Abstract
Introduction
Decreased immunosuppression has been proposed for kidney transplant recipients infected with coronavirus disease 2019 (COVID-19), but the impact on the alloreactive immune response during and after infection has been poorly investigated. We evaluated the occurrence of antihuman leukocyte antigen (HLA) donor-specific antibodies (DSAs) (post–COVID-19) and rejection episodes after COVID-19 with particular focus on immunosuppression modulation.
Methods
Kidney transplant recipients from 2 French institutions had anti-HLA antibody screening before and after COVID-19. Management of immunosuppression, rejection episodes, COVID-19 severity, inflammatory markers, and antiviral therapies were recorded.
Results
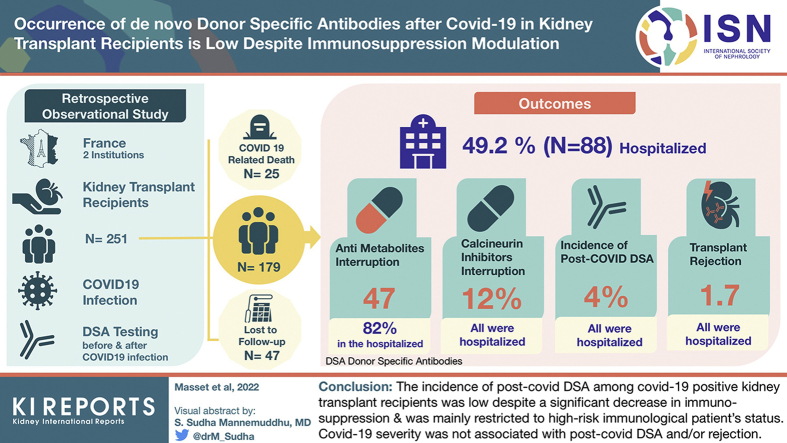
From 251 recruited patients, 72 were excluded because of COVID-19–related death (n = 25) and incomplete immunologic follow-up (n = 47). Among the remaining 179 included patients, almost half were hospitalized (49.2%). Antimetabolites were interrupted in 47% of patients (82% in hospitalized, median time of resumption of 23 days and in 15% nonhospitalized, median time of resumption of 7 days). Calcineurin inhibitors were interrupted in 12% of patients (all hospitalized, median time of resumption of 11 days). The incidence of post–COVID-19 DSA was 4% (8% and 0% in hospitalized and nonhospitalized, respectively). Allograft rejection occurred in 3 patients (1.7%) and all were hospitalized. Younger age, transplantation <1 year, and preexisting DSA were more frequently observed in patients with post–COVID-19 DSA, whereas inflammatory markers, lymphopenia, and use of antiviral therapies were not.
Conclusion
The incidence of post–COVID-19 DSA among COVID-19–positive kidney transplant recipients was low (4%) despite a significant decrease in immunosuppression and was mainly restricted to high-risk immunologic patient’s status. COVID-19 severity was not associated with post–COVID-19 DSA and/or rejection.
Keywords: allograft rejection, COVID-19, DSA
Graphical abstract
See Commentary on Page 937
COVID-19 infection/disease is a pandemic caused by SARS-CoV-2 with >5 million deaths attributable so far (end 2021). Greater severity of COVID-19 has been reported in kidney transplant recipients and is most likely owing to comorbidities and immunosuppressive therapy.1, 2, 3 The management of immunosuppression in kidney transplant recipients with COVID-19 varies between centers.4,5 For nonhospitalized patients with nonsymptomatic forms, immunosuppression can be slightly decreased or even maintained.6 For hospitalized patients, antimetabolite drugs such as mycophenolate mofetil and mycophenolic acid are often reduced or stopped whereas calcineurin inhibitors (CNIs) such as tacrolimus or cyclosporine are continued to avoid the occurrence of acute rejection, but also possibly because of potential anti–SARS-CoV-2 properties in vitro.7 For patients admitted to an intensive care unit (ICU) with severe forms, no consensus exists and immunosuppression may be completely discontinued. During SARS-CoV-2 infection, viral antigenic stimulation causes a cytokine storm involving high interleukin-6 levels, potentially leading to an alloreactive reaction against the graft.8,9 Nevertheless, adjunction of immunosuppressive therapies such as high-dose steroids or tocilizumab may prevent this alloimmune response.10 There are currently no data on the occurrence of HLA DSA, rejection episodes, or graft loss after COVID-19 infection in kidney transplant recipients. Our study aimed to search the occurrence of DSA, documented acute rejection episodes, and graft losses during and after COVID-19 in kidney transplant recipients, according to the clinical disease severity and the way immunosuppression was managed.
Methods
Studied Population
All kidney transplant recipients who were transplanted and followed-up in 2 French University Hospitals and who contracted COVID-19 between March 1, 2020, and April 30, 2021, were recruited. COVID-19 diagnosis was confirmed by real-time polymerase chain reaction on nasal or bronchoalveolar sampling or by positive SARS-CoV-2 serology result (presence of antinucleocapsid antibodies superior to laboratory threshold determined by electrochemiluminescence immunoassay (Roche Elecsys).
Study Design
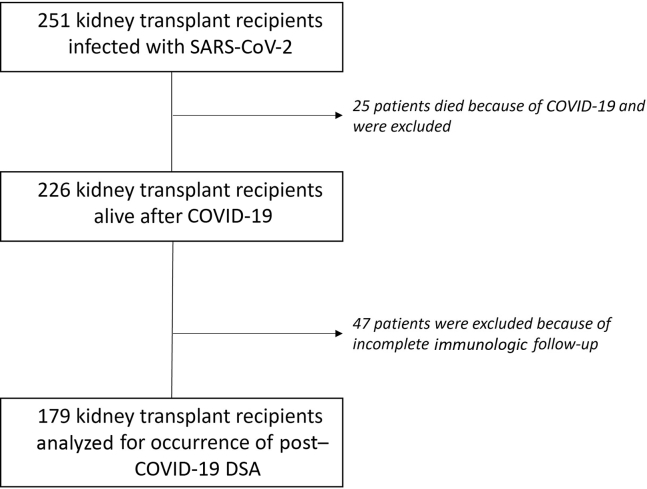
We conducted a retrospective cohort analysis of kidney transplant recipients and evaluated occurrence of new DSAs (post–COVID-19 DSA) and allograft rejection after COVID-19. As part of their standard follow-up, all included patients had regular anti-HLA screening. Deceased patients were excluded from the final analysis, as were patients with incomplete immunologic follow-up (defined as anti-HLA screening >24 months before COVID-19 and/or no anti-HLA screening after COVID-19). After COVID-19 resolution, patients underwent a complete check-up by their usual physician and were screened for anti-HLA antibodies. Anti-HLA immunization was compared before (last screening before COVID-19) and after COVID-19 (screening at disease resolution) for all patients with complete immunologic follow-up; notably, occurrence of post–COVID-19 DSA with their mean fluorescence index (MFI) and MFI evolution of pre-existing DSA. For patients diagnosed by serologic screening, we compared anti-HLA immunization before March 2020 (i.e., before occurrence of COVID-19) and after serologic diagnosis. Patients suspected of allograft rejection were indicated for allograft biopsy.
Anti-HLA Antibody Screening
We defined DSA according to the time of appearance as follows:
-
1.
Pre-existing DSA: presence of a DSA with MFI ≥1000 before transplantation.
-
2.
Post-transplant DSA: occurrence of a de novo DSA with MFI ≥1000 after transplantation but before SARS-CoV-2 infection.
-
3.
Post–COVID-19 DSA: occurrence of a de novo DSA with MFI ≥1000 after SARS-CoV-2 infection with no description in patient history at any MFI level.
Class I and II anti-HLA antibodies were measured by Luminex screening (Immucor or LABScreen—One lambda). Single antigen screening was then performed for positive cases, and the DSA’s MFI was evaluated (LABScreen—One lambda). All MFIs >1000 were included and noted. All sera were treated with EDTA to mitigate interference and the prozone effect. Patients with DSA before COVID-19 (pre-existing or post-transplant) were described based on the evolution of the MFI values, which were considered significant when the MFI values varied ≥25%.11
Management of Immunosuppressive Drugs
Global management of patients in both institutions was based on current guidelines, suggesting antimetabolite withdrawal for cases of COVID-19 requiring hospitalization and CNI withdrawal for patients admitted to the ICU. Nevertheless, management of immunosuppressive therapies during and after COVID-19 was left to the physicians’ discretion, balancing their patients’ risk for severe COVID-19 and immunologic complication. Treatment reduction, withdrawal, and resumption were recorded. If treatment had not been reintroduced yet, we considered the time from interruption to the time of anti-HLA antibody assessment.
Statistical Analysis
Comorbidities, clinical and biological characteristics, baseline immunosuppressive therapy, and COVID-19–specific therapies were also noted. Immunologic follow-up of patients was analyzed depending on COVID-19 severity (nonhospitalized patients followed by videoconference or phone call and hospitalized patients). Continuous variables were expressed as mean or median and categorical variables as total number (n) and percentage (%). For continuous variables, Student t tests or Wilcoxon tests were used; χ2 tests were used for qualitative variables. The significance threshold was set at 0.05 (2 tailed), and analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA) and R software.
Ethical Statement
Patients were included in the French SOT COVID Registry (approval number 02.26 of the Strasbourg University; registered at clinicaltrial.gov NCT04360707). Although the requirement for informed consent was waived, patients were informed on their inclusion in the registry, and those who declined to participate were deemed ineligible. The clinical and research activities reported here are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Results
Baseline Characteristics
In the study period, 251 transplant recipients were infected with SARS-CoV-2. A total of 25 died owing to COVID-19 (10%, average age of 67 years old), and 47 (18.7%) were excluded because of incomplete immunologic follow-up (1 patient because of anti-HLA screening >24 months before COVID-19 infection, all others because of lack of post–COVID-19 DSA screening). Among the 47 patients, 28 were not hospitalized (average age of 54 years old) and 19 were hospitalized (average age of 69 years old). None of them was suspected of allograft rejection during or after COVID-19.
Final analyses were conducted on 179 patients (Figure 1). There were 88 hospitalized (49%), and 21 (24%) of these required admission to the ICU because of mechanical ventilation or hemodialysis. Most (90%) of the patients were recipients of kidney transplant alone with 82% of primary transplantations, and an average time since transplantation of 7.5 years. Patients’ average age was 55 years old, 68% were male, and body mass index was 25.7 for nonhospitalized patients and 27.3 for hospitalized ones (P = 0.04). For hospitalized patients, the highest mean C-reactive protein (CRP) level was 80 mg/l (n = 81, SD = 65 mg/l), interleukin-6 was 249 pg/ml (n = 40, SD = 1243 pg/ml), and the mean lowest total lymphocyte count was 654/mm3 (n = 83, SD = 1043/mm3). Nearly all patients (90%) were under CNI maintenance therapy, 80% under antimetabolites, 15% under mTOR inhibitors, and 51% under oral steroids. In addition, 25 (14%) patients had preformed DSA before COVID-19 infection (i.e., pre-existing DSA and/or post-transplant DSA) and 38 (21%) had a history of biopsy-proven rejection.
Figure 1.
Flowchart of the study.
Among hospitalized patients, 45% (n= 40) received high-dose steroids (dexamethasone), 6% tocilizumab (n = 5), and 33% (n = 29) a combination of i.v. immunoglobulin (n = 5), chloroquine (n = 29), lopinavir/ritonavir (n = 17), remdesivir (n = 6), beta-interferon (n = 1) and eculizumab (n = 1). Overall, 35 hospitalized (40%) and 85 nonhospitalized patients (93%) did not receive any specific treatment for COVID-19. Table 1 summarizes the characteristics of the patients included in the study.
Table 1.
Clinical and biological characteristics of 179 analyzed patients with complete immune screening
| Characteristics | All (N = 179) |
Nonhospitalized (n = 91) |
Hospitalized (n = 88) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NA | n | % | NA | n | % | NA | n | % | ||
| Male recipient | 0 | 121 | 67.6 | 0 | 57 | 62.6 | 0 | 64 | 72.7 | 0.1997 |
| Transplant rank ≥2 | 0 | 32 | 17.9 | 0 | 13 | 14.3 | 0 | 19 | 21.6 | 0.2801 |
| Kidney transplant alone | 0 | 161 | 89.9 | 0 | 82 | 90.1 | 0 | 79 | 89.8 | 1 |
| Deceased donor | 0 | 146 | 81.6 | 0 | 68 | 74.7 | 0 | 78 | 88.6 | 0.0273 |
| HLA incompatibilities >4 | 9 | 29 | 17.1 | 6 | 17 | 20.0 | 3 | 12 | 14.1 | 0.4147 |
| Depleting induction | 3 | 90 | 51.1 | 3 | 44 | 50.0 | 0 | 46 | 52.3 | 0.8801 |
| Calcineurin inhibitor treatment | 1 | 161 | 90.4 | 1 | 81 | 90.0 | 0 | 80 | 90.9 | 1 |
| Belatacept treatment | 0 | 8 | 4.5 | 0 | 4 | 4.4 | 0 | 4 | 4.5 | 1 |
| mTOR inhibitor treatment | 0 | 27 | 15.1 | 0 | 11 | 12.1 | 0 | 16 | 18.2 | 0.3524 |
| Antimetabolite treatment | 0 | 144 | 80.4 | 0 | 76 | 83.5 | 0 | 68 | 77.3 | 0.3873 |
| Steroid treatment | 1 | 91 | 51.1 | 0 | 41 | 45.1 | 1 | 50 | 57.5 | 0.1319 |
| Diabetes history | 0 | 64 | 35.8 | 0 | 30 | 33.0 | 0 | 34 | 38.6 | 0.5253 |
| Cardiovascular history | 0 | 67 | 37.4 | 0 | 33 | 36.3 | 0 | 34 | 38.6 | 0.8623 |
| Neoplasia history | 0 | 44 | 24.6 | 0 | 21 | 23.1 | 0 | 23 | 26.1 | 0.7629 |
| Previous DSA | 0 | 14 | 7.8 | 0 | 7 | 7.7 | 0 | 7 | 8.0 | 1 |
| Episode of rejection | 0 | 38 | 21.2 | 0 | 17 | 18.7 | 0 | 21 | 23.9 | 0.5062 |
| Positive SARS-CoV-2 RT-PCR | 16 | 158 | 98.1 | 16 | 75 | 100 | 0 | 83 | 96.5 | 0.2488 |
| COVID-19 pneumonia | 1 | 84 | 47.2 | 1 | 7 | 7.8 | 0 | 77 | 87.5 | <0.0001 |
| AKI episode | 5 | 48 | 27.6 | 1 | 5 | 5.6 | 4 | 43 | 51.2 | <0.0001 |
| Requiring dialysis | 6 | 11 | 6.4 | 1 | 0 | 0 | 5 | 10 | 12.0 | — |
| Intensive care unit | 1 | 21 | 11.8 | 1 | 0 | 0 | 0 | 21 | 23.9 | — |
| Mechanical ventilation | 0 | 17 | 9.5 | 0 | 0 | 0 | 0 | 17 | 19.3 | — |
| Use of SARS-CoV-2–specific therapy | 1 | 59 | 33.1 | 1 | 6 | 6.7 | 0 | 53 | 60.2 | <0.0001 |
| High-dose steroids | 1 | 41 | 23 | 1 | 1 | 1.1 | 0 | 40 | 45.5 | <0.0001 |
| Tocilizumab | 3 | 5 | 2.8 | 1 | 0 | 0 | 2 | 5 | 5.8 | 0.0262 |
| Other |
1 |
34 |
19.1 |
1 |
5 |
5.6 |
0 |
29 |
33.0 |
<0.0001 |
| NA |
Mean |
SD |
NA |
Mean |
SD |
NA |
Mean |
SD |
P value |
|
| Recipient age (yr) | 1 | 55.5 | 13.5 | 0 | 54.1 | 13.0 | 1 | 57.0 | 13.9 | 0.1636 |
| Recipient BMI (kg m2) | 1 | 26.5 | 5.2 | 0 | 25.7 | 4.9 | 1 | 27.3 | 5.5 | 0.0433 |
| Time from transplantation (yr) | 0 | 7.4 | 7.6 | 0 | 8.4 | 8.1 | 0 | 6.5 | 7.0 | 0.0932 |
| Allograft function by MDRD (ml/min) | 3 | 58.5 | 58.3 | 0 | 64.6 | 77.8 | 3 | 52.0 | 22.5 | 0.1434 |
| Creatininemia (μmol/l) | 3 | 133.8 | 53.5 | 0 | 124.3 | 48.6 | 3 | 144.0 | 56.9 | 0.0148 |
| CRP highest level (mg/l) | 66 | 61.6 | 63.5 | 59 | 14.6 | 18.8 | 7 | 80.1 | 65.4 | <0.0001 |
| Lymphocyte lowest level (/mm3) | 60 | 793.6 | 974.6 | 54 | 1101.8 | 729.1 | 6 | 654.5 | 1041.4 | 0.0084 |
| IL-6 dosage (pg/ml) | 138 | 249.3 | 1211.9 | 90 | — | — | 48 | 249.3 | 1211.9 | — |
AKI, acute kidney injury defined by an increase of ≥50% of basal creatininemia; BMI, body mass index; CRP, C-reactive protein; DSA, donor-specific antibody; HLA, human leucocyte antigen; IL-6, interleukin-6; MDRD, modification of diet in renal diseases; RT-PCR, real-time polymerase chain reaction.
Bold emphasis has been used to clarify the statistical significant differences between groups.
Management of Immunosuppression Therapy During COVID-19 Infection
Among the deceased patients, CNI was interrupted in 54% and antimetabolites in 80% of the cases. Among patients with incomplete immunologic follow-up, CNI was never interrupted in nonhospitalized patients and in 25% of hospitalized ones (median resumption time was 8 days). Antimetabolites were interrupted in 5% of nonhospitalized patients and 73% of hospitalized ones (median resumption time was 27 days).
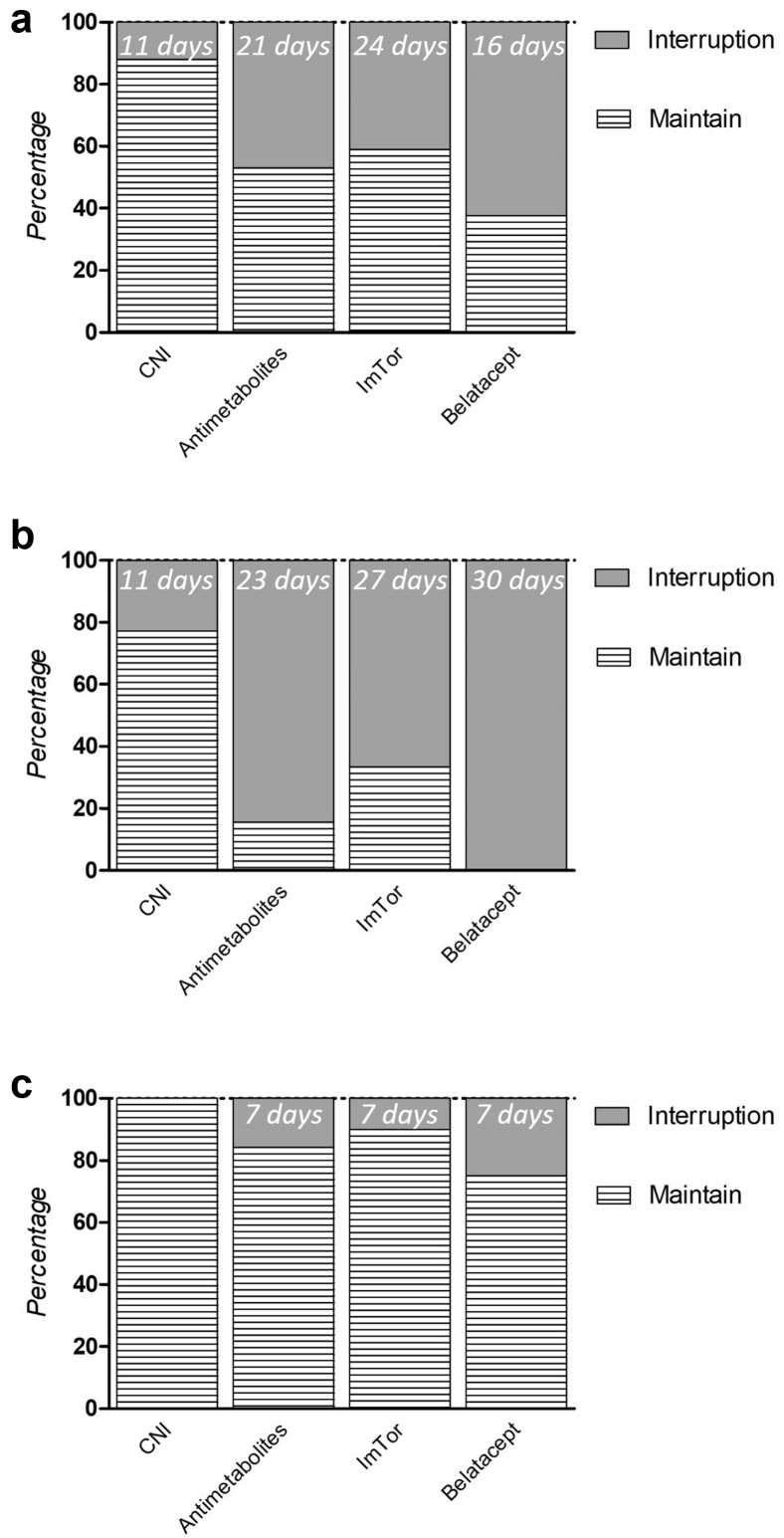
Among patients with complete immunologic follow-up, CNI interruption occurred in 12% (all hospitalized, representing 24% [n = 19] of hospitalized patients) with a median interruption time of 11 days and in none of the nonhospitalized patients. Interruption of antimetabolites occurred in 47% of the patients; 82% were hospitalized patients (n = 58) with a median interruption time of 23 days and in 15% of nonhospitalized ones (n = 12) with a median interruption time of 7 days. Of note, neither CNI nor antimetabolite dosage was reduced in patients without treatment interruption. mTOR inhibitors were interrupted in 41%; 62% of the hospitalized patients (n = 10) with a median interruption time of 27 days and in 9% of the nonhospitalized patients (n = 1) with an interruption time of 7 days. Finally, belatacept was discontinued in all hospitalized patients (n = 4) with a median resumption time of 30 days (i.e., belatacept perfusion was performed 30 days after than the theoretical day of injection) and discontinued in 1 nonhospitalized patient and resumed 7 days after (Figure 2a–c).
Figure 2.
Management of maintenance immunosuppressive drugs among 179 kidney transplant recipients infected with SARS-CoV-2 (a), depending on hospitalized status (b) or not (c). The percentage of immunosuppression withdrawal for each drug and their respective median time for resumption are illustrated. CNI, calcineurin inhibitor.
Occurrence of DSA, Rejection Episodes, and Graft Loss After COVID-19
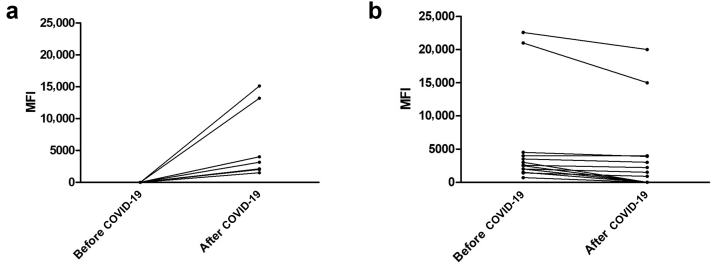
There were 7 patients (4%) who developed post–COVID-19 DSA, and 4 of them had a MFI >3000 (2.2%) (Figure 3a). Pre–COVID-19 screening for DSA was performed at a median of 212 days before SARS-CoV-2 infection (ranging from 2 to 701 days, Q25 = 95.5; Q75 = 309). Of note, only 1 patient of the 47 was excluded because of pre–COVID-19 screening longer than 24 months. Post–COVID-19 screening for DSA was performed at a median of 45 days after SARS-CoV-2 infection (ranging from 4 to 412, Q25 = 27; Q75 = 94.5). The large majority of excluded patients because of “incomplete immunologic follow-up” were because of lack of postinfection DSA screening (46 of 47). Of note, 4 patients had DSA screening <15 days after COVID-19 diagnosis because their diagnosis was made by serologic assessment. In the large majority of cases, the usual immunosuppressive treatment was resumed at post–COVID-19 DSA screening, with only 2 patients still on antiproliferative drug interruption. Screening for post–COVID-19 DSA occurred at a median of 66 days after CNI resumption for patients who had CNI withdrawal, and at a median of 18 days after antiproliferative resumption for patients who had antiproliferative withdrawal.
Figure 3.
(a) Occurrence of de novo anti-HLA DSA with their corresponding MFI in 7 hospitalized patients. (b) Evolution of the MFI of previously formed DSA after COVID-19 in 14 kidney transplant recipients. DSA, donor-specific antibody; HLA, human leucocyte antigen; MFI, mean fluorescence index.
All patients with post–COVID-19 DSA were hospitalized (2 were admitted in the ICU) resulting in an 8% incidence in this specific population. These were younger (45 vs. 58 years old, P = 0.17) and were considered at higher immunologic risk (shorter time from transplantation, 2.6 vs. 6.8 years, P = 0.06 and with a higher prevalence of previously formed DSA, 29% vs. 7%, P = 0.09). Inflammatory markers (C-reactive protein and interleukin-6 levels), total lymphocyte count, and COVID-19 severity (i.e., admission to the ICU) did not seem to differ between patients with or without post–COVID-19 DSA. The comparison between hospitalized patients with occurrence of post–COVID-19 DSA and those without is summarized in Table 2.
Table 2.
Characteristics of hospitalized patients depending on occurrence of post–COVID-19 DSA
| Characteristics | Hospitalized (n = 88) |
No post–COVID-19 DSA (n = 81) |
Post–COVID-19 DSA (n = 7) |
P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NA | n | % | NA | n | % | NA | n | % | ||
| Male recipient | 0 | 64 | 72.7 | 0 | 57 | 70.4 | 0 | 7 | 100 | 0.1825 |
| Calcineurin inhibitor treatment | 0 | 80 | 90.9 | 0 | 74 | 91.4 | 0 | 6 | 85.7 | 0.4996 |
| Belatacept treatment | 0 | 4 | 4.5 | 0 | 4 | 4.9 | 0 | 0 | 0 | 1 |
| mTOR inhibitor treatment | 0 | 16 | 18.2 | 0 | 16 | 19.8 | 0 | 0 | 0 | 0.3417 |
| Antimetabolite treatment | 0 | 68 | 77.3 | 0 | 63 | 77.8 | 0 | 5 | 71.4 | 0.6552 |
| Steroid treatment | 1 | 50 | 57.5 | 1 | 46 | 57.5 | 0 | 4 | 57.1 | 1 |
| Previous DSA | 0 | 7 | 8.0 | 0 | 5 | 6.2 | 0 | 2 | 28.6 | 0.0944 |
| Episode of rejection | 0 | 21 | 23.9 | 0 | 19 | 23.5 | 0 | 2 | 28.6 | 0.6699 |
| COVID-19 Pneumonia | 0 | 77 | 87.5 | 0 | 71 | 87.7 | 0 | 6 | 85.7 | 1 |
| AKI episode | 4 | 43 | 51.2 | 3 | 41 | 52.6 | 1 | 2 | 33.3 | 0.4274 |
| Requiring dialysis | 5 | 10 | 12.0 | 4 | 9 | 11.7 | 1 | 1 | 16.7 | 0.5490 |
| Intensive care unit | 0 | 21 | 23.9 | 0 | 19 | 23.5 | 0 | 2 | 28.6 | 0.6699 |
| Mechanical ventilation | 0 | 17 | 19.3 | 0 | 15 | 18.5 | 0 | 2 | 28.6 | 0.6165 |
| Use of SARS-CoV-2–specific therapy | 0 | 53 | 60.2 | 0 | 49 | 60.5 | 0 | 4 | 57.1 | 1 |
| High-dose steroids | 0 | 40 | 45.5 | 0 | 37 | 45.7 | 0 | 3 | 42.9 | 1 |
| Tocilizumab | 2 | 5 | 5.8 | 2 | 5 | 6.3 | 0 | 0 | 0 | 1 |
| Other |
0 |
29 |
33.0 |
0 |
27 |
33.3 |
0 |
2 |
28.6 |
1 |
| NA |
Mean |
SD |
NA |
Mean |
SD |
NA |
Mean |
SD |
P value |
|
| Recipient age (yr) | 1 | 57.0 | 13.9 | 1 | 58.0 | 12.8 | 0 | 45.3 | 21.4 | 0.1794 |
| Time from transplantation (yr) | 0 | 6.5 | 7.0 | 0 | 6.8 | 7.1 | 0 | 2.6 | 3.3 | 0.0610 |
| Allograft function by MDRD (ml/min) | 3 | 52.0 | 22.5 | 2 | 50.2 | 21.4 | 1 | 75.6 | 25.8 | 0.0201 |
| Creatininemia (μmol/l) | 3 | 144.0 | 56.9 | 2 | 146.2 | 57.0 | 1 | 115.4 | 51.0 | 0.1068 |
| CRP highest level (mg/l) | 7 | 80.1 | 65.4 | 6 | 80.8 | 66.4 | 1 | 72.0 | 55.1 | 0.8357 |
| Lymphocyte lowest level (/mm3) | 6 | 654.5 | 1041.4 | 5 | 655.9 | 1072.6 | 1 | 636.7 | 556.8 | 0.8100 |
| IL-6 dosage (pg/ml) | 48 | 249.3 | 1211.9 | 44 | 264.3 | 1260.1 | 4 | 65.1 | 67.0 | — |
| Time from CNI interruption | 0 | 13.7 | 11.3 | 0 | 13.9 | 11.9 | 0 | 12.7 | 9.1 | 1 |
| Time from antimetabolite interruption | 0 | 27.4 | 19.1 | 0 | 27.3 | 19.7 | 0 | 27.8 | 13.4 | 0.8407 |
AKI, acute kidney injury defined by an increase of 50% or more of basal creatininemia; BMI, body mass index; CNI, calcineurin inhibitor; CRP, C-reactive protein; DSA, donor-specific antibody; IL-6, interleukin 6; MDRD, Modification of Diet in Renal Diseases.
Bold emphasis has been used to clarify the statistical significant differences between groups.
Among the 7 patients with post–COVID-19 DSA, mycophenolate mofetil or mycophenolic acid was interrupted for all cases (average time of resumption was 27 days), and CNI was interrupted for 3 of 6 (average time of resumption was 13 days). There was 1 patient who did not receive conventional maintenance therapy (neither tacrolimus nor antimetabolites) owing to a recent life-threatening acute varicella-zoster virus (VZV) infection, followed by a chronic varicella-zoster virus replication complicated by severe cerebral vasculitis. There were 2 patients with post–COVID-19 DSA who received dexamethasone for treatment of COVID-19 and none received tocilizumab. In addition, 5 patients with post–COVID-19 DSA underwent kidney biopsy, 2 presented no sign of antibody-mediated rejection (ABMR), 2 had ABMR, and 1 had simultaneous ABMR and T-cell mediated rejection. Of the 3 patients with ABMR, 2 were treated resulting in clearance of DSA and stable allograft function, and the last patient was not treated owing to recent opportunistic infection (varicella-zoster virus infection). Table 3 summarizes the evolution and main clinical characteristics of all 7 patients with post–COVID-19 DSA. Of note, allograft biopsies were performed on 17 patients without post–COVID-19 DSA (11 indicated biopsies for sera creatininemia increase > 25% and/or occurrence of proteinuria12; 6 systematic post-transplant biopsies). Of those, only 3 histologic lesions were compatible with borderline rejection (no T-cell mediated rejection nor ABMR), but among them, only 1 was considered clinically relevant thus requiring steroid treatment (Supplementary Table S1).
Table 3.
Evolution of hospitalized patients with occurrence of post–COVID-19 DSA
| Characteristics | P1 | P2 | P3 | P4 | P5 | P6 | P7 |
|---|---|---|---|---|---|---|---|
| Sex | M | M | M | M | M | M | M |
| Age (yr) | 32 | 19 | 61 | 72 | 20 | 61 | 52 |
| Transplant rank | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Time from transplantation (yr) | 5 | 1 | 0 | 9 | 2 | 1 | 0 |
| Maintenance therapy | |||||||
| CNI | Yes | No | Yes | Yes | Yes | Yes | Yes |
| MMF | No | No | Yes | Yes | Yes | Yes | Yes |
| Steroids | Yes | Yes | No | No | Yes | Yes | No |
| Other | No | IV-Ig | No | No | No | No | No |
| ICU admission | No | No | Yes | No | No | No | No |
| CRP highest level (mg/l) | 16 | 5.2 | 95 | 61 | 147 | NA | 108 |
| Lymphocyte lowest count (/mm3) | 750 | 1600 | 120 | 550 | 710 | NA | 80 |
| Management of immunosuppression | |||||||
| CNI | Continue | — | Stop | Stop | Continue | Stop | Continue |
| MMF/MPA | — | — | Stop | Stop | Stop | Stop | Stop |
| Time from CNI resumption (d) | 0 | — | 14 | 3 | 0 | 21 | 0 |
| Time from MPA resumption (d) | — | — | 42 | 21 | 13 | 21 | 42 |
| COVID-19 treatment | None | None | DXM | Other | Other | None | DXM |
| Increase in SCr | No | Yesa | NAb | No | No | No | No |
| Proteinuria (g/24 h) | No | 0.4 | 1 | No | No | No | No |
| De novo DSA | |||||||
| Anti HLA class I | No | Yes | No | Yes | No | No | Yes |
| Anti HLA class II | Yes | No | Yes | No | Yes | Yes | Yes |
| Highest MFI | 4450 | 13180 | 2200 | 4000 | 2000 | 2000 | 11600 |
| Biopsy | No | Yes | Yes | Yes | Yes | Yes | No |
| Rejection | NA | ABMR | ABMR + TCMR | cABMR | None | None | NA |
| Treatment | None | None | Yesc | Yesd | IV-Ig | None | None |
| Evolution of allograft function | CKD I | CKD IV | CKD III | CKD II | CKD II | CKD I | CKD III |
ABMR, antibody-mediated rejection; cABMR, chronic antibody-mediated rejection; CNI, calcineurin inhibitor; CRP, C-reactive protein; DSA, donor-specific antibody; DXM, dexamethasone; ICU, intensive care unit; MFI, mean fluorescence index; MMF, mycophenolate mofetil; MPA, mycophenolic acid; TCMR, T-cell mediated rejection.
Serum creatininemia increased from 90 μmol/l to 130 μmol/l.
COVID-19 developed immediately after transplantation with delayed graft function during the first weeks post-transplantation.
Steroid pulse + plasma exchange + i.v. immunoglobulin.
Reinforcement of maintenance immunosuppressive therapy (i.e., increasing in CNI trough level objectives and antimetabolite dosage plus oral steroid therapy).
There were 14 patients who had DSA before COVID-19 (4 anti-HLA class I and 10 anti-HLA class II). After COVID-19, 8 of them experienced a significant decrease of their MFI value (≥25%) and 6 became negative (Figure 3b). Surprisingly, post–COVID-19 DSA occurred in 2 patients who had experiment disappearance of another post-transplant DSA. In the remaining 6 patients, MFI values remained similar before and after COVID-19.
Among the 179 evaluated and 47 excluded patients, only 3 allografts were lost: all of them owing to COVID-19–induced acute tubular necrosis requiring immediate dialysis because of previously impaired allograft function (chronic kidney disease stage 4 or 5).
Discussion
Modulation of immunosuppression (i.e., interruption or withdrawal) seems logical in cases of COVID-19 infection/disease after kidney transplantation. Whether this attitude results in a HLA alloreactive response and graft rejection is not known. We report here the outcomes of a large cohort of kidney transplant recipients with COVID-19, with special reference to post–COVID-19 DSA occurrence, graft rejection, graft loss, and immunosuppression reduction. The global incidence of post–COVID-19 DSA was 4% (7 from 179 patients). No post–COVID-19 DSA was observed in patients who were monitored by videoconference or by phone (nonhospitalized), whereas the incidence was 8% in patients who were hospitalized. In fact, the criteria for hospitalization were not defined and were mostly based on patient’s demand, not always reflecting the severity of the disease. Therefore, this difference should be interpreted with caution and may be just a result of hazard. In non–COVID-19 kidney transplant recipients, the average occurrence of de novo DSA was 5%, and this varied according to individual immune status (HLA mismatches, previous anti-HLA sensitization, history of rejection).13,14 In our COVID-19–positive population, younger age, onset of infection within the first year after transplantation, and presence of DSA before transplantation were possible risk factors for developing post–COVID-19 DSA, but we cannot exclude that those appeared after the last screening and before COVID-19 infection, especially because DSAs appeared in high immunologic risk patients. In contrary, inflammatory markers (interleukin-6 and C-reactive protein levels), total lymphocyte count, COVID-19 severity, and administration of antiviral therapies did not affect the occurrence of post–COVID-19 DSA. Regarding previously formed DSA (pre-existing and/or post-transplant), MFI value did not increase after infection and significantly decreased (and even vanished) in most of the cases thereafter, suggesting COVID-19 itself did not seem to be a major immunologic trigger. The use of tocilizumab was very low in our cohort (5 patients on 179, all were hospitalized). Its potential benefit for reducing anti-HLA antibodies is still on debate with various results lying on a small number of series.15,16 The low use of tocilizumab and the small number of post–COVID-19 DSA patients preclude any firm conclusion in our study, and further observations will be needed to evaluate this interesting point.
Since the beginning of the COVID-19 era, we took the decision to interrupt antimetabolite immunosuppressants for COVID-19–infected kidney transplant recipients. This was done according to clinical disease severity and after evaluating the risk and benefit balance in each case. Concerning CNI, no changes were made except for ICU admissions, where immunosuppression was almost always totally stopped. It is well demonstrated that early occurrence of dnDSA and acute rejection follows CNI withdrawal17 or CNI reduction18 in stable, low-immunologic risk kidney transplant recipients. In cases of BK virus-induced (BKV) nephropathy19,20 and/or post-transplant lymphoproliferative disorders,21, 22, 23 the reduction of immunosuppression was also followed by an increased risk of dnDSA and ABMR. Therefore, any change in immunosuppressive therapy during COVID-19 could also be associated with an immune response against the graft.
Despite a significant but almost transient decrease of immunosuppression, our global incidence of acute rejection was 1.7%. These episodes were only found in patients with post–COVID-19 DSA, except for 1 patient with borderline lesions and glomerular double contours, which appeared several months after COVID-19 and immunosuppression resumption. All episodes except one (a patient with concomitant severe infections) were treated and reverted, and no graft loss was observed. In addition, among the 47 patients with COVID-19 excluded because of incomplete immunologic follow-up, none experienced acute rejection. This low incidence of rejection may be however underestimated because no protocol biopsies were performed during COVID-19 infection when immunosuppression was modified. The occurrence of post–COVID-19 DSA in fact motivated the indication of graft histology (with or without renal impairment). Nevertheless, clinical outcomes and renal function remained uncomplicated in nearly all patients (with or without post–COVID-19 DSA) suggesting the absence of a potential alloreactive response. Finally, no immunologic-related graft loss was noted up until the patient’s last check-up.
Current guidelines for immunosuppression management in COVID-19 kidney transplant recipients are mainly based on expert opinion and mostly depend on infection severity. Interruption of antimetabolites seems legitimate during an active infection with deep lymphopenia, especially if CNI is maintained.24 The recent TANGO cohort analysis did not find any survival benefit for immunosuppression withdrawal in kidney transplant recipients with COVID-19.25 After liver transplantation, continuation of tacrolimus (but not antimetabolites) has been associated with better survival.26 Analysis from kidney transplant COVID-19 registries around the world did not reveal a clear benefit of immunosuppression withdrawal in occurrence with severe COVID-19 pneumonia.1,27, 28, 29 Moreover, it has been suggested that continuation of CNI during COVID-19 may have direct antiviral effects and could help to reduce the deleterious cytokine storm.30 Nevertheless, maintenance of immunosuppression during life-threatening infectious episodes may also increase nosocomial infections and cause death.31 Furthermore, pharmacodynamics and pharmacokinetics of immunosuppressants can be modified during severe infections which can lead to potential toxicity.32 In short, this question still remains without a clear answer and would need prospective evaluation.
Although we evaluated a large number of patients, we can unfortunately only provide a descriptive analysis with clinical significance. Statistics were not suitable because of the low number of events. Nevertheless, to our knowledge, this is the largest kidney transplant cohort to date, with a complete immunologic and immunosuppression management follow-up after COVID-19. A small series of 47 kidney transplant recipients recently described the absence of DSA 3 months after COVID-19 infection despite immunosuppression withdrawal.33
A major limitation of our study is the absence of a control group without COVID-19 infection. Nevertheless, such a control cohort is somewhat counterintuitive, as reducing or interrupting immunosuppression is not routinely done in the absence of a major reason. In addition, unnoticed COVID-19 infection during follow-up can be possible in some patients, unless repeated real-time polymerase chain reaction or serologic assessments are systematically performed, which in practice was not the case in our institutions. Regarding the occurrence of post–COVID-19 DSA, as alloantibody responses are a dynamic phenomenon, we could have missed transient changes in DSAs in the immediate post–COVID-19 period owing to the heterogeneous time of post–COVID-19 anti-HLA antibody screening. Moreover, we cannot exclude occurrence of another DSA at a later time (i.e., 1-year post–COVID-19) in patients evaluated in the early post–COVID-19 period, thus increasing the global 1-year incidence of post–COVID-19 DSA.11 Nevertheless, the possible appearance of these new DSAs would not necessarily appear to be related to the COVID-19 episode but could be considered as the described incidence of DSA in kidney transplant recipients, because their immunosuppressive therapy had been resumed post–COVID-19 DSA screening. Another limitation is the retrospective nature of our study. This is highlighted by the incomplete immunologic follow-up in approximately 20% of the total cohort and lack of uniform hospital admission criteria, which may have resulted in a bias in our final description of DSA occurrence after COVID-19. Finally, the absence of systematic biopsy protocols prevents any information on possible subclinical rejections after COVID-19.
In conclusion, kidney transplant patients with COVID-19 had an incidence of post–COVID-19 DSA and acute rejection of 4% and 1.7%, respectively. No immunologic-related graft loss was observed in the total cohort. The occurrence of post–COVID-19 DSA seemed more likely to be related to an immunologically higher patient’s risk status rather than immunosuppression modulation, alloimmune response induced by the virus, and any adjunctive antiviral therapy used. Transient interruption of immunosuppression for kidney transplant patients infected by COVID-19 seems to be safe, at least within the evaluated time of this study.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank all medical staff that took care of patients during the current COVID-19 pandemic. The authors also thank the clinical research associates who participated in the data collection. The analysis and interpretation of these data are the responsibility of the authors.
Footnotes
Table S1. Description of main histologic diagnosis in biopsies after COVID-19 in patients without DSA post–COVID-19 (note that 1 patient could have several histologic diagnoses).
Supplementary Material
Table S1. Description of main histologic diagnosis in biopsies after COVID-19 in patients without DSA post–COVID-19 (note that 1 patient could have several histologic diagnoses) (PDF).
References
- 1.Caillard S., Anglicheau D., Matignon M., et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillard S., Chavarot N., Francois H., et al. Is COVID-19 infection more severe in kidney transplant recipients? Am. J. Transplant. 2021;21:1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaunat O., Legeai C., Anglicheau D., et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT) Kidney Int. 2020;98:1568–1577. doi: 10.1016/j.kint.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kant S., Menez S.P., Hanouneh M., et al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol. 2020;21:449. doi: 10.1186/s12882-020-02112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maggiore U., Abramowicz D., Crespo M., et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant. 2020;35:899–904. doi: 10.1093/ndt/gfaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubetzky M., Aull M.J., Craig-Schapiro R., et al. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35:1250–1261. doi: 10.1093/ndt/gfaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbajo-Lozoya J., Müller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagedal S., Nordal K.P., Hartmann A., et al. The impact of Cytomegalovirus infection and disease on rejection episodes in renal allograft recipients: CMV infection and renal transplant rejection. Am J Transplant. 2002;2:850–856. doi: 10.1034/j.1600-6143.2002.20907.x. [DOI] [PubMed] [Google Scholar]
- 9.Jordan S.C., Choi J., Kim I., et al. Interleukin-6, a cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: therapeutic implications of IL-6 receptor blockade. Transplantation. 2017;101:32–44. doi: 10.1097/TP.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tambur A.R., Campbell P., Claas F.H., et al. Sensitization in transplantation: assessment of risk (STAR) 2017 working group meeting report. Am J Transplant. 2018;18:1604–1614. doi: 10.1111/ajt.14752. [DOI] [PubMed] [Google Scholar]
- 12.Special Issue: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 13.Hourmant M., Cesbron-Gautier A., Terasaki P.I., et al. Frequency and clinical implications of development of donor-specific and non–donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16:2804–2812. doi: 10.1681/ASN.2004121130. [DOI] [PubMed] [Google Scholar]
- 14.Senev A., Coemans M., Lerut E., et al. Eplet mismatch load and de novo occurrence of donor-specific anti-HLA antibodies, rejection, and graft failure after kidney transplantation: an observational cohort study. J Am Soc Nephrol. 2020;31:2193–2204. doi: 10.1681/ASN.2020010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J., Aubert O., Vo A., et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17:2381–2389. doi: 10.1111/ajt.14228. [DOI] [PubMed] [Google Scholar]
- 16.Jouve T., Laheurte C., Noble J., et al. Immune responses following tocilizumab therapy to desensitize HLA-sensitized kidney-transplant candidates. Am. J. Transplant. 2022;22:71–84. doi: 10.1111/ajt.16709. [DOI] [PubMed] [Google Scholar]
- 17.Dugast E., Soulillou J.P., Foucher Y., et al. Failure of calcineurin inhibitor (tacrolimus) weaning randomized trial in long-term stable kidney transplant recipients. Am J Transplant. 2016;16:3255–3261. doi: 10.1111/ajt.13946. [DOI] [PubMed] [Google Scholar]
- 18.Gatault P., Kamar N., Büchler M., et al. Reduction of extended-release tacrolimus dose in low-immunological-risk kidney transplant recipients increases risk of rejection and appearance of donor-specific antibodies: a randomized study. Am J Transplant. 2017;17:1370–1379. doi: 10.1111/ajt.14109. [DOI] [PubMed] [Google Scholar]
- 19.Dieplinger G., Everly M.J., Briley K.P., et al. Onset and progression of de novo donor-specific anti-human leukocyte antigen antibodies after BK polyomavirus and preemptive immunosuppression reduction. Transpl Infect Dis. 2015;17:848–858. doi: 10.1111/tid.12467. [DOI] [PubMed] [Google Scholar]
- 20.Cheungpasitporn W., Kremers W.K., Lorenz E., et al. De novo donor-specific antibody following BK nephropathy: the incidence and association with antibody-mediated rejection. Clin Transpl. 2018;32 doi: 10.1111/ctr.13194. [DOI] [PubMed] [Google Scholar]
- 21.Dierickx D., Habermann T.M. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–562. doi: 10.1056/NEJMra1702693. [DOI] [PubMed] [Google Scholar]
- 22.Reshef R., Vardhanabhuti S., Luskin M.R., et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder★: reduction of immunosuppression for PTLD. Am J Transplant. 2011;11:336–347. doi: 10.1111/j.1600-6143.2010.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swinnen L.J., LeBlanc M., Grogan T.M., et al. Prospective study of sequential reduction in immunosuppression, interferon alpha-2b, and chemotherapy for posttransplantation lymphoproliferative disorder. Transplantation. 2008;86:215–222. doi: 10.1097/TP.0b013e3181761659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mourer J.S., Hartigh Jd, van Zwet E.W., Mallat M.J.K., Dubbeld J., de Fijter J.W. Randomized trial comparing late concentration-controlled calcineurin inhibitor or mycophenolate mofetil withdrawal. Transplantation. 2012;93:887–894. doi: 10.1097/TP.0b013e31824ad60a. [DOI] [PubMed] [Google Scholar]
- 25.Cravedi P., Mothi S.S., Azzi Y., et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belli L.S., Fondevila C., Cortesi P.A., et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology. 2021;160:1151–1163.e3. doi: 10.1053/j.gastro.2020.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kates O.S., Haydel B.M., Florman S.S., et al. Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 2021;73:e4090–e4099. doi: 10.1093/cid/ciaa1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santeusanio A.D., Menon M.C., Liu C., et al. Influence of patient characteristics and immunosuppressant management on mortality in kidney transplant recipients hospitalized with coronavirus disease 2019 (COVID-19) Clin Transpl. 2021;35 doi: 10.1111/ctr.14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kute V.B., Bhalla A.K., Guleria S., et al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. 2021;105:851–860. doi: 10.1097/TP.0000000000003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willicombe M., Thomas D., McAdoo S. COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol. 2020;31:1145–1146. doi: 10.1681/ASN.2020030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canet E., Zafrani L., Azoulay É. The critically ill kidney transplant recipient. Chest. 2016;149:1546–1555. doi: 10.1016/j.chest.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 32.McMinn J., Black H., Harrison L.L., Geddes C. SARS-CoV-2 and tacrolimus blood concentration in kidney transplant recipients. Kidney Int Rep. 2021;6:2694–2697. doi: 10.1016/j.ekir.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anton Pampols P., Trujillo H., Melilli E., et al. Immunosuppression minimization in kidney transplant recipients hospitalized for COVID-19. Clin Kidney J. 2021;14:1229–1235. doi: 10.1093/ckj/sfab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.