Abstract
Background
Cognitive manifestations associated with Severe Acute Respiratory Syndrome by Coronavirus 2 (SARS-CoV-2) are yet to be described in the existing literature. The aim of this exploratory study is to analyze the impact of severe SARS-CoV-2 infection on neuropsychological performance 6 months following hospital discharge, and to identify which medical variables predict worse outcome. In this context, we study if cognitive reserve (CR) may play a protective role on cognitive impairment.
Methods
We enrolled a cohort of 102 severe SARS-CoV-2 survivors who had been admitted to the Intensive Care Unit (ICU) and were contacted 6-months post discharge. A total of 58 agreed to participate in this 6-month follow-up study. Patients with previously known cognitive impairment were excluded. Demographic, clinical and laboratory data were collected. Firstly, to test the magnitude of neurocognitive sequalae two standard deviations below normative group were considered. Secondly, to analyze the main effects of medical variables on cognition and the interaction with cognitive reserve, ANCOVA analyses were performed.
Results
53.4% obtained a score below the cutoff point (<26) in the screening test MOCA. ICU variables including mechanical ventilation, days of sedation or high CRP days were related with cognition. Cognitive Reserve (CR) interacted with delirium (F = 6.8, p = 0.01) and sedation days (F = 9.40, p = 0.003) to predict verbal memory and interacted with high CRP to predict phonemic fluency (F = 6.47, p = 0.01). Finally, no differences in neuropsychological performance were found depending on subjective cognitive impairment (SCI). However, patients with SCI had a higher score in the HAD anxiety subscale (t = −2.2; p < 0.05).
Conclusions
In our cohort, cognitive dysfunction was related with ICU variables such as delirium, mechanical ventilation, and inflammation. CR modulated the impact of these variables on cognition. Cognitive complaints were related with anxiety but not with cognitive performance. Despite some limitations, including the need of replication of the findings with larger samples and control groups, our study suggests that high CR may be protective for severe COVID-19-related cognitive impairment.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Neurological manifestations, Cognitive impairment, Neuropsychology, Cognitive reserve, ICU, PICS
1. Introduction
The disease resulting from the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), commonly known as COVID-19, has become a global pandemic causing a worldwide public health crisis. Symptoms of the disease include Acute Respiratory Distress Syndrome (ARDS), but also cardiac, renal, hematological, intestinal, and neurological problems (Wadman et al., 2020).
Approximately 34% of patients with COVID-19 have presented neurological or psychiatric diagnosis in the following 6 months, and for those admitted to an ICU the estimated incidence of a diagnosis increased to 46% (Taquet et al., 2021). Hyperinflammatory response called “cytokine storm” has been related to neurological symptoms and to an increased risk for neurodegenerative diseases (Serrano-Castro et al., 2020; Iwashyna et al., 2020; Widmann and Heneka, 2014). Among the neurological alterations described are fatigue, dizziness, vertigo, anosmia, seizures, stroke, myopathies, encephalitis, and Guillain-Barre syndrome (Khatoon et al., 2020). Neuropsychiatric and neuropsychological symptoms have also been reported (Rogers et al., 2020; Llach and Vieta, 2021). In longitudinal studies, COVID-19 patients showed more cognitive decline than controls and this effect was higher in severe cases (Liu et al., 2021). Moreover, SARS-CoV-2 symptoms were associated with neuropathological correlates (Ramani et al., 2020; Xu et al., 2020; Mattioli et al., 2021).
The cognitive manifestations of COVID-19 have been explained by different mechanisms, including the direct effect of the virus in the Central Nervous System (CNS) via the olfactory bulb (Guedj et al., 2021), neuroinflammation, systemic infection, and prolonged hypoxia (Steardo et al., 2020; Miskowiak et al., 2021). Patients with severe COVID-19 admitted to ICU are also at risk of developing post-intensive care syndrome (PICS), characterized by physical, cognitive, and psychological alterations (Inoue et al., 2019). PICS increases the risk of long-term cognitive impairment in roughly 6–51% of post-ICU patients (Collet et al., 2021). Moreover, delirium is one of the main factors related with cognitive impairment (Kotfis et al., 2020) and was one of the most frequent behavioral manifestations of COVID-19 in up to 11% of all associated hospitalizations (Steardo et al., 2020).
Studies published thus far have demonstrated varying degrees of cognitive impairment in patients with severe SARS-CoV-2 manifestations (Kotfis et al., 2020; Helms et al., 2020; Negrini et al., 2020). Sustained attention (Filatov et al., 2020; Zhou et al., 2020), processing speed, verbal memory (Almeria et al., 2020; Mazza et al., 2021), language (Beaud et al., 2020) and executive function (Beach et al., 2020; Zhou et al., 2020; Almeria et al., 2020) were the most affected. Regarding the clinical variables related to cognitive impairment; the number of days admitted to ICU (Negrini et al., 2020), presence of delirium (Beaud et al., 2020), oxygen therapy (Almeria et al., 2020), systemic inflammation at baseline (Mazza et al., 2021), neurological symptoms (like headache, anosmia or dysgeusia) (Almeria et al., 2020) or psychiatric symptoms (Filatov et al., 2020; Mazza et al., 2021; Mattioli et al., 2021) have been described as risk factors in COVID-19 patients.
Previous studies have focused on risk factors for cognitive impairment in post-COVID patients. However, the role of protective factors on cognition is understudied in this population. Cognitive Reserve (CR) is a construct which refers to the plasticity of the brain and is related to factors such as lifestyle, educational or intellectual level (Stern et al., 2012). CR is a protective factor for the risk of developing dementia (Valenzuela et al., 2006; Cheng et al., 2016), a predictor of better cognitive functioning in patients with psychiatric disorders (Amoretti et al., 2021; Forcada et al., 2015; Grande et al., 2016) and less risk of cognitive impairment after ICU discharge (Fernández-Gonzalo et al., 2020). Nevertheless, to our knowledge the possible protective effect of CR on patients who survived SARS-CoV-2 infection has not been studied yet.
As such the aim of this study is to address this gap in the literature in order to make more conclusive remarks regarding the role of CR in survivors of SARS-CoV-2. Based on previous clinical observations, we expect that patients who developed a serious form of SARS-CoV-2 infection and required ICU admission present some form of cognitive impairment 6 months after hospital discharge. A 6 month period was considered necessary in order to avoid the effect of other possible confounding factors which could interfere with cognition in the early post-ICU phase after hospital discharge. Moreover, we hypothesize that CR has a protective role on the negative effects of clinical severity variables on cognition.
Our main aims are:
-
1)
To study the neuropsychological function in severe SARS-CoV-2 patients admitted to ICU services at 6-month after hospital discharge.
-
2)
To analyze the relationship between cognitive performance and ICU severity variables that were described as potential risk factors for cognitive impairment.
-
3)
To study the role of CR as a protective factor on the impact of SARS-CoV-2 on cognition.
2. Material and methods
2.1. Study design and participants
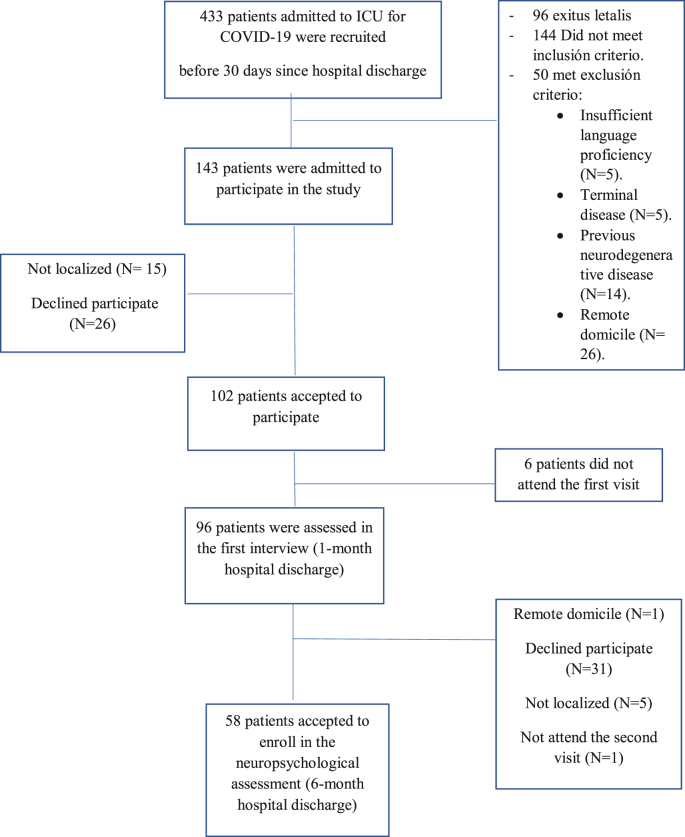
This is a prospective cohort study developed in a tertiary university hospital. We included adult patients who were admitted to the Intensive Care Unit (ICU) for SARS-CoV-2 infection from April to December 2020. From an initial cohort of 102 COVID-19 survivors that were evaluated one-month follow-up after hospital discharge by the PAIN-COVID protocol (see Ojeda et al., 2021); 58 COVID-19 survivors agreed to participate in our study and were assessed 6 months after hospital discharge (See Flowchart 1).
Flowchart 1.
Follow-up of patients admitted to ICU for COVID-19.
Adult patients were enrolled if they had SARS-CoV-2 infection, confirmed with a respiratory tract sample using PCR-based tests and they fulfill at least one of the following inclusion criteria: 1) had an Acute Physiology and Chronic Health Evaluation (APACHE II) score over 14, 2) ICU stay over 10 days, 3) acquired weakness in ICU, 4) Delirium during ICU and acceptance to participate in the study by signing the informed consent form. The exclusion criteria were: 1) patients with non-confirmed SARS-CoV-2 infection according to WHO guidance, 2) previous diagnosis of cognitive impairment or Central Nervous System diseases, 3) terminal illness, 4) insufficient understanding of the Spanish language, 5) patients with whom it would be difficult to complete follow-up, 6) not willing to sign the informed consent form.
The local ethical committee approved the study protocol in accordance with the principles in the Declaration of Helsinki –approval number: HCB/2020/1194. Written informed consent was obtained from all participants.
2.2. Clinical and neuropsychological assessment
Demographic data were collected at Baseline Visit (4–6 weeks after hospital discharge); including age, gender, socio-cultural and socioeconomic status, work status and marital status. Barthel and Charlson indexes and medical history were also recorded. The Barthel index (BI) is a measure of previous functional status and predicts healthcare outcomes, such as length of stay or in-hospital mortality (Ocagli et al., 2021). The Charlson comorbidity index (CCI) is a method of estimating the risk of death from comorbid disease and it is related with poor outcomes in COVID-19 patients (Kuswardhani et al., 2020).
The following data regarding ICU and hospital admission were also collected: Acute Physiology and Chronic Health disease Classification System (APACHE II) and Sequential Organ Failure Assessment Score (SOFA) severity scores, days under mechanical ventilation, presence of sepsis, days under sedation, delirium presence, maximum value of ferritin, d-dimer and C reactive protein, and ICU and hospital length of stay.
A validated self-report questionnaire, the Hospital Anxiety and Depression Scale (HADS), was used to assess psychopathology. Generally accepted standard cut-off scores were used to consider the presence of psychopathology in each subscale score (HADS≥ 8).
To assess neurocognitive function, a set of subtests was selected to create a neuropsychological battery specific designed for this population. The battery included: The Montreal Cognitive Assessment (MOCA) screening test, Digit Span Forward (DSF) and Backward (DSB) and Vocabulary from Wechsler Adult Intelligence Scale- III (WAIS-III), The Stroop Test, The Free and Cued Selective Reminding Test (FCSRT), The Benton Judgment of Line Orientation (JLO), The Trail Making Test (TMT), The Controlled Oral Word Association Test (COWAT), The Animal Fluency Test (ANF) and The Boston Naming Test (BNT).
CR was estimated with proxy measures such as premorbid IQ or CR questionnaires (Rami et al., 2011), so, we included the Cognitive Reserve Questionnaire (CRQ) which is a validated instrument for assessing the degree of cognitive reserve in the Spanish population. Finally, patients were asked an ad-hoc question with a Likert type scale format (ranging from 0-None to3–Much) about subjective cognitive impairment related to SARS-CoV-2 infection.
Direct scores were transformed to z-scores (mean 0 points and standard deviation (SD) of 1 points), using the standardized normative data for each test, to normalize data correcting the effects of the subjects ‘age and education, and providing a better compliance with the Normal Distribution.
All the assessments were performed 6 months from hospital discharge by trained clinical psychologists and explorations lasted approximately 60 min.
2.3. Statistical analysis
Descriptive statistics were calculated for each demographic, medical, clinical and neuropsychological variable. Continuous variables are presented as the mean value ± standard deviation and as the median ± interquartile range when variables are not normally distributed. To compare these variables, we used the Student's t-test or Mann-Whitney U test as appropriate. Categorical variables were expressed as total number (percentages) and compared between groups using Chi-square test.
The primary outcome was the magnitude of neurocognitive sequalae. The proportion of subjects with a score 2 SD under the population mean was calculated for each test score. Secondly, to examine the relationship between cognitive performance and measures of illness severity, negative affect, subjective cognitive impairment and demographic variables we performed correlation analyses. To test if clinical variables predicted the neuropsychological function regression analyses were conducted. We selected those cognitive variables expected to be more impaired according to previous studies.
Finally, ANCOVA analyses were used to test the effect of interaction between medical variables and cognitive reserve on neurocognitive performance. In all analyses we controlled the effect of age, sex and previous health status (Charlson Index). Scores of CR were classified in two groups (High CR ≥ 10 or Low CR < 10).
Statistical analyses were performed using R version 4.1.1. CRAN. Oficina de software libre (CIXUG). Spanish National Research Network. http://cran.es.r-project.org/
3. Results
3.1. Demographic and clinical variables
A total of fifty-eight patients were included in the study (29% women, mean age 65 ± 9.32; range age from 37 to 81). The six-month follow-up cohort did not differ from the drop-out group in terms of sociodemographic characteristics and clinical severity. Their demographic and clinical characteristics are described in Table 1.
Table 1.
Demographical and clinical variables vaariablesvariables
| Characteristics |
Whole sample |
Sex |
t or U | p | |
|---|---|---|---|---|---|
| Continuum variables | (n = 58) | Male (n = 41) | Female (n = 17) | ||
| Age | 67 ± 9.31 | 64.80 ± 9.85 | 65.59 ± 8.14 | −0.28 | 0.774 |
| CRQ | 15 [10.75–18] | 14.73 ± 4.47 | 13 ± 4.56 | 272.5 | 0.193 |
| BMI | 27.93 ± 4.86 | 26 [24–30.4] | 29.38 ± 6.66 | −1.26 | 0.214 |
| Charlson Index | 3 [1–3] | 3 [1.5–4] | 2 [1–3] | 260.5 | 0.373 |
| Barthel Index | 100 [95–100] | 100 [95–100] | 95 [90–100] | 237.5 | 0.134 |
| APACHE II | 12.47 ± 4.74 | 12.73 ± 4.60 | 11.80 ± 5.19 | 0.64 | 0.525 |
| SOFA | 5 [4–7] | 4.5 [3.25–7] | 6.27 ± 2.37 | 380 | 0.126 |
| Ferritin | 1982.5 [1409–2902.25] | 2125 [1454–2983] | 2140.04 ± 1622.88 | 247 | 0.263 |
| D-Dimer | 6400 [3700–10000] | 7300 [3175–10000] | 6620 ± 3159.83 | 302.5 | 0.962 |
| Hospitalization days | 41.11 ± 17.93 | 39.85 ± 18.61 | 44.53 ± 16.03 | −0.86 | 0.392 |
| ICU days | 26.38 ± 14.21 | 25.17 ± 14.69 | 29.67 ± 12.33 | −1.01 | 0.299 |
| Mechanical ventilation days | 15 [8.5–20.5] | 14.35 ± 10.71 | 15 [12–21] | 205 | 0.277 |
| Nº days of high CRP | 8 [5–17] | 8 [5–17] | 10.67 ± 7.25 | 292 | 0.774 |
| HAD anxiety | 4 [2–6] | 3.5 [1.25–6] | 5 [3–8] | 326.5 | 0.240 |
| HAD depression |
3 [1–4] |
3 [1–3.75] |
4 [2–5] |
305 |
0.464 |
| Categorical variables | χ2 | p | |||
| Education level | |||||
|
5 (8.6%) | 2 (4.9%) | 3 (17.6%) | 5.15 | 0.161 |
|
12 (21%) | 9 (22%) | 3 (17.6%) | ||
|
40 (69%) | 30(73.2%) | 10 (58.8%) | ||
|
1 (1.4%) | ||||
| Employment status | |||||
|
10 (17.2%) | 8 (19.5%) | 2 (11.8%) | 6.04 | 0.196 |
|
1 (1.7%) | – | 1 (5.9%) | ||
|
26 (44.8%) | 20 (48.8%) | 6 (35.3%) | ||
|
20 (34.5%) | 13 (31.7%) | 7 (41.2%) | ||
| Civil Status | |||||
|
39 (67.2%) | 33 (80.5%) | 6 (35.3%) | 12.93 | 0.005 |
|
11 (19%) | 4 (9.8%) | 7 (41.2%) | ||
|
7 (12.1%) | 4 (9.8%) | 3 (17.6%) | ||
| Tobacco consumption | |||||
|
36 (62.1%) | 22 (53.7%) | 14 (82.4%) | 5.60 | 0.061 |
|
20 (34.5) | 18 (43.9%) | 2 (11.8%) | ||
| Psychiatric history | |||||
|
9 (15.5%) | 4 (9.8%) | 5 (29.4%) | 9.28 | 0.01 |
|
47 (81%) | 37 (90.2%) | 10 (58.8%) | ||
| Sepsis | |||||
|
36 (62.1%) | 23 (56.1%) | 13 (76.5%) | 4.47 | 0.034 |
|
20 (34.5%) | 18 (43.9%) | 2 (11.8%) | ||
|
2 (3.4%) | 2 (11.8%) | |||
| Delirium | |||||
|
22 (37.9%) | 16 (39%) | 6 (35.3%) | 0.004 | 0.947 |
|
34 (58.6%) | 25 (61%) | 9 (52.9%) | ||
| Hypoxemia maintained >24 h | |||||
|
43 (74.1%) | 30 (73.2%) | 13 (76.5%) | 1.12 | 0.289 |
|
13 (22.4%) | 11 (26.8%) | 2 (11.8%) | ||
| SCI after COVID-19 infection | |||||
|
32 (55.2%) | 27 (65.9%) | 5 (29.4%) | 7.47 | 0.024 |
|
22 (37.9%) | 11 (26.8) | 11 (64.7%) | ||
|
4 (6.9%) | 3 (7.3%) | 1 (5.9%) | ||
Abbreviations: CRC, Cognitive Reserve Questionnaire; BMI, Body Mass Index; AHT, Arterial Hypertension; APACHE-II, Acute Physiology and Chronic Health disease Classification System II; SOFA, Sepsis related Organ Failure Assessment; ICU, Intensive Care Unit; CRP, C-Reactive Protein; HAD, Hospital Anxiety and Depression Scale; SCI, Subjetive Cognitive Impairment.
Most patients had higher education (69%), were retired (45%) and were married (67%). They were mostly functionally independent before the admission in ICU services (BI mean: 95.62 ± 8.26), had low comorbidity (CCI mean 2.55 ± 1.6), were overweight (mean 27.93 ± 4.8), AHT (46%) and didn't smoke (62%). The mean of ICU stay days was 26.38 ± 14.10 and mean hospitalization days 41.11 ± 17.93. Most of them developed sepsis (62%), sustained hypoxia (74%) and required mechanical ventilation (74%), while 38% presented delirium during ICU admission.
Laboratory findings didn't find gender differences in ferritin or D-Dimer, but said differences were observed in sepsis (χ2 = 4.47, p = 0.034), with high prevalence for women (76.5% vs. 56.1% in men).
When patients were asked about cognitive complaints after hospital discharge, 84% denied cognitive complaints before COVID-19 infection, whereas almost 50% referred some type of cognitive complaint after the hospitalization. Women tended to report more cognitive complaints (70.6% vs 34.1% in men) and to have more psychiatric comorbidity (29.4% vs. 9.8% in men).
3.2. Neuropsychological functioning in post-ICU patients after COVID-19
More than half of sample (53.4%) obtained a score below the cutoff point (<26) for normal performance in the screening test MOCA and 19% below of the cutoff for mild cognitive impairment (see Table 2.).
Table 2.
Neuropsychological performance of patients with severe COVID-19.
| Neuropsychological test | Minimum | Maximum | Mean (SD) |
|---|---|---|---|
| MOCA | 12 | 29 | 24.4 (3.89) |
| Digits WAIS-III (Z) | −1 | 3 | 0.81 (0.96) |
| TMT-A (Z) | −2 | 2 | 0.58 (0.89) |
| TMT-B (Z) | −2.7 | 2 | 0.54 (1.01) |
| Phonemic fluency (Z) | −3.3 | 2.4 | −0.31 (0.97) |
| Semantic fluency (Z) | −1.3 | 3.3 | 0.39 (1.04) |
| BNT (Z) | −1.33 | 2.67 | 0.86 (1.03) |
| JLO (Z) | −2.67 | 2.67 | 0.14 (0.89) |
| FCSRT FR1 (Z) | −2 | 2.33 | 0.36 (0.81) |
| FCSRT FR Total (Z) | −2.67 | 2.67 | 0.14 (0.87) |
| FCSRT total (Z) | −2.67 | 2.67 | 0.57 (1.16) |
| FCSRT FR Delayed (Z) | −2.67 | 2.67 | 0.24 (0.9) |
| FCSRT total Delayed (Z) | −2.67 | 2.67 | 0.87 (1.56) |
| Stroop W (Z) | −3 | 1.4 | −0.78 (0.97) |
| Stroop C (Z) | −2.7 | 2.6 | −0.79 (1.08) |
| Stroop Interference (Z) | −2.1 | 1.7 | −0.21 (0.84) |
| Vocabulary WAIS-III (Z) | −0.67 | 3 | 1 (0.77) |
Abbreviations: MOCA, Montreal Cognitive Assessment; WAIS-III, Wechsler Adult Intelligence Scale; TMT-A, Trail Making Test A; TMT-B, Trail Making Test B; BNT, Boston Naming Test; JLO, Judgement Line Orientation; FCSRT FR, Free and Cued Selective Reminding Test Free Recall; Stroop W, Stroop test word; Stroop C, Stroop test color.
When the battery tests were analyzed with normative data (Z scores), pathological scores (<Z = −2) were seen in Stroop Word (7 [12.7%]), Stroop Color (9 [16.7%]), Stroop Interference (1 [1.9%]), TMT-A (1 [1.7%], TMT-B (1 [1.7%]), Phonemic fluency (3 [5.2%]), FCSRT FR1 (1 [1.8%]), FCSRT FR total (1 [1.8%]), FCSRT total (2 [3.6%]), FCSRT FR delay (2 [3.6]), FCSRT total delay (3 [5.4%]), JLO (2 [3.5%]).
No differences in neuropsychological performance were found between patients expressing cognitive complaints after COVID-19 infection in comparison to patients who didn't. However, there were differences between those groups in HADS anxiety subscale (t = −2.2; p < 0.05) but not in HADS depression subscale (t = −0.8; p = 0.4).
3.3. Relationship between neuropsychological and clinical variables
Results of regression analyses showed that most clinical variables had no significant direct effects on neurocognitive variables (see Table 3).
Table 3.
Main effects of ICU variables in cognitive performance.
| TMT-B | FAS | STROOP W | STROOP Interference |
FCRST Total | FCRST Total delayed | |
|---|---|---|---|---|---|---|
| ICU days | β = −0,14 (3,20–0,89); p = 0,26 | β = 0.17 (−0.82–0.42); p = 0.18 | β = −0.16 (−0.57 – 0.15); p = 0.25 | β = 0.40 (0.72–0.34); p = 0.00 | β = 0.16 (−0.19 – 0.04); p = 0.19 | β = −0.05 (−0.07 – 0.05); p = 0.73 |
| High CRP days | β = −0,09 (-3 – 1,44); p = 0,48 | β = −0.01 (−0.28 – 0.27); p = 0.96 | β = −0.24 (−0.72–0.04); p = 0.08 | β = 0.33 (0.03–0.32); p = 0.02 | β = −0.18 (−0.21 – 0.04); p = 0.16 | β = −0.15 (−0.09 – 0.02); p = 0.24 |
| Delirium | β = −0.11 (−86.69–32.03); p = 0.36 | β = −0.01 (−7.78 – 7.09); p = 0.96 | β = 0.17 (−3.8 – 16.7); p = 0.21 | β = −0.02 (−4.39 – 3.92); p = 0.90 | β = −1.82 (−6.26 – 0.30); p = 0.07 | β = −0.18 (−2.67 –0.51); p = 0.18 |
| MV days | β = −0.74 (−3.87 – 2.21); p = 0.59 | β = 0.08 (−0.25 – 0.45); p = 0.57 | β = −0.15 (−0.86 – 0.30); p = 0.33 | β = 0.30 (−0.01 – 0.45); p = 0.05 | β = −0.31 (−0.34 to −0.03); p = 0.02 | β = −0.18 (−0.13 – 0.03); p = 0.20 |
| Sedation days | β = 0.16 (−0.67 – 2.91); p = 0.21 | β = −0.62 (−0.42 – 0.27); p = 0.67 | β = −0.15 (−0.99 – 0.34); p = 0.33 | β = 0.31 (0.01–0.51); p = 0.04 | β = −0.40 (−0.39 to −0.10); p = 0.00 | β = −0.29 (−0.16 to −0.01); p = 0.03 |
| Sepsis | β = −0.18 (−106.19 – 16.35); p = 0.15 | β = 0.13 (−4.01 – 11.33); p = 0.34 | β = −0.16 (−16.42 – 4.80); p = 0.28 | β = 0.27 (−0.12 – 8.23); p = 0.06 | β = −0.75 (−4.56 – 2.49); p = 0.56 | β = 0.63 (−1.29 – 2.09); p = 0.63 |
Abbreviations: TMT-B, Trail Making Test B; Stroop W, Stroop test word; Free and Cued Selective Reminding Test; ICU, Intensive Care Unit; CRP, C-reactive protein; MV, Mechanical Ventilation.
Days of mechanical ventilation were related with lower FCRST total score (β = −0.31, p = 0.02) and Stroop interference score (β = 0.3, p = 0.05), days of sedation were related to lower FCRST total score (β = −0.40, p < 0.005), lower FCRST delayed score (β = −0.29, p = 0.03) and more Stroop interference score (β = 0.31, p = 0.04). On the other hand, ICU days and high PCR days were related with more Stroop interference score (β = 0.4 p < 0.005; β = 0.33, p = 0.02).
3.4. Role of cognitive reserve (CR)
The interaction effects between CR and ICU variables are described in Table 4.
Table 4.
Interaction effect between CR and ICU variables.
| CR↑ |
CR↓ |
Effect |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delirium |
No Delirium |
Delirium |
No Delirium |
Group |
CR |
GroupXCR |
||||
| Mean (IC 95%) | Mean (IC 95%) | Mean (IC 95%) | Mean (IC 95%) | F | p | F | p | F | p | |
| Verbal Memory | ||||||||||
| FCSRT total | 43.1 (40.8–45.4) | 44.6 (42.7–46.5) | 28.2 (23.5–32.9) | 38.2 (34.2–42.2) | 5.62 | 0.02 | 31.2 | <0.001 | 6.80 | 0.01 |
| FCSRT total delay | 14.78 (13.66–15.9) | 15.09 (14.16–16) | 7.52 (5.25–9.8) | 12.23 (10.29–14.2) | 3.09 | 0.08 | 29.37 | <0.001 | 7.63 | 0.01 |
| Processing Speed | ||||||||||
| SCWT W | 107.9 (98.9–117) | 101 (93.5–108) | 89.4 (68.6–110) | 90.1 (73.1–107) | 1.45 | 0.23 | 3.58 | 0.06 | 0.29 | 0.58 |
| Language | ||||||||||
| BNT | 53.4 (50.8–56) | 54.9 (52.8–57) | 44.7 (39.5–49.9) | 48.2 (44.1–52.3) | 1.43 | 0.23 | 16.04 | <0.001 | 0.30 | 0.58 |
| Executive function | ||||||||||
| TMT-B | 99.2 (48.1–150) | 122.1 (79.7–164) | 209 (105.6–312) | 247.5 (159.4–336) | 0.94 | 0.33 | 9.25 | 0.003 | 0.04 | 0.82 |
| Phonemic Fluency | 38.6 (33.22–43.9) | 38.1 (33.67–42.5) | 11.3 (0.53–22.1) | 18.9 (10.47–27.3) | 0.02 | 0.89 | 33.37 | <0.001 | 1.19 | 0.29 |
| SCWT interference |
3.58 (−0.13–7.29) |
1.36 (−1.72–4.44) |
−7.98 (−16.55–0.59) |
5.11 (−1.93–12.14) |
0.006 |
0.98 |
0.68 |
0.41 |
6.87 |
0.01 |
| Sepsis | No Sepsis | Sepsis | No Sepsis | Group | CR | GroupXCR | ||||
| Mean (IC 95%) |
Mean (IC 95%) |
Mean (IC 95%) |
Mean (IC 95%) |
F |
p |
F |
p |
F |
p |
|
| Verbal Memory | ||||||||||
| FCSRT total | 25.2 (23–27.3) | 26.2 (22.9–29.6) | 19 (14–24.1) | 17.2 (11.4–22.9) | 0.42 | 0.51 | 26.38 | <0.001 | 0.008 | 0.92 |
| FCSRT total delay | 9.84 (8.94–10.73) | 10.08 (8.71–11.44) | 6.41 (4.34–8.47) | 6.82 (4.46–9.17) | 0.16 | 0.68 | 23.78 | <0.001 | 0.43 | 0.51 |
| Processing Speed | ||||||||||
| SCWT P | 103.4 (96.6–110.2) | 105.7 (95.2–116.2) | 79.4 (60.1–98.7) | 98.4 (80.3–116.5) | −1.46 | 0.15 | 0.72 | 0.47 | 1.20 | 0.23 |
| Language | ||||||||||
| BNT | 54.6 (52.6–56.6) | 53.2 (50.2–56.3) | 48.2 (43.6–52.8) | 45.5 (40.7–50.2) | 2.69 | 0.10 | 13.79 | <0.001 | 0.15 | |
| Executive function | ||||||||||
| TMT-B | 101 (62.2–140) | 147 (88.1–206) | 219(130.1–308) | 252 (149.8–353) | 3.37 | 0.07 | 8.35 | <0.001 | 0.03 | 0.84 |
| Phonemic Fluency | 37.75 (33.76–41.7) | 38.12 (32.08–44.1) | 22.55 (13.39–31.7) | 9.13 (−0.36–18.6) | 2.05 | 0.04 | 5.41 | <0.001 | −1.92 | 0.06 |
| SCWT interference | 3.24 (0.43–6.05) | −0.96 (−5.29–3.37) | 6.35 (−1.59–14.3) | −5.99 (−13.43–1.45) | 6.53 | 0.013 | 0.11 | 0.73 | 2.04 | 0.15 |
|
Days in ICU |
Days in ICU |
Group |
CR |
GroupXCR |
||||
|---|---|---|---|---|---|---|---|---|
| Mean (IC 95%) | Mean (IC 95%) | F | p | F | p | F | p | |
| Verbal Memory | ||||||||
| FCSRT total | 44.10 (42.3–45.9) | 34.4 (30.9–37.9) | 2.44 | 0.12 | 24.73 | <0.001 | 0.39 | 0.53 |
| FCSRT total delay | 15 (14.2–15.9) | 10.4 (8.7–12.1) | 0.25 | 0.61 | 23.98 | <0.001 | 0.91 | 0.34 |
| Processing Speed | ||||||||
| SCWT P | 103.5 (97.3–110) | 89.6 (76.1–103) | 1.21 | 0.27 | 3.66 | 0.06 | 0.41 | 0.52 |
| Language | ||||||||
| BNT | 54.3 (52.5–56.1) | 46.9 (43.6–50.3) | 0.09 | 0.76 | 15.09 | <0.001 | 0.10 | 0.74 |
| Executive function | ||||||||
| TMT-B | 112 (77.3–147) | 234 (165–303) | 1.77 | 0.18 | 9.99 | <0.001 | 0.12 | 0.72 |
| Phonemic Fluency | 37.6 (34.07–41.2) | 15.7 (9.12–22.3) | 3.83 | 0.05 | 36.16 | <0.001 | 1.11 | 0.29 |
| SCWT interference |
1.95 (−0.46–4.37) |
−0.39 (−5.66–4.88) |
11.37 |
0.001 |
0.80 |
0.37 |
1.83 |
0.18 |
| MV days | MV days | |||||||
| Mean (IC 95%) |
Mean (IC 95%) |
F |
p |
F |
p |
F |
p |
|
| Verbal Memory | ||||||||
| FCSRT total | 43.8 (42–45.6) | 34.8 (31.3–38.4) | 8.29 | 0.006 | 20.38 | <0.001 | 0.03 | 0.85 |
| FCSRT total delay | 15 (14.1–15.9) | 10.7 (8.9–12.5) | 2.96 | 0.09 | 19.36 | 0.001 | 2.40 | 0.12 |
| Processing Speed | ||||||||
| SCWT P | 103.3 (96.9–110) | 88.4 (74.3–102) | 0.92 | 0.34 | 3.59 | 0.06 | 0.37 | 0.54 |
| Language | ||||||||
| BNT | 54.3 (52.4–56.2) | 48.4 (44.7–52.2) | 0.28 | 0.59 | 8.46 | 0.005 | 1.64 | 0.20 |
| Executive function | ||||||||
| TMT-B | 113 (74.8–152) | 238 (160.9–315) | 0.45 | 0.50 | 8.31 | 0.006 | 0 | 0.99 |
| Phonemic Fluency | 37.7 (33.85–41.6) | 16.3 (8.65–24) | 0.72 | 0.39 | 24.15 | <0.001 | 0.17 | 0.67 |
| SCWT interference |
2.50 (−0.27–5.28) |
−0.84 (−6.99–5.23) |
3.71 |
0.06 |
0.97 |
0.32 |
0.05 |
0.81 |
| Sedation days | Sedation days | |||||||
| Mean (IC 95%) |
Mean (IC 95%) |
F |
p |
F |
p |
F |
p |
|
| Verbal Memory | ||||||||
| FCSRT total | 44 (42.4–45.6) | 36.3 (32.5–39.9) | 15.14 | <0.001 | 16.53 | <0.001 | 1.11 | 0.29 |
| FCSRT total delay | 15 (14.2–15.7) | 11.9 (10.1–13.6) | 8.54 | 0.005 | 14.5 | <0.001 | 9.40 | 0.003 |
| Processing Speed | ||||||||
| SCWT P | 103.9 (97.3–111) | 86.2 (69.1–103) | 0.66 | 0.41 | 3.44 | 0.07 | 0.25 | 0.61 |
| Language | ||||||||
| BNT | 54.6 (52.9–56.4) | 49.2 (45.2–53.2) | 2.49 | 0.12 | 7.88 | 0.007 | 2.62 | 0.11 |
| Executive function | ||||||||
| TMT-B | 102 (85.1–119) | 214 (175.5–253) | 0.92 | 0.34 | 29.04 | 0.001 | 0.21 | 0.64 |
| Phonemic Fluency | 37.9 (34.2–41.6) | 19.4 (10.8–28) | 0.006 | 0.93 | 14.82 | <0.001 | 0.26 | 0.60 |
| SCWT interference |
1.93 (−0.75–4.62) |
3.12 (−3.81–10.6) |
5.22 |
0.02 |
0.14 |
0.70 |
0.09 |
0.76 |
| High CRP days | High CRP days | |||||||
| Mean (IC 95%) |
Mean (IC 95%) |
F |
p |
F |
p |
F |
p |
|
| Verbal Memory | ||||||||
| FCSRT total | 43.8 (42.1–45.5) | 34.4 (30.9–37.8) | 3.14 | 0.08 | 24.55 | 0.001 | 0.65 | 0.42 |
| FCSRT total delay | 14.8 (14.02–15.6) | 10.5 (8.84–12.1) | 1.89 | 0.17 | 24.96 | 0.001 | 3.31 | 0.07 |
| Processing Speed | ||||||||
| SCWT P | 103.3 (97.2–109) | 89.5 (75.7–103) | 4.14 | 0.04 | 2.98 | 0.09 | 0.28 | 0.59 |
| Language | ||||||||
| BNT | 54.3 (52.5–56.1) | 46.8 (43.5–50.2) | 0.06 | 0.79 | 15.14 | <0.001 | 0.68 | 0.41 |
| Executive function | ||||||||
| TMT-B | 113 (78.7–148) | 229 (160–298) | 0.43 | 0.51 | 9.88 | 0.001 | 1.21 | 0.27 |
| Phonemic Fluency | 37.7 (34.3–41.2) | 16.7 (10.2–23.3) | 0.06 | 0.80 | 36.6 | <0.001 | 6.47 | 0.01 |
| SCWT interference | 2.5 (0.06–4.93) | 0.44 (−5.11–6) | 8.47 | 0.005 | 1.50 | 0.22 | 2.88 | 0.09 |
CR, Cognitive Reserve; FCSRT FR, Free and Cued Selective Reminding Test; SCWT W, Stroop Color and Word Test; BNT, Boston Naming Test; TMT-B, Trail Making Test B; SCWT interference, Stroop Color and Word Test Interference; ICU, Intensive Care Unit; MV, Mechanical Ventilation; CRP (C-Reactive Protein).
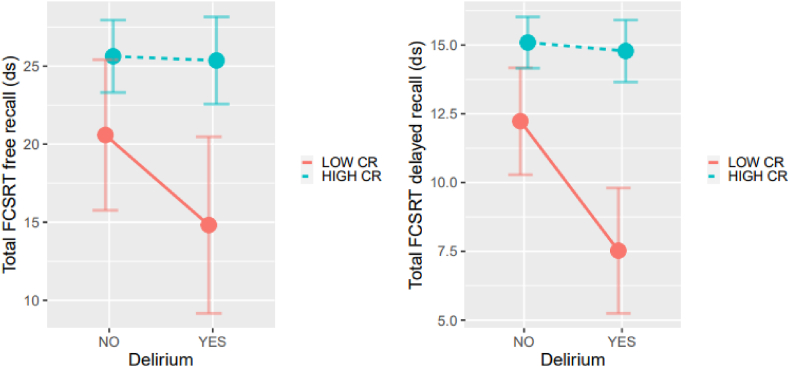
CR interacted with delirium in FCSRT total (F = 6.8, p = 0.01) and FCSRT total delay (F = 7.63, p = 0.01) performance in a way that those with low CR who suffer delirium obtained the worst performance (see Fig. 1). A similar finding occurred with the interaction between CR and Stroop interference (F = 6.87, p = 0.01), in which those with low CR and delirium obtained the worst performance.
Fig. 1.
Interaction between cognitive reserve (CR) and delirium in memory (short and delayed recall tasks).
The number of sedation days also interacted with CR in FCSRT total delay (F = 9.40, p = 0.003), with a poorer performance in those with low CR and a high number of sedation days.
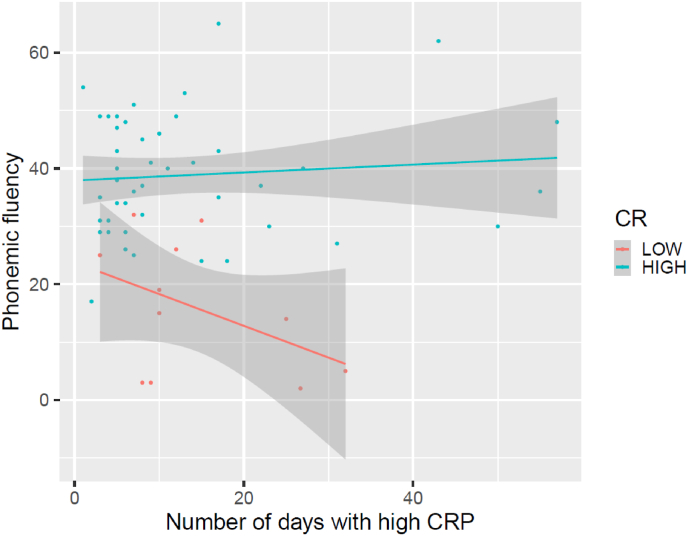
Finally, the number of days with high PCR interacted with CR in phonemic fluency (F = 6.47, p = 0.01) with worse performance in patients with low CR and more days with high PCR during ICU stay (see Fig. 2).
Fig. 2.
Interaction between cognitive reserve (CR) and number of days with high CRP (C-Reactive Potein) in a task of phonemic fluency.
4. Discussion
This is the first study to prospectively investigate the neurocognitive sequelae in post-ICU COVID-19 survivors and the role of cognitive reserve as a protective factor.
We found the SARS-CoV-2 infection profile to be consistent with that described in general population. Contrary to other studies (Almeria et al., 2020), we didn't find differences between sexes in levels of ferritin or D-dimer but sex differences were found in sepsis. Additionally, women had more psychiatric comorbidity and tended to refer more cognitive complaints.
According to the literature (Negrini et al., 2020), some type of cognitive sequelae is common, with 19% of the total sample performance below the cutoff for mild cognitive impairment in the screening test. Besides verbal memory, speed processing and executive function were the most affected domains, coinciding with findings from similar studies (Kotfis et al., 2020).
The number of days admitted to the ICU did not predict cognitive decline as demonstrated in the literature (Beaud et al., 2020), but mechanical ventilation predicted worse verbal memory function. This finding is similar to other studies that suggested oxygen therapy could be explained by the continuous hypoxia caused by pulmonary disease related COVID-19 infection (Almeria et al., 2020). Hypoxia is a potential risk factor for brain damage, especially in limbic regions like the hippocampus. The hippocampal CA3 neurons are particularly susceptible to hypoxic damage (Biswal et al., 2016) and this area is related with episodic memory (Zammit et al., 2017). While CA2, CA3 and dentate gyrus are involved in encoding, CA1 and subiculum are responsible for retrieval (Eldridge et al., 2005; Zammit et al., 2017). Taking into account hypoxia and ARDS are core symptoms in COVID-19 infection, it seems plausible to consider them as risk factors for memory impairment in these patients.
Other ICU variables such as the presence of delirium or days of sedation also predicted worse memory performance. Although sedation was described as a potential risk factor for cognitive impairment after ICU discharge (Inoue et al., 2019); delirium at ICU seems to be the most important predictor variable for long-term cognitive impairment (Collet et al., 2021; Beaud et al., 2020) and is linked to abnormalities of brain structure (Kohler et al., 2019). In our study, similar to previous findings (Pandharipande et al., 2013; Inoue et al., 2019), delirium in the ICU predicted worse verbal memory performance at 6 months follow-up. Thus, it seems delirium could be a risk factor for long-term memory impairment in COVID-19 patients who required ICU services. Therefore, it is required to follow-up and monitor the cognitive function of these patients to promote early detection of possible long-term cognitive disorders.
Mazza et al. (2021) suggested that another risk factor for cognitive impairment could be inflammatory mechanisms that occur in the first stages of COVID-19 infection. They found that systemic inflammation at baseline predicted not only the cognitive functioning at 3 months follow-up, but also the severity of depression; suggesting a possible common cerebral alteration. In our study, we found that number of days with high PCR predict higher scores in interference and interacted with CR to predict worse phonemic fluency, suggesting a relationship between inflammation and cognitive performance in COVID-19 patients admitted in ICU.
Almost half of our sample expressed subjective cognitive impairment (SCI), but cognitive complaints were not related to neuropsychological performance. However, there was a relationship between SCI and anxiety scores, with more SCI in those patients with high levels of anxiety. These results are similar to those of previous studies (Almeria et al., 2020; Mazza et al., 2021) which pointed out the relevance of exploring the affective and anxiety levels of patients with cognitive complaints after COVID-19 infection to plan a holistic treatment, addressing not only the neuropsychological sequels but also the psychological symptoms that can be related to functional impairments with high disease burden (Liu et al., 2017).
Finally, as our study recruited an incidental sample, most patients had a high sociocultural level due to the socioeconomical level of the hospital reference area. Sociocultural level; as well as premorbid intelligence (IQ) and other proxy measures, is associated with the construct of Cognitive Reserve (CR). As previously described, this construct refers to individual differences in susceptibility to age-related brain changes and pathologic changes (Stern et al., 2012). CR can act as a moderator between pathology and clinical outcome, in which brain actively cope with brain damage by using pre-existing cognitive processing approaches or by enlisting compensatory approaches. This means that individuals with high CR probably cope better with brain damage than individuals with low CR (Stern et al., 2012). In this context, we hypothesized CR would play a role in the relation between medical risk factors for brain damage and neurocognition. We found that most of our sample with severe SARS-CoV-2 had a preserved global performance in spite of multiple medical risk factors. For this reason, we decided to split up the sample in two groups (high and low CR) to analyze the differences in cognitive performance when they were exposed to different medical risk factors. In all analyses, patients with high CR tended to perform better in neuropsychological tests than patients with low CR. In addition, we found that medical risk factors like delirium, sedation days or inflammation level interacted with CR to predict cognitive performance in COVID-19 patients. Moreover, high CR exposed to these risk factors were less cognitively affected than those with low CR; especially in domains like verbal memory or executive function. To our knowledge, this is the first study to analyze the role of CR in cognitive sequelae in post-ICU COVID-19 patients. Our results are consistent with other studies that analyze the role of CR in the risk of developing Alzheimer dementia (Cheng et al., 2016).
Our study has limitations that warrant comment. First of all, due to the pandemic crisis it was hard to plan a rigorous method to select the population group and an incidental sample was recruited. Although this method is considered to be more ecological it can also be susceptible to selection biases. In our study, most of the patients belonged to a district with a high socio-cultural level and it is possible that this fact affected the low rates of cognitive impairments found (mediated by high CR). Second, our study does not include a control group of ICU patients without COVID-19 infection and we cannot determine if our findings are explained entirely by severe COVID-19 infection or by other risk factors associated with ICU admission. Third, the relatively small sample size of the study suggests that results should be interpreted with caution, specifically in terms of drawing concrete scientific conclusions. Fourth, alternative and more comprehensive instruments for CR assessment could have been used, such as the CRASH (Amoretti et al., 2019; de la Serna et al., 2021). Further studies with severe COVID-19 patients are needed to replicate the results. Finally, we assessed the patients 6 months after hospital discharge and it is possible that some cognitive deficits would have improved by then.
Future studies are necessary to conclude if severe SARS-CoV-2 infection can be a risk factor for cognitive impairment or if the deficits are mainly due to other medical conditions associated with it (i.e., ICU admission, delirium, etc.). For this purpose, comparing severe COVID-19 patients admitted to ICU services with control groups admitted in ICU by other causes is necessary. Moreover, including a larger sample size would allow capturing the heterogeneity of CR in the population and improving understanding of how CR can be a protective factor of the effect of SARS-CoV-2 on cognition. Furthermore, psychological variables like anxiety and depression can be related with cognitive complaints in these patients and could explain some of the functional difficulties found in some cases. Therefore, to explore the psychological status of these patients is as important as exploring their cognitive or physical status.
Furthermore, a long-term follow-up of these patients is necessary to determine the extent of the deficits caused by the COVID-19 and whether this population could have an increased risk for neurodegenerative diseases as some researchers have suggested.
Finally, a future line of investigation could be the study of cognitive protector factors for severe COVID-19 patients and other severe patients who required ICU admission. Therefore, interventions to promote cognitive reserve in the community and cognitive stimulation respectively as primary and secondary prevention actions for severe COVID-19 patients could reduce the taxes of cognitive impairment in this population, as well as the long-term health and economic consequences of this illness.
5. Conclusions
In conclusion, patients with severe COVID-19 infection who required ICU admission could have some type of cognitive impairment in the long-term, especially in domains like processing speed, verbal memory, or executive function. Medical risk factors like delirium, sedation, inflammation, hypoxia and mechanical ventilation during hospitalization can be risk factors for worse cognitive performance in the long term in this population.
CR mediates the relationship between these medical risk factors and cognitive performance at the follow-up. Likewise, those patients with high CR at the baseline demonstrate less risk to develop cognitive impairment than those with low CR; especially in verbal memory or executive functioning.
Our data suggest that there are not only risk factors for cognitive impairment in severe COVID-19 patients but also protective factors, and public health policies should invest in interventions that could reduce the long-term disease burden.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
A. Costas-Carrera reports no disclosures relevant to the manuscript. M.M. Sánchez-Rodríguez reports no disclosures relevant to the manuscript. S. Cañizares reports no disclosures relevant to the manuscript. A. Ojeda reports no disclosures relevant to the manuscript. I. Martín-Villalba reports no disclosures relevant to the manuscript. M. Primé-Tous reports no disclosures relevant to the manuscript. M. A. Rodríguez-Rey reports no disclosures relevant to the manuscript. X. Segú-Rosa reports no disclosures relevant to the manuscript. F. Valdesoiro-Pulido reports no disclosures relevant to the manuscript. R. Borras reports no disclosures relevant to the manuscript. J.M. Peri reports no disclosures relevant to the manuscript. EV has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, AbbVie, Angelini, Biogen, Boehringer-Ingelheim, Celon, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, Janssen, Lundbeck, Novartis, Organon, Otsuka, Sanofi-Aventis, Sunovion, and Takeda, outside the submitted work.
Declaration of competing interest
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Acknowledgements
The authors thank Derek Clougher for his collaboration in the translation and revision of the manuscript. Authors also appreciate the patients and all the physicians and health professionals of HCB involved in the care of these patients during COVID-19 pandemic. EV also acknowledges the support of Fundación Soria Melguizo and CIBER.
References
- Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoretti S., Cabrera B., Torrent C., Bonnín C.D.M., Mezquida G., Garriga M., Jiménez E., et al. Cognitive reserve assessment scale in health (CRASH): its validity and reliability. J. Clin. Med. 2019;8(5):586. doi: 10.3390/jcm8050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoretti S., Ramos-Quiroga J.A. Cognitive reserve in mental disorders. Eur. Neuropsychopharmacol. 2021;49:113–115. doi: 10.1016/j.euroneuro.2021.04.011. [DOI] [PubMed] [Google Scholar]
- Beach R.S., Praschan N.C., Hogan C., Dotson S., Merideth F., Kontos N., et al. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen. Hosp. Psychiatr. 2020:6547–6553. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaud V., Crottaz-Herbette S., Dunet V., et al. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp2020-325173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal S., Sharma D., Kumar K., Nag T.C., Barhwal K., Hota S.K., Kumar B. Global hypoxia induced impairment in learning and spatial memory is associated with precocious hippocampal aging. Neurobiol. Learn. Mem. 2016;133:157–170. doi: 10.1016/j.nlm.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Cheng S.T. Cognitive reserve and the prevention of dementia: the role of physical and cognitive activities. Curr. Psychiatr. Rep. 2016;18(9):85. doi: 10.1007/s11920-016-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet M.O., Egerod I., Thomsen T., Wetterslev J., Lange T., Ebdrup B.H., Perner A. Risk factors for long-term cognitive impairment in ICU survivors: a multicenter, prospective cohort study. Acta Anaesthesiol. Scand. 2021;65(1):92–99. doi: 10.1111/aas.13692. [DOI] [PubMed] [Google Scholar]
- De la Serna E., Montejo L., Solé B., Castro-Fornieles J., Camprodon-Boadas P., Sugranyes G., Rosa-Justicia M., et al. Effectiveness of enhancing cognitive reserve in children, adolescents and young adults at genetic risk for psychosis: study protocol for a randomized controlled trial. Rev. Psiquiatía Salud Ment. 2021;22:S1888–S9891. doi: 10.1016/j.rpsm.2021.02.003. (21)00029-X. [DOI] [PubMed] [Google Scholar]
- Eldridge L.L., Engel S.A., Zeineh M.M., Bookheimer S.Y., Knowlton B.J. A dissociation of encoding and retrieval processes in the human hippocampus. J. Neurosci. 2005;25(13):3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Gonzalo S., Navarra-Ventura G., Bacardit N., Gomà Fernández G., de, Haro C., Subirà C., López-Aguilar J., et al. Cognitive phenotypes 1 month after ICU discharge in mechanically ventilated patients: a prospective observational cohort study. Crit. Care. 2020;24(1):618. doi: 10.1186/s13054-020-03334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19) encephalopathy. Cureus. 2020;12:7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcada I., Mur M., Mora E., Vieta E., Bartrés-Faz D., Portella M.J. The influence of cognitive reserve on psychosocial and neuropsychological functioning in bipolar disorder. Eur. Neuropsychopharmacol. 2015;25(2):214–222. doi: 10.1016/j.euroneuro.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Grande I., Sanchez-Moreno J., Sole B., Jimenez E., Torrent C., Bonnin C.M., Varo C., et al. High cognitive reserve in bipolar disorders as a moderator of neurocognitive impairment. J. Affect. Disord. 2016;208:621–627. doi: 10.1016/j.jad.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Guedj E., Million M., Dudouet P., Tissot-Dupont H., Bregeon F., Cammilleri S., Raoult D. 18F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur. J. Nucl. Med. Mol. Imag. 2021;48(2):592–595. doi: 10.1007/s00259-020-04973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Hatakeyama J., Kondo Y., Hifumi T., Sakuramoto H., Kawasaki T., Taito S., et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019;6(3):233–246. doi: 10.1002/ams2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon F., Prasad K., Kumar V. Neurological manifestations of COVID-19: available evidences and a new paradigm. J. Neurovirol. 2020;26(5):619–630. doi: 10.1007/s13365-020-00895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler J., Borchers F., Endres M., Weiss B., Spies C., Emmrich J.V. Cognitive deficits following intensive care. Dtsch Arztebl Int. 2019;116(38):627–634. doi: 10.3238/arztebl.2019.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotfis K., Roberson S., Wilson J.E., Dabrowski W., Pun B.T., Ely E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care. 2020;24(1):176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswardhani R.A., Henrina J., Pranata R., Lim A.M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llach C.D., Vieta E. Mind long COVID: psychiatric sequelae of SARS-CoV-2 infection. Eur. Neuropsychopharmacol. 2021;49:119–121. doi: 10.1016/j.euroneuro.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., He H., Yang J., Feng X., Zhao F., Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J. Psychiatr. Res. 2017;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Liu Y.H., Wang Y.R., Wang Q.H., Chen Y., Chen X., Li Y., Cen Y., Xu C., Hu T., Liu X.D., Yang L.L., Li S.J., Liu X.F., Liu C.M., Zhu J., Li W., Zhang L.L., Liu J., Wang Y.J. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol. Neurodegener. 2021;16(1):48. doi: 10.1186/s13024-021-00469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Righetti F., Sala E., Tomasi C., De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J. Neurol. 2021;1:1–7. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrini F., de Sire A., Andrenelli E., Lazzarini S.G., Patrini M., Ceravolo M.G. International multiprofessional steering committee of cochrane rehabilitation REH-COVER action. Rehabilitation and COVID-19: the cochrane rehabilitation 2020 rapid living systematic review. Eur. J. Phys. Rehabil. Med. 2020;56(5):652–657. doi: 10.23736/S1973-9087.20.06539-9. [DOI] [PubMed] [Google Scholar]
- Ocagli H., Cella N., Stivanello L., Degan M., Canova C. The Barthel index as an indicator of hospital outcomes: a retrospective cross-sectional study with healthcare data from older people. J. Adv. Nurs. 2021;77(4):1751–1761. doi: 10.1111/jan.14708. [DOI] [PubMed] [Google Scholar]
- Ojeda A., Calvo A., Cuñat T., et al. Rationale and study design of an early care, therapeutic education, and psychological intervention program for the management of post-intensive care syndrome and chronic pain after COVID-19 infection (PAIN-COVID): study protocol for a randomized controlled trial. Trials. 2021;22:486. doi: 10.1186/s13063-021-05463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandharipande P.P., Girard T.D., Jackson J.C., et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Müller L., Ostermann P.N., Gabriel E., Abida-Islam P., Müller- Schiffmann A., Mariappan A., et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39(20) doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami L., Valls-Pedret C., Bartres-Faz D., Caprile C., Sole-Padulles C., Castellvi M., Olives J., et al. Cuestionario de reserva cognitiva. Valores obtenidos en población anciana sana y con enfermedad de Alzheimer. Rev. Neurol. 2011;52:195–201. doi: 10.33588/rn.5204.2010478. [DOI] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Castro P.J., Estivill-Torrus G., Cabezudo-García P., Reyes-Bueno J., Petersen N.C., Aguilar-Castillo M.J., Suarez-Pérez J., et al. Influencia de la infección SARS-Cov2 sobre Enfermedades Neurodegenerativas y Neuropsiquiatricas: ¿Una pandemia demorada? Neurologia. 2020;35:245–251. doi: 10.1016/j.nrl.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steardo L., Steardo L., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020;229(3) doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236379survivors of COVID -19:a retrospective cohort study using electronic health records .The lancet. Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M.J., Sachdev P. Brain reserve and cognitive decline: a non- parametric systematic review. Psychol. Med. 2006;36(8):1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- Wadman M., et al. A rampage through the body. Science. 2020;368:356–360. doi: 10.1126/science.368.6489.356. [DOI] [PubMed] [Google Scholar]
- Widmann C.N., Heneka M.T. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13(6):630–636. doi: 10.1016/s1474-4422(14)70017-1. [DOI] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit A.R., Ezzati A., Zimmerman M.E., Lipton R.B., Lipton M.L., Katz M.J. Roles of hippocampal subfields in verbal and visual episodic memory. Behav. Brain Res. 2017;317:157–162. doi: 10.1016/j.bbr.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., et al. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588(7836):E6. doi: 10.1038/s41586-020-2951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]