimodality approach using surgery and radiotherapy.
-
•
We report the clinical course and management of a recurrent grade II EPM.
1. Introduction
Meningiomas are dural-based tumours arising from meningothelial cells (arachnoid ‘cap’ cells) [1,2]. They represent 37.6% of all primary brain tumours in adults, making them the most common type of intracranial tumour [2]. The incidence of meningioma increases with age, with a median age of diagnosis of 65 years [3]. Meningiomas are usually slow-growing and not infiltrative, and their symptoms are variable and depend on their location [1,4]. En-plaque-meningioma (EPM) is a rare type of meningioma defined by a sheet-like lesion that infiltrates the dura and at times invades the bone [[5], [6], [7]]. EPM accounts for 2–9% of all meningiomas and is usually located in the sphenoid wing [5,6]. EPM arises much more commonly in females than males [8,9]. Most of the reported cases of EPM are WHO grade I, with very few cases being grade II or III [5,10].
Surgical resection with the goal of total removal of the tumour when feasible is the main therapeutic strategy for meningioma. The extent of resection greatly impacts the rate of recurrence [11]. Surgical resection of sphenoid wing EPM is especially challenging as the tumour tends to invade the cavernous sinus and/or the orbit and their neurovascular structures. Consequently, tumours in such locations have a higher rate of recurrence [10].
In this article, we present a case and literature review of surgically challenging recurrent WHO grade II En-plaque-meningioma of the sphenoid wing with emphasis on the management of multiple recurrences during an 11-year follow-up period. This work has been reported in line with the SCARE criteria [12].
2. Case report
Our patient is a 53-year-old right-handed male with a history of three previous surgeries for EPM complicated by right-sided weakness, left eye blindness, and aphasia (Table 1 lists all the surgeries the patient had in chronological order, including the most recent ones). Most recently, the patient presented with decreased vision in the right eye. Brain magnetic resonance imaging (MRI) showed progression of a multifocal meningioma seen along the left anterior clinoid process, left sphenoid wing, anterior temporal fossa, and planum sphenoidale with an invasion of the left pre-chiasmatic optic nerve, indicating a second recurrence of the tumour (Fig. 1).
Table 1.
Lists of surgeries the patient had in chronological order.
| Date | Age at time of surgery | Presenting symptoms | Diagnosis | Extent of Resection | Histopathology and/or Microbiology | Radiotherapy |
|---|---|---|---|---|---|---|
| 2010 | 42 | Seizures | SWEPM* | Debulking- Simpson grade 4 | Grade II meningioma, Mitotic rate is 2/10HPF. Ki-67 proliferation index is 15%. Brain invasion is highlighted with GFAP | External beam radiotherapy as 55.8Gy/31Fx. |
| 2016 | 48 | Right-sided weakness, dysphasia, and medically refractory seizures | SWEPM- First Recurrence | GTR- Simpson grade 2 | Atypical Meningioma | None |
| 2016 | 48 | Decreased level of consciousness and weakness in the right hand | Subdural empyema | NA | Culture results: scant growth of coagulase-negative staphylococcus | None |
| 2020 | 53 | Decreased vision in right eye | Sphenoid Wing- clinoidal meningioma. 2nd recurrence | Near-Total with residual over the cavernous sinus and petroclival region | Atypical meningioma | None |
| 2020 | 53 | Wound dehiscence | Wound dehiscence with CSF leak | NA | NA | None |
| 2021 | 53 | Headache | left temporal fossa pseudomningocele | Left cystoperitoneal shunting | NA | None |
SWEPM: sphenoid wing en-plaque meningioma, NA: not available.
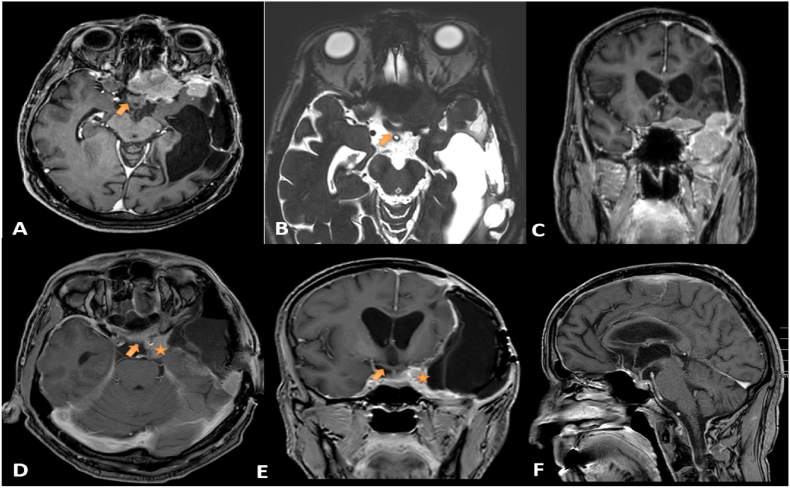
Fig. 1.
A, B, and C: MRI with contrast showing multifocal meningioma seen along the left anterior clinoid process, left sphenoid wing, anterior temporal fossa, and planum sphenoidale with an invasion of the left pre-chiasmatic optic nerve and optic chiasm (arrow) compression (A: Axial sequence, B: CISS sequence, C: Coronal sequence).
D, E, and F: MRI after Tumor resection with residual lesion marked with a star (*) over the cavernous sinus and petroclival area. Both optic nerve-intracranial parts and optic chiasm (arrow) were freed. Temporal fossa pseudomningocele is seen in E (D: Axial sequence, E: Coronal sequence, F: Sagittal sequence).
The patient's first surgery dates back to 2010 when he presented with seizures. MRI of the brain is shown in Fig. 2. Simpson grade 4 Surgical resection was performed. Histopathology revealed a grade II meningioma with a mitotic rate of 2/10 HPF and a Ki-67 proliferation index of 15%. Brain invasion is highlighted with GFAP (Fig. 3). The patient underwent external beam radiotherapy at 55.8Gy/31Fractions.
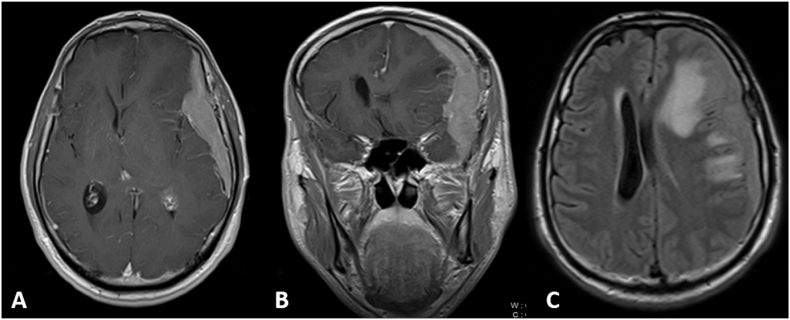
Fig. 2.
Brain MRI with contrast at the patient's first presentation on August 2010: A, B and FLAIR sequence (C) showing extensive extra-axial dural based contrast enhancement along the left cerebral convexity centred over the sphenoid ridge. There is a mass effect on the underlying brain parenchyma with resultant vasogenic oedema with a midline shift to the right.
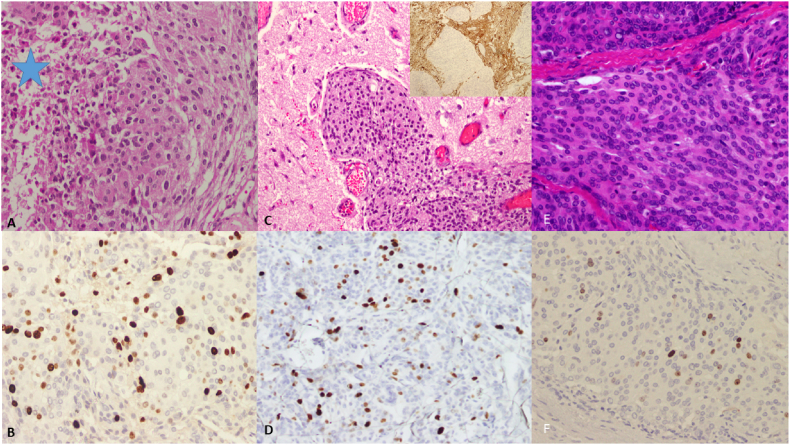
Fig. 3.
A and B. Initial diagnosis in 2010. A; There is a sheet-like growth pattern with necrosis (star) and scattered mitotic figures (H&E, X40). B; Ki-67 proliferative index is estimated at 15.0% (X40). C and D; First recurrence in 2016. C; There is clear evidence of brain invasion (H&E, X40), insert; further highlighted with GFAP immunostain (X40), D; Ki-76 proliferative index is estimated at 15.0% (X40). E and F; Second recurrence in 2020. E; There is sheet-like growth, with no evidence of brain invasion (H&E, X40), F; Ki-67 proliferative index is 5.0% (X40).
On follow-up, the tumour progressed over the years, but the patient refused surgical intervention. Six years after the initial surgery, the patient presented to the emergency department with right-sided weakness, dysphasia, and medically refractory seizures. MRI of the brain revealed a left frontal-temporal en-plaque-meningioma (Fig. 4). A frontotemporal craniotomy was performed and achieved Simpson grade 2 resection. 11 weeks later, the patient presented with a decreased level of consciousness and weakness in the right hand; brain MRI showed a subdural collection with diffusion restriction consistent with a subdural empyema (Fig. 4). A left frontotemporal craniotomy and evacuation of subdural empyema were performed. Culture results revealed scant growth of coagulase-negative staphylococcus.
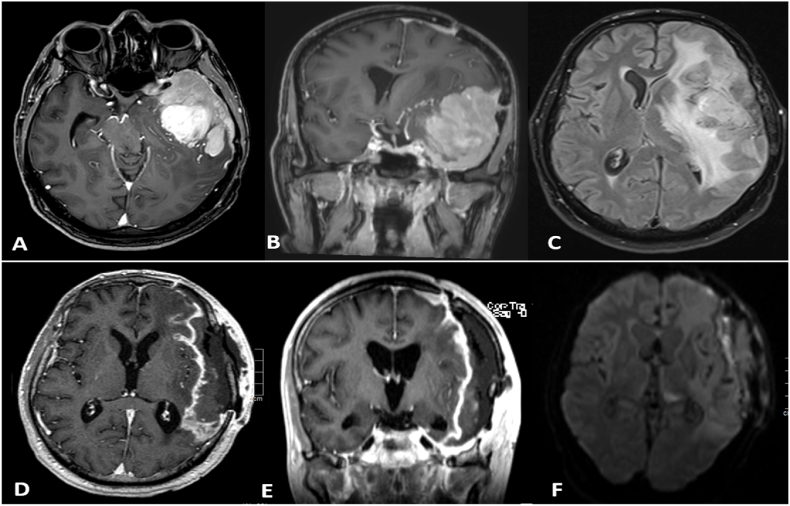
Fig. 4.
Brain MRI with contrast (images A-C) for the first recurrence in 2016: Infiltrative avidly contrast-enhancing extra-axial dural mass at the left frontotemporal convexity with medial extension along the left lesser wing of the sphenoid bone and the lateral wall of the left cavernous sinus. the mass infiltrates the lateral surface of the left frontal and temporal lobes causing vasogenic oedema in the left cerebral hemisphere with resultant mass effect causing rightward midline shift and left uncal herniation.
8 weeks after surgery (images D-F): shows abnormal enhancement in the surgical cavity (D, E) with diffusion restricted subdural fluid collection underneath the craniotomy flap (F), most suggestive of subdural empyema.
Although tumour progression was detected on follow-up 12 months later, the patient became symptomatic with decreased vision in the right eye 59 months after the surgery for the first recurrence.
3. Management of the second recurrence
The mainstay management of refractory meningioma is surgery and radiotherapy. In this particular case, the decision to re-operate with a known history of 3 previous major surgeries, infection, and radiotherapy treatment, has its risks. The large volume of the tumour makes radiotherapy, not the best option. Therefore, surgery was the only suitable way to remove the tumour and address the decreased vision in the right eye.
The preoperative assessment of the visual field had shown complete blindness in the left eye and right temporal hemianopia. Optic canals invasion was assessed with TI weighted VIBE and CISS sequence which showed complete obliteration of the left optic canal and sparing of the right canal. This means the defect in the right field correlates with the compression on chiasm which was obvious in the preoperative MRI. Optic canal invasion in the skull base meningioma is usually assessed preoperatively with contrast-enhanced T1 Weighted VIBE or CISS sequence [13]. The primary goal of reoperation was to remove the tumour close to the optic chiasm and the right optic nerve, as well as maximally resecting the tumour over the anterior and middle skull base.
A modified orbitozygomatic craniotomy was utilized over the previous frontotemporoparietal approach. The modified orbitozygomatic craniotomy provided better exposure of the anterior and middle cranial base, including the cavernous sinus. Given the history of complete left eye blindness for 10 months; anterior clinoidectomy was not done as it will prolong the procedure and increase the risks of internal carotid artery injury. The orbitozygomatic approach is an important armamentarium for tumours in the cavernous sinus region [14,15]. The postoperative course was uneventful. A small residual tumour was left in the posterior cavernous sinus and petroclival region for treatment with stereotactic radiosurgery after ensuring a distance between the optic chiasm and tumour, separation surgery, as the patient did not have any trigeminal neuralgia before surgery [16,17]. Histopathology is shown in Fig. 2.
MRI on the first follow-up after surgical resection for the second recurrence showed a tiny residual lesion in the cavernous sinus and left temporal fossa pseudomeningocele managed by Cystoperitoneal shunting (Fig. 1).
4. Discussion
En-plaque-meningioma (EPM) is defined by its characteristic flat ‘carpet-like’ growth along the bony contour. EPM may also be associated with hyperostosis. These features differentiate it from the more common Meningioma En-masse [10,18]. EPM is three to six times more common in females than in males. The mean age of presentation of EPM is in the fifth decade. EPM more commonly arises in the sphenoidal wing and orbital regions and less frequently along the cerebral convexity, temporal bone, and foramen magnum [5,10,19,20].
The clinical presentation of EPM depends on its location and spread. The symptoms arise either due to the direct neural compression and invasion by the tumour or due to the bony hyperostosis which may narrow foramina and fissures in which neural structures pass through. In sphenoid wing EPM, such neural compression can result in decreased visual acuity and visual field defects. Hyperostosis of the orbital bones can result in proptosis. Some patients also complain of retrobulbar pressure, orbital pain, and headache [5,6,10,20].
EPM presents a diagnostic challenge due to its unusual radiologic appearance. First-line diagnostic imaging studies include computed tomography (CT) to delineate bony involvement and hyperostosis, and magnetic resonance imaging (MRI) to identify dural and intradural involvement. Orbital invasion can be evaluated on postcontrast fat suppression T1-weighted MRI. Hyperostosis often is seen in a periosteal pattern with surface irregularity of involved structures and inward bulging of the lesion [[5], [6], [7],10]. Bony hyperostosis is seen in 13–49% of EPMs [[21], [22], [23], [24]]. A review by Yao and colleagues (2016) concluded that hyperostosis is neither exclusive nor wholly indicative of EPM [21].
Despite the locally invasive nature, the majority of EPM cases still are classified as WHO Grade I tumours due to a low proliferative index [10]. The clinical course of patients with grade II EPM is scarcely discussed in the literature. Samadian et al. (2020) reported grade I EPM in 96% and grade II tumours in 4% of their patients [5]. Kiyofuji et al. (2020) reported grade II in 2 (4.3%) of their patients. One of the patients suffered a second recurrence 47 months after gross-total resection of the tumour. The other patient with a grade II tumour had gross-total resection without suffering any recurrence during the 83 months of follow up [19]. Honeybul et al. (2001) reported a patient with grade II EPM that died due to disease progression despite surgery and radiotherapy [18].
EPM tends to arise in complex and sensitive cranial regions, and it tends to invade bony structures and infiltrate fissures and foramina. Thus, its surgical resection is challenging. Anatomical constraints and tumour involvement of cranial nerves, superior orbital fissure, or cavernous sinus make gross total resection difficult. Therefore, the benefits of achieving gross total resection should be weighed against the increased risk of surgical morbidity. Subtotal resection is commonly preferred when the tumour is intra-orbital, invading the cavernous sinus, extending beyond the tentorial notch, and invading the superior orbital fissure. All the patients reported by Samadian et al. (2020) who had cavernous sinus involvement (9%) had sub-total resection. Adjuvant radiotherapy was utilized in cases of residual tumours and cases of grade II tumours with varying results between studies [5,6,10,19].
Samdian et al. (2020) reported recurrence in ≈12% of cases; ≈4% after complete tumour removal; ≈9% had progressive growth of the residual tumour. In ≈4% of patients with relapse, grade II EPM was reported [5]. All the patients reported by Simas et al. (2013) had grade I EPM, 28% of which suffered a recurrence [6]. A literature review by Elder and colleagues (2021) analysed primary studies on sphenoid wing EPM; they found that recurrence rates were significantly higher in EPMs that involved the orbit or cavernous sinus [10].
EPM is liable to recur even after total resection [8]. Our patient suffered 2 recurrences. Surgery was the only option to save his vision in the second recurrence. Management of recurrences includes surgery, radiotherapy, and gamma knife radiosurgery [25]. Mirone et al. (2009) reported a patient who underwent 4 operations during an 8-year period. He had an extensive spheno-orbital meningioma involving the cavernous sinus, superior orbital fissure, and infratemporal fossa. Although complete removal was achieved with the first operation (Simpson grade II), he experienced multiple recurrences. During the most recent operation, cavernous sinus exenteration was associated with complex craniofacial reconstruction but, despite aggressive surgery and normal histopathological results, this patient continued to harbour residual tumour in the posterior orbit at the most recent follow-up examination [20]. Boari et al. (2013) reported 4 patients with recurrent tumours after total resection in their cohort. Three patients underwent Gamma-Knife treatment on the recurrent tumour. One patient underwent a second surgical operation. Only one patient who underwent Gamma-Knife radiosurgery experienced tumour recurrence 18 months after the treatment. The tumour growth was detected outside the radiosurgical target. Further Gamma-Knife treatment was performed. The mean follow-up period in the radiosurgical group was 54.8 months (range 18–102) [25]. Moreover, Simas et al. (2013) reported tumour recurrence in two patients treated with surgery alone. One of these patients with an intraorbital tumour presented with a second recurrence which was submitted to combined treatment (surgery and radiotherapy) allowing a 5-year progression-free survival since this combined treatment. These reported cases show that although uncommon, multiple surgical interventions may be necessary to control disease progression and symptoms in this disease.
5. Conclusion
In this article, we report the clinical course and surgical management of a second recurrence in a patient with grade II En-plaque-meningioma. Furthermore, we reviewed the management of sphenoid wing EPM patients with multiple recurrences reported in the literature. Sphenoid wing EPM is a very challenging pathology to manage especially when dealing with WHO grade II tumours which are rarely encountered. Treatment and approach should be individualized. Multimodality treatment with surgery and radiotherapy offers EPM patients the best chance of treatment. Management of recurrences is insufficiently discussed in the literature. By this article, we hope to shed light on this complex pathology. Further studies are required to gain more insight regarding the appropriate management of grade II En-plaque sphenoid wing meningiomas.
Sponsers
There are no sponsors involved in the study.
Ethical approval
N/A.
Sources of funding
No funding or grants were recieved by any of the authors for this work.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Research registration (for case reports detailing a new surgical technique or new equipment/technology)
N/A.
Declaration of competing interest
All authors declare that they have no competing interests.
Acknowledgements
We would like to thank our patient for giving permission to publish the case.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103322.
Contributor Information
Baha'eddin A. Muhsen, Email: bmuhsen08@gmail.com.
Abdelmajid I. Aljariri, Email: abdelmajeed-aljariri@live.com.
Hasan Hashem, Email: hasankamalhashem@yahoo.com.
Qasem Alzoubi, Email: dr_qasem87@hotmail.com.
Nasim Sarhan, Email: nsarhan@khcc.jo.
Maysa Al-Hussaini, Email: mhussaini@khcc.jo.
Abdellatif Al Mousa, Email: Aalmousa@khcc.jo.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Buerki R.A., Horbinski C.M., Kruser T., Horowitz P.M., James C.D., Lukas R.V. An overview of meningiomas. Futur. Oncol. 2018;14:2161–2177. doi: 10.2217/fon-2018-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huntoon K., Toland A.M.S., Dahiya S. Meningioma: a review of clinicopathological and molecular aspects. Front. Oncol. 2020;10:1–14. doi: 10.3389/fonc.2020.579599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qt O., G C., H G., N P., K W., C K., Js B.-S. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016, neuro. Oncol. 21. 2019. V1–V100. [DOI] [PMC free article] [PubMed]
- 4.Magill S.T., Young J.S., Chae R., Aghi M.K., Theodosopoulos P.V., McDermott M.W. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg. Focus. 2018;44:E4. doi: 10.3171/2018.1.FOCUS17752. [DOI] [PubMed] [Google Scholar]
- 5.Samadian M., Sharifi G., Mousavinejad S.A., Amin A.A., Ebrahimzadeh K., Tavassol H.H., Borghei-Razavi H., Rezaei O. Surgical outcomes of sphenoorbital en plaque meningioma: a 10-year experience in 57 consecutive cases. World Neurosurg. 2020;144:e576–e581. doi: 10.1016/j.wneu.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Simas N., Farias J. Sphenoid wing en plaque meningiomas: surgical results and recurrence rates. Surg. Neurol. Int. 2013;4:86. doi: 10.4103/2152-7806.114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Jesús O., Toledo M.M. Surgical management of meningioma en plaque of the sphenoid ridge. Surg. Neurol. 2001;55:265–269. doi: 10.1016/S0090-3019(01)00440-2. [DOI] [PubMed] [Google Scholar]
- 8.Schick U., Bleyen J., Bani A., Hassler W. Management of meningiomas en plaque of the sphenoid wing. J. Neurosurg. 2006;104:208–214. doi: 10.3171/jns.2006.104.2.208. [DOI] [PubMed] [Google Scholar]
- 9.Amirjamshidi A., Abbasioun K., Amiri R.S., Ardalan A., Hashemi S.M.R. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg. Neurol. Int. 2015;6 doi: 10.4103/2152-7806.157074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder T.A., Yokoi H., Chugh A.J., Lagman C., Wu O., Wright C.H., Ray A., Bambakidis N. En plaque meningiomas: a narrative review. J. Neurol. Surg. Part B Skull Base. 2021;82 doi: 10.1055/s-0039-3402012. E33–E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D S. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry. 1957;20:22–39. doi: 10.1136/JNNP.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., Beamish A.J., Noureldin A., Rao A., Vasudevan B., Challacombe B., Perakath B., Kirshtein B., Ekser B., Pramesh C.S., Laskin D.M., Machado-Aranda D., Miguel D., Pagano D., Millham F.H., Roy G., Kadioglu H., Nixon I.J., Mukhejree I., McCaul J.A., Chi-Yong Ngu J., Albrecht J., Rivas J.G., Raveendran K., Derbyshire L., Ather M.H., Thorat M.A., Valmasoni M., Bashashati M., Chalkoo M., Teo N.Z., Raison N., Muensterer O.J., Bradley P.J., Goel P., Pai P.S., Afifi R.Y., Rosin R.D., Coppola R., Klappenbach R., Wynn R., De Wilde R.L., Surani S., Giordano S., Massarut S., Raja S.G., Basu S., Enam S.A., Manning T.G., Cross T., Karanth V.K., Kasivisvanathan V., Mei Z. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/J.IJSU.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Borghei-Razavi H., Lee J., Ibrahim B., Muhsen B.A., Raghavan A., Wu I., Poturalski M., Stock S., Karakasis C., Adada B., Kshettry V., Recinos P. Accuracy and interrater reliability of CISS versus contrast-enhanced T1-weighted VIBE for the presence of optic canal invasion in tuberculum sellae meningiomas. World Neurosurg. 2021;148 doi: 10.1016/j.wneu.2021.01.015. e502–e507. [DOI] [PubMed] [Google Scholar]
- 14.Najera E., Muhsen B.A., Borghei-Razavi H., obrzut M., Adada B. Transcavernous approach for microsurgical clipping of ruptured right superior cerebellar artery aneurysm with cadaveric stepwise video illustration. J. Neurol. Surg. Part B Skull Base. 2021 doi: 10.1055/s-0041-1727178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najera E., Muhsen B.A., Borghei-Razavi H., Adada B. Cavernous sinus meningioma resection through orbitozygomatic craniotomy. World Neurosurg. 2021;148:205. doi: 10.1016/j.wneu.2021.01.069. [DOI] [PubMed] [Google Scholar]
- 16.Joshi K.C., Raghavan A., Muhsen B., Hsieh J., Borghei-Razavi H., Chao S.T., Barnett G.H., Suh J.H., Neyman G., Kshettry V.R., Recinos P.F., Mohammadi A.M., Angelov L. Fractionated Gamma Knife radiosurgery for skull base meningiomas: a single-institution experience. Neurosurg. Focus. 2019;46:E8. doi: 10.3171/2019.3.FOCUS1963. [DOI] [PubMed] [Google Scholar]
- 17.Muhsen B.A., Ali A.M., Jain A., Ibrahim B., Nagera E., Borghei-Razavi H., Adada B. Microsurgical resection of petroclival meningiomas treated with stereotactic radiosurgery to address persistent post-treatment trigeminal pain. Clin. Neurol. Neurosurg. 2021;202:106533. doi: 10.1016/j.clineuro.2021.106533. [DOI] [PubMed] [Google Scholar]
- 18.Honeybul S., Neil-Dwyer G., Lang D.A., Evans B.T., Ellison D.W. Sphenoid wing meningioma en plaque: a clinical review. Acta Neurochir. 2001;143:749–758. doi: 10.1007/s007010170028. [DOI] [PubMed] [Google Scholar]
- 19.Kiyofuji S., Casabella A.M., Graffeo C.S., Perry A., Garrity J.A., Link M.J. Sphenoorbital meningioma: a unique skull base tumor. Surgical technique and results. J. Neurosurg. 2020;133:1044–1051. doi: 10.3171/2019.6.JNS191158. [DOI] [PubMed] [Google Scholar]
- 20.Mirone G., Chibbaro S., Schiabello L., Tola S., George B. En plaque sphenoid wing meningiomas: recurrence factors and surgical strategy in a series of 71 patients. Neurosurgery. 2009;65:100–109. doi: 10.1227/01.NEU.0000345652.19200.D5. [DOI] [PubMed] [Google Scholar]
- 21.Yao A., Sarkiss C.A., Lee J., Zarzour H.K., Shrivastava R.K. Surgical limitations in convexity meningiomas en-plaque: is radical resection necessary? J. Clin. Neurosci. 2016;27:28–33. doi: 10.1016/j.jocn.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Cushing H. The cranial hyperostoses produced BY meningeal endotheliomas. Arch. Neurol. Psychiatr. 1922;8:139–154. doi: 10.1001/ARCHNEURPSYC.1922.02190140030003. [DOI] [Google Scholar]
- 23.Akutsu H., Sugita K., Sonobe M., Matsumura A. Parasagittal meningioma en plaque with extracranial extension presenting diffuse massive hyperostosis of the skull. Surg. Neurol. 2004;61:165–169. doi: 10.1016/S0090-3019(03)00521-4. [DOI] [PubMed] [Google Scholar]
- 24.Bouguila J., Khonsari R.H., Ayashi K., Neji N.B., Yacoub K., Besbes G. Méningiome en plaque frontal : forme rare d’une tumeur commune. Rev. Stomatol. Chir. Maxillofac. 2009;110:309–311. doi: 10.1016/J.STOMAX.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Boari N., Gagliardi F., Spina A., Bailo M., Franzin A., Mortini P. Management of spheno-orbital en plaque meningiomas: clinical outcome in a consecutive series of 40 patients. Br. J. Neurosurg. 2013;27:84–90. doi: 10.3109/02688697.2012.709557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.