Summary
Background
Advanced age is accompanied by a decline of immune functions, which may play a role in increased vulnerability to emerging pathogens and low efficacy of primary vaccinations in elderly people. The capacity to mount immune responses against new antigens is particularly affected in this population. However, its precise determinants are not fully understood. We aimed here at establishing the influence of persistent viral infections on the naive T-cell compartment and primary immune responsiveness in older adults.
Methods
We assessed immunological parameters, related to CD8+ and CD4+ T-cell responsiveness, according to the serological status for common latent herpesviruses in two independent cohorts: 1) healthy individuals aged 19y to 95y (n = 150) and 2) individuals above 70y old enrolled in a primo-vaccination clinical trial (n = 137).
Findings
We demonstrate a prevalent effect of age and CMV infection on CD8+ and CD4+ naive T cells, respectively. CMV seropositivity was associated with blunted CD4+ T-cell and antibody responses to primary vaccination.
Interpretation
These data provide insights on the changes in adaptive immunity over time and the associated decline in vaccine efficacy with ageing. This knowledge is important for the management of emerging infectious diseases in elderly populations.
Keywords: Naive T lymphocytes, Thymus, Elderly, T-cell responses, Vaccines
Research in context.
Evidence before this study
Age-related decline of the immune system, or immunosenescence, is thought to play an important role in the increased rate of severe infections or cancer, and reduced responsiveness to vaccination observed in the elderly. Improving our understanding of immune aging has broad implications in disease prevention and optimization of vaccine immunogenicity with advanced age. While recall responses to pathogens encountered earlier in life are usually uncompromised, there is an established paradigm that the ability to mount primary cellular immune responses from naive T cells is waning in older people. For instance, reduced immunogenicity to SARS-CoV-2 vaccination has recently been demonstrated in elderly people. However, the factors that can impact the naive T-cell compartment and primary immune responsiveness in older adults are not fully understood. The potential influence of persistent viral infections, known to continuously stimulate and consume immune resources, remains controversial due to conflicting evidence.
Added value of this study
In the present study, we address the question of the cumulative and differential impact of infections with common persistent herpesviruses (i.e. CMV, EBV, HSV-1 and HSV-2) on the naive T-cell compartment and de novo T-cell responsiveness. We studied immune parameters in two cohorts of healthy adults: a cohort of healthy individuals aged 19y to 95y (n = 150) and a cohort of old people (n = 137) who received a primary vaccination in the context of a clinical trial designed to address the question of the influence of CMV infection on vaccine responsiveness. We show that infection with these viruses, in particular CMV, results in a reduction of the naive CD4+ T-cell compartment with advanced age. Consistently, CMV infection is associated with reduced cellular as well as humoral responsiveness to a primary vaccination in old people.
Implications of all the available evidence
Collectively, these findings provide clear insights into the alterations of adaptive immunity over time and the decline in vaccine efficacy with ageing. In particular, we show that infections with persistent viruses, like CMV, which is common in the general population, can affect primary immune responsiveness with old age. This knowledge is important for the follow up of elderly populations in settings of infectious disease outbreaks and vaccine campaigns.
Alt-text: Unlabelled box
Introduction
It is common nowadays to live beyond 80 years old, which has an important impact on human demographics and represents a growing challenge in terms of health care. Advanced age is accompanied by the increase in acute and chronic diseases, resulting in an important decline in quality of life. In turn, this is associated with the need to care for elderly individuals with poor health. In particular, elderly people suffer more often and more severely from infections than young individuals.1, 2, 3 The current SARS-CoV-2 pandemic represents a particularly relevant example of the issues that can be encountered in this context.4,5 It highlights the importance of understanding better the evolution of the immune system with ageing, which is key in the vulnerability of old people to many diseases including COVID-19. Moreover the response of older people to vaccination is considered generally suboptimal.6 A recent study shows that the immunogenicity to the SARS-CoV-2 vaccine BNT162b2 is indeed reduced in the elderly.7 The need for in-depth knowledge on immune responsiveness to emerging infections and primary vaccinations in the elderly has never been greater.
Studying primary immune responses, in particular to vaccination, in older humans poses several challenges. Most vaccinations are given to boost preexisting immunity in this population. The responses to several recall vaccines, such as those against influenza virus, varicella zoster virus,8,9 Streptococcus pneumoniae10,11 or diphtheria and tetanus antigens,12 have been studied in older individuals. These studies have revealed differential responses upon vaccination in elderly populations. Some vaccines provided an adequate boosting of immunological memory while others presented a waning capacity to maintain strong and protective responses.13 In contrast, available data sets from studies on yellow fever,14,15 hepatitis B,16 or Japanese encephalitis virus17 vaccines, indicate that primary responses in older individuals are mostly compromised. Vaccinated older people presented delayed and reduced primary antibody responses compared with young adults, and both their CD8+ and CD4+ T-cell responses were impaired. Overall, these data support a general impairment of de novo immune responses, while recall immunity seems more preserved in some cases.
Immunosurveillance against emerging viruses and de novo vaccine responsiveness are crucially dependent on the efficacy to mount primary responses,18 and thus the activation of naive T cells.19 Several independent studies have confirmed that ageing results in general defects of the quality (e.g. activation, proliferation, telomere length, and differentiation) and the quantity of naive CD8+ and CD4+ T cells, reducing their ability to respond to previously unencountered antigens.20, 21, 22 The quantitative loss of naive T cells is a key feature of immune ageing and can by itself be a cause of impaired vaccine-specific immune response. Several factors play a role in the age-related contraction of the naive T-cell compartment: (i) the naive T-cell pool is used and consumed throughout life to generate effector and memory T cells; (ii) old age-associated impaired lymphopoiesis and thymic involution result in diminished naive T-cell production and replenishment; (iii) naive T-cell maintenance is affected by an altered homeostasis due to an unbalanced naive vs memory cell ratio.
Whether common chronic and latent viral infections impact immune responsiveness, e.g against emerging viruses or vaccines, is an important issue in this context. Persistent viruses, such as those of the herpesvirus family, are highly prevalent in the human population and, although classically benign in immunocompetent individuals, exert a long-term stress on host's immune cells. From a biological point of view, the herpesvirus family represents the most classic model of viruses that establish a latent infection, a strategy that allows them to remain in the host for the duration of its life. After primary acute infection, herpesviruses induce a persistent infection characterized by successive cycles of reactivation and authentic virological latency, established only in particular cell types and anatomical sites, different for each herpesvirus. Latent infection constitutes a reduced immunogenic stimulus for the infected host, while the reactivation phase is characterized by the initiation of the lytic viral cycle, the rapid destruction of target cells and the induction of inflammatory and virus-specific immune responses. The prevalence of these infections increases proportionally with age, and their role in age-related immune decline remains a matter of debate. One persistent virus has been mainly studied in this context: cytomegalovirus (CMV). CMV is a β-herpesvirus, whose prevalence is high in the human population and increases with age. Despite a strong immune response, CMV is not eliminated and remains as a latent infection. This virus is known to leave a major imprint on our immune system, in particular reflected by the accumulation of CD27−CD28−CCR7−CD45RA+CD57+ T cells, identified as late differentiated effector memory (EM) T cells which produce pro-inflammatory cytokines.23 CMV infection has also been associated with changes of naive T-cell frequencies, though not in all studies,24 and with consistently increased levels of systemic inflammatory cytokines.23 Its impact on vaccine responsiveness has been controversial. It has been mostly studied in the context of vaccination against influenza,25, 26, 27, 28 with some studies reporting suboptimal influenza vaccine responses in CMV-infected patients, and other studies showing no effect or even an enhancement of vaccine humoral and cellular immune responses or heterologous immunity by CMV.29, 30, 31 Lack of consensus may be related to the variability of the parameters investigated in different studies, including: CD8+ versus CD4+ T cells, T-cell frequencies versus absolute counts, young versus old subjects, primary versus recall vaccine responses. Therefore the influence on adaptive immunity by latent infection with CMV and other persistent viruses remains an open question with several unresolved issues.
In the present work, we aimed to identify cumulative and differential effects on the CD8+ and CD4+ T-cell compartments by persistent long-term viral infections in an elderly population. We focused on the influence of herpesvirus infections including CMV, EBV, HSV-1 and HSV-2 on the counts, maintenance and responsiveness of naive T cells. To this end, two cohorts were studied: a cohort of 150 healthy adults aged between 19 and 95 years old, and a cohort of 137 older healthy adults (aged more than 70 years old) who received a primary vaccination against tick-borne encephalitis (TBE). We found that long-term infections with herpesviruses, in particular CMV, impacted the naive CD4+, but not CD8+, T-cell compartment, and yielded reduced adaptive and humoral responses to a primary vaccination in the elderly.
Methods
Study design, setting and participants
Two cohorts of healthy volunteers were enrolled in this study. The first cohort (observational study - cohort 1) consisted of 150 healthy adults (aged between 19 and 95 years old) with serology data for four common herpesviruses. Individuals <65y were recruited among blood donors (Etablissement francais du sang) in Paris (France) while those aged >-5y were recruited at the geriatric department of the Pitié-Salpêtrière Hospital (Paris, France) between 2015 and 2016. Individuals with malignancies, acute diseases, or severe chronic diseases, such as atherosclerosis, congestive heart failure, poorly controlled diabetes mellitus, renal or hepatic disease, various inflammatory conditions, or chronic obstructive pulmonary disease, as well as individuals on immunosuppressive therapy, were excluded from the study. The second cohort (cohort 2) consisted of 137 individuals aged more than 70 years old, who received a full TBE vaccination course of three injections at week 0, 4 and 24 with a licensed inactivated whole virus vaccine (FSME Immun® CC, Baxter) as part of a clinical trial (registered through ClinicalTrials.gov: NCT00461695) conducted at the Epidemiology, Biostatistics and Prevention Institute (EBPI) at the University of Zürich Switzerland between 2007 and 2010 (see Table S1 in supplemental materials). Here, we report the core data of this study including immunogenicity of TBE vaccination in healthy elderly stratified by CMV serostatus. Previous reports had included additional data derived from this cohort.21,32, 33, 34 Included individuals were healthy according to a health questionnaire and a physical evaluation at study entry; 57% reported no co-morbidities and no long-term medical treatment, 43% reported one co-morbidity requiring drug treatment (mostly hypertension). All individuals were immunocompetent and TBEv-naive and -seronegative. Immune response assays were conducted prior to vaccination, and at weeks 4, 8, 24 and 28 post-vaccination for humoral and week 26 for cellular immune responses. PBMCs were isolated from venous blood samples via density gradient centrifugation according to standard protocols and cryopreserved in complete medium supplemented with dimethyl sulfoxide (DMSO; 10% v/v; Sigma-Aldrich) and fetal calf serum (FCS; 20% v/v; Sigma-Aldrich) or as dry pellet for DNA extraction. Complete medium (R+) consisted of RPMI 1640 supplemented with non-essential amino acids (1% v/v), penicillin/streptomycin (100 U/mL), L-glutamine (2 mM), and sodium pyruvate (1 mM) (all from Thermo Fisher Scientific).
Sample size
Sample size calculation for the interventional study was based on published data on efficacy and variability of the antibody response after TBEv-vaccination.35 To detect a two-fold difference in the geometric mean titer (GMT) of TBEv-neutralizing antibodies after the total TBEv vaccination course between the CMV+ and CMV- population, 84 patients would need to be recruited in each group to reach a power of 80% with a one-sided significance level of 5%. Considering the measure of TBEv-specific immune responses was planned longitudinally in all patients with separate measurements after one, two and three doses of the vaccine, recruiting 60 patients for each group was calculated to be sufficient to reach a power of > 80% with a one-sided significance level of < 5%. This calculation includes a safety margin for drop-outs before study completion of 10 subjects per group (15%). There was no missing data in the study.
Ethics
The studies were approved by the local institutional ethics committees (i.e. Comité de Protection des Personnes of the Pitié Salpétrière Hospital, Paris and cantonal ethics committee, Zürich, Switzerland - number of the ethics approval is EK1309) and all participants provided written informed consent.
Variables, biases and limitations
The aim of this study was to assess if the previous exposure to one or multiple herpesvirus infections could impact on the properties of T lymphocytes and on cellular and humoral responsiveness to a primary vaccination, according to the age of the subjects. To address the potential bias of co-morbidities in this setting, we excluded subjects with major diseases and ongoing co-infections, as detailed above. The present study has a number of potential limitations. In cohort 1, the number of elderly donors with 0 or 1 co-infections is relatively low, resulting in an underpowered analysis. Middle aged adults subjects in this cohort have been recruited at the local blood bank, which might constitute a selection bias as blood donors are supposed to be healthier than the general population. The precise co-morbidity status and medication use of these subjects are unknown, not allowing to control for these potential confounders. The female/male ratio is similar between cohorts 1 and 2, although not equivalent in all subgroups.
Herpesvirus serological assays
Herpesvirus serological tests were performed using clinical-grade assays measuring IgG levels, according to the manufacturer's instructions. Anti-CMV IgGs were measured using the BioPlex 2200 ToRC IgG kit on the BioPlex 2200 analyzer (Bio-Rad). Anti-HSV1 and anti-HSV2 IgGs were measured using the BioPlex 2200 HSV-1 and HSV-2 IgG kit on the BioPlex 2200 analyzer (Bio-Rad).
Measurement of inflammation associated cytokines
Plasma cytokines were measured by Simoa digital ELISA using a commercial triplex assay for IL-6, TNFα, and IL-10 (Quanterix) and a homebrew assay for IFNα2 as previously described.36
Phenotypic analysis
PBMCs were stained for surface markers using combinations of the following directly conjugated monoclonal antibodies: anti-CCR7–PE-Cy7 (clone 3D12; BD Biosciences), anti-CD3–BV605 (clone SK7; BD Biosciences), anti-CD8–APC-Cy7 (clone SK1; BD Biosciences), anti-CD4 BUV395 (clone SK3; BD Biosciences) anti-CD27–AF700 (clone O323; BioLegend), anti-CD45RA–ECD (clone 2H4LDH11LDB9; Beckman Coulter) and anti-CD95–FITC (clone DX2; BD Biosciences). Non-viable cells were eliminated from the analysis using LIVE/DEAD Fixable Aqua (Thermo Fisher Scientific). Samples were acquired using an LSR Fortessa (BD Biosciences). Data were analyzed using FACSDiva software version 7 (BD Biosciences) and/or FlowJo software version 10 (FlowJo LLC).
Analysis of TBEv-specific humoral and cellular immune responses
TBEv-specific antibody titers were measured before (week 0) during (week 4, 8 and 24) and after (week 28) the tick-borne encephalitis virus (TBE) vaccination course by ELISA and TBEv-neutralization assay according to published protocols.32,37,38 The TBEv-specific cellular immune response was assessed at week 0 and 26 by IFNγ enzyme-linked immunosorbent spot assay (ELISpot) using pools of overlapping peptides for all structural proteins of TBEv. Briefly, 2 × 105 thawed PBMCs / well from week 0 and week 26 of the same donor were stimulated in anti-IFNγ (clone 1-D1K, Mabtech) coated 96-well ELISpot-plates (MAIP S45, Millipore) for 18 h with 2 × 104 freshly generated autologous monocyte-derived DCs. For antigen-specific stimulation, five pools of overlapping peptides encompassing all structural proteins of TBEv were used at 1mg/ml final peptide concentration (15-mers overlapping by 5; BMC Microcollections, Germany). Washed plates were incubated with anti-IFNγ-Biotin (7-B6-1, Mabtech) followed by Streptavidin-alkaline Phosphatase (Mabtech), developed with color reagents (170-6432, Biorad) and analyzed in an automated ELISpot reader (AID). The number of total spot forming units (SFU) was calculated after background subtraction of the unstimulated control. For intracellular cytokine staining (ICS) 1 × 106 PBMCs from week 26 were stimulated with pools of TBEv overlapping peptides (1mg/ml final peptide concentration) in the presence of Brefeldin A overnight at 37 °C. Stimulation with Staphylococcus enterotoxin B (SEB) or varicella-zoster virus (VZV) lysate were also performed as comparison. Medium alone was used as a negative control. On the next day, cells were surface stained with anti-CD3-PerCP (clone SK7; BD Biosciences), anti-CD4-AmCyan (clone SK3; BD Biosciences), and anti-CD8-APC-Cy7 (clone SK1; BD Biosciences). Samples were then fixed and permeabilized, before staining with anti-IFNγ-APC (clone B27; BD Biosciences). Samples were acquired using an LSR Fortessa (BD Biosciences) and data were analyzed using FACSDiva software version 7 (BD Biosciences) and/or FlowJo software version 10 (FlowJo LLC). Percentages of antigen-specific T cells were calculated after background subtraction of the unstimulated control.
DNA extraction and TREC analysis on PBMCs
DNA was extracted from dry pellet using RSC Blood DNA according to manufacturer instructions (Promega). sjTREC were quantified by multiplex quantitative PCR together with the Albumin for cell normalization on a ViiA7 (Thermofisher Scientific) as described previously.39
DNA and RNA extraction on sorted cell populations
After thawing, PBMCs were stained with CCR7 BV650 (Clone 3D12; BD Biosciences), CD95 FITC (Clone DX2; BD Biosciences), CD27 PE (Clone M-T271; BD Biosciences), CD8 APC (Clone RPA-T8; BD Biosciences), CD4 APC-Cy7 (Clone RPA-T4; BD Biosciences), CD45RA ECD (Clone IM271111; Beckman Coulter), CD57 PB (Clone HCD57; Biolegend), CD49d PeCY7 (Clone 9F10; Biolegend), and eFluor 506 for viability (Biolegend). Naive CD4+ and CD8+ T cells, defined as CD45RA+, CD27+ CCR7+, CD95−, CD49d−, were sorted on an ARIAIII (Becton Dickinson). DNA and RNA from sorted cells were extracted using AllPrep DNA/RNA Micro kit (Qiagen) according to manufacturer instruction.
sjTREC digital droplet PCR
Ten µl of genomic DNA (50-200 ng) were mixed in 1x Master Mix Takyon (Eurogentec) 200 nM of sjTREC FAM-DarkQuencher Probe, 400 nM of sjTREC Forward and Reverse Primers, 200 nM of BCKDHA YakimaYellow-TAMRA probe, 900 nM of BCKDHA Forward and Reverse primers40 and 1.2 µL of surfactant. Droplets were generated with a RainDance Source system. PCR amplification (95 °C 5 min then 45 cycles of 95 °C 15 s and 60 °C, 1 min) was performed and read using the RainDance Sense apparatus. SjTREC were normalized for the number of cell using BCKDHA quantification.
TCR repertoire analysis
Fifty ng of RNA were used with the human QIAseq Immune Repertoire RNA Library Kit (Qiagen) according to manufacturer instruction. Libraries were read on Myseq sequencing platform (Illumina). TCR repertoires were first obtained with the Qiagen NGS data analysis pipeline and analyzed with VDJtools and tcR package.
Statistical analysis
Scattered plot representations were used to describe changes in immunological parameters according to the age (Figures 1 to 4, cohort 1) or CMV serostatus (Figures 5 and 6, cohort 2) of subjects. Data from the first cohort were divided in subgroups based on the number of herpesvirus infections. Univariate statistical analyses were performed using GraphPad Prism, R41 and Rstudio42 softwares with the packages Tidyverse43 and Scales.44 Groups were compared using the non-parametric Mann-Whitney test, as indicated in Figure legends. There is no allowance for multiplicity. Bonferroni correction was applied when performing multiple group comparisons. Spearman's rank test was used to determine correlations. P values below 0.05 were considered statistically significant.
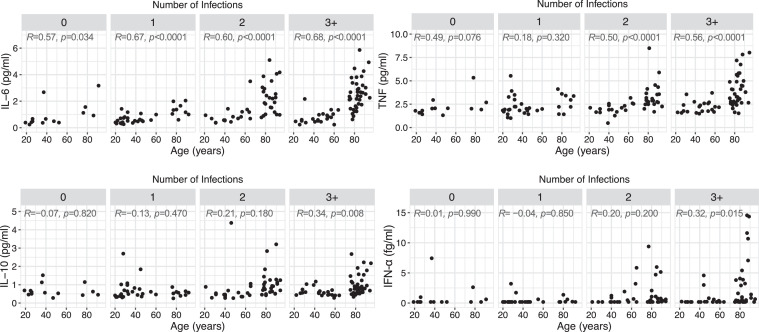
Figure 1.
Impact of age and herpesvirus infections on inflammation levels. Correlation between inflammatory cytokine levels and age according to the number of infections with herpesviruses (n = 14, 33, 43 and 59 for 0, 1, 2, and 3+ infections). Statistical significance was determined by Spearman's rank correlation. Spearman's R and p values are shown for each panel.
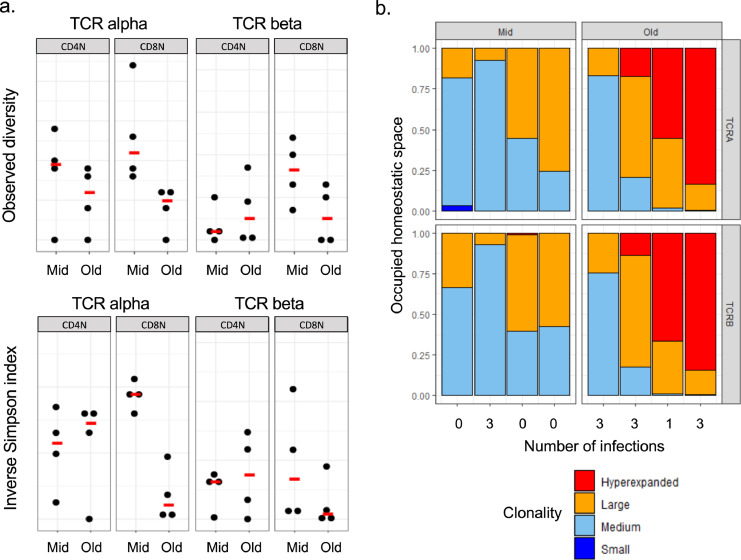
Figure 4.
TCR repertoire diversity of CD4+ and CD8+ naive T-cell compartments. (a) Observed diversity and inverse Simpson indexes calculated in CD4+ and CD8+ naive T cells from middle aged (< 65y) and old (> 75y) subjects. Each dot represents one donor and line median values. (b) Clonal space homeostasis in CD8+ naive T cells from middle aged (< 65y) and old (> 75y) subjects. Each bar represents an individual donor, identified on the x axis by the number of infections with herpesviruses.
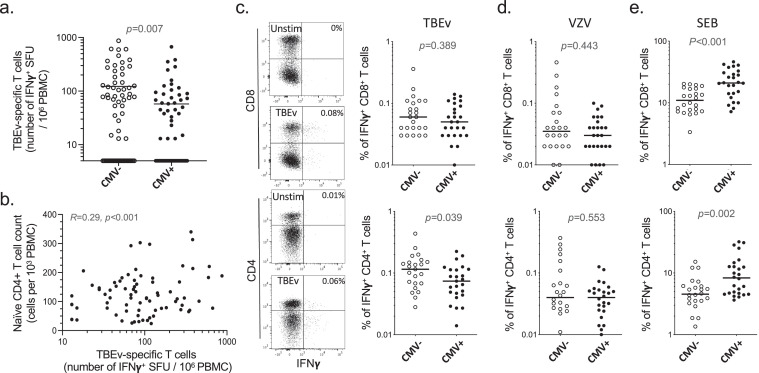
Figure 5.
Impact of CMV on TBEv vaccine T-cell responsiveness in older subjects. (a) Frequency of TBEv specific T cells determined by IFNγ Elispot upon stimulation with TBEv overlapping peptides at week 26 after the first vaccination in CMV-seronegative and CMV-seropositive subjects (> 70y) (n = 68 for CMV- and n = 69 CMV+). Data shown are subtracted of no-stimulation background values. (b) Correlation between naive CD4+ T-cell counts prior to vaccination and the frequency of TBEv-specific T cells at week 26 after the first vaccination (n = 132). (c, d, e) Frequencies of IFNγ producing CD4+ or CD8+ T cells upon stimulation with TBEv antigens (c), VZV antigens (d) or SEB (e), determined by intracellular cytokine staining at week 26 after the first vaccination in TBEv Elispot positive CMV-seronegative or CMV-seropositive subjects (> 70y) (n = 68 for CMV- and n = 69 CMV+). Each dot represents one donor and line median values (a, c–e). Representative flow cytometry plots are shown for unstimulated control and TBEv stimulation conditions (percentages of IFNγ producing CD4+ or CD8+ T cells are indicated). Data shown are subtracted of no-stimulation background values. Statistical significance was determined by Mann-Whitney test (a, c–e) or Spearman's rank correlation (b).
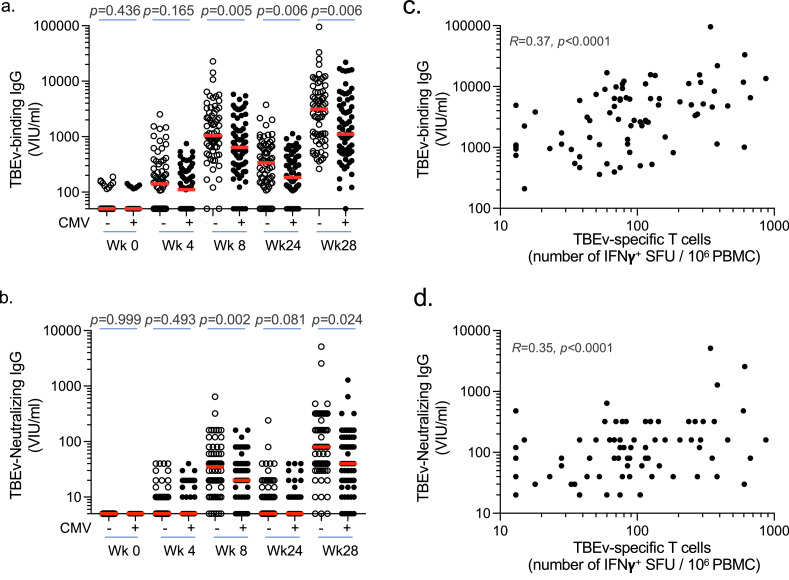
Figure 6.
Impact of CMV on TBEv vaccine humoral responsiveness in older subjects. (a, b) Levels of TBEv specific binding (a) and neutralizing (b) antibodies (IgG) determined at week 0, 4, 8, 24 and 28 after the first vaccination in CMV-seronegative or CMV-seropositive subjects (> 70y). All individuals received three vaccine doses at week 0, 4 and 24 (n = 68 for CMV- and n = 69 CMV+). Each dot represents one donor and line median values. (c, d) Correlation between the frequency of TBEv-specific T cells at week 26 after the first vaccination and the levels of TBEv-specific binding (c) and neutralizing (d) antibodies (IgG) determined at week 28 (n = 137). Statistical significance was determined by Mann-Whitney test (a, b) or Spearman's rank correlation (c, d). Spearman's R and p values are shown for each panel.
Supplemental material
The supplemental material file includes supplementary information for cohort 2, study flow diagrams for cohorts 1 and 2, STROBE checklists for abstract and observational studies, as well as the study protocol for the interventional study (i.e. cohort 2).
Role of funders
The funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Different influence of age and persistent infections on systemic inflammation and the T-cell compartment
We studied a cohort of healthy adults (n = 150, named “cohort 1”) aged from 19 to 95 years old, grouped according to the number of persistent viruses they were infected with on the basis of the serology data for four common herpesviruses: CMV, EBV, HSV-1 and HSV-2 (see Table 1 for a distribution of donors according to their serology). Persistent infections with these viruses may induce sustained inflammatory responses in their host. To verify this hypothesis, we measured by digital ELISA circulating cytokines (IFN-α, IL-6, TNF and IL-10) commonly associated with inflammatory manifestations. We observed an age-dependent increase in all four inflammatory markers, also associated with increasing numbers of herpesvirus infections (Figure 1 and Table S2). However, the cumulative effect of latent infections on inflammatory cytokines could only be found in the long term, i.e. in older adults (above 75 years old), while younger subjects stratified by number of infections displayed similar cytokine levels (Figure S1a). This rise in inflammatory cytokine levels in older people was strongly associated with CMV infection, except for IL-6 levels, which presented a modest increase in CMV-negative older donors (Figure S1b). Of note, apart from IL-6, the increase in inflammatory cytokines with ageing was barely noticeable in the absence of infections (Figure S1b). These data indicate that herpesvirus infections, especially CMV, exacerbate the inflammatory profile of adults only in the long term, i.e. in older people.
Table 1.
Baseline characteristics of study participants.
| Cohort 1 N = 150 |
Cohort 2 N = 137 |
||||||
|---|---|---|---|---|---|---|---|
| Mid |
Old |
Old |
|||||
| Parameter | CMV-neg N = 36 | CMV-pos N = 37 | CMV-neg N = 23 | CMV-pos N = 54 | CMV-neg N = 68 | CMV-pos N = 69 | |
| Age (year) | (median; range) | 32 (19–65) | 44 (20–64) | 82 (76–94) | 83 (75–95) | 73 (70–86) | 74 (70–87) |
| Gender | (female: male) | 16:20 | 20:17 | 16:7 | 31:23 | 30:38 | 38:31 |
| Body Mass Index (BMI) | (kg/m2, mean, range) | N/A | N/A | 21 (17–29) | 24 (16–32) | 24 (18–30) | 24 (18–30) |
| Co-morbidities | with/without | N/A | N/A | 13/10 | 37/17 | 26/42 | 33/36 |
| Medication | with/without | N/A | N/A | 16/7 | 42/12 | 26/42 | 32/37 |
| Number of drugs | 0 | N/A | N/A | 7 | 12 | 42 | 37 |
| 1 | N/A | N/A | 4 | 13 | 19 | 26 | |
| 2+ | N/A | N/A | 12 | 29 | 7 | 6 | |
These common herpesviruses, in particular CMV, are known to induce strong T-cell responses through the activation of naive T cells and differentiation into effector memory (EM) T cells. Total CD4+ and CD8+ T-cell counts were unaffected by age while only the number of CD8+ T cells was increased by the presence of multiple infections (Figure S2). In line with the inflammatory cytokine profile, an increase in both CD4+ and CD8+ EM T cells (defined as CD3+ CD27− CD45RA+/- CCR7−) was observed overtime with herpesvirus infections (Figure 2a, Table S2 and Figs. S3 and S4a). This rise, which was mainly driven by CD45RA+ EM among CD8+ T cells and CD45RA− EM among CD4+ T cells (Figure S5), could be attributed again almost-exclusively to CMV, especially in the CD8 compartment, as it was not observed in CMV uninfected donors (Figure S6a). Of note, this infection-driven accumulation of EM T cells was statistically significant in both old and young age (Figure S6b). Increased expression of CD57, a marker of late T cell differentiation, on CD4+ and CD8+ T cells was also associated with CMV seropositivity, but not age (Figure S6c). These data add to and confirm the multiple lines of evidence that CMV is the main cause of a disturbed EM T-cell compartment and inflation of this subset, which is observable at both younger and older ages.
Figure 2.
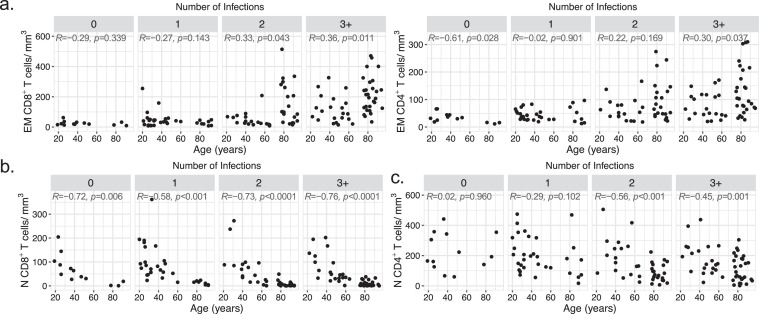
Impact of age and herpesvirus infections on effector memory and naive T-cell counts. Correlation between effector memory CD8+ and CD4+(a), and naive CD8+(b) and CD4+(c) T-cell absolute counts and age according to the number of infections with herpesviruses (n = 13, 32, 39 and 49 for 0, 1, 2, and 3+ infections). Statistical significance was determined by Spearman's rank correlation. Spearman's R and p values are shown for each panel.
The influence of persistent viral infections on the CD8+ and CD4+ naive T-cell pools has certainly more relevance in the context of de novo responsiveness, as naive T cells are required for mounting adaptive immune responses to newly encountered antigens. Naive T cells were defined here as CD3+ CD27+ CD45RA+ CCR7+ CD95−, thus excluding so-called CD95+ “stem cell memory T cells”. Interestingly, the evolution of the naive T-cell pool did not mirror that of the EM T-cell pool, and there was a clear difference between the evolution of CD8+ and CD4+ naive T-cell counts with ageing and herpesvirus infections. The loss of the naive CD8+ T-cell compartment was profound in older subjects (reaching numbers below 10 cells/mm3 of blood in oldest individuals), irrespective of infection number or CMV serostatus (Figure 2b, Table S2 and Figs. S4b and S7a). In contrast, the reduction in naive CD4+ T-cell counts with ageing was more moderate and associated with herpesvirus infections (Figures 2c and S4b and S7b). While CMV had the strongest association with reduced naive CD4+ T cells, CMV-negative donors presented nonetheless a modest but statistically significant reduction (R = -0.48, p = 0.049, Spearman's rank correlation) of these cells if infected with two other herpesviruses (Figure S7b). Altogether, these data highlight a differential influence of ageing and persistent viral infections on the naive CD8+ and CD4+ T-cell compartments, and raise two questions: the first one related to the basis of these differences, and the second one related to the eventual impact of common herpesvirus infections on the capacity of older people to mount T-cell responses against previously unencountered antigens.
Distinct evolution of the naive CD8+ and CD4+ T-cell compartments with increasing age
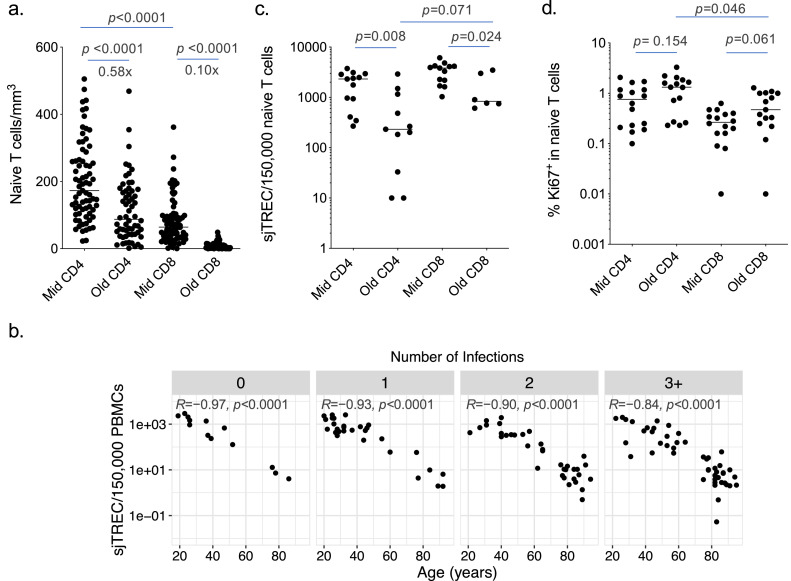
Our data highlight a stronger contraction of naive CD8+ T cells (i.e. 10-time reduction) over naive CD4+ T cells (i.e. less than 2-time reduction) with ageing, comparing adults versus elderly subjects (Figure 3a and Table S3). This is in line with the preservation of the CD4+ naive T-cell pool in healthy individuals of 20–69 years of age comparatively to naive CD8+ T cells.24 This is also consistent with a higher thymic output of CD4+ T cells in the adult age-range45 and in elderly individuals,46 although in these previous studies the impact of infections was not considered. Here, production of new naive T cells or thymic output, as measured by T-cell receptor excision circle (TREC) levels in total PBMCs, was strongly associated with ageing and was profoundly reduced in older individuals, independently of the number of herpesvirus infections (Figure 3b and Table S2). This pattern was similar to the decline of naive CD8+ T-cell counts (Figure 2b), which suggests a strong dependency of naive CD8+ T-cell maintenance on thymic production. The naive CD8+ T-cell compartment seems to contract as the thymus involutes and thymopoiesis wanes with ageing. The lack of influence of CMV or EBV seropositivity on the thymic production of new T cells per se was documented in a large-scale population study of healthy adults.39
Figure 3.
Thymic output and naive T-cell homeostatic proliferation. (a) CD4+ and CD8+ naive T-cell absolute counts in middle aged (< 65y) and old (> 75y) subjects (n = 73 for Mid CD4 and CD8, n = 60 for Old CD4 and CD8). The magnitude of the reduction in the number of naive CD4+ and CD8+ T cells between Mid and Old is indicated. (b) Correlation between sjTRECs levels measured in total PBMCs and age according to the number of infections with herpesviruses (n = 13, 29, 35 and 48 for 0, 1, 2, and 3+ infections). (c) TRECs levels determined by digital PCR in FACS sorted CD8+ and CD4+ naive T-cell subsets from middle aged (< 65y) and old (> 75y) subjects (n = 13 for Mid CD4 and CD8, n = 11 for Old CD4, n = 6 for Old CD8). (d) Ki67 expression levels determined by flow cytometry in CD4+ and CD8+ naive T-cell subsets from middle aged (< 65y) and old (> 75y) subjects (n = 16 for Mid CD4 and CD8, n = 15 for Old CD4 and CD8). Each dot represents one donor and line median values (a, c, d). Statistical significance was determined by Mann-Whitney test and Bonferroni adjustment (a, c, d) or Spearman's rank correlation (b). Spearman's R and p values are shown for each panel (b).
As the maintenance of naive CD4+ T cells showed a different dynamic to that of naive CD8+ T cells, we hypothesized that they are less dependent on thymic output, but more linked to homeostatic proliferation. To examine this possibility, we measured TREC levels directly in naive T cells, using sensitive digital PCR assays on FACS-sorted cells. We observed reduced TREC levels in naive T cells from old donors. This reveals a dilution of TRECs in the naive cells through cell division, likely reflecting higher homeostatic proliferation in older compared to younger subjects (Figure 3c and Table S3). Interestingly, the reduction in TREC levels was more pronounced in the CD4+ than the CD8+ pool. Moreover, higher levels of the proliferation marker Ki67 were found in naive CD4+ T cells compared to naive CD8+ T cells, in particular from older individuals (Figure 3d and Table S3). Together, these data indicate that the pool of naive CD8+ T cells contracts with ageing due to reduced thymic production, while the pool of naive CD4+ T cells is maintained to some extent through robust homeostatic proliferation. The naive CD4+ T-cell pool is nonetheless affected by infections with persistent viruses, in particular CMV, but also other herpesviruses.
We next wanted to assess if the contraction of the CD8+ and CD4+ T-cell pools with ageing could be reflected in their TCR repertoire diversity. To answer this question, we performed high throughput sequencing of TCRα and TCRβ regions using target specific RNASeq, on FACS-sorted naive CD4+ and CD8+ T cells from younger (n = 4) and older donors (n = 4), and calculated observed diversity and inverse Simpson index (Figure 4a). This analysis did not reveal any evident difference in naive CD4+ T-cell TCR repertoire diversity between young and old subjects, although this observation should be mitigated due to the limited number of donors studied. In contrast, there is an obvious loss in TCR repertoire diversity within the naive CD8+ T-cell compartment of older individuals, in line with its profound contraction with advanced age, unlike the naive CD4+ T cell pool. TCR repertoire alterations of the naive CD8+ T cells did not present a direct association with the number of infections (Figure 4b), in line with a virus infection independent contraction of this compartment with ageing. However, the small number of subjects studied limits our conclusions on a potential effect of herpesvirus infections on naive T-cell TCR repertoire. Overall, these data inform on the basis of the differential evolutions between naive CD4+ and CD8+ T cells with increasing age.
Reduced responsiveness to de novo immunization in CMV-infected older adults
To understand the impact of persistent viral infections on the ability to mount T-cell responses against neoantigens in older people, we concentrated on CMV infection, being the virus with the strongest impact on the T-cell compartment in older people. We previously used an in vitro priming assay to demonstrate the reduced capacity of older people to prime antigen-specific CD8+ T-cell responses from the naive pool.21 Using the same in vitro approach, we found no influence of CMV serostatus on CD8+ T-cell priming efficacy in older individuals (Figure S8a), in accordance with a loss of naive CD8+ T cells with ageing that is largely independent of CMV infection. This in vitro approach nonetheless may not grasp the full complexity of the primary immune response in vivo, and it cannot be used to study the induction of CD4+ T-cell responses from the naive pool, as there is no equivalence of this assay for CD4+ T cells.
We therefore studied the induction of de novo immune responses upon vaccination in a second cohort of older people. Healthy elderly individuals (n = 137, named “cohort 2”, Table 1) were recruited in a clinical trial to receive for the first time a vaccination against tick-borne encephalitis (TBE) using the recommended prime-boost regimen with three injections (at week 0, 4 and 24). The individuals selected for this study had no serum anti-TBE virus (TBEv) antibodies prior to vaccination, indicating that they had never been exposed to nor vaccinated against TBEv. In line with the cohort 1 studied above, CMV-seropositive subjects presented lower CD4+ (but not CD8+) naive T-cell counts than CMV-seropositive subjects, confirming the association between CMV infection and lower naive CD4+ T-cell counts with old age (Figure S8b). De novo cellular immune responses to TBE vaccination were monitored at week 26 post-first immunization (2 weeks after the last boost), and compared to baseline values, using IFNγ ELISpot with pools of overlapping peptides against all structural proteins of TBEv. Total TBEv-specific T-cell responses were statistically significantly lower in CMV+ compared to CMV− elderly individuals (Figure 5a and Table S4). The frequency of TBEv-specific T cells induced upon vaccination correlated with the counts in naive CD4+ T cells prior to vaccination (Figure 5b), but not naive CD8+ T cells (data not shown). This indicates a reliance of the capacity to mount de novo vaccine T-cell responses on the pool of naive CD4+ T cells, and suggests that CMV infection may therefore impact this capacity due to its effect on the naive CD4+ T-cell pool.
To get further insights into these observations, intracellular IFNγ staining and assessment by flow cytometry was performed on Elispot-positive donors in order to distinguish TBEv-specific CD8+ and CD4+ T cells. This revealed that the TBEv-specific cellular response was mostly dominated by CD4+ rather than CD8+ T cells in vaccinated individuals (Figure S8c), consistent with its reliance on the naive CD4+ T-cell pool. CMV seropositivity had no impact on TBEv-specific CD8+ T-cell levels, in line with the in vitro CD8+ T-cell priming data. However, it was associated with lower TBEv-specific CD4+ T-cell responses (Figure 5c and Table S4), consistent with the impact of CMV on the naive CD4+, but not CD8+, T-cell compartment in older donors. For comparison, we assessed the influence of CMV on memory T-cell reactivity. In contrast to TBEv-specific de novo responses, VZV-specific memory CD8+ and CD4+ T-cell levels were equivalent regardless of the CMV serostatus (Figure 5d and Table S4). Of note, we observed a higher number of IFNγ producing CD8+ and CD4+ T cells upon stimulation with Staphylococcus enterotoxin B (SEB) in CMV+ donors (Figure 5e and Table S4), reflecting higher levels of effector memory (thus IFNγ producing) cells in CMV+ donors, that can be activated with this superantigen. These results support a reduced CD4+ T-cell responsiveness to neoantigens in CMV-infected older adults.
We next assessed vaccine-induced humoral immunity measuring TBEv-specific binding antibody levels as well as neutralizing antibody activity at different time points after vaccination. We observed that both TBEv-specific binding and neutralizing antibody responses were delayed and decreased in elderly vaccinees with latent CMV infection (Figure 6a,b and Table S5). Of note, there were clear associations between the induction of TBEv-specific T-cell responses and the levels of both TBEv-specific binding and neutralizing antibody levels upon vaccination (Figure 6c,d). Altogether, these data indicate that CMV infection has a negative impact on the mounting of de novo cellular and humoral immune responses in the elderly.
Discussion
A reduced capacity to mount effective de novo immune responses is a major issue in older human populations and the consequence of multiple factors. Infection with persistent viruses, which represent a substantial and long-lasting burden for our immune system, have been suspected to play a role in this decline. Through chronic activation and mobilization of immune resources, herpesviruses, in particular CMV, result in continuous naive T-cell activation and inflation of EM T cells23. However, we observed here that the associations of naive and EM T-cell numbers with ageing and chronic infections did not mirror each other and that the contraction or maintenance of the naive T-cell pool depends on multiple factors. In a context of limited or even absent T-cell renewal capacity, the naive T-cell compartment can be particularly affected, as shown by studies in young adults thymectomized during early childhood. In this model of T-cell ageing independent of chronological age, the combination of reduced thymic output (i.e. due to thymectomy) and chronic immune activation (i.e. due to infection with a persistent virus like CMV) resulted in the alteration of naive T-cell frequencies and homeostasis.47,48 With ageing, thymic involution and long-term immunity against persistent viruses may combine to exhaust immune resources.
In the present work, we observed that the contraction of the naive CD8+ T-cell compartment with old age was independent of infections by herpesviruses, including CMV, in line with a previous study.49 We propose that the naive CD8+ T-cell pool maintenance with increasing age relies strongly on cell replenishing capacity due to thymic output, so much that the influence of persistent virus infections, although expected, was not observed. In contrast, the naive CD4+ T-cell pool is maintained more by homeostatic proliferation, allowing for the long-term effects of persistent viral infections to be observed. Previous studies suggest that CD8+ and CD4+ naive T-cell repertoires are altered in old people.50,51 The different rates of decline between CD8+ and CD4+ naive T-cell compartments likely translates into a major loss of TCR repertoire diversity for the CD8+ naive T cells, in contrast to CD4+ naive T cells, with advanced age. Overall, the strong contraction of the naive CD8+ T-cell repertoire likely results in an important reduction of naive CD8+ T-cell responsiveness to new antigens in elderly human populations, explaining our previous findings.21,52 On the same line, diminished yellow fever vaccine-specific CD8+ T-cell responses were found to be correlated with baseline naive T-cell frequencies.15 Older individuals may thus become more vulnerable to diseases for which the induction of CD8+ T-cell immunity plays a protective role, such as against emerging viruses and tumors.
Nonetheless we observed that herpesvirus infections, in particular CMV but also EBV/HSV, were associated with decreased absolute counts of naive CD4+ T cells with ageing. Considering that the frequency of naive cell precursors specific for a given antigen are typically very low, ranging approximately between one cell in 105 to 106 T lymphocytes in adults,53,54 this quantitative reduction in naive cells, including potentially unique antigen-reactive ones, may affect the mounting of neoantigen-specific immune responses. Indeed, we found that CMV seropositivity was associated with a reduction in CD4+ T-cell responsiveness to neoantigens (i.e. TBEv antigens), but not to recall antigens (VZV antigens), in older people. With advanced age, de novo CD4+ T-cell responses appear to be affected by a persistent virus like CMV, in line with the impact on naive CD4+ T-cell counts.
Interestingly, distinct patterns were observed regarding T-cell subsets and inflammatory markers that were associated with ageing and persistent infections. For example, the naive CD8+ T-cell decline and IL-6 level increase are inversely related (p < 0.0001, not shown), mostly dependent on ageing but not latent infections. Ageing-related increased levels of IL-6, which is known to contribute to thymic atrophy,55,56 may thus play an important role in the contraction of the naive CD8+ T cells by inhibiting their production in the thymus. On the other hand, the inflation of EM CD8+ T cells and increased levels of inflammation associated cytokines such as TNF, IFNα and IL-10 in older individuals followed a similar pattern, and both were clearly associated with CMV infection. Strikingly, in healthy older subjects, the increase in systemic inflammatory cytokines was strongly associated with CMV serostatus. CMV by itself has therefore a central part in the increased inflammatory status characteristic of the elderly subjects. However, the fact that all recruited donors were healthy volunteers of French or Swiss nationality might render these findings not completely generalizable. We cannot ascertain whether the phenomena described in this manuscript are similar in subjects with major co-morbidities or co-infections.
Previous studies have found conflicting evidence concerning the influence of latent CMV-infection on influenza vaccine immunogenicity in different populations. Some showed decreased vaccine immunogenicity in CMV-seropositive elderly,25,27 while others did not show any substantial influence.29,57 A beneficial effect of CMV infection on influenza vaccine immunogenicity was even reported in younger individuals.30 By using influenza vaccine immunogenicity as a readout these studies mainly analyzed the influence of latent CMV on recall or memory responses. Using TBE vaccine in TBEv-naive elderly we were able to assess the influence of latent CMV infection on a true neoantigen, and we found a delay and reduction in TBE-vaccine induced humoral immunity in CMV-infected older subjects. As previously proposed,58 CMV-related increased inflammation, possibly mediated by the action of TNF on B-cells, might be a mechanism through which CMV impacts the humoral response and antibody production upon vaccination. Moreover, CMV infection might indirectly affect germinal center and antibody formation through defective generation of follicular T helper (Tfh) cells due to lower naive T-cell numbers in CMV-seropositive subjects. Although it would have been interesting to evaluate antigen specific Tfh CD4+ T cells directly, our study was limited to TBEv-specific IFNγ producing, i.e. Th1, CD4+ T cells. Previous studies suggest nonetheless that Tfh cells are even more dampened than Th1 cells in older individuals, affecting the related humoral response.59, 60, 61 In elderly adults, the reduced quality of Tfh cells and ability to provide help to B cells were reported to be partly responsible for declined influenza specific antibody titers upon vaccination.62, 63, 64, 65, 66 From these studies, we may infer that the induction of TBEv-specific Tfh cells should be lower upon vaccination in older people, in particular if they are CMV seropositive, resulting in impaired humoral immune responses. It will be important to decipher the exact role of CMV in the impaired humoral responsiveness with ageing.
The present study provides potential insights underlying the vulnerability of elderly people to emerging pathogens. Related to the contraction of the naive T-cell compartment, older CMV positive individuals may indeed present blunted adaptive immune responses to new antigens, such as those of SARS-CoV-2, possibly affecting their capacity to control viral replication. This is in line with a recent report of a favorable impact of thymic rebound during COVID lymphopenia.67 The observation that CMV infection plays an important role in the establishment of a systemic inflammatory status in elderly subjects might even add to its hypothetical role in COVID-19 severity.68,69 Future studies will be necessary to establish if serology to herpesviruses, in particular CMV, may inform on the capacity to mount a good immune response against SARS-CoV-2, and if it may help to identify patients more at risk to develop severe disease. Our results are also relevant in the context of vaccination campaigns focused on elderly populations. Vaccine immunogenicity may be blunted in CMV infected individuals, resulting in a lower induction of vaccine-specific antibodies. Of note though, CMV serology was not found to be associated with lower vaccine immunogenicity in a study of elderly people vaccinated with SARS-CoV-2 vaccine BNT162b2.7 Moreover, SARS-CoV-2 vaccine clinical efficacy appears overall as effective in young and older people. These apparently discordant findings highlight the complexity of the issue and the need for specific studies to test the exact impact of CMV on SARS-CoV-2 vaccines immunogenicity and efficacy. An active role of CMV infection in depleting host immune resources, that may affect immune response efficacy, would support strategies to limit CMV infection in the general population.
Contributors
Conceptualization, D.D. J.B. D.S. A.T. U.K. V.A.;
Methodology, F.N. E.C. K.W. A.V.B. V.B. C.A. C.D. C.L. M.B.;
Formal analysis, F.N. E.C. K.W. A.V.B. V.B. C.A. C.D. C.L. M.B. P.M. K.S. F.X.H. M.M. D.D. J.B. D.S. A.T. U.K. V.A.;
Verification of the underlying data, F.N. E.C. U.K. V.A.;
Investigation, F.N. E.C. K.W. A.V.B. V.B. C.A. C.D. C.L. M.B. P.M. K.S. F.X.H. M.M. D.D. J.B. D.S. A.T. U.K. V.A.;
Resources: J.B. U.K.;
Writing – Original Draft, F.N. U.K. V.A.;
Writing – Review & Editing, F.N. E.C. K.W. A.V.B. V.B. C.A. C.D. C.L. M.B. P.M. K.S. F.X.H. M.M. D.D. J.B. D.S. A.T. U.K. V.A.;
Supervision, D.D. J.B. A.T. U.K. V.A.;
Funding Acquisition, J.B. A.T. U.K. V.A.
Data sharing statement
All data reported in this paper will be shared by the lead contact upon request.
Declaration of interests
Dr Wanke reports having received personal fees as medical advisor from Novartis Pharma Schweiz AG, outside of the submitted work. Dr Stiasny reports having received a research grant from Pfizer corporation Austria Ges.m.b.H for the period 2018-2020, outside the submitted work. The other authors declare no conflict of interest.
Acknowledgments
We are indebted to Matthew Albert (Insitro, San Francisco, CA) for constructive scientific discussion. We are very grateful to the researchers and technical staff of the Division of Infectious Diseases and Hospital Epidemiology of the University Hospital of Zurich (Switzerland), the Institute of Social and Preventive Medicine of the University of Zurich (Switzerland), the Center for Virology of the Medical University of Vienna (Austria), and the Laboratoire d'Immunologie et d‘Histocompatibilité of the Hopital Saint-Louis (Paris, France) for their scientific advice and expert technical assistance.
This work was supported by the ANR (Project ANR-14-CE14-0030-01) and by Università ItaloFrancese/Univeristé FrancoItalienne (Galileo Project G10-718; PHC Galilee Project 39582TJ), by the Swiss National Science Foundation (grant PP0033-110737 to UK), by the Heuberg Foundation (Zurich, Switzerland), by the AETAS Foundation (Geneva, Switzerland) and by a Senior IdEx Chair of the University of Bordeaux (France). EC, VB, CA, MA, DD and AT were supported by the French Government's Investissement d'Avenir Program, Laboratoire d'Excellence “Milieu Intérieur” Grant ANR-10-LABX-69-01. EC and AT are supported by the Agence Nationale de la Recherche (Project RANKLthym ANR-19- CE18-0021-02).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103852.
Contributor Information
Urs Karrer, Email: urs.karrer@ksw.ch.
Victor Appay, Email: victor.appay@immuconcept.org.
Appendix. Supplementary materials
References
- 1.Hayes E.B., Komar N., Nasci R.S., Montgomery S.P., O'Leary D.R., Campbell G.L. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11(8):1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia N., Feng D., Fang L.Q., et al. Case fatality of SARS in mainland China and associated risk factors. Trop Med Int Health. 2009;14(Suppl 1):21–27. doi: 10.1111/j.1365-3156.2008.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kline K.A., Bowdish DM. Infection in an aging population. Curr Opin Microbiol. 2016;29:63–67. doi: 10.1016/j.mib.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Gustafson C.E., Kim C., Weyand C.M., Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145(5):1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collier D.A., Ferreira I., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng H.F., Harpaz R., Luo Y., et al. Declining effectiveness of herpes zoster vaccine in adults aged >/=60 years. J Infect Dis. 2016;213(12):1872–1875. doi: 10.1093/infdis/jiw047. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg A., Canniff J., Rouphael N., et al. Varicella-zoster virus-specific cellular immune responses to the live attenuated zoster vaccine in young and older adults. J Immunol. 2017;199(2):604–612. doi: 10.4049/jimmunol.1700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenkein J.G., Park S., Nahm M.H. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine. 2008;26(43):5521–5526. doi: 10.1016/j.vaccine.2008.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Werkhoven C.H., Huijts S.M., Bolkenbaas M., Grobbee D.E., Bonten M.J. The impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clin Infect Dis. 2015;61(12):1835–1838. doi: 10.1093/cid/civ686. [DOI] [PubMed] [Google Scholar]
- 12.Grasse M., Meryk A., Schirmer M., Grubeck-Loebenstein B., Weinberger B. Booster vaccination against tetanus and diphtheria: insufficient protection against diphtheria in young and elderly adults. Immun Ageing. 2016;13(1):26. doi: 10.1186/s12979-016-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugan H.L., Henry C., Wilson P.C. Aging and influenza vaccine-induced immunity. Cell Immunol. 2020;348 doi: 10.1016/j.cellimm.2019.103998. [DOI] [PubMed] [Google Scholar]
- 14.Roukens A.H., Soonawala D., Joosten S.A., et al. Elderly subjects have a delayed antibody response and prolonged viraemia following yellow fever vaccination: a prospective controlled cohort study. PLoS One. 2011;6(12):e27753. doi: 10.1371/journal.pone.0027753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz A.R., Malzer J.N., Domingo C., et al. Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J Immunol. 2015;195(10):4699–4711. doi: 10.4049/jimmunol.1500598. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger B., Haks M.C., de Paus R.A., Ottenhoff T.H.M., Bauer T., Grubeck-Loebenstein B. Impaired immune response to primary but not to booster vaccination against hepatitis B in older adults. Front Immunol. 2018;9:1035. doi: 10.3389/fimmu.2018.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner A., Garner-Spitzer E., Jasinska J., et al. Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep. 2018;8(1):9825. doi: 10.1038/s41598-018-28111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicoli F., Solis-Soto M.T., Paudel D., et al. Age-related decline of de novo T cell responsiveness as a cause of COVID-19 severity. Geroscience. 2020;42(4):1015–1019. doi: 10.1007/s11357-020-00217-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appay V., Sauce D. Naive T cells: the crux of cellular immune aging? Exp Gerontol. 2014;54:90–93. doi: 10.1016/j.exger.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Li G., Yu M., Lee W.W., et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18(10):1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briceno O., Lissina A., Wanke K., et al. Reduced naive CD8(+) T-cell priming efficacy in elderly adults. Aging Cell. 2016;15(1):14–21. doi: 10.1111/acel.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafson C.E., Cavanagh M.M., Jin J., Weyand C.M., Goronzy J.J. Functional pathways regulated by microRNA networks in CD8 T-cell aging. Aging Cell. 2019;18(1):e12879. doi: 10.1111/acel.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klenerman P., Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol. 2016;16(6):367–377. doi: 10.1038/nri.2016.38. [DOI] [PubMed] [Google Scholar]
- 24.Patin E., Hasan M., Bergstedt J., et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19(3):302–314. doi: 10.1038/s41590-018-0049-7. [DOI] [PubMed] [Google Scholar]
- 25.Derhovanessian E., Theeten H., Hahnel K., Van Damme P., Cools N., Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine. 2013;31(4):685–690. doi: 10.1016/j.vaccine.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 26.Frasca D., Diaz A., Romero M., Landin A.M., Blomberg B.B. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine. 2015;33(12):1433–1439. doi: 10.1016/j.vaccine.2015.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trzonkowski P., Mysliwska J., Szmit E., et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination–an impact of immunosenescence. Vaccine. 2003;21(25-26):3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 28.Derhovanessian E., Maier A.B., Hahnel K., McElhaney J.E., Slagboom E.P., Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol. 2014;193(7):3624–3631. doi: 10.4049/jimmunol.1303361. [DOI] [PubMed] [Google Scholar]
- 29.den Elzen W.P., Vossen A.C., Cools H.J., Westendorp R.G., Kroes A.C., Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29(29-30):4869–4874. doi: 10.1016/j.vaccine.2011.03.086. [DOI] [PubMed] [Google Scholar]
- 30.Furman D., Jojic V., Sharma S., et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med. 2015;7(281):281ra43. doi: 10.1126/scitranslmed.aaa2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smithey M.J., Venturi V., Davenport M.P., et al. Lifelong CMV infection improves immune defense in old mice by broadening the mobilized TCR repertoire against third-party infection. Proc Natl Acad Sci U S A. 2018;115(29):E6817–E6E25. doi: 10.1073/pnas.1719451115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stiasny K., Aberle J.H., Chmelik V., Karrer U., Holzmann H., Heinz FX. Quantitative determination of IgM antibodies reduces the pitfalls in the serodiagnosis of tick-borne encephalitis. J Clin Virol. 2012;54(2):115–120. doi: 10.1016/j.jcv.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Vratskikh O., Stiasny K., Zlatkovic J., et al. Dissection of antibody specificities induced by yellow fever vaccination. PLoS Pathog. 2013;9(6) doi: 10.1371/journal.ppat.1003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradt V., Malafa S., von Braun A., et al. Pre-existing yellow fever immunity impairs and modulates the antibody response to tick-borne encephalitis vaccination. npj Vaccines. 2019;4:38. doi: 10.1038/s41541-019-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zent O., Hennig R., Banzhoff A., Broker M. Protection against tick-borne encephalitis with a new vaccine formulation free of protein-derived stabilizers. J Travel Med. 2005;12(2):85–93. doi: 10.2310/7060.2005.12205. [DOI] [PubMed] [Google Scholar]
- 36.Bondet V., Rodero M.P., Posseme C., et al. Differential levels of IFNalpha subtypes in autoimmunity and viral infection. Cytokine. 2021;144 doi: 10.1016/j.cyto.2021.155533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiasny K., Holzmann H., Heinz F.X. Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine. 2009;27(50):7021–7026. doi: 10.1016/j.vaccine.2009.09.069. [DOI] [PubMed] [Google Scholar]
- 38.Holzmann H., Kundi M., Stiasny K., et al. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J Med Virol. 1996;48(1):102–107. doi: 10.1002/(SICI)1096-9071(199601)48:1<102::AID-JMV16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 39.Clave E., Araujo I.L., Alanio C., et al. Human thymopoiesis is influenced by a common genetic variant within the TCRA-TCRD locus. Sci Transl Med. 2018;10(457) doi: 10.1126/scitranslmed.aao2966. eaao2966. [DOI] [PubMed] [Google Scholar]
- 40.Zhong Q., Bhattacharya S., Kotsopoulos S., et al. Multiplex digital PCR: breaking the one target per color barrier of quantitative PCR. Lab Chip. 2011;11(13):2167–2174. doi: 10.1039/c1lc20126c. [DOI] [PubMed] [Google Scholar]
- 41.R Core Team . R Core Team, R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 42.RStudio . RStudio Team, RStudio, Inc.; Boston, MA: 2016. RStudio: integrated development environment for R. [Google Scholar]
- 43.Wickham H., Averick M., Bryan J., et al. Welcome to the tidyverse. Journal of Open Source Software. 2019;4(43):1686–1691. [Google Scholar]
- 44.https://scales.r-lib.org/, Accessed 17 July 2018
- 45.Mold J.E., Reu P., Olin A., et al. Cell generation dynamics underlying naive T-cell homeostasis in adult humans. PLoS Biol. 2019;17(10) doi: 10.1371/journal.pbio.3000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrando-Martinez S., Ruiz-Mateos E., Hernandez A., et al. Age-related deregulation of naive T cell homeostasis in elderly humans. Age. 2011;33(2):197–207. doi: 10.1007/s11357-010-9170-8. (Dordrecht, Netherlands) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauce D., Larsen M., Fastenackels S., et al. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119(10):3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauce D., Larsen M., Fastenackels S., et al. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J Immunol. 2012;189(12):5541–5548. doi: 10.4049/jimmunol.1201235. [DOI] [PubMed] [Google Scholar]
- 49.Wertheimer A.M., Bennett M.S., Park B., et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192(5):2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi Q., Liu Y., Cheng Y., et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci USA. 2014;111(36):13139–13144. doi: 10.1073/pnas.1409155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egorov E.S., Kasatskaya S.A., Zubov V.N., et al. The changing landscape of naive T cell receptor repertoire with human aging. Front Immunol. 2018;9:1618. doi: 10.3389/fimmu.2018.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallerani E., Proietto D., Dallan B., et al. Impaired priming of SARS-CoV-2-specific naive CD8(+) T cells in older subjects. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.693054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alanio C., Lemaitre F., Law H.K., Hasan M., Albert M.L. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 2010;115(18):3718–3725. doi: 10.1182/blood-2009-10-251124. [DOI] [PubMed] [Google Scholar]
- 54.Su L.F., Kidd B.A., Han A., Kotzin J.J., Davis M.M. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38(2):373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sempowski G.D., Hale L.P., Sundy J.S., et al. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164(4):2180–2187. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 56.Carbajosa S., Gea S., Chillon-Marinas C., et al. Altered bone marrow lymphopoiesis and interleukin-6-dependent inhibition of thymocyte differentiation contribute to thymic atrophy during Trypanosoma cruzi infection. Oncotarget. 2017;8(11):17551–17561. doi: 10.18632/oncotarget.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wald A., Selke S., Magaret A., Boeckh M. Impact of human cytomegalovirus (CMV) infection on immune response to pandemic 2009 H1N1 influenza vaccine in healthy adults. J Med Virol. 2013;85(9):1557–1560. doi: 10.1002/jmv.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frasca D., Blomberg B.B. Aging, cytomegalovirus (CMV) and influenza vaccine responses. Hum Vaccin Immunother. 2016;12(3):682–690. doi: 10.1080/21645515.2015.1105413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefebvre J.S., Lorenzo E.C., Masters A.R., et al. Vaccine efficacy and T helper cell differentiation change with aging. Oncotarget. 2016;7(23):33581–33594. doi: 10.18632/oncotarget.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lefebvre J.S., Masters A.R., Hopkins J.W., Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci Rep. 2016;6:25051. doi: 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herati R.S., Reuter M.A., Dolfi D.V., et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J Immunol. 2014;193(7):3528–3537. doi: 10.4049/jimmunol.1302503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eaton S.M., Burns E.M., Kusser K., Randall T.D., Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200(12):1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson S.A., Cambier J.C. Ageing, autoimmunity and arthritis: senescence of the B cell compartment - implications for humoral immunity. Arthritis Res Ther. 2004;6(4):131–139. doi: 10.1186/ar1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song H., Price P.W., Cerny J. Age-related changes in antibody repertoire: contribution from T cells. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 65.Frasca D., Landin A.M., Riley R.L., Blomberg B.B. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008;180(5):2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- 66.Hill D.L., Whyte C.E., Innocentin S., et al. Impaired HA-specific T follicular helper cell and antibody responses to influenza vaccination are linked to inflammation in humans. Elife. 2021;10 doi: 10.7554/eLife.70554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cuvelier P., Roux H., Couedel-Courteille A., et al. Protective reactive thymus hyperplasia in COVID-19 acute respiratory distress syndrome. Crit Care. 2021;25(1):4. doi: 10.1186/s13054-020-03440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moss P. The ancient and the new": is there an interaction between cytomegalovirus and SARS-CoV-2 infection? Immun Ageing. 2020;17:14. doi: 10.1186/s12979-020-00185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadambari S., Klenerman P., Pollard A.J. Why the elderly appear to be more severely affected by COVID-19: the potential role of immunosenescence and CMV. Rev Med Virol. 2020;30(5):e2144. doi: 10.1002/rmv.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.